Abstract
Background:
Various adjuvants are being used with local anesthetics for prolongation of intraoperative and postoperative analgesia. Dexmedetomidine, the highly selective 2 adrenergic agonist is a new neuraxial adjuvant gaining popularity.
Settings and Design:
The study was conducted in prospective, double blind manner. It included 120 American Society of Anesthesiology (ASA) class I and II patients undergoing lower limb surgery under spinal anesthesia after approval from hospital ethics committee with written and informed consent of patients.
Materials and Methods:
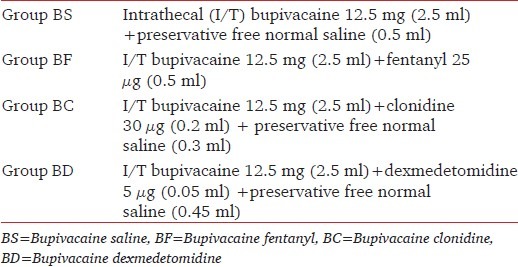
The patients were randomly allocated into four groups (30 patients each). Group BS received 12.5 mg hyperbaric bupivacaine with normal saline, group BF received 12.5 mg bupivacaine with 25 g fentanyl, group BC received 12.5 mg of bupivacaine supplemented 30 g clonidine, and group BD received 12.5 mg bupivacaine plus 5 g dexmedetomidine. The onset time to reach peak sensory and motor level, the regression time of sensory and motor block, hemodynamic changes, and side effects were recorded.
Results:
Patients in Group BD had significantly longer sensory and motor block times than patients in Groups BC, BF, and BS with Groups BC and BF having comparable duration of sensory and motor block. The mean time of two segment sensory block regression was 147 ± 21 min in Group BD, 117 ± 22 in Group BC, 119 ± 23 in Group BF, and 102 ± 17 in Group BS (P > 0.0001). The regression time of motor block to reach modified Bromage zero (0) was 275 ± 25, 199 ± 26, 196 ± 27, 161 ± 20 in Group BD, BC, BF, and BS, respectively (P > 0.0001). The onset times to reach T8 dermatome and modified Bromage 3 motor block were not significantly different between the groups. Dexmedetomidine group showed significantly less and delayed requirement of rescue analgesic.
Conclusions:
Intrathecal dexmedetomidine is associated with prolonged motor and sensory block, hemodynamic stability, and reduced demand of rescue analgesics in 24 h as compared to clonidine, fentanyl, or lone bupivacaine.
Keywords: α2, adrenoreceptor agonist, bupivacaine, clonidine, dexmedetomidine, fentanyl, spinal anesthesia
Introduction
Subarachnoid blockade is the most commonly used regional anesthetic technique for lower limb surgery. Various adjuncts are being used with local anesthetics for prolongation of intraoperative and postoperative analgesia. However, there use is thwarted either due to the adverse effects of adjuvants or unreliable postoperative analgesia.
Most of the clinical studies about the intrathecal α2 adrenergic agonist are related to clonidine.[1] Dexmedetomidine, a highly selective α2 adrenergic agonist has evolved as a panacea for various applications and procedures in the perioperative and critical care settings.[2] It is also emerging as a valuable adjunct to regional anesthesia and analgesia, where gradually evolving studies can build the evidence for its safe use in central neuraxial blocks.[3] Based on earlier human studies, it is hypothesized that intrathecal 5 μ g dexmedetomidine would produce more postoperative analgesic effect with hyperbaric bupivacaine in spinal anesthesia with minimal side effects.[4,5,6,7]
In view of few evidences[4,5,6,7] of dexmedetomidine's efficacy as an adjuvant to hyperbaric bupivacaine in spinal anesthesia, we strived to explore its usefulness and also compare this new α2 adrenergic agonist with the previously established and widely used adjuncts clonidine and fentanyl on the spinal block characteristics in patients scheduled for lower limb surgery.
Materials and Methods
After obtaining approval from the Hospital Ethics Committee alongwith written and informed consent, 120 adults of either sex belonging to American Society of Anesthesiology (ASA) classI and II and scheduled for lower limb surgery under subarachnoid block, were enrolled in this prospective, randomized, and double blinded study. Patients with contraindication to regional anesthesia, history of significant coexisting diseases like ischemic heart disease, hypertension, impaired renal functions, rheumatoid arthritis, and severe liver disease where excluded from the study. Presence of pregnant patients, chronic alcoholics and malnourished patients, atrioventricular block, incomplete or partial heart blocks, intake of -blockers also precluded us from considering these patients for the study.
All patients were examined and investigated a day prior to surgery, and were familiarized with visual analogue scale (VAS)[8] and its use for measuring the postoperative pain. They were advised fasting for 6 h and received alprazolam 0.5 mg as premedication a night before and 0.25 mg in morning on the day of the surgery.
Intraoperative
In the operation theatre electrocardiogram (ECG), pulse oximetry, and noninvasive blood pressure were attached and baseline parameters were recorded and monitoring was initiated. Intravenous (IV) access was secured and all patients were preloaded with ringer lactate 10 ml/kg. These patients were randomly assigned using sealed envelope technique to either of the four groups in a double blind manner. The various treatment groups were as per Table 1.
Table 1.
Grouping for the study
The study solutions were prepared in a 5 ml syringe by an anesthesiologist who then handed them over in a coded form to the attending anesthesiologist blinded to the nature of drug given to him/her. Subarachnoid block was administered at the L2-3 or L3-4 vertebral level using 26-gauge Quincke spinal needle with patients in the sitting position under all aseptic precautions. Patients were made supine following the block. The anesthesiologist performing the block recorded the intraoperative data.
The onset and duration of sensory block, highest level of sensory block, time to reach the highest dermatomal level of sensory block, motor block onset, time to complete motor block recovery, and duration of spinal anesthesia were recorded. The onset of sensory block was defined as the time between injection of intrathecal anesthetic and the absence of pain at the T8 dermatome assessed by sterile pinprick every 2 min till T8 dermatome was achieved. The highest level of sensory block was evaluated by pinprick at midclavicular line anteriorly every 5 min for 20 min after the injection, thereafter every 15 min.
The duration of sensory block was defined as the time of regression of two segments in the maximum block height, evaluated by pinprick. The motor level was assessed according to modified Bromage score:[9] Bromage 0, the patient is able to move the hip, knee, and ankle; Bromage 1, the patient is unable to move the hip, but is able to move the knee and ankle; Bromage 2, the patient is unable to move hip and knee, but is able to move the ankle; and Bromage 3, the patient is unable to move the hip, knee, and ankle. Time for motor block onset was defined as modified Bromage score of 3. Complete motor block recovery was assumed when modified Bromage score was 0.
The duration of spinal anesthesia was defined as the period from spinal injection to the first occasion when the patient complained of pain in the postoperative period. All durations were calculated considering the time of spinal injection as time zero.
Surgery was allowed to commence on achieving adequate sensory block height (T8). Vitals were recorded 5 min before intrathecal injection; 5, 10, 15, 20, and 25 minutes after and subsequently every 15 minutes. Pain scores using VAS were recorded 5 min before intrathecal injection, after the start of surgery, and subsequently every 15 min till the surgery was over; and thereafter VAS was assessed in the postoperative period. IV fluids were given to maintain the blood pressure.
Hypotension was defined as a decrease in systolic blood pressure (SBP) by 30% from baseline and was treated with IV boluses of 6 mg ephedrine or crystalloid fluids. Heart rate (HR) less than 50 beats/min was corrected using 0.6 mg of IV atropine sulfate. The incidence of pruritus, nausea, vomiting, and sedation were recorded. De Kock sedation scale[10] was used: 1 = patient somnolent but responding to verbal commands; 2 = patient somnolent, not responding to verbal commands but responding to manual stimulation; and 3 = patient somnolent, not responding to verbal commands or manual stimulation.
Postoperative
Motor block recovery (modified Bromage score of zero), sensory block regression were assessed every 15 min after completion of surgery till the time of regression of two segments in maximum block in the post-anesthetic care unit (PACU) along with the vital signs and VAS scores. Any patient showing VAS more than or equal to 3 was administered a supplemental dose of IV. tramadol 50 mg. The amount required by the patients in the next 24 h was recorded in all the groups.
Statistical analysis
Data obtained were tabulated and analyzed using Statistical Package for Social Science (SPSS 15.0 evaluation version). To calculate the sample size, a power analysis of = 0.05 and = 1.00 showed that 30 patients were needed per study group to detect an increase of 30 min difference between the median duration of spinal sensory block between the groups. Data was expressed as means and standard deviation (SD), medians and ranges, or numbers and percentages. For categorical covariates (sex, ASA class, nausea/vomiting, use of additive analgesia, hypotension, and bradycardia) Chi-square test or Fisher's exact test was used as appropriate, with P value reported at the 95% confidence interval (CI). Continuous covariates (age, duration of surgery) were compared using analysis of variance (ANOVA). If P value was significant, then Tukey's honestly significant difference (HSD) post hoc multi-comparison test was applied to see the significance between each pair of groups.
Results
All patients (n = 120) completed the study; there was no statistical difference in patients demographics or duration of surgery as shown in Table 2. Table 3 shows the number of patients in each group undergoing different types of lower limb surgeries. The numbers of patients under each type of surgery performed on the lower limb were similar amongst the groups thereby keeping the comparison unbiased.
Table 2.
Patients demographics
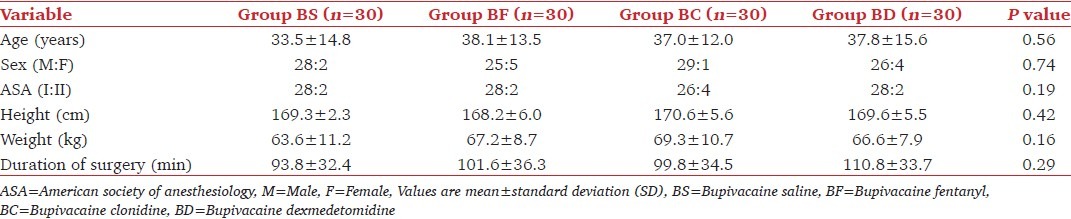
Table 3.
Type of lower limb surgeries performed
When compared the time of onset of both, sensory and motor block was statistically insignificant in all the four groups (P < 0.05) [Table 4]. T6 was the highest level of sensory block attained at 10.1 ± 3.5, 9.6 ± 2.9, 9.5 ± 3.0, 10.3 ± 3.3 min after injection in 26.6, 13.3, 23.3, and 26.7% patients in group BS, BF, BC, and BD; respectively. However; 63.3, 80.0, 73.3, and 70.0% of patients in groups BS, BF, BC, and BD had sensory block to a level of T8 at 7.8 ± 1.8, 8.6 ± 1.5, 8.3 ± 2.8, 8.3 ± 2.4 min after the injection (statistically insignificant). T8 sensory level was achieved in all patients. However, there were patients with level progressing further to the highest sensory level of T6.
Table 4.
Characteristics of spinal block

The duration of sensory and motor block was significantly prolonged in group BD as compared to other groups (P > 0.0001). Group BS had a statistically significant shorter duration of both sensory and motor block when compared with BF, BC, and BD (P > 0.0001). However, group BC and BF were comparable with no statistical differences between these two groups [Table 4].
The duration of spinal anesthesia was shorter in group BS as compared to the other groups with significantly delayed requirement in the group BD (P > 0.0001) [Table 4].
The mean values of mean arterial pressure (MAP) and heart rate (HR) were comparable between the four groups throughout the intraoperative and postoperative periods [Figures 1 and 2]. None of the patients experienced respiratory distress at any point of time. All patients had peripheral oxygen saturation (SpO2) greater than 96% at all the times and did not require additional oxygen in PACU.
Figure 1.
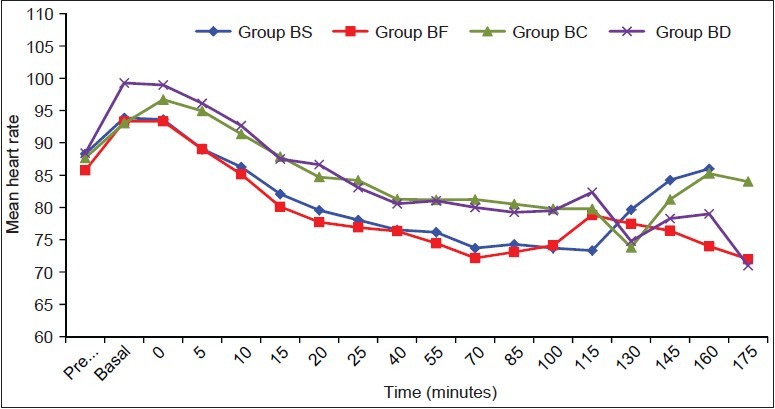
Heart rate (HR) values are mean ± standard deviation (SD). No significant differences were noted between the groups
Figure 2.
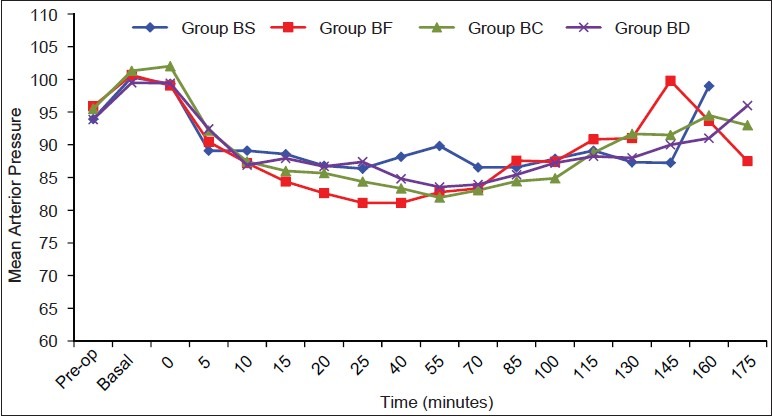
Mean arterial pressure (MAP) values are mean ± SD. No significant differences were noted between the groups
No significant difference was observed in the sedation scores with patients in all groups having score of 1. Pruritus was observed only in group BF in four patients (13.3%) at different intervals of time, but it did not reach statistical significance (P = 0.10). In group BS, one patient had nausea score = 4 at 5 min and two patients in group BC had nausea score = 4 at 15 and 55 min and required treatment intraoperatively (P = 0.36). However, one patient in group BF had postoperative vomiting requiring treatment with ondansetron. Two of the patients in the group BC and one patient in group BD had bradycardia and required treatment with atropine (P > 0.05). There was no incidence significant hypotension or respiratory depression in patientsin any of the groups.
Lower VAS values (>3) were observed in all the groups during the whole duration of the surgery and none of the patients required additional analgesics intraoperatively. Postoperative VAS scores and total analgesic requirement in 24 h were minimal in group BD (P value: BD vs BF - 0.009, BD vs. BC - 0.05). Group BS had a statistically significant requirement of rescue analgesic as compared to group BF, BC, and BD with the P value of 0.04, 0.008, and 0.005, respectively. Group BF and BC were comparable in total analgesic requirement over 24 h [Figure 3].
Figure 3.
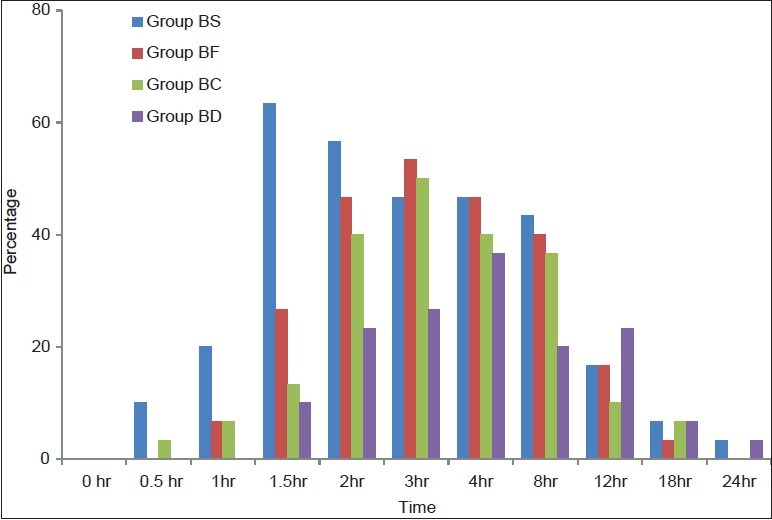
Trends in postoperative requirement of rescue analgesic. Values are in percentage of patients requiring rescue analgesics at different time intervals
Discussion
Our study compared three drugs in comparison to studies of other investigators who have compared dexmedetomidine with either one of the adjuncts only. We also evaluated the analgesic efficacy of intrathecal dexmedetomidine which has been hitherto report in literature previously by only one study.[7] The results of our study show that supplementation of spinal bupivacaine with 5 g dexmedetomidine significantly prolonged both sensory and motor block compared with intrathecal 25 g fentanyl and 30 g clonidine. Quality of analgesia significantly improved with use of dexmedetomidine as an adjuvant when compared to groups containing fentanyl and clonidine or lone bupivacaine.
The mechanism by which intrathecal α2 adrenoreceptor agonists prolong the motor and sensory block of local anesthetics is at the best, speculative. It may be an additive or synergistic effect secondary to the different mechanisms of action of the local anesthetics and intrathecal α2 adrenoreceptor agonists. Local anesthetics act by blocking sodium channels. α2 adrenoreceptor agonists act by binding to the presynaptic C-fibers and postsynaptic dorsal horn neurons. They produce analgesia by depressing release of C-fiber transmitters and by hyperpolarization of post synaptic dorsal horn neurons.[4,5,11] The complementary action of local anesthetics and α2 adrenoreceptor agonists accounts for their profound analgesic properties. The prolongation of the motor block of spinal anesthetics may be the result of binding of α2 adrenoreceptor agonists to the motor neurons in the dorsal horn.[4,5] Dexmedetomidine is eight times more specific andhighly selective α2 adrenoreceptor agonists compared to clonidine, thereby making it a useful and safe adjunct in diverse clinical applications.[12,13]
The use of dexmedetomidine has been studied as an epidural adjunct by various authors who have observed its synergism with local anesthetics. It is observed to prolong the motor/sensory block duration time and postoperative analgesia without any additional morbidity.[14,15] Clinical studies exhibit potentiation of neuraxial local anesthetics, decrease in intraoperative anesthetic requirements with prevention of intraoperative awareness, improved intraoperative oxygenation, and postoperative analgesia when epidural or caudal dexmedetomidine was used in conjunction with general anesthesia.[16,17,18]
Most of the clinical experience gained in the use of intrathecal α2 adrenoreceptor agonists has been described with clonidine[19,20,21,22] and there has been a need for clinical studies related to intrathecal dexmedetomidine to prove its efficacy, safety, and the suitable dose for supplementation to spinal local anesthetics. In our study, the intrathecal dose of dexmedetomidine selected was based on previous human studies wherein no neurotoxic effects have been observed.[4,5,6] Kanazi et al.,[4] found that 3g dexmedetomidine or 30 g clonidine added to 13 mg spinal bupivacaine produced same duration of sensory and motor block with minimal side effects in urological surgical patients. On the basis of this, we assumed that 3-5 g of dexmedetomidine is equipotent to 30-45 g clonidine when used for supplementation of spinal bupivacaine.
Time of onset of sensory block was comparably similar among all the groups. These findings were in concordance with the results of Al Ghanem et al.,[5] who observed no difference in the onset time in patients receiving dexmedetomidine (7.5 ± 7.4 min) and fentanyl (7.4 ± 3.3 min) as adjuvants to isobaric bupivacaine (P = 0.95). The onset times observed in the study conducted by Al Ghanem et al., were relatively shorter than those observed by us which can be attributed to their use of isobaric bupivacaine, difference in definition of onset time (T10 dermatome vs T8 in our study), and differences in patient positioning (lithotomy vs supine in our study). Similarly, comparable time of onset of sensory block among study groups was also observed by Kanazi et al.,[4] when comparing 3 g of dexmedetomidine with 30 g of clonidine and Gupta et al.,[7] on comparison of 5 g dexmedetomidine with 25 g fentanyl when used as adjuvants to isobaric and hyperbaric bupivacaine, respectively. These authors also observed significantly prolonged two sensory segment regressions in the dexmedetomidine group as observed in our study.
The intrathecal 5 g dexmedetomidine used in our study had shown comparable onset of motor block with significantly prolonged duration ofmotor block, which is in consonance with the results observed by investigators in comparison to various adjuvants (clonidine, fentanyl, and sufentanil) used in their studies.[4,5,6,7,23] The duration of motor block as observed in our study was markedly prolonged (273.3 ± 24.6 min) when compared to the duration of motor block of 250 ± 76 min in Kanazi et al.,'s study (P > 0.001) and 240 ± 64 min in Al Ghanem et al.,'s study (P > 0.001), which could be attributed to higher intrathecal volume of drug (3 ml) used in our study as compared to 1.9 and 2.5 ml drug used in the respective studies.
We noted significantly delayed requirement of rescue analgesic and significantly reduced 24 h rescue analgesic requirement with 5 μg dexmedetomidine when compared to 30 μg clonidine (P = 0.05) and 25 μg fentanyl (P = 0.009) which supports the analgesic efficacy of dexmedetomidine as an intrathecal adjunct. Similarly, significantly improved analgesic efficacy was seen by Gupta et al., on comparison of dexmedetomidine and fentanyl as intrathecal adjuvant (P > 0.001).[7] Al-Mustafa et al.,[6] and Hala EA Eid et al.,[24] observed dose dependent prolongation of motor and sensory blockade with reduced analgesic requirement with increasing dosages of intrathecal dexmedetomidine (5, 10, and 15 μg).
The most significant side effects reported about the use of intrathecal α2 adrenoreceptor agonists are bradycardia and hypotension.[25] In the present study, these side effects were not significant probably because we used small dose of intrathecal dexmedetomidine, clonidine, and fentanyl with high dose local anesthetics. These doses of adjuvants used in our study did not affect the near maximal sympatholysis caused by local anesthetics. Small dosages of adjuvants may also be responsible for minimal or no sedation observed in any of the groups in the study. The 15 μg intrathecal dose of dexmedetomidine used by Hala EA Eid et al.,[24] showed significantly higher sedation scores which can be beneficial for patients undergoing lengthy complex surgeries as an alternative to epidural or prolonged general anesthetics and can preclude the use of IV sedatives. However, such high sedation scores may be harmful in elderly and high risk surgical patients owing to the risk associated with excessive sedation and respiratory depression. Pruritus after intrathecal fentanyl is known and was observed in a few patients but was not significant.
Although this study adds to the current knowledge on dexmedetomidine, the results should be considered taking into consideration the obvious limitations: The population involved includes the young and otherwise healthy patients and the effect in older patients with cardiovascular comorbidities are yet to be investigated. This study also lacks an active control for systemic dexmedetomidine effect. Hence, further studies that compare the effect of intrathecal and IV dexmedetomidine on the spinal bupivacaine may also be warranted.
Thus, as the renewed interest in regional anesthesia techniques grows, especially for the prolongation of excellent quality of intraoperative and postoperative analgesia with minimal side effects, use of intrathecal dexmedetomidine as an adjuvant to local anesthetics is evolving gradually and further clinical studies are proving its efficacy and safety and will be determining the suitable dosages of dexmedetomidine required for supplementation of spinal local anesthetics.
To conclude, our study report shows that the use of intrathecal dexmedetomidine as an adjuvant to bupivacaine seems it to be an attractive alternative to fentanyl and clonidine for long duration surgical procedures due to its profound intrathecal anesthetic and analgesic properties combined with minimal side effects. However, prolonged duration of motor blockade with dexmedetomidine may be undesirable for short-term surgical procedures or ambulatory surgeries.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Elia N, Culebras X, Mazza C, Schiffer E, Tramer MR. Clonidine as an adjuvant to intrathecal local anesthetics for surgery: Systematic review of randomized trials. Reg Anesth Pain Med. 2008;33:159–67. doi: 10.1016/j.rapm.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Grewal A. Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol. 2011;27:297–302. doi: 10.4103/0970-9185.83670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantz J, Josserand J, Hamada S. Dexmedetomidine: New insights. Eur J Anaesthesiol. 2011;8:3–6. doi: 10.1097/EJA.0b013e32833e266d. [DOI] [PubMed] [Google Scholar]
- 4.Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50:222–7. doi: 10.1111/j.1399-6576.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 5.Al Ghanem SM, Massad IM, Al-Mustafa MM, Al-Zaben KR, Qudaisat IY, Qatawneh AM, et al. Effect of adding dexmedetomidine versus fentanyl to intrathecal bupivacaine on spinal block characteristics in gynecological procedures: A double blind controlled study. Am J Appl Sci. 2009;6:882–7. [Google Scholar]
- 6.Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, Murshidi MM, Ammari BA, Awwad ZM, et al. Effect of dexmedetomidine added to spinal bupivacaine for urological procedures. Saudi Med J. 2009;30:365–70. [PubMed] [Google Scholar]
- 7.Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK. A Comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to Bupivacaine. J Anaesthesiol Clin Pharmacol. 2011;27:339–43. doi: 10.4103/0970-9185.83678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katz J, Melzack R. Measurement of pain. Surg Clin North Am. 1999;79:231–52. doi: 10.1016/s0039-6109(05)70381-9. [DOI] [PubMed] [Google Scholar]
- 9.Bromage PR. A comparison of the hydrochloride and carbon dioxide salts of lidocaine and prilocaine in epidural analgesia. Acta Anaesthesiol Scand Suppl. 1965;16:55–69. doi: 10.1111/j.1399-6576.1965.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 10.De Kock M, Wiederkher P, Laghmiche A, Schottes JL. Epidural clonidine used as a sole analgesic agent during and after abdominal surgery: A dose-response study. Anesthesiology. 1997;86:285–92. doi: 10.1097/00000542-199702000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Lawhead RG, Blaxall HS, Bylund BD. Alpha-2A is the predominant -2 adrenergic receptor subtype in human spinal cord. Anesthesiology. 1992;77:983–91. doi: 10.1097/00000542-199211000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: A novel sedative- analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murthy TV, Singh R. Alpha 2 adrenoceptor agonist-dexmedetomidine role in anaesthesia and intensive care: A clinical review. J Anaesth Clin Pharmacol. 2009;25:267–72. [Google Scholar]
- 14.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salgado PF, Sabbag AT, Silva PC, Brienze SL, Dalto HP, Modolo NS, et al. Synergistic effect between dexmedetomidine and 0.75% ropivacaine in epidural anesthesia. Rev Assoc Med Bras. 2008;54:110–5. doi: 10.1590/s0104-42302008000200011. [DOI] [PubMed] [Google Scholar]
- 16.Elhakim M, Abdelhamid D, Abdelfattach H, Magdy H, Elsayed A, Elshafei M. Effect of epidural dexmedetomidine on intraoperative awareness and post-operative pain after one-lung ventilation. Acta Anaesthesiol Scand. 2010;54:703–9. doi: 10.1111/j.1399-6576.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- 17.Saadawy I, Boker A, Elshahawy MA, Almazrooa A, Melibary S, Abdellatif AA, et al. Effect of dexmedetomidine on the characteristics of bupivacaine in a caudal block in pediatrics. Acta Anaesthesiol Scand. 2009;53:251–6. doi: 10.1111/j.1399-6576.2008.01818.x. [DOI] [PubMed] [Google Scholar]
- 18.El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth. 2009;103:268–74. doi: 10.1093/bja/aep159. [DOI] [PubMed] [Google Scholar]
- 19.Racle JP, Benkhadra A, Poy JY, Gleizal B. Prolongation of isobaric bupivacaine spinal anesthesia with epinephrine and clonidine for hip surgery in the elderly. Anesth Analg. 1987;66:442–6. doi: 10.1213/00000539-198705000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Niemi L. Effects of intrathecal clonidine on duration of bupivacaine spinal anesthesia, hemodynamics, and postoperative analgesia in patients undergoing knee arthroscopy. Acta Anaesthesiol Scand. 1994;38:724–8. doi: 10.1111/j.1399-6576.1994.tb03985.x. [DOI] [PubMed] [Google Scholar]
- 21.De Kock M, Gautier P, Fanard L, Hody JL, Lavand’homme P. Intrathecal ropivacaine and clonidine for ambulatory arthroscopy: A dose-response study. Anesthesiology. 2001;94:574–8. doi: 10.1097/00000542-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Mervivirta R, Kuusniemi K, Jaakkola P, Pihlajamaki K, Pitkanen M. Unilateral spinal anesthesia for outpatient surgery: A comparison between hyperbaric bupivacaine and bupivacaine-clonidine combination. Acta Anaesthesiol Scand. 2009;53:788–93. doi: 10.1111/j.1399-6576.2009.01955.x. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim FA. A comparative study of adding intrathecal dexmedetomidine versus sufentanil to heavy bupivacaine for postoperative analgesia in patients undergoing inguinal hernia repair. Benha M.J. 2009;26:207–17. [Google Scholar]
- 24.Hala EA, Shafie MA, Youssef H. Dose-related prolongation of hyperbaric bupivacaine spinal anesthesia by dexmedetomidine. Ain Shams J Anesthesiol. 2011;4:83–95. [Google Scholar]
- 25.Eisenach JC, De Kock M, Klimscha W. Alpha 2 -adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995) Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]


