Abstract
Inflammatory skin diseases such as atopic dermatitis (AD) and rosacea were complicated by barrier abrogation and deficiency in innate immunity. The first defender of epidermal innate immune response is the antimicrobial peptides (AMPs) that exhibit a broad-spectrum antimicrobial activity against multiple pathogens, including Gram-positive and Gram-negative bacteria, viruses, and fungi. The deficiency of these AMPs in the skin of AD fails to protect our body against virulent pathogen infections. In contrast to AD where there is a suppression of AMPs, rosacea is characterized by overexpression of cathelicidin antimicrobial peptide (CAMP), the products of which result in chronic epidermal inflammation. In this regard, AMP generation that is controlled by a key ceramide metabolite S1P-dependent mechanism could be considered as alternate therapeutic approaches to treat these skin disorders, i.e., Increased S1P levels strongly stimulated the CAMP expression which elevated the antimicrobial activity against multiple pathogens resulting the improved AD patient skin.
Keywords: Cathelicidin antimicrobial peptide, Atopic dermatitis, Endoplasmic reticulum stress, Sphingosine 1-phosphate, Innate immunity
INTRODUCTION
The skin of atopic dermatitis (AD) is highly susceptible to colonization by Staphylococcus aureus. The defense system of the skin against bacterial invasion is significantly disrupted in AD. The high incidence of S. aureus colonization was also observed in the decrease of IgA and the slight change toward an alkaline pH in AD skin. Recently, another important association between sphingolipid metabolites and the defense mechanism against S. aureus colonization has been ascribed to decreased levels of ceramide which reflects possible alteration of sphingolipid metabolites in the AD skin. Endoplasmic reticulum (ER) stress stimulates the production of cathelicidin antimicrobial peptide (CAMP), the proapoptotic sphingolipids, ceramide and sphingosine as well as an antiapoptotic sphingosine 1-phosphate (S1P). In previous studies, a variety of external perturbations including ER stress stimulated the production of ceramides (Uchida et al., 2003; Lei et al., 2008; Uchida et al., 2010). Recently, we also suggested the CAMP production via a novel NF-κB- and C/EBPα-mediated pathway independent of vitamin D receptor mediated mechanism (Park et al., 2011). Interestingly, sphingolipid metabolites especially ceramides were converted into sphingosine by ceramidase or dephosphorylated from S1P by S1P phosphohydrolase-1 in subtoxic levels of ER stress (Lepine et al., 2011). Therefore, sphingolipid metabolites ceramides, sphingosine and S1P were possibly involved in triggering innate immunity in skin.
Here, we introduce the antimicrobial peptides released from skin epithelial cells in relation to the activation or upregulation of their defense mechanism mediated by the sphingolipid signaling molecules in AD.
FUNCTION AND IMPORTANCE OF KEY EPIDERMAL ANTIMICROBIAL PEPTIDES
Mammalian epithelial tissues, such as intestine, respiratory tract, reproductive tract, mucosal epithelia and skin, are positioned at the interface with the external environment, where they continuously encounter exogenous microbial pathogens. These tissues have developed protective mechanisms that include antimicrobial peptides (AMP) (also called host defense peptides) that exhibit a broad-spectrum antimicrobial activity against multiple pathogens, including Gram-positive and Gram-negative bacteria, fungi, enveloped viruses and even transformed or cancerous cells. (Liu et al., 2013). In addition to antimicrobial activity, it has been highlighted that AMPs have the ability to enhance innate/adaptive immunity by functioning as immunomodulators (Beignon et al., 2001). In human and other mammals, the two main AMP families are defensin and CAMP. Defensins are widely distributed in mammalian epithelial cells and phagocytes (Ali et al., 2001). CAMP is a structurally and evolutionarily distinct antimicrobial peptide that is similar to defensins in abundance and distribution. In addition to CAMP and defensins, additional antimicrobial peptides also exist which including histatins (de Sousa-Pereira et al., 2013), dermcidin (Schittek et al., 2001; Zeth, 2013) and anionic peptides. However, these antimicrobial peptides are restricted to a few animal species and tissues. In this review, the major characteristics of two major epidermal AMP families, β-defensins and CAMP will be present and discussed.
Defensins
The defensins, which have six conserved cysteine residues, are small and cationic peptides that are divided into three subfamilies, α-, β-, and θ-defensin based on the pairing of the cysteine residues to form three disulfide bridges (α-defensin [cys1-cys6, cys2-cys4, cys3-cys5], β-defensin [cys1-cys5, cys2-cys4, cys3-cys6], θ-defensin [cys1-cys1, cys2-cys3, cys2-cys3]) (Ali et al., 2001; Huh et al., 2002; Fazakerley et al., 2010). Six α-defensins have been identified from five genes in human, human neutrophil peptide (HNP)-1, HNP-2, HNP-3, HNP-4, and human defensin (HD)-5, and HD-6. The α-defensins are mainly produced by neutrophils and to our knowledge are not found in keratinocytes, which are human epidermal skin cells. Whereas, human β-defensins (hBDs) are encoded by more than 30 β-defensin genes (DEFBs) and the keratinocytes express hBD-1 through hBD-3 (Table 1). Unlike α- and β-defensins those are found in humans, the θ-defensin protein product has not been identified in humans.
Table 1.
Antimicrobial peptides in the skin
| Family | Representative peptide | Main cells or tissues | Main stimulators | Antimicrobial activity | Reference |
|---|---|---|---|---|---|
|
| |||||
| Defensin | β-defensin1 | Epidermis and other epithelia | Constitutive | Gram-positive bacteria Gram-negative bacteria Viruses, and Fungi | Ali et al., 2001 |
| β-defensin2 | Epidermis and other epithelia | IL-1 | Gram-negative bacteria and Fungi | Huh et al., 2002 | |
| β-defensin3 | Epidermis and other epithelia | IL-6, Growth factor | Gram-positive bacteria Gram-negative bacteria Viruses, and Fungi | Fazakerley et al., 2010 | |
| Cathelicidin | LL-37 | Epidermis and other epithelia | Vitamin D, ER stress | Gram-positive bacteria Gram-negative bacteria Viruses, and Fungi | Segaert, 2008 |
| Psoriasin | N/A | Epidermis | Unknown | Escherichia coli | Glaser et al., 2005 |
| RNase | RNase7 | Epidermis and other epithelia | Unknown | Gram-positive and Gram-negative bacteria | Wittersheim et al., 2013 |
| Catestatin | N/A | Epidermis | Injury | Gram-positive bacteria Gram-negative bacteria yeast, and Fungi | Radek et al., 2008 |
hBD-1 is constitutively expressed in human skin, whereas both hBD-2 and hBD-3 are inducible AMP in epidermis dependent on a number of factors, including pathogenic bacteria, cytokines, growth factors or external stresses such as UVB irradiation. These factors have shown to differentially induce hBDs production in the skin. Toll-like receptor (TLR) 4 has been noted to recognize lipopolysaccharides (LPS) that induce hBD-2, not hBD-3, expression in keratinocytes (Luo et al., 2012). Moreover, hBD-2 production was mainly regulated by IL-1, whereas hBD-3 induction is influenced primarily by IL-6 and epidermal growth factors, indicating that different stimulators and regulatory mechanisms are responsible for the generation of various hBDs (Harder et al., 2000; Pioli et al., 2006; Ahn et al., 2013). hBD3 has potent antimicrobial activity against exogenous microbial pathogens, including Gram-negative and Gram-positive bacteria, while hBD2 has no noted effects against Gram-positive Staphylococcus (S.) aureus, which is the virulent bacteria that colonizes approximately 90% of AD patients (Fazakerley et al., 2010; Ong, 2010).
Cathelicidins
The properties of CAMP were first discovered in porcine (Ritonja et al., 1989), CAMP has been continuously investigated and found in multiple other mammalian species, e.g., rabbit (Larrick et al., 1991), humans (Larrick et al., 1995), sheep (Bagella et al., 1995), mice (Popsueva et al., 1996), monkeys (Bals et al., 2001), and recently in dogs (Sang et al., 2007). These mammalian CAMPs are small, cationic, and amphipathic peptides, which range in size <100 amino acid residues. In humans, there is a single CAMP gene, which encodes the inactive precursor protein, known as CAP18. This inactive precursor consists of an N-terminal cathelin domain and a C-terimal peptide domain. Local proteases, e.g., serine protease 3, kalikerin-5 or -7, cleave the C-terminal domain from the inactive precursor CAP18 to form active AMP such as LL-37, which is responsible for antimicrobial activity (Table 1) (Glaser et al., 2005; Radek et al., 2008; Segaert, 2008; Wittersheim et al., 2013).
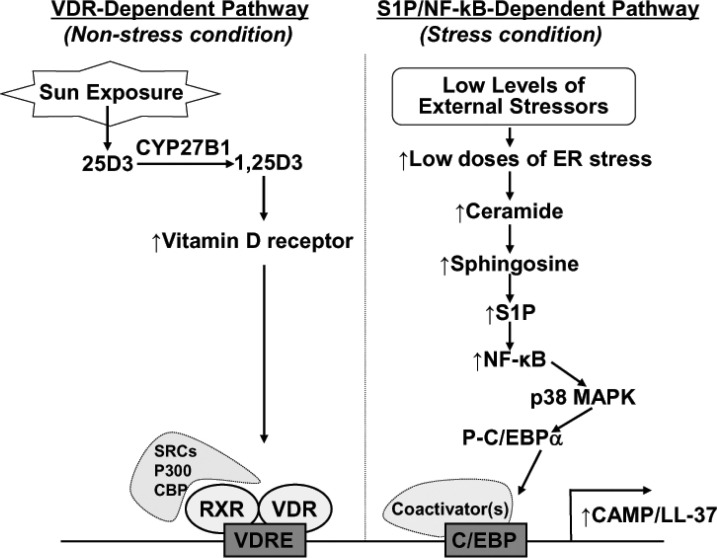
Epidermal CAMP is minimally detected in intact, unperturbed skin but significantly upregulated in response to exogenous infections, wounding, and permeability barrier perturbation. It has been well established that the Vitamin D3-induced Vitamin D Receptor (VDR) activation is the primary transcriptional regulatory mechanism of CAMP production in the skin (Martineau et al., 2007; Heilborn et al., 2010; Kanda et al., 2012). In general, while Vitamin D3 is synthesized from 7-dehydrocholesterol in human skin upon exposure of UVB irradiation, with the two key enzymes being vitamin D 25-hydroxylase (CYP27A1) in liver and 25-hydroxyvitamin D3 1-α-hydroxylase (CYP27B1) in kidney. Both enzymes are required to form active Vitamin D3, 1,25 dihydroxy vitamin D3 (1,25D3) (Lehmann et al., 1999; Kragballe, 2002; Vantieghem et al., 2006). In particular, CYP27B1 which can activate 1,25D3 is also expressed in keratinocytes and is under the control of the signals that occur in bacterial infection or injury in the skin. Activated 1,25D3 binds to the VDR and recruits transcriptional coactivator proteins such as steroid receptor coactivator (SRC) 3. This result is in the formation of a complex with activated VDR, leading to initiate CAMP expression and function (Fig. 1).
Fig. 1. Regulatory mechanisms of VDR- or S1P/NF-κB-dependent induction of CAMP/LL-37 in human skin.
In addition to VDR-dependent mechanism, we have discovered that the subtoxic external perturbations such as UVB irradiation that induce endoplasmic reticulum (ER) stress and increase cellular ceramide production in parallel with stimulated metabolic conversion of sphingosine to sphingosine-1-phosphate (S1P) lead to enhanced CAMP generation via an NF-κB activation, independent of the VDR pathway (Fig. 1) (Uchida et al., 2010). In addition to VDR, several consensus transcription factor-binding sequences, including C/EBP, are present on the promoter region of CAMP, but an NF-κB binding element has not been identified in the region that includes the 693 bp upstream from the transcription initiating site. Since NF-κB binding site(s) is not present within the CAMP promoter, the ER stress-induced activation of NF-κB could not directly bind to the promoter region; instead, NF-κB activation induces C/EBPα via p38 MAP kinase (Hayakawa et al., 2010; Garcia-Garcia et al., 2012). Although a detailed mechanism of how the ER stress-mediated increases in S1P activate NF-κB still remains unresolved, our recent studies further demonstrated that CAMP generation likely occurs by an S1P receptor independent mechanism (Park et al., 2013). Moreover, VDR- and S1P/NF-κB-dependent mechanism are not operating simultaneously in response to ER stress, both pathways could alternatively regulate CAMP expression depending upon cellular environments, i.e. under basal (unstressed) vs. under stressed conditions in the skin (Fig. 1).
A number of previous studies have revealed that CAMP is a major epidermal AMP with the most potent antimicrobial activity against virulent S. aureus, and a multifunctional peptide that also modulates cytokine production, cellular differentiation, and adaptive immunity (Table 1). Furthermore, recent studies revealed that CAMP combined with hBD-2 has synergistic antimicrobial activity by effectively killing S. aureus (Kim et al., 2009; Goo et al., 2010).
Role of antimicrobial peptides in inflammatory skin disorders
AD is the most common inflammatory skin disease and is characterized by T helper type 2 (Th2) cytokine-mediated inflammations and significant barrier disruption. Since Th2 cytokines such as IL-4 and IL-13, which are highly expressed in AD skin and suppress key epidermal AMPs, e.g., hBD-2, hBD-3, and CAMP, expression levels of these AMPs decline in AD skin as compared to healthy skin (Reinholz et al., 2012; Lancto et al., 2013). Decreased expression of AMPs can explain the increased susceptibility of AD patients to bacterial infection, particularly methicillin-resistant S. aureus (MRSA), which poses a significant community health problem for atopic dermatitis patients since treatment is difficult. In contrast to AD, CAMP is strongly overexpressed in the skin of rosacea patient compared to the skin of healthy controls (Meyer-Hoffert and Schroder, 2011). However, levels of hBDs in rosacea patient do not differ from healthy controls. As presented above, the active form LL-37, which is cleaved from the inactive precursor CAP18 by serine proteases, can be further processed to smaller peptide fragments and has pro-inflammatory response. In addition, increased expression/activity of serine proteases such as serine protease 3 and kalikerin-5 have been found in skin lesions of rosacea patients (Yamasaki et al., 2007; Morizane et al., 2010). Although further studies are needed to show how cutaneous serine proteases are activated in rosacea, increased CAMP expression due to enhanced serine protease activity can explain why patients suffering from rosacea show chronic inflammation.
MAIN SPHINGOLIPID METABOLITES IN SKIN
Ceramide structure in skin
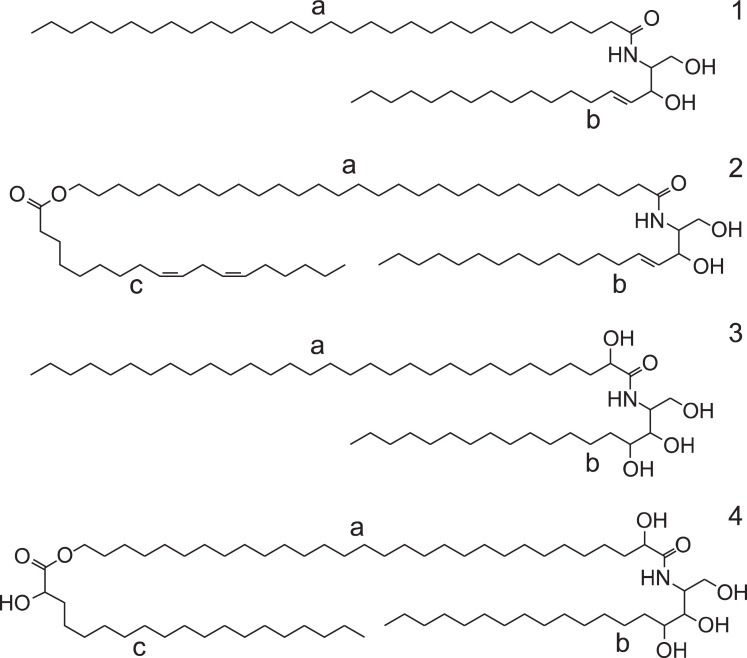
The stratum corneum (SC) is the outmost upper layer of the skin and provides the rate-limiting barrier function for penetration into and out of the skin. The SC of the skin contains relatively high amounts of ceramides as much as 50% of total skin lipids (Bouwstra et al., 1999). Ceramides in skin exist both in the free form and bound to proteins. Interestingly, skin ceramides have unique structures which are not reported in other tissues. Typical skin ceramides structures contain the long-chain fatty acids linked to sphingosine or phytosphingosine (Stewart and Downing, 1999). Some of fatty acids with 2-hydroxyl group were also observed in skin ceramides structure (Huang and Chang, 2008). In addition, there are O-acyl ceramides structure consisted with a long-chain fatty acids, having a terminal hydroxyl group which esterified either with linoleate or a 2-hydroxy acid (Janusova et al., 2011) (Fig. 2).
Fig. 2. Distinctive structures of skin ceramides. Sphingosine (1b,2b) or phytosphingosine (3b,4b) backbone were linked to a long-chain fatty acid (typically C30;1a, 3a) or has a terminal hydroxy group (2a,4a) which esterified with linoleate (2c) or 2-hydroxy acid (4c).
These ceramides have an essential function in the skin barrier properties, inhibiting water loss and protecting ingress of potentially harmful substances. This barrier functions have been impaired in skin diseases such as psoriasis and AD, presumably caused by an altered lipid composition and organization (Farwanah et al., 2005). Indeed, the skin barrier disruption has been attributed to decreased levels of ceramides in SC of AD patients (Kita et al., 2002).
Antimicrobial sphingosine in skin
Among various sphingoid bases, only sphingosine was clearly and profoundly effective against Staphylococcus aureus (4-log reduction at 20 μM; 2-log reduction at 2.5 μM) (Arikawa et al., 2002). The sphingosine was similarly active against Streptococcus pyogenes, Micrococcus luteus, Propionibacterium acnes, Brevibacterium epidermidis, and Candida albicans, moderately active against Pseudomonas aeruginosa, and ineffective against Escherichia coli and Serratia marcescens (Bibel et al., 1992a). The positively charged structure of 2-amino position in sphingosine is responsible for enzyme inhibition such as a protein-kinase system because there was no activity at pH higher than 8.0 and N-acetyl sphingosine was ineffective, suggesting this chemical structure is important in antimicrobial activity (Bottega et al., 1989; Igarashi et al., 1989). In addition, both erythro- and threo-isomers were effective and essentially required Ca2+ ion for antimicrobial activity. Therefore, the existence of sphingosine in the SC suggests that sphingosine may have a major role in the resistance of skin to colocalization and infection by microorganisms (Bibel et al., 1992b, Bibel et al., 1993). The levels of sphingosine in the SC are regulated by two factors; the hydrolysis of ceramides to sphingosine by acidic ceramidase (CDase) and ceramides levels as a substrate pool. Actually, the levels of sphingosine are significantly down-regulated to 140-160 μM in SC of AD patients, compared with healthy controls (268 μM). This suggests that the decreased levels of ceramides and reduced activities of acidic CDase are in part associated with the deficiency of sphingosine in the skin of AD patients (Arikawa et al., 2002).
Sphingosine 1-phosphate (S1P) in skin
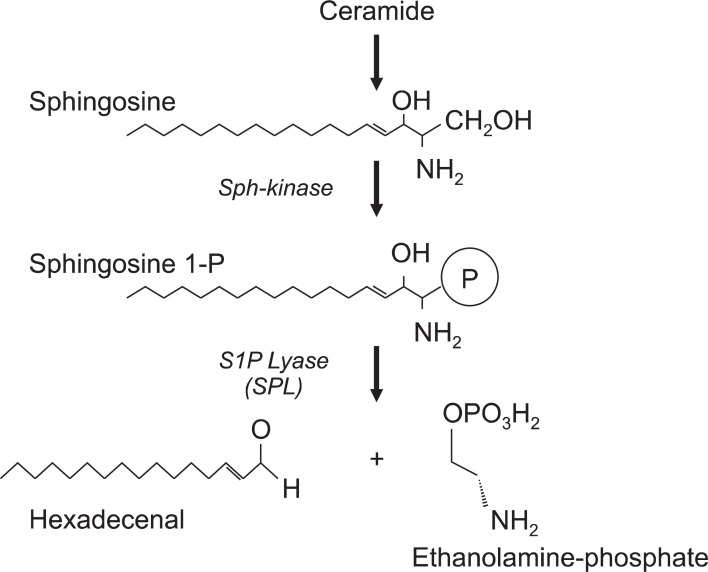
In skin, sphingosine can be phosphorylated by sphingosine kinases into S1P, which acting both the maintenance of the epidermal permeability and as a critical signaling molecule, which binds to a family of G-protein coupled receptors, termed S1PR1-S1PR5 (Maceyka et al., 2009). This S1P signaling via S1P receptors (S1PRs) significantly regulate the immunosuppressive action by causing lymphophenia as demonstrated by a S1PR1 agonist, FTY720 (Fingolimod) treatment in vivo (Bohler et al., 2003). The immunosuppressive ligand S1P is irreversibly metabolized into hexadecenal and ethanolamine phosphate by S1P lyase (Schwab et al., 2005) (Fig. 3). Therefore, the regulation of S1P lyase activity may mainly control the intracellular S1P concentrations (Berdyshev et al., 2011). Therefore, S1P lyase activity may also exhibit a central role for the regulation of immunological disorders, suggesting that an imbalance in the S1P-S1P lyase axis may directly related to the incidence of skin diseases. Interestingly, the S1P lyase mRNA and S1P lyase activity were greatly enhanced in atopic skin lesions in humans, supposing that S1P concentration in AD patients skin may be much less compared to healthy skin (Baumer et al., 2011). In this report, the S1P concentration in lesional skin of dogs suffering from AD was significantly lower, 0.18 nmol/mg protein while healthy skin was 0.63 nmol/mg protein.
Fig. 3. S1P lyase (SPL) play a central role for regulating endogenous S1P concentrations.
Sphingolipid metabolism in ER stress
In a recent paper, we demonstrated that ER stresses by cell-permeable ceramide (C2Cer) in human keratinocytes upregulated the mRNA expression of CAMP as well as the endogenous sphingosine in 10-fold concentrations and S1P in 1.3-fold increase (Park et al., 2013). In this conditions, the inhibition of S1P lyase activity by siRNA treatment profoundly increased S1P level, indicating the possible involvement of sphingolipid metabolites, ceramides, sphingosine and S1P for the induction of CAMP mRNA and protein expression. Especially the enzyme of sphingosine kinase type 1 (SK-1) which produce S1P was significantly activated in CAMP expression in human keratinocytes which were treated with C2Cer. In addition, a pharmaceutical inhibitor (SKI) or siRNA treatment of SK-1 attenuated CAMP expression in cells. From in vivo studies using S1P lyase-deficient mouse skin, C2Cer (7.5 μM) treatment induced ER stress and subsequently increased the protein and mRNA levels of CAMP compared to vehicle. Exogenously added S1P or synthetic S1PR1 ligands (SEW 2871) did not enhanced CAMP mRNA expression. These results suggested that S1P regulation of enhanced CAMP expression may not be related to S1P receptor-mediated S1P signaling pathway.
CONCLUSIONS
Taken together, the antimicrobial peptide, CAMP is an indispensable factor of the skin’s innate immune defense system against microorganisms’ infection. Previously, the CAMP protein in keratinocytes is regulated by vitamin D3 through the vitamin D receptor (Gombart et al., 2005). We newly identified S1P as an ER-stress signaling molecule which increases production of CAMP when the epidermis is under stress; e.g., UV exposure (D'Orazio et al., 2013; Wallingford et al., 2013), wound healing (Kawanabe et al., 2007), or attack by microorganisms (Arikawa et al., 2002). Identification of this new signaling mechanism will allow the development of new topical compounds, both synthetic and natural, that could be used to enhance epidermal innate immunity and antimicrobial defense, when the skin is under microbial attack from the outside.
Acknowledgments
This work was supported by research grant of the Chungbuk National University in 2010.
References
- 1.Ahn J. K., Huang B., Bae E. K., Park E. J., Hwang J. W., Lee J., Koh E. M., Cha H. S. The role of alpha-defensin-1 and related signal transduction mechanisms in the production of IL-6, IL-8 and MMPs in rheumatoid fibroblast-like synoviocytes. Rheumatology (Oxford) (2013);52:1368–1376. doi: 10.1093/rheumatology/ket147. [DOI] [PubMed] [Google Scholar]
- 2.Ali R. S., Falconer A., Ikram M., Bissett C. E., Cerio R., Quinn A. G. Expression of the peptide antibiotics human beta defensin- 1 and human beta defensin-2 in normal human skin. J. Invest. Dermatol. (2001);117:106–111. doi: 10.1046/j.0022-202x.2001.01401.x. [DOI] [PubMed] [Google Scholar]
- 3.Arikawa J., Ishibashi M., Kawashima M., Takagi Y., Ichikawa Y., Imokawa G. Decreased levels of sphingosine, a natural antimicrobial agent, may be associated with vulnerability of the stratum corneum from patients with atopic dermatitis to colonization by Staphylococcus aureus. J. Invest. Dermatol. . (2002);119:433–439. doi: 10.1046/j.1523-1747.2002.01846.x. [DOI] [PubMed] [Google Scholar]
- 4.Bagella L., Scocchi M., Zanetti M. cDNA sequences of three sheep myeloid cathelicidins. FEBS Lett. (1995);376:225–228. doi: 10.1016/0014-5793(95)01285-3. [DOI] [PubMed] [Google Scholar]
- 5.Bals R., Lang C., Weiner D. J., Vogelmeier C., Welsch U., Wilson J. M. Rhesus monkey (Macaca mulatta) mucosal antimicrobial peptides are close homologues of human molecules. Clin. Diagn. Lab. Immunol. (2001);8:370–375. doi: 10.1128/CDLI.8.2.370-375.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumer W., Rossbach K., Mischke R., Reines I., Langbein-Detsch I., Luth A., Kleuser B. Decreased concentration and enhanced metabolism of sphingosine-1-phosphate in lesional skin of dogs with atopic dermatitis: disturbed sphingosine-1-phosphate homeostasis in atopic dermatitis. J. Invest. Dermatol. (2011);131:266–268. doi: 10.1038/jid.2010.252. [DOI] [PubMed] [Google Scholar]
- 7.Beignon A. S., Briand J. P., Muller S., Partidos C. D. Immunization onto bare skin with heat-labile enterotoxin of Escherichia coli enhances immune responses to coadministered protein and peptide antigens and protects mice against lethal toxin challenge. Immunology . (2001);102:344–351. doi: 10.1046/j.1365-2567.2001.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berdyshev E. V., Gorshkova I., Usatyuk P., Kalari S., Zhao Y., Pyne N. J., Pyne S., Sabbadini R. A., Garcia J. G., Natarajan V. Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: role of sphingosine kinase 1 and S1P lyase. PLoS One . (2011);6:e16571. doi: 10.1371/journal.pone.0016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bibel D. J., Aly R., Shah S., Shinefield H. R. Sphingosines: antimicrobial barriers of the skin. Acta Derm. Venereol. (1993);73:407–411. doi: 10.2340/0001555573407411. [DOI] [PubMed] [Google Scholar]
- 10.Bibel D. J., Aly R., Shinefield H. R. Antimicrobial activity of sphingosines. J. Invest. Dermatol. (1992a);98:269–273. doi: 10.1111/1523-1747.ep12497842. [DOI] [PubMed] [Google Scholar]
- 11.Bibel D. J., Aly R., Shinefield H. R. Inhibition of microbial adherence by sphinganine. Can. J. Microbiol. (1992b);38:983–985. doi: 10.1139/m92-158. [DOI] [PubMed] [Google Scholar]
- 12.Bohler T., Waiser J., Schutz M., Schumann B., Neumayer H. H., Budde K. Pharmacodynamics of FTY720, the first member of a new class of immune-modulating therapeutics in transplantation medicine. Int. J. Clin. Pharmacol. Ther. (2003);41:482–487. doi: 10.5414/cpp41482. [DOI] [PubMed] [Google Scholar]
- 13.Bottega R., Epand R. M., Ball E. H. Inhibition of protein kinase C by sphingosine correlates with the presence of positive charge. Biochem. Biophys. Res. Commun. . (1989);164:102–107. doi: 10.1016/0006-291X(89)91688-4. [DOI] [PubMed] [Google Scholar]
- 14.Bouwstra J. A., Dubbelaar F. E., Gooris G. S., Weerheim A. M., Ponec M. The role of ceramide composition in the lipid organisation of the skin barrier. Biochim. Biophys. Acta . (1999);1419:127–136. doi: 10.1016/S0005-2736(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 15.D'Orazio J., Jarrett S., Amaro-Ortiz A., Scott T. UV Radiation and the Skin. Int. J. Mol. Sci. (2013);14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Sousa-Pereira P., Amado F., Abrantes J., Ferreira R., Esteves P.J., Vitorino R. An evolutionary perspective of mammal salivary peptide families: cystatins, histatins, statherin and PRPs. Arch. Oral Biol. . (2013);58:451–458. doi: 10.1016/j.archoralbio.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 17.Farwanah H., Raith K., Neubert R. H., Wohlrab J. Ceramide profiles of the uninvolved skin in atopic dermatitis and psoriasis are comparable to those of healthy skin. Arch. Dermatol.Res. (2005);296:514–521. doi: 10.1007/s00403-005-0551-2. [DOI] [PubMed] [Google Scholar]
- 18.Fazakerley J., Crossley J., McEwan N., Carter S. In vitro antimicrobial efficacy of beta-defensin 3 against Staphylococcus pseudintermedius isolates from healthy and atopic canine skin. Vet. Dermatol. (2010);21:463–468. doi: 10.1111/j.1365-3164.2010.00893.x. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Garcia F. J., Mullol J., Perez-Gonzalez M., Pujols L., Alobid I., Roca-Ferrer J., Picado C. Signal transduction pathways (MAPKs, NF-kappaB, and C/EBP) regulating COX-2 expression in nasal fibroblasts from asthma patients with aspirin intolerance. PLoS One . (2012);7:e51281. doi: 10.1371/journal.pone.0051281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glaser R., Harder J., Lange H., Bartels J., Christophers E., Schroder J. M. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat. Immunol. (2005);6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 21.Gombart A. F., Borregaard N., Koeffler H. P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. . (2005);19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 22.Goo J., Ji J. H., Jeon H., Kim M. J., Jeon S. Y., Cho M. Y., Lee S. H., Choi E. H. Expression of antimicrobial peptides such as LL-37 and hBD-2 in nonlesional skin of atopic individuals. Pediatr. Dermatol. . (2010);27:341–348. doi: 10.1111/j.1525-1470.2010.01122.x. [DOI] [PubMed] [Google Scholar]
- 23.Harder J., Meyer-Hoffert U., Teran L. M., Schwichtenberg L., Bartels J., Maune S., Schroder J. M. Mucoid Pseudomonas aeruginosa, TNF-alpha, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am. J. Respir. Cell Mol. Biol. (2000);22:714–721. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- 24.Hayakawa K., Nakajima S., Hiramatsu N., Okamura M., Huang T., Saito Y., Tagawa Y., Tamai M., Takahashi S., Yao J., Kitamura M. ER stress depresses NF-kappaB activation in mesangial cells through preferential induction of C/EBP beta. J. Am. Soc. Nephrol. (2010);21:73–81. doi: 10.1681/ASN.2009040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heilborn J. D., Weber G., Gronberg A., Dieterich C., Stahle M. Topical treatment with the vitamin D analogue calcipotriol enhances the upregulation of the antimicrobial protein hCAP18/ LL-37 during wounding in human skin in vivo. Exp. Dermatol. (2010);19:332–338. doi: 10.1111/j.1600-0625.2009.00997.x. [DOI] [PubMed] [Google Scholar]
- 26.Huang H. C., Chang T. M. Ceramide 1 and ceramide 3 act synergistically on skin hydration and the transepidermal water loss of sodium lauryl sulfate-irritated skin. Int. J. Dermatol. (2008);47:812–819. doi: 10.1111/j.1365-4632.2008.03687.x. [DOI] [PubMed] [Google Scholar]
- 27.Huh W. K., Oono T., Shirafuji Y., Akiyama H., Arata J., Sakaguchi M, Huh N. H., Iwatsuki K. Dynamic alteration of human beta-defensin 2 localization from cytoplasm to intercellular space in psoriatic skin. J. Mol. Med. (Berl) . (2002);80:678–684. doi: 10.1007/s00109-002-0373-z. [DOI] [PubMed] [Google Scholar]
- 28.Igarashi Y., Hakomori S., Toyokuni T., Dean B., Fujita S., Sugimoto M., Ogawa T., el-Ghendy K., Racker E. Effect of chemically well-defined sphingosine and its N-methyl derivatives on protein kinase C and src kinase activities. Biochemistry . (1989);28:6796–6800. doi: 10.1021/bi00443a002. [DOI] [PubMed] [Google Scholar]
- 29.Janusova B., Zbytovska J., Lorenc P., Vavrysova H., Palat K., Hrabalek A., Vavrova K. Effect of ceramide acyl chain length on skin permeability and thermotropic phase behavior of model stratum corneum lipid membranes. Biochim. Biophys. Acta. (2011);1811:129–137. doi: 10.1016/j.bbalip.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Kanda N., Hau C. S., Tada Y., Sato S., Watanabe S. Decreased serum LL-37 and vitamin D3 levels in atopic dermatitis: relationship between IL-31 and oncostatin M. Allergy . (2012);67:804–812. doi: 10.1111/j.1398-9995.2012.02824.x. [DOI] [PubMed] [Google Scholar]
- 31.Kawanabe T, Kawakami T, Yatomi Y, Shimada S., Soma Y. Sphingosine 1-phosphate accelerates wound healing in diabetic mice. J. Dermatol. Sci. (2007);48:53–60. doi: 10.1016/j.jdermsci.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Kim B. J., Rho Y. K., Lee H. I., Jeong M. S., Li K., Seo S. J., Kim M. N., Hong C. K. The effect of calcipotriol on the expression of human beta defensin-2 and LL-37 in cultured human keratinocytes. Clin. Dev. Immunol. . (2009);2009:645898. doi: 10.1155/2009/645898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kita K., Sueyoshi N., Okino N., Inagaki M., Ishida H., Kiso M., Imayama S., Nakamura T., Ito M. Activation of bacterial ceramidase by anionic glycerophospholipids: possible involvement in ceramide hydrolysis on atopic skin by Pseudomonas ceramidase. Biochem. J. . (2002);362:619–626. doi: 10.1042/0264-6021:3620619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kragballe K. Vitamin D and UVB radiation therapy. Cutis . (2002);70:9–12. [PubMed] [Google Scholar]
- 35.Lancto C. A., Torres S. M., Hendrickson J. A., Martins K. V., Rutherford M. S. Altered expression of antimicrobial peptide genes in the skin of dogs with atopic dermatitis and other inflammatory skin conditions. Vet. Dermatol. (2013);24:414–e90. doi: 10.1111/vde.12034. [DOI] [PubMed] [Google Scholar]
- 36.Larrick J. W., Hirata M., Balint R. F., Lee J., Zhong J., Wright S.C. Human CAP18: a novel antimicrobial lipopolysaccharidebinding protein. Infect. Immun. (1995);63:1291–1297. doi: 10.1128/iai.63.4.1291-1297.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larrick J. W., Morgan J. G., Palings I., Hirata M., Yen M. H. Complementary DNA sequence of rabbit CAP18--a unique lipopolysaccharide binding protein. Biochem. Biophys. Res. Commun. (1991);179:170–175. doi: 10.1016/0006-291X(91)91350-L. [DOI] [PubMed] [Google Scholar]
- 38.Lehmann B., Tiebel O., Meurer M. Expression of vitamin D3 25-hydroxylase (CYP27) mRNA after induction by vitamin D3 or UVB radiation in keratinocytes of human skin equivalents--a preliminary study. Arch. Dermatol. Res. (1999);291:507–510. doi: 10.1007/s004030050445. [DOI] [PubMed] [Google Scholar]
- 39.Lei X., Zhang S., Bohrer A., Ramanadham S. Calciumindependent phospholipase A2 (iPLA2 beta)-mediated ceramide generation plays a key role in the cross-talk between the endoplasmic reticulum (ER) and mitochondria during ER stress-induced insulin-secreting cell apoptosis. J. Biol. Chem. . (2008);283:34819–34832. doi: 10.1074/jbc.M807409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lepine S., Allegood J. C., Park M., Dent P., Milstien S., Spiegel S. Sphingosine-1-phosphate phosphohydrolase-1 regulates ER stress-induced autophagy. Cell Death Differ. (2011);18:350–361. doi: 10.1038/cdd.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J. J., Zamany A., Sniezko R. A. Anti-microbial peptide (AMP): nucleotide variation, gene expression, and host resistance in the white pine blister rust (WPBR) pathosystem. Planta . (2013);237:43–54. doi: 10.1007/s00425-012-1747-2. [DOI] [PubMed] [Google Scholar]
- 42.Luo W., Wang C. Y., Jin L. Baicalin downregulates Porphyromonas gingivalis lipopolysaccharide-upregulated IL-6 and IL-8 expression in human oral keratinocytes by negative regulation of TLR signaling. PLoS One. (2012);7:e51008. doi: 10.1371/journal.pone.0051008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maceyka M., Milstien S., Spiegel S. Sphingosine-1-phosphate: the Swiss army knife of sphingolipid signaling. J. Lipid Res. (2009);50 Suppl:S272–276. doi: 10.1194/jlr.R800065-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martineau A. R., Wilkinson K. A., Newton S. M., Floto R. A., Norman A. W., Skolimowska K., Davidson R. N., Sorensen O. E., Kampmann B., Griffiths C. J., Wilkinson R. J. IFN-gammaand TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J. Immunol. . (2007);178:7190–7198. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 45.Meyer-Hoffert U., Schroder J. M. Epidermal proteases in the pathogenesis of rosacea. J. Investig. Dermatol. Symp. Proc. (2011);15:16–23. doi: 10.1038/jidsymp.2011.2. [DOI] [PubMed] [Google Scholar]
- 46.Morizane S., Yamasaki K., Kabigting F. D., Gallo R. L. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D(3), and retinoic acid. J. Invest. Dermatol. (2010);130:1297–1306. doi: 10.1038/jid.2009.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ong P. Y. Human beta defensin-2: too good to be dismissed in atopic dermatitis. J. Invest. Dermatol. (2010);130:2138–2139. doi: 10.1038/jid.2010.101. [DOI] [PubMed] [Google Scholar]
- 48.Park K., Elias P. M., Oda Y., Mackenzie D., Mauro T., Holleran W. M., Uchida Y. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J. Biol. Chem. (2011);286:34121–34130. doi: 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park K., Elias P. M., Shin K. O., Lee Y. M., Hupe M., Borkowski A.W., Gallo R. L., Saba J., Holleran W. M., Uchida Y. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol. Cell Biol. . (2013);33:752–762. doi: 10.1128/MCB.01103-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pioli P. A., Weaver L. K., Schaefer T. M., Wright J. A., Wira C. R., Guyre P. M. Lipopolysaccharide-induced IL-1 beta production by human uterine macrophages up-regulates uterine epithelial cell expression of human beta-defensin 2. J. Immunol. (2006);176:6647–6655. doi: 10.4049/jimmunol.176.11.6647. [DOI] [PubMed] [Google Scholar]
- 51.Popsueva A. E., Zinovjeva M. V., Visser J. W., Zijlmans J. M., Fibbe W. E., Belyavsky A. V. A novel murine cathelin-like protein expressed in bone marrow. FEBS Lett. (1996);391:5–8. doi: 10.1016/0014-5793(96)00692-8. [DOI] [PubMed] [Google Scholar]
- 52.Radek K. A., Lopez-Garcia B., Hupe M., Niesman I. R., Elias P.M., Taupenot L., Mahata S. K., O'Connor D. T., Gallo R. L. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J. Invest. Dermatol. (2008);128:1525–1534. doi: 10.1038/sj.jid.5701225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reinholz M, Ruzicka T., Schauber J. Cathelicidin LL-37: an antimicrobial peptide with a role in inflammatory skin disease. Ann. Dermatol. (2012);24:126–135. doi: 10.5021/ad.2012.24.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ritonja A., Kopitar M., Jerala R., Turk V. Primary structure of a new cysteine proteinase inhibitor from pig leucocytes. FEBS Lett. (1989);255:211–214. doi: 10.1016/0014-5793(89)81093-2. [DOI] [PubMed] [Google Scholar]
- 55.Sang Y., Teresa Ortega M., Rune K., Xiau W., Zhang G., Soulages J. L., Lushington G. H., Fang J., Williams T. D., Blecha F., Melgarejo T. Canine cathelicidin (K9CATH): gene cloning, expression, and biochemical activity of a novel pro-myeloid antimicrobial peptide. Dev. Comp. Immunol. . (2007);31:1278–1296. doi: 10.1016/j.dci.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Schittek B., Hipfel R., Sauer B., Bauer J., Kalbacher H., Stevanovic S., Schirle M., Schroeder K., Blin N., Meier F., Rassner G., Garbe C. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. . (2001);2:1133–1137. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 57.Schwab S. R., Pereira J. P., Matloubian M., Xu Y., Huang Y., Cyster J. G. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. (2005);309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 58.Segaert S. Vitamin D regulation of cathelicidin in the skin: toward a renaissance of vitamin D in dermatology? J. Invest. Dermatol. (2008);128:773–775. doi: 10.1038/jid.2008.35. [DOI] [PubMed] [Google Scholar]
- 59.Stewart M. E., Downing D. T. A new 6-hydroxy-4-sphingenine- containing ceramide in human skin. J. Lipid. Res. (1999);40:1434–1439. [PubMed] [Google Scholar]
- 60.Uchida Y., Houben E., Park K., Douangpanya S., Lee Y. M., Wu B. X., Hannun Y. A., Radin N. S., Elias P. M., Holleran W.M. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J. Invest. Dermatol. (2010);130:2472–2480. doi: 10.1038/jid.2010.153. [DOI] [PubMed] [Google Scholar]
- 61.Uchida Y., Nardo A. D., Collins V., Elias P. M., Holleran W. M. De novo ceramide synthesis participates in the ultraviolet B irradiation-induced apoptosis in undifferentiated cultured human keratinocytes. J. Invest. Dermatol. (2003);120:662–669. doi: 10.1046/j.1523-1747.2003.12098.x. [DOI] [PubMed] [Google Scholar]
- 62.Vantieghem K., Kissmeyer A. M., De Haes P., Bouillon R., Segaert S. UVB-induced production of 1,25-dihydroxyvitamin D3 and vitamin D activity in human keratinocytes pretreated with a sterol delta7-reductase inhibitor. J. Cell Biochem. (2006);98:81–92. doi: 10.1002/jcb.20756. [DOI] [PubMed] [Google Scholar]
- 63.Wallingford S. C., Jones G., Kobayashi L. C., Grundy A., Miao Q., Tranmer J., Aronson K. J. UV and dietary predictors of serum 25-hydroxyvitamin D concentrations among young shiftworking nurses and implications for bone density and skin cancer. Public Health Nutr.; (2013). pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wittersheim M., Cordes J., Meyer-Hoffert U., Harder J., Hedderich J., Glaser R. Differential expression and in vivo secretion of the antimicrobial peptides psoriasin (S100A7), RNase 7, human beta-defensin-2 and -3 in healthy human skin. Exp. Dermatol. (2013);22:364–366. doi: 10.1111/exd.12133. [DOI] [PubMed] [Google Scholar]
- 65.Yamasaki K., Di Nardo A., Bardan A., Murakami M., Ohtake T., Coda A., Dorschner R. A., Bonnart C., Descargues P., Hovnanian A., Morhenn V. B., Gallo R. L. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. (2007);13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 66.Zeth K. Dermcidin: what is its antibiotic potential? Future Microbiol. (2013);8:817–819. doi: 10.2217/fmb.13.67. [DOI] [PubMed] [Google Scholar]