Abstract
We demonstrate herein that silibinin, a polyphenolic flavonoid compound isolated from milk thistle (Silybum marianum), inhibits LPS-induced activation of macrophages and production of nitric oxide (NO) in RAW 264.7 cells. Western blot analysis showed silibinin inhibits iNOS gene expression. RT-PCR showed that silibinin inhibits iNOS, TNF-α, and IL1β. We also showed that silibinin strongly inhibits p38 MAPK phosphorylation, whereas the ERK1/2 and JNK pathways are not inhibited. The p38 MAPK inhibitor abrogated the LPS-induced nitrite production, whereas the MEK-1 inhibitor did not affect the nitrite production. A molecular modeling study proposed a binding pose for silibinin targeting the ATP binding site of p38 MAPK (1OUK). Collectively, this series of experiments indicates that silibinin inhibits macrophage activation by blocking p38 MAPK signaling.
Keywords: Silibinin, Macrophages, p38 MAPK, Nitric oxide
INTRODUCTION
Silibinin is the major active constituent of silymarin, a standardized extract isolated from the fruit and seeds of the milk thistle, Silybum marianum (Pliskova et al., 2005). It is well known that silymarin and silibinin protect against hepatotoxicity caused by a variety of agents including ethanol, phenylhydrazine, acetaminophen, microcystin, and ochratoxin (Mereish et al., 1991; Valenzuela and Garrido, 1994; Al-Anati et al., 2009). Various studies also indicate that silibinin exhibits anticarcinogenic effects (Hogan et al., 2007; Mokhtari et al., 2008). Moreover, silibinin possesses a number of additional biological effects such as anti-inflammatory effects (Kang et al., 2002; Cristofalo et al., 2013). Although the mechanism or mechanisms of action are largely unknown, silymarin and silibinin have been shown to have direct antioxidant activity mediated through the scavenging of free radicals, and modulations of antioxidant and inflammatory enzymes (Letteron et al., 1990; Zhao et al., 1999).
Macrophage stimulation by external stimuli including lipopolysaccharides, a cell wall component of gram-negative bacteria, results in the phosphorylation of mitogen-activated protein kinases (MAPK) family, which includes extracellular signal-regulated kinase 1/2 (ERK1/2), p38 mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (JNK) (Su and Karin, 1996). ERK1/2, p38 MAPK, and JNK are serine threonine kinases that are located in the cytoplasm until activated by dual phosphorylation of their Thr and Tyr residues at Thr-Gly-Tyr, Thr-Glu-Tyr, or Thr-Pro-Tyr, respectively (Raingeaud et al., 1995). MAPK activation then stimulates transcription factors including NF-κB and AP-1 (Weinstein et al., 1992; Whitmarsh and Davis, 1996). In particular, the p38 MAPK is an important mediator of stress-induced gene expression and plays a key role in LPS-induced signal transduction pathways leading to cytokine synthesis (Raingeaud et al., 1995; Lee and Young, 1996). The p38 MAPK activation was reported to be involved in iNOS expression in tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1)-stimulated mouse astrocytes, as well as in LPS-stimulated mouse macrophages (Da Silva et al., 1997; Chen and Wang, 1999). It has been shown that MAPKs are potential targets of silymarin and silibinin (Singh et al., 2002; Kim et al., 2009). Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice by modulation of MAPKs and induction of apoptosis (Singh et al., 2002). Silibinin blocks the activation of NF-κB, JNK, p38 MAPK, and ERK in osteoclast precursors in response to RANKL (Kim et al., 2009).
In the study presented herein, we investigated the effect of silibinin on macrophage activation and production of NO, an important indicator of inflammation. To further investigate the mechanism by which silibinin inhibits activation of macrophage, we examined the possibility that silibinin targets p38 MAPK for its anti-inflammatory effects. The present study demonstrates that silibinin inhibits macrophage activation through the inhibition of p38 MAPK pathways.
MATERIALS AND METHODS
Materials
Silibinin and LPS from Salmonella thyposa was purchased from Sigma (St. Louis, MO, USA). Reagents used for cell culture were purchased from Gibco BRL (Grand Island, NY, USA). PD98059 (2'-amino-3'-methoxyflavone) and SB203580 (4-(4-fl uorophe-nyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl) 1Himidazole) were purchased from Calbiochem (San Diego, CA, USA). Anti-iNOS was purchased from Upstate Biotechnology (Lake Placid, NY, USA). Antibodies (anti-phospho-ERK1/2 antibody, anti-phospho p38 MAPK antibody, and anti-JNK antibody) were purchased from New England Biolabs Inc. (Beverly, MA, USA).
Cell culture
RAW 264.7 cells (murine macrophage line) were purchased from American Type Culture Collection (Bethesda, MD, USA). Cells were grown in DMEM supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were cultured in the presence of 5% CO2 at 37℃.
Nitrite determination
RAW 264.7 cells were treated with the indicated concentrations of silibinin in the presence of LPS (200 ng/ml) for 18 h. Culture supernatants were collected, and the accumulation of NO2- in culture supernatants was measured as an indicator of NO production in the medium as previously described (Green et al., 1982; Huong et al., 2012).
Western immunoblot analysis
Whole cell lysates were separated by 10% SDS-PAGE, then electro-transferred to nitrocellulose membranes (Amersham International, Buckinghamshire, UK). The membranes were preincubated for 1 h at room temperature in Tris-buffered saline (TBS) pH 7.6 containing 0.05% Tween-20 and 3% bovine serum albumin. The nitrocellulose membranes were then incubated with primary antibodies. Immunoreactive bands were then detected by incubation with conjugates of anti-rabbit IgG with horseradish peroxidase and enhanced chemiluminescence reagents (Amersham).
RT-PCR
Total RNA was isolated with Tri Reagent (Molecular Research Center, Cincinnati, OH, USA). Forward and reverse primer sequences were as follows: iNOS: 5'-CTG CAG CAC TTG GAT CAG GAA CCT G-3', 5'-GGG AGT AGC CTG TGT GCA CCT GGA A-3'; TNF-α: 5’-CCT GTA GCC CAC GTC GTA GC-3’, 5’-TTG ACC TCA GCG CTG AGT TG-3’, IL-1β: 5’-TGC AGA GTT CCC CAA CTG GTA CAT C-3’, 5’-GTG CTG CCT AAT GTC CCC TTG AAT C-3’, and β-actin: 5'-TGG AAT CCT GTG GCA TCC ATG AAA C-3', 5'-TAA AAC GCA GCT CAG TAA CAG TCC G-3'. Equal amounts of RNA were reversetranscribed into cDNA with oligo(dT)15 primers. PCR was performed with cDNA and each primer. Samples were heated to 94℃ for 5 min and cycled 30 times at 94℃ for 1 min, 55℃for 1.5 min, and 94℃ for 1 min, after which an additional extension step at 72℃ for 5 min was conducted. PCR products were separated by 8% SDS-PAGE and visualized by staining with ethidium bromide.
Molecular modeling
The p38 MAPKα (PDB ID: 1OUK) was chosen for docking studies. Its X-ray diffraction structure had a resolution of 2.5 ao (Fitzgerald et al., 2003) and pyridinylimidazole inhibitor was bound to the ATP-binding site of the p38 MAPK. p38 MAPK was prepared for docking using the Protein Preparation Wizard in the Schrödinger Suite 2010 by a standard procedure. Silibinin was prepared using MacroModel of Schrödinger and minimized, and the lowest energy conformations for docking were determined by using default parameters. The proteinligand docking analysis was conducted using the Induced Fit docking program of Schrödinger, which can provide the ligand binding flexibility with the binding pocket residues. Images were generated using the UCSF Chimera program (Pettersen et al., 2004).
Statistical analysis
The mean ± SD was determined for each treatment group in a given experiment. When significant differences occurred, treatment groups were compared to the vehicle control using a Dunnett's two-tailed t test (Dunnett, 1955).
RESULTS
Effect of silibinin on morphological change of macrophage
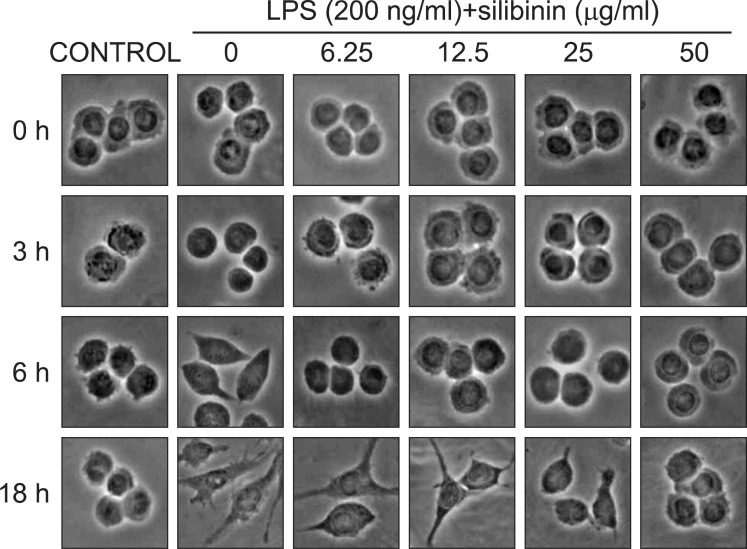
To investigate the effects of silibinin on macrophage activation, we analyzed the morphological changes in the mouse macrophage cell line, RAW 264.7 induced by LPS. The RAW 264.7 cells (5×105 cells/ml) were incubated with silibinin in the presence of LPS (200 ng/ml) for 3, 6, or 18 hours on cover slides in 12 well plates. When RAW 264.7 cells were exposed to LPS for 6 hours, pseudopodia extended from one or two sides of the cells (Fig. 1). More pseudopodia were extended at 18 hours of LPS-stimulated macrophages. Whereas untreated cells did not show these morphological changes at any time point. Treatment with silibinin inhibited LPS-induced morphological changes of macrophage at 18 h in a dose-dependent manner. Notably, no morphological change was found until 6 h at any dose of silibinin (Fig. 1). These data suggest that silibinin inhibits macrophage activation in LPS-stimulated RAW 264.7 cells.
Fig. 1. Inhibition of macrophage activation by silibinin. RAW 264.7 cells (5×105 cells/ml) were incubated with silibinin in the presence of LPS (200 ng/ml) for the indicated time on cover slides in 12 well plates. Cells were then subjected to microscopic analysis.
Inhibition of nitric oxide production by silibinin in LPSstumulated macrophages
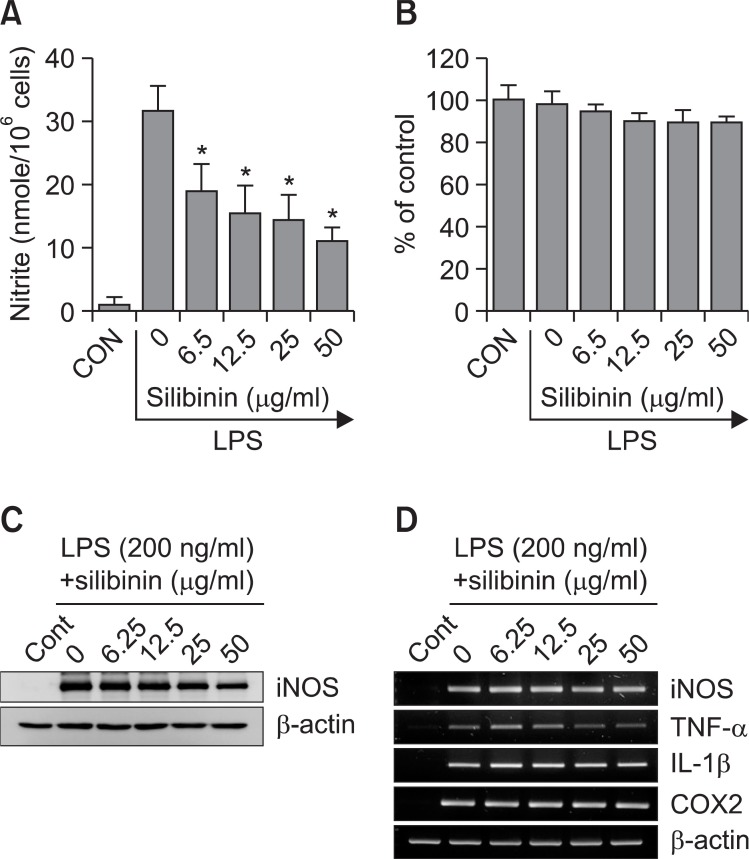
We investigated the effect of silibinin on NO production in LPS-stimulated RAW 264.7 cells since macrophages play a pivotal role in a host's defense against microbial infection through the production of variety of chemicals including NO (Hibbs et al., 1987; Palmer et al., 1988). LPS increased production of nitrite ≥20-fold over basal levels in RAW 264.7 cells (Fig. 2A). This induction of nitrite generation by LPS was inhibited by silibinin in a dose-dependent manner. No effect on cell viability was observed in any of the treatment groups and always exceeded 90% as determined by MTT assay (Fig. 2B). After RAW 264.7 cells were exposed to silibinin in the presence of LPS, the expression levels of iNOS gene were monitored by Western immunoblot analysis. As shown in Fig. 2C, iNOS protein production was inhibited by silibinin treatment. Control β-actin was constitutively expressed and was not affected by the treatment of silibinin. These results indicate that silibinin decreases the gene expression of iNOS, which is involved in inflammation (Hibbs et al., 1987). RT-PCR analysis of iNOS, TNF-α, IL-1β and COX-2 mRNA of silibinin-treated macrophages showed no significant changes in transcription of these inflammatory mediators induced by LPS, although silibinin induced mild inhibition of iNOS and TNF-α mRNA production (Fig. 2D).
Fig. 2. Inhibition of iNOS expression by silibinin. (A, B) RAW 264.7 cells (5×105 cells/ml) were treated with the indicated concentrations of silibinin in the presence of LPS (200 ng/ml) for 18 h. (A) The supernatants were subsequently isolated and analyzed for nitrite. (B) Cells were subjected to MTT assay. (C, D) RAW 264.7 cells were treated with the indicated concentrations of silibinin in the presence of LPS for 18 h (C) or 8 h (D). (C) Expression of iNOS was analyzed by Western immunoblotting using an antibody specific for iNOS. (D) Total RNA was isolated and analyzed for mRNA expression levels of iNOS, TNF-α, IL-1β, and β-actin. Each column shows the mean ± S.D. of triplicate determinations. An *, indicates a response that is significantly different from the control group as determined by Dunnett's two-tailed t test at p<0.05.
Inhibition of p38 MAPK phosphorylation by silibinin in LPSstumulated macrophages
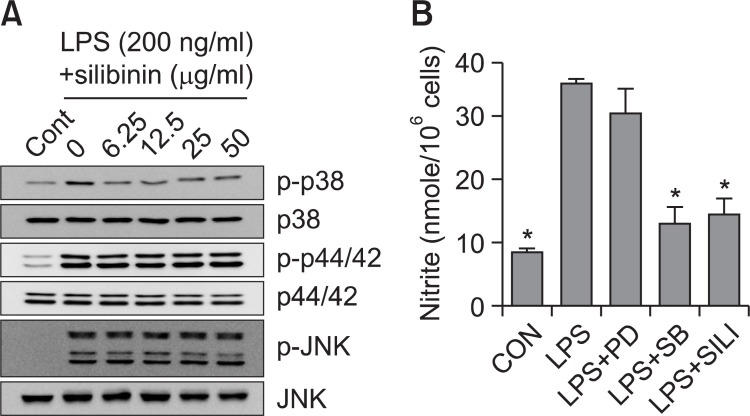
To further investigate the mechanism by which silibinin inhibits macrophage activation, we analyzed the effect of silibinin on MAPK signal transduction in LPS-stimulated macrophages. p38 MAPK phosphorylation is induced by LPS. In RAW 264.7 cells that were treated with silibinin in the presence of LPS for 30 min, the LPS-induced p38 MAPK phosphorylation was significanlty inhibited by silibinin (Fig. 3A). However, LPS-induced phosphorylation of ERK1/2 and JNK was not changed by silibinin.
Fig. 3. Inhibition of p38 MAPK phosphorylation by silibinin in LPSstimulated RAW 264.7 cells. (A) RAW 264.7 cells were treated with silibinin for 30 min in the presence of LPS. The phosphorylation of p38 MAPK and ERK1/2 (p44/p42) was analyzed using Western blot assay. (B) RAW 264.7 cells were treated with PD98059 (50 μDM), SB203580 (30 μM), or silibinin (SILI, 50 μg/ml) for 18 h in the presence of LPS. The supernatants were subsequently isolated and analyzed for nitrite. Each column shows the mean ± S.D. of triplicate determinations. An *, indicates a response that is significantly different from the control group as determined by Dunnett's two-tailed t test at p<0.05.
Since we showed that p38 MAPK is a possible target for silibinin, we further investigated whether the p38 MAPK pathway is involved in LPS-induced macrophage activation. Therefore, we blocked the p38 MAPK pathway and monitored macrophage activation when RAW 264.7 cells were challenged with LPS. SB203580, a bicyclic imidazole compound, is a specific inhibitor of p38 MAPK (Cuenda et al., 1995). PD98059 is a specific inhibitor of MEK-1, mitogen activated protein kinase/ extracellular signal-regulated kinase 1, which is responsible for ERK1/2 activation (Dudley et al., 1995). SB203580 inhibited LPS-induced nitrite generation, while PD98059 did not inhibit (Fig. 3B). These results suggest that silibinin inhibits p38 MAPK pathway, which is important in the regulation of macrophage activation by LPS.
Molecular docking
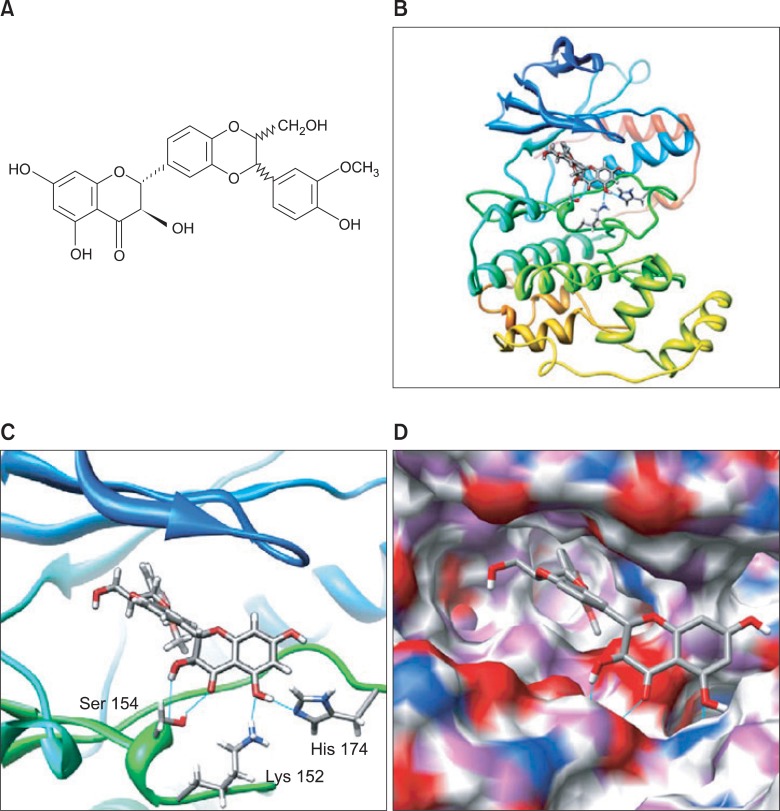
Because the activation of p38 MAPK, which plays an important role in macrophage activation, was inhibited by silibinin we performed a molecular docking of silibinin in silico to the ATP binding pocket of p38 MAPK using several protocols in the Schrödinger Suite of software. The chemical structure of silibinin, 2,3-Dihydro-3-(4-hydroxy-3-methoxyphenyl)-2- (hydroxymethyl)-6-(3,5,7-trihydroxy-4-oxobenzopyran-2-yl) benzodioxin, is shown in Fig. 4A. By studying all the models returned, we found that silibinin formed some favorable connections and docked nicely within the p38 MAPK ATP binding site (Fig. 4B). Some important hydrogen bonds were formed between silibinin and both backbone and side chains of p38 MAPK including Lys152, Ser154, and His174 (Fig. 4C). A space filling model of p38 MAPK represented silibinin binding at the ATP binding pocket of p38 MAPK (Fig. 4D). These images were generated using the UCSF Chimera program (Pettersen et al., 2004).
Fig. 4. Molecular Docking and Pose Generation. (A) Chemical structure of silibinin is shown. (B) A docking study was performed as described in Materials and methods. Silibinin was docked with p38 MAPK structure (PDB code: 1OUK). The protein residues are shown in a ribbon model. (C) The proposed binding pose of silibinin shows an interaction with Lys152, Ser154, and His174. (D) Space filling model showing the binding of silibninin in the ATP binding pocket of p38 MAPK.
DISCUSSION
We demonstrate that silibinin treatment significantly attenuates LPS-induced macrophage activation by blocking p38 MAPK activation in the macrophage line RAW 264.7 cells. Our molecular modeling study suggests that silibilin interacts with amino acid residues (Lys152, Ser154, and His174) of p38 MAPK (1OUK) and occupies the ATP binding pocket of p38 MAPK. The p38 MAPK plays an important role in the LPS-induced signal pathway leading to the expression of a number of proinflammatory molecules (Lee and Young, 1996; Da Silva et al., 1997; Chen and Wang, 1999). In the present study, we also showed that the p38 MAPK pathway is specifically involved in LPS-induced NO generation since NO production in the presence of p38 MAPK specific inhibitor, SB203580 was dramatically diminished. In contrast, PD98059, a specific inhibitor of MEK1 had no effect on NO production. This is further supported by our previous results showing the role of p38 MAPK in iNOS expression (Jeon et al., 2000). The p38 MAPK also regulates LPS-induced production of cytokines including TNF-α, IL-1, and IL-10 and TNF-induced IL-6 production in fibroblasts (Beyaert et al., 1996; Foey et al., 1998). Thus, silibinin, like SB203580, inhibits NO production and macrophage activation at least in part by blocking the p38 MAPK pathway. MAPKs have been suggested to be potential targets of silymarin and silibinin (Singh et al., 2002; Kim et al., 2009). Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of MAPKs and induction of apoptosis (Singh et al., 2002). Silibinin block the activation of NF-κB, JNK, p38 MAPK, and ERK in osteoclast precursors in response to RANKL (Kim et al., 2009).
Macrophages can be activated by cytokines or by microbial components (Stuehr and Nathan, 1989; Higuchi et al., 1990), and play pivotal roles in both nonspecific and specific immunity. For nonspecific immunity, macrophages engulf and digest microorganisms and release inflammatory mediators such as NO. In specific immunity, macrophages act as antigen presenting cells and release cytokines such as TNF-α, IL-1, IL-6, and IL-12 to regulate helper T cells (Billack, 2006). Furthermore, activated macrophages suppress the growths of a variety of microbes and tumor cells (Lowenstein et al., 1993). It was previously reported that silibinin has cancerpreventive and anticarcinogenic effects (Hogan et al., 2007; Mokhtari et al., 2008). Although macrophage activation and NO production are essential for proper defense mechanism, strong activation of macrophages causes the releases excessive cytokines and mediators, including NO, which has been implicated in many pathophysiological conditions including inflammation, atherosclerosis, and septic shock (Cohen, 2002). Immunomodulating compounds target macrophages due to the critical role that macrophage activation plays in innate immune response, (Li et al., 2010; Vo et al., 2012).
In summary, these experiments demonstrate that silibinin, a polyphenolic compound isolated from Silybum marianum, inhibits LPS-induced macrophage activation and NO generation. Based on our findings, the most likely mechanism that can account for this biological effect involves the negative regulation of the p38 MAPK pathway. Due to the critical role that macrophage activation plays in mediating inflammatory responses, the inhibitory effects of silibinin on macrophage activation suggest that silibinin may be a useful agent for inflammatory disease such as insulin-dependent Diabetes mellitus, rheumatoid arthritis, and sepsis.
Acknowledgments
This research was supported by research funds from Chosun University, 2013.
References
- 1.Al-Anati L., Essid E., Reinehr R., Petzinger E. Silibinin protects OTA-mediated TNF-alpha release from perfused rat livers and isolated rat Kupffer cells. Mol. Nutr. Food Res. (2009);53:460–466. doi: 10.1002/mnfr.200800110. [DOI] [PubMed] [Google Scholar]
- 2.Beyaert R., Cuenda A., Vanden Berghe W., Plaisance S., Lee J. C., Haegeman G., Cohen P., Fiers W. The p38/RK mitogen- activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. (1996);15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- 3.Billack B. Macrophage activation: role of toll-like receptors, nitric oxide, and nuclear factor kappa B. Am. J. Pharm. Educ. (2006);70:102. doi: 10.5688/aj7005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C. C., Wang J. K. p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 cells. Mol. Pharmacol. (1999);55:481–488. [PubMed] [Google Scholar]
- 5.Cohen J. The immunopathogenesis of sepsis. Nature. (2002);420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 6.Cristofalo R., Bannwart-Castro C. F., Magalhaes C. G., Borges V. T., Peracoli J. C., Witkin S. S., Peracoli M. T. Silibinin attenuates oxidative metabolism and cytokine production by monocytes from preeclamptic women. Free Radic. Res. (2013);47:268–275. doi: 10.3109/10715762.2013.765951. [DOI] [PubMed] [Google Scholar]
- 7.Cuenda A., Rouse J., Doza Y. N., Meier R., Cohen P., Gallagher T. F., Young P. R., Lee J. C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. (1995);364:229–233. doi: 10.1016/0014-5793(95)00357-F. [DOI] [PubMed] [Google Scholar]
- 8.Da Silva J., Pierrat B., Mary J. L., Lesslauer W. Blockade of p38 mitogen-activated protein kinase pathway inhibits inducible nitric-oxide synthase expression in mouse astrocytes. J. Biol. Chem. . (1997);272:28373–28380. doi: 10.1074/jbc.272.45.28373. [DOI] [PubMed] [Google Scholar]
- 9.Dudley D. T., Pang L., Decker S. J., Bridges A. J., Saltiel A. R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. U.S.A. (1995);92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunnett C. W. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. (1955);50:1096–1121. doi: 10.1080/01621459.1955.10501294. [DOI] [Google Scholar]
- 11.Fitzgerald C. E., Patel S. B., Becker J. W., Cameron P. M., Zaller D., Pikounis V. B., O'Keefe S. J., Scapin G. Structural basis for p38alpha MAP kinase quinazolinone and pyridol-pyrimidine inhibitor specificity. Nat. Struct. Biol. (2003);10:764–769. doi: 10.1038/nsb949. [DOI] [PubMed] [Google Scholar]
- 12.Foey A. D., Parry S. L., Williams L. M., Feldmann M., Foxwell B. M., Brennan F. M. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J. Immunol. (1998);160:920–928. [PubMed] [Google Scholar]
- 13.Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J.S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. (1982);126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 14.Hibbs J. B. Jr., Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science . (1987);235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi M., Higashi N., Taki H., Osawa T. Cytolytic mechanisms of activated macrophages. Tumor necrosis factor and Larginine- dependent mechanisms act synergistically as the major cytolytic mechanisms of activated macrophages. J. Immunol. (1990);144:1425–1431. [PubMed] [Google Scholar]
- 16.Hogan F. S., Krishnegowda N. K., Mikhailova M., Kahlenberg M. S. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J. Surg. Res. (2007);143:58–65. doi: 10.1016/j.jss.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 17.Huong P. T., Lee M. Y., Lee K. Y., Chang I. Y., Lee S. K., Yoon S. P., Lee D. C., Jeon Y. J. Synergistic induction of iNOS by IFN-γ and glycoprotein isolated from Dioscorea batatas. Korean J. Physiol. Pharmacol. (2012);16:431–436. doi: 10.4196/kjpp.2012.16.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon Y. J., Kim Y. K., Lee M., Park S. M., Han S. B., Kim H.M. Radicicol suppresses expression of inducible nitric-oxide synthase by blocking p38 kinase and nuclear factor-kappaB/Rel in lipopolysaccharide-stimulated macrophages. J. Pharmacol. Exp. Ther. (2000);294:548–554. [PubMed] [Google Scholar]
- 19.Kang J. S., Jeon Y. J., Kim H. M., Han S. H., Yang K. H. Inhibition of inducible nitric-oxide synthase expression by silymarin in lipopolysaccharide-stimulated macrophages. J. Pharmacol. Exp. Ther. . (2002);302:138–144. doi: 10.1124/jpet.302.1.138. [DOI] [PubMed] [Google Scholar]
- 20.Kim J. H., Kim K., Jin H. M., Song I., Youn B. U., Lee J., Kim N. Silibinin inhibits osteoclast differentiation mediated by TNF family members. Mol. Cells . (2009);28:201–207. doi: 10.1007/s10059-009-0123-y. [DOI] [PubMed] [Google Scholar]
- 21.Lee J. C., Young P. R. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J. Leukoc. Biol. . (1996);59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 22.Letteron P., Labbe G., Degott C., Berson A., Fromenty B., Delaforge M., Larrey D., Pessayre D. Mechanism for the protective effects of silymarin against carbon tetrachlorideinduced lipid peroxidation and hepatotoxicity in mice. Evidence that silymarin acts both as an inhibitor of metabolic activation and as a chain-breaking antioxidant. Biochem. Pharmacol. (1990);39:2027–2034. doi: 10.1016/0006-2952(90)90625-U. [DOI] [PubMed] [Google Scholar]
- 23.Li M. H., Kothandan G., Cho S. J., Huong P. T., Nan Y. H., Lee K.Y., Shin S. Y., Yea S. S., Jeon Y. J. Magnolol inhibits LPS-induced NF-kappaB/Rel activation by blocking p38 kinase in murine macrophages. Korean J. Physiol. Pharmacol. (2010);14:353–358. doi: 10.4196/kjpp.2010.14.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowenstein C. J., Alley E. W., Raval P., Snowman A. M., Snyder S. H., Russell S. W., Murphy W. J. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc. Natl. Acad. Sci. U.S.A. (1993);90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mereish K. A., Bunner D. L., Ragland D. R., Creasia D. A. Protection against microcystin-LR-induced hepatotoxicity by silymarin: biochemistry, histopathology, and lethality. Pharm. Res. (1991);8:273–277. doi: 10.1023/A:1015868809990. [DOI] [PubMed] [Google Scholar]
- 26.Mokhtari M. J., Motamed N., Shokrgozar M. A. Evaluation of silibinin on the viability, migration and adhesion of the human prostate adenocarcinoma (PC-3) cell line. Cell Biol. Int. (2008);32:888–892. doi: 10.1016/j.cellbi.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature . (1988);333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 28.Pettersen E. F., Goddard T. D., Huang C. C., Couch G. S., Greenblatt D. M., Meng E. C., Ferrin T. E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. . (2004):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 29.Pliskova M., Vondracek J., Kren V., Gazak R., Sedmera P., Walterova D., Psotova J., Simanek V., Machala M. Effects of silymarin flavonolignans and synthetic silybin derivatives on estrogen and aryl hydrocarbon receptor activation. Toxicology. (2005);215:80–89. doi: 10.1016/j.tox.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 30.Raingeaud J., Gupta S., Rogers J. S., Dickens M., Han J., Ulevitch R. J., Davis R. J. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. (1995);270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 31.Singh R. P., Tyagi A. K., Zhao J., Agarwal R. Silymarin inhibits growth and causes regression of established skin tumors in SENCAR mice via modulation of mitogen-activated protein kinases and induction of apoptosis. Carcinogenesis. (2002);23:499–510. doi: 10.1093/carcin/23.3.499. [DOI] [PubMed] [Google Scholar]
- 32.Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. . (1989);169:1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su B., Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr. Opin. Immunol. (1996);8:402–411. doi: 10.1016/S0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 34.Valenzuela A., Garrido A. Biochemical bases of the pharmacological action of the flavonoid silymarin and of its structural isomer silibinin. Biol. Res. (1994);27:105–112. [PubMed] [Google Scholar]
- 35.Vo V. A., Lee J. W., Chang J. E., Kim J. Y., Kim N. H., Lee H. J., Kim S. S., Chun W., Kwon Y. S. Avicularin inhibits lipopolysaccharide-induced inflammatory response by suppressing ERK phosphorylation in RAW 264.7 macrophages. Biomol. Ther. (2012);20:532–537. doi: 10.4062/biomolther.2012.20.6.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinstein S. L., Sanghera J. S., Lemke K., DeFranco A. L., Pelech S. L. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J. Biol. Chem. (1992);267:14955–14962. [PubMed] [Google Scholar]
- 37.Whitmarsh A. J., Davis R. J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. (1996);74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 38.Zhao J., Sharma Y., Agarwal R. Significant inhibition by the flavonoid antioxidant silymarin against 12-O-tetradecanoylphorbol 13-acetate-caused modulation of antioxidant and inflammatory enzymes, and cyclooxygenase 2 and interleukin-1alpha expression in SENCAR mouse epidermis: implications in the prevention of stage I tumor promotion. Mol. Carcinog. (1999);26:321–333. doi: 10.1002/(SICI)1098-2744(199912)26:4&lt;321::AID-MC11&gt;3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]