Abstract
Silymarin has been introduced fairly recently as a hepatoprotective agent. But its mechanisms of action still have not been well established. The aim of this study was to make alcoholic fatty liver model of rats in a short time and investigate silymarin’s protective effects and possible mechanisms on alcoholic fatty liver for rats. The model of rat’s alcoholic fatty liver was induced by intragastric infusion of ethanol and high-fat diet for six weeks. Histopathological changes were assessed by hematoxylin and eosin staining (HE). The activities of alanine transarninase (ALT) and aspartate aminotransferase (AST), the levels of total bilirubin (TBIL), total cholesterol (TC) and triglyceride (TG) in serum were detected with routine laboratory methods using an autoanalyzer. The activities of superoxide dismutase (SOD) and glutathione peroxidase (GPx) and the level of malondialdehyde (MDA) in liver homogenates were measured by spectrophotometry. The TG content in liver tissue was determined by spectrophotometry. The expression of nuclear factor-κB (NF-κB), intercellular adhesion molecule-1 (ICAM-1) and interleukin-6 (IL-6) in the liver were analyzed by immunohistochemistry. Silymarin effectively protected liver from alcohol-induced injury as evidenced by improving histological damage situation, reducing ALT and AST activities and TBIL level in serum, increasing SOD and GPx activities and decreasing MDA content in liver homogenates and reducing TG content in liver tissue. Additionally, silymarin markedly downregulated the expression of NF-κB p65, ICAM-1 and IL-6 in liver tissue. In conclusion, Silymarin could protect against the liver injury caused by ethanol administration. The effect may be related to alleviating lipid peroxidation and inhibiting the expression of NF-κB.
Keywords: Alcoholic, Fatty liver, ICAM-1, IL-6, NF-κB, Silymarin
INTRODUCTION
Long-term intake and abuse of alcohol results in various liver abnormalities ranging from simple fatty liver or steatosis to steatohepatitis, fibrosis, cirrhosis and hepatocellular carcinoma (Tilg and Diehl, 2000). Liver injury from alcohol has many cases, including intrahepatic events resulting in cellular oxidative stress, loss of protective enzymes and transporters and extrahepatic stimuli, such as ethanol inducing gut-derived endotoxin (Eagon, 2010). Although much progress has been made in understanding the pathogenesis of alcoholic liver disease (ALD), there is still no widely accepted drug therapy for any stage of ALD. Therefore, efforts to understand pathogenesis of ALD primarily focuses on events that lead to the early accumulation of the scar in the hope of identifying therapeutic targets to hinder its progression. Silymarin is a flavonoid extracted from the milk thistle Silybum marianum. Although silymarin has been described to possess antioxidant, immunomodulatory, antiproliferative, antifibrotic, and antiviral activities (Saller et al., 2001), its mechanisms of action still have not been well established.
The aim of this study is to evaluate the protective effects of silymarin on alcoholic fatty liver model of rats, and to explore the possible mechanisms involved in the protection.
MATERIALS AND METHODS
Chemicals
Silymarin (lot number: J20050094) used in our study was kindly presented by Madaus AG (Cologne, Germany). Its appropriate proportion could achieve maximum efficiency. Silymarin was dissolved into water by 20 mg/ml, 30 mg/ml and 40 mg/ml. Anhydrous ethanol was purchased from Shang-hai Semi-works Production Chemical Corporation (Shanghai, China). Commercial kits, used for determining superoxide dismutase (SOD), glutathione peroxidase (GPx), malondialdehyde (MDA) and triglyceride (TG), were obtained from Jiancheng Institute of Biotechnology (Nanjing, China). Diaminobenzidine (DAB) chromogenic agent was obtained from Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd. (Beijing, China). Monoclonal anti-ICAM-1 antibody and polyclonal anti-NF-κB p65 antibody were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Polyclonal rabbit anti-IL-6 antibody was purchased from Beijing Boisynthsis Biotechnology Co. Ltd. (Beijing, China). IHC Biotin Block kit was purchased from Fuzhou Maixin Biotechnology Co. Ltd. (Fuzhou, China).
Animal experiments and drug treatment
Female Sprague-Dawley rats, weighing 180-220 g, were purchased from the Experimental Animal Center of Anhui Medical University. The animal study protocol was in compliance with the Guidelines of China for Animal Care, which conformed to the internationally accepted principles in the care and use of experimental animals. After acclimation for 1 week, rats were randomly divided into five groups: normal group (n=10), model group (n=15) treated with ethanol for 6 weeks, silymarin groups (100 mg/kg,150 mg/kg, 200 mg/kg; n=15, respectively) treated with ethanol and different silymarin doses for 6 weeks. Five groups of rats were fed with same fat-rich diet (84% common animal feeds+1% cholesterol+5% lard+10% corn oil). The rats of model group and silymarin groups received ethanol by gavage twice a day, whereas normal group received equal amount of physiological saline. The daily doses and concentrations of ethanol for the rats of the model and silymarin groups were 6 g/kg/d (40%, v/v) in the first week and then increased progressively every week up to a maximum of 8 g/kg/d (60%, v/v) in a further 5 weeks. In the silymarin groups, silymarin was gavaged two hours after the administration of the first ethanol every day at the doses of 100 mg/kg/d, 150 mg/kg/d, 200 mg/kg/d. Body weight for each rat was measured weekly. At the end of the experimental period, the rats were anesthetized by 10% hydral (3 ml/ kg) and blood was collected into tubes and centrifuged. The plasma was stored at -80oC till analysis. Liver tissue was fixed in formalin for routine histological examination. The remaining livers were frozen in liquid nitrogen and stored at -80oC in case of requiring.
Histopathological examination
Liver tissues were cut and fixed with 4% paraformaldehyde. The tissue slices were embedded in paraffi n. Tissue sections of 5 μm were stained with hematoxylin and eosin (HE) and observed under a light microscope. The severity of liver pathology was assessed as follows (Nanji et al., 2003): steatosis (the percentage of liver cells containing fat) was scored F0 with less than 5% of the cells containing fat; F1 with 5% to 30% of cells containing fat; F2 with 30% to 50% of the cells containing fat; F3 with 50% to 75% of the cells containing fat; and F4 with greater than 75% of the cells containing fat.
Serum liver function and blood lipids assay
Serum activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and serum levels of total bilirubin (TBIL), total cholesterol (TC) and triglyceride (TG) were measured with routine laboratory methods using an autoanalyzer (Hitachi Automatic Analyzer, Japan).
Liver MDA content and SOD, GPx activities assaying
Liver samples were weighed and homogenized (1:9, w:v) in 0.9% saline. The homogenates were then centrifuged at 1,000×g for 10 min at 4℃, and the supernatant was taken for MDA content, SOD and GPx activities assaying, total protein determination with a commercial kit following manufacturer’s instructions.
Hepatic triglyceride content determination
For determination of total triglyceride content, 100 mg of liver tissue was homogenized in 2 ml of buffer that contains 18 mM Tris, pH 7.5, 300 mM mannitol, 50 mM EGTA, and 0.1 mM phenylmethysulfonyl fluoride (Zhou et al., 2006). Tissue lipids were extracted with methanol: chloroform (1:2). Triglyceride content was determined by using commercially available kit.
Immunohistochemical detection of NF-κB p65, ICAM-1 and IL-6 in liver
The expression of NF-κB p65, ICAM-1 and IL-6 were determined by standard immunohistochemical procedure. Briefly, sections (5 μm thick) were cut from formalin-fixed and paraffi n embedded liver samples. After a standard dehydration-rehydration procedure, liver sections were incubated with 3% H2O2 to quench endogenous peroxidase activity. The sections were then heated using a microwave oven for 20 min in 10 mM sodium citrate (pH 6.0) buffer to retrieve antigen. After cooling, blocking buffer (goat serum) was applied at room temperature for 20 min followed by application of rabbit anti-rat NF-κB p65 antibody (diluted 1:100) or rabbit anti-rat ICAM-1 antibody (diluted 1:100) or rabbit anti-rat IL-6 antibody (diluted 1:100). Finally, sections were incubated in diaminobenzidine and then counterstained with hematoxylin. The sections incubated with PBS instead of the primary antibody were used as negative controls. Brown-yellow granules in cytoplasm or nuclei were recognized as positive staining for NF-κB p65, and brown-yellow granules in cytoplasm was recognized as positive staining for ICAM-1 or IL-6. Evaluation of sections was undertaken by assessing the optical density (OD). Five randomly areas on each section were selected using a light microscope Nikon E400. All measurements were carried out using an image analyzer Image-Pro Plus, while the staining was expressed as density of unit area.
Statistical analysis
Data were analyzed with SPSS software. Quantitative data were expressed as mean ± SD and analyzed by one way ANOVA analysis. Frequency data (pathologic grading of fatty liver) were analyzed by Ridit analysis. A p-value<0.05 was considered significant.
RESULTS
General appearance
During the experiment, 28 rats died because of alcohol’s poison or fluids poured mistakenly into trachea. At the beginning of study, there were no differences in appearance among the groups. However, the appearance of lethargy, anorexia, sluggishness, dim fur occurred in model group at the end of the study. The surface of the liver was smooth and red-brown in normal group, whereas enlarged hepatic volume, increased weight, dimmer color were noted in model group. The weight gain was significantly lower in model group than that in normal group (p<0.01). Treatment with silymarin (200 mg/kg) showed significantly higher in the weight gain than in model group (p<0.05). Indices of liver in model group was significantly higher than that in normal group (p<0.05), whereas no significant difference in the indices of liver was observed among model group and silymarin groups (Table 1).
Table 1.
Body weight gain and indices of liver in different groups (mean ± SD)
| Groups | n | Weight gain (g) | Indices of liver (%) |
|---|---|---|---|
|
| |||
| Normal | 8 | 57.88 ± 28.79 | 3.57 ± 0.23 |
| Model | 9 | 30.33 ± 13.31** | 4.06 ± 0.53* |
| Silymarin (mg/kg) | |||
| 100 | 8 | 41.13 ± 10.53 | 3.87 ± 0.46 |
| 150 | 8 | 41.13 ± 17.90 | 3.76 ± 0.34 |
| 200 | 9 | 49.33 ± 10.01▲ | 3.93 ± 0.58 |
*p<0.05 ,**p<0.01 vs normal group, ▲p<0.05 vs model group.
Pathological changes
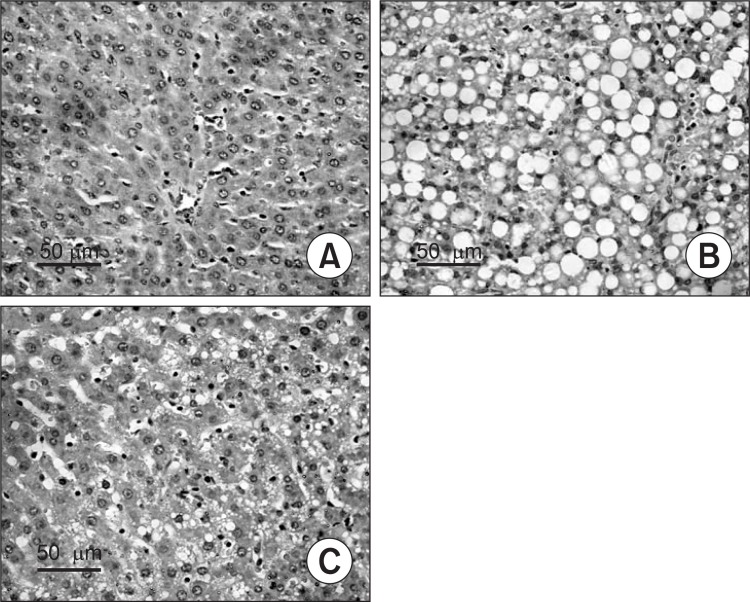
Liver tissue samples of rats in normal group showed slight steatosis, normal lobular architecture with central veins, but no obvious inflammation or necrosis was observed (Fig. 1A). In model group, the structure of hepatic cord was deranged, and a greater accumulation of fat droplets, various degrees of diffuse hepatic steatosis and intralobular inflammation could be found obviously (Fig. 1B). In the silymarin groups, less degree and less extensive of fatty liver were observed than those in livers from the model group (Fig. 1C). Statistical analysis revealed that steatosis was much less severe in silymarin groups (150 mg/kg, 200 mg/kg) than that in model group (p<0.05, p<0.01, respectively) (Table 2, Fig. 1).
Fig. 1. Histopathological changes in the liver of different groups (HE staining, original magnification, 200). (A) Normal group. (B) Model group, alcohol was administrated for 6 weeks. (C) Silymarin group (150 mg/kg), alcohol and silymarin (150 mg/kg) were administrated for 6 weeks.
Table 2.
Pathological changes in different groups
| Groups | n | Pathologic grading of alcoholic fatty liver | u value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| F0 | F1 | F2 | F3 | F4 | |||
|
| |||||||
| Normal | 8 | 5 | 3 | 0 | 0 | 0 | 3.5645▲▲ |
| Model | 9 | 0 | 0 | 3 | 4 | 2 | - |
| Silymarin (mg/kg) | |||||||
| 100 | 8 | 0 | 0 | 4 | 4 | 0 | 0.9901 |
| 150 | 8 | 0 | 3 | 3 | 2 | 0 | 2.1288▲ |
| 200 | 9 | 0 | 5 | 3 | 1 | 0 | 2.8124▲▲ |
p<0.05 indicate u>1.96, p<0.01 indicate u>2.58, ▲p<0.05, ▲▲p< 0.01 vs model group.
Liver function and blood lipids
The results showed that the administration of ethanol induced a greater increase in activities of AST and ALT (p<0.05, p<0.01, respectively) and levels of TBIL and TG (p<0.05, p<0.01, respectively) in serum as compared to normal group. Treatment with silymarin (200 mg/kg) showed a greater decrease in serum activities of ALT and AST and the level of TBIL than in model group (p<0.05), whereas no significant difference in the level of TC was observed among normal group, model group and silymarin groups (Table 3).
Table 3.
Serum biochemical parameters in different groups (mean ± SD)
| Groups | n | ALT (U/L) | AST (U/L) | TG (mmol/L) | TC (mmol/L) | TBIL (umol/L) |
|---|---|---|---|---|---|---|
|
| ||||||
| Normal | 8 | 43.75 ± 17.46 | 121.13 ± 42.37 | 0.55 ± 0.12 | 2.64 ± 0.62 | 0.41 ± 0.14 |
| Model | 9 | 86.00 ± 41.37** | 168.33 ± 65.51* | 1.18 ± 0.56** | 3.05 ± 1.13 | 0.75 ± 0.31* |
| Silymarin (mg/kg) | ||||||
| 100 | 8 | 73.88 ± 29.78 | 140.25 ± 34.52 | 0.93 ± 0.39 | 2.76 ± 0.58 | 0.53 ± 0.37 |
| 150 | 8 | 59.62 ± 7.60▲ | 139.75 ± 40.85 | 0.88 ± 0.47 | 2.71 ± 0.99 | 0.52 ± 0.29 |
| 200 | 9 | 54.22 ± 14.86▲ | 117.00 ± 39.20▲ | 0.87 ± 0.20 | 2.42 ± 1.35 | 0.44 ± 0.16▲ |
*p<0.05 ,**p<0.01 vs normal group, ▲p<0.05 vs model group.
MDA content and SOD, GPx activity in liver homogenates
The MDA level was significantly higher in model group than that in normal group in liver homogenates (p<0.01). Compared with the model group, silymarin could decrease the MDA level obviously in silymarin groups (p<0.01). Furthermore, ethanol gavage led to a marked reduction in activities of SOD, GPx compared with normal group (p<0.01). Compared with the model group, silymarin (100 mg/kg, 150 mg/kg, 200 mg/kg) significantly increased GPx activity (p<0.05), and similarly silymarin (150 mg/kg, 200 mg/kg) significantly increased the activity of SOD (p<0.01) (Table 4).
Table 4.
Effects of silymarin on the content of MDA and the activities of SOD and GPx in liver homogenates of different groups (mean ± SD)
| Groups | n | MDA (nmol/mg prot) | SOD (U/mg prot) | GPx (U/mg prot) |
|---|---|---|---|---|
|
| ||||
| Normal | 8 | 1.07 ± 0.71 | 102.29 ± 5.24 | 47.21 ± 11.30 |
| Model | 9 | 2.31 ± 0.65** | 78.68 ± 7.04** | 30.40 ± 5.06** |
| Silymarin (mg/kg) | ||||
| 100 | 8 | 0.82 ± 0.65▲▲ | 84.45 ± 9.20 | 41.59 ± 13.77▲ |
| 150 | 8 | 0.74 ± 0.19▲▲ | 91.70 ± 9.87▲▲ | 41.76 ± 9.04▲ |
| 200 | 9 | 0.53 ± 0.29▲▲ | 93.46 ± 8.58▲▲ | 41.85 ± 4.17▲ |
**p<0.01 vs normal group, ▲p<0.05 , ▲▲p<0.01 vs model group.
Hepatic triglyceride content
Compared with the normal group, ethanol administration induced a significantly higher triglyceride content in the the liver (p<0.01); however, this accumulation was attenuated by silymarin (150 mg/kg, 200 mg/kg) supplementation (p<0.01) (Fig. 2).
Fig. 2. Effects of silymarin on the content of the triglyceride (TG) in alcoholic fatty liver in rats. Values are mean ± SD. **p<0.01 vs normal group, ▲▲p<0.01 vs model group.
Expression of NF-κB p65, ICAM-1 and IL-6 in liver
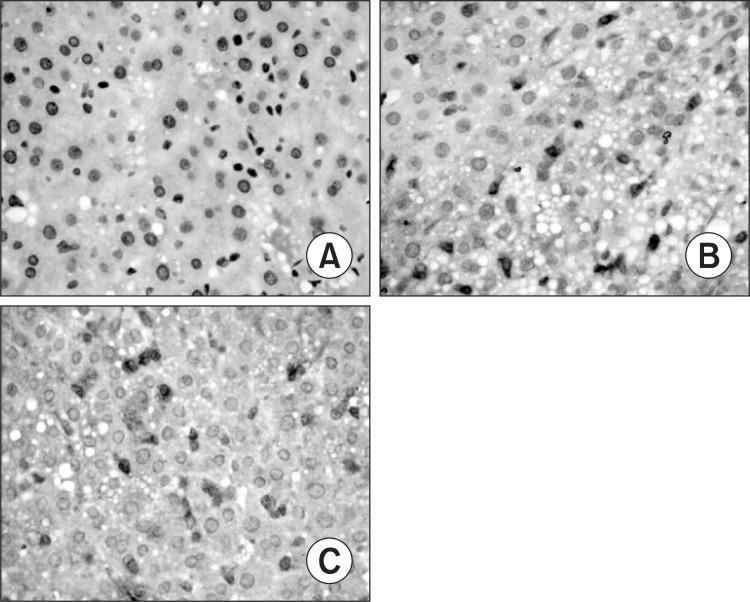
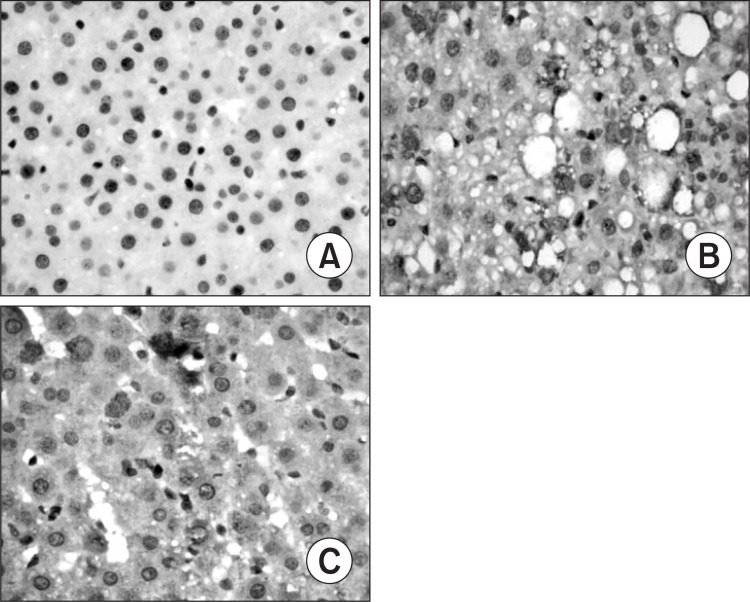
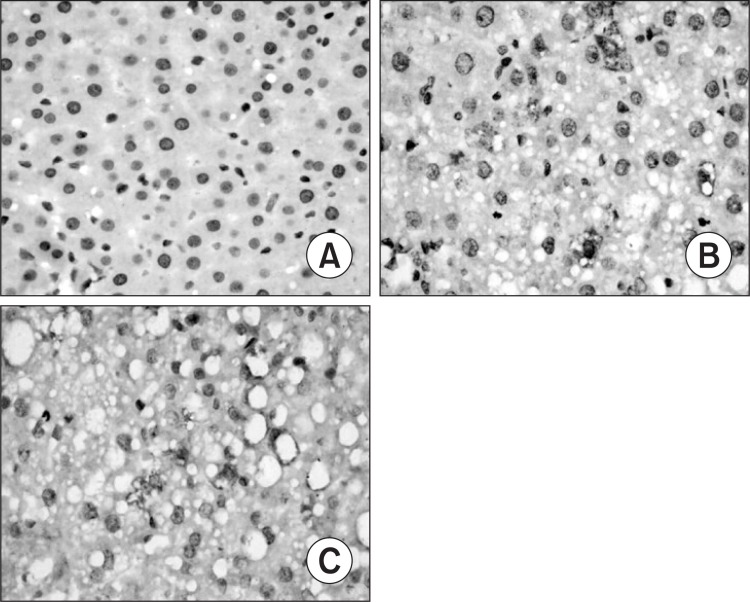
Only faint immunoreactive staining of NF-κB p65, ICAM-1 and IL-6 were detected in the liver from normal group. More intense nuclear staining of NF-κB p65 and intense cytoplasm staining of ICAM-1 and IL-6 were observed in model group as compared to those in normal group (p<0.01). Compared with the model group, less intense staining of NF-κB p65, ICAM-1 and IL-6 were found in silymarin groups (150 mg/kg, 200 mg/ kg) (p<0.01) (Table 5, Fig. 3–5).
Table 5.
Effects of silymarin on expression of NF-κB, ICAM-1 and IL-6 in liver of different groups (mean ± SD)
| Groups | n | NF-κB p65 | ICAM-1 | IL-6 |
|---|---|---|---|---|
|
| ||||
| Normal | 8 | 0.298 ± 0.055 | 0.278 ± 0.018 | 0.359 ± 0.040 |
| Model | 9 | 0.463 ± 0.095** | 0.679 ± 0.132** | 0.507 ± 0.063** |
| Silymarin (mg/kg) | ||||
| 100 | 8 | 0.419 ± 0.023 | 0.597 ± 0.093 | 0.474 ± 0.036 |
| 150 | 8 | 0.352 ± 0.040▲▲ | 0.435 ± 0.082▲▲ | 0.385 ± 0.029▲▲ |
| 200 | 9 | 0.327 ± 0.023▲▲ | 0.347 ± 0.059▲▲ | 0.381 ± 0.041▲▲ |
**p<0.01 vs normal group, ▲▲p<0.01 vs model group.
Fig. 3. Hepatic nuclear factor-κB (NF-κB p65) expression in different groups (original magnification, 400). (A) Normal group. (B) Model group, alcohol was administrated for 6 weeks. (C) Silymarin group (150 mg/kg), alcohol and silymarin (150 mg/kg) were administrated for 6 weeks.
Fig. 5. Hepatic interleukin-6 (IL-6) expression in different gro-ups (original magnification, 400). (A) Normal group. (B) Model group, alcohol was administrated for 6 weeks. (C) Silymarin group (150 mg/kg), alcohol and silymarin (150 mg/kg) were administrated for 6 weeks.
DISCUSSION
Although there are many methods, ethanol gavage is widely used to induce alcoholic fatty liver in rats. This method could avoid rejection of rat to alcohol and consist with human drinking. Excessive alcohol intake could maintain blood high alcohol levels, and increase the levels of circulating endotoxin in the portal blood (Shi et al., 2010) and this is the key factor for the progression of alcoholic liver diseases. It has been reported that polyunsaturated fatty acids play an important role in the pathogenesis of alcoholic liver disease (Polavarapu et al., 1998; Nanji, 2004; Purohit et al., 2004). Female rats in the alcohol-induced liver injury model experience signifi-cantly more severe liver injury than the males (Polavarapu et al., 1998; Banerjee et al., 2009; Eagon, 2010). In view of the above, hepatic steatosis was induced by ethanol gavage plus diet such as corn oil which is rich in unsaturated fatty acids for 6 weeks in our study. Our results showed that obvious liver injury and hepatic steatosis were observed in model group. The alcoholic fatty liver model was successful and this model could mimic key aspects of alcoholic liver disease in humans, and could be useful for exploring the effects of silymarin on hepatic steatosis. Compared with other studies, the duration of making model was significantly shortened. But the animal death rate were increased. We shall improve the mothod of making alcoholic fatty liver model for reducing the animal death rate.
Oxidant stress has been reported to play a role in the pathogenesis of alcohol-induced liver injury (Arteel, 2003). Liver content of MDA, a marker of lipid peroxidation, is gener-ally presented as the total level of lipid peroxidation products. MDA can produce ozone which leading to peroxidation and denaturation of membranes (Ajamieh et al., 2004). Oxidative stress is determined by increased ROS and enzymatic antioxidant systems including SOD, GPx, and so on, which act as protectors of oxidative stress. So MDA and SOD, GPx are a manifestation of peroxidation and activities of hepatic antioxidants. It has been reported that administration of silymarin significantly decreased lipid peroxidation and increased endogenous antioxidants, such as SOD, CAT, and GSH (Kiruthiga et al., 2007). Silymarin is probably able to antagonise the depletion of the two main detoxifying mechanisms, GSH and SOD, by reducing the free radical load, increasing GSH levels and stimulating SOD activity (Fraschini et al., 2002). In this study, treatment with silymarin (150 mg/kg, 200 mg/kg) could significantly increase the activities of SOD and GPx as well as decrease MDA level, which alleviates deleterious effects induced by alcohol. These results are in agreement with previously reported (Fraschini et al., 2002). So silymarin may play a role of protection of liver by inhibiting oxidative stress.
Fig. 4. Hepatic intercellular adhesion molecule-1 (ICAM-1) expression in different groups (original magnification, 400). (A) Normal group. (B) Model group, alcohol was administrated for 6 weeks. (C) Silymarin group (150 mg/kg), alcohol and silymarin (150 mg/kg) were administrated for 6 weeks.
NF-κB is a ubiquitous transcription factor that is implicated in the activation of many genes including those involved in alcoholic liver injury (Barnes and Karin,1997). In unstimulated cells, NF-κB exists in cytoplasm in an inactive form associated with regulatory proteins called IκB. When these cells are stimulated, it is translocated to the nuclei and bound to decameric DNA sequences, and activates transcription of target gene. NF-κB can be activated by lesion-induced oxidative stress, bacterial endotoxin, or cytokines and subsequently transactivate the expression of many cytokines and adhesion molecules (Chen et al., 1999). NF-κB activation enhances the transcription of proinflammatory cytokines, and the cytokines are known to in turn activate NF-κB (Neurath et al., 1996). The positive feedback is believed to serve to amplify inflammatory signals and exacerbate liver injury. One pathway of liver injury by oxidative stress is via NF-κB (Nanji et al., 1999). The activation of NF-κB was associated with necrosis and inflammation in the liver (Nanji et al., 1999). Following activation, NF-κB causes the expression of inflammatory mediators, including cytokines (particularly TNF-α, IL-6, IL-12), chemokines, inducible nitric oxide synthase (iNOS) and enzymes such as adhesion molecules (Barnes and Karin, 1997). Few reports involved whether hepatoprotective effects of silymarin on alcoholic fatty liver were related to inhibiting NF-κB activation. Our data showed that ethanol administration significantly enhanced the expression of NF-κB, IL-6 and ICAM-1 in the liver, and the expression were attenuated by silymarin supplementation (150 mg/kg, 200 mg/kg). Our results suggested that silymarin supplementation can attenuate expression of NF-κB in the liver, then inhibit the proinflammatory cytokines production. So silymarin may play a role of protecting liver by inhibiting expression of NF-κB and reducing proinflammatory cytokines production.
Silymarin, an extract from the seeds of the milk thistle plant, has been introduced fairly recently as a hepatoprotec-tive agent. Two major mechanisms that have been proposed include functioning as an antioxidative scavenger of free radicals and as a regulator of immune functions by modulating cytokine production (Luper, 1998). Song et al. showed that silymarin may prove to be an effective therapeutic agent in toxin-induced liver injuries, such as those induced by ethanol (Song et al., 2006). Polyak et al. demonstrated that four activities of silymarin: antiviral, antioxidant, anti-inflammatory, and immunomodulatory, were all likely to be directly related to its well-described hepatoprotective actions (Polyak et al., 2010). Hepatoprotective effects of silymarin on acute alcohol-induced liver injury have been reported previously (Song et al., 2006; Chen et al., 2010). In our study, liver injury was assessed with histological and biochemical parameters. The results showed that silymarin could improve the degree of hepatic steatosis and inflammation in liver tissues, and decrease the levels of serum ALT, AST, TBIL and level of TG in liver tissue. The results suggested that silymarin could inhibit the alcohol-induced fatty liver effectively, through decreasing fat accumulation and improving the liver function.
In conclusion, silymarin may significantly attenuated the rat liver injury induced by alcohol. Its mechanisms may be related to inhibiting expression of NF-κB and reducing proinfl ammatory cytokines production and alleviate lipid peroxidation through scavenging of free radicals, or by enhancing the activity of antioxidants.
Acknowledgments
This work was supported by the annual scientific research project of Anhui Province No.10020503084. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Arteel G. E. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. (2003);124:778–790. doi: 10.1053/gast.2003.50087. [DOI] [PubMed] [Google Scholar]
- 2.Ajamieh H. H., Menéndez S., Marténez-Sánchez G., Candelario-Jalil E., Re L., Giuliani A., Fernández O. S. Effects of ozone oxidative preconditioning on nitric oxide generation and cellular redox balance in a rat model of hepatic ischaemia-reperfusion. Liver Int. (2004);24:55–62. doi: 10.1111/j.1478-3231.2004.00885.x. [DOI] [PubMed] [Google Scholar]
- 3.Barnes P. J., Karin M. Nuclear factor κB-a pivotal factor in chronic inflammatory diseases. N. Engl. J. Med. (1997);336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee A., Rose R., Johnson G. A., Burghardt R. C., Ramaiah S. K. The influence of estrogen on hepatobiliary osteopontin (SPP1) expression in a female rodent model of alcoholic steatohepatitis. Toxicol. Pathol. (2009);37:492–501. doi: 10.1177/0192623309335633. [DOI] [PubMed] [Google Scholar]
- 5.Chen F., Castranova V., Shi X., Demers L. M. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the initiation of diseases. Clin. Chem. (1999);45:7–17. [PubMed] [Google Scholar]
- 6.Chen S. L., Hong R. T., Diao L., Ruan H. L., Xu J., Mei Q. Silymarin protects against acute ethanol-induced liver injury in rats. Acta Universitatis Medicinalis Anhui. . (2010);45:209–212. [Google Scholar]
- 7.Eagon P. K. Alcoholic liver injury: Influence of gender and hormones. World J. Gastroenterol. (2010);16:1377–1384. doi: 10.3748/wjg.v16.i11.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraschini F., Demartini G., Esposti D. Pharmacology of silymarin. Clin. Drug Invest. (2002);22:51–65. doi: 10.2165/00044011-200222010-00007. [DOI] [Google Scholar]
- 9.Kiruthiga P. V., Shafreen R. B., Pandian S. K., Devi K. P. Silymarin protection against major reactive oxygen species released by environmental toxins: exogenous H2O2 exposure in erythrocytes. Basic Clin. Pharmacol. Toxicol. . (2007);100:414–419. doi: 10.1111/j.1742-7843.2007.00069.x. [DOI] [PubMed] [Google Scholar]
- 10.Luper S. A review of plants used in the treatment of liver disease: part 1. Altern. Med. Rev. (1998);3:410–421. [PubMed] [Google Scholar]
- 11.Nanji A. A. Role of different dietary fatty acids in the pathogenesis of experimental alcoholic liver disease. Alcohol . (2004);34:21–25. doi: 10.1016/j.alcohol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Nanji A. A., Jokelainen K., Rahemtulla A., Miao L., Fogt F., Matsumoto H., Tahan S. R., Su G. L. Activation of nuclear factor κappa b and cytokine imbalance in experimental alcoholic liver disease in the rat. Hepatology . (1999);30:934–943. doi: 10.1002/hep.510300402. [DOI] [PubMed] [Google Scholar]
- 13.Nanji A. A., Jokelainen K., Tipoe G. L., Rahemtulla A., Thomas P., Dannenberg A. J. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-κappa Bdependent genes. Am. J. Physiol. Gastrointest. Liver Physiol. (2003);284:G321–327. doi: 10.1152/ajpgi.00230.2002. [DOI] [PubMed] [Google Scholar]
- 14.Neurath M. F., Pettersson S., Meyer zum Büschenfelde K. H., Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-κappa B abrogates established experimental colitis in mice. Nat. Med. (1996);2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 15.Polyak S. J., Morishima C., Lohmann V., Pal S., Lee D. Y., Liu Y., Graf T. N., Oberlies N. H. Identification of hepatoprotective flavonolignans from silymarin. Proc. Natl. Acad Sci. U.S.A. (2010);107:5995–5999. doi: 10.1073/pnas.0914009107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purohit V., Russo D., Coates P. M. Role of fatty liver, dietary fatty acid supplements, and obesity in the progression of alcoholic liver disease: introduction and summary of the symposium. Alcohol. (2004);34:3–8. doi: 10.1016/j.alcohol.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Polavarapu R., Spitz D. R., Sim J. E., Follansbee M. H., Oberley L.W., Rahemtulla A., Nanji A. A. Increased lipid peroxidation and impaired antioxidant enzyme function is associated with pathological liver injury in experimental alcoholic liver disease in rats fed diets high in corn oil and fish oil. Hepatology. (1998);27:1317–1323. doi: 10.1002/hep.510270518. [DOI] [PubMed] [Google Scholar]
- 18.Song Z., Deaciuc I., Song M., Lee D. Y., Liu Y., Ji X., McClain C. Silymarin protects against acute ethanol-induced hepatotoxicity in mice. Alcohol Clin. Exp. Res. (2006);30:407–413. doi: 10.1111/j.1530-0277.2006.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saller R., Meier R., Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. (2001);61:2035–2063. doi: 10.2165/00003495-200161140-00003. [DOI] [PubMed] [Google Scholar]
- 20.Shi Q. Z., Wang L. W., Zhang W., Gong Z. J. Betaine inhibits Toll-like receptor 4 expression in rats with ethanol-induced liver injury. J. Gastroenterol. . (2010);16:897–903. doi: 10.3748/wjg.v16.i7.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilg H., Diehl A. M. Cytokines in alcoholic and nonalcoholic steatohepatitis. N. Engl. J. Med. . (2000);343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J., Zhai Y., Mu Y., Gong H., Uppal H., Toma D., Ren S., Evans R. M., Xie W. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J. Biol. Chem. (2006);281:15013–15020. doi: 10.1074/jbc.M511116200. [DOI] [PMC free article] [PubMed] [Google Scholar]