Abstract
In this study, we investigated the effects of a selective urotensin II (UII) receptor antagonist, SB-657510, on the inflammatory response induced by UII in human umbilical vein endothelial cells (EA.hy926) and human monocytes (U937). UII induced inflammatory activation of endothelial cells through expression of proinflammatory cytokines (IL-1β and IL-6), adhesion molecules (VCAM-1), and tissue factor (TF), which facilitates the adhesion of monocytes to EA.hy926 cells. Treatment with SB-657510 significantly inhibited UII-induced expression of IL-1β, IL-6, and VCAM-1 in EA.hy926 cells. Further, SB-657510 dramatically blocked the UII-induced increase in adhesion between U937 and EA.hy926 cells. In addition, SB-657510 remarkably reduced UII-induced expression of TF in EA.hy926 cells. Taken together, our results demonstrate that the UII antagonist SB-657510 decreases the progression of inflammation induced by UII in endothelial cells.
Keywords: Urotensin II, Urotensin II receptor antagonist, Adhesion molecules, Cytokines, Tissue factor, Endothelial cells
INTRODUCTION
Atherosclerosis has been implicated as the cause of most cardiovascular diseases such as heart failure and myocardial infarction. Atherosclerosis is associated with endothelial cell injury and/or dysfunction, which results in lipid and leukocyte deposition in the arterial wall (Ross, 1993; Harrison and Ohara, 1995). Endothelial cells are the first cell line to be damaged during atherosclerosis, and endothelial dysfunction is a key event in the development of atherosclerotic lesions and remodeling of the heart after injury (Ross, 1993). Therefore, endothelial cell dysfunction can be regarded as an early marker for cardiovascular disease (Chatterjee et al., 2008).
Urotensin II (UII), a cyclic peptide of 11 amino acids, has been identified as a potential mediator in the pathogenesis of cardiovascular diseases. UII is highly expressed in many tissues, including the heart and vasculature (Douglas et al., 2002). The expression of UII is positively correlated with the severity of heart function and atherosclerosis. The expression of UII is increased in the cardiac tissue of patients with congestive heart failure (CHF) (Douglas et al., 2002) and in the endothelial cells, smooth muscle cells, and infiltrating macrophages in atherosclerotic lesions of human coronary arteries (Bousette et al., 2004; Hassan et al., 2005). The plasma UII levels are high in patients with atherosclerosis and coronary artery disease (Suguro et al., 2007; Loirand et al., 2008). Additionally, UII increases the expression of inflammatory mediators (adhesion molecules and tissue factor [TF]), which are well-known risk factors for the development of atherosclerosis or thrombosis in human coronary endothelial cells (Cirillo et al., 2008). Taken together, the increased expression of UII may contribute to the pathogenesis of atherosclerosis. In fact, UII gene deletion or UII receptor (UT) blockade reduces initiation and progression of atherosclerosis in vivo (You et al., 2012). Therefore, inhibition of UII signaling has been suggested as a potential therapeutic target for atherosclerosis.
Recent studies have shown that a highly specific UT antagonist, SB-657510, inhibits the progression of high-fat dietinduced atherosclerosis and diabetes-associated atherosclerosis in vivo (Papadopoulos et al., 2009; You et al., 2012; Watson et al., 2013). However, the anti-inflammatory effects of SB-657510 at a cellular level have not been evaluated to date. In this study, we investigated the role of UII and UT antagonist SB-657510 in UII-induced inflammatory responses in human vascular endothelial cells.
MATERIALS AND METHODS
Cell culture
EA.hy926 cells, a human umbilical vein endothelial cell line, were grown in Dulbecco’s modified Eagle’s medium (Gibco- BRL, Gaithersberg, MD, USA) supplemented with 10% fetal bovine serum (FBS) and antibiotics. U937 cells, a human monocyte cell line, were maintained in Roswell Park Memorial Institute medium (Gibco-BRL) supplemented with 10% FBS and antibiotics. Cells were incubated in an atmosphere of 95% air and 5% CO2 at 37oC. EA.hy926 cells were pretreated with SB-657510 (Sigma-Aldrich, St Louis, MO, USA) for 30 min. Subsequently, the cells were treated with 100 nM UII (Sigma- Aldrich) in the presence or absence of SB-657510.
Cell-cell adhesion assay
EA.hy926 cells (5×104 cells/well) were seeded in 24-well plates and grown to 80-85% confluence. Then, the EA.hy926 cells were treated with 100 nM UII in the presence or absence of SB-657510. After washing, U937 monocytes (6×105 cells/ well) were added to the UII-treated EA.hy926 cells. After incubation for 1 h, non-adherent U937 monocytes were removed through washing with PBS. Cells were counted in 5 fields per well using an optical microscope. Adherent cells were calculated by expressing the numbers of adherent U937 monocytes as a percentage of total EA.hy926 cells.
Table 1.
Oligonucleotide sequences of primers for RT-PCR
| Gene | Accession number | Sequences (5’-3’) | Tm (℃) |
|---|---|---|---|
|
| |||
| Actin | NM_001101 | CCC AGA TCA TGT TTG AGA CCT | 57 |
| ATG TCA CGC ACG ATT TCC C | |||
| ICAM-1 | NM_000201 | CGA CTG GAC GAG AGG GAT TG | 57 |
| TTA TGA CTG CGG CTG CTA CC | |||
| IL-1β | NM_000576 | TAC CTG AGC TCG CCA GTG AAA | 57 |
| GGA AGA CAC AAA TTG CAT GG | |||
| IL-6 | NM_000600 | AGA GTA GTG AGG AAC AAG CC | 57 |
| TAC ATT TGC CGA AGA GCC CT | |||
| IL-8 | NM_000584 | TGT TCC ACT GTG CCT TGG TT | 57 |
| CTG GCA ATG ACA AGA CTG GG | |||
| PECAM-1 | NM_000442 | AGC CAA CTT CAC CAT CCA GAA | 57 |
| CGT TGT TGG AGT TCA GAA GTG | |||
| Tissue factor | NM_001993 | TGC TAT ATT GCA CTG TGA CCG | 60 |
| CAC CTG AGG TCA GGA ATT CAA | |||
| TNF-α | NM_000594 | AGG CCA AGC CCT GGT ATG AGC | 57 |
| CAC AGG GCA ATG ATC CCA AAG TAG | |||
| Urotensin II | NM_021995 | CTT TCA ACT CTC AGC ACC TCA | 57 |
| CCT AGT TTT TCT CCA CAC TGT T | |||
| Urotensin receptor | NM_018949 | CGC AAC CCT CAA CAG CTC CT | 57 |
| CCT TGG TGA CGT AGG TGG CC | |||
| VCAM-1 | NM_001078 | ACG AAC ACT CTT ACC TGT GCA | 57 |
| AAT TTA GCT CGG CAA ACA AGA | |||
Reverse transcription-polymerase chain reaction (RT-PCR)
The expressions of adhesion molecules, cytokines, and TF mRNA were measured using RT-PCR as previously described (Lee et al., 2011). cDNAs were synthesized from total RNA using M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Then, the cDNAs were amplified by PCR using gene-specific primers. All PCR primers are listed in Table 1. Relative quantifi cation of mRNA was performed by densitometry using QuantityOne software (Biorad Laboratories, Philadelphia, PA, USA).
Western blot analysis
Protein expression of TF was measured using western blot analysis as previously described (Lee et al., 2012). Cells were lysed using lysis buffer (50 mmol/L Tris-HCl, pH 7.4, 1% NP-40, 150 mmol/L NaCl, 0.25% Na-deoxycholate, 2 mmol/L EDTA, 1 mmol/L NaF, 1 mmol/L Na3VO4, 1 mmol/L PMSF, 10 μg/mL of aprotinin, and 10 μmol/L leupeptin). Lysates were subsequently centrifuged at 14,000 rpm for 15 min and supernatants were collected. Then, equal amounts of protein were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and reacted with anti-tissue factor antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-β-actin antibody (Sigma-Aldrich). After probing with horseradish peroxidase (HRP)-conjugated secondary antibody, proteins were visualized using LAS 1000 (Fuji Photo Film, Tokyo, Japan). Densitometric analyses were performed using QuantityOne software (Biorad Laboratories).
Statistical analysis
All data were expressed as the mean ± SEM from at least 3 different experiments. The comparisons were performed using Student’s t-tests. A p value of <0.05 was considered statistically significant.
RESULTS
Effect of SB-657510 on UII-induced attachment of monocytes to endothelial cells
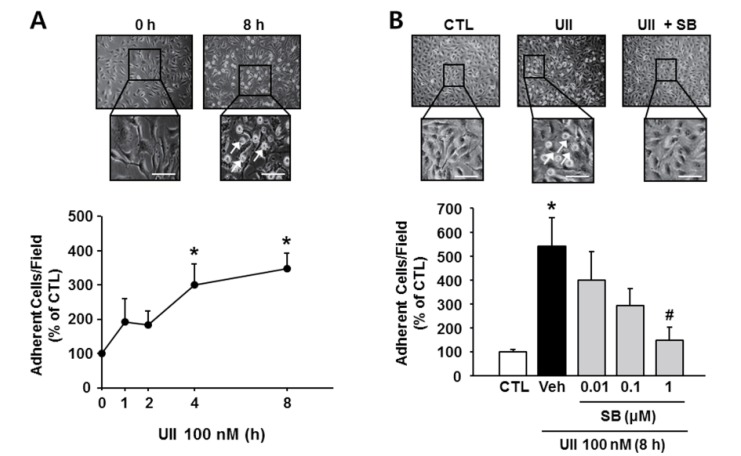
One of the earliest events in inflammation is binding and infiltration of monocytes through the endothelium. Therefore, monocyte-endothelial interactions are important in the development of atherosclerosis (Grenon et al., 2012). To determine whether SB-657510 inhibits a primary process of inflammation, we performed cell-cell adhesion assay. Treatment of EA.hy926 cells with 100 nM UII significantly increased the number of U937 attached to the activated endothelial cells in a time-dependent manner (Fig. 1A). SB-657510 treatment inhibited the adhesion of monocytes to UII-stimulated EA.hy926 cells (544.4 ± 117.7%) in a dose-dependent manner (Fig. 1B). In particular, 1 μM SB-657510 (non-cytotoxic dose, data not shown) dramatically decreased the attachment of U937 to that of the control level (148.6 ± 54.8%). This finding indicates that SB-657510 inhibits the attachment of monocytes to endothelial cells by blocking the stimulation of endothelial cells with UII.
Fig. 1. Effect of SB-657510 (SB) on urotensin II (UII)-induced attachment of monocytes to endothelial cells. After treatment with 100 nM UII for the indicated times in the presence or absence of SB, EA.hy926 cells were incubated with U937 monocytes for 1 h. Representative images and quantitative analysis are shown in top and bottom, respectively. (A) Adhesion assay after UII treatment. Arrow, attached U937 monocyte; scale bar, 100 μm. *p<0.05 vs. 0 h. n=3. (B) Adhesion assay after UII and SB treatment. Arrow, attached U937 monocyte, scale bar, 100 μm. *p<0.05 vs. control (CTL), #p<0.05 vs. vehicle (Veh). n=3.
Effect of SB-657510 on UII-induced expression of adhesion molecules
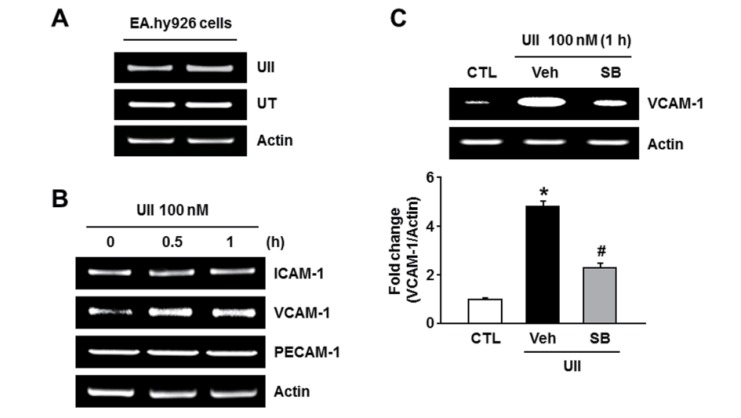
As shown in Fig. 2A, we found that UII and UT mRNA were expressed in EA.hy926 cells. Cell-cell interaction is regulated by the expression of adhesion molecules on the endothelial cells, which occurs as a result of inflammatory activation of the endothelium (Grenon et al., 2012). To evaluate the effect of SB-657510 on the expression of adhesion molecules in endothelial cells, we investigated the mRNA expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and platelet endothelial cell adhesion molecule 1 (PECAM-1) in EA.hy926 cells. Although the expressions of ICAM-1 and PECAM-1 were not changed by 100 nM UII, the expression of VCAM-1 was increased by UII (Fig. 2B). And the UII-induced 4.8-fold increase in VCAM- 1 expression was markedly inhibited to 2.3-fold upon treatment with 1 μM SB-657510 (Fig. 2C). This finding indicates that SB-657510 blocks VCAM-1 expression induced by UII in endothelial cells.
Fig. 2. Effect of SB-657510 (SB) on urotensin II (UII)-induced expression of adhesion molecules. (A) RT-PCR of UII and UII receptor (UT) in EA.hy926 cells. n=4. (B) RT-PCR of adhesion molecules after UII treatment. EA.hy926 cells were treated with 100 nM UII for 0.5 or 1 h. n=4. (C) RT-PCR of VCAM-1 after UII and SB treatment. After pretreatment with 1 μM SB, cells were treated with 100 nM UII for 1 h in the presence or absence of SB. Quantitative analysis is shown in the bottom. *p<0.05 vs. control (CTL), #p<0.05 vs. vehicle (Veh). n=4.
Effect of SB-657510 on UII-induced expression of cytokines
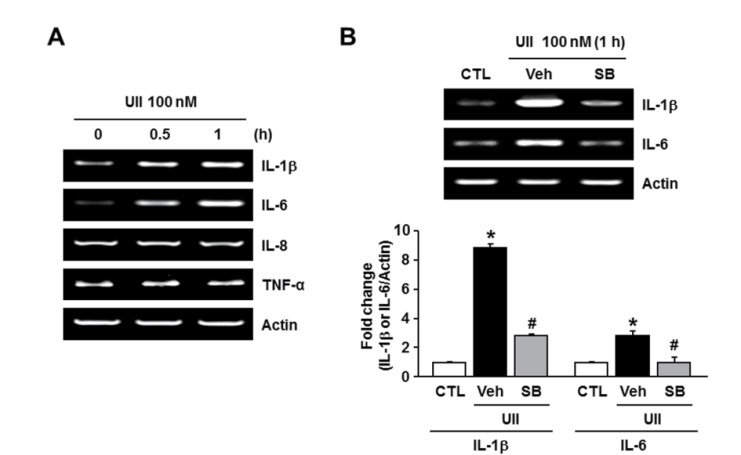
Proinflammatory cytokines are well known to regulate the expression of adhesion molecules in various cells (Chen et al., 2001; Kwon et al., 2007). To examine the effect of SB-657510 on the expression of cytokines in endothelial cells, we investigated the mRNA expression of cytokines (IL-1β, IL-6, IL-8, and TNF-β) in EA.hy926 cells. The expression of IL-8 and TNF-β was unchanged by 100 nM UII. However, the expression of IL-1β and IL-6 increased after treatment with UII (Fig. 3A). The UII-induced 8.9 fold increase in the expression of IL-1β and 2.8 fold increase in the expression of IL-6 were markedly inhibited to 2.8 fold and 1 fold, respectively, upon treatment with 1 μM SB-657510 (Fig. 3B). This finding indicates that SB- 657510 blocks IL-1β and IL-6 expression induced by UII in endothelial cells.
Fig. 3. Effect of SB-657510 (SB) on urotensin II (UII)-induced expression of inflammatory cytokines. (A) RT-PCR of inflammatory cytokines after UII treatment. EA.hy926 cells were treated with 100 nM UII for 0.5 or 1 h. n=4. (B) RT-PCR of IL-1β and IL-6 after UII and SB treatment. After pretreatment with 1 μM SB, cells were treated with 100 nM UII for 1 h in the presence or absence of SB. Quantitative analysis is shown in the bottom. *p<0.05 vs. control (CTL), #p<0.05 vs. vehicle (Veh). n=4.
Effect of SB-657510 on UII-induced expression of TF
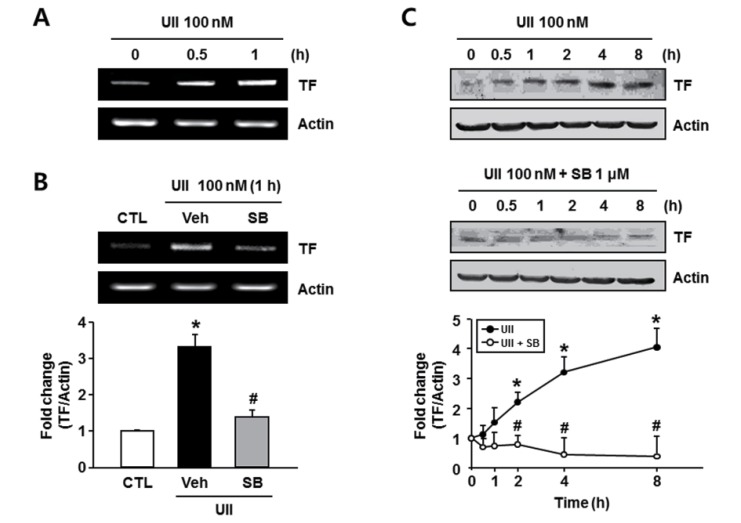
Inflammatory mediators activate induction of the TF by the endothelium, which could contribute to both hemostasis and the formation of an occlusive thrombus (Lwaleed et al., 2007), and, the TF molecule has strong proinflammatory properties, which cause arthritis (Bokarewa et al., 2002). Therefore, we investigated the effect of SB-657510 on the mRNA expres-sion of TF in endothelial cells. The mRNA expression of TF increased after treatment with 100 nM UII (Fig. 4A). And the UII-induced 3.3 fold increase in the mRNA expression of TF was significantly inhibited to 1.4 fold upon treatment with 1 μM SB-657510 (Fig. 4B). This finding indicates that SB-657510 blocks IL-1β and IL-6 expression induced by UII in endothelial cells. Next, we investigated the effect of SB-657510 on the protein expression of TF in endothelial cells. Treatment with 100 nm UII induced a time-dependent increase in the expression of TF protein similar to the results of mRNA expression (Fig. 4C). And treatment with 1 μM SB-657510 remarkably decreased the UII-induced protein expression of TF to that of the control level. This finding indicates that SB-657510 blocks the expression of TF induced by UII in endothelial cells.
Fig. 4. Effect of SB-657510 (SB) on urotensin II (UII)-induced expression of tissue factor (TF). (A) RT-PCR of TF after UII treatment. EA.hy926 cells were treated with 100 nM UII for 0.5 or 1 h. n=4. (B) RT-PCR of TF after UII and SB treatment. After pretreatment with 1 μM SB, cells were treated with 100 nM UII for 1 h in the presence or absence of SB. Quantitative analysis is shown in the bottom. *p<0.05 vs. control (CTL), #p<0.05 vs. vehicle (Veh). n=4. (C) Western blots of TF after UII and SB treatment. After pretreatment with 1 μM SB, cells were treated with 100 nM UII for the indicated times in the presence or absence of SB. Quantitative analysis is shown in the bottom. *p<0.05 vs. 0 h, #p<0.05 vs. UII. n=4.
DISCUSSION
This study shows that SB-657510, a selective UT antagonist, exerts anti-inflammatory effects by inhibiting UII-induced upregulation of inflammatory mediators such as adhesion molecules, cytokines, and TF in human vascular endothelial cells.
Inflammation is a well-known risk factor for the development of atherosclerosis and plays a pivotal role in all stages of atherosclerosis (Steinberg, 2002). Several experimental studies and clinical observations have confirmed that a variety of inflammatory mediators such as adhesion molecules, cytokines, and TF, participate in the progression of atherosclerotic lesions (Tremoli et al., 1999; Kleemann et al., 2008). Adhesion molecules, including VCAM-1, ICAM-1, PECAM-1, and E-selectin, play pivotal roles in mediating the migration of leucocytes to the site of inflammation and its subsequent adhesion to endothelial cells, which is one of the major mechanisms underlying the pathogenesis of atherosclerosis (O’ Brien et al., 1996; Price and Loscalzo, 1999; Libby, 2002). In particular, VCAM- 1 is expressed by endothelial cells in atherosclerotic lesions, and it promotes the adhesion of monocytes on the vascular endothelium (Cybulsky et al., 2001; Tedgui and Mallat, 2006). Furthermore, deletion of VCAM-1 reduces atherosclerotic lesions in hyperlipidemic mouse models (Ramos et al., 1999). These studies indicate that expression of VCAM-1 is essential for the inflammatory process in atherosclerosis lesions (Galkina and Ley, 2007). In the present study, UII increased VCAM- 1 mRNA expression but not ICAM-1 and PECAM-1 mRNA expression in human umbilical vein endothelial cells. On the other hand, not only VCAM-1 but also ICAM-1 was increased by UII in human coronary endothelial cells (Cirillo et al., 2008). These distinct results can be explained by differences in cell-type and species. In this study, SB-657510 inhibited the function of VCAM-1 induced by UII, shown by its inhibition of attachment between monocytes and endothelial cells. These results suggest that SB-657510 has anti-inflammatory effects by reducing UII-induced VCAM-1 expression and attachments between monocytes and endothelial cells.
Proinflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8, are key players in the pathogenesis of atherosclerosis and regulate the expression of adhesion molecules (Tedgui and Mallat, 2006; Kleemann et al., 2008). Several studies showed that TNF-α and IL-1β induce VCAM-1 expression in vascular endothelial cells (Haraldsen et al., 1996; Min et al., 2005). At a cellular level, UII stimulated mRNA expressions of IL-1β and IL-6, and these increases were inhibited by SB- 657510. A recent study of You et al. showed that SB-657510 decreased the serum level of other cytokines, including tissue inhibitor of metalloproteinase-1, M-CSF, and MIG in apoE KO mice (You et al., 2012). In that study, they further demonstrated that SB-657510 inhibited aortic ERK1/2 activation, suggesting its ameliorating effect on the experimental atherosclerosis (You et al., 2012). In this point of view, it will be meaningful to extend our study to confirm those parameters at the vascular smooth muscle cell level.
Various studies suggest that TF, a key initiator of the coagulation cascade, is involved in the pathogenesis of atherosclerosis by promoting thrombus formation (Steffel et al., 2006). The expression of TF in endothelial cells can be induced by cytokines such as TNF-α and IL-1β, which could promote a prothrombotic phenotype of the endothelium and finally lead to the occurrence of acute coronary syndromes (Steffel et al., 2006; Cirillo et al., 2008). A recent study showed that UII induces the expression of TF in human coronary artery endothelial cells, which indicate the pro-atherothrombotic effects of UII (Cirillo et al., 2008). Consistent with findings reported in previous study, our study showed that UII increased the expression of TF in human umbilical vein endothelial cells, and the UII-induced TF expression was significantly suppressed by SB-657510. Thus, our results suggest that SB-657510 has the potential to block UII-induced endothelial pro-thrombotic phenotype through inhibition of TF expression.
In conclusion, the present study showed that SB-657510 inhibits UII-induced expression of VCAM-1, IL-1β, IL-6, and TF in human vascular endothelial cells, supporting the in vivo anti-atherosclerotic effect of SB-657510 reported previously. Our results will provide insights in understanding the protective role of SB-657510 against atherosclerosis.
Acknowledgments
This research was supported by the Bio & Medical Technology Development Program (2011-0019397) of the National Research Foundation (NRF) funded by the Korean government (MEST). This work was also supported by the Bio Medicine of the Chungcheong Leading Industry Office of the Korean Ministry of Knowledge Economy (MKE).
References
- 1.Bokarewa M. I., Morrissey J. H., Tarkowski A. Tissue factor as a proinflammatory agent. Arthritis Res. (2002);4:190–195. doi: 10.1186/ar405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousette N., Patel L., Douglas S. A., Ohlstein E. H., Giaid A. Increased expression of urotensin II and its cognate receptor GPR14 in atherosclerotic lesions of the human aorta. Atherosclerosis. (2004);176:117–123. doi: 10.1016/j.atherosclerosis.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee A., Black S. M., Catravas J. D. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul. Pharmacol. (2008);49:134–140. doi: 10.1016/j.vph.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H., Liu C., Sun S., Mei Y., Tong E. Cytokine-induced cell surface expression of adhesion molecules in vascular endothelial cells in vitro. J. Tongji. Med. Univ. . (2001);21:68–71. doi: 10.1007/BF02888042. [DOI] [PubMed] [Google Scholar]
- 5.Cirillo P., De Rosa S., Pacileo M., Gargiulo A., Angri V., Fiorentino I., Prevete N., Petrillo G., De Palma R., Leonardi A., De Paulis A., Chiariello M. Human urotensin II induces tissue factor and cellular adhesion molecules expression in human coronary endothelial cells: an emerging role for urotensin II in cardiovascular disease. J. Thromb. Haemost. (2008);6:726–736. doi: 10.1111/j.1538-7836.2008.02923.x. [DOI] [PubMed] [Google Scholar]
- 6.Cybulsky M. I., Iiyama K., Li H., Zhu S., Chen M., Iiyama M., Davis V., Gutierrez-Ramos J. C., Connelly P. W., Milstone D. S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Invest. (2001);107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas S. A., Tayara L., Ohlstein E., Halawa N., Giaid A. Congestive heart failure and myocardial expression of urotensin II. Lancet . (2002);359:1990–1997. doi: 10.1016/S0140-6736(02)08831-1. [DOI] [PubMed] [Google Scholar]
- 8.Galkina E., Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. (2007);27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 9.Grenon S. M., Aguado-Zuniga J., Hatton J. P., Owens C. D., Conte M. S. Effects of fatty acids on endothelial cells: inflammation and monocyte adhesion. J. Surg. Res. (2012);177:e35–e43. doi: 10.1016/j.jss.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haraldsen G., Kvale D., Lien B., Farstad I. N., Brandtzaeg P. Cytokine-regulated expression of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule- 1 (VCAM-1) in human microvascular endothelial cells. J. Immunol. (1996);156:2558–2565. [PubMed] [Google Scholar]
- 11.Harrison D. G., Ohara Y. Physiologic consequences of increased vascular oxidant stresses in hypercholesterolemia and atherosclerosis: implications for impaired vasomotion. Am. J. Cardiol. (1995);75:75B–81B. doi: 10.1016/0002-9149(95)80018-N. [DOI] [PubMed] [Google Scholar]
- 12.Hassan G. S., Douglas S. A., Ohlstein E. H., Giaid A. Expression of urotensin-II in human coronary atherosclerosis. Peptides. (2005);26:2464–2472. doi: 10.1016/j.peptides.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Kleemann R., Zadelaar S., Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc. Res. (2008);79:360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon H. M., Choi Y. J., Choi J. S., Kang S. W., Bae J. Y., Kang I. J., Jun J. G., Lee S. S., Lim S. S., Kang Y. H. Blockade of cytokine-induced endothelial cell adhesion molecule expression by licorice isoliquiritigenin through NF-kappaB signal disruption. Exp. Biol. Med. (Maywood). (2007);232:235–245. [PubMed] [Google Scholar]
- 15.Lee H. J., Lee S. Y., Cho K., Jeon B. K., Lee J. Y., Bae H. S., Lee C. J. Effect of ambroxol on secretion, production and gene expression of mucin from cultured airway epithelial cells. Biomol. Ther. . (2011);19:65–69. doi: 10.4062/biomolther.2011.19.1.065. [DOI] [Google Scholar]
- 16.Lee N. Y., Rieckmann P., Kang Y. S. The changes of pglycoprotein activity by interferon-γ and tumor necrosis factor-α in primary and immortalized human brain microvascular endothelial cells. Biomol. Ther. (2012);20:293–298. doi: 10.4062/biomolther.2012.20.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Libby P. Inflammation in atherosclerosis. Nature. (2002);420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 18.Loirand G., Rolli-Derkinderen M., Pacaud P. Urotensin II and atherosclerosis. Peptides. (2008);29:778–782. doi: 10.1016/j.peptides.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Lwaleed B. A., Cooper A. J., Voegeli D., Getliffe K. Tissue factor: a critical role in inflammation and cancer. Biol. Res. Nurs. (2007);9:97–107. doi: 10.1177/1099800407305733. [DOI] [PubMed] [Google Scholar]
- 20.Min J. K., Kim Y. M., Kim S. W., Kwon M. C., Kong Y. Y., Hwang I.K., Won M. H., Rho J., Kwon Y. G. TNF-related activation induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells. J. Immunol. (2005);175:531–540. doi: 10.4049/jimmunol.175.1.531. [DOI] [PubMed] [Google Scholar]
- 21.O’ Brien K. D., McDonald T. O., Chait A., Allen M. D., Alpers C. E. Neovascular expression of E-selectin, intercellular adhesion molecule-1 and vascular adhesion molecule-1 in human atherosclerosis and their relation to intinal leukocyte content. Circulation. (1996);93:672–682. doi: 10.1161/01.CIR.93.4.672. [DOI] [PubMed] [Google Scholar]
- 22.Papadopoulos P., Bousette N., Al-Ramli W., You Z., Behm D. J., Ohlstein E. H., Harrison S. M., Douglas S. A., Giaid A. Targeted overexpression of the human urotensin receptor transgene in smooth muscle cells: effect of UT antagonism in ApoE knockout mice fed with Western diet. Atherosclerosis. (2009);204:395–404. doi: 10.1016/j.atherosclerosis.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 23.Price D. T., Loscalzo J. Cellular adhesion molecules and atherogenesis. Am. J. Med. (1999);107:85–97. doi: 10.1016/S0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- 24.Ramos C. L., Huo Y., Jung U., Ghosh S., Manka D. R., Sarembock I. J., Ley K. Direct demonstration of P-selectinand VCAM-1-dependent mononuclear cell rolling in early atherosclerotic lesions of apolipoprotein E-deficient mice. Circ. Res. (1999);84:1237–1244. doi: 10.1161/01.RES.84.11.1237. [DOI] [PubMed] [Google Scholar]
- 25.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature . (1993);362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 26.Steffel J., Lüscher T. F., Tanner F. C. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation . (2006);113:722–731. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat. Med. . (2002);8:1211–1217. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- 28.Suguro T., Watanabe T., Ban Y., Kodate S., Misaki A., Hirano T., Miyazaki A., Adachi M. Increased human urotensin II levels are correlated with carotid atherosclerosis in essential hypertension. Am. J. Hypertens. (2007);20:211–217. doi: 10.1016/j.amjhyper.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Tedgui A., Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. . (2006);86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 30.Tremoli E., Camera M., Toschi V., Colli S. Tissue factor in atherosclerosis. Atherosclerosis. (1999);144:273–283. doi: 10.1016/S0021-9150(99)00063-5. [DOI] [PubMed] [Google Scholar]
- 31.Watson A. M., Olukman M., Koulis C., Tu Y., Samijono D., Yuen D., Lee C., Behm D. J., Cooper M. E., Jandeleit-Dahm K. A., Calkin A. C., Allen T. J. Urotensin II receptor antagonism confers vasoprotective effects in diabetes associated atherosclerosis: studies in humans and in a mouse model of diabetes. Diabetologia. (2013);56:1155–1165. doi: 10.1007/s00125-013-2837-9. [DOI] [PubMed] [Google Scholar]
- 32.You Z., Genest J. Jr., Barrette P. O., Hafiane A., Behm D. J., D'Orleans-Juste P., Schwertani A. G. Genetic and pharmacological manipulation of urotensin II ameliorate the metabolic and atherosclerosis sequalae in mice. Arterioscler. Thromb. Vasc. Biol. (2012);32:1809–1816. doi: 10.1161/ATVBAHA.112.252973. [DOI] [PubMed] [Google Scholar]