Abstract
Antidiabetic and beta cell-protection activities of purple corn anthocyanins (PCA) were examined in pancreatic beta cell culture and db/db mice. Only PCA among several plant anthocyanins and polyphenols showed insulin secretion activity in culture of HIT-T15 cells. PCA had excellent antihyperglycemic activity (in terms of blood glucose level and OGTT) and HbA1c-decreasing activity when compared with glimepiride, a sulfonylurea in db/db mice. In addition, PCA showed efficient protection activity of pancreatic beta cell from cell death in HIT-T15 cell culture and db/db mice. The result showed that PCA had antidiabetic and beta cell-protection activities in pancreatic beta cell culture and db/db mice.
Keywords: Antidiabetes, Purple corn anthocyanins, Beta cell-protection, Antihyperglycemic activity, Pancreatic beta cell, db/db Mice
INTRODUCTION
Type 2 diabetes mellitus is characterized by insufficient insulin secretion and insulin resistance (Goldstein, 2002). The insulin resistance results in hyperinsulinemia, amyloid deposits, and inflammation in pancreatic beta cells (Lebovitz and Banerji, 2004). Consequently, pancreatic beta cells mass and insulin secretion are gradually decreased. Sulfonylureabased hypoglycemic agents that have been most prescribed for type-2 diabetic patients, directly stimulate insulin secretion from pancreatic beta cells sufficiently to lower blood glucose level through blocking potassium channel (Ashcroft, 1988; Ashcroft and Ashcroft, 1992). The sulfonylurea has well been prescribed with the medicine for insulin resistance like metformin. Disadvantages of using sulfonylurea-based medicines are insulin oversecretion and induction of pancreatic beta cells death (Efanova et al., 1998; Iwakura et al., 2000; Del Guerra et al., 2005; Maedler et al., 2005). Therefore, better medicine with sufficient insulin secretion and maintenance of pancreatic beta cell mass has been searched.
Anthocyanins are a class of antioxidant polyphenols and are present in various colored fruits, crops, beans and vegetables. The anthocyanins found most commonly are the glycosides of anthocyanidins. More than 400 naturally occurring anthocyanins have been identified (Kong et al., 2003). Dietary intake of anthocyanins is associated with various therapeutic benefits including anti-inflammatory, cardioprotective, neuroprotective, and anticarcinogenic properties (Bickford et al., 1999; Juranic and Zizak, 2005; Bobe et al., 2006; Cirico and Omaye, 2006). Purple corn anthocyanins also reported to contain insulin secretion activity (Jayaprakasam et al., 2005). Cyanidine 3-glucoside-rich purple corn anthocyanins improved the high fat diet-induced obesity and hyperglycemia in mice (Tsuda et al., 2003). Cyanidine 3-glucoside reported to ameliorate hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mouse (Sasaki et al., 2007). Dietary anthocyanin-rich bilberry extract reported to ameliorate hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice (Takikawa et al., 2010). However, it has not been tested whether purple corn anthocyanins induce apoptotic cell death like sulfonylurea and how insulin secretion is activated. We tested the effect of purple corn anthocyanins on survival of pancreatic beta cells in db/db mouse. Here, we report that purple corn anthocyanins not only improve secretion of insulin from pancreatic beta cells but also protect beta cells from death in pancreatic beta cell culture and db/db mice.
MATERIALS AND METHODS
Materials
Powder of purple corn (Zea mays var. kculli) anthocyanin extract (extracted with ethanol, total anthocyanin content: 10%) was purchased from Zana Export Co. (Peru). Glimepiride (a sulfonylurea), dideoxyadenosine (DDA, an inhibitor of adenylate cyclase activity) and H89 (a PKA inhibitor) were purchased from Sigma-Aldrich Co. Various anthocyanins and polyphenols from plants were prepared for measuring insulin secretion activity as follows. 200 g of dried plants were crushed in thermomix then 400 ml of water were added and the mixture was heated to 80℃ for 5 min. After 5 min the heating was turn off, and 600 ml of methanol (with 10 ml of acetic acid) was added. The anthocyanins were extracted for 2.5 h. The extract was then centrifuged (4℃, 30 min) and the supernatants were removed. The sample was then extracted again in 600 ml of 60% methanol (1% acetic acid) using the same procedure, and the supernatants were then combined. The supernatants were then evaporated at 40℃ under vacuum to remove solvents. The residue was dissolved in water (0.1% acetic acid) and applied to a column of nonionic polymeric absorbent (Amberlite XAD-16). After washing with water (0.1% acetic acid) the anthocyanins were collected by elution with methanol (0.1% acetic acid) and then evaporated to dryness using a rotary evaporator. The residue was dissolved with HPLC grade water (0.1% acetic acid). Polyphenols were extracted with ethanol. Polyphenols were applied to Amberlite XAD-16 column and collected by elution with ethanol. Polyphenols were then evaporated to dryness using a rotary evaporator.
Animals
Our study was reviewed and approved by the Animal Care and Use Committee of Hallym University (Hallym 2009-87). Six-week-old male C57BL/KsJ db/db mice were purchased from Orient Bio (Sungnam, Korea), and they were acclimatized for 1 week before being randomly assigned into the experimental groups. The animals were housed in a room with a 12-12 h light-dark cycle (8:00 AM to 8:00 PM), a temperature of 23 ± 1℃, and a humidity of 55 ± 5%. During the acclimatization period, animals were fed standard rodent chow (LabDiet, Richmond, VA, USA) and water ad libitum.
Measurement of insulin secretion from pancreatic beta cells
HIT-T15 cells (ATCC CRL-1777, hamster pancreatic beta cell line) were cultured in RPMI 1640 media containing 11.1 mM glucose with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. The medium was changed every 2 days, and cells were subcultured every 5-6 days. HIT-T15 cells were seeded into a 24-well plate at a density of 2×105 cells per well and grown for 24 h. The cells were washed twice and preincubated for 30 min in Krebs-Ringers bicarbonate (KRB) buffer (115 mM NaCl, 4.7 mM KCl, 2.56 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 20 mM NaHCO3, 16 mM HEPES, and 0.3% bovine serum albumin, pH 7.4). Various anthocyanins and polyphenols were added to see insulin secretion activity. Cells were then incubated with KRB buffer containing 11.1 mM glucose for 1 h at 37℃. After incubation, cells were centrifuged and aliquots of supernatants were stored at -20℃ until insulin measurement. Insulin levels were determined by mouse insulin ELISA kit (Shibayagi Co.).
Oral glucose tolerance test (OGTT) in db/db mice
The db/db mice were allowed to fast for 12 h prior to experiment and PCA (purple corn anthocyanins, 10 mg/kg of body weight) was administered orally 30 min prior to the glucose challenge. Glucose (2 mg/kg) was orally administered at 0 min, and the blood was withdrawn from the orbital venous plexus at 0, 30, 60, and 120 min after glucose administration. Blood glucose and insulin levels were determined by blood glucose meter (Accu-check Active, Roche) and mouse insulin ELISA kit (Shibayagi Co.), respectively.
Administration of purple corn extract to animals
Seven week old, C57BL/KsJ db/db mice were randomly divided into four groups (8 mice per group, the diabetic control group and three treatment groups. Purple corn (Zea mays var. kculli) anthocyanin extracts (Zana Export Co. Peru) were fed to the animal by dissolving in drinking water in the concentration of 10 mg/kg/day for 7 weeks. As a positive control, glimepiride, a sulfonylurea was added to drinking water and administered at a dose of 10 mg/kg/day. No addition to drinking water was a negative control. During the experiment, body weight and blood glucose levels were measured every week. OGTT, HbA1C, blood insulin level was measured at the first day and the last day of experiment.
Immunostaining
The pancreas was removed at the last day of experiment and fixed in 10% neutral buffered formalin. The tissues were subsequently embedded in paraffin and sectioned with thickness of 5 μm using a microtome (Leica, Wetzlar, Germany). The sections prepared onto aminosilane-treated slides were deparaffinized and rehydrated through graded alcohols to distilled water, and incubated with 0.1% trypsin and normal rabbit serum blocking solution for 30 min to block nonspecific binding of immunoglobulin. The sections were incubated with goat anti-insulin A (C-12) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 h at room temperature and treated with 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. The tissues were then incubated with biotinylated rabbit anti-goat IgG for 30 min at room temperature. The tissues were labeled using a modification of the avidin-biotin complex immunoperoxidase staining procedure (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, USA). Positive staining was visualized using DAB peroxidase substrate solution for 5-10 min, and tissues were counterstained with hematoxylin.
Cell viability and apoptosis analysis in HIT-T15 cells
The viability of HIT-T15 cells was estimated by measuring the rate of mitochondrial reduction of a methylthiazoletetrazolium (MTT, Sigma) after treatment of PCA and glimepiride (100 μg/ml) for 2 days. 1×104 HIT-T15 cells were seeded in each well of 24-well plate containing 200 μl of medium and 1 mg/ml of MTT. After 4 h incubation at 37℃, the medium was replaced with 200 μl of dimethylsulfoxide (DMSO, Sigma). The optical density was measured at 570 nm by using a microplate spectrophotometer (SPECTRA-max PLUS384, Molecular Devices). Apoptosis of HIT-T15 cells after treatment of PCA and glimepiride (100 μg/ml) for 2 days was measured by immunoblotting of cleaved caspase 3 using anti-cleaved caspase 3 antibody (Cell Signaling Technology).
Statistical analysis
Data were analyzed using Sigma Plot (Ver. 8.0, SPSS Inc., Chicago, IL). All data are expressed as mean (standard deviation and comparisons of data have been performed by unpaired Student’s t test or ANOVA). Mean values were considered significantly different when p<0.05 or p<0.01.
RESULTS
Insulin secretion activity of PCA in hamster pancreatic beta cells (HIT-T15)
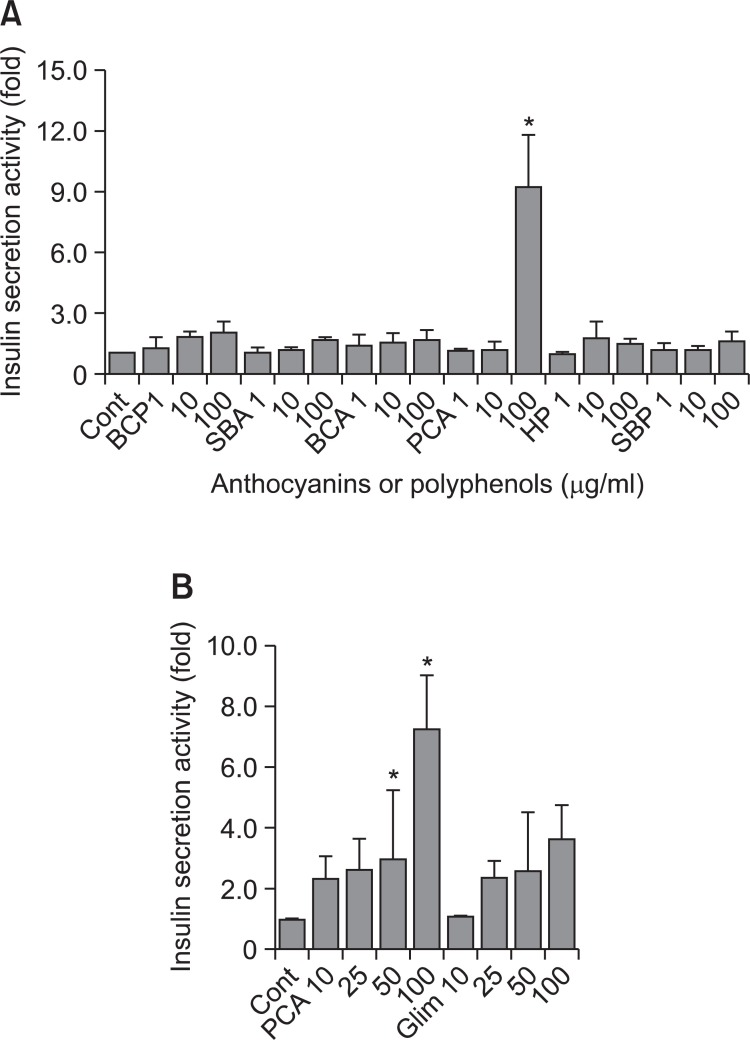
Anthocyanins or polyphenols were extracted from black currant, Saskatoon berries, purple corn, and honeysuckle, using same extraction procedure and tested for insulin secretion at various concentrations (1-100 μg/ml) in hamster pancreatic cells HIT-T15. Only PCA (purple corn anthocyanins) among six plant-extracted anthocyanins or polyphenols showed insulin secretion activity at 100 μg/ml (Fig. 1A). PCA also showed insulin secretion activity at lower concentration (25 and 50 μg/ml). Insulin secretion activity of PCA appeared to be a little better than glimepiride, a widely-prescribed sulfonylurea (Fig. 1B). These results indicate that PCA (purple corn anthocyanins) has high insulin secretion activity.
Fig. 1. Insulin secretion activity of anthocyanins and polyphenols. (A) Hamster pancreatic beta-cell line, HIT-T15 cells were cultured in RPMI 1640 media containing 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. HIT-T15 cells were seeded into a 24-well plate at a density of 2×105 cells per well and grown for 24 h. The cells were used for measuring insulin secretion activity as described in Materials and Methods. Abbreviations represent follows. BCP: black currant polyphenols, SBA: saskatoon berries anthocyanins, BCA: black currant anthocyanins, PCA: purple corn anthocyanins, HP: honeysuckle polyphenols, SBP: saskatoon berries polyphenols. Anthocyanins and polyphenols were added at the concentrations of 1-100 μg/ml in culture media. Control was no addition. Insulin secretion for control was counted as 1. The fold increase over control was calculated for each samples and plotted. (B) HIT-15 cells were seeded into a 24-well plate. Cells were treated with PCA and glimepiride as indicated. Insulin secretion activity was measured as described in Materials and Methods. The error bars indicate standard deviations of five different results and the statistical significance is indicated as *p<0.05 by Student’s t test. Glim: Glimipiride.
Antidiabetic activity of PCA in db/db mice
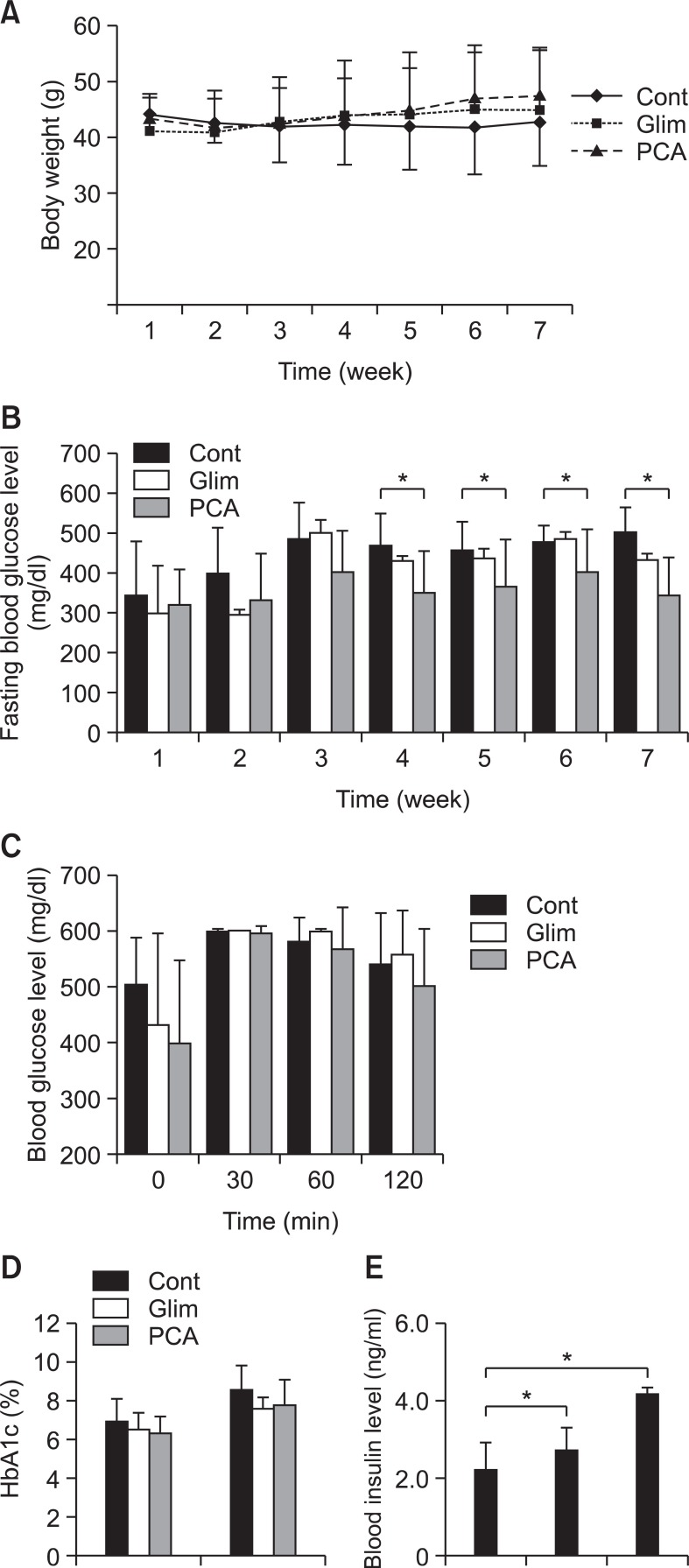
Body weight and fasting blood glucose level were measured every week during experimental period. Glimepiride was used for positive control group and untreated was used for negative control group. Both PCA and glimepiride were treated at 10 mg/kg/day. While body weight of mice maintained similar in PCA group, positive control group (glimepiride group) and negative control group during experimental period (Fig. 2A), fasting blood glucose level was increased to 500 mg/dl at third weeks and maintained until 7 th week in negative control group. PCA group did not show any abrupt increase of fasting blood glucose but rather maintained at about 350-400 mg/dl. Glimepiride group showed a little higher fasting glucose level at about 450 mg/dl (Fig. 2B). The result suggests that PCA has more efficient antihyperglycemic activity when compared with glimepiride in db/db mice.
Fig. 2. Effect of PCA on fasting blood glucose level, OGTT, HbA1c, and insulin level in db/db mice. Seven week old, C57BL/KsJ db/db mice were randomly divided into four groups (8 mice per group), the diabetic control group and three treatment groups. Purple corn anthocyanin extracts (Zana Export Co. Peru) were fed to the animal by dissolving in drinking water in the concentration of 10 mg/kg/day for 7 weeks. As a positive control, glimepiride was administered at a dose of 10 mg/kg/day. No addition to drinking water was a negative control. (A) Body weights were measured every week. (B) Blood glucose levels were measured every week. (C) OGTT (oral glucose tolerance test) was performed at the last week of treatment as described in Materials and Methods. (D) HbA1C was measured at the first and last weeks of treatment. (E) Blood insulin level was measured at the last week of experiment. The error bars indicate standard deviations of five different results and the statistical significance is indicated as *p<0.05 by Student’s t test. ‘Cont’ represents control. ‘Glim’ represents glimepiride. ‘PCA’ represents purple corn anthocyanins.
OGTT (oral glucose tolerance test) was examined for seven week-treated and fasted db/db mice at every 30 min for 120 min after administrating glucose. Blood glucose level at the beginning was low in PCA-treated db/db mice as expected. PCA group showed decrease of blood glucose level to 500 mg/dl at 120 min but glimepiride and negative control group maintained blood glucose level at 550 mg/dl at 120 min (Fig. 2C), suggesting that PCA showed excellent shot term antihyperglycemic activity. The level of HbA1c, glycated hemoglobin was increased to 8.3 in negative control group but to 7.7-7.8 in glimepiride and PCA group at 7th week, indicating that long term expose of hemoglobin to high blood glucose level was also decreased in glimepiride and PCA group (Fig. 2D). The result indicates that PCA-treated db/db mice showed efficient short and long term blood glucose control. Blood insulin levels were tested at 7th week. PCA group showed much higher blood insulin level than negative control group and glimepiride group (Fig. 2E), indicating that PCA is an excellent insulin secreting agent.
Pancreatic beta cell-protective activity of PCA
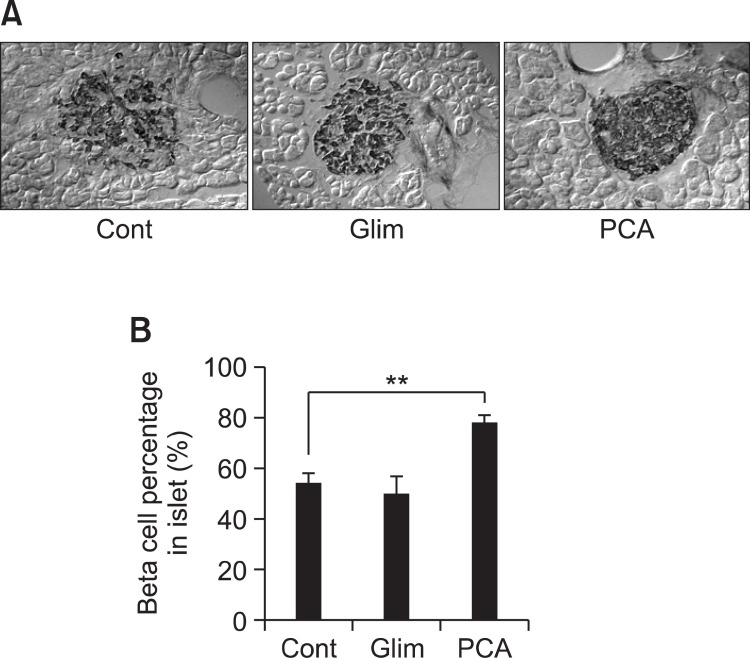
The population of insulin secreting cells (beta cells) in pancreatic islets was examined using immunohistochemical staining with anti-insulin antibody. Interestingly, while negative control group and glimepiride group showed decreased beta cell population, PCA group showed higher population of beta cells in pancreatic islets (Fig. 3A). The population of pancreatic insulin secreting cells (beta cells) in PCA-treated mice was dramatically increased up to about 80% while negative control and glimepiride-treated mice showed 54% and 50%, respectively. Beta cells content of glimepiride-treated mice appeared to be slightly lower than negative control, although that result did not give statistical significance (Fig. 3B). The result indicates that PCA has efficient activity of protection from beta cell death observed in db/db mice although the underlying mechanism is unknown.
Fig. 3. Effect of PCA on survival of pancreatic beta cells. The pancreas was removed at the last day of experiment and fixed in 10% neutral buffered formalin. The tissues were subsequently embedded in paraffin and sectioned using a microtome. The sections were incubated with goat anti-insulin A (C-12), incubated with biotinylated rabbit anti-goat IgG and then labeled using a modification of the avidin-biotin complex immunoperoxidase staining as described in Materials and Methods. Positive staining was visualized using DAB peroxidase substrate solution and tissues were counterstained with hematoxylin. (A) Photos of typical anti-insulin staining of pancreatic islet. (B) Insulin-positive area over entire area of each islet was measured for 10 islets per group. Percentage of insulin positive cells was calculated and plotted. The error bars indicate standard deviations of five different results and the statistical significance is indicated as **p<0.01 by Student’s t test. ‘Cont’ represents control. ‘Glim’ represents glimepiride. ‘PCA’ represents purple corn anthocyanins.
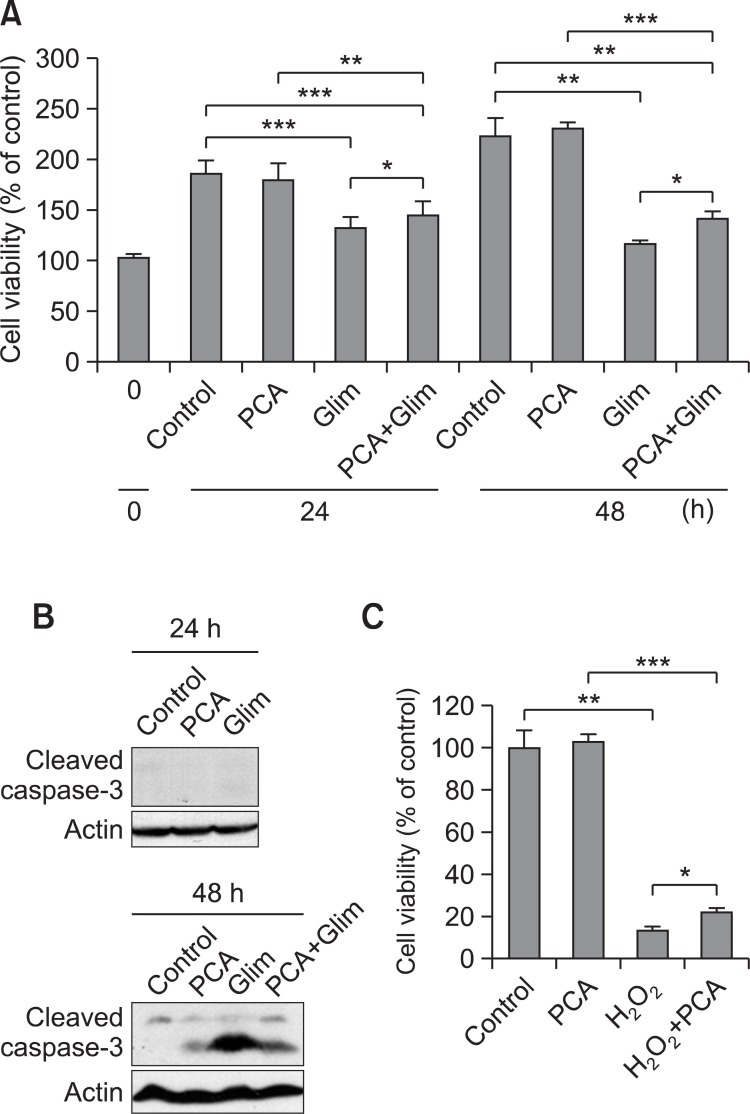
Further, cell viability was tested by MTT assay after treatment of HIT-T15 with either 100 μg/ml of glimepiride or PCA for 48 h. PCA-treated cells showed no cell death up to 48 h similar to untreated control while glimepiride-treated pancreatic cells showed about 52% of cell viability at 48 h. When cells were treated with PCA and glimepiride at the same time, cells showed about 63% of cell viability in comparison to control at 48 h (Fig. 4A), indicating that PCA helped to enhance a little cell viability decreased by glimepiride. Glimepiride-induced cell death was turned out to be apoptosis since cleavage of caspase-3 is increased in glimepiride-treated cells at 48 h. The level of cleaved caspase-3 was decreased in PCA and glimepiride co-treated cells (Fig. 4B). In addition, 1 μM H2O2- treated cells showed about 13% survival whereas PCA and H2O2 co-treated cells showed about 22% survival (Fig. 4C). The results indicate that glimepiride-induced apoptosis is apparent in culture of pancreatic beta cells while PCA protected beta cells from apoptosis induced by glimepiride.
Fig. 4. Effect of PCA on viability and apoptosis pancreatic beta cells. (A) Cell viability of HIT-T15 cells was measured by MTT assay after treatment of PCA and glimepiride (100 μg/ml) as indicated. Control was untreated cell culture. Cell viability of 0 h was considered as 100%. Relative cell viability percentages of treated cells were calculated and plotted. (B) HIT-T15 cells were treated with PCA and glimepiride (100 μg/ml) as indicated. Apoptosis was measured by immunoblotting with anti-cleaved caspase 3 antibody (Cell Signaling Technology). (C) Cell viability of HIT-T15 cells was measured by MTT assay after treatment of PCA and H2O2 (1 μM) as indicated. The error bars indicate standard deviations of five different results and the statistical significance is indicated as *p<0.05, **p< 0.01, ***p<0.001 by Student’s t test. ‘Cont’ represents control. ‘Glim’ represents glimepiride. ‘PCA’ represents purple corn anthocyanins.
The mechanism of insulin secretion by PCA in HIT-T15 cells
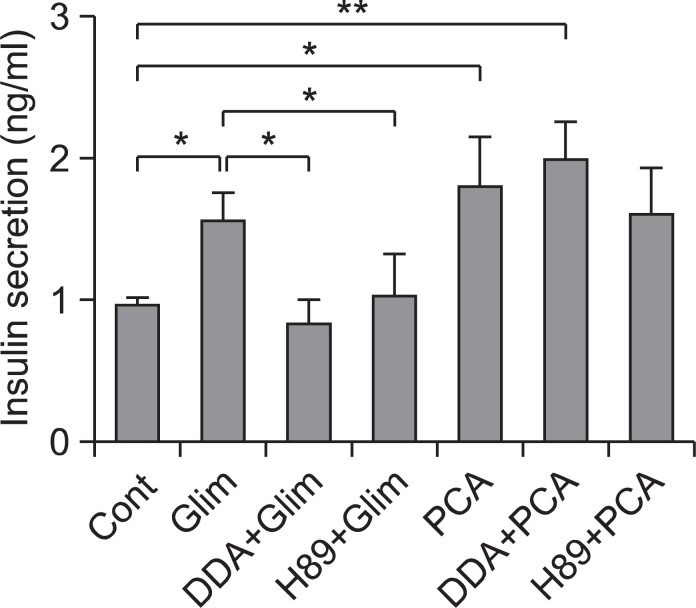
GLP-1R-mediated cAMP/PKA activation has been reported to be one of major mechanism of insulin secretion in addition to K channel blocking (Szecowka et al., 1982; Drucker et al., 1987; Fehmann et al., 1995; Holz, 2004). To elucidate the insulin secretion mechanism of PCA, involvement of cAMP-mediated GLP-1R/Adenylate cyclase-mediated cAMP production and PKA (Protein kinase A) activation was tested in culture of HIT-T15 cells by treating an adenylate cyclase inhibitor, dideoxyadenosine (DDA) and a PKA inhibitor, H89. Insulin secretion by glimepiride, a well known K channel blocker, showed dramatic inhibition by DDA or H89 (10 μM), while insulin secretion by PCA was not affected at all (Fig. 5). The result indicates that insulin secretion by PCA does not involve GLP-1R-mediated cAMP/PKA activation but insulin secretion by glimepiride somehow involves GLP-1R-mediated cAMP/ PKA activation. Although the mechanism of insulin secretion by PCA was not elucidated clearly, GLP-1R-mediated cAMP/PKA activation and K channel blocking does not appear to be involved.
Fig. 5. Effect of an adenylate cyclase inhibitor or a PKA inhibitor on PCA-induced insulin secretion enhancement. HIT-T15 cells were washed twice and preincubated for 30 min in Krebs-Ringers bicarbonate (KRB) buffer. An adenylyl cyclase inhibitor, dideoxyadenosine (DDA, 10 μM) or a PKA inhibitor, H89 (10 μM) with combination PCA or glimepiride (100 μg/ml) were added. Cells were then incubated with KRB buffer containing 11.1 mM glucose for 1 h at 37℃. After incubation, insulin levels were determined as described in Materials and Methods. The error bars indicate standard deviations of five different results and the statistical significance is indicated as *p<0.05 and **p<0.01 by Student’s t test. ‘Cont’ represents control. ‘Glim’ represents glimepiride. ‘PCA’ represents purple corn anthocyanins.
DISCUSSION
Although antihyperglycemic activity of purple corn anthocyanin (PCA) has been tested in type-2 diabetic rodents (Tsuda et al., 2003; Sasaki et al., 2007), beta cell-protection activity of PCA has not been reported. Since gradual deterioration of pancreatic beta cell function and mass has been one of most prominent phenomena in type II diabetes, beta cell protection is one of effective therapeutic approach to managing type II diabetes (Butler et al., 2003), the proper protection is one of most important criteria to develop the medicine for type II diabetes. To test the beta cell protection activity of PCA, db/db mice were examined. First, insulin secretion activity of PCA was tested in hamster pancreatic beta cells, HIT-T15. Only PCA among several plant anthocyanins and polyphenols had insulin secretion activity in HIT-T15 cells (Fig. 1). Antidiabetic activity of PCA was tested by administration to 7 weeks-old db/db mice with dose of 10 mg/kg/day for seven weeks. The db/db mice (C57BLKS db/db) lack a functional leptin receptor by homozygous mutation of leptin receptor gene. They spontaneously develop obesity, hyperinsulinemia, and glucose intolerance at 4-6 week of age, progressing to diabetes by about 10 weeks of age (Leiter, 1989). The decrease in plasma insulin level and increase in blood glucose level are correlated to a degeneration of the pancreatic islets and destruction of the islet beta cells. They have thus been used extensively as a model for Type II diabetes. PCA showed efficient lowering activity of blood glucose level, OGTT (Fig. 2B and 2C) and HbA1c (Fig. 2D) when compared with glimepiride, a sulfonylurea in db/db mice. PCA also showed increased secretion of insulin (Fig. 2E). Interestingly, the population of pancreatic insulin secreting cells (beta cells) was dramatically increased up to about 80% while negative control and glimepiride-treated mice showed 54% and 50% (Fig. 3). As reported earlier, sulfonylurea could not protect from gradual deterioration of pancreatic beta cell function and mass. Rather, it reported to accelerate somewhat dysfunction of beta cells by inducing cell death (Efanova et al., 1998; Iwakura et al., 2000; Del Guerra et al., 2005; Maedler et al., 2005). Sustained enhancement of Ca2+ influx by sulfonylurea caused beta cell apoptosis. Sulfonylurea, glibenclamide as well as high glucose levels, stimulated production of ROS in the pancreatic beta cell line MIN6 (Tsubouchi et al., 2005). If ROS production by sulfonylurea is the mechanism for deterioration of beta cell death, PCA may have protection activity of beta cell death since anthocyanins are well known to be powerful antioxidant. Beta cell protection activity of PCA was confirmed in HIT-T15 cell culture co-treated with PCA and glimepiride (100 μg/ml) (Fig. 4A and 4B). In addition, PCA protected cells from H2O2-induced cell death (Fig. 4C).
PCA stimulated insulin secretion was apparent in culture of HIT-T15 cells and db/db mice (Fig. 1 and 2E). However the mechanism for insulin secretion stimulation has not been verified. It has been reported that both GIP and GLP-1 incretins exert their insulinotropic effects by binding to GIP and GLP-1 receptors expressed on pancreatic beta cells. Incretin bound receptors increased intracellular cAMP levels (Szecowka et al., 1982; Drucker et al., 1987), thereby activating protein kinase A (PKA) (Fehmann et al., 1995) and Epac2/cAMP-GEFII (Holz, 2004). PCA may activate GLP/GLP receptor signal to stimulate insulin secretion in beta cells. Therefore, adenylate cyclase inhibitor (DDA) and PKA inhibitor (H89) were treated to examine the effect on insulin secretion in HIT-T15 cells. Interestingly, stimulation of insulin secretion by glimepiride was inhibited by both DDA and H89. But stimulation of insulin secretion by PCA was not inhibited at all by either DDA or H89 (Fig. 5). Although sulfonylurea has been well known to stimulate insulin secretion of beta cells by blocking potassium channel, it also affects GLP/GLP receptor/cAMP/PKA signal for insulin secretion. However, PCA does not appear to involve GLP-1R-mediated cAMP/PKA activation and K channel blocking. Further characterization how PCA affects the growth, differentiation and insulin secretion of pancreatic beta cells at molecular level will provide better understanding of PCA-mediated stimulation of insulin secretion and beta cell protection.
Acknowledgments
This study was supported in part by the Inter-ER Cooperation Projects funded by the Ministry of Knowledge Economy (MKE) and Korea Institute for Advancement of Technology (KIAT). This study was also supported in part by the Priority Research Centers Program (NRF-2009-0094071).
References
- 1.Ashcroft F. M. Adenosine 5'-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci. (1988);11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft S. J., Ashcroft F. M. The sulfonylurea receptor. Biochim. Biophys. Acta . (1992);1175:45–59. doi: 10.1016/0167-4889(92)90008-Y. [DOI] [PubMed] [Google Scholar]
- 3.Bickford P. C., Shukitt-Hale B., Joseph J. Effects of aging on cerebellar noradrenergic function and motor learning: nutritional interventions. Mech. Ageing Dev. (1999);111:141–154. doi: 10.1016/S0047-6374(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 4.Bobe G., Wang B., Seeram N. P., Nair M. G., Bourquin L. D. Dietary anthocyanin-rich tart cherry extract inhibits intestinal tumorigenesis in APC (Min) mice fed suboptimal levels of sulindac. J. Agric. Food Chem. . (2006);54:9322–9328. doi: 10.1021/jf0612169. [DOI] [PubMed] [Google Scholar]
- 5.Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. (2003);52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 6.Cirico T. L., Omaye S. T. Additive or synergetic effects of phenolic compounds on human low density lipoprotein oxidation. Food Chem. Toxicol. (2006);44:510–516. doi: 10.1016/j.fct.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Del Guerra S., Marselli L., Lupi R., Boggi U., Mosca F., Benzi L., Del Prato S., Marchetti P. Effects of prolonged in vitro exposure to sulphonylureas on the function and survival of human islets. J. Diabetes Complications . (2005);19:60–64. doi: 10.1016/j.jdiacomp.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Drucker D. J., Philippe J., Mojsov S., Chick W. L., Habener J. F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc. Natl. Acad. Sci. U.S.A. (1987);84:3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efanova I. B., Zaitsev S. V., Zhivotovsky B., Kohler M., Efendic S., Orrenius S., Berggren P. O. Glucose and tolbutamide induce apoptosis in pancreatic beta-cells. A process dependent on intracellular Ca2+ concentration. J. Biol. Chem. . (1998);273:33501–33507. doi: 10.1074/jbc.273.50.33501. [DOI] [PubMed] [Google Scholar]
- 10.Fehmann H. C., Goke R., Goke B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucosedependent insulin releasing polypeptide. Endocr. Rev. (1995);16:390–410. doi: 10.1210/edrv-16-3-390. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein B. J. Insulin resistance as the core defect in type 2 diabetes mellitus. Am. J. Cardiol. (2002);90:3G–10G. doi: 10.1016/S0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- 12.Holz G. G. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes. (2004);53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwakura T., Fujimoto S., Kagimoto S., Inada A., Kubota A., Someya Y., Ihara Y., Yamada Y., Seino Y. Sustained enhancement of Ca2+ influx by glibenclamide induces apoptosis in RINm5F cells. Biochem. Biophys. Res. Commun. (2000);271:422–428. doi: 10.1006/bbrc.2000.2616. [DOI] [PubMed] [Google Scholar]
- 14.Jayaprakasam B., Vareed S. K., Olson L. K., Nair M. G. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J. Agric. Food Chem. . (2005);53:28–31. doi: 10.1021/jf049018. [DOI] [PubMed] [Google Scholar]
- 15.Juranic Z., Zizak Z. Biological activities of berries: from antioxidant capacity to anti-cancer effects. BioFactors . (2005);23:207–211. doi: 10.1002/biof.5520230405. [DOI] [PubMed] [Google Scholar]
- 16.Kong J. M., Chia L. S., Goh N. K., Chia T. F., Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. (2003);64:923–933. doi: 10.1016/S0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- 17.Lebovitz H. E., Banerji M. A. Treatment of insulin resistance in diabetes mellitus. Eur. J. Pharmacol. . (2004);490:135–146. doi: 10.1016/j.ejphar.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 18.Leiter E. H. The genetics of diabetes susceptibility in mice. FASEB J. (1989);3:2231–2241. doi: 10.1096/fasebj.3.11.2673897. [DOI] [PubMed] [Google Scholar]
- 19.Maedler K., Carr R. D., Bosco D., Zuellig R. A., Berney T., Donath M. Y. Sulfonylurea induced beta-cell apoptosis in cultured human islets. J. Clin. Endocrinol. Metab. . (2005);90:501–506. doi: 10.1210/jc.2004-0699. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki R., Nishimura N., Hoshino H., Isa Y., Kadowaki M., Ichi T., Tanaka A., Nishiumi S., Fukuda I., Ashida H., Horio F., Tsuda T. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem. Pharmacol. (2007);74:1619–1627. doi: 10.1016/j.bcp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Szecowka J., Grill V., Sandberg E., Efendic S. Effect of GIP on the secretion of insulin and somatostatin and the accumulation of cyclic AMP in vitro in the rat. Acta Endocrinol. . (1982);99:416–421. doi: 10.1530/acta.0.0990416. [DOI] [PubMed] [Google Scholar]
- 22.Takikawa M., Inoue S., Horio F., Tsuda T. Dietary anthocyanin- rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. (2010);140:527–533. doi: 10.3945/jn.109.118216. [DOI] [PubMed] [Google Scholar]
- 23.Tsubouchi H., Inoguchi T., Inuo M., Kakimoto M., Sonta T., Sonoda N., Sasaki S., Kobayashi K., Sumimoto H., Nawata H. Sulfonylurea as well as elevated glucose levels stimulate reactive oxygen species production in the pancreatic beta-cell line, MIN6- a role of NAD(P)H oxidase in beta-cells. Biochem. Biophys. Res. Commun. (2005);326:60–65. doi: 10.1016/j.bbrc.2004.10.201. [DOI] [PubMed] [Google Scholar]
- 24.Tsuda T., Horio F., Uchida K., Aoki H., Osawa T. Dietary cyanidin 3-O-beta-D-glucoside-rich purple corn color prevents obesity and ameliorates hyperglycemia in mice. J. Nutr. . (2003);133:2125–2130. doi: 10.1093/jn/133.7.2125. [DOI] [PubMed] [Google Scholar]