Abstract
Quetiapine is an atypical or second-generation antipsychotic agent and has been a subject of a series of case report and suggested to have the potential for misuse or abuse. However, it is not a controlled substance and is not generally considered addictive. In this study, we examined quetiapine’s dependence potential and abuse liability through animal behavioral tests using rodents to study the mechanism of quetiapine. Molecular biology techniques were also used to find out the action mechanisms of the drug. In the animal behavioral tests, quetiapine did not show any positive effect on the experimental animals in the climbing, jumping, and conditioned place preference tests. However, in the head twitch and self-administration tests, the experimental animals showed significant positive responses. In addition, the action mechanism of quetiapine was found being related to dopamine and serotonin release. These results demonstrate that quetiapine affects the neurological systems related to abuse liability and has the potential to lead psychological dependence, as well.
Keywords: Quetiapine, Dopamine system, Serotonin system, Drug dependence, Animal behavioral tests
INTRODUCTION
Drug dependence is defined as the loss of control over drug use or the compulsive seeking and taking of drugs despite adverse consequences (Koob, 1999). It is caused by drug activity in the brain. However, it is also related to physiological and social factors. Once a person develops drug dependence, it might last their whole life. In this context, research to evaluate drugs regarding their potential for dependence, before the drugs to go into common usage, is important.
Animal experiment can be one of the tools for indirectly measuring drug dependence (Acheson et al., 1999; Varlinskaya and Spear, 2002; Doremus et al., 2003; Chung et al., 2008; Morris et al., 2010). There are two types of drug dependence: physical dependence and psychological dependence. Physical dependence refers to the state resulting from chronic use of a drug-to the point of tolerance-in which negative physical symptoms or withdrawal result from abrupt drug discontinuation or dosage reduction (Landry et al., 1992). Psychological dependence refers to a lack of self restraint regarding drug use. Two important concepts pertain to this phenomenon are reinforcement and reward (Taylor, 2002; Koob and Kreek, 2007). “Reinforcement” refers to an event that increases the probability of a given action. The meaning of “reward” is similar, but reward usually refers to a positive sensation, such as pleasure (Koob, 1992).
Several laboratory experiments are commonly used to validate a drug’s dependence potential (Chung et al., 2008). Researchers examine the climbing and head twitch behaviors in pre-evaluation experiments to evaluate a drug’s dopaminergic and serotonergic effects, respectively. The jumping behavior test is typically used to determine a drug’s potential to lead to physical dependence, especially for the opioids (Way et al., 1969; Saelens et al., 1971; Smits, 1975; Ritzmann, 1981; El-Kadi and Sharif, 1994; Kest et al., 2001). To evaluate and validate a substance’s potential to result in psychological dependence, researchers make considerable use of the conditioned place preference test and self-administration test, for investigating the substance’s rewarding effect and reinforcing effect respectively (Mucha et al., 1982; Gorelick et al., 2004).
Quetiapine is an atypical second-generation antipsychotic agent that has an approved labeling from the Food and Drug Administration in 1997 for the on-label treatment of schizo-phrenia and the on-label short-term treatment (as monotherapy or adjunct therapy to lithium or valporic acid) of acute manic episodes associated with bipolar I disorder (Morin, 2007; Sansone and Sansone, 2010). Quetiapine is sometimes used off-label, often as an augmentation agent, to treat conditions such as obsessive-compulsive disorder, post-traumatic stress disorder, restless legs syndrome, autism, alcoholism, depression, and Tourette syndrome. Additionally, physicians have used it as a sedative for those with sleep disorders or anxiety disorders (FDA, 2007). Quetiapine is a dibenzothiazepine derivative, and known to be an antagonist of serotonin, dopamine, histamine, and adrenergic receptors. However the mode of action has not been clearly elucidated yet. Distinctively, quetiapine’s transient occupancy and rapid dissociation from postsynaptic dopamine receptors appear sufficient for antipsychotic action but insufficient to induce extrapyramidal symptoms or hyperprolactinemia (Kapur et al., 2000; Tauscher et al., 2004). This is one reason quetiapine is the most frequently- prescribed antipsychotic agent in the United States of America (USA). Though quetiapine is not a controlled substance and is not considered addictive, its drug dependence potential has been described in several case reports (Pinta, 2007). The available misuse/abuse routes were oral, intranasal, or intravenous, and most cases occurred in people with some history of multi-substance abuse (Pinta, 2007; Morin, 2007). Some are the cases of inmates in jails or prisons (Pierre et al., 2004; Hussain et al., 2005). However, little or no scientific evidence revealing its action of mechanism and dependence or abuse liability, including animal behavioral experiments, presently exists (Sansone and Sansone, 2010). We therefore performed various animal behavioral experiments, and used molecular biology techniques to elucidate the abuse liability and the mechanisms of action of quetiapine in the present study.
MATERIALS AND METHODS
Animals and drugs
Sprague-Dawley rats (180-220 g) and ICR mice (15-20 g) were obtained from Korea Food and Drug Administration (AAALAC member, Seoul, Korea) and they were housed in groups, of adequate size, in a temperature-controlled 23 ± 2℃ room with a 12 h light/dark cycle (lights on 08:00 to 20:00). The animals received a solid diet and tap water ad libitum, and their treatment conformed to the Guide for the Care and Use of Laboratory Animals (NRC 1996). We performed all experiments between 09:00 and 18:00. Methamphetamine HCl, cocaine, and quetiapine were obtained from Sigma (St. Louis, MO, USA).
Apparatus
The climbing behavior test apparatus was a stainless steel cylinder with many vertical bars, which an experimental mouse could climb. Its floor diameter was 12 cm, and each vertical bar’s length was 24 cm. To evaluate jumping behavior and head twitch responses, a transparent box, sans ceiling, measuring 30×30×40 cm was used.
The conditioned place preference test chamber had three distinct compartments (white, black, and grey) separated by automatic guillotine doors. To automate data collection, infrared photo-beam detectors were added. The overall inside dimensions were 21×21×68 cm, and the unit’s base measured 86.4×25.4 cm. The manufacturer provided the mounting holes for the ENV-013 IR Infrared Sensor Package (Med Associates Inc., Georgia, VT, USA), which places six photo-beams across the white and black zones, 1.25 cm from each end wall, with 5 cm intervals between the beams. The choice compartments were 28 cm long. One choice compartment was all black, with a stainless steel grid rod floor consisting of 4.8 mm rods on 16 mm centers. The other compartment was all white, with a 1.25×1.25 cm stainless steel mesh floor.
The self-administration test chamber was purchased from Med Associates Inc. (Georgia, VT, USA) and measured 29×21×24 cm. The chambers contained two levers, an active lever to deliver a drug dose, via the jugular vein, through a connected catheter and an inactive lever, not connected to the experimental animal. Infusion pumps were placed outside the chamber and connected to a 10 ml syringe. We connected the chamber to a computer, to record test data and control the experimental processes.
Methods
Climbing behavior test: One group of mice was administered with the negative control (saline, 1 mg/kg, i.p.) or one of the three doses of quetiapine (5, 7, or 10 mg/kg, i.p.) for 40 to 90 min, respectively. Then for 1 min, their climbing duration was checked, using a stopwatch. The other group of mice was pre-treated with the negative control (saline, 1 mg/kg, i.p.) or one of the three doses of quetiapine (5, 7, or 10 mg/kg, i.p.) for 40 to 90 min before the test. Then just before testing, apomorphine (2 mg/kg, i.p.) was administered to each subject and timed their climbing duration as above. The tests were repeated three times, with a time-out period of 10 min.
Jumping test: One group of mice was administered the negative control (saline, 1 mg/kg, i.p.), or one of the three doses of quetiapine (5, 7, or 10 mg/kg, i.p.) for 40 to 90 min and followed by naloxone (10 mg/kg, i.p.). Then for 15 min, the jumping numbers of the animals were counted. The other group of mice was pre-treated with the negative control (saline, 1 mg/kg, i.p.) or one of the three doses of quetiapine (5, 7, or 10 mg/kg, i.p.) for 40 to 90 min before the test. Next, morphine (150 mg/kg, s.c.) was administered and followed by naloxone administration (10 mg/kg, i.p.) 4 h after the morphine treatment. The jumping number was counted for 15 min. The experiment was repeated three times.
Head twitch response: One group of mice was administered with the negative control (saline, 1 mg/kg, i.c.v.) and one of the three doses of quetiapine (5, 7, or 10 mg/kg, i.c.v.) for 40 to 90 min before the test, and the numbers of their head twitches were counted for 2 min. The other group of mice was pre-treated with the negative control (saline, 1 mg/kg, i.p.) and one of the three doses of quetiapine (5, 7, and 10 mg/kg, i.p.) for 40 to 90 min before the test. Then 5-HT (3-4 mg/kg, i.p.) was administered and the numbers of head twitches were counted for 2 min. The test was repeated three times, at 10 min intervals.
Conditioned place preference test: Before starting the experiment, the mice were acclimated to the experimental apparatus and handled for 6 days. The procedure was similar to that described previously (Bozarth, 1987; Narita et al., 2004).
Each experiment consisted of three phases, as follows.
Pre-conditioning: For 2 days (days 1 and 2) the rats were allowed free access to both compartments of the apparatus for 15 min (900 s) each day. On day 2, the time spent by the mice in each compartment was recorded and served as a baseline. The mice showed preference for the black compartment was selected for further experiments and divided into two groups.
Conditioning: Conditioning was conducted for 8 days (days 3 to 10), for one session per day. On day 3, one group of the selected mice was treated with drugs (methamphetamine, 1 mg/kg, i.p., one of the three doses of quetiapine, 0.1, 0.5, and 1 mg/kg, i.p.), and placed in the non-preferred compartment (white) for 30 min. The other group of mice was treated with saline, and placed in the preferred compartment (black) for 30 min. The groups were switched everyday and the same procedure was conducted.
Post-conditioning: On day 11, the mice were allowed to access freely both compartments of the apparatus for 15 min (900 s). The time spent by the mice in each compartment was recorded, with these values serving as a test line.
Self-administration test: Surgical procedures were as follows. The rats were anesthetized with pentobarbital sodium (Entobar®, Hanlim pharmaceuticals). The surgical procedures adhered to aseptic conditions described previously (Weeks, 1972; Mucha et al., 1982). Briefly, a catheter was inserted into each rat’s right jugular vein. The catheter exited on the rat’s shoulder. The rats received heparin everyday of the experimental periods. After surgery, each rat recovered for at least 14 days in a controlled cage, receiving a solid diet and tap water ad libitum.
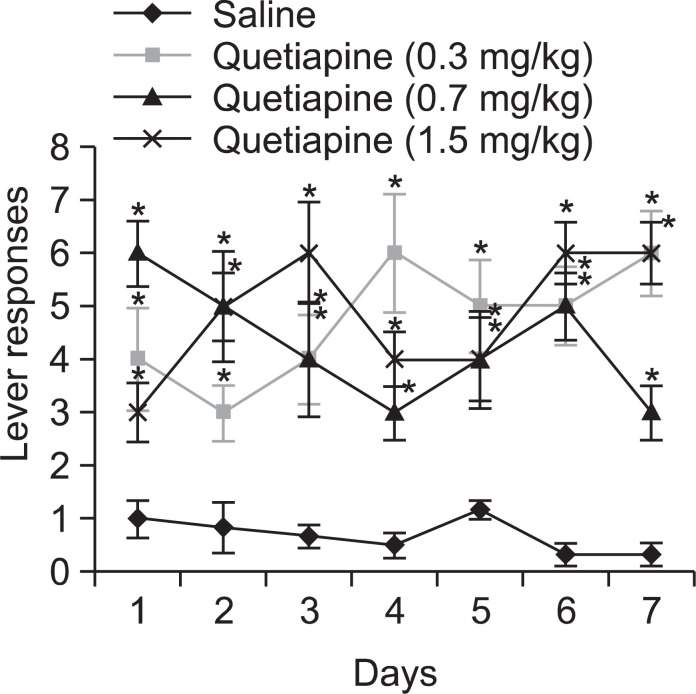
The testing procedures were as follows. The rats could self-administer with one of the three doses of quetiapine (0.3, 0.7, and 1.5 mg/kg/0.1 ml per infusion), and a negative control substance (saline, 0.1 ml per infusion) for 6 s followed by 20 s of time-out, during daily 2 h sessions on a fixed-ratio 1 (FR1) reinforcement schedule. With this schedule, when a rat presses the active lever, it receives a certain drug dose (0.1 ml) injected into the jugular vein through the catheter. The selfadministration chamber contains two levers linked to a computer program which records the experimental data. The test was carried out for more than 7 days.
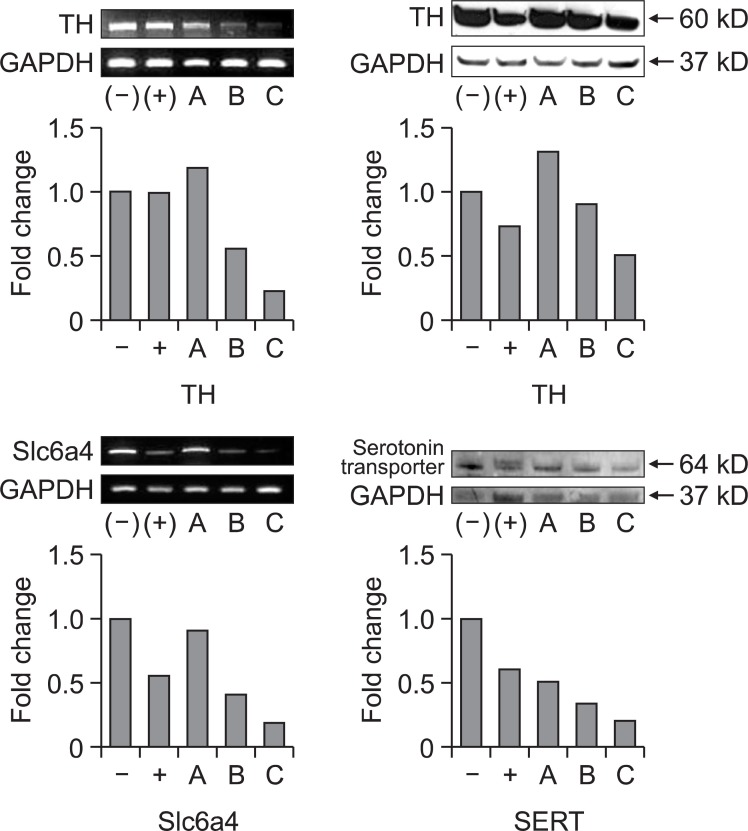
PCR and western blotting: The brain samples for the PCR analysis and western blotting were obtained from striatum part of the rats administered negative control (saline), positive control (methamphetamine) and three different doses of quetiapine (5, 7, 10 mg/kg) for 8 days. Two genes [tyrosine hydroxylase (TH) and serotonin transporter (Slc6a4)] were detected using PCR technique, and the same proteins were detected using western blotting technique. GAPDH was used as control. Antibodies were purchased from Chemicon International (Bilerica, MA, USA).
Statistics: The data are expressed as the mean ± S.E. The climbing, jumping, and head twitch data were analyzed via paired t-tests. Likewise, paired t-tests were used to analyze the CPP and self-administration data (p<0.05).
RESULTS
Climbing behavior test and jumping test
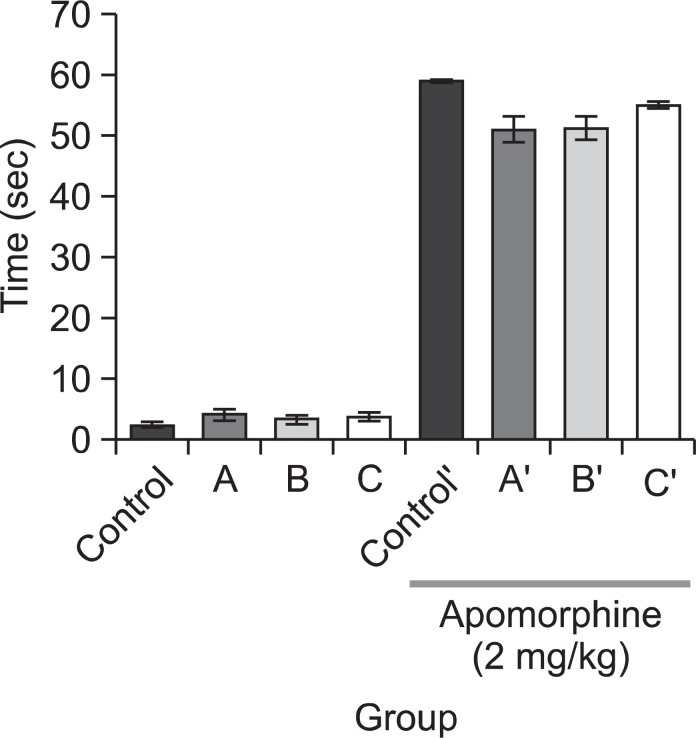
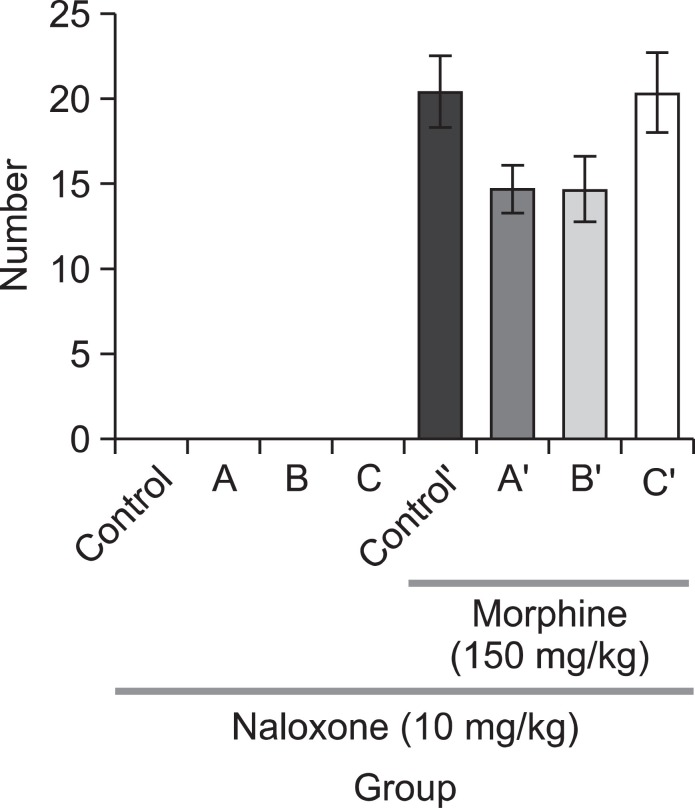
We measured climbing behavior in experimental mice with or without pre-treatment of the negative control (saline, 1 mg/kg, i.p.) or quetiapine (5, 7, or 10 mg/kg, i.p.) to figure out whether quetiapine affects dopaminergic system. In the group without apomorphine treatment, there were no differences between the saline treated group and the quetiapine treated groups, regardless of drug concentration. In the apomorphinetreated group, on the other hand, the quetiapine treated group tended to spend less time for climbing as compared to the saline treated group. However, the difference between these two groups was not statistically significant (Fig. 1). In the jumping test, we administered the negative control (saline, 1 mg/kg, i.p.) or one of the three doses of quetiapine (5, 7, or 10 mg/kg, i.p.) prior to administrating morphine. The mice received morphine (150 mg/kg, s.c.) 4 h before naloxone (10 mg/kg, i.p.) administration. As shown in Fig. 2, no mice in the saline or quetiapine treated groups jumped without morphine administration. Though animals in the two quetiapine treated groups (the 5 and 10 mg/kg dosages) which were treated with morphine showed a tendency of decreasing number of jumps compared with the corresponding saline treated animals but it was not statistically significant.
Fig. 1. Climbing behaviors were measured after injection of apomorphine to each subject (2 mg/kg, s.c.). The pre-treatments were quetiapine (A (5 mg/kg), B (7 mg/kg) or C (10 mg/kg), i.p.), administered before the apomorphine treatment (after apomorphine treatment: control’, A’ (5 mg/kg), B’ (7 mg/kg), C’ (10 mg/kg), i.p.). Data are expressed as mean ± S.E. (n=15).
Fig. 2. Quetiapine (A) 5 mg/kg, (B) 7 mg/kg or (C) 10 mg/kg, i.p. was administered prior to morphine administration. Morphine (150 mg/kg, s.c.) was administered 4 h prior to naloxone administration (after morphine treatment: control’, A’ (5 mg/kg), B’ (7 mg/kg), C’ (10 mg/kg), i.p.). The jumping score in groups (n=15) was measured for 15 min immediately after the injection of naloxone (10 mg/kg, i.p.). Each value is the mean ± S.E.
Head twitch response
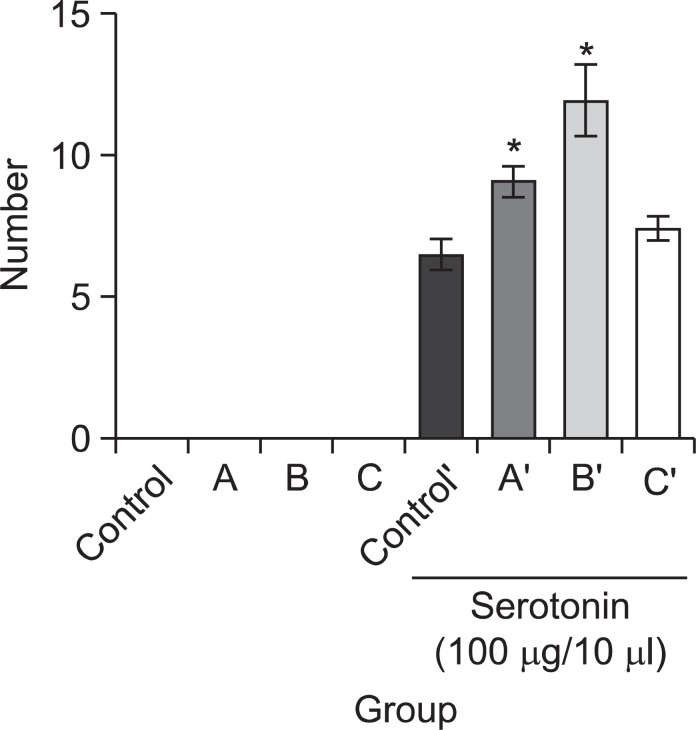
Head twitch response was observed for evaluation of quetiapine’s serotonergic effect. One of the three doses of quetiapine (5, 7, or 10 mg/kg, i.p.) was treated prior to administering the serotonin (5-HT, 3-4 mg/kg, i.c.v.). We counted responses in each of the groups three times, for 2 min each time, with 10 min intervals. As shown in Fig. 3, no mice in either the negative control (saline-treated) group or the quetiapine treated groups showed a head twitch response in the absence of serotonin. On the other hand, Fig. 3 also shows that, with serotonin, two quetiapine-treated groups (5 and 7 mg/kg dosages) showed increased head twitch responses as compared to the saline treated group and the differences were statistically significant.
Fig. 3. Quetiapine (A) 5 mg/kg, (B) 7 mg/kg or (C) 10 mg/kg, i.p. was injected prior to serotonin (5-HT, 3-4 mg/kg, i.c.v.) administration (after serotonin treatment: control’, A’ (5 mg/kg), B’ (7 mg/kg), C’ (10 mg/kg), i.p.). The score of response in groups (n=15) was measured for 2 min, 3 times, 10 min of interval. Each value is the mean ± S.E. *p<0.05, compared with saline treated group.
Conditioned place preference
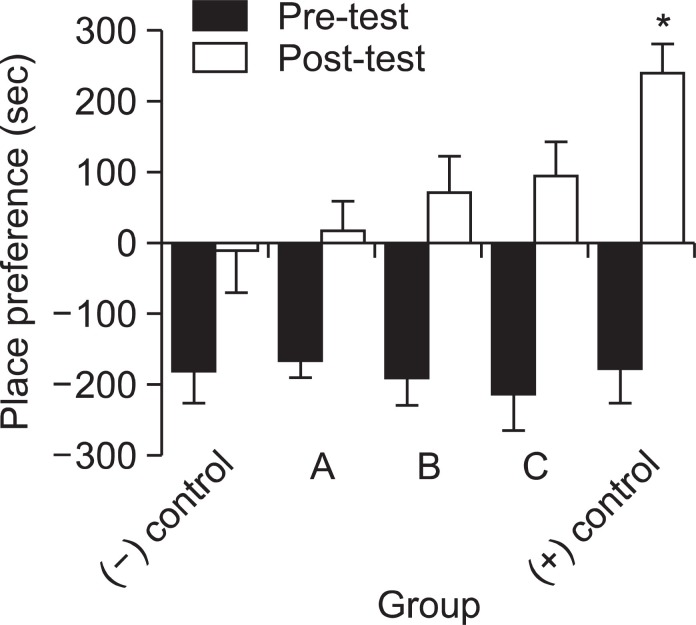
Possibilities of psychological dependency or abuse liability were evaluated through the two known methods: conditioned place preference and self-administration. Considering the overall results, the animal’s place preference clearly changed in every group during the 8 day-conditioning period. In contrast with the mice treated with saline, the entire group treated with drugs (quetiapine and methamphetamine) spent more time in the undesirable room after the conditioning period. When compared the differences between the negative control and drug-treated groups, the animals received quetiapine showed a dose-dependent place preference pattern. However, the differences were not statistically significant. Moreover, the positive control group (methamphetamine, 1 mg/kg) showed a markedly increased place preference, spending more than 200 s longer in the undesirable compartment compared to the negative control (saline) group. Fig. 4 shows these results.
Fig. 4. These mice were preconditioned for 2 days without drug treatment. Then quetiapine quetiapine (A) 0.1 mg/kg, (B) 0.5 mg/kg or (C) 1.0 mg/kg), i.p., saline ((-) control, 1 ml, i.p.) and methamphetamine ((+) control, 1 mg/kg, i.p.) were administered to the mice once a day for 8 days. Place preference was measured on the next day after the conditioning period. Data are expressed as mean ± S.E. (n=10). *p<0.05, compared with saline treated group.
Self-administration
The self-administration test was maintained on a fixed-ratio (FR) 1 schedule for more than 7 days, and the responses on the active lever were checked on a daily basis. The negative control (saline-treated) group did not show active responses. Interestingly, the experimental rats in all the three groups of quetiapine treatment showed increased self-administration and statistically significant active responses compared with that of the negative control (saline-treated) group. The selfadministration test result is depicted in Fig. 5.
Fig. 5. The animals were administered quetiapine (at 0.3, 0.7 or 1.5 mg/kg/0.1 ml per infusion) for 6 s followed by a 20 s time-out period in the way of self-administration. Lever responses were performed everyday for more than 7 days. Data are expressed as mean ± S.E. (n=6 or 8), and t-test was used for statistical analysis (*p<0.05 as compared with the saline treated group).
PCR and western blotting
To verify quetiapine’s effects on dopaminergic and serotonergic system, the expression levels of tyrosine hydroxylase (TH) and serotonin transporter (Slc6a4) were analyzed by PCR and western blotting. Both gene and protein expression were decreased in dose dependent manner in nucleus accumbens by quetiapine treatment as well as methamphetamine treatment. The PCR and western blotting results are shown in Fig. 6.
Fig. 6. Quetiapine (A) 5 mg/kg, (B) 7 mg/kg or (C) 10 mg/kg, i.p., negative control ((-), saline 1 ml, i.p.) and positive control ((+), methamphetamine 1 mg/kg, i.p.) were treated to rats for 8 days, and the striatum part of the brain was collected. Two genes and proteins (Tyrosine hydroxylase (TH), serotonin transporter (Slc6a4)) were detected using PCR and western blotting techniques. GAPDH was used as control. Quantitative measures were calculated from the density of each area using Image J program (NIH, USA).
DISCUSSION
Quetiapine is frequently prescribed in various psychological conditions including schizophrenia, manic episodes associated with bipolar I disorder, obsessive-compulsive disorder, post traumatic stress disorder and so forth. However, little has been known about its abuse liability and receptors that it is binding yet. In the present study, we performed various animal behavioral experiments to demonstrate quetiapine’s mode of action and potential for inducing physical or psychological dependence. Climbing behavior and head twitch experiments were performed as pre-evaluating experiments to see if quetiapine has effects on dopaminergic and serotonergic system, respectively. In climbing behavior experiment, no significant change was observed. This suggests that quetiapine has low binding affinity to dopamine receptor, otherwise it dissociates easily from dopamine receptors (Sumegi, 2008; Erdogan, 2010). According to our experiments on the gene and protein expression of tyrosine hydroxylase, it turned out obvious that quetiapine affects dopaminergic system in the brain. In the mean time, a confident decrease of head twitch response was observed in the quetiapine treated group (5 and 7 mg/kg), which reconfirmed the effect of quetiapine on serotonergic system, and this is coincident with our results of PCR and western blotting. These results were well concurred with previous reports that quetiapine enhances central serotonergic neurotransmission through its high affinity for serotonergic receptors especially for 5-HT2A (Goldstein, 1999; Pae et al., 2010).
The jumping behavior test was conducted to check if there is any physical dependence potential of quetiapine. In our result, there was a tendency for decrease in the number of jumping in the two doses of quetiapine treated animals (5 and 10 mg/kg) with morphine, compared with saline treated animals. Studies have commonly noted that withdrawal jumping behavior is the most reliable and generally useful for measuring physical dependence in rodents, especially with regard to opioids (Way et al., 1969; Saelens et al., 1971; Smits, 1975; Ritzmann, 1981; El-Kadi and Sharif, 1994; Kest et al., 2001). However, there is a report that has indicated that different neural substrates may contribute to the various signs and symptoms of withdrawal syndrome (Koob et al., 1992). Therefore, additional research will be needed to confirm quetiapine’s tendency to cause physical dependence.
To evaluate quetiapine’s psychological dependence potential, the conditioned place preference test and self-administration test were performed. For the conditioned place preference test, the mice that stayed longer in the black chamber during the preconditioning test were selected. Though statistically meaningful data were not obtained, dose-dependent increases in place preference were observed in the quetiapine administered group. Moreover, in the self-administration test, significant increases of self-administration were observed in all groups of quetiapine treated rats. In this experiment, the experimental rats in all the three groups of quetiapine acquired self-administration and demonstrated statistically significant active responses compared with that of the negative control (saline-treated) group. Based on these results, it seems clear that quetiapine has potentials for causing psychological dependence in animals.
Concerning quetiapine’s dependency potential and/or abuse liability, several studies have focused on the drug’s clinical benefits in patients with mood disorders, anxiety disorders, aggression, etc. (Adityanjee and Schulz, 2002; Brown et al., 2002; Weisnam, 2003; Monnelly et al., 2004; Sattar et al., 2004; Pinkofsky et al., 2005). The antihistaminergic, antidopaminergic and antiadrenergic properties of quetiapine most likely explain its calming and sedating effects (Arango and Bobes, 2004; Cohrs et al., 2004). It seems that quetiapine’s property of rapid dissociation from dopamine receptors plays a role in its abuse potential but not in either euphoria or the dysphoria enhancement associated with drug withdrawal (Morin, 2007; Sumegi, 2008; Erdogan, 2010).
Drug abuse generally exerts its addictive properties via the release of dopamine in the reward pathway of limbic system. Furthermore, stimulation of both the dopaminergic and serotonergic system is implicated in substance abuse. Taken together the results from conditioned place preference and selfadministration tests, we conclude that quetiapine might have a potential to induce dependence. This suggests that it would be worthwhile monitoring usage of quetiapine with precaution to prevent possible drug abuse in the future.
Acknowledgments
This work was supported by the National Institute of Food and Drug Safety Evaluation (09171KFDA667).
References
- 1.Acheson S. K., Richardson R., Swarzwelder H. S. Developmental changes in seizure susceptibility during ethanol withdrawal. Alcohol. (1999);18:23–26. doi: 10.1016/S0741-8329(98)00063-9. [DOI] [PubMed] [Google Scholar]
- 2.Adityanjee, Schulz S. C. Clinical use of quetiapine in disease states other than schizophrenia. J. Clin. Psychiatry. (2002);63:32–38. [PubMed] [Google Scholar]
- 3.Arango C., Bobes J. Managing acute exacerbations of schizophrenia: focus on quetiapine. Curr. Med. Res. Opin. (2004);20:619–626. doi: 10.1185/030079904125003430. [DOI] [PubMed] [Google Scholar]
- 4.Bozarth M. A. Conditioned place preference: a parametric analysis using system heroin injection. In Methods of assessing the reinforcing properties of abused drugs (M. A. Bozarth, Ed.) Springer; New York: (1987). pp. 241–273. [Google Scholar]
- 5.Brown E. S., Nejtek V. A., Perantie D. C., Bobadilla L. Quetiapine in bipolar disorder and cocaine dependence. Bipolar Disord. (2002);4:406–411. doi: 10.1034/j.1399-5618.2002.02229.x. [DOI] [PubMed] [Google Scholar]
- 6.Chung C. S., Wang J., Wehman M., Rhoads D. E. Severity of alcohol withdrawal symptoms depends on developmental stage of Long-Evans rats. Pharmacol. Biochem. Behav. (2008);89:137–144. doi: 10.1016/j.pbb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohrs S., Rodenbeck A., Guan Z., Pohlmann K., Jordan W., Meier A., Ruther E. Sleep promoting properties of quetiapine in healthy subjects. Psychopharmacology. (2004);174:421–429. doi: 10.1007/s00213-003-1759-5. [DOI] [PubMed] [Google Scholar]
- 8.Doremus T. L., Brunell S. C., Varlinskaya E. L., Spear L. P. Anxiogenic effects during withdrawal from acute ethanol in adolescent and adult rats. Pharmacol. Biochem. Behav. (2003);75:411–418. doi: 10.1016/S0091-3057(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 9.El-Kadi A. O., Sharif S. I. The influence of various experimental conditions on the expression of naloxone-induced withdrawal symptoms in mice. Gen. Pharmacol. (1994);25:1505–1510. doi: 10.1016/0306-3623(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 10.Erdogan S. Quetiapine in substance use disorders, abuse and dependence possibility: a review. Turk. Psikiyatri Derg. (2010);21:167–175. [PubMed] [Google Scholar]
- 11.Goldstein J. M. Quetiapine fumarate (Seroquel): a new atypical antipsychotic. Drugs Today. (1999);35:193–210. doi: 10.1358/dot.1999.35.3.533849. [DOI] [PubMed] [Google Scholar]
- 12.Gorelick D. A., Gardner E. L., Xi Z. X. Agents in development for the management of cocaine abuse. Drugs. (2004);64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hussain M. Z., Waheed W., Hussain S. Intravenous quetiapine abuse (letter). Am. J. Psychiatry. (2005);162:1755–1756. doi: 10.1176/appi.ajp.162.9.1755-a. [DOI] [PubMed] [Google Scholar]
- 14.Kapur S., Zipursky R., Jones C., Shammi C. S., Remington G., Seeman P. A positron emission tomography study of quetiapine in schizophrenia. Arch. Gen. Psychiatry. (2000);57:553–559. doi: 10.1001/archpsyc.57.6.553. [DOI] [PubMed] [Google Scholar]
- 15.Kest B., Palmese C. A., Hopkins E., Adler M., Juni A. Assessment of acute and chronic morphine dependence in male and female mice. Pharmacol. Biochem. Behav. (2001);70:149–156. doi: 10.1016/S0091-3057(01)00600-1. [DOI] [PubMed] [Google Scholar]
- 16.Koob G. F. Drug addiction: the yin and yang of hedonic homeostasis. Neuron. (1999);16:893–896. doi: 10.1016/S0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- 17.Koob G. F. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol. Sci. (1992);13:177–184. doi: 10.1016/0165-6147(92)90060-J. [DOI] [PubMed] [Google Scholar]
- 18.Koob G., Kreek M. J. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry. (2007);164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koob G. F., Maldonado R., Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. . (1992);15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- 20.Landry M. J., Smith D. E., McDuff D. R., Baghman O. L. Benzodiazepine dependence and withdrawal: identification and medical management. J. Am. Board Fam. Pract. (1992);5:167–175. [PubMed] [Google Scholar]
- 21.Monnelly E. P., Ciraulo D. A., Knapp C., LoCastro J., Sepulvedal Quetiapine for treatment of alcohol dependence. J. Clin. Psychopharmacol. (2004);24:532–535. doi: 10.1097/01.jcp.0000138763.23482.2a. [DOI] [PubMed] [Google Scholar]
- 22.Morin A. K. Possible intranasal quetiapine misuse. Am. J. Health Syst. Pharm. (2007);64:723–725. doi: 10.2146/ajhp060226. [DOI] [PubMed] [Google Scholar]
- 23.Morris S. A., Kelso M. L., Liput D. J., Mrshall K., Nixon K. Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol. (2010);44:89–98. doi: 10.1016/j.alcohol.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mucha R. F., van der Kooy D., O'Shaughnessy M., Bucenieks P. Drug reinforcement studied by the use of place conditioning in rat. Brain Res. . (1982);243:91–105. doi: 10.1016/0006-8993(82)91123-4. [DOI] [PubMed] [Google Scholar]
- 25.Narita M., Akai H., Nagumo Y., Sunagawa N., Hasebe K., Nagase H., Kita T., Hara C., Suzuki T. Implications of protein kinase C in the nucleus accumbens in the development of sensitization to methamphetamine in rats. Neuroscience. (2004);127:941–948. doi: 10.1016/j.neuroscience.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Pae C. U., Sohi M. S., Seo H. J., Serretti A., Patker A. A., Steffens D. C., Masand P. S. Quetiapine XR: current status for the treatment of major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. (2010);34:1165–1173. doi: 10.1016/j.pnpbp.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Pierre J. M., Shnayder I., Wirshing D. A., Wirshing W. C. Intranasal quetiapine abuse (letter). Am. J. Psychiatry. (2004);161:1718. doi: 10.1176/appi.ajp.161.9.1718. [DOI] [PubMed] [Google Scholar]
- 28.Pinkofsky H. B., Hahn A. M., Campbell F. A., Fueda J., Daley D. C., Doualihy A. B. Reduction of opioid-withdrawal symptoms with quetiapine. J. Clin. Psychiatry. (2005);66:1285–1288. doi: 10.4088/JCP.v66n1011. [DOI] [PubMed] [Google Scholar]
- 29.Pinta E. R. Quetiapine addiction? Am J. Psychiatry. (2007);164:174–175. doi: 10.1176/appi.ajp.164.1.174. [DOI] [PubMed] [Google Scholar]
- 30.Quetiapine fumarate. http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm. Food and Drug Administration: Electronic Orange Book. (2007)
- 31.Ritzmann R. F. Opiate dependence following acute injections of morphine and naloxone: the assessment of various withdrawal signs. Pharmacol. Biochem. Behav. (1981);14:575–577. doi: 10.1016/0091-3057(81)90320-8. [DOI] [PubMed] [Google Scholar]
- 32.Saelens J. K., Granat F. R., Sawyer W. K. The mouse jumping test - a simple screening method to estimate the physical dependence capacity of analgesics. Arch. Int. Pharmacol. Ther. (1971);190:213–218. [PubMed] [Google Scholar]
- 33.Sansone R. A., Sansone L. A. Is seroquel developing an illicit reputation for misuse/abuse? Psychiatry. (2010);7:13–16. [PMC free article] [PubMed] [Google Scholar]
- 34.Sattar S. P., Bhatia S. C., Petty F. Potential benefits of quetiapine in the treatment of substance dependence disorders. J. Psychiatry Neurosci. (2004);29:452–457. [PMC free article] [PubMed] [Google Scholar]
- 35.Smits S. E. Quantitation of physical dependence in mice by naloxone-precipitated jumping after a single dose of morphine. Res. Commun. Chem. Pathol. Pharmacol. (1975);10:651–661. [PubMed] [Google Scholar]
- 36.Sumegi A. Quetiapine in bipolar disorders. Neuropsychopharmacol. Hung. (2008);10:281–291. [PubMed] [Google Scholar]
- 37.Tauscher J., Hussain T., Agid O., Verhoeff N. P., Wilson A. A., Houle S., Remington G., Zipursky R. B., Kapur S. Equivalent occupancy of dopamine D1 and D2 receptors with clozapine: differentiation from other atypical antipsychotics. Am. J. Psychiatry. (2004);161:1620–1625. doi: 10.1176/appi.ajp.161.9.1620. [DOI] [PubMed] [Google Scholar]
- 38.Taylor C. Z. Religious addiction: obsession with spirituality. Pastoral Psychol. (2002);50:291–315. doi: 10.1023/A:1014074130084. [DOI] [Google Scholar]
- 39.Varlinskaya E. I., Spear L. P. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin. Exp. Res. (2002);26:1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- 40.Way E. L., Loh H. H., Shen F. H. Simultaneous quantitative assessment of morphine tolerance and physical dependence. J. Pharmacol. Exp. Ther. (1969);167:1–8. [PubMed] [Google Scholar]
- 41.Weeks J. R. Long term intraveneous infusion. In Methods in psychology (R. D. Myers, Ed.) 4th ed. Academic press; New York: (1972). pp. 155–168. [Google Scholar]
- 42.Weisnam R. L. Quetiapine in the successful treatment of schizophrenia with comorbid alcohol and drug dependence: a case report. Int. J. Psychiatry Med. (2003);33:85–89. doi: 10.2190/WM5V-D9Y2-2HYW-VGWX. [DOI] [PubMed] [Google Scholar]