Abstract
Chemical cues play an important role in the host-seeking behavior of blood-feeding mosquitoes (Diptera: Culicidae). A field study was carried out in The Gambia to investigate the effects of human odor or synthetic odor blends on the attraction of mosquitoes. MM-X traps baited with 16 odor blends to which carbon dioxide (CO2) was added were tested in four sets of experiments. In a second series of experiments, MM-X traps with 14 odor blends without CO2 were tested. A blend of ammonia and l-lactic acid with or without CO2 was used as control odor in series 1 and 2, respectively. Centers for Disease Control and Prevention (CDC) traps were placed in a traditional house and an experimental house to monitor mosquito densities during the experiments. The MM-X traps caught a total number of 196,756 mosquitoes, with the most abundant species belonging to the genera Mansonia (70.6%), Anopheles (17.5%), and Culex (11.5%). The most abundant mosquito species caught by the CDC traps (56,290 in total) belonged to the genera Mansonia (59.4%), Anopheles (16.0% An. gambiae s.l. Giles, and 11.3% An. ziemanni Grünberg), and Culex (11.6%). MM-X traps baited with synthetic blends were in many cases more attractive than MM-X traps baited with human odors. Addition of CO2 to synthetic odors substantially increased the catch of all mosquito species in the MM-X traps. A blend of ammonia + L-lactic acid + CO2 + 3-methylbutanoic acid was the most attractive odor for most mosquito species. The candidate odor blend shows the potential to enhance trap collections so that traps will provide better surveillance and possible control.
Keywords: mosquito sampling, odor baits, carbon dioxide, human odor
In Africa, major problems are caused by anopheline mosquitoes that act as vectors of human malarias. One of the most important species is Anopheles gambiae s.s. Giles, distinctive for its strong preference for human blood and close association with human habitation. Host seeking and blood feeding of An. gambiae take place during the night. Physical cues, and, more importantly, chemical cues emanating from humans, mediate host-seeking behavior (Takken and Knols 1999). Gillies and Wilkes (1968) reported that CO2 could attract mosquitoes to hosts at distances of 18–36 m and skin odors at distances of 54–73 m. Human skin odors were demonstrated to account for most of the attractiveness to An. gambiae (Costantini et al. 1996, 1998), whereas breath was shown to be only slightly attractive or even repellent (Mboera et al. 1997, Mukabana et al. 2004). Evidence has accumulated that the variation of the attractiveness between human individuals to An. gambiae is odor mediated (Brady et al. 1997, Costantini et al. 1998, Mukabana 2002, Qiu et al. 2006b).
Although hundreds of components have been identified from human odors, only a few compounds were tested singly and proven to be attractive to mosquitoes (Bernier et al. 2000, 2002; Healy et al. 2002). Ammonia, a compound present in incubated human sweat, was repeatedly reported to be attractive in an olfactometer (Geier et al. 1999, Braks et al. 2001, Smallegange et al. 2005). Although attractive to Aedes aegypti L. (Davis 1984), l-lactic acid was not or only slightly attractive to An. gambiae. However, synergism between ammonia, l-lactic acid, and a blend of carboxylic acids was found, whereas binary blends of these compounds were less attractive or even repellent (Smallegange et al. 2005).
Carbon dioxide, which is exhaled by all vertebrates, is considered to contribute partially to the attraction of humans to An. gambiae (Snow 1970, Healy and Copland 1995, Costantini et al. 1996, Mboera and Takken 1997). A study in West Africa demonstrated that CO2 is more attractive to zoophilic than to anthropophilic mosquito species (Costantini et al. 1996), suggesting that anthropophilic mosquito species rely to a greater extent on skin odors in addition to CO2 (Dekker and Takken 1998, Takken and Knols 1999). Addition of the human-specific 7-octenoic acid to CO2 attracted a greater number of An. gambiae s.l. Giles to traps in Burkina Faso (Costantini et al. 2001).
The attraction of mosquitoes to humans cannot be ascribed to single components, but to a blend of components in specific ratios (Eiras and Jepson 1991). In olfactometer studies, several compounds were found to increase mosquito attraction when added to ammonia and l-lactic acid (Qiu 2005, Smallegange et al. 2005). It is important to know whether blends of synthetic odors that are bioactive to mosquitoes in the laboratory are attractive to mosquitoes in the field by using odor-baited traps. Although many field studies have shown that the efficiency of mosquito traps can be substantially improved by adding odors that are attractive to mosquitoes (Kline et al. 1991; Costantini et al. 1996, 1998, 2001; Mboera et al. 1997; Njiru et al. 2006), an odor-baited trap that can compete with a human has yet to be developed. Most odor-baited mosquito traps operate with CO2 as bait, but the results with this compound alone are highly variable. The present article reports on a field study carried out in The Gambia to investigate the effects of synthetic odor blends on mosquito catches by modified MM-X traps either with or without CO2 as an additional stimulus.
Materials and Methods
Study Site
The study was carried out near Wali Kunda (13° 34′ N, 14° 5′ W) in the Central River Division of The Gambia (West Africa). Wali Kunda is a small village located at the south bank of the River Gambia, ≈180 km from the coast. This is the same village where Gillies conducted many behavioral studies on mosquitoes >30 yr ago (Gillies and Wilkes 1972). The area is a flat Sudan Savanna. Rice, Oryza sativa L., fields in the surroundings of the village and temporary pools of rainwater are major breeding sites of mosquitoes. The experiments were conducted during the last part of the rainy season, between August and October. Malaria transmission is at its maximum during this period of the year in The Gambia (Lindsay et al. 1991). This study was approved by the Gambian Government/Medical Research Council Joint Ethics Committee.
Mosquito Traps
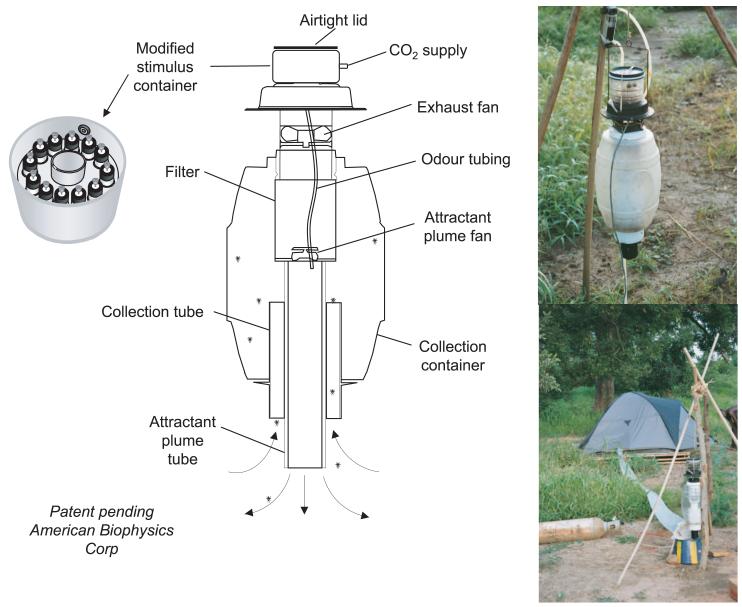
The MM-X trap (American Biophysics Corp., North Kingstown, RI) was used to assess the effect of candidate odors (Kline 1999, Mboera et al. 2000b, Njiru et al. 2006). The odor delivery system was modified to allow the release of blends of odors (Fig. 1). A sealed airtight container was mounted on top of the MM-X trap. The container had two plastic plates with 12 holes in each to hold glass vials. Single odor compounds were contained in 4-ml screw type clear glass vials with a hole-cap and a PTFE/silicone septum inside (Supelco, Sigma-Aldrich Chemie B.V., Zwijndrecht, The Netherlands). A medical injection needle (21G × 5/8 in., 0.8 × 16 cm, Terumo, Leuven, Belgium) that was pierced through the septum, with one end above the surface of the compound, served as a vent for the volatiles. The volatiles from the different vials volatilized through the vent to form a blend in the container (A.M.G. and R.C.S., unpublished data). CO2 from a pressurized cylinder passed a flowmeter through silicone tubing, and it was led into the odor container where it mixed with the candidate odors. The headspace of the test compounds in the containers was propelled to the “attractant plume tube” by one of the two fans. A rechargeable lead-acid battery (12 V, 3 A, Super Sona YP3-12, Eijlander Electronics, Ede, The Netherlands) was used to power the electric ventilators. Grease was used around the suspension cable and electrical cables to prevent ants from reaching the mosquitoes caught in the MM-X trap. In spite of these precautions, we occasionally found ants eating mosquitoes in our traps. Data from these traps were discarded.
Fig. 1.
Schematic drawing of the MM-X (alias Counterflow Geometry) trap (after Kline 1999) with an extra stimulus container. The top photograph shows the MM-X trap, and the bottom photo shows how odors from a human occupied tent were led to the MM-X trap via lay-flat tubing.
The Centers for Disease Control miniature light trap (CDC trap, model 512, John W. Hock Company, Gainesville, FL) (Sudia and Chamberlain 1962, Garrett-Jones 1964) was used to provide back-ground information on the seasonal abundance of mosquitoes.
Test Odors
Synthetic Odor Compounds
Compounds selected for this study were all components of human emanations and were found to cause either behavioral or electrophysiological activities in An. gambiae s.s. in the laboratory (Bernier et al. 2000, Meijerink et al. 2000, Braks et al. 2001, Costantini et al. 2001, Healy et al. 2002, Qiu 2005, Smallegange et al. 2005, Qiu et al. 2006a). Chemicals used in our experiments, their purity, producer as well as the bioactivity reported for An. gambiae are listed in Table 1. One milliliter or 1 g of a pure compound was put in a glass vial (see above). Carbon dioxide (100%) was provided by a pressurized cylinder connected to the MM-X traps by polythene tubing (8 mm in outer diameter) and released via a gas regulator (Tornado 2000, Messer Cutting & Welding AG, Frankfurt, Germany) and the flow rate was fine-regulated to 230 ml/min (an amount equivalent to that exhaled by a breathing human at rest) with a flow meter (Brooks Instruments, Rijswijk, The Netherlands)(Gillies and Wilkes 1968).
Table 1. Chemicals used in the field study.
| Compound | Purity | Supplier | Bioactivitya | Reference |
|---|---|---|---|---|
| CO2 | 100% | Banjul Oxygen Ltd.b | B | Costantini et al. 1996 |
| Ammonia | 25% aqueous solution |
Riedel-de Haënc | B, E |
Van den Broek and den Otter 2000, Braks et al. 2001, Meijerink et al. 2001, Smallegange et al. 2005 |
| l-Lactic acid | >85% | Aldrichd | B |
Braks et al. 2001, Dekker et al. 2002, Smallegange et al. 2005 |
| Propionic acid | ±99% | Sigmad | B | Qiu 2005 |
| 3-Methylbutanoic acid | 99% | Aldrich | B | Qiu 2005 |
| Butanoic acid | >99% | Aldrich | B | Qiu 2005 |
| Hexanoic acid | ≥99% | Sigma | B | Qiu 2005, Smallegange et al. 2005 |
| Heptanoic acid | ≥99% | Sigma | B | Qiu 2005 |
| Octenoic acid | ≥99% | Sigma | B | Qiu 2005 |
| Tridecanoic acid | ≥99% | Sigma | B | Qiu 2005 |
| Tetradecanoic acid | 99–100% | Sigma | B | Qiu 2005 |
| Hexadecanoic acid | ±99% | Sigma | B | Qiu 2005 |
| 1-Dodecanol | ≥99.5% | Fluka Chemikad | B | Qiu 2005 |
| 6-Methyl-5-hepten-2-one | 99% | Aldrich | E | Meijerink et al. 2001, Qiu 2005 |
| 3-Methyl-1-butanol | ≥99.5% | Fluka Chemika | E | Meijerink et al. 2001, Qiu 2005 |
| Indole | ≥99% | Fluka Chemika | E | Meijerink et al. 2001, Qiu 2005 |
| Geranylacetone | ≥97% | Aldrich | E | Meijerink et al. 2001, Qiu 2005 |
| 4-Ethylphenol | 99% | Aldrich | E |
Van den Broek and Den Otter 1999, Qiu 2005 |
| 7-Octenoic acid | >99% | IACR-Rothamstede | B, E | Costantini et al. 2001, Qiu 2005 |
| Ethanol, absolute | 100% | Riedel-de Haën |
B, behavioral response; E, electrophysiological response.
Location: Kanifing, The Gambia.
Location: Seelze, Germany.
Location: Zwijndrecht, the Netherlands.
Location: Harpenden, Hertfordshire, United Kingdom.
Human Odor
Human odor was fed to a MM-X trap by drawing air from a human-occupied tent through lay-flat tubing (11 cm in diameter). For this purpose, a Gambian male volunteer (22 yr old) slept in a nylon tent each experimental night. A hole was made at one side of the tent in which an electric ventilator was fixed (12 V, 2.8 W, Sunon, Brea, CA). During the experimental time (from 7 p.m. to 7 a.m.), air was drawn from the tent through the lay-flat tubing to the MM-X trap 10 m away from the tent. The outlet of the lay-flat tubing was ≈5 cm from the odor outlet of the MM-X trap (Fig. 1).
Mosquito Population Dynamics
To monitor the population dynamics of mosquitoes during the experimental period in the nearby village, one CDC trap was suspended every night during the experimental period in a house in Wali Kunda village and one trap in an experimental house within the MRC field station (Lindsay et al. 1993, 2003). Two Gambian males (24 and 27 yr old) slept in either house under a nonimpregnated bed net, next to which a CDC trap was placed ≈50 cm above the ground. The village house was a traditional one with adobe walls and a thatched roof. As there was no door, the house entrance remained open at all times. The experimental house was designed in traditional African style. The indoor-floor area was ≈6.25 m2. Mosquitoes could enter the houses via an aperture under the two opposite eaves. The volunteers were offered prophylaxis with chloroquine.
Experimental Setup
Experimental Design
The effects of candidate odors released from MM-X traps were tested following a replicated 6 by 6 Latin square design. Six odor blends were tested in each experimental set, and this was repeated for 12 nights. Sixteen odor blends with CO2 were tested in four sets of experiments (Table 2), and 14 odor blends without CO2 were tested in three sets of experiments (Table 3). The MM-X traps baited with CO2 were placed along a dirt road parallel to the River Gambia; MM-X traps without CO2 were placed along a road leading from the River Gambia. MM-X traps were placed 50 m apart and were >100 m from the nearest house. Both roads were along the edge of a large rice field. The MM-X traps were suspended on a wooden tripod; the lowest point of the trap was ≈50 cm above the ground (Fig. 1). In each set a control odor, ammonia + l-lactic acid + CO2 (odor A) was tested simultaneously with other odor blends. Ammonia + l-lactic acid (odor B) was included in set one for comparison with odor A, the control odor. A human odor-baited MM-X trap was included in three (sets 1, 2, and 3) of the four sets of experiments. Traps were operated from 7 p.m. to 7 a.m., corresponding with the hours between sunset and sunrise.
Table 2. Mean ± SD mosquitoes trapped by MM-X trap baited with human odors or synthetic blends with CO2.
| Set | Code | Test odora | n |
An.
gambiae |
An.
pharoensis |
An.
ziemanni |
Culex spp. |
Mansonia spp. |
Aedes spp. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | A | LA + NH3 + CO2 | 10 | 2.2 ± 2.2 | 4.5 ± 5.9 | 86.6 ± 106 | 73.6 ± 84.5 | 680 ± 734 | 12.5 ± 11.6 |
| B | LA + NH3 | 10 | 0 ± 0 | 0.2 ± 0.4 | 0.3 ± 0.5 | 4.7 ± 3.9 | 11.8 ± 8.8 | 0.5 ± 1.0 | |
| C | Human in tent | 5 | 1.2 ± 1.1 | 2.2 ± 3.3 | 6.0 ± 6.4 | 14.8 ± 10.6 | 147 ± 100 | 1.6 ± 2.1 | |
| D | A + 3MC4 | 12 | 1.8 ± 2.0 | 8.3 ± 8.8 | 179 ± 154 | 127 ± 169 | 743 ± 711 | 12.9 ± 13.7 | |
| F | A + 3MC4 + C3 + C4 | 11 | 1.7 ± 1.5 | 5.2 ± 6.8 | 119 ± 94.0 | 74.2 ± 59.4 | 589 ± 415 | 7.2 ± 6.9 | |
| G | A + C14 | 10 | 1.4 ± 2.5 | 6.3 ± 6.7 | 102 ± 89.0 | 65.2 ± 65.2 | 632 ± 464 | 12.4 ± 10.5 | |
| 2 | A | LA + NH3 + CO2 | 12 | 4.1 ± 3.9 | 26.7 ± 61.7 | 262 ± 401 | 146 ± 262 | 549 ± 627 | 2.6 ± 2.9 |
| C | Human in tent | 12 | 3.3 ± 4.6 | 1.7 ± 3.4 | 14.3 ± 27.2 | 26.5 ± 39.2 | 316 ± 303 | 1.1 ± 1.4 | |
| H | F + C14 | 12 | 3.7 ± 4.1 | 10.4 ± 10.5 | 173 ± 155 | 93.6 ± 81.0 | 520 ± 342 | 5.0 ± 9.0 | |
| I | A + C6 | 11 | 4.2 ± 4.8 | 20.7 ± 24.6 | 277 ± 289 | 163 ± 183 | 709 ± 650 | 2.8 ± 2.1 | |
| J | A + C6 + 7OA | 12 | 4.0 ± 3.3 | 12.1 ± 9.7 | 91.8 ± 76.8 | 69.8 ± 52.8 | 420 ± 259 | 2.7 ± 3.2 | |
| E | CO2 | 12 | 2.1 ± 3.3 | 12.7 ± 18.3 | 200 ± 201 | 85.3 ± 100 | 548 ± 485 | 1.9 ± 2.4 | |
| 3 | A | LA + NH3 + CO2 | 12 | 1.8 ± 1.8 | 5.2 ± 5.8 | 340 ± 272 | 178 ± 197 | 766 ± 542 | 3.1 ± 1.8 |
| C | Human in tent | 12 | 2.1 ± 2.7 | 0.4 ± 0.8 | 7.6 ± 11.2 | 17.6 ± 20.7 | 551 ± 557 | 0.9 ± 2.0 | |
| K | J + C7 + C8 | 11 | 1.2 ± 1.4 | 8.4 ± 6.3 | 130 ± 116 | 140 ± 178 | 757 ± 532 | 2.1 ± 2.1 | |
| L | J + 3MC4 + C14 | 11 | 2.0 ± 2.6 | 5.2 ± 7.3 | 124 ± 97.3 | 97.1 ± 58.9 | 624 ± 337 | 1.3 ± 1.3 | |
| M | A + 3MC4 + C6 | 12 | 1.3 ± 1.4 | 5.2 ± 6.0 | 173 ± 151 | 114 ± 92.8 | 630 ± 409 | 1.7 ± 1.3 | |
| N | A+ GA + IND + 4EP | 12 | 2.0 ± 3.0 | 5.2 ± 7.0 | 112 ± 163 | 132 ± 123 | 568 ± 521 | 0.8 ± 1.3 | |
| 4 | A | LA + NH3 + CO2 | 11 | 0.2 ± 0.4 | 1.6 ± 3.5 | 83.5 ± 97.9 | 50.4 ± 96.4 | 300 ± 398 | 0.2 ± 0.6 |
| O | N + 3MC4 | 11 | 1.0 ± 1.4 | 0.5 ± 1.0 | 32.5 ± 40.6 | 20 ± 10.4 | 152 ± 82.5 | 0.5 ± 0.8 | |
| P | N + C6 | 12 | 0.8 ± 1.4 | 0.8 ± 0.8 | 32.4 ± 45.6 | 24.3 ± 19.5 | 179 ± 148 | 0 ± 0 | |
| Q | N + C6 + 7OA | 10 | 0.6 ± 0.9 | 1.9 ± 2.5 | 95.5 ± 145 | 43.4 ± 62.7 | 313 ± 356 | 0.4 ± 0.7 | |
| R | N + 3MC4 + C6 | 11 | 0.1 ± 0.3 | 0.3 ± 0.9 | 22.4 ± 24.6 | 21.3 ± 11.6 | 194 ± 104 | 0.1 ± 0.3 | |
| S | N + 3MC4 + C6 + 7OA | 12 | 0.2 ± 0.4 | 1.3 ± 2.1 | 61.1 ± 86.2 | 37.0 ± 44.2 | 281 ± 357 | 0.4 ± 0.7 |
Odors in the same sets were tested in the same experimental nights.
LA, L-lactic acid; NH3, ammonia; 3MC4,3-methylbutanoic acid; C3, propionic acid; C4, butanoic acid; C14, tetradecanoic acid; C6, hexanoicacid; 7OA, 7-octenoic acid; C7, heptanoic acid; C8, octenoic acid; GA, geranylacetone; IND, indole; and 4EP, 4-ethylphenol.
Table 3. Mean ± SD mosquitoes trapped by MM-X trap baited with human odors or synthetic blends without CO2.
| Set | Code | Test odora | n | An. gambiae |
An. pharoensis |
An. ziemanni |
Culex spp. |
Mansonia spp. |
Aedes spp. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | B | LA + NH3 | 12 | 0 | 0 | 3.3 ± 5.0 | 4.4 ± 3.4 | 19.8 ± 18.6 | 0.3 ± 0.5 |
| Y | No odor | 12 | 0 | 0.2 ± 0.4 | 1.8 ± 2.8 | 8.6 ± 17.9 | 17.6 ± 29.7 | 0.2 ± 0.4 | |
| D′ | B + 3MC4 | 11 | 0 | 0 | 1.5 ± 2.1 | 7.7 ± 7.5 | 11.7 ± 8.4 | 0.5 ± 0.5 | |
| F′ | B + 3MC4 + C3 + C4 | 12 | 0 | 0.1 ± 0.3 | 3.0 ± 3.7 | 8.3 ± 8.9 | 24.2 ± 25.9 | 0.1 ± 0.3 | |
| G′ | B + C14 | 12 | 0 | 0.1 ± 0.3 | 2.2 ± 3.5 | 4.6 ± 3.5 | 12.8 ± 10.2 | 0.3 ± 0.6 | |
| H′ | F′ + C14 | 10 | 0 | 0.1 ± 0.3 | 0.6 ± 0.8 | 5.2 ± 6.3 | 15.3 ± 17.7 | 0.1 ± 0.3 | |
| 2 | B | LA + NH3 | 12 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.9 ± 1.4 | 2.6 ± 5.4 | 8.2 ± 11.7 | 0 |
| I′ | B + C6 | 12 | 0 | 0.2 ± 0.4 | 1.5 ± 2.1 | 2.1 ± 2.9 | 13.7 ± 19.3 | 0.1 ± 0.3 | |
| J′ | B + C6 + 7OA | 12 | 0 | 0.1 ± 0.3 | 1.1 ± 0.8 | 0.9 ± 0.8 | 5.5 ± 4.5 | 0 | |
| K′ | J′ + C7 + C8 | 12 | 0 | 0.2 ± 0.6 | 2.3 ± 3.6 | 1.8 ± 3.0 | 15.8 ± 22.2 | 0.1 ± 0.3 | |
| L′ | J′ + 3MC4 + C14 | 12 | 0 | 0.1 ± 0.3 | 0.9 ± 1.1 | 1.8 ± 2.5 | 7.3 ± 10.0 | 0 | |
| N′ | B + GA + IND + 4EP | 12 | 0.1 ± 0.3 | 0.2 ± 0.4 | 1.3 ± 2.3 | 1.1 ± 1.4 | 6.4 ± 7. | 0.1 ± 0.3 | |
| 3 | B | LA + NH3 | 6 | 0 | 0 | 3.3 ± 4.8 | 1.2 ± 1.3 | 10.5 ± 11.4 | 0.2 ± 0.4 |
| T | N′ + 3MC4 + C6 + C14 | 6 | 0 | 0.2 ± 0.4 | 1.2 ± 1.2 | 1.5 ± 1.1 | 6.3 ± 5.2 | 0 | |
| U | N′ + C13 + C16 | 6 | 0 | 0.2 ± 0.4 | 0.8 ± 1.6 | 2.2 ± 3.3 | 11.3 ± 8.1 | 0.2 ± 0.4 | |
| V | N′ + DD | 6 | 0 | 0.3 ± 0.8 | 3.2 ± 4.3 | 4.2 ± 7.4 | 10.7 ± 12.0 | 0 | |
| W | N′ + 6M5H | 6 | 0 | 0 | 2.3 ± 4.3 | 1.8 ± 2.1 | 10.8 ± 16.2 | 0 | |
| X | N′ + 3MB | 6 | 0 | 0.2 ± 0.4 | 3.0 ± 2.0 | 3.5 ± 1.9 | 14.0 ± 7.1 | 0 |
Odors in the same set were tested in the same experimental nights.
LA, L-lactic acid; NH3, ammonia; 3MC4,3-methylbutanoic acid; C3, propionic acid; C4, butanoic acid; C14, tetradecanoic acid; C6, hexanoic acid; 7OA, 7-octenoic acid; C7, heptanoic acid; C8, octenoic acid; GA, geranyl acetone; IND, indole; and 4EP, 4-ethylphenol. The prime symbol is used for the code of the same mixtures but without CO2, e.g., D′ = LA + NH3 + 3MC4 and D = LA + NH3 + 3MC4 + CO2.
Odor-Baited MM-X Traps
Chemicals were placed in the vials 3–4 h before the start of the experiments. All chemicals and vials were replaced daily. The odor container of the MM-X trap was rinsed with 100% ethanol before a new odor blend was tested. The traps were placed at the experimental locations at ≈7 p.m. every evening. At ≈7 a.m. the next day the traps were taken back to the laboratory and placed into a freezer for 1 h to kill the mosquitoes that were caught during the night. Subsequently, mosquitoes were removed from the traps, identified, and counted.
Identification of Mosquitoes
The mosquitoes caught in each trap during one night were morphologically identified and counted (Gillies and Wilkes 1968, Gillies and Coetzee 1987). Anophelines were identified to species, but culicines were not. Female An. gambiae s.l. mosquitoes were placed in a 4-ml Eppendorf tube with dry silica gel. These mosquitoes were transported to the Laboratory of Entomology of Wageningen University for species identification by polymerase chain reaction (PCR) (Scott et al. 1993).
Meteorological Data Collection
Daily rainfall was registered with a rain gauge (Remote Rain Gauge model RGR122, OR Scientific, Berkshire, United Kingdom). The average and maximum wind speed was recorded during experimental nights by an anemometer (Windmaster 2, Kaindl Electronic, Rohrbach, Germany). Daily temperature and humidity were registered by data loggers (Tiny Tag Plus, INTAB Benelux, Cuijk, The Netherlands).
Data Analysis
For each species or genus, the number of mosquitoes (only females) caught in the traps were analyzed by regression analysis by using a generalized linear model (GLM) with logarithmic link function and a negative binomial distribution (McCullagh and Nelder 1989) with the procedure RNEGBINOMIAL in the statistical package Genstat 7.1 (Genstat Committee 2003). Preliminary analyses assuming a Poisson distribution showed strong overdispersion for the more abundant mosquitoes. The negative binomial distribution models this overdispersion with a mean-variance relation of variance = mean + a mean2, with a ≥ 0, the aggregation parameter. If a = 0, then the Poisson distribution is obtained. The predictors in this log-linear regression were the factor site, a smoothing of day of capture with 4 degrees of freedom (Hastie and Tibshirani 1990) and the factor odor blend. The smooth function of day naturally accounted for the differences in numbers caught between sets as judged by preliminary deviance tests. Therefore, the factor set was not included in further analyses. The factor odor blend was coded in such a way that the regression coefficients of the blends expressed the (natural) logarithm of the relative attractiveness of each blend with respect to the control odor (odor A for experiments with CO2 and odor B for those without CO2), where relative attractiveness is defined as the ratio of the expected numbers of mosquitoes caught in traps with the odor blend and those with the control odor. Approximate 95% confidence intervals were calculated from the coefficients and their standard errors in the usual way (Jongman et al. 1995), which were then converted to base 10 logarithms. If the interval embraces 0, the odor does not differ significantly in attractiveness from the control odor at the 5% significance level. Additional analyses were carried out in which the factor odor was replaced by a set of 0/1 variables representing the presence of 3-methylbutanoic acid, hexanoic acid, tetradecanoic acid, and the combination of geranylacetone, indole, and 4-ethylphenol in different blends. Regression analysis using all possible subsets revealed which compounds influenced the number of mosquitoes caught. For the compounds in the model with minimum Akaike Information Criterion (AIC) (McCullagh and Nelder 1989), the relative attractiveness was calculated together with approximate 95% confidence intervals.
To display patterns in the relative attractiveness of the odor blends for the different species, a principal components biplot (Jongman et al. 1995) was produced from the species-by-mixture table containing all estimates of log attractiveness obtained by the separate regression analyses. The principal component analysis was noncentered and the rows (columns) of the table were weighted inverse with the row (column) average variance of the estimate. Because odor B had in this way such a large weight, it was down-weighted by a factor 40; this downweighting did not change the biplot much.
Redundancy analysis (Jongman et al. 1995) was used to detect the effects of the weather conditions on the number of mosquitoes of the different species (females and males) caught. The response variables of this analysis were the log(y + 1)-transformed numbers caught, and the predictor variables were five weather variables: relative humidity, rainfall during the day and night, average and maximum wind speed. The analysis was adjusted for the linear and quadratic effect of day (season), site and odor blend by specifying these variables as covariables. The results are presented in a biplot of weather variables and mosquito species. Redundancy analysis also was used to display in a biplot the difference in species composition of CDC traps in the experimental house and village house. In this analysis day, dM =−(day − 24)2, and the weather variables were entered as supplementary variables. All biplots were produced with Canoco for Windows 4.5 (ter Braak and Šmilauer 2002).
Results
The MM-X traps caught 196,756 mosquitoes, with the most abundant species being Mansonia spp. (70.6%), Anopheles spp. (17.5%), and Culex spp. (11.5%). The anophelines comprised of Anopheles ziemanni Grünberg (16.3%), An. pharoensis Theobald (0.9%), An. gambiae s.l. (0.3%), and An. wellcomei Theobald (0.02%). In all experiments, 94.7% of mosquitoes trapped were females. Less than 1% of the trapped mosquitoes was Aedes spp. More mosquitoes were caught in traps with CO2 than without this compound.
Experiments with CO2
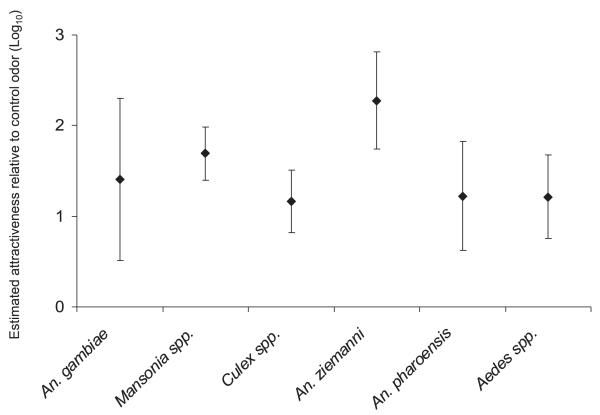
For all species odor B (ammonia + l-lactic acid) was the least attractive (Fig. 2). The control odor A (ammonia + l-lactic acid + CO2) attracted a greater number of mosquitoes than natural human odor (odor C) for all mosquito species except for An. gambiae (Fig. 2). For Aedes species, the control odor (A) attracted more mosquitoes than CO2 alone (odor E), but for the other mosquito groups the control odor A attracted similar numbers of mosquitoes as CO2 alone (Fig. 2).
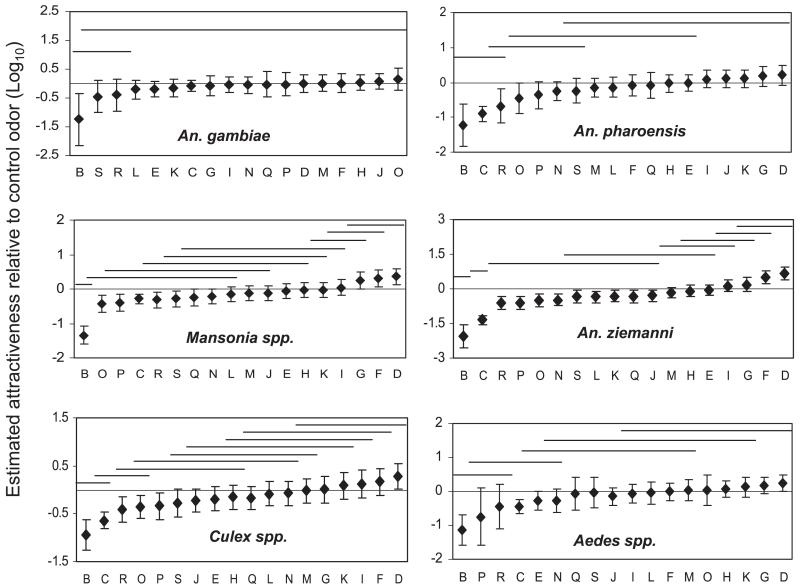
Fig. 2.
Attractiveness of odors in the experiment with CO2 relative to the control odor A, on a 10log scale, as estimated by log linear regression. The vertical error bars indicate the approximate 95% confidence intervals for the attractiveness. An odor differs significantly (P < 0.05) in attractiveness from the control if the error bars do not cross the horizontal line at a log attractiveness of 0. The horizontal lines at the top indicate groups of odors that do not differ significantly as judged by a t-test on the difference of their regression coefficients. See Table 2 for the codes of the odors.
All odor blends tested attracted only a few An. gambiae mosquitoes (less than five mosquitoes per night on average; Table 2). Odor B attracted fewer An. gambiae than all other odors, but the difference was not significant for odors S and R (Fig. 2). No differences in attraction of An. gambiae were found between other odors.
For Mansonia species odors blends D, F, and G were significantly more attractive than the control odor A (Fig. 2). Eight odor blends, including all blends containing the combination of geranylacetone, indole and 4-ethylphenol (odor N, O, P, Q, R, and S) were significantly less attractive than the control odor (Fig. 2). Culex species preferred odor D to the control, and were less attracted to odor blends O, P, and R (Fig. 2). For An. pharoensis none of the blends tested was significantly more attractive than the control odor, and four odors, including odors R and O, were significantly less attractive than the control odor (Fig. 2). For An. ziemanni most of the odors tested were less attractive than the control odor, except odors D and F, which were more attractive than the control odor (Fig. 2).
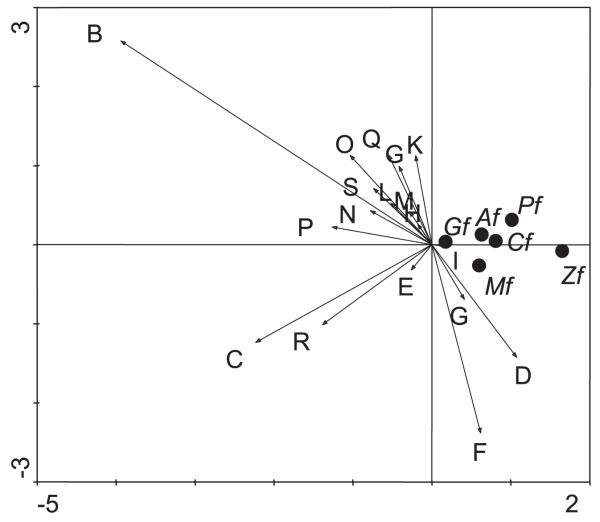
Figure 3 summarizes the pattern of attractiveness graphically. All mosquito species were less attracted to odor B than to the control (odor A) and were not attracted to all other odors, except odors D, F, and G. The horizontal dimension of Fig. 3 shows that females of An. gambiae (Gf) and An. ziemanni (Zf) differed most in their odor preference: the response to odors was much stronger in An. ziemanni than in An. gambiae. The other species were intermediate in response strength. The vertical dimension distinguishes females of Mansonia species (Mf) from those of An. pharoensis (Pf), with the other groups being intermediate. An. pharoensis females were less attracted by human odor and odor R than Mansonia females, and they were less attracted to odors G, D, and F than Mansonia females.
Fig. 3.
Biplot display of the log-relative attractiveness of species groups to odors. The biplot is based on a weighted noncentered principal components analysis, displaying 92% of the sum of squared data values. The scaling is such that distances among species groups display their Pythagorean dissimilarity. Arrows are odors; see Table 2 for the codes of the odors. Black dots stand for females (f) of mosquito species, A, Aedes spp.; C, Culex spp.; G, Anopheles gambiae s.l.; M, Mansonia spp.; P, An. pharoensis; and Z, An. ziemanni.
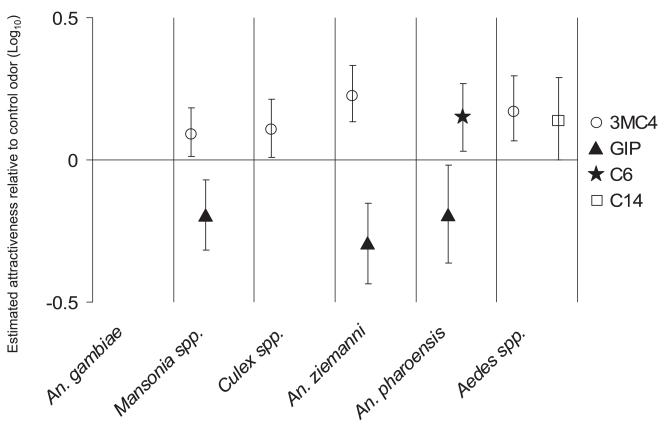
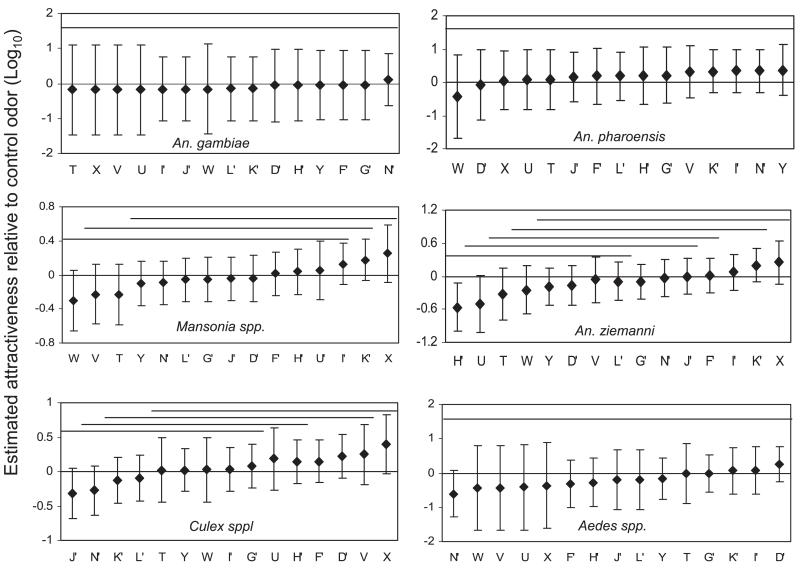
Odor blends containing 3-methylbutanoic acid attracted more female mosquitoes of An. ziemanni, Mansonia, Culex, and Aedes species than the blends without this compound (Fig. 4). An. pharoensis showed a preference for odor blends containing hexanoic acid. A similar preference was found for Aedes mosquitoes to odor blends containing tetradecanoic acid (Fig. 4). Odor blends containing the combination of geranylacetone, indole, and 4-ethylphenol were less attractive than the control odor for Mansonia spp., An. ziemanni, and An. pharoensis.
Fig. 4.
Attractiveness of odor blends in which certain compounds were present in the experiments using release of CO2. Attractiveness of odor blends in which certain compounds were present in the experiments using release of CO2 relative to the control odor A, on a 10log scale, as estimated by log linear regression. The vertical error bars indicate the approximate 95% confidence intervals for the attractiveness. An odor blend had a significant (P < 0.05) effect on the attraction if the error bars do not cross the horizontal line at a log attractiveness of 0. 3MC4, odor blends containing 3-methylbutanoic acid; GIP, odor blends containing geranylacetone, indole and 4-ethylphenol; C6, odor blends containing hexanoic acid; and C14, odor blends containing tetradecanoic acid.
Experiments without CO2
The numbers of mosquitoes caught in MM-X traps without CO2 were lower than the numbers caught with the same odors to which CO2 had been added (Tables 2 and 3). For all species, adding CO2 to any odor blend increased the catch significantly (P < 0.001; GLM) (Fig. 5). The effect was independent of the odor blends tested.
Fig. 5.
Attractiveness of odor blends in the experiments in which CO2 was released from traps relative to the same odor blends in experiments without CO2, on a 10log scale, as estimated by log linear regression. The vertical error bars indicate the approximate 95% confidence intervals for the attractiveness. Odors with and without CO2 differed significantly (P < 0.05) in attractiveness if the error bars do not cross the horizontal line at a log-attractiveness of 0. See Table 2 for the codes of the odor blends.
Few An. gambiae, An. pharoensis, and Aedes species were trapped by the test odor blends, and no significant differences were found among the blends. (Table 3; Fig. 6), and none of the odors tested caught a greater number of mosquitoes than the control odor. For An. ziemanni odor H′ and U attracted fewer mosquitoes than the control odor B (Fig. 6).
Fig. 6.
Attractiveness of odors relative to the control odor B, on a 10log scale, in the experiments without CO2, as estimated by log linear regression. The vertical error bars indicate the approximate 95% confidence intervals for the attractiveness. An odor differs significantly (P < 0.05) in attractiveness from the control if the error bars do not cross the horizontal line at a log attractiveness of zero. The horizontal lines at the top indicate groups of odors that do not differ significantly as judged by a t-test on the difference of their regression coefficients. See Table 2 for the codes of the odors.
For Mansonia species, Culex species, and An. ziemanni, odor X showed the highest attractiveness among all the blends tested, but it was not significantly more attractive than no odor (Fig. 6).
Effect of Weather Conditions on Mosquito Catches
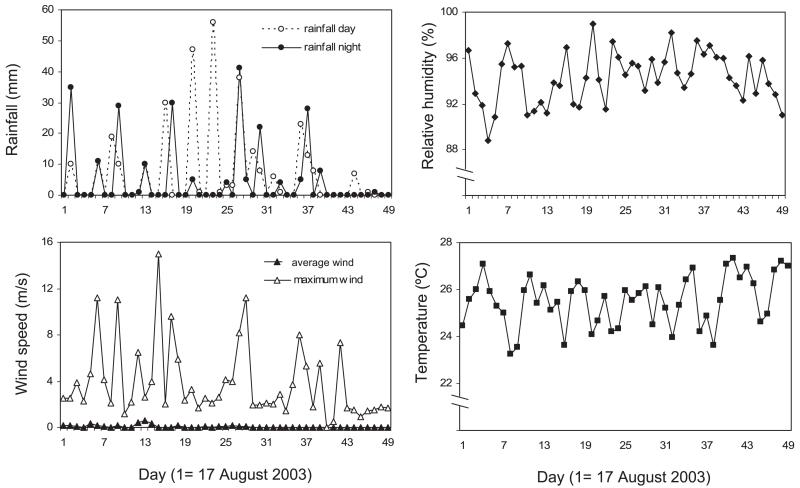
The temperature in the period of the study varied between 20.5 and 33.3°C with an average value of 27.6°C during the day and 25.5°C during the night. The relative humidity varied between 91 and 99.9%, with an average of 94.1% during the experimental nights (Fig. 7). Total rainfall during the study period was 1171 mm, varying from 0 to 116 mm per 24 h. The average wind speed during the experimental nights was 0.064 m/s with a minimum of 0 and maximum of 11.2 m/s.
Fig. 7.
Meteorological data showing daily average values of rainfall during the day and at night, average and maximum wind speed, relative humidity and temperature during the experimental period in Wali Kunda.
A noticeable decrease in rainfall was observed during the last 10 d of the experiment, at the end of the rainy season. The maximum wind speed decreased also during this period.
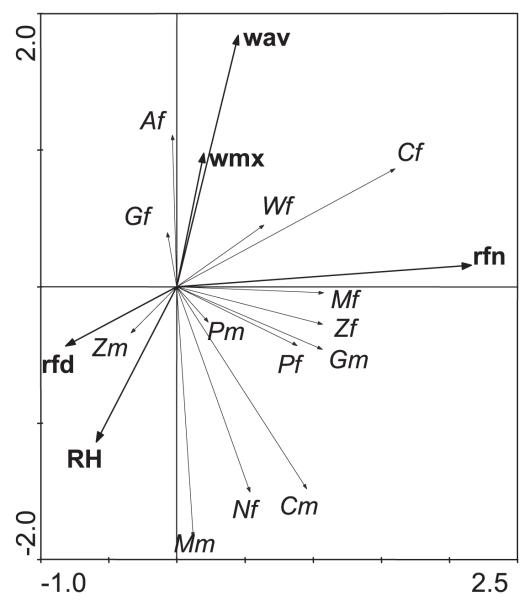
The effects of weather variables on the catches for different groups of mosquitoes are shown in Fig. 8. Weather variables had a statistically significant effect on the trap results, but their effects were small, and they explain only 5% of the variance that remains after accounting for date and site.
Fig. 8.
Biplot of the effects of weather variables on species groups. The biplot is based on a partial redundancy analysis of log counts on the weather variables (see text), displaying 93% of the variance in the effects. The weather variables are rainfall during the day (rfd) and at night (rfn), average (wav), and maximum wind speed (wmx) and relative humidity (RH). Although the weather variables have a statistically significant effect on the trapping results, they explain only 5% of the variance that remains after accounting for season and site. Codes for females (f) and males (m) of mosquito species are A, Aedes spp.; C, Culex spp.; G, Anopheles gambiae s.l.; M, Mansonia spp.; P, An. pharoensis; W, An. wellcomei; Z, An. ziemanni; and N, unidentified Anopheles spp.
Rainfall during the day (7 a.m.−7 p.m.) and night caused an opposite effect (Fig. 8). Nocturnal rainfall affected the catch of all mosquito species positively, with the exception of female An. gambiae and male An. ziemanni (Fig. 8). In contrast, rainfall during the day affected the catch of female mosquitoes negatively except for some unidentified Anopheles species (280 mosquitoes), whereas the catches of males of Mansonia species, Culex species, and An. ziemanni were positively affected (Fig. 8).
Wind speed, be it measured either as the maximum or average wind speed on a day, affected the catches of female Culex species, Aedes species, An. gambiae, An. wellcomei, and Mansonia species positively, whereas it affected the catch of males of all mosquito groups and females of An. pharoensis and An. ziemanni negatively (Fig. 8). The maximum and average wind speed on a day correlated with each other.
High humidity had a negative effect on the catch of females of most mosquito species, but it exerted a positive effect on the catch of most male mosquitoes (Fig. 8).
CDC Traps and Population Dynamics
The most abundant mosquito species caught by the CDC traps (56,290 in total) were Mansonia spp. (59.4%), An. gambiae s.l. (16.0%), Culex spp. (11.6%), and An. ziemanni (11.3%). Of all mosquitoes caught by CDC traps, 98.4% were females.
The average number of mosquitoes trapped by the CDC traps suspended next to a sleeping human in the experimental house and in a village house were compared (Fig. 9). The CDC trap in the village house trapped more mosquitoes of all species than the CDC trap in the experimental house, except for male An. gambiae (Fig. 9: horizontal axis). The difference between the two locations was greatest for female An. ziemanni, An. pharoensis, and Culex spp., less for An. gambiae and small for Mansonia spp. and Aedes spp.
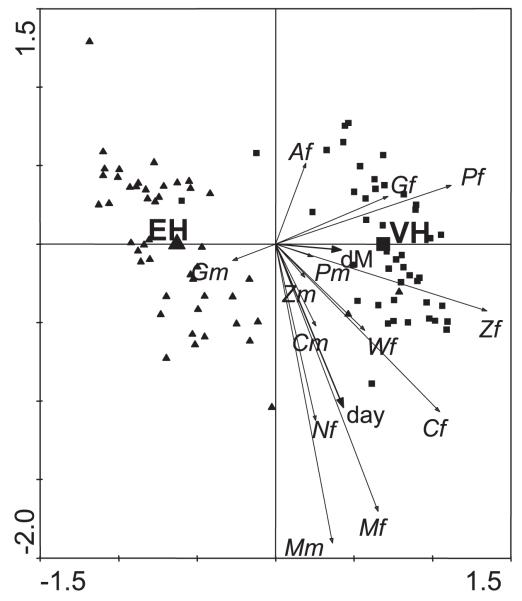
Fig. 9.
Species composition in different locations and change during experimental period. Triplot showing the difference in the species composition between traps in the experimental house (EH, triangles) and the village house (VH, squares) and the changes in species composition with season based on a redundancy analysis of log counts on house indicator variables with the season variables (day and dM) as supplementary variables. The constrained horizontal axis (EH versus VH) explains 43% of the variance; the unconstrained vertical axis displays 18% of the variance. Codes for females (f) and males (m) of mosquito species are A, Aedes spp.; C, Culex spp.; G, Anopheles gambiae s.l.; M, Mansonia spp.; P, An. pharoensis; W, An. wellcomei; Z, An. ziemanni; and N, unidentified Anopheles spp.
The mosquito populations varied over the experimental period. Aedes spp. were most abundant at the start of the experimental period (Fig. 9, top). By contrast, Mansonia spp., Culex spp. and some unidentified Anopheles spp. were most abundant at the end of the study period, as shown in Fig. 9 by their long arrows that point in the same direction as the arrow for day. The short arrow for dM (which marks day 24 in Fig. 9) suggests a weak abundance peak in the middle of the study period for An. gambiae, An. pharoensis, and An. ziemanni (Gf, Pf, and Zf) that points in this direction. By inspecting scatter plots of abundance against day (data not shown) the peak is confirmed for Gf, but Pf and Zf were about equally abundant across the study period.
Species Composition of An. gambiae s.l.
Results from the DNA-PCR showed that of the An. gambiae complex only An. gambiae s.s. and An. arabiensis were recovered from the traps, although An. melas and An. quadriannulatus primers also were included. The average proportion of the two species was 84.9 and 15.1%, respectively, with a slight variation over the experimental period (Table 4).
Table 4. PCR species identification of An. gambiae complex.
| Trap | An. gambiae s.s. | An. arabiensis |
|---|---|---|
| 17 Aug.–1 Sept. (CDC) | 188 (80%) | 48 (20%) |
| 2–18 Sept. (CDC) | 26 (90%) | 3 (10%) |
| 19 Sept.–4 Oct. (CDC) | 16 (76%) | 5 (24%) |
| All traps (CCD + MM-X) | 444 (85%) | 79 (15%) |
Discussion
The overall results of the study demonstrate the feasibility of using odor-baited traps for the collection of blood-feeding mosquitoes in the field. This was particularly interesting because the odor baits tested consisted of synthetic blends, whereas previously only live hosts or CO2 had been used (Takken and Knols 1999). Njiru et al. (2006), using the same traps, demonstrated the basic principle of such odor-baited traps in a semifield situation, with laboratory-reared mosquitoes of An. gambiae. In the current study, this principle has been taken outdoors in a natural environment, catching wild mosquitoes. It was encouraging that many of the odor blends tested were as attractive as or more attractive than natural odor from a human host.
Experiments with CO2
Large numbers of mosquitoes from various taxonomic groups were collected in this study, with Mansonia and Culex spp. being the most abundant. Because of their high abundance and because the main targets of the study were anopheline mosquitoes, Culex and Mansonia mosquitoes were not identified to species, but they most likely existed of Cx. poicilipes Theobald, Cx. bitaeniorhynchus Giles, Mansonia uniformis Theobald, and M. africana Theobald, because these species had previously been recorded from the same area (Gillies and Wilkes 1968). Mansonia spp. and Culex spp. are vectors of arboviruses and heartworm (Diallo et al. 2005), and they cause nuisance because of aggressive biting behavior. Therefore, the combination of MM-X traps and some of the odor blends tested in this study, such as D, F, and G, may be good candidates to be used in the control of these mosquito species.
The most abundant anophelines were An. ziemanni, An. pharoensis, and An. gambiae s.l. With PCR analysis, we identified the An. gambiae s.l. trapped in our experiments to be either An. gambiae s.s. or An. arabiensis, with slight fluctuations in species composition over the experimental period. However, not all catches were subjected to PCR tests, and therefore separate analysis of all trap data for both sibling species cannot be done.
For all species except for Aedes spp., the reference odor, ammonia + l-lactic acid + CO2, was not more attractive than CO2 alone. Previous research had shown that ammonia alone and the combination of ammonia and l-lactic acid was attractive to An. gambiae s.s. in a dual-choice olfactometer when tested against no odor (Smallegange et al. 2005). Furthermore, the combination of ammonia + l-lactic acid + CO2 was shown to be more attractive than CO2 alone (Spitzen et al. 2007). It is possible that the concentration or ratios of the odors released from the trap was not within the range that is most effective for attracting An. gambiae or that the density of An. gambiae was too low to demonstrate this effect.
The control odor attracted more mosquitoes of all species than the odor from a human sleeping in a tent with the exception of An. gambiae. Meanwhile, according to the mean number of mosquitoes trapped with CO2 alone and with the odor from a human-occupied tent, CO2 accounted for 63% of the attractiveness of the human odor to An. gambiae, whereas CO2 alone caught more mosquitoes of the other species than the odor from a human-occupied tent (Table 2). The contribution of CO2 to the attractiveness of a human to An. gambiae s.l. was estimated to be 9 and 50% by Costantini et al. (1996) and Mboera et al. (2000a), respectively. Because we used a similar experimental design as described by Costantini et al. (1993), we are confident that the human odors reached the trap in sufficient quantity to be attractive to mosquitoes, demonstrating that our control odor blend was compatible to natural human odor.
The most attractive blend for all mosquito groups except An. gambiae s.l. was odor D: CO2 + ammonia + l-lactic acid + 3-methylbutanoic acid. The latter compound was detected in the volatiles of Limburger cheese and human sweat, and these volatiles were found to be attractive to female An. gambiae s.s. (Cork and Park 1996, Knols et al. 1997, Meijerink et al. 2000, Healy et al. 2002). Olfactometry experiments showed that certain concentrations of 3-methylbutanoic acid increased the attractiveness of ammonia + l-lactic acid (Qiu 2005). Electrophysiological responses to 3-methylbutanoic acid were found at the antennal and the single olfactory neuron level in An. gambiae (Cork and Park 1996, Meijerink and van Loon 1999, Qiu 2005). In addition, 3-methylbutanoic acid is also a component of mosquito oviposition sites, pig manure, and various plant odors, and it was considered a potential kairomone for various insects (Kramer et al. 1980, Cossé and Baker 1996, Larsson et al. 2003). Our results suggest that the blend of ammonia, l-lactic acid, CO2, and 3-methylbutanoic acid (odor D) produces a broad-ranging attractant for many species of nocturnal African mosquitoes. The above-mentioned blend in combination with propanoic acid and butanoic acid (odor F) was the second most attractive mixture for An. ziemanni, Culex spp., and Mansonia spp. The other compounds tested in our experiments reduced the attraction. The attractiveness decreased further when tetradecanoic acid was added to odor F (odor H) or when hexanoic acid was added to odor D (odor M). These latter two compounds apparently cause repellency, masking effects, or both at the concentrations tested.
Olfactory receptor neurons in subpopulations of sensilla trichodea were found to be strongly tuned to indole, geranylacetone, or 4-ethylphenol (Qiu 2005). Certain concentrations of these compounds, when tested in combination with ammonia and l-lactic acid against ammonia in a dual-choice olfactometer, showed a repellent effect in An. gambiae s.s. (Qiu 2005). The results of the current study suggest again that indole, geranylacetone, or 4-ethylphenol, alone or as blend, may render human odors less attractive.
Synthetic Blends without CO2
A comparison of the number of mosquitoes trapped by odor blends with and without CO2 revealed that CO2 contributes substantially to the trapping efficiency of the MM-X traps (Fig. 5). Carbon dioxide accounted for >97% of the attraction of the control odor for all mosquito species except for Culex spp. For the latter species, CO2 accounted for 79% of the attraction. Carbon dioxide is considered a general cue used by almost all blood-feeding mosquito species in their host-seeking behavior (Takken 1996). For highly anthropophilic species such as An. gambiae s.s., CO2 was considered to account for only a minor part of the attractiveness of a human (Costantini et al. 1996, Mboera and Takken 1997, Takken et al. 1997, Dekker et al. 2005). Part of the reason that CO2 plays such a dominant role in the current study might be due to the design of the MM-X odor delivery system, in which CO2 functioned as an extra carrier gas that carried the odor blend to the odor release point at the bottom of the trap.
CDC Traps
The number of An. gambiae s.l. caught in the MM-X traps was lower than the number caught by the CDC traps placed in a human-occupied house. We do not consider this to be due to an effect of a lower trapping efficiency of the MM-X traps, because it was demonstrated previously that a MM-X trap caught more An. gambiae and Cx. quinquefasciatus than a CDC trap (Mboera et al. 2000a). Because of the strong association of An. gambiae with humans (Coluzzi et al. 1979), female An. gambiae rarely venture outside a village and the population of An. gambiae in rural Africa is concentrated near human dwellings (Smith et al. 2005). To compare the population near to with that further away from human occupied houses, two CDC traps were placed outdoors: one suspended at the MRC research station and the other in the field, along the farm road where the MM-X traps with CO2 were tested. A higher number of An. gambiae s.l. was found at the MRC research station (2.8 ± 2; n = 9) than in the field (0.1 ± 0.3; n = 9). In this study, MM-X traps were deliberately placed as far as possible away from the village to avoid interference of the human odors from the houses. This also may explain the low number of An. gambiae collected in our human-odor baited trap, and it suggests that such olfactory studies should be conducted within the village, i.e., close to human dwellings.
The average number of mosquitoes trapped in the CDC trap in a village house was higher for all species of mosquitoes than the CDC trap in the experimental house. The difference might have been caused by the differential attractiveness of the two human individuals sleeping under the bed-net adjacent to the trap. Another possible reason for the difference is that the population level of blood-feeding mosquitoes in the village was higher than in the experimental house because of the constant availability of humans or the presence of more potential hosts in the village. A third explanation might be a structural difference between the two houses: the village house had an open door, which was probably the main mosquito entrance; the experimental house had a door that was closed during the experiments, and the main mosquito entrance was the eaves. When comparing the species composition of the CDC traps in these two different houses, the percentage of the endophilic species An. gambiae s.l. and Mansonia spp. was higher in the experimental house, whereas the exophilic mosquitoes An. pharoensis, An. ziemanni, and Culex spp. were more abundant in the village house. A fourth possible reason for the difference in mosquito species composition in the two trapping locations is that a number of goats were kept in the village house at night, which might have contributed to the high proportion of zoophilic mosquitoes.
The current study shows that MM-X traps baited with synthetic odor blends were in many cases more attractive than human odors collected from a tent. Carbon dioxide substantially increased the catches of the MM-X traps of all mosquito species. MM-X traps baited with the blend of ammonia + l-lactic acid + CO2 + 3-methylbutanoic acid was the most attractive odor for most mosquito species. This blend is a promising starting point for further studies to develop potent attractants for African mosquitoes, to reduce their nuisance biting and transmission of vector-borne diseases.
Acknowledgments
We thank Lamin Jaiteh for hard work as field assistant. Our thanks also go to Mbemba Ceesay for making the research team mobile in The Gambia. We also thank Demba Wadu, Ousmane Chulanta, the late Momodou Baldeh, and Janko Tambedou for being volunteers in the experiments. We thank Gradus Leenders for adjusting the MM-X traps. Gerard Nagtegaal helped with the An. gambiae complex PCR identification. We thank Prof. S. Lindsay for constructive suggestions and advice. Our thanks also go to M. Birkett and Prof. J. Pickett for kindly supplying the 7-octenoic acid sample. The Medical Research Council (MRC) of The Gambia, especially Malcolm Guy, Ignatius Baldeh, and Kebba Keita, is acknowledged for facilitating the research. We thank the people that worked in the MRC Wali Kunda field station for support, and the residents in Wali Kunda village for help in several respects during the fieldwork. We thank J. Moskal and A. den Braber (Denka International, Barneveld, The Netherlands) for giving technical advice about the odor release system. The MM-X traps were generously provided by American Biophysics Corp., and we are grateful for the support received. The Technology Foundation of the Netherlands Organization for Scientific Research is acknowledged for funding this project (WBI.4834).
References Cited
- Bernier UR, Kline DL, Barnard DR, Schreck CE, Yost RA. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for yellow fever mosquito (Aedes aegypti) Anal. Chem. 2000;72:747–756. doi: 10.1021/ac990963k. [DOI] [PubMed] [Google Scholar]
- Bernier UR, Kline DL, Schreck CE, Yost RA, Barnard DR. Chemical analysis of human skin emanations: comparison of volatiles from humans that differ in attraction of Aedes aegypti (Diptera: Culicidae) J. Am. Mosq. Control Assoc. 2002;18:186–195. [PubMed] [Google Scholar]
- Brady J, Costantini C, Sagnon N, Gibson G, Coluzzi M. The role of body odours in the relative attractiveness of different men to malarial vectors in Burkina Faso. Ann. Trop. Med. Parasitol. 1997;91:S121–S122. [Google Scholar]
- Braks MAH, Meijerink J, Takken W. The response of the malaria mosquito, Anopheles gambiae, to two components of human sweat, ammonia and L-lactic acid, in an olfactometer. Physiol. Entomol. 2001;26:142–148. [Google Scholar]
- Coluzzi M, Sabatini A, Petrarca V, di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans. R. Soc. Trop. Med. Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- Cork A, Park KC. Identification of electrophysiological-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med. Vet. Entomol. 1996;10:269–276. doi: 10.1111/j.1365-2915.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Cossé AA, Baker TC. House flies and pig manure volatiles: wind tunnel behavioral studies and electrophysiological evaluations. J. Agric. Entomol. 1996;13:301–317. [Google Scholar]
- Costantini C, Gibson G, Brady J, Merzagora L, Coluzzi M. A new odour-baited trap to collect host-seeking mosquitoes. Parassitologia. 1993;35:5–9. [PubMed] [Google Scholar]
- Costantini C, Gibson G, Sagnon N, Della Torre A, Brady J, Coluzzi M. Mosquito responses to carbon dioxide in a West African Sudan savanna village. Med. Vet. Entomol. 1996;10:220–227. doi: 10.1111/j.1365-2915.1996.tb00734.x. [DOI] [PubMed] [Google Scholar]
- Costantini C, Sagnon N, Dalla Torre A, Diallo M, Brady J, Gibson G, Coluzzi M. Odor-mediated host preferences of West-African mosquitoes, with particular reference to malaria vectors. Am. J. Trop. Med. Hyg. 1998;58:56–63. doi: 10.4269/ajtmh.1998.58.56. [DOI] [PubMed] [Google Scholar]
- Costantini C, Birkett MA, Gibson G, Ziesmann J, Sagnon NF, Mohammed HA, Coluzzi M, Pickett JA. Electroantennogram and behavioural responses of the malaria vector Anopheles gambiae to human-specific sweat components. Med. Vet. Entomol. 2001;15:259–266. doi: 10.1046/j.0269-283x.2001.00297.x. [DOI] [PubMed] [Google Scholar]
- Davis EE. Development of lactic acid receptor sensitivity and host-seeking behavior in newly emerged female Aedes aegypti mosquitos. J. Insect Physiol. 1984;30:211–215. [Google Scholar]
- Dekker T, Steib B, Cardé RT, Geier M. l-Lactic acid, a human-signifying host cue for the anthropophilic mosquito Anopheles gambiae sensu stricto. Med. Vet. Entomol. 2002;16:91–98. doi: 10.1046/j.0269-283x.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- Dekker T, Takken W. Differential responses of mosquito sibling species Anopheles arabiensis and An. quadriannulatus to carbon dioxide, a man or a calf. Med. Entomol. 1998;12:136–140. doi: 10.1046/j.1365-2915.1998.00073.x. [DOI] [PubMed] [Google Scholar]
- Dekker T, Geier M, Gardé RT. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J. Exp. Biol. 2005;208:2963–2972. doi: 10.1242/jeb.01736. [DOI] [PubMed] [Google Scholar]
- Diallo M, Nabeth P, Ba K, Sall AA, Ba Y, Mondo M, Girault L, Abdalahi MO, Mathiot C. Mosquito vectors of the 1998–1999 outbreak of Rift Valley Fever and other arboviruses (Bagaza, Sanar, Wesselsbron and West Nile) in Mauritania and Senegal. Med. Vet. Entomol. 2005;19:119–126. doi: 10.1111/j.0269-283X.2005.00564.x. [DOI] [PubMed] [Google Scholar]
- Eiras AE, Jepson PC. Host location by Aedes aegypti (Diptera, Culicidae)–a wind tunnel study of chemical cues. Bull. Entomol. Res. 1991;81:151–160. [Google Scholar]
- Garrett-Jones C. The human blood index of malaria vectors in relation to epidemiological assessment. Bull. W.H.O. 1964;30:241–261. [PMC free article] [PubMed] [Google Scholar]
- Geier M, Bosch OJ, Boeckh J. Ammonia as an attractive component of host odour for the yellow fever mosquito Aedes aegypti. Chem. Senses. 1999;24:647–653. doi: 10.1093/chemse/24.6.647. [DOI] [PubMed] [Google Scholar]
- Genstat Committee . Genstat release 7.1 The guide to GenStat. VSN International Ltd; Oxford, United Kingdom: 2003. [Google Scholar]
- Gillies MT, Wilkes TJ. A comparison of the range of attraction of animal baits and of carbon dioxide for some West African mosquitoes. Bull. Entomol. Res. 1968;59:441–456. doi: 10.1017/s0007485300003412. [DOI] [PubMed] [Google Scholar]
- Gillies MT, Wilkes TJ. The range of attraction of animal baits and carbon dioxide for mosquitoes. Studies in a freshwater area of West Africa. Bull. Entomol. Res. 1972;61:389–404. [Google Scholar]
- Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. The South African Institute for Medical Research; Johannesburg, South Africa: 1987. [Google Scholar]
- Hastie T, Tibshirani R. Generalized additive models. Chapman & Hall; London, United Kingdom: 1990. [Google Scholar]
- Healy TP, Copland MWJ. Activation of Anopheles gambiae mosquitoes by carbon-dioxide and human breath. Med. Vet. Entomol. 1995;9:331–336. doi: 10.1111/j.1365-2915.1995.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Healy TP, Copland MJW, Cork A. Landing responses of Anopheles gambiae elicited by oxocarboxylic acids. Med. Vet. Entomol. 2002;16:126–132. doi: 10.1046/j.1365-2915.2002.00353.x. [DOI] [PubMed] [Google Scholar]
- Jongman RHG, ter Braak CJF, van Tongeren OFR. Data analysis in community and landscape ecology. Cambridge University Press; Cambridge, United Kingdom: 1995. [Google Scholar]
- Kline DL. Comparison of two American biophysics mosquito traps: the professional and a new counterflow geometry trap. J. Am. Mosq. Control Assoc. 1999;15:276–282. [PubMed] [Google Scholar]
- Kline DL, Wood JR, Cornell JA. Interactive effects of 1-octen-3-ol and carbon dioxide on mosquito (Diptera: Culicidae) surveillance and control. J. Med. Entomol. 1991;28:254–258. doi: 10.1093/jmedent/28.2.254. [DOI] [PubMed] [Google Scholar]
- Knols BGJ, van Loon JJA, Cork A, Robinson RD, Adam W, Meijerink J, de Jong R, Takken W. Behavioural and electrophysiological responses of the female malaria mosquito Anopheles gambiae (Diptera: Culicidae) to Limburger cheese volatiles. Bull. Entomol. Res. 1997;87:151–159. [Google Scholar]
- Kramer WL, Hwang YS, Mulla MS. Oviposition repellents of mosquitoes: negative responses elicited by lower aliphatic carboxylic acids. J. Chem. Ecol. 1980;6:415–424. [Google Scholar]
- Larsson MC, Stensmyr MC, Bice SB, Hansson BS. Attractiveness of fruit and flower odorants detected by olfactory receptor neurons in the fruit chafer Pachnoda marginata. J. Chem. Ecol. 2003;29:1253–1268. doi: 10.1023/a:1023893926038. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Wilkins HA, Zieler HA, Daly RJPV, Byass P. Ability of Anopheles gambiae mosquitoes to transmit malaria during the dry and wet seasons in an area of irrigated rice cultivation in The Gambia. J. Trop. Med. Hyg. 1991;94:313–324. [PubMed] [Google Scholar]
- Lindsay SW, Adiamah JH, Miller JE, Pleass RJ, Armstrong JRM. Variation in attractiveness of human subjects to malaria mosquitoes (Diptera: Culicidae) in The Gambia. J. Med. Entomol. 1993;30:308–373. doi: 10.1093/jmedent/30.2.368. [DOI] [PubMed] [Google Scholar]
- Lindsay SW, Jawara M, Paine K, Pinder M, Walraven GEL, Emerson PM. Changes in house design reduce exposure to malaria mosquitoes. Trop. Med. Int. Health. 2003;8:512–517. doi: 10.1046/j.1365-3156.2003.01059.x. [DOI] [PubMed] [Google Scholar]
- Mboera LEG, Takken W. Carbon dioxide chemotropism in mosquitoes (Diptera: Culicidae) and its potential in vector surveillance and management programmes. Rev. Appl. Entomol. Ser. B Med. Vet. Entomol. 1997;85:355–368. [Google Scholar]
- Mboera LEG, Knols BGJ, Takken W, DellaTorre A. The response of Anopheles gambiae s.l. and A. funestus (Diptera: Culicidae) to tents baited with human odour or carbon dioxide in Tanzania. Bull. Entomol. Res. 1997;87:173–178. [Google Scholar]
- Mboera LEG, Knols BGJ, Braks MAH, Takken W. Comparison of carbon dioxide-baited trapping systems for sampling outdoor mosquito populations in Tanzania. Medical and Veterinary Entomology. 2000a;14:257–263. doi: 10.1046/j.1365-2915.2000.00239.x. [DOI] [PubMed] [Google Scholar]
- Mboera LEG, Takken W, Sambu EZ. The response of Culex quinquefasciatus (Diptera: Culicidae) to traps baited with carbon dioxide, 1-octen-3-ol, acetone, butyric acid and human foot odour in Tanzania. Bull. Entomol. Res. 2000b;90:155–159. doi: 10.1017/s0007485300000262. [DOI] [PubMed] [Google Scholar]
- McCullagh P, Nelder JA. Generalized linear models. 2nd ed. Chapman & Hall; London, United Kingdom: 1989. [Google Scholar]
- Meijerink J, van Loon JJA. Sensitivity of antennal olfactory neurons of the malaria mosquito, Anopheles gambiae, to carboxylic acids. J. Insect Physiol. 1999;45:365–373. doi: 10.1016/s0022-1910(98)00135-8. [DOI] [PubMed] [Google Scholar]
- Meijerink J, Braks MAH, van Loon JJA. Olfactory receptors on the antennae of the malaria mosquito Anopheles gambiae are sensitive to ammonia and other sweat-borne components. J. Insect Physiol. 2001;47:455–464. doi: 10.1016/s0022-1910(00)00136-0. [DOI] [PubMed] [Google Scholar]
- Meijerink J, Braks MAH, Brack AA, Adam W, Dekker T, Posthumus MA, van Beek TA, van Loon JJA. Identification of olfactory stimulants for Anopheles gambiae from human sweat samples. J. Chem. Ecol. 2000;26:1367–1382. [Google Scholar]
- Mukabana WR. Differential attractiveness of humans to the African malaria vector Anopheles gambiae Giles: effects of host characteristics and parasite infection. Wageningen University; Wageningen, The Netherlands: 2002. [Google Scholar]
- Mukabana WR, Takken W, Killeen GF, Knols BGJ. The role of breath and body odour in the relative attractiveness of humans to Anopheles gambiae. Malar. J. 2004;3:1–8. doi: 10.1186/1475-2875-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njiru BN, Mukabana WR, Takken W, Knols BGJ. Trapping of the malaria vector Anopheles gambiae with odour-baited MM-X traps in semi-field conditions in western Kenya. Malar. J. 2006;5:1–8. doi: 10.1186/1475-2875-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YT. Sensory and behavioural responses of the malaria mosquito Anopheles gambiae to human odours. Plant Science Department. Wageningen University; Wageningen: 2005. p. 208. [Google Scholar]
- Qiu YT, van Loon JJA, Takken W, Meijerink J, Smid HM. Olfactory coding in antennal neurons of the malaria mosquito, Anopheles gambiae. Chemical Senses. 2006a;31:845–863. doi: 10.1093/chemse/bjl027. [DOI] [PubMed] [Google Scholar]
- Qiu YT, Smallegange RC, van Loon JJA, Ter Braak CJF, Takken W. Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s. s. Med. Vet. Entomol. 2006b;20:280–287. doi: 10.1111/j.1365-2915.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- Smallegange RC, Qiu YT, van Loon JJA, Takken W. Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae) Chem. Senses. 2005;30:145–152. doi: 10.1093/chemse/bji010. [DOI] [PubMed] [Google Scholar]
- Smith DL, Dushoff J, Snow RW, Hay SI. The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature (Lond.) 2005;438:492–495. doi: 10.1038/nature04024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow WF. The effect of a reduction in expired carbon dioxide on the attractiveness of human subjects to mosquitoes. Bull. Entomol. Res. 1970;60:43–48. [Google Scholar]
- Spitzen J, Smallegange RC, Takken W. Effects of positioning of carbon dioxide release point and human odours on catches of the malaria mosquito Anopheles gambia s.s. in an olfactometer. Physiol. Entomol. 2007 in press. [Google Scholar]
- Sudia WD, Chamberlain RW. Battery-operated light trap, an improved model. Mosq. News. 1962;22:126–129. [PubMed] [Google Scholar]
- Takken W. Olfaction in mosquito host interactions. Wiley; Chichester, United Kingdom: 1996. Synthesis and future challenges: the response of mosquitoes to host odours; pp. 302–320. [DOI] [PubMed] [Google Scholar]
- Takken W, Knols BGJ. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu. Rev. Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- Takken W, Dekker T, Wijnholds YG. Odor-mediated flight behavior of Anopheles gambiae Giles sensu stricto and A. stephensi Liston in response to CO2, acetone, and 1-octen-3-ol (Diptera: Culicidae) J. Insect Behav. 1997;10:395–407. [Google Scholar]
- Ter Braak CJF, Šmilauer P. CANOCO reference manual and canodraw for Windows user’s guide: software for canonical community ordination (version 4.5) Microcomputer Power; Ithaca, NY: 2002. [Google Scholar]
- Van den Broek IVF, den Otter CJ. Olfactory sensitivities of mosquitoes with different host preferences (Anopheles gambiae s.s., An. arabiensis, An. quadriannulatus, An. m. atroparvus) to synthetic host odours. J. Insect Physiol. 1999;45:1001–1010. doi: 10.1016/s0022-1910(99)00081-5. [DOI] [PubMed] [Google Scholar]
- Van den Broek IVF, den Otter CJ. Odour sensitivity of antennal olfactory cells underlying grooved pegs of Anopheles gambiae s.s. and An. quadriannulatus. Entomol. Exp. Appl. 2000;96:167–175. [Google Scholar]