Abstract
Conformational thermostabilisation of G protein-coupled receptors (GPCRs) is a successful strategy for their structure determination. The thermostable mutants tolerate short-chain detergents, such as octylglucoside and nonylglucoside, which are ideal for crystallography and, in addition, the receptors are preferentially in a single conformational state. The first thermostabilised receptor to have its structure determined was the β1-adrenoceptor mutant, β1AR-m23, bound to the antagonist cyanopindolol and recently additional structures have been determined with agonist bound. Here we describe further stabilisation of β1AR-m23 by the addition of three thermostabilising mutations (I129V, D322K, Y343L) to make a mutant receptor that is 31°C more thermostable than the wild-type receptor in dodecylmaltoside and 13°C more thermostable than β1AR-m23 in nonylglucoside. Although a number of thermostabilisation methods were tried, including rational design of disulphide bonds and engineered zinc bridges, the two most successful strategies to improve the thermostability of β1AR-m23 were an engineered salt bridge and leucine scanning mutagenesis. The three additional thermostabilising mutations did not significantly affect the pharmacological properties of β1AR-m23, but the new mutant receptor was significantly more stable in short-chain detergents such as heptylthioglucoside and denaturing detergents such as SDS.
Introduction
G protein-coupled receptors (GPCRs) are members of a large and important class of membrane proteins that are involved in transmembrane signal transduction that control many aspects of human physiology1. Their key role in mediating transmembrane signalling upon hormone-binding make GPCRs ideal drug targets for the alleviation or treatment of many afflictions and diseases2; 3. GPCRs also have complex pharmacologies associated with multiple conformational states4; 5. Structures of GPCRs are therefore required, preferably in different conformations, to understand how they function and to design new therapeutic compounds2; 3. In an ideal world, pharmaceutical companies would like structures of GPCRs to be available bound to different agonists and antagonists, or bound to many different small organic compounds for fragment screening procedures. There is therefore considerable interest in developing mutant GPCRs that reproducibly give diffraction-quality crystals, but there are many problems to be overcome before this becomes a reality.
Structures of membrane proteins are being determined at an accelerating rate, but to crystallize and solve the structure of a membrane protein is still not a trivial process with a certain outcome6. Experiences from many laboratories worldwide indicate that strategies which select for membrane proteins that are more stable in detergent during solubilisation and purification are more likely to lead to crystallisation and structure determination7. There are two main strategies currently used in membrane protein crystallisation projects. The first is to identify membrane proteins that are sufficiently stable in short-chain detergents to allow structure determination, usually using a rapid method to assess the homogeneity and stability of the impure solubilised membrane protein by, for example, fluorescence-detection size exclusion chromatography (FSEC)8 or by light scattering or sedimentation procedures after purification9; 10. This approach has been augmented for the study of GPCRs by choosing ligands that significantly increase the stability of the detergent-solubilised receptor (see for example reference 11) as assessed through a thermal unfolding assay using a fluorophore12. In addition, the use of T4 lysozyme fused into cytoplasmic loop 3 greatly facilitates crystal formation in lipid mesophases13 and, in combination with high-affinity ligands, a number of GPCR structures have been determined11; 14-21. The second strategy is to choose an important membrane protein target and to alter its properties by mutagenesis to make it sufficiently stable in short-chain detergents for crystallisation22-27. This latter strategy, termed conformational thermostabilisation, has been used successfully to stabilise three G protein-coupled receptors (GPCRs) in at least two different conformational states and has resulted in high-resolution structures of both the turkey β1-adrenoceptor (β1AR)28-30 and the human adenosine A2A receptor31; 32.
The structure of a thermostabilised β1AR mutant, β1AR-m23, in complex with the high-affinity antagonist cyanopindolol was determined by X-ray crystallography to 2.7 Å resolution29. β1AR-m23 contained 6 thermostabilising mutations identified by alanine scanning mutagenesis coupled with a thermostability assay23. These mutations increased the apparent Tm of the receptor in the detergent dodecylmaltoside (DDM) by 21°C and made the receptor more conformationally homogenous by biasing it towards the ground state conformation (R) that does not couple to G proteins. Although the structure of β1AR-m23 has been determined bound to both antagonists29; 30 and agonists28, there have been difficulties crystallising it in the presence of some ligands or in the absence of any ligand. This suggests that perhaps an even more highly stabilised receptor is required. Crystals of receptors produced in the absence of ligands would allow the crystals to be soaked with small fragments whose structures could then be used in the design of new drugs. Here we describe four methodologies used to try and further stabilise β1AR-m23, namely engineering disulphide bonds, zinc bridges or salt bridges and performing leucine scanning mutagenesis.
Results
The β1AR-m23 receptor23 that was used in all the experiments below consists of residues 34-424 of the turkey β1-adrenoceptor33; 34 and contains the thermostabilising mutations R68S, M90V, Y227A, A282L, F327A and F338M. In addition, β1AR-m23 contains the mutation C116L, which increases expression.
Engineering disulphide bonds to stabilise flexible regions
In the structure of β1AR-m23 (PDB code 2VT4)29, the C-terminal amphipathic helix 8 (H8) has a high B-factor due to disorder in this part of the receptor, while transmembrane helix 1 (H1) is bent through 60° in two of the four molecules in the unit cell. This suggests that these regions are potentially the most dynamic when the receptor is in detergent solution and they may thus represent initial sites of unfolding, which could potentially initiate global unfolding of the whole receptor. Therefore, both these areas were targeted for stabilisation by anchoring the flexible regions with disulphide bonds to neighbouring regions (Fig. 1). The aim was to anchor the potentially flexible extracellular end of H1 to H2, whereas H8 could be anchored to the intracellular side of H1. Pairs of amino acid residues in the desired regions with the Cα atoms less than 8 Å apart35 were identified as possible positions for disulphide bonds and the residues mutated to Cys. The six pairs of amino acid residues mutated were E41-V103, M44-V103, M48-A99, V53-V95, L61-F353 and A65-A352. Eleven single cysteine mutants and six double mutants combining these mutations were therefore constructed and expressed in HEK293T cells. The stability of the expressed mutants was subsequently determined by measuring radioligand binding to receptors solubilised in nonylglucoside (NG) as a function of temperature (Fig. 1). If the disulphide bond formed, it was expected that it would increase receptor stability by tethering the potentially flexible H1 or H8 regions to H2 or H1, respectively. Unfortunately, most of the single Cys mutations destabilised the receptor by 3-7°C, with only one of the single mutants, V52C having similar stability to the wild type receptor. Only one double mutant, M44C+V103C, showed significant stabilisation in relation to the single Cys mutants, which suggested that at least in this instance a thermostabilising disulphide bond had probably formed. However, each of the individual mutations M44C and V103C destabilised the receptor by 4°C and 7°C, respectively, resulting in the double mutant containing the potential disulphide bond having a similar stability to the wild-type receptor (Fig 1). Incubation of membranes containing the receptor mutants with the oxidising agent Cu2+-phenanthroline to promote disulphide bond formation improved marginally the stability of the M44C+V103C mutant, although it also destabilised β1AR-m23, but none of the other double cysteine mutants became more stable (results not shown). In contrast, the reducing agent dithiothreitol (DTT) had a similar destabilizing effect on both β1AR-m23 and the mutant containing M44C+V103C, presumably because it reduces the two native disulphide bonds in addition to the engineered M44C+V103C potential disulphide bond (results not shown). Thus, although strengthening the interactions between H1 and H2 may indeed stabilize a receptor, in the case of β1AR-m23 this stabilisation was offset by the destabilisation of the introduced Cys mutations.
Figure 1.
Engineering disulphide bonds in β1AR-m23. (a) The positions of potential disulphide bonds engineered into β1AR-m23 are shown as dashed lines: a, E41C-V103C (red); b, M44C-V103C (orange); c, M48C-A99C (yellow); d, V52C-V95C (green); e, L61C-F353C (blue); f, A65C-A352C (purple). The structure of β1AR-m23 is shown in rainbow coloration (N-terminus red, C-terminus blue) with transmembrane helices discussed in the main text labelled (H1, H2, H8). (b) The apparent thermostability of each double Cys mutant is represented relative to the thermostability of β1AR-m23, with the thermostability of each of the constituent single Cys mutants shown alongside. The colour code is identical to that depicted in (a).
Improved thermostability of β1AR-m23 by engineering a salt bridge
It is known that the human β2AR is considerably more thermostable than either the human or avian β1AR26. Comparison of the sequences and structures of β1AR29 and β2AR14 shows that β2AR contains a salt bridge between Asp192 and Lys305 that is absent in β1AR, because the equivalent residues are Asp200 and Asp322 (Fig. 2). We hypothesised that engineering this salt bridge into β1AR would increase the stability of the protein, because it would link extracellular loop 2 (EL2) to the top of H7 in an analogous manner to the native thermostabilising disulphide bond Cys114-Cys221 that anchors EL2 to the top of H3. A number of combinations of mutations at both Asp200 and Asp322 were therefore constructed and tested for thermostability. If the native Asp200 remained unchanged, the mutants D322R and D322K both showed an increase in thermostability of 5°C after solubilisation in NG (Fig. 2), consistent with the formation of a salt bridge between EL2 and H7. The thermostability of β1AR by this salt bridge was not improved by including the mutant D200E, even though D200E alone did not adversely affect the thermostability of β1AR-m23. Thus, regardless of the combination of salt bridge-forming residues (Asp or Glu paired with either Arg or Lys), the salt bridge always gave a similar degree of thermostabilisation to the receptor.
Figure 2.
Engineering a salt bridge in β1AR-m23. (a) The structure of β1AR-m23 with D200 and D322 in space-filling representation (C, green; O, red; N, blue) is compared to (b) β2AR where the corresponding residues, D192 and K305 form a salt bridge. (c) Apparent thermostability of various salt bridge forming mutations: β1AR-m23 (blue circles); D200E (orange squares); D322K (green triangles); D322R (cyan inverted triangles); D200E+D322K (purple diamonds); D200E+D322R (pink stars). Data points are from duplicate measurements in a representative experiment with error bars representing the SEM. (d) Change in the apparent Tm of the salt bridge mutants relative to β1AR-m23 measured in DDM and NG (±SEM).
Leucine scanning mutagenesis of β1AR-m23 to identify residues that affect stability
Alanine scanning mutagenesis was used previously to identify amino acid residues that could be mutated to improve the thermostability of GPCRs22-24; 27 and this approach was used to create the thermostable mutant β1AR-m23. We thought that using this method on β1AR-m23, but using leucine scanning mutagenesis rather than alanine, might identify new mutations that would enable us to further improve the thermostability of the receptor. Leucine was chosen because it has a high propensity for forming α-helices36; 37 and, as the majority of the receptor consists of transmembrane helices, this hydrophobic amino acid could have a stabilising effect. A total of 295 leucine mutants (or alanine if the residue was already leucine) were made throughout β1AR-m23 between residues 34-372 but excluding CL3 (242-271); 16 mutations could not be obtained due to strong secondary structure in the DNA template. The Leu/Ala mutants were expressed in E.coli, solubilised in 1% NG and assayed for stability by measuring 3H-DHA binding after heating the samples for 30 minutes at 25°C. The most thermostable 27 mutants were further characterised by determining thermostability curves by heating NG-solubilised receptors for 30 minutes at a range of temperatures and then using the 3H-DHA binding assay to determine the amount of receptor remaining capable of ligand binding. The best 12 mutants increased the apparent Tm of β1AR-m23 by 2-5°C (Fig. 3, blue bars). In addition to the leucine mutations, the mutation I129V which had been previously identified23 as a thermostabilising mutant of wild-type β1AR, was similarly found to thermostabilise β1AR-m23.
Figure 3.
(a) Thermostability changes of leucine mutations and combinations of mutations compared to β1AR-m23 (solid baseline). The stability of each single mutant relative to β1AR-m23 is shown (blue bars) adjacent to the stability of each double mutant i.e. the mutant indicated plus Y343L (red bars). The dashed line at 5°C indicates the stability of β1AR-m23+Y343L. (b) Thermostability of β1AR-m23 (blue circles) and β1AR-JM3 (green triangles) in NG.
Combining thermostable mutations to make β1AR-JM3
The most stabilising single mutation identified by the leucine scan was Y343L, which improved the stability by 5°C. This mutation was combined with the 11 other stabilising mutations identified by the leucine scan, in addition to I129V identified by previous Ala scanning mutagenesis experiments23, V52C identified during the introduction of disulphide bonds and the salt bridge, D200E+D322K. Five of these combinations were additive, while combinations with the other mutations produced a less stable mutant (Fig. 3). Other combinations of these mutations were tried (results not shown), but they did not give reliably favourable results. The best mutant was β1AR-JM3, which contained the mutations D200E, D322K, I129V and Y343L, and was 12°C more stable than β1AR-m23 in NG.
Engineering Zn2+-bridges to potentially reduce conformational heterogeneity
After β1AR-JM3 was constructed, Martin et al38 demonstrated a significant improvement in the thermostability of the leukotriene B4 receptor BLT1 by engineering a Zn2+-bridge that was designed to prevent the formation of the activated receptor R*. This was achieved by the introduction of two His residues at the cytoplasmic ends of H3 and H6 that, in combination with other co-ordinating side chains, was able to bind Zn2+ with high-affinity. Such mutants had previously been described in rhodopsin39, the parathyroid hormone receptor40 and β2-adrenoceptor (β2AR)40 where they lock the receptor in the inactive conformation (R) upon the addition of Zn2+, thus defining regions of the receptor that are involved in the conformation change to R*. The usefulness of this strategy for the thermostabilisation of GPCRs rests on the observation that the R* conformation is invariably less stable than the R state41. Therefore, in theory, it should be possible to thermostabilise any GPCR by engineering in a Zn2+-bridge to prevent the formation of R*, although the thermostability of these engineered β2AR Zn2+-bridge mutants, only the stability of BLT1 was measured38.
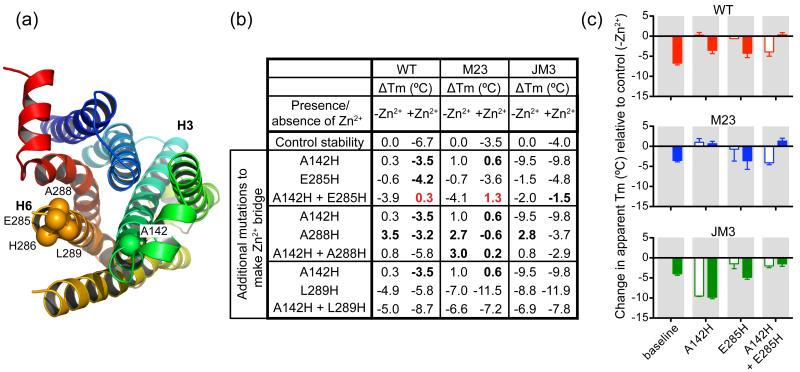
To engineer a Zn2+-bridge in wild-type β1AR at the cytoplasmic ends of H3 and H6 the equivalent mutations to those introduced in the β2AR were made. Thus, the mutations A142H and I143H in H3, and E285H, A288H and L289H in H6 were constructed singly and in all possible combinations of one mutation in H3 and one in H6. The third co-ordinating residue required for formation of the Zn2+-bridge in β2AR was suggested40 to be Asp1303.49, which is also found in wild-type β1AR. Initial thermostability data for the combinations of mutations that included I143H showed that none of the double His mutants were more thermostable than wild-type (results not shown), so further work was only performed on the A142H series of mutants. The only double mutant that appeared to be more stable in the presence of Zn2+ than in its absence was A142H+E282H (Fig. 4), suggesting that in this instance a Zn2+-bridge had probably formed, although the thermostability of the double mutant was similar to the wild-type receptor. Two significant factors prevented the engineered Zn2+-bridge from improving the thermostability of the receptor. Firstly, addition of Zn2+ to the wild-type receptor decreased its stability by up to 7°C. Secondly, the combination of His142 and His285 decreased the stability of the wild type receptor by 4°C in the absence of Zn2+. These effects were also seen when the His residues were engineered into the thermostabilised mutants β1AR-m23 and β1AR-JM3 (Fig. 4). These data show that the transfer of His mutants, known to form a Zn2+-bridge in one receptor, does not necessarily lead to thermostabilisation in a homologous receptor and, in this case, did not do so for any choice of mutations.
Figure 4.
Engineering Zn2+-bridges in β1AR-WT, β1AR-m23 and β1AR-JM3. (a) The positions of residues mutated to histidine for potential Zn2+-bridge formation. The structure of β1AR is viewed from the cytoplasmic side and is shown in rainbow colouration (N-terminus red, C-terminus blue) with transmembrane helices H3 and H6 labelled. Cα atoms of the histidine mutants A142H, E285H, H286, A288H and L289H, are shown as spheres. (b) The apparent Tm of each mutant compared to its parental construct (WT, m23 or JM3) is shown without Zn2+ and with 1 mM ZnCl2 added. Bold indicates mutants that had increased thermostability compared to the parental receptor under the same conditions, whilst red indicates mutants where the addition of Zn2+ significantly improved the thermostability. (c) The apparent Tm of the best mutant combination from (b), A142H+E285H, together with its constituent mutants, A142H and E285H, are plotted in relation to the stability of the parental receptor; WT (red), m23 (blue) and JM3 (green) in the absence of Zn2+ (open bars) or in the presence of 1 mM Zn2+ (solid bars). Results are expressed as mean change in stability relative to their parental receptor together with error bars representing the SEM for two or three independent experiments performed in duplicate.
Characterisation of β1AR-JM3
The effect of the thermostabilising mutations in β1AR-JM3 on its ligand binding properties were determined by saturation binding assays and competition binding experiments performed using membranes prepared from E. coli. The affinity of β1AR-JM3 for the antagonist 3H-DHA (KD = 10.6 ± 1.6 nM, n = 4) is similar but slightly weaker than β1AR-m23 (KD = 5.5 ± 1.1 nM, n = 4), which in turn is weaker than the wild type receptor (KD = 1.92 ± 0.07 nM, n = 4) (Fig. 5). In competition experiments, where 3H-DHA was displaced by increasing concentrations of antagonists (alprenolol and cyanopindolol) or agonists (noradrenaline and isoprenaline), β1AR-JM3, like β1AR-m23, showed a similar affinity for antagonist binding. However, β1AR-JM3 bound the agonists noradrenaline and isoprenaline 4960-fold and 990-fold more weakly, respectively, compared to wild-type β1AR (Fig. 5). β1AR-JM3 also bound agonists 4-fold weaker than β1AR-m23, suggesting that the three additional thermostabilising mutations in β1AR-JM3 have further biased the receptor towards the antagonist-bound conformation.
Figure 5.
Pharmacological analysis of β1AR-JM3. Binding assays and competition assays were performed on receptors expressed in E. coli membranes and solubilised in DDM: β1AR (WT, red squares); β1AR-m23 (m23, blue circles); β1AR-JM3 (JM3, green triangles). (a) Saturation binding curve of WT, m23 and JM3 performed using 3H-DHA. Competition binding assays were performed by measuring the displacement of 3H-DHA with the agonists noradrenaline (b) and isoprenaline (c), and the antagonists alprenolol (e) and cyanopindolol (f). Experiments are the average of 2-4 experiments performed in duplicate with error bars representing the SEM. (d) Table of KD and Ki values calculated by non-linear regression from the data in panels a-c, e and f (GraphPad Prism).
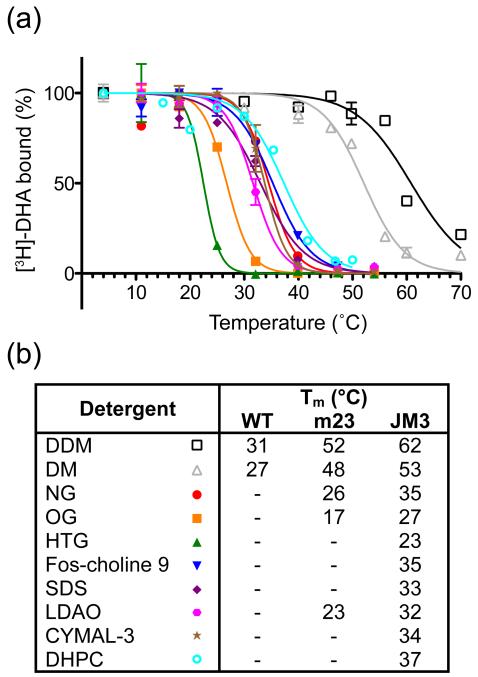
Short chain detergents are better for crystallisation of small membrane proteins because they expose hydrophilic parts of the membrane protein essential for the formation of crystal contacts. However, these detergents are also more denaturing (harsh) and therefore significantly decrease the stability of membrane proteins. The additional thermostability of β1AR-JM3 led to improved stability in a wide range of detergents compared to β1AR or β1AR-m23 (Fig. 6). To avoid any potential discrepancies arising due to different experimental conditions, β1AR-JM3 and β1AR-m23 were compared under identical conditions in the presence of 0.8% NG, which gave an apparent Tm for β1AR-JM3 of 36.3 ± 0.4°C (n = 3) and an apparent Tm for β1AR-m23 of 23.4 ± 0.8°C (n = 4).
Figure 6.
Thermostability of β1AR-JM3 in different detergents. (a) E. coli membranes containing β1AR-JM3 were solubilised in the respective detergents and the apparent Tms determined from the thermostability curves: dodecylmaltoside (black empty squares), Tm 61.7 ± 0.9°C; decylmaltoside (grey empty triangles), Tm 52.8 ± 0.5°C; nonylglucoside (red circles), Tm 34.5 ± 0.7°C; octylglucoside (orange squares), Tm 26.8 ± 0.2°C; heptylthioglucoside (green triangles), Tm 22.5 ± 0.7°C; fos-choline 9 (blue inverted triangles), Tm 35.3 ± 0.5°C; SDS (purple diamonds), Tm 33.0 ± 0.7°C; LDAO (pink hexagons), Tm 31.6 ± 0.3°C; CYMAL-3 (brown stars), Tm 33.8 ± 0.5°C; DHPC (cyan empty circles), Tm 37.4 ± 1.0°C. Detergent concentrations were all 2% except for SDS (1%) and LDAO (0.9%). Data are from a single experiment performed simultaneously in duplicate with errors representing the SEM. (b) Comparison of the stability of β1AR-JM3 with β1AR-m23 and wild-type β1AR (data for β1AR-m23 and β1AR are from reference 23). Symbols refer to the graph in panel (a).
Discussion
In recent years there has been a flurry of GPCR structures11; 14-20; 28; 29; 31; 32; 42; 43, driven mainly by different genetic engineering or crystallisation methodologies to improve the probability of them crystallizing44. However, in every instance, stability of the GPCR during crystallisation has been a key factor in determining whether well-ordered crystals are formed. The structure determination of some receptors has been facilitated by using high-affinity antagonists, preferably with a slow off-rate, in combination with increasing the area of hydrophilic surface by making T4L fusions13. An interesting approach that has also contributed to a structure of β2AR was to make a covalently bound agonist43. However, because it is necessary for the ligand to impart stability to the receptor to allow crystal formation, this methodology reduces the possibilities for crystallisation with weakly bound ligands that do not stabilise the receptor. Conformational thermostabilisation of GPCRs stabilises the receptor in a specific conformation and it appears that the receptor can then be sufficiently stable for crystallisation with some weakly-bound ligands28. However, it has not yet been possible to produce crystals in the absence of ligands, which would allow crystal-soaking strategies to be used for high-throughput fragment screening that is currently popular in some drug design platforms. We have therefore studied a series of approaches to see if it is possible to make the thermostabilised receptor β1AR-m23 even more thermostable.
Two broad strategies to thermostabilisation were attempted, which can be described as either rational engineering or systematic scanning mutagenesis coupled to selection. The rational engineering approaches introduced either disulphide bonds, zinc bridges or a salt bridge in regions of β1AR-m23 that were known to be dynamic, as suggested either from biochemical data or directly from the crystal structure of β1AR-m23. The rationale behind this strategy is that flexible regions are thought to be more likely to initiate unfolding processes compared to non-flexible regions, because they can potentially access many different conformations, which may be considerably less stable than the native structure. However, engineering disulphide bonds or Zn2+ bridges into β1AR-m23 did not improve the overall thermostability of the receptor. In both instances, the introduction of the mutations required for the formation of either the disulphide bonds or the Zn2+ bridges decreased the stability of the receptor and, in addition, the presence of Zn2+ destabilised the receptor. Thus, although an engineered disulphide bond stabilised opsin45 and Zn2+ bridges have been shown to improve the thermostability of BLT138, improved thermostability is not guaranteed.
An engineering approach that did improve the stability of β1AR-m23 was the introduction of a salt bridge on the extracellular surface, linking EL2 and the extracellular end of H7. Regardless of whether the salt bridge contained Asp or Glu paired with Arg or Lys, there was a 5°C improvement in the thermostability of β1AR-m23. The improvement in thermostability by the single mutation D322K is equivalent to the improvement seen for the best thermostabilising mutations observed by Ala/Leu scanning procedures22-24, suggesting that further efforts to alter the extracellular surface are warranted.
The systematic scanning approach we used for the thermostabilisation of β1AR-m23 utilised a Leu scan throughout the receptor and the testing of the thermostability of each of the mutants. The best single mutations gave an improvement in thermostability of 2-5°C, which was similar to that observed previously in Ala scans of the wild-type receptor. Combination of the best single mutant Y343L from the Leu scan with I129V and the mutations to form the extracellular salt bridge gave a 12°C improvement in thermostability as measured in nonylglucoside. This mutant, β1AR-JM3, showed a similar pharmacological profile to β1AR-m23, with a 1.5 fold decrease in affinity for antagonists and a 4-fold decrease in affinity for agonists. The slightly greater decrease in affinity for agonists compared to antagonists suggests that there is a further bias in the equilibrium towards the inactive R state. β1AR-JM3 also showed significant stability in very denaturing detergents like SDS and in very short chain detergents such as heptylthioglucoside.
The work described here demonstrates a number of points. Firstly, even though β1AR-m23 was highly thermostabilised compared to wild-type β1AR23, it is still possible to improve the thermostability even further. In fact, we believe that it is possible to continue to improve thermostability if time and resources are unlimited. Secondly, rational engineering approaches involving attempts to make disulphide or zinc bridges by mutating residues in transmembrane domains were less successful than mutating residues in the extracellular domains. This appears to be due primarily to the decrease in stability of Cys and His mutants in the transmembrane regions. The good improvement in thermostability upon engineering a salt bridge between EL2 and the end of H7 suggests that focusing rational engineering approaches on the soluble regions of GPCRs will be a useful strategy for future work. In addition, it may be that orthogonal approaches, such as binding antibody fragments to these regions, may also improve the thermostability of GPCRs. Finally, we have demonstrated that Leu scanning identifies thermostable mutations as observed in Ala scanning mutagenesis. Indeed, it is likely that scans with other amino acid residues would identify other thermostabilising mutations.
The significant improvement in thermostability of β1AR-JM3 compared to β1AR-m23 suggests that it will show improved qualities in crystallisation trials. Indeed, initial data suggest that in lipidic cubic phase crystallisation trials β1AR-JM3 forms crystals that are 10 times larger than obtained for β1AR-m23 and they also diffract commensurately better and to higher resolution. Crystallisation trials with ligand fragments and in the absence of ligands are ongoing.
Methods
Materials
The construct β1AR-WT consists of the residues 34-424 of the turkey β1AR33. Mutant β1AR6-m23 encodes residues 34-424 of the turkey β1AR33; 34 and additionally contains 6 thermostabilising point mutations (R68S, M90V, Y227A, A282L, F327A and F338M)23, plus the mutation C116L to improve expression. Plasmid pcDNA3.1 was used to express receptors in mammalian cells and plasmid pRGIII was used to express receptors in E. coli46. 1-[4,6-propyl-3H]dihydroalprenolol (3H-DHA) was supplied by GE Healthcare and PerkinElmer. L-(-)-norepinephrine bitartrate salt monohydrate, (–)-isoproterenol hydrochloride, alprenolol hydrochloride and S(-)-cyanopindolol hemifumarate were from Sigma.
Site-directed mutagenesis
Mutants were generated by PCR using either the plasmid pcDNA3.1-β1AR-m23 (cysteine mutations) or pRGIII-β1AR-m23 (all other mutants). The QuikChange II methodology was employed (Stratagene) using KOD Hot start polymerase (Novagen). PCR reactions were transformed into XL1 competent cells (Stratagene) and the receptor cDNAs in individual clones fully sequenced to ascertain the presence of the desired mutations.
Protein Expression and Membrane Preparation
For expression of the cysteine mutants, HEK293T cells were transiently transfected with the plasmid using GeneJuice (Novagen). After transfection, cells were incubated for 48 h and then harvested; cell pellets containing 1.44 million cells were frozen in liquid nitrogen and stored at −20°C. For the thermostability assays, cells were resuspended in 360 μl of buffer [75 mM Tris pH 8, 12.5 mM MgCl2, 2 mM EDTA, protease inhibitors (Complete; Roche)] incubated on ice for 1 h and solubilised in 2% DDM for 30 minutes at 4°C. Insoluble material was removed by centrifugation (13 000 g, 1 minute, 4°C).
Expression of all the other mutants was performed in E. coli strain XL1 (Stratagene) using auto-induction media [2×TY, 100 μg/ml ampicillin, 0.5% glycerol, 0.05% glucose, 0.2% lactose]. Cultures (7 ml) were grown in 24-well deep-well plates (Promega) with shaking at 37°C for 6 hours followed by 25°C overnight. Aliquots of cultures (2 ml, approximately 6.6 million cells) were pelleted and frozen. Cells were resuspended in 200 μl lysis buffer [20 mM Tris pH 7.5, 0.4 M NaCl, 1 mM EDTA (EDTA was excluded for the histidine mutants), 260 μg/ml lysozyme, DNAse and protease inhibitors (Complete; Roche)] and incubated on ice for 1 hour. Samples were solubilised with 2% detergent (DDM or NG) on ice for 30 min and then sonicated briefly and centrifuged to remove insoluble material.
For large-scale membrane preps, 500 ml of E. coli culture expressing the desired mutant were grown as described above. The cells were harvested by centrifugation (5 000 g, 20 minutes, 20°C) and resuspended in 25 ml buffer [20 mM Tris pH 7.5, 1 mM EDTA, protease inhibitors (Complete; Roche)], frozen in liquid nitrogen and stored at −80°C. Thawed cells were disrupted using a French Press (two passages, 20 000 psi) and cell debris removed by centrifugation (10 000 g, 45 minutes, 4°C). The supernatant (membranes) was centrifuged (65 000 g, 90 minutes, 4°C) and the pellet frozen. The pellet was resuspended in 8 ml buffer [20 mM Tris pH 7.5, 0.4 M NaCl, 1 mM EDTA] and stored in 1 ml aliquots at −20°C. For radioligand binding assays, membranes were solubilised in 20 mM Tris (pH 8), 0.4 M NaCl, protease inhibitors (Complete; Roche) and 1% detergent for 1 hour on ice.
Radioligand Binding and Thermostability Assays
For thermostability assays 40 μl solubilised cells were diluted to 108 μl with buffer [20 mM Tris pH 8, 0.4 M NaCl, ± 1 mM ZnCl2 as required for histidine mutants] and heated for 30 minutes at the relevant incubation temperature in the absence of ligand. 3H-DHA was added to a final concentration of 80 nM, followed by an incubation at 4°C for 1 hour. Bound and free radioligand were separated on mini gel filtration columns by brief centrifugation33. Liquid scintillation counting was used to determine amount of bound 3H-DHA and hence expressed, folded protein. Non-specific binding was determined either using membranes prepared from cells not expressing β1AR or in the presence of 1 mM alprenolol.
For KD and Ki determination, solubilised membranes containing the receptors β1AR-WT, β1AR-m23 or β1AR-JM3 were incubated in a final volume of 120 μl buffer [20 mM Tris pH 8, 0.4 M NaCl, 0.1% DDM and 3H-DHA]. Saturation binding curves were obtained using 1.25-160 nM 3H-DHA. Competition assays were performed by first incubating (1 h, 4°C) the sample with 3H-DHA at a concentration of 1 to 3 times the KD of the mutant, followed by incubation (1 h, 4°C) with various concentrations of the competitors norepinephrine, isoprenaline, alprenolol and cyanopindolol. Radioactivity was counted on a MicroBeta TriLux scintillation counter (PerkinElmer) and data were analysed by nonlinear regression using Prism software (GraphPad).
Acknowledgements
JLM is the recipient of a Commonwealth Scholarship and we thank R. Henderson for comments on the manuscript.
References
- 1.Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, Spedding M, Harmar AJ. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacological Reviews. 2005;57:279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- 2.Congreve M, Marshall F. The impact of GPCR structures on pharmacology and structure-based drug design. British Journal of Pharmacology. 2010;159:986–996. doi: 10.1111/j.1476-5381.2009.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–30. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 4.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Vauquelin G, Van Liefde I. G protein-coupled receptors: a count of 1001 conformations. Fundam Clin Pharmacol. 2005;19:45–56. doi: 10.1111/j.1472-8206.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 6.Bill RM, Henderson PJ, Iwata S, Kunji ER, Michel H, Neutze R, Newstead S, Poolman B, Tate CG, Vogel H. Overcoming barriers to membrane protein structure determination. Nat Biotechnol. 2011;29:335–340. doi: 10.1038/nbt.1833. [DOI] [PubMed] [Google Scholar]
- 7.Sonoda Y, Newstead S, Hu NJ, Alguel Y, Nji E, Beis K, Yashiro S, Lee C, Leung J, Cameron AD, Byrne B, Iwata S, Drew D. Benchmarking membrane protein detergent stability for improving throughput of high-resolution x-ray structures. Structure. 2011;19:17–25. doi: 10.1016/j.str.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawate T, Gouaux E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure. 2006;14:673–81. doi: 10.1016/j.str.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Gutmann DAP, Mizohata E, Newstead S, Ferrandon S, Henderson PJF, van Veen HW, Byrne B. A high-throughput method for membrane protein solubility screening: The ultracentrifugation dispersity sedimentation assay. Protein Sci. 2007;16:1422–1428. doi: 10.1110/ps.072759907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Postis VLG, Deacon SE, Roach PCJ, Wright GSA, Xia X, Ingram JC, Hadden JM, Henderson PJF, Phillips SEV, McPherson MJ, Baldwin SA. A high-throughput assay for membrane protein stability. Mol. Memb. Biol. 2008;25:617–624. doi: 10.1080/09687680802530469. [DOI] [PubMed] [Google Scholar]
- 11.Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332:322–7. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexandrov AI, Mileni M, Chien EY, Hanson MA, Stevens RC. Microscale fluorescent thermal stability assay for membrane proteins. Structure. 2008;16:351–9. doi: 10.1016/j.str.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007;318:1266–73. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 14.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–65. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chien EY, Liu W, Zhao Q, Katritch V, Han GW, Hanson MA, Shi L, Newman AH, Javitch JA, Cherezov V, Stevens RC. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science. 2010;330:1091–5. doi: 10.1126/science.1197410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaakola VP, Griffith MT, Hanson MA, Cherezov V, Chien EY, Lane JR, Ijzerman AP, Stevens RC. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–7. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, Schnapp A, Konetzki I, Sunahara RK, Gellman SH, Pautsch A, Steyaert J, Weis WI, Kobilka BK. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature. 2011;469:175–80. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen SG, Devree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah ST, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the beta(2) adrenergic receptor-Gs protein complex. Nature. 2011 doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wacker D, Fenalti G, Brown MA, Katritch V, Abagyan R, Cherezov V, Stevens RC. Conserved binding mode of human beta2 adrenergic receptor inverse agonists and antagonist revealed by X-ray crystallography. J Am Chem Soc. 2010;132:11443–5. doi: 10.1021/ja105108q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Kuhn P, Handel TM, Cherezov V, Stevens RC. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–71. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimamura T, Shiroishi M, Weyand S, Tsujimoto H, Winter G, Katritch V, Abagyan R, Cherezov V, Liu W, Han GW, Kobayashi T, Stevens RC, Iwata S. Structure of the human histamine H1 receptor complex with doxepin. Nature. 2011;475:65–70. doi: 10.1038/nature10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magnani F, Shibata Y, Serrano-Vega MJ, Tate CG. Co-evolving stability and conformational homogeneity of the human adenosine A2a receptor. Proc Natl Acad Sci U S A. 2008;105:10744–9. doi: 10.1073/pnas.0804396105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serrano-Vega MJ, Magnani F, Shibata Y, Tate CG. Conformational thermostabilization of the beta1-adrenergic receptor in a detergent-resistant form. Proc Natl Acad Sci U S A. 2008;105:877–82. doi: 10.1073/pnas.0711253105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata Y, White JF, Serrano-Vega MJ, Magnani F, Aloia AL, Grisshammer R, Tate CG. Thermostabilization of the neurotensin receptor NTS1. J Mol Biol. 2009;390:262–77. doi: 10.1016/j.jmb.2009.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson N, Jazayeri A, Errey J, Baig A, Hurrell E, Zhukov A, Langmead CJ, Weir M, Marshall FH. The properties of thermostabilised G protein-coupled receptors (StaRs) and their use in drug discovery. Neuropharmacology. 2010;60:36–44. doi: 10.1016/j.neuropharm.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Serrano-Vega MJ, Tate CG. Transferability of thermostabilizing mutations between beta-adrenergic receptors. Mol. Memb. Biol. 2009;26:385–96. doi: 10.3109/09687680903208239. [DOI] [PubMed] [Google Scholar]
- 27.Lebon G, Bennett K, Jazayeri A, Tate CG. Thermostabilisation of an Agonist-Bound Conformation of the Human Adenosine A(2A) Receptor. J Mol Biol. 2011;409:298–310. doi: 10.1016/j.jmb.2011.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warne T, Moukhametzianov R, Baker JG, Nehme R, Edwards PC, Leslie AG, Schertler GF, Tate CG. The structural basis for agonist and partial agonist action on a beta(1)-adrenergic receptor. Nature. 2011;469:241–4. doi: 10.1038/nature09746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AG, Tate CG, Schertler GF. Structure of a beta1-adrenergic G-protein-coupled receptor. Nature. 2008;454:486–91. doi: 10.1038/nature07101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moukhametzianov R, Warne T, Edwards PC, Serrano-Vega MJ, Leslie AG, Tate CG, Schertler GF. Two distinct conformations of helix 6 observed in antagonist-bound structures of a beta1-adrenergic receptor. Proc Natl Acad Sci U S A. 108:8228–32. doi: 10.1073/pnas.1100185108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, Tate CG. Agonist-bound adenosine A(2A) receptor structures reveal common features of GPCR activation. Nature. 2011;474:521–525. doi: 10.1038/nature10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dore AS, Robertson N, Errey JC, Ng I, Hollenstein K, Tehan B, Hurrell E, Bennett K, Congreve M, Magnani F, Tate CG, Weir M, Marshall F. Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure. 2011 doi: 10.1016/j.str.2011.06.014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warne T, Chirnside J, Schertler GF. Expression and purification of truncated, non-glycosylated turkey beta-adrenergic receptors for crystallization. Biochim Biophys Acta. 2003;1610:133–40. doi: 10.1016/s0005-2736(02)00716-2. [DOI] [PubMed] [Google Scholar]
- 34.Warne T, Serrano-Vega MJ, Tate CG, Schertler GF. Development and crystallization of a minimal thermostabilized G protein-coupled receptor. Protein Expr Purif. 2009;65:204–213. doi: 10.1016/j.pep.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 35.O’connor BD, Yeates TO. GDAP: a web tool for genome-wide protein disulfide bond prediction. Nucleic Acids Research. 2004;32:W360–W364. doi: 10.1093/nar/gkh376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blaber M, Zhang XJ, Matthews BW. Structural Basis of Amino-Acid Alpha-Helix Propensity. Science. 1993;260:1637–1640. doi: 10.1126/science.8503008. [DOI] [PubMed] [Google Scholar]
- 37.Pace CN, Scholtz JM. A helix propensity scale based on experimental studies of peptides and proteins. Biophysical Journal. 1998;75:422–427. doi: 10.1016/s0006-3495(98)77529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin A, Damian M, Laguerre M, Parello J, Pucci B, Serre L, Mary S, Marie J, Baneres JL. Engineering a G protein-coupled receptor for structural studies: Stabilization of the BLT1 receptor ground state. Protein Science. 2009;18:727–734. doi: 10.1002/pro.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheikh SP, Zvyaga TA, Lichtarge O, Sakmar TP, Bourne HR. Rhodopsin activation blocked by metal-ion-binding sites linking transmembrane helices C and F. Nature. 1996;383:347–350. doi: 10.1038/383347a0. [DOI] [PubMed] [Google Scholar]
- 40.Sheikh SP, Vilardarga JP, Baranski TJ, Lichtarge O, Iiri T, Meng EC, Nissenson RA, Bourne HR. Similar structures and shared switch mechanisms of the beta(2)-adrenoceptor and the parathyroid hormone receptor - Zn(II) bridges between helices III and VI block activation. Journal of Biological Chemistry. 1999;274:17033–17041. doi: 10.1074/jbc.274.24.17033. [DOI] [PubMed] [Google Scholar]
- 41.Gether U, Ballesteros JA, Seifert R, Sanders-Bush E, Weinstein H, Kobilka BK. Structural instability of a constitutively active G protein-coupled receptor. Agonist-independent activation due to conformational flexibility. J Biol Chem. 1997;272:2587–90. doi: 10.1074/jbc.272.5.2587. [DOI] [PubMed] [Google Scholar]
- 42.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–7. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 43.Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SG, Choi HJ, Devree BT, Sunahara RK, Chae PS, Gellman SH, Dror RO, Shaw DE, Weis WI, Caffrey M, Gmeiner P, Kobilka BK. Structure and function of an irreversible agonist-beta(2) adrenoceptor complex. Nature. 2011;469:236–40. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tate CG, Schertler GF. Engineering G protein-coupled receptors to facilitate their structure determination. Curr Opin Struct Biol. 2009;19:386–95. doi: 10.1016/j.sbi.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Standfuss J, Xie G, Edwards PC, Burghammer M, Oprian DD, Schertler GF. Crystal structure of a thermally stable rhodopsin mutant. J. Mol. Biol. 2007;372:1179–88. doi: 10.1016/j.jmb.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tucker J, Grisshammer R. Purification of a rat neurotensin receptor expressed in Escherichia coli. Biochem. J. 1996;317:891–9. doi: 10.1042/bj3170891. [DOI] [PMC free article] [PubMed] [Google Scholar]