Summary
Background
To date, only two agents, imatinib and sunitinib, have shown clinical benefit in patients with gastrointestinal stromal tumours (GISTs), but almost all metastatic GISTs eventually develop resistance to these agents, resulting in fatal disease progression. This phase 3 trial assessed efficacy and safety of regorafenib in patients with metastatic and/or unresectable GIST progressing after failure of at least imatinib and sunitinib.
Methods
Patients were randomised 2:1 to receive either regorafenib 160 mg orally daily or placebo, plus best supportive care in both arms, for the first 3 weeks of each 4-week cycle. The primary endpoint was progression-free survival (PFS). Upon disease progression, patients on placebo could cross over to regorafenib. Secondary endpoints included overall survival (OS), objective response rate, disease control rate (DCR: rate of durable stable disease lasting for ≥12 weeks plus complete or partial responses), and safety. This trial is registered at ClinicalTrials.gov (NCT01271712).
Results
From January to August 2011, 240 patients were screened at 57 centres in 17 countries, and 199 patients were randomised to receive regorafenib (n=133) or matching placebo (n=66). Median PFS per independent blinded central review was 4·8 months and 0·9 months, respectively (hazard ratio [HR] 0·27, 95% confidence interval [CI] 0·19–0·39; p<0·0001). Following progression, 56/66 patients (84·8%) on placebo crossed over to regorafenib, resulting in no significant difference in OS between study arms (HR 0·77, 95% CI 0·42–1·41; p=0·199). A best response of partial response or stable disease was observed in 101/133 patients (75·9%) on regorafenib and 23/66 patients (34·8%) on placebo. DCR was 52·6% (70/133 patients) and 9·1% (6/66 patients), respectively. Drug-related adverse events were reported in 130 (98·5%) of 132 regorafenib patients and 45 (68·2%) of 66 placebo patients. The most common grade ≥3 regorafenib-related adverse events were hypertension (31/132, 23·5%), hand–foot skin reaction (26/132, 19·7%), and diarrhoea (7/132, 5·3%).
Interpretation
Regorafenib significantly improved PFS and DCR, compared with placebo, in patients with advanced GIST progressing after failure of at least imatinib and sunitinib.
Introduction
Gastrointestinal stromal tumours (GISTs) are the most common sarcomas arising in the gastrointestinal tract. Worldwide, the annual incidence of GISTs is approximately 10 cases per million people,1 corresponding to at least 8,000 new cases per year in Europe. Early-stage disease can be surgically resected, but over 40% of cases may recur and metastasise.2
Cytotoxic chemotherapy, although active in other subtypes of sarcomas, is ineffective in metastatic GISTs.3,4 Elucidation of GIST molecular pathophysiology as a mutation-driven cancer has facilitated the development of targeted, kinase inhibitor therapies that have revolutionised the treatment options and clinical outcomes of this disease.5 About 85% of GISTs are caused by gain-of-function mutations in the receptor tyrosine kinase (RTK)-encoding proto-oncogene KIT,6 which result in constitutive ligand-independent activation of KIT intracellular signalling.1,7,8 Approximately 8% of metastatic GISTs are associated with gain-of-function mutations in the structurally similar RTK gene PDGFRA, encoding the platelet-derived growth factor receptor-α.6,8,9 Other rare subtypes of GIST exist which harbour no mutations in KIT or PDGFRA, but are likely driven by other mutations in genes such as BRAF, NF1, or those encoding subunits of the succinate dehydrogenase (SDH) complex.9
Imatinib mesylate, a relatively selective tyrosine kinase inhibitor (TKI) of KIT, PDGFRα, and Abl, significantly improves clinical outcomes in GIST both as therapy for advanced metastatic disease and in the postsurgical adjuvant setting.10-13 However, imatinib therapy is limited by primary resistance to the drug in approximately 15% of patients,5,14-16 and over 80% of patients eventually develop disease progression driven by secondary-resistance mutations located in additional KIT exons.16-20
The first drug shown definitively to provide clinical benefit in GIST following resistance to imatinib was sunitinib malate, which has more potent activity against the wild-type KIT kinase than the first-line treatment, and also inhibits a number of other RTK-related signalling pathways including the vascular endothelial growth factor receptors (VEGFR1-3), Fms-like tyrosine kinase-3 (FLT3), and the receptor encoded by the proto-oncogene RET.21-25 A randomised, placebo-controlled phase 3 trial evaluating sunitinib in imatinib-resistant patients showed a significant improvement in median time to tumour progression for sunitinib compared with placebo (all patients also received best supportive care).26 However, clinical progression and drug resistance to sunitinib subsequently evolve, generally within 1 year of treatment, and no other effective therapy has been developed to date for TKI-resistant GIST. Structural biology studies have explained mechanistically that the smaller sunitinib molecule may demonstrate activity in imatinib-resistant patients due to avoidance of steric hindrance by gatekeeper mutations which block entrance of the larger imatinib molecule to the ATP-binding pocket of the KIT protein.27
Regorafenib is a novel, oral multikinase inhibitor that blocks the activity of multiple protein kinases, including those involved in the regulation of tumour angiogenesis (VEGFR-1, -2, and -3, and TIE2), oncogenesis (KIT, RET, RAF-1, BRAF, and BRAFV600E), and the tumour microenvironment (PDGFR and FGFR).28 In preclinical studies, regorafenib demonstrated antitumour activity against human GIST and other tumour models.28
Following the phase 1 study that defined the safety, tolerability, and recommended dose of regorafenib in unselected solid tumour patients,29 a phase 2 multicentre trial was designed and conducted under independent academic sponsorship to evaluate regorafenib in GIST patients with metastatic disease, following failure of at least prior imatinib and sunitinib.30 In that phase 2 study, regorafenib demonstrated activity against TKI-resistant GIST, including some partial responses, a high rate of durable stable disease, and median progression-free survival (PFS) of 10 months, along with the expected grade 3 toxicities of hypertension and hand–foot skin reaction.30 Based on these data and the preclinical rationale of targeting the pathogenic mutant kinases with a structurally distinct small-molecule inhibitor, this randomised, placebo-controlled, phase 3 trial (GIST–Regorafenib In Progressive Disease, acronym GRID; NCT01271712) was conducted to definitively evaluate the efficacy and safety of regorafenib in patients with metastatic and/or unresectable GIST progressing after failure of at least prior imatinib and sunitinib. We report here the final results of this international phase 3 trial.
Methods
Study design
The study was a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial, involving 57 hospital sites in 17 countries (Austria, Belgium, Canada, China, Finland, France, Germany, Israel, Italy, Japan, Netherlands, Poland, Singapore, South Korea, Spain, UK, and USA).
The study protocol was approved by the institutional review board of each participating institution and complied with the Declaration of Helsinki, current Good Clinical Practice guidelines, and local laws and regulations. An independent data monitoring committee, comprising three oncologists and a statistician, ensured the overall integrity of the trial and safety of participants. All participants provided informed consent before enrolment.
Participants
Eligibility included histologically confirmed, metastatic and/or unresectable GIST, with failure of at least: (1) prior imatinib (due to either disease progression or intolerance) and (2) prior sunitinib (due solely to progression to decrease heterogeneity, since the definition of intolerance is more variable with this agent). Patients could have received other systemic therapies, including investigational agents, except any VEGFR inhibitors other than sunitinib. Additional inclusion criteria included: at least one measurable lesion on computed tomography or magnetic resonance imaging; resolution of all toxic effects of prior therapy to grade 1 or less; adequate haematological, hepatic, cardiac, and renal function; and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1.
Additional details on the inclusion and exclusion criteria are provided in Online Supplemental Table 1.
Interventions
Enrolled patients were randomised in a 2:1 ratio to receive either regorafenib 160 mg orally once daily or matching placebo, for the first 3 weeks of each 4-week cycle. All patients also received best supportive care (defined as any method to preserve the comfort and dignity of the patient, excluding disease-specific antineoplastic therapy such as TKI therapy other than study drug, chemotherapy, radiation therapy or surgical intervention). Blinded study drug administration was continued until disease progression, occurrence of unacceptable toxicity, or patient withdrawal from the study.
In the event of centrally assessed tumour progression, treatment assignment could be unblinded. Patients originally assigned to the placebo arm were offered the option to cross over to receive open-label regorafenib, and patients originally assigned to the regorafenib arm could continue to receive open-label regorafenib, both at the discretion of the investigator. Throughout both the blinded and the open-label phases of the trial, the dose of study drug could be delayed or reduced according to a prespecified schedule in the case of unacceptable toxicities (see Online Supplemental Tables 2–5).
Tumour assessments were made at baseline, then every 4 weeks for the first 3 months, every 6 weeks for the next 3 months, and subsequently every 8 weeks until the end of study drug administration. Intervening tumour assessments could be made more frequently if clinically indicated. In addition to central review, an investigator assessment was also made at each evaluation. During the open-label period, only investigator assessments were made.
Safety and tolerability were assessed by analysis of adverse events, physical examinations, vital signs, ECOG performance status, and laboratory assessments, on days 1 and 15 of each treatment cycle for the first 6 cycles. Cardiac function was assessed with 12-lead electrocardiogram at screening, day 1 of the first two treatment cycles (and subsequent cycles at the discretion of the investigator), and at treatment end. Severity of adverse events was rated by investigators according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4·0.
Outcomes
The primary endpoint was PFS per modified Response Evaluation Criteria In Solid Tumors (RECIST) 1·1, assessed by central radiology reviewer blinded to patient data. The prospectively defined RECIST modifications, which were unique to this study and developed to apply specifically to GIST, included the following criteria: (a) no lymph nodes were chosen as target lesions; enlarged lymph nodes were followed as non-target lesions; (b) no bone lesions were chosen as target lesions; (c) positron emission tomography was not acceptable for radiological evaluation. A progressively growing new tumour nodule within a pre-existing tumour mass had to meet the following criteria to be considered as unequivocal evidence of progression on our modification to RECIST 1·1: (a) the lesion was at least 2 cm in size and definitively a new active GIST lesion (e.g. enhancing with contrast or other criteria to rule out artefact); or (b) the lesion had to be expanding on at least two sequential imaging studies. The blinded central radiology review was performed according to a prospectively agreed central imaging charter and conducted by an external imaging contract research organisation. Two readers reviewed the images. Adjudication was used when only one reader assessed a progression or when the date of progression was discordant between the two independent readers.
Secondary endpoints included overall survival (OS), time to progression (TTP), objective response rate (ORR), and disease control rate (DCR), defined as rate of complete response or partial response (PR) plus stable disease (SD) lasting for at least 12 weeks. Exploratory endpoints (not reported here) included health-related quality of life, pharmacokinetics, and biomarker evaluation.
Sample size
With 199 patients randomised, assuming a target treatment effect of 100% improvement in PFS, a randomisation ratio of 2:1 (regorafenib to placebo), a one-sided alpha of 0·01, and a power of 0·94, 144 events were needed for the final PFS analysis. A preplanned interim analysis of OS was performed at the time of the final PFS analysis.
Randomisation and masking
Patients were randomly assigned on a 2:1 basis to regorafenib or placebo using a computer-generated randomisation list prepared by the study sponsor (preallocated block design). Investigators received the randomisation number for each participant through an interactive voice response system (IVRS), which was also used to manage study drug supply.
Randomisation was stratified by (1) treatment line (failure of prior imatinib and sunitinib [“true third-line”] versus failure of prior imatinib, sunitinib, and other GIST therapies) and (2) geographical region (Asia versus rest of world).
Randomisation was blinded so that neither the patient, nor the investigator, nor the sponsor knew which agent was being administered. To maintain blinding, study medication was labelled with a unique drug pack number pre-printed on each bottle, which was assigned to the patient through the IVRS. Unblinding for individual patients could occur via the IVRS for emergencies; serious adverse events did not necessarily precipitate immediate unblinding.
Statistical analysis
Statistical analyses were performed using Statistical Analysis System software version 9·1 or higher (SAS Institute Inc, Cary, NC, USA).
The null hypothesis that both treatment arms would have the same PFS distribution was tested against the alternative hypothesis that the distribution of PFS times in the regorafenib arm would differ from that of the control arm.
PFS and OS estimates were calculated using the Kaplan–Meier method. Hazard ratios (HRs) and 95% confidence intervals (CIs) were derived from a Cox proportional hazard model with stratified log-rank test. ORR and DCR were analysed using the Cochran–Mantel–Haenszel test.
Role of funding source
The study sponsor (Bayer HealthCare) provided regorafenib and matching placebo, and collaborated with the principal investigator, G. D. Demetri, and an international steering committee of academic investigators on protocol design, data collection and interpretation, and preparation of this report. All logistical study operations were managed by the sponsor. Data were collected by the sponsor and analysed by the principal investigator, steering committee, and sponsor. All authors had full access to all data and vouch for the accuracy and completeness of the data presentation and analysis. The authors had final responsibility to submit for publication.
Results
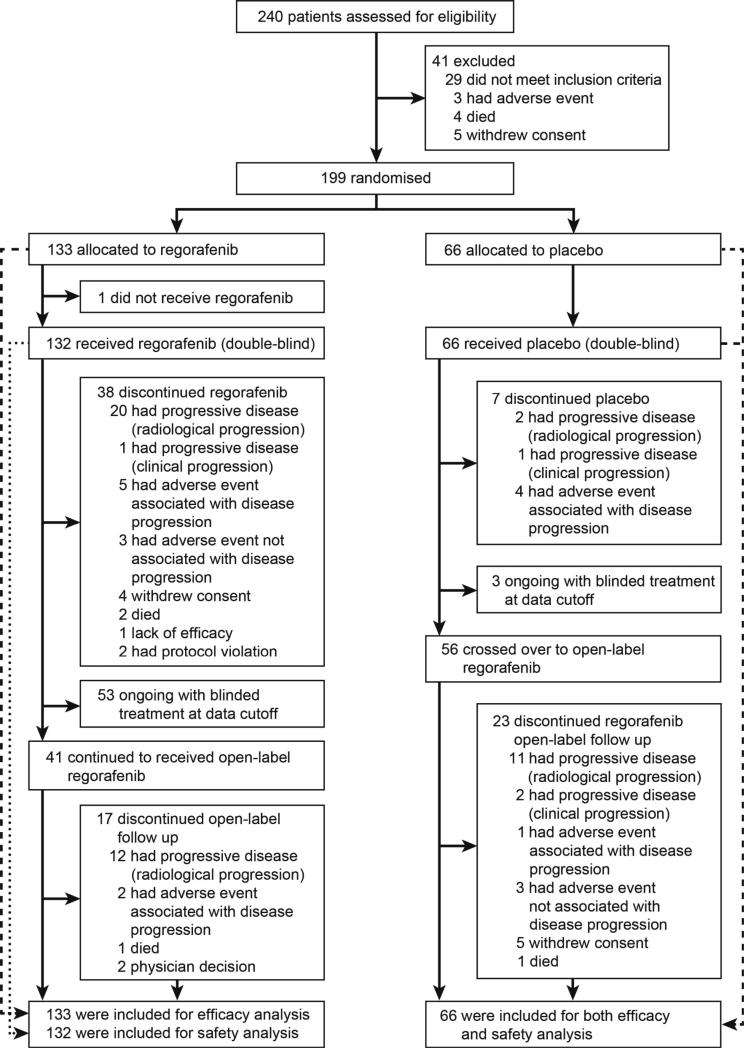
Between January and August, 2011, 240 patients were screened and 199 patients were randomised to receive regorafenib (n=133) or placebo (n=66; Figure 1). One patient randomised to the regorafenib group did not receive treatment. Baseline characteristics and previous treatments were similar between the two groups (Table 1); 193 of the 199 patients (97·0%) had previous disease progression while on both imatinib and sunitinib, with only six patients (3·0%) entered with a history of intolerance to imatinib. Notably, 86 of the 199 patients (43·2%) had received three or more prior lines of anticancer therapy for GIST.
Figure 1.
Trial profile
Table 1.
Baseline patient characteristics
| Regorafenib N=133 | Placebo N=66 | All N=199 | ||
|---|---|---|---|---|
| Age, median years (range) | 60 (18–82) | 61 (25–87) | 60 (18–87) | |
| Sex, n (%) | Male | 85 (63·9) | 42 (63·6) | 127 (63·8) |
| Female | 48 (36·1) | 24 (36·4) | 72 (36·2) | |
| Race, n (%) | White | 90 (67·7) | 45 (68·2) | 135 (67·8) |
| Black or African American | 0 | 1 (1·5) | 1 (0·5) | |
| Asian | 34 (25·6) | 16 (24·2) | 50 (25·1) | |
| Not reported or missing | 9 (6·8) | 4 (6·1) | 13 (6·5) | |
| ECOG performance status, n (%) | 0 | 73 (54·9) | 37 (56·1) | 110 (55·3) |
| 1 | 60 (45·1) | 29 (43·9) | 89 (44·7) | |
| Previous systemic anticancer therapy, n (%) | 2 lines | 74 (55·6) | 39 (59·1) | 113 (56·8) |
| >2 lines | 59 (44·4) | 27 (40·9) | 86 (43·2) | |
| Duration of previous imatinib therapy, n (%) | ≤6 months | 18 (13·5) | 4 (6·1) | 22 (11·1) |
| 6–18 months | 26 (19·5) | 7 (10·6) | 33 (16·6) | |
| >18 months | 89 (66·9) | 55 (83·3) | 144 (72·4) |
ECOG: Eastern Cooperative Oncology Group
Final analysis was done when the predetermined criteria of 144 PFS events was reached: 81 events among the 133 patients (60·9%) in the regorafenib group and 63 events among the 66 patients (95·5%) in the placebo group. During the double-blind period, 38 (28·6%) of the 133 patients in the regorafenib group and seven (10·6%) of the 66 patients in the placebo group discontinued study treatment (see Online Table 6). The most common reason for termination of study treatment was radiologically confirmed disease progression.
At the data cutoff (26 January 2012), 53 (39·8%) of the 133 patients in the regorafenib group and three (4·5%) of the 66 patients in the placebo group were still receiving double-blind treatment. A further 41 patients (30·8%) in the regorafenib group continued to receive open-label regorafenib after disease progression, and 24 (18·0%) were still receiving regorafenib at the time of analysis. In the placebo group, 56 patients (84·8%) crossed over to receive open-label regorafenib following progression, and 33 (50·0%) were still receiving treatment at data cutoff (see Online Table 6).
During the double-blind period, patients who were assigned to receive regorafenib had a median treatment duration of 22·9 weeks (mean 20·2 weeks), and patients who were assigned to receive placebo had a median treatment duration of 7·0 weeks (mean 9·1 weeks). The median daily dose during the double-blind treatment period was 146·8 mg for regorafenib-treated patients (mean 139·8 mg) and 160 mg for placebo recipients (mean 159·5 mg). In the regorafenib group, patients received 78·0% of the planned dose; in the placebo group, patients received 83·8% of the planned dose.
Efficacy
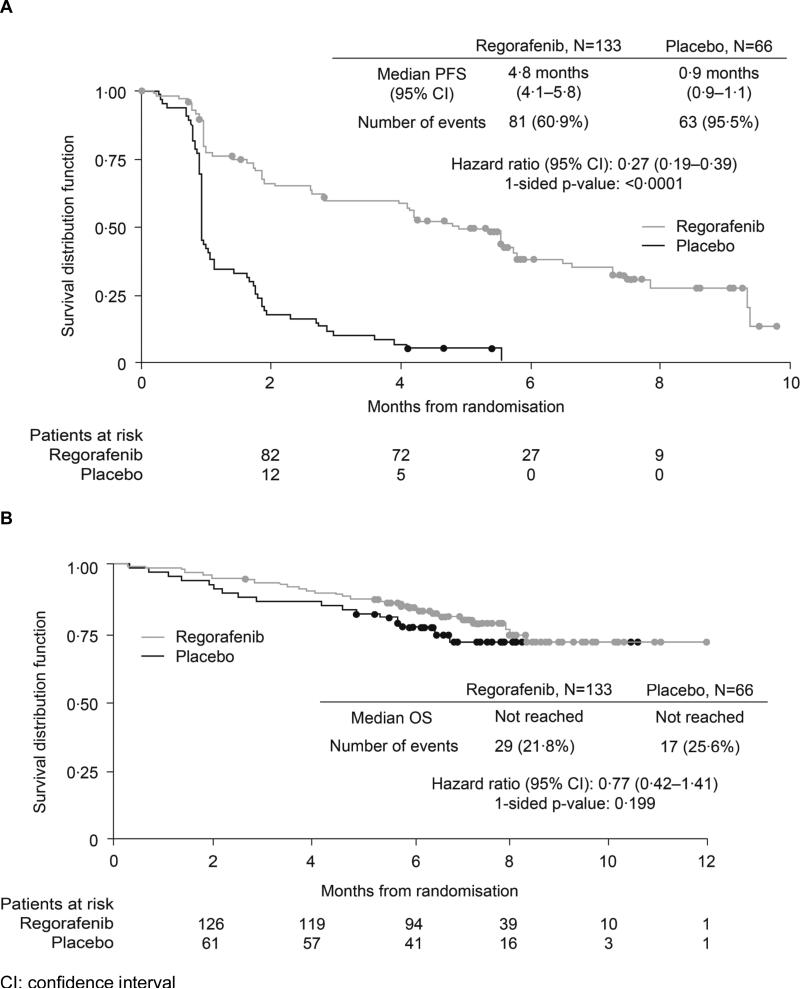
Median PFS was 4·8 months in the regorafenib group and 0·9 months in the placebo group, according to blinded central review (HR 0·27, 95% CI 0·19–0·39; p<0·0001; Figure 2A), meeting the primary endpoint of the study. PFS rates at 3 and 6 months were 60% and 38%, respectively, for regorafenib, and 11% and 0%, respectively, for placebo. Investigator assessment showed a median PFS of 7·4 months in the regorafenib group and 1·7 months in the placebo group (HR 0·22, 95% CI 0·14–0·35; p<0·001; see Online Figure 1). Median PFS for the 56 patients in the placebo group who crossed over to open-label regorafenib following progression was 5·0 months (per investigator assessment). There was no statistically significant difference in OS between the regorafenib and placebo groups (HR 0·77, 95% CI 0·42–1·41; p=0·199; Figure 2B).
Figure 2.
Survival following treatment with regorafenib or placebo. (A) Kaplan–Meier curves for progression-free survival (PFS) per central review (primary endpoint, final analysis). (B) Kaplan–Meier curves for overall survival (OS; interim analysis)
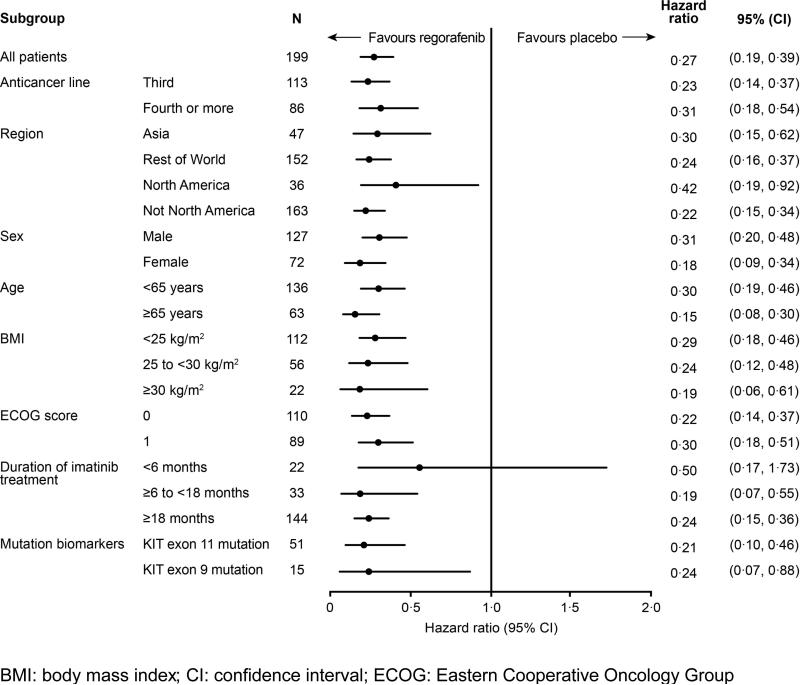
The impact of baseline factors on the treatment effect was analysed using a Cox proportional hazards model (Figure 3). The benefits of regorafenib on centrally assessed PFS were observed across all subgroups, including treatment line, duration of previous treatment, geographical region, age, ECOG performance status, and body mass index. Similar benefits of regorafenib were also observed in patients whose tumours harboured the two most common primary KIT mutations (exon 11 mutation, HR 0·212, 95% CI 0·098–0·458, n=51; exon 9 mutation, HR 0·239, 95% CI 0·065–0·876, n=15).
Figure 3.
Progression-free survival by subgroup
No patients in either group had a complete response, while six of the 133 patients in the regorafenib group and one of the 66 patients in the placebo group had a PR, giving ORRs of 4·5% and 1·5%, respectively. The rate of SD as best response (occurring at any time and for any duration) was 71·4% (95/133 patients) in the regorafenib group and 33·3% (22/66 patients) in the placebo group. The more clinically meaningful DCR (defined as the sum of rates of durable SD lasting for at least 12 weeks plus objective tumour response) was 52·6% (70/133 patients) and 9·1% (6/66 patients), respectively.
Safety
During the double-blind period, all 132 evaluable patients in the regorafenib group and 61 (92·4%) of the 66 patients in the placebo group experienced adverse events. Drug-related adverse events were reported in 130 (98·5%) of the 132 patients in the regorafenib group and 45 (68·2%) of the 66 patients in the placebo group (Table 2). Drug-related adverse events of grade 3 or higher were reported in 81 (61·4%) of the regorafenib-treated patients and nine (13·6%) of the placebo patients. The most common regorafenib-related adverse events of grade 3 or higher were hypertension (31/132 patients, 23·5%), hand–foot skin reaction (26/132 patients, 19·7%), and diarrhoea (7/132 patients, 5·3%).
Table 2.
Drug-related adverse events in ≥10% of patients during double-blind treatment period
| Regorafenib |
Placebo |
|||||
|---|---|---|---|---|---|---|
| Adverse event | Any grade n (%) | Grade 3 n (%) | Grade 4 n (%) | Any grade n (%) | Grade 3 n (%) | Grade 4 n (%) |
| Any event | 130 (98·5) | 77 (58·3) | 2 (1·5) | 45 (68·2) | 5 (7·6) | 1 (1·5) |
| Hand–foot skin reaction | 74 (56·1) | 26 (19·7) | 0 | 9 (13·6) | 0 | 0 |
| Hypertension | 64 (48·5) | 30 (22·7) | 1 (0·8) | 11 (16·7) | 2 (3·0) | 0 |
| Diarrhoea | 53 (40·2) | 7 (5·3) | 0 | 3 (4·5) | 0 | 0 |
| Fatigue | 51 (38·6) | 3 (2·3) | 0 | 18 (27·3) | 0 | 0 |
| Oral mucositis | 50 (37·9) | 2 (1·5) | 0 | 5 (7·6) | 1 (1·5) | 0 |
| Alopecia | 31 (23·5) | 2 (1·5) | 0 | 1 (1·5) | 0 | 0 |
| Hoarseness | 29 (22·0) | 0 | 0 | 3 (4·5) | 0 | 0 |
| Anorexia | 27 (20·5) | 0 | 0 | 5 (7·6) | 0 | 0 |
| Rash, maculopapular | 24 (18·2) | 3 (2·3) | 0 | 2 (3·0) | 0 | 0 |
| Nausea | 21 (15·9) | 1 (0·8) | 0 | 6 (9·1) | 1 (1·5) | 0 |
| Constipation | 20 (15·2) | 1 (0·8) | 0 | 4 (6·1) | 0 | 0 |
| Myalgia | 18 (13·6) | 1 (0·8) | 0 | 6 (9·1) | 0 | 0 |
| Voice alteration | 14 (10·6) | 0 | 0 | 2 (3·0) | 0 | 0 |
Serious adverse events were reported in 38 (28·8%) of 132 patients in the regorafenib group and 14 (21·2%) of 66 patients in the placebo group during the double-blind phase. Although dose modifications (see Online Tables 2–5) were more frequent in the regorafenib group (95/132 patients, 72·0%, vs 17/66 patients, 25·8%, in the placebo group), the incidence of adverse events that led to permanent discontinuation of treatment was similar between the groups (6·1%, 8/132 patients on regorafenib; 7·6%, 5/66 patients on placebo), indicating that adverse events were largely manageable by dose modification without the need to discontinue treatment in most cases. Grade 5 adverse events were reported in seven (5·3%) of the 132 patients in the regorafenib group and three (4·5%) of the 66 patients in the placebo group. In three patients, the grade 5 adverse events were considered by the investigator to be drug-related: two (1·5%) in the regorafenib group (cardiac arrest and hepatic failure) and one (1·5%) in the placebo group (fatigue).
Discussion
GIST is now recognised as the most common sarcoma subtype.31 Elucidation of GIST molecular pathogenesis has allowed rational translation of basic science into clinical therapies targeting the root cause of the disease, usually KIT or PDGFRA mutations. Inhibiting these driver mutations has improved disease control, leading to increased survival of patients with GIST.10,11,26 Despite these advances, only two TKIs, imatinib and sunitinib, have been proven to be clinically beneficial to GIST patients, and resistance to these agents eventually leads to disease progression and death in the majority of patients with advanced GIST. Several other structurally distinct inhibitors of KIT and PDGFRα kinases have been developed, but, despite promise in controlling TKI-resistant disease in early-phase trials, none has demonstrated proven benefit in prospective phase 3 trials.32,33
Regorafenib is a structurally unique small molecule with differential kinase binding and pharmacological properties from TKIs currently approved for GIST. Following encouraging data from preclinical models and an exploratory phase 2 clinical trial, this phase 3 GRID trial was designed to assess definitively the safety and efficacy of regorafenib in patients with advanced GIST with progressive disease refractory to both imatinib and sunitinib. The results of the study show that, when added to best supportive care, regorafenib significantly improves PFS in a population of patients with progressive disease following failure of all approved prior therapies, compared with matching placebo. Median PFS with regorafenib was more than five times that with placebo, reducing the risk of progression or death by 73%. While, in theory, the regorafenib group could have included patients with more indolent disease, we feel that the robust results argue against any such confounding influence of disease-specific variables and instead represent evidence of regorafenib activity to arrest disease progression. Crossover to open-label regorafenib was allowed following objective progression, leading to 56 (85%) of the 66 placebo patients accessing regorafenib, which could have confounded any potential difference in OS between groups.
Similar to other effective kinase inhibitors in TKI-resistant disease, regorafenib did not induce high rates of objective tumour response per modified RECIST.26 However, DCR (defined as the sum of objective tumour response plus SD for at least 12 weeks) was higher in regorafenib-treated patients (52·6%, 70/133 patients) than in placebo-treated patients (9·1%, 6/66 patients), suggesting that regorafenib was associated with clinically meaningful tumour control in patients with advanced GIST following failure of all other approved TKI therapies.
Efficacy analysis in prespecified subgroups demonstrated robustness in the benefit of regorafenib over placebo in nearly all subgroups. In particular, regorafenib had a similar benefit over placebo for patients receiving treatment either as third line or as fourth or later line of therapy. This result indicates that regorafenib can achieve therapeutic benefit independent of prior treatment regimens. A possible explanation is that regorafenib targets multiple disease pathways, which may block resistance mechanisms.28
The safety profile of regorafenib in this study was similar to that observed in previous clinical trials.29,30 Regorafenib dosing was reasonably well tolerated with the predefined rules for dose modification (dose delays or reductions, with an option to dose escalate again based on tolerability; see Online Tables 2–5). Adverse events leading to permanent treatment discontinuation were comparable in the two study arms. The most common drug-related adverse events in the regorafenib group were hypertension, hand–foot skin reaction, and diarrhoea. Drug-related grade 3 or higher hypertension was reported in 31 (23·5%) of 132 regorafenib-treated patients and, similarly to other therapies targeting the VEGF/VEGFR pathway,26,32 is likely related to antiangiogenic effects. This adverse event could be managed with dose modification and appropriate antihypertensive intervention. Drug-related hand–foot skin reaction occurred in 74 of (56·1%) of the 132 patients treated with regorafenib (26 patients, 19·7%, with grade 3 events). This skin condition is also commonly associated with other multitargeted kinase inhibitors.26,32 In GRID, this adverse event was generally manageable using dose modifications and proper care of the affected skin area.
Future studies of regorafenib in GIST will investigate further the molecular mechanisms by which the treatment can induce disease control after failure of both imatinib and sunitinib. Specifically, predictive tumour biomarkers will be studied in an effort to correlate molecular subtypes of the disease with regorafenib activity. Increased understanding of the key pathways involved in successful treatment of GIST refractory to both imatinib and sunitinib may provide new insight into mechanisms of resistance to molecularly targeted therapies.
In conclusion, regorafenib significantly improved PFS compared with placebo in patients with advanced GIST who experienced disease progression following treatment with at least imatinib and sunitinib or were unable to tolerate those agents. This is the first small-molecule kinase inhibitor to provide positive phase 3 efficacy data in patients with metastatic GIST resistant to other kinase-targeted therapies.
Supplementary Material
Panel: Research in context.
Systematic review
We searched PubMed articles added since 2010, the abstracts of relevant oncology congresses (American Society of Clinical Oncology [ASCO] annual meeting, ASCO Gastrointestinal Cancer Symposium, Connective Tissue Oncology Society conference, European Multidisciplinary Cancer Congress, European Society for Medical Oncology conference, Molecular Markers in Cancer, Molecular Targets and Cancer Therapeutics, Targeted Anticancer Therapies, and the World Congress on Gastrointestinal Cancers) and ClinicalTrials.gov. For the literature and congress searches, we used MeSH and full-text search terms for metastatic or unresectable gastrointestinal stromal tumours (GISTs). We restricted the ClinicalTrials.gov search to agents identified in the literature and congress searches, including inhibitors of KIT, PDGFRα, heat-shock protein 90, mammalian target of rapamycin, RAF, VEGF, and Abl kinases. However, two agents failed to demonstrate clinical activity in phase 3 trials (nilotinib and retaspimycin), while others have not entered phase 3 trials in this specific indication (e.g. everolimus, masitinib, motesanib, sorafenib, vatalanib, dasatanib, ganetespib, and pazopanib). Our discussion reflects the lack of available or investigational agents for patients with metastatic GIST who require further treatment beyond imatinib and sunitinib.
Interpretation
Our work is a translation of the evolving scientific understanding of aberrant intracellular signalling in GIST. Since 1998, it has been recognised that most GIST lesions have constitutively activated signalling through either the KIT or PDGRFA kinase pathways. The current treatment options for patients with metastatic GIST are limited to only two kinase-inhibiting drugs, imatinib and sunitinib. Once a patient has disease progressing despite therapy with these two agents, there are currently no therapeutic options of proven efficacy. Our translational science has previously indicated that structurally distinct kinase-inhibiting agents with novel activities may overcome resistance to imatinib and sunitinib, and thereby offer clinical benefits to patients with this life-threatening disease. Following promising clinical phase 2 evidence of antitumour activity of regorafenib in pretreated patients, this international, randomised, placebo-controlled phase 3 trial demonstrates that oral regorafenib can indeed provide a significant progression-free survival benefit over placebo in patients with pretreated, progressive metastatic GIST. The study confirms that drug-resistant GIST remains an oncogene-addicted disease that can be therapeutically targeted by new structural inhibitory attacks on the pathogenic mutated kinase. To our knowledge, this is the first clinical trial to show benefit from a kinase inhibitor following objective resistance to two prior kinase-inhibiting therapies in a disease driven by an oncogenic kinase mechanism.
Acknowledgements
We would like to thank the participating patients and staff at each of the study centres. The trial was supported by Bayer HealthCare Pharmaceuticals. Administrative and minor editorial assistance in the preparation of this manuscript was provided by Succinct Healthcare Communications, with financial support from Bayer HealthCare Pharmaceuticals; the authors retained complete editorial control over the content.
Additional support for this work has been provided to GDD from the following sources: The Virginia and Daniel K. Ludwig Trust for Cancer Research, Team Russo, Paul's Posse and the Pan Mass Challenge, and Gastrointestinal Cancer SPORE Grant 1P50CA127003-05 from the US National Cancer Institute.
Funding Bayer HealthCare Pharmaceuticals as well as additional support to GDD from the following sources: The Virginia and Daniel K. Ludwig Trust for Cancer Research, Team Russo, Paul's Posse and the Pan Mass Challenge, and Gastrointestinal Cancer SPORE Grant 1P50CA127003-05 from the US National Cancer Institute.
Footnotes
Contributors
GDD, IK, DL, HJ, PR, J-YB, PC, Y-KK contributed to trial conception and design. All authors contributed to data collection. GDD, PC, PR, Y-KK, J-YB, HJ, CK, IK, DL, JC, RM contributed to data analysis and interpretation. All authors reviewed the manuscript and agreed on submission to The Lancet.
GRID lead investigators
AUSTRIA: Hellmut Samonigg, Thomas Brodowicz, Wolfgang Eisterer; BELGIUM: Patrick Schöffski; CANADA: Martin Blackstein, Karen Mulder, Jawaid Younus; CHINA: Jin Li, Shukui Qin, De Sen Wan, Jianming Xu; FINLAND: Heikki Joensuu; FRANCE: Jean-Yves Blay, Binh Bui Nguyen, Antoine Adenis, Axel Le Cesne; GERMANY: Peter Reichardt, Jens Chemnitz, Sebastian Bauer, Peter Hohenberger, Viktor Grünwald, Frank Mayer, Jochen Schütte; ISRAEL: Ofer Merimsky; ITALY: Paolo Casali, Guido Biasco, Massimo Aglietta, Giuseppe Badalamenti; JAPAN: Toshihiko Doi, Tatsuo Kanda, Toshirou Nishida, Yasuhide Yamada, Yoshito Komatsu, Akira Sawaki; NETHERLANDS: Hans Gelderblom, Winette Van der Graaf; POLAND: Piotr Rutkowski; SINGAPORE: Richard Quek; SOUTH KOREA: Yoon-Koo Kang, Hyuk Chan Kwon, Seock-Ah Im, Joon Oh Park, Sun Young Kim; SPAIN: Claudia M Valverde Morales, Xavier Garcia Del Muro; UK: Ian Judson, Michael Leahy, Anne Thomas; USA: George Demetri, Mary Louise Keohan, Michael Heinrich, Margaret von Mehren, Robin Jones, Bruce Brockstein, Pamela Kaiser, Keith Skubitz, Michael Gordon
Conflict of interest
GDD has served as scientific adviser/consultant to Novartis, Pfizer, Lilly, Infinity, GlaxoSmithKline, Plexxikon, Kolltan, and Blueprint Medicines. PReichardt sits on advisory boards for and has received honoraria from Novartis, Pfizer, and Bayer. JYB received compensation from Bayer to serve as a member of the GRID steering committee. PRutkowski has received honoraria and travel grants from Novartis and Pfizer and has served as an advisory board member for Novartis. MvM has served as a scientific adviser to Novartis and Pfizer. ALC has received honoraria received from Novartis, Pfizer, and Pharmamar. PS has been a member of speaker bureaus and received grants for translational and clinical research for Novartis, Pfizer, and Bayer. RGM has provided consultancy for Bayer. SB has received honoraria from Novartis and Pfizer and research support from Novartis. TN has received research funding from Novartis, sits on an advisory board for Novartis, and has received honoraria for speaking from Novartis and Pfizer. CK, JC, DL, and IK are employees of Bayer, and CK owns 34 shares in Bayer. All other authors declare no conflicts of interest.
References
- 1.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8:S1–41. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joensuu H. Adjuvant treatment of GIST: patient selection and treatment strategies. Nat Rev Clin Oncol. 2012;9:351–58. doi: 10.1038/nrclinonc.2012.74. [DOI] [PubMed] [Google Scholar]
- 3.Edmonson JH, Marks RS, Buckner JC, Mahoney MR. Contrast of response to dacarbazine, mitomycin, doxorubicin, and cisplatin (DMAP) plus GM-CSF between patients with advanced malignant gastrointestinal stromal tumors and patients with other advanced leiomyosarcomas. Cancer Invest. 2002;20:605–12. doi: 10.1081/cnv-120002485. [DOI] [PubMed] [Google Scholar]
- 4.Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33:466–77. doi: 10.1053/hupa.2002.124122. [DOI] [PubMed] [Google Scholar]
- 5.Caram MV, Schuetze SM. Advanced or metastatic gastrointestinal stromal tumors: systemic treatment options. J Surg Oncol. 2011;104:888–95. doi: 10.1002/jso.21930. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–49. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 7.Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–80. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–10. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 9.Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:865–78. doi: 10.1038/nrc3143. [DOI] [PubMed] [Google Scholar]
- 10.Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–80. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 11.Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–34. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- 12.Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373:1097–104. doi: 10.1016/S0140-6736(09)60500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265–72. doi: 10.1001/jama.2012.347. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase 3 Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360–67. doi: 10.1200/JCO.2008.17.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Debiec-Rychter M, Sciot R, Le Cesne A, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42:1093–103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 16.Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res. 2005;11:4182–90. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 17.Chen LL, Trent JC, Wu EF, et al. A missense mutation in KIT kinase domain 1 correlates with imatinib resistance in gastrointestinal stromal tumors. Cancer Res. 2004;64:5913–19. doi: 10.1158/0008-5472.CAN-04-0085. [DOI] [PubMed] [Google Scholar]
- 18.Debiec-Rychter M, Cools J, Dumez H, et al. Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology. 2005;128:270–79. doi: 10.1053/j.gastro.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216:64–74. doi: 10.1002/path. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinrich MC, Corless CL, Blanke CD, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24:4764–74. doi: 10.1200/JCO.2006.06.2265. [DOI] [PubMed] [Google Scholar]
- 21.Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471–78. [PubMed] [Google Scholar]
- 22.Mendel DB, Laird AD, Xin X, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- 23.O'Farrell AM, Abrams TJ, Yuen HA, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 24.Osusky KL, Hallahan DE, Fu A, Ye F, Shyr Y, Geng L. The receptor tyrosine kinase inhibitor SU11248 impedes endothelial cell migration, tubule formation, and blood vessel formation in vivo, but has little effect on existing tumor vessels. Angiogenesis. 2004;7:225–33. doi: 10.1007/s10456-004-3149-y. [DOI] [PubMed] [Google Scholar]
- 25.Broutin S, Ameur N, Lacroix L, et al. Identification of soluble candidate biomarkers of therapeutic response to sunitinib in medullary thyroid carcinoma in preclinical models. Clin Cancer Res. 2011;17:2044–54. doi: 10.1158/1078-0432.CCR-10-2041. [DOI] [PubMed] [Google Scholar]
- 26.Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–38. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 27.Gajiwala KS, Wu JC, Christensen J, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A. 2009;106:1542–47. doi: 10.1073/pnas.0812413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245–55. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 29.Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:2658–67. doi: 10.1158/1078-0432.CCR-11-1900. [DOI] [PubMed] [Google Scholar]
- 30.George S, Wang Q, Heinrich MC, et al. Efficacy and safety of regorafenib in patients with metastatic and/or uresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol. 2012;30:2401–07. doi: 10.1200/JCO.2011.39.9394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ducimetière F, Lurkin A, Ranchère-Vince D, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6:e20294. doi: 10.1371/journal.pone.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demetri GD. Differential properties of current tyrosine kinase inhibitors in gastrointestinal stromal tumors. Semin Oncol. 2011;38(suppl 1):S10–9. doi: 10.1053/j.seminoncol.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Reichardt P, Blay JY, Gelderblom H, et al. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol. 2012;23:1680–87. doi: 10.1093/annonc/mdr598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.