Abstract
Human metapneumovirus (hMPV) is a common cause of lung and airway infections in infants and young children. Recently, we and others have shown that hMPV infection induces Toll-like receptor (TLR)-dependent cellular signaling. However, the contribution of TLR-mediated signaling in host defenses against pulmonary hMPV infection and associated disease pathogenesis has not been elucidated. In this study, mice deficient in MyD88, a common adaptor of TLRs, was used to investigate the contribution of TLRs to in vivo pulmonary response to hMPV infection. MyD88−/− mice have significantly reduced pulmonary inflammation and associated disease compared with wild-type (WT) C57BL/6 mice after intranasal infection with hMPV. hMPV-induced cytokines and chemokines in bronchoalveolar lavage fluid (BALF) and isolated lung conventional dendritic cells (cDC) are also significantly impaired by MyD88 deletion. In addition, we found that MyD88 is required for the recruitment of DC, T cells, and other immune cells to the lungs, and for the functional regulation of DC and T cells in response to hMPV infection. Taken together, our data indicate that MyD88-mediated pathways are essential for the pulmonary immune and pathogenic responses to this viral pathogen.
Keywords: hMPV, MyD88, Cytokines/chemokines, Pulmonary immune response
1. Introduction
Human metapneumovirus (hMPV) belongs to the Paramyxovirus family of negative single stranded, non-segmented RNA viruses. It is a common cause of lung and airway infections in infants and young children. Most infants have had this infection by age 5 (Vicente, Cilla et al. 2003;Crowe, Jr. and Williams, 2003;Falsey, Erdman et al. 2003). However, repeated infections with hMPV occur throughout life, suggesting insufficient innate and/or adaptive immune responses (Williams, Wang et al. 2006). Currently, there is a paucity of knowledge on host molecules that contribute to the overall immune response to hMPV infection.
We and others have shown that several pattern recognition receptors (PRRs) mediate hMPV-induced innate immune responses. Examples include the retinoic-acid-inducible protein I (RIG-I) in airway epithelial cells (Liao, Bao et al. 2008), the helicase melanoma differentiation-associated gene 5 (MDA5) and toll-like receptor (TLR)-4 in human monocyte-derived dendritic cells (MoDC) and mouse bone marrow-derived DC (BMDC) (Banos-Lara, Ghosh et al. 2012;Kolli, Bao et al. 2011), and TLR-7 in plasmacytoid DC (pDC) (Goutagny, Jiang et al. 2010). However, the contribution of the above described PRR-mediated signaling to hMPV-induced host immunity and disease pathogenesis has not been elucidated and confirmed in vivo. Myeloid differentiation primary response gene (88) (MyD88) is essential for signaling via all TLRs with the exception of TLR3 (Kawai and Akira, 2006b). It is vital to combat a wide array of pathogens both in human and in experimental mouse models (Isnardi, Ng et al. 2008;Picard, von et al. 2010;von, Picard et al. 2012). Following the activation of TLRs, MyD88 binds to the TLRs via its Toll-interleukin-1 receptor (TIR) domain, then recruits interleukin-1 receptor-associated kinase (IRAK)-4 to the receptor (Lin, Lo et al. 2010), mediating the activation of various transcription factors, including IRF5 and IRF7, AP-1 and NF-κB. This activation depends in part on the cell type and the cell surface receptor (Kawai and Akira, 2005). In this study, we used mice deficient in MyD88 as a model to investigate the contribution of TLRs to pulmonary response to hMPV infection.
Following the initial innate immune response to viral infection, activation of adaptive responses is driven by DC (Banchereau and Steinman, 1998;Banchereau, Briere et al. 2000). Upon the activation by viral pathogens through PRRs, DC upregulate the expression of costimulatory molecules, major histocompatibility complex (MHC), and/or immune mediators. Activated DC then use these molecules to prime T cells to shape adaptive immune responses. It is commonly known that CD4+ and CD8+ T cells are two major types of T cells that play a significant role in mediating adaptive immune responses to viral infection. In addition to their commonly known role, these two cell types have been shown to control hMPV eradication, clinical disease and lung pathology during primary infection (Kolli, Bataki et al. 2008). We found that MyD88 contributes to hMPV-induced pulmonary inflammation and associated disease. Following hMPV infection, MyD88 is essential for the production of IFN-β and inflammatory cytokines, both in bronchoalveolar lavage fluid (BALF) and in isolated lung conventional DC (cDC). MyD88 also controls the lung infiltration of DC, neutrophils, macrophages, and T cells in response to hMPV infection. In addition to its role in mediating innate immune response, MyD88 is critical to initiating T cell responses, thereby modulating adaptive immune response as well.
2. Materials and Methods
2.1. hMPV preparation and titer determination
The Canadian isolate hMPV 97-83 were propagated in LLC-MK2 cells at 35°C in the absence of serum and in the presence of 1 µg of trypsin/ml (Worthington, Lakewood, NJ), and were sucrose purified, as previously described (Bao, Liu et al. 2008;Bao, Sinha et al. 2008;Junping Ren, 2012). Viral titer of purified hMPV was determined by immunostaining in LLC-MK2 cells, as previously described (Bao, Liu et al. 2008;Bao, Sinha, et al. 2008;Junping Ren, 2012). Viral titers of infected lung were determined by immunostaining as well. In brief, tissue samples were homogenized in 1 ml of Dulbecco's modified Eagle's medium and centrifuged twice at 10,000× g for 1 min at 4°C. Serial two-fold dilutions of the supernatant were applied onto LLC-MK2 cells. Two days later, LLC-MK2 cells were fixed and lung titers were then determined by immunostaining.
2.2. Mice
MyD88−/− mice were kindly provided by Dr. Richard Flavell (Howard Hughes Medical Institute, Yale University School of Medicine, New Haven) and Dr. Shizuo Akira (Osaka University, Japan). They were bred to the B6 background by backcrossing for 10 successive generations and then housed under specific pathogen-free conditions at the University of Texas Medical Branch. Age- and sex-matched control wild-type mice were purchased from the Jackson Laboratory (The Jackson Laboratory, Bar Harbor, Maine). Mice were anesthetized and infected intranasally (i.n.) with 107 PFU of hMPV diluted in Dulbecco phosphate-buffered saline (D-PBS) (Invitrogen, Grand Island, NY) as described (Guerrero-Plata, Casola et al. 2005). As mock treatment, mice were inoculated with an equivalent volume of sucrose diluted in D-PBS.
2.3. Bronchioalveolar lavage (BAL) analysis
At different days p.i., BALF was collected by washing the lungs two times with 1 ml of D-PBS. An aliquot of BAL samples was used to determine total-cell counts and counts of different types of cells as described elsewhere (Guerrero-Plata, Baron et al. 2005;Kolli, Bataki et al. 2008). IFN-β and other cytokines/chemokines from BAL sample aliquots were determined by ELISA (PBL, Piscataway, NJ) and Luminex-based Bio-Plex system (Bio-Rad Laboratories, Hercules, CA) respectively, according to the manufacturer’s instructions.
2.4. Real-time PCR
Total lung RNAs were extracted using TRIzol reagents (Invitrogen). First strand cDNA was synthesized by using TaqMan RT reagents (ABI, Carlsbad, CA). The RT reaction was performed under the following conditions: 25 °C, 10 minutes; 48 °C, 30 minutes; 95 °C, 5 minutes. Quantitative RT-PCR amplification was performed by using SYBR. Sequence information on green-labeled primers for target genes and internal control 18S rRNA is available upon request. Quantitative PCR reactions were performed with the FastStart Universal SYBR Green Master (ROX) (Roche, San Francisco, CA) in the ABI 7500 Sequence Detection System using following conditions: initial steps: 50 °C, 2 minutes and 95 °C, 10 minutes; PCR steps: 95 °C, 15 seconds and 60 °C, 1 minute for 40 cycles.
2.5 Airway obstruction and hyperresponsiveness (AHR)
AHR was assessed in unrestrained mice using whole-body barometric plethysmography (Buxco, Troy, NY) to record enhanced pause (Penh), as previously described (Kolli, Bataki, Spetch,et al. 2008;Castro, Guerrero-Plata et al. 2006). Penh is a dimensionless value that represents a function of the ratio of peak expiratory flow to peak inspiratory flow and a function of the timing of expiration. Penh has previously been validated in animal models of AHR and infection-associated airway obstruction (Hamelmann, Schwarze et al. 1997; Hamelin, Prince et al. 2006;van Schaik, Enhorning et al. 1998). Respiratory activity was recorded for 4 min in order to establish baseline Penh values. Mice were subsequently exposed to increasing doses of nebulized methacholine (3, 12, and 25 mg/ml) for 1.5 min, and data were recorded for another 3 min.
2.6. Fluorescence-activated cell sorter (FACS) analysis of leukocytes in lungs
Lungs were harvested at different time points up to 8 days after mock or hMPV infection as described (Guerrero-Plata, Kolli et al. 2009). In brief, mice were anesthetized, followed by lung excision. The lungs were then cut into small pieces and incubated with collagenase A (0.5 mg/ml PBS; Sigma-Aldrich, St. Louis, MO) and type IV bovine pancreatic DNase (20 µg/ml PBS; Sigma-Aldrich) for 1 h at 37°C. After digestion, lung cells were dispersed by shearing through a 20-gauge needle, followed by filtration through a nylon screen cell strainer (100 µm). Single cell suspensions were washed and contaminating erythrocytes were lysed using ACK lysis buffer (Invitrogen). Isolated lung cells were incubated with anti-FcγRII/FcγRIII mAb (24G2; BD Biosciences, San Diego, CA). For cell surface marker staining, an aliquot of cells was stained with the following anti-mouse antibodies: anti-CD11c, anti-I-A/I-E (MHC-II), anti-CD11b, (all from BD-Pharmingen, San Diego, CA) and anti-mPDCA1 (Miltenyi Biotec, Auburn, CA). In a separate aliquot of samples, cells were stained with anti-CD11c, anti-MHC-II in combination with anti-CD80 and anti-CD86, or stained with a combination of antibodies: anti-CD3, anti-CD4 and anti-CD8. Samples were stained at 4°C in PBS with 1% FBS and analyzed with a FACS Canto flow cytometer equipped with BD FACSDiva software (both from Becton Dickinson Immunocytometry Systems, San Jose, CA). Analysis was performed using WinMDI2.8 (Scripps, La Jolla, CA).
2.7. CD8+ T cell analysis
Intracellular cytokine staining was performed as described (Wang, Welte et al. 2008). In brief, lung cells were isolated on day 8 after mock or hMPV infection and then stimulated at 2.5×106 cells/ml with 50 ng/ml PMA and 500 ng/ml ionomycin (Sigma-Aldrich) or an hMPV-derived peptide (VGALIFTKL, 10 µg/ml) or its control, RSV-derived peptide (NAITNAKII) for 5 h at 37°C (Rutigliano, Rock et al. 2005;Herd, Mahalingam et al. 2006). Golgi Stop (BD Biosciences) was added during the final 3.5 h. The cells were then harvested, stained with anti-CD3 and -CD8 antibodies, and fixed in 2% paraformaldehyde. The cells were then stained for IFN-γ using the Becton Dickinson fix/perm reagent according to the manufacturer's instructions. Samples were run on a Becton Dickinson FACS Canto flow cytometer equipped with BD FACSDiva software. Analysis was performed using WinMDI2.8 (Scripps, La Jolla, CA).
Cytotoxicity assays were performed as described (Rutigliano, Rock et al. 2005). EL4 targets (a gift from Dr. Gregg Milligan, University of Texas Medical Branch) were incubated with either RSV- or hMPV-derived peptides (10 µg/ml) overnight. hMPV peptide-loaded targets were then labeled with 5 µM carboxyfluorescein diacetate succinimidyl ester (CFSE) and control targets with 0.5 µM CFSE before mixing them at a 1:1 ratio. Mixed targets were then incubated at the indicated effector/target (E/T) ratios with enriched lung CD8+ T cells from mice infected with hMPV for 8 days. Cells were incubated at 37°C for 4 h and then were analyzed by flow cytometry. Percent specific lysis was calculated as 100 − (100 × Percent CFSEhigh [at E/T of 20)/Percent CFSEhigh [at 0:1]).
2.8. Statistical analysis
Statistical significance was determined by ANOVA with a Student-Neu-man-Kuhl posttest, Values of P<0.05 were considered significant (GraphPad Instat Software, Inc., San Diego, CA). Values for phenotype analysis, viral replication, and cytokine production experiments were presented as Mean ± standard error of the mean (SEM).
3. Results
3.1. MyD88 mediates hMPV-induced clinical disease
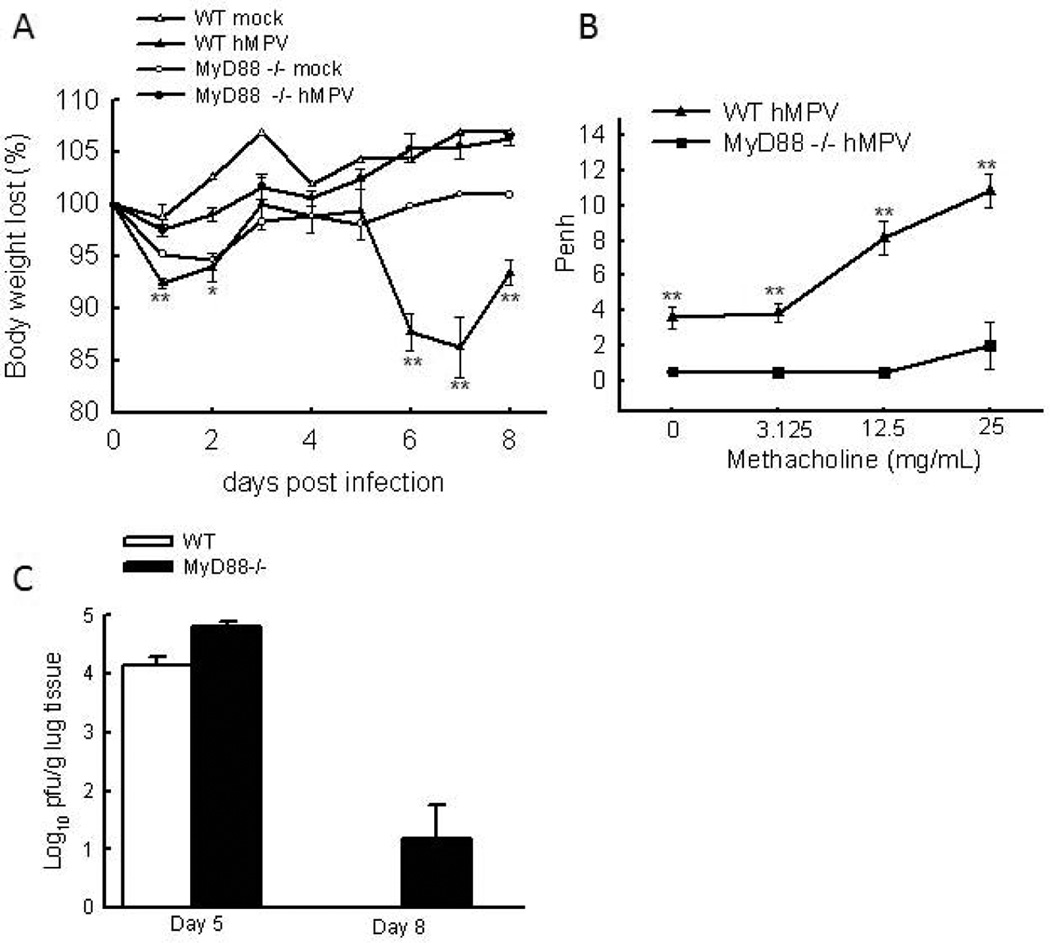
During infection, TLRs play an important role in triggering immune response and associated pathophysiology (Aderem and Ulevitch, 2000;Akira, Uematsu et al. 2006;Akira and Takeda, 2004;Kawai and Akira, 2006a;Takeda and Akira, 2005). Among TLRs, TLR4 and TLR7are shown to be involved in the response of cDC and pDC to hMPV stimulation, respectively (Kolli, Bao et al. 2011;Goutagny, Jiang et al. 2010). However, the role of TLRs in regulating hMPV-induced host immunity has not been confirmed in vivo. In this study, mice deficient in MyD88 was used to investigate the contribution of TLRs to pulmonary response to hMPV infection, as MyD88 is the most crucial adaptor in TLR signaling (except for TLR-3)(Liew, Xu et al. 2005). To test whether MyD88 is essential in generating an appropriate pulmonary response, we first compared disease phenotype in WT and MyD88−/− mice infected with hMPV. WT and MyD88−/− mice were inoculated i.n. with hMPV (107 PFU/mouse), UV-inactivated hMPV, or mock medium. WT mice showed modest body weight loss at an initial phase (days 1 and 2) and rapid recovery at day 3. hMPV-infected WT mice consistently showed significant body weight loss starting around day 5, with a peak at days 7 and a recovery starting at day 8 (Fig. 1A), consistent with what was reported previously (Guerrero-Plata, Kolli et al. 2009;Kolli, Bataki et al. 2008). Mice inoculated with UV-inactivated hMPV did not show significant body weight loss and behaved similarly to the mock-infected control group (data not shown). Compared to hMPV-infected WT mice, hMPV-infected MyD88−/− mice did not experience body weight loss (Fig. 1A), suggesting milder illness in hMPV-infected MyD88−/− mice.
Figure 1. The effect of MyD88 in disease severity and lung viral replication in hMPV-infected mice.
(A) WT and MyD88−/− mice were infected i.n. with 1×107 PFU of hMPV or mock-infected, then monitored daily and body weight is expressed as a percentage of baseline weight. n=six mice/group. (B) Lung function in hMPV-infected mice, either WT or MyD88, at day 7 p.i. was assayed by post-methacholine challenge. Penh values were determined by unrestrained plethysmography. n=6 mice/group. (C) Total lung was harvested at indicated time points p.i., and lung infectious viral particles were titrated on LLC-MK2 cell monolayers by methylcellulose plaque assay. n = 3–5 mice/group. The results are representative of 2–4 independent experiments. Asterisks indicate levels of significance, *, P < 0.05 and **, P < 0.01 for comparison to hMPV-infected samples from MyD88−/− mice.
Next, we investigated the role of MyD88 in the pulmonary function of mice by comparing the AHR of infected WT and MyD88−/− mice. AHR was assessed in unrestrained mice using whole-body barometric plethysmography to record enhanced pause (Penh), as previously described (Li, Gottwein et al. 2011;Castro, Guerrero-Plata et al. 2006). Since the increase in Penh values in hMPV-infected mice correlates with body weight loss and clinical disease severity (van Schaik, Enhorning et al., 1998), we measured the Penh values in WT- and MyD88−/− mice. As shown in Fig. 1B, hMPV-infected WT mice had a dose-dependent increase in AHR in response to aerosolized methacholine. At each dose, WT-infected mice exhibited higher Penh than MyD88−/− mice, suggesting that WT-infected mice had severer pulmonary dysfunction than MyD88−/−-infected mice. The mice without hMPV infection had similar response to methacholine challenge as infected MyD88−/− mice (data not shown).
It has been previously shown that hMPV replication in the lung starts around day 2 with a peak viral titer by day 4 to 5. p.i. No replicating virus was detected in the lungs of infected mice after day 7 or 8 p.i.(Kolli, Bataki et al.2008). To determine the replication of hMPV in WT and MyD88−/− mice, lungs from WT and MyD88−/− were harvested at days 5, 8 and 9 p.i. As shown in Fig. 1C, MyD88−/− mice had a slightly increase in viral loads at days 5 and 8 than WT mice, but not at a significant level. At day 8 p.i., the viral loads in WT-infected mice reached an undetectable level while the lungs from one third of infected MyD88−/− mice still had some infectious particles. By the day 9 p.i., no replicating viruses were detected in the lungs of infected WT and MyD88−/− mice, suggesting MyD88 deficiency did not significantly affect hMPV clearance. Consistent with hMPV replication in WT and MyD88−/− mice, the viral genome synthesis in the mice lungs showed evidence of minor enhancement in viral genome copies in MyD88−/− mice (data not shown).
3.2. MyD88 is necessary for the induction of inflammatory and antiviral mediators
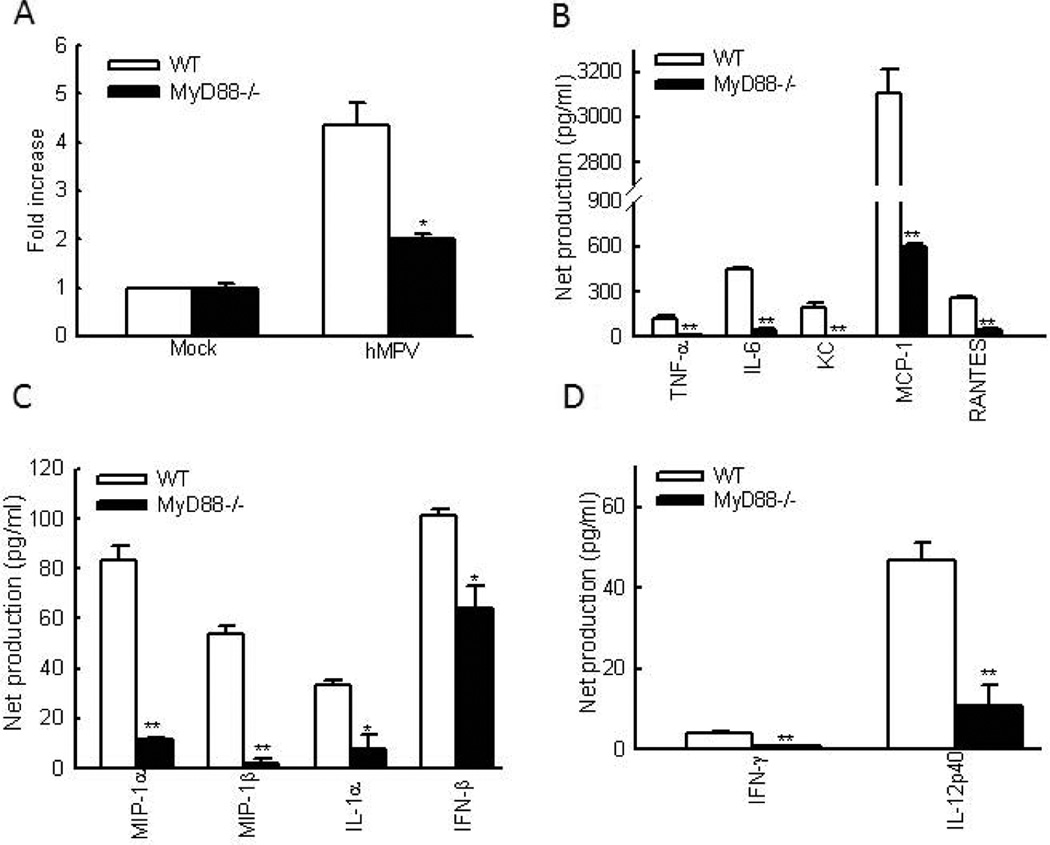
Another hallmark of severe hMPV disease is lung inflammation with excessive mucus production. MUC5AC gene is a primary mucin gene upregulated during severe airway diseases associated with mucus overproduction (Voynow, Gendler et al. 2006;Morcillo and Cortijo, 2006). Expression of this gene has been used to assess the inflammation in r espiratory syncytial virus (RSV)-infected mice (Rudd, Schaller et al. 2007). We found that hMPV-infected WT C57BL/6 mice showed an increased expression of MUC5AC mRNA. However, MUC5AC expression was substantially down-regulated in MyD88−/− mice infected with hMPV (Fig.2A), demonstrating that MyD88 augments lung inflammation in response to hMPV infection,
Figure 2. The effect of MyD88 on pulmonary inflammation and cytokine/chemokines production in hMPV-infected lung.
(A) RNA was isolated from the lungs of mock- and hMPV-infected mice, WT and MyD88−/− at day 8 p.i. Samples were assessed for expression of MUC5AC using qRT-PCR by SYBR green. Each sample was normalized using 18S control. Fold increase in MUC5AC gene expression was shown after comparing the gene expression of infected mice with that of uninfected mice in a strain respective manner. n = 3 lungs/group for two independent experiments. *P<0.05, levels of significance for comparison to WT mice. (B–D) BAL samples, at day 1 p.i., were collected from each group of mice and assessed for IFN-β by ELISA. Other cytokine/chemokine production was measured by a multi-Plex Cytokine detection system. Net induction was calculated for infected WT and MyD88−/− mice. n = 7–8 mice/group from two independent experiments.*P<0.05 and **P<0.01 for comparison to hMPV-infected samples from WT mice.
The role of MyD88 in controlling lung inflammation was also investigated by comparing the induction of inflammatory mediators in lungs of WT and MyD88−/− mice. WT C57BL/6 and MyD88−/− mice were infected i.n. with hMPV, and BALF was analyzed at day 1 and 5 p.i. As shown in Figs. 2B and C. the release of many pro-inflammatory and antiviral cytokines and chemokines occurred as early as day 1 after hMPV infection. The production of these immune mediators was significantly reduced in MyD88−/− mice. At day 5 p.i., IFN-β induction was undetectable in WT and MyD88−/− mice, while the production of other chemokines and cytokines was still detectable in WT mice, but was significantly less in MyD88 knockout mice (Fig. S1). Overall, the data from Figs.2A–C demonstrated that MyD88 contributes to hMPV-induced lung inflammation. MyD88 was also involved in producing Th1-type cytokines. As shown in Figs. 2D and S1C, the induction of several Th1 type cytokines, such as IFN-γ and IL-12p40, required MyD88. We did not observe the induction of IL-4 and IL-5 by hMPV infection in WT and MyD88−/− mice (data not shown).
3.3. MyD88 is necessary for the trafficking of immune cells after hMPV infection
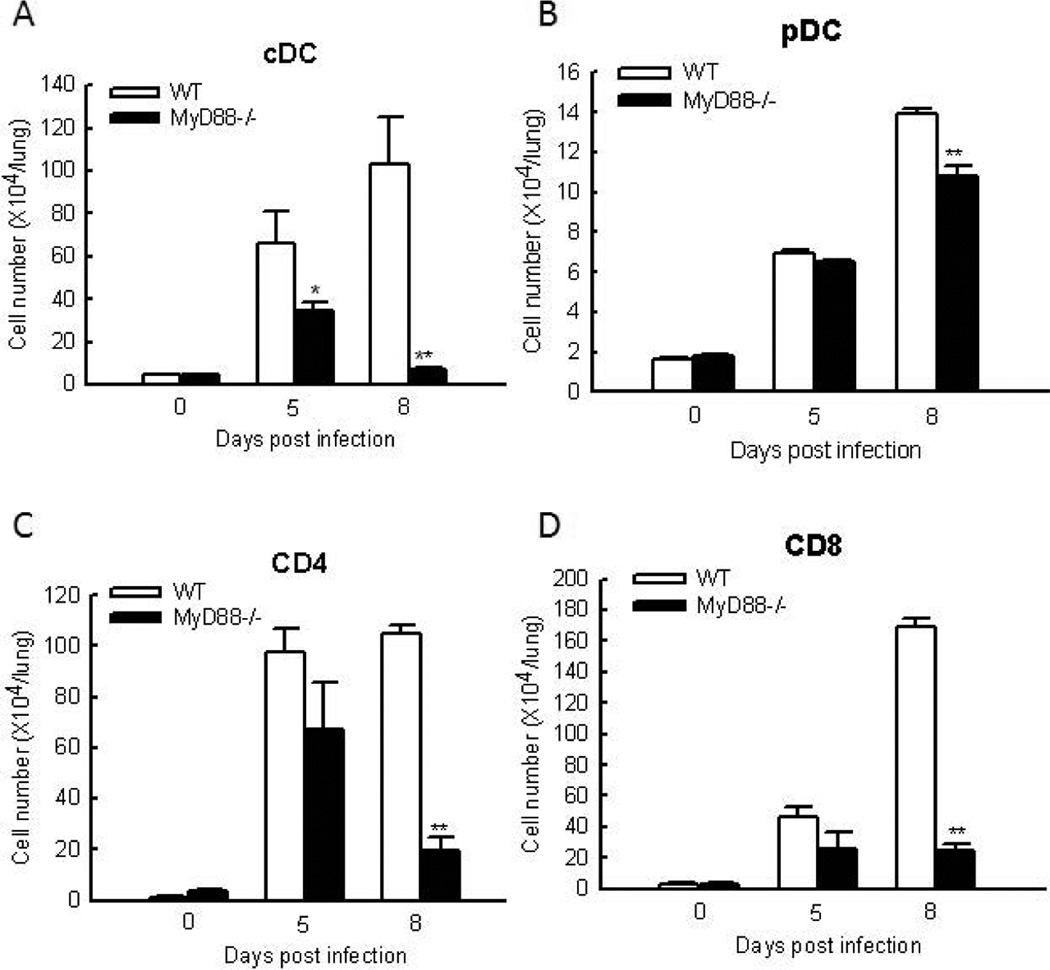
It has been previously shown that, compared to sham-inoculated mice, hMPV-infected mice have a rapid increase in the number of immune cells recovered from BALF, lung and draining lymph nodes (LN), which is important for both innate and adaptive immunity (Kolli, Bataki et al. 2008;Guerrero-Plata, Kolli et al. 2009). In this study, we used cytospin to quantify total cell count, polymorphonuclear neutrophils (PMN), macrophages (Macs), and lymphocytes (Lym) in BALF as described (Kolli, Bataki et al. 2008). As shown in Table I, hMPV-infected WT mice had significant increase in the number of neutrophils in the BALF at day 1 p.i. Compared to WT mice, MyD88−/− mice exhibited less neutrophils migration. The difference in the number of neutrophils between WT and MyD88−/− mice persisted at day 5 p.i. The number of macrophage in the BAL started to increase at day 5 p.i. in the infected mice, both WT and MyD88−/− mice. However MyD88−/− mice had much less migrated macrophages than WT mice. The inhibited migration of macrophage by MyD88 deficiency sustained at day 8 p.i. (data not shown). Similarly, MyD88 was necessary for lymphocytes to migrate to BAL.
Table I.
Quantification of total cell count, polymorphonuclear neutrophils (PMN), macrophages (Macs), and lymphocytes (Lym) in BAL fluid cytospins.
| Days of infection |
PMNa (104) | Macs (104) | Lym (104) | |||
|---|---|---|---|---|---|---|
| WT | MyD88 | WT | MyD88 | WT | MyD88 | |
| 0 | ND | ND | 15±1 | 15±1.3 | ND | ND |
| 1 | 66± 6b | 0.47± 0.37 | 17±1.8 | 15.6±1.2 | 0.8±0.31 | 0.08±0.08 |
| 5 | 3.6±0.4b | 0.11±0.06 | 379±1b | 84±3.6 | 77±21b | 0.46±0.13 |
PMN, polymorphonuclear neutrophils
Significantly higher at p<0.05, WT vs MyD88−/− mice at same day p.i.
Resident and infiltrating DC in the lung are important for innate immune system to sense and respond to hMPV infection. Studies in the mice model for hMPV infection have shown that infection of naive mice with hMPV is accompanied by immune cell infiltration in the lungs, with an influx of pDC and cDC at its peak at day 8 p.i (Guerrero-Plata, Kolli et al. 2009). Herein, we determined the role of MyD88 in the recruitment of the pDC and cDC to the lung after hMPV infection. As demonstrated in Fig. 3A, MyD88 deficiency significantly impaired the recruitment of cDC to the lung. MyD88 was also important to the recruitment of pDC to the lung at day 8 p.i. (Fig. 3B). Compared to the effect of MyD88 on the recruitment of cDC, its effect on the recruitment of pDC was less significant.
Figure 3. The effect of MyD88 on DC and T cell infiltration.
Mice were either infected with 1×107 PFU of hMPV or mock infected, and at days of 5 and 8 p.i., cells were isolated from the lung by collagenase digestion, stained with antibodies for lineage-specific markers, and analyzed by FACS. Increased cell numbers was calculated by subtracting cell numbers of mock infected mice from those of infected mice in a strain respective manner. Data are means ± SEM from four mice per group per time point and are representative of three independent experiments. *P<0.05 and **P<0.01, relative to WT mice.
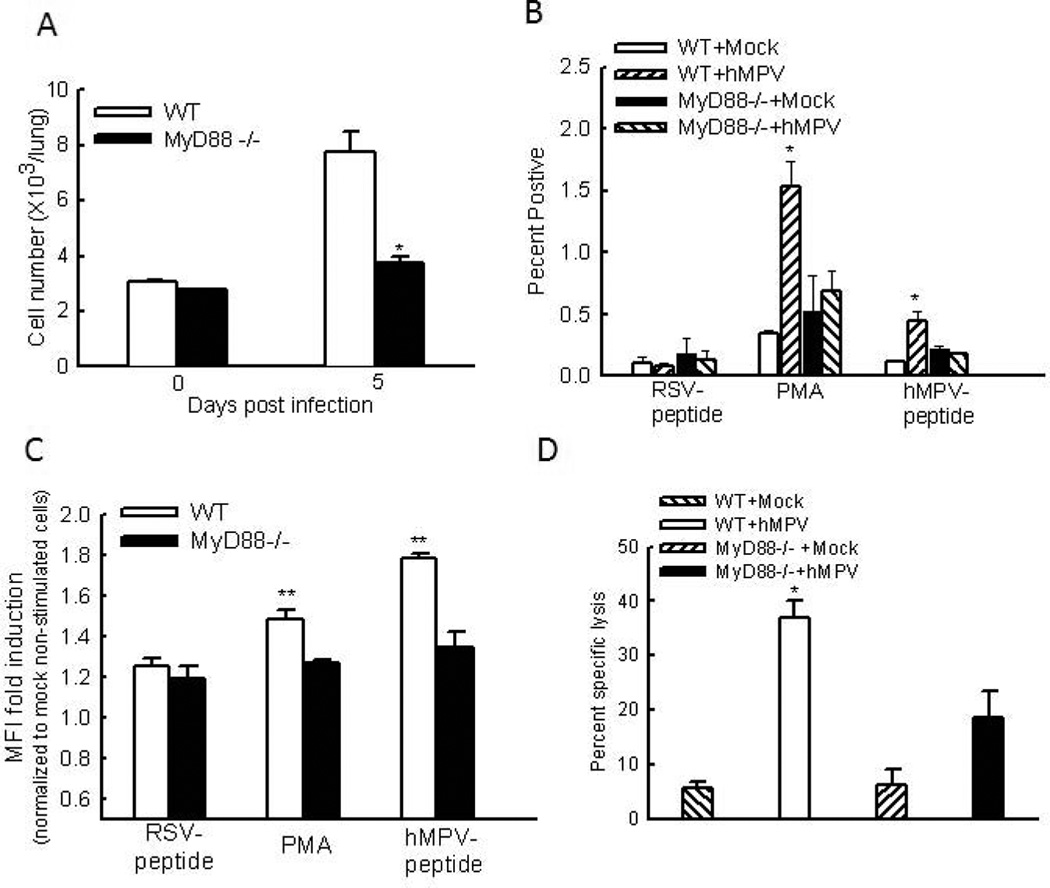
Since an initial infiltration of DC and macrophages is always accompanied by an influx of T cells and MyD88 controls DC infiltration in response to hMPV infection (Kolli, Bataki et al. 2008), it is likely that MyD88 affects the T cell infiltration. To test this hypothesis, FACS analysis of lung cells was performed. Compared to uninfected WT mice, hMPV-infected WT mice had a significant increase in the infiltration of CD4+ and CD8+ T cells into the lung at day 8 p.i. This enhancement was greatly inhibited by MyD88 deficiency (Figs. 3C and D). It has been previously shown that the numbers of T lymphocytes are increased in the lung during primary infection, which, consequently, contributes to hMPV eradication and enhances clinical disease and lung pathology (Alvarez, Harrod et al. 2004;Kolli, Bataki et al. 2008). Therefore, the impairment of T cell migration by MyD88 knockout might contribute to the reduced disease in MyD88−/− mice as shown in Fig. 1
3.4. MyD88 is required for lung DC maturation
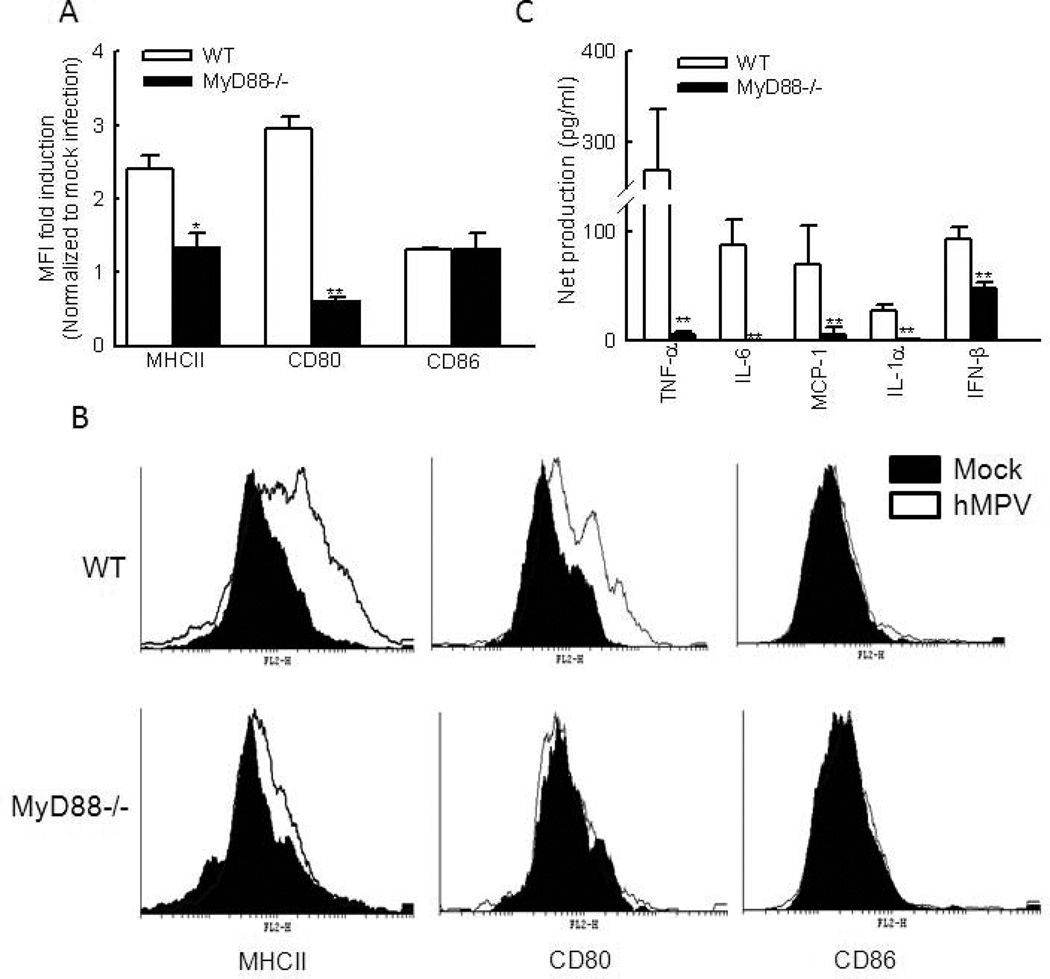
The ability of DC to regulate immunity is dependent on DC maturation. During their conversion from immature to mature cells, DC undergo a number of phenotypical and functional changes, including a redistribution of MHC molecules from intracellular endocytic compartments to the DC surface and an increase in the surface expression of costimulatory molecules, such as CD80 and CD86 (Banchereau and Steinman, 1998;Banchereau, Briere et al. 2000). To investigate whether MyD88 plays a role in regulating DC maturation, we assessed lung DC phenotype and functionality in WT and MyD88−/− mice following infection with hMPV.
At day 8 p.i., the expression of CD80 and MHC class II on CD11c+ lung cells of WT mice was significantly increased as measured by mean fluorescence intensity (MFI), which was about 2.5–3 fold compared to mock-infected WT mice. In hMPV-infected MyD88−/− mice, the induction of these two surface molecules was significantly lower (Fig. 4A). Compared to mock-infected WT mice, hMPV-infected WT mice had only a minimal increase in CD86 MFI, so did hMPV-infected MyD88−/− mice. The histogram of DC maturation markers also demonstrated that cDCs from WT mice had greater expression of CD80 and MHC class II than cDCs from MyD88−/− mice (Fig. 4B).
Figure 4. The effect of MyD88 on DC maturation and function.
(A). Mice were infected with hMPV or mock infected as described above. At day 8 p.i., cell surface molecule expression of MHCII, CD80 or CD86 of lung CD11c+ cells as determined by MFI. The increases in MFI were calculated by normalizing MFI of hMPV-infected samples by MFI of mock-infected samples in a strain respective manner. Data are presented as means ± SEM, n = 6/group from two independent experiments. *P < 0.05 or ** P < 0.01 for increased MFI by hMPV infection in WT DC vs that in MyD88−/− DC. (B). Representative histograms to elucidate the expression of DC maturation markers were also shown. (C). cDC were isolated from the lungs of WT or MyD88−/− mice. Enriched CD11c+ populations by MACS were stained with anti-MHC-II-FITC and anti-CD11c-PE-Cy7 and sorted by FACS. cDC (105 cells/well) were infected with hMPV at an MOI of 5 for 24 h. Concentration of IFN-β was determined by ELISA and other cytokines by Bio-Plex assay. Net induction was calculated for infected WT and MyD88−/− lung cDC. Data are means ± SEM from four lungs per group and are representative of two independent experiments. **P < 0.01, relative to WT cDC.
We also investigated the role of MyD88 in regulating the function of DC by comparing the ability of WT- and MyD88−/−-derived lung cDC to produce cytokines in response to hMPV infection. The lung cDC were isolated as described (Guerrero-Plata, Kolli et al. 2009), followed by mock and hMPV infection. The supernatant was harvested at 24 h p.i. Measurement of cytokines, chemokines and IFN-β revealed that induction of TNF-α and IL-6 MCP-1, IL-1α and IFN-β were dependent on MyD88 (Fig. 4C). Similarly, we found that MyD88 was important for the induction of these immune mediators in BMDC in response to hMPV infection (data not shown). Because the observed difference in the production of cytokines and chemokines could be due to differential infectivity of the virus to WT and MyD88−/− DC, we determined hMPV antigens in these cells using intracellular staining for hMPV proteins. A similar percentage of cells with Ag staining was observed (37.77 % WT DC vs 36.45 % MyD88−/− DC) at the time when cells were harvested for chemokine/cytokine measurement (24 h p.i.), demonstrating no differential infectivity between WT and MyD88−/− cDC.
3.5. MyD88 is required for T cell function
Next, we examined the pulmonary T cell responses after intranasal infection of hMPV. We found that mock-infected mice, WT or MyD88−/−, had few IFN-γ-producing CD8+ pulmonary T cells. Upon infection, the pulmonary CD8+IFN-γ+ T cells were significantly enhanced in WT mice, but not in MyD88 KO mice (Fig. 5A), which is consistent with the measurement of IFN-γ in the BALF of WT and MyD88−/− mice after infection (Fig. S1C).
Figure 5. The effect of MyD88 on T cell function.
(A). Lung cells from infected mice were harvested at day 8 p.i., followed by no treatment or the stimulation with PMA/ ionomycin or an hMPV-derived peptide. A RSV-derived peptide was used as a control. Five hours later, intracellular IFN-γ levels in CD3+CD8+ cells were measured by FACS. (A) The total IFN-γ+CD3+CD8+ cell number without stimulation. (B) The percentage of IFN-γ-producing cells after the stimulation. (C) MFI of IFN-γ+CD3+CD8+ population from infected cells was normalized to the MFI of IFN-γ+CD3+CD8+ population from uninfected and non-stimulated cells, and data are plotted for each genotype. Data presented are representative of three similar experiments with n=6 mice/group. **P < 0.01 compared to RSV-peptide-treated and hMPV-infected WT mice. (D) Pulmonary CD8+ cells (effectors) from day 8 hMPV- or mock-infected mice were incubated with EL4 target cells loaded with a peptide (hMPV peptide or RSV control peptide). The target cells were differentially labeled with CFSE and incubated with the effector cells at the indicated effector: target (E:T) ratios for 5 hours. Cells were then analyzed by flow- cytometry and the specific killing of hMPV peptide-loaded targets was calculated. Data points are means ± SEM from three-four mice per group and are representative of two independent experiments *, P < 0.05 relative to WT hMPV-infected mice.
To investigate T cell memory function, we also prepared total lung cells after infection and cultured them with PMA plus ionomycin, or an hMPV-derived peptide. An RSV-derived peptide was used as a negative control. Both peptides bind to H2-Db (Herd, Mahalingam et al. 2006; Rutigliano, Rock et al. MT, 2005). The percentage and MFI of IFN-γ-producing CD8+ pulmonary T cells was quantified by FACS. We found that mock-infected mice, WT or MyD88−/−, had few IFN-γ-producing CD8+ pulmonary T cells (about 0.1%) and low MFI. Upon stimulation of hMPV-peptide, both the percentage and MFI of IFN-γ+ CD8 cells were not significantly increased in infected MyD88−/− mice. On the other hand, lung cells, isolated from hMPV-infected WT mice, showed a significant increase in the percentage and MFI of IFN-γ-producing CD8+ pulmonary T cells by hMPV-peptide simulation, but not by RSV-peptide stimulation (Figs. 5B and C). In this study, we also investigated the role of MyD88 in regulating CD8+ T cell cytolytic activity. Analysis of CD8+ T cell cytolytic activity showed that CD8+ cells from mock-treated animals, WT or MyD88−/−, killed about 5% target cells which were loaded with the MHC-I-binding hMPV epitope. Compared with cells from mock-treated animals, lung cells from hMPV-infected WT mice demonstrated increased ability to specifically kill about 40 % target cells (Fig. 5C). Cells from hMPV-infected MyD88−/− mice also tended to increase its target killing, but not at a significant level compared to cells from paired mock-infected mice, demonstrating that CTL activation was impaired in MyD88−/− mice.
4. Discussion
hMPV was identified as the third major human-pathogenic member of the Paramyxovirinae in 2001, besides RSV and parainfluenzaviruses (PIV) (van den Hoogen, de Jong et al. 2001). In this study, we have provided both in vivo and in vitro evidence that MyD88 is essential for the production of antiviral and inflammatory cytokines in response to hMPV infection (Figs. 2 and 4C). Following hMPV infection, fewer macrophages and neutrophils were present in BALF samples of MyD88−/− mice (Table I). The migration of other immune cells, such as DC and T cells, was also significantly inhibited by the absence of MyD88 (Fig. 3). Moreover, the functions of DC and T cells in response to hMPV infection were impaired in MyD88−/− mice (Figs. 4 and 5). All of these data suggested that MyD88 plays a critical role in controlling pulmonary immune responses to hMPV infection.
We observed that hMPV-induced airway inflammation (Fig. 2), bodyweight loss (Fig. 1A) and AHR (Fig. 1B) were eased in the absence of MyD88. Reduced inflammation in hMPV-infected MyD88−/− mice was associated with decreased inflammatory cell populations, such as neutrophils and macrophages (Table I), downregulation of inflammatory cytokines, such as IL-6, TNF-α and IL-12p40 (Beutler, 1995;Wang, Bao et al. 2004;Chakraborty, Zhou et al. 2012) (Figs. 2B–D and Fig.S1), and the reduced expression of MUC5AC (Fig.2A), a hallmark of airway mucosal inflammation (Voynow, Gendler, and Rose, 2006;Song, Kim et al. 2009), in the BAL or lung samples from hMPV-infected MyD88−/− mice. MyD88-mediated MUC5AC expression in response to RSV and hMPV infection is somewhat opposite. It has been previously shown that MyD88 deficiency leads to higher expression of MUC5AC in response to RSV infection (Rudd, Schaller et al.,2007), even though the proinflammatory cytokines are reduced (Bhoj, Sun et al. 2008). Other than proinflammatory cytokines, allergic lung inflammation associated with Th2 responses has been previously shown to further increase MUS5AC (Hashimoto, Graham et al. 2004). Compared to WT mice, MyD88−/− mice have enhanced secretion of IL-4, IL-5 and IL-13 in response to RSV (Rudd, Schaller et al., 2007). However, MyD88 deficiency did not affect Th2 cytokine secretion in response to hMPV (data not shown). Therefore, even though MyD88 deficiency reduces the secretion of certain types of proinflammatory cytokines/chemokines in the context RSV infection, enhanced Th2 secretion might lead to more expression of MUS5AC. Overall, reduced inflammation in MyD88 KO mice was consistent with less bodyweight loss and AHR in MyD88−/− mice in response to hMPV infection (Figs. 1A and B). It has been previously shown that T cell infiltration also contributes to hMPV-induced pulmonary disease, as concurrent depletion of CD4+ and CD8+ T cells completely blocked airway obstruction as well as AHR (Kolli, Bataki et al. 2008). Therefore, decreased infiltration of T cells (Fig.3) might contribute to disease alleviation. Overall, our data demonstrates, for the first time, that MyD88 is a key molecule that controls hMPV-induced pulmonary inflammation and disease pathology of mice.
It was recently shown that IL-12p40 deficiency exacerbates pulmonary inflammatory response and mucus production in response to hMPV infection, with mechanism(s) unclear (Chakraborty, Zhou, Wakamatsu, and Guerrero-Plata, 2012). In this study, we found that MyD88 deficiency impaired IL-12p40 induction (Fig. 2D and Fig. S1–C). However, this impairment did not lead to exacerbated pulmonary inflammation. Following hMPV infection, IL-12p40 and MyD88 deficiency led to varied chemokines induction. IL-12p40 KO enhances induction of Eotaxin and KC, two chemokines that regulate the chemotaxis and function of neutrophils and eosinophils respectively (Chakraborty, Zhou, Wakamatsu, and Guerrero-Plata, 2012), while MyD88 deletion significantly reduced the secretion of the majority of proinflammatory cytokines, including KC (Figs. 2B and S1-A). In addition, MyD88 did not play a role in Eotaxin induction and in Th2 response. It is known that the types, relative abundance, chemotaxis ability of virus-induced proinflammatory cytokines/chemokines determine the overall pulmonary inflammation. Although a varied impact of IL-12p40 and MyD88 on chemokine induction may explain the difference in disease outcome of IL12p40 KO and MyD88-impaired IL12p40 secretion, the relative importance of IL12p40 and other chemokines in hMPV-induced pulmonary inflammation is currently unknown and needs to be determined. As mentioned, Th1/Th2 balance and T cell infiltration are also major factors determining hMPV-induced pulmonary disease. Whether these two factors are regulated by IL12p40 is also currently unknown.
Although RSV is a close family member of hMPV, and hMPV and RSV infections share a similar clinical spectrum from mild to severe and sometimes life threatening affliction, we and others have shown that that these two viruses use different PRRs in airway epithelial cells to initiate innate immune responses (Liao, Bao et al. 2008;Liu, Jamaluddin et al. 2007). hMPV and RSV also induce a distinct profile of lung cytokines in vivo (Guerrero-Plata, Casola, and Garofalo, 2005), and different DC responses (Guerrero-Plata, Casola et al. 2006). In this study, we found that the role of MyD88 in controlling hMPV- and RSV-induced immune responses and their associated pulmonary diseases is also distinct. In addition to the above discussed difference in MyD88-controlled pulmonary diseases and T cell responses, we found that MyD88-mediated chemokines, DC infiltration and adaptive responses in RSV- and hMPV-infected animals are also different. It has been shown recently that MyD88 is required for the induction of TNF-α, MCP-1 and IL-1β by RSV. However, the induction of type I IFN secretion by RSV infection is MyD88 independent (Bhoj, Sun et al. 2008). In this study, we have provided in vivo and in vitro evidence that MyD88 is indispensable for the induction of IFN-β and several other antiviral and proinflammatory cytokines in response to hMPV infection. Regarding pulmonary DC infiltration, MyD88-controlled pDC migration following RSV infection is somewhat different than that in hMPV-infected mice. We observed that MyD88 is critical for hMPV-induced pDC migration (Fig.3). However, such role of MyD88 does not exist in the context of RSV infection (Rudd, Schaller et al.,2007). This regulatory difference may be explained by different testing time points picked up by two groups, and/or the distinct role of MyD88 in mediating RSV- and hMPV-induced pDC chemoattractants. Whether MyD88 plays a different role in producing pDC chemoattractants, e.g. SDF-1 and chemerin, in RSV- and hMPV-infected animals needs to be investigated (Hartmann, Graefe et al. 2006;Skrzeczynska-Moncznik, Wawro et al. 2009;McKenna, Beignon et al. 2005). MIP-1α, MIP-1β, MCP-1, and RANTES are commonly known as cDC chemoattractants (Xu, Warren et al. 1996;McKenna, Beignon, and Bhardwaj, 2005). The reduced cDC migration in hMPV-infected MyD88−/− mice (Fig.3A) was consistent with the impaired induction of these cDC chemoattractants by MyD88 deficiency (Figs. 2B and C). The downregulation of RSV-induced MCP-1 expression in MyD88−/− mice is also consistent with less cDC migration due to MyD88 deletion. It is not clearly whether other cDC chemoattractants are reduced by MyD88 KO following RSV infection (Bhoj, Sun et al.,2008). We also observed that the role of MyD88 in controlling T cell responses to RSV and hMPV infection is also different. MyD88 is dispensable for RSV-induced CTL activation (Bhoj, Sun et al. 2008), but plays an essential role in CTL activation by hMPV infection (Fig. 5C). The activation of CTL is dependent on several simultaneous interactions between molecules expressed on the surface of the T cell and molecules on the surface of the antigen-presenting cell (APC), e.g. T-cell receptors with peptide-bound MHC class I molecule, and CD28 on T cells with CD80/CD86. MyD88 may differentially affect APC-T cells interaction in response to RSV and hMPV infection. Overall, our results suggest that the role of MyD88 in controlling disease development and immune responses is pathogen-specific.
We have previously reported that hMPV-induced innate signaling in DC is TLR4 dependent (Kolli, Bao et al.2011). In this study, we observed that the expression of many genes, such as KC, TNF-α and MCP-1, whose expression is dominantly regulated by NF-κB, was significantly reduced in lung cDC from MyD88 deficient mice. This is in line with the previous study demonstrating that TLR-4 activates NF-κB through MyD88-dependent pathway (Kawai and Akira, 2006b;Yamamoto, Sato et al. 2003). We also found that MyD88 played a role in regulating IFN-β induction. There was still significant induction of IFN-β in lung cDC from MyD88−/− mice (Fig.4C). It has been recently showed that MDA5-dependent pathway is essential in inducing type I IFN in DC (Banos-Lara, Ghosh et al. 2012). Therefore, IFN-β induction in MyD88−/− mice by hMPV was likely achieved through activating unaffected MDA5-dependent pathway in MyD88−/− DC.
In summary, our results provide both in vitro and in vivo evidence on the contribution of MyD88, an important adaptor in TLR-mediated signaling, to hMPV-induced pulmonary immune responses and inflammation. Therefore, this study has potential to therapeutically control hMPV pathogenesis and host immune defense against hMPV infection. Whether MyD88-mediated signaling is targeted by hMPV is currently under investigation, as it is important to elucidate why effective adaptive immune memory is difficult to obtain, which may contribute to repeated hMPV infection. Because MyD88 is required for immune response, agents that activate TLR-MyD88 pathway may serve as important adjuvants in future hMPV vaccines and therapeutics.
5. Conclusion
Comparisons of pulmonary lung function, body weight loss, immune mediator induction and immune cell function between hMPV-infected WT and MyD88−/− mice provide evidence of the importance of MyD88 in regulating hMPV-induced pulmonary immune responses and associated disease development.
Supplementary Material
Highlights.
MyD88 contributes to hMPV-induced pulmonary inflammation.
MyD88 plays an important role in hMPV-induced innate antiviral signaling.
MyD88 is required for lung DC and T cell function in response to hMPV infection.
Acknowledgements
This work was supported by grants from the National Institutes of Health-National Institute of Allergy and Infectious DiseasesKAI074829A, the American Lung Association RG232529N, American Heart Association 12BGIA12060008 to X.B, and National Institute of Health grants AI079246 to AC. Authors thank Dr. Animesh Chandra for editing the manuscript. We also thank Qingrong Wang for the technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors concur there are no conflicts of interest associated in this published work.
References
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Akira S, Takedah K. Functions of toll-like receptors: lessons from KO mice. C.R.Biol. 2004;327:581–589. doi: 10.1016/j.crvi.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Alvarez R, Harrod KS, Shieh WJ, Zaki S, Tripp RA. Human metapneumovirus persists in BALB/c mice despite the presence of neutralizing antibodies. J.Virol. 2004;78:14003–14011. doi: 10.1128/JVI.78.24.14003-14011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu.Rev.Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Banos-Lara MD, Ghosh A, Guerrero-Plata A. Critical role of MDA5 in the interferon response induced by hMPV infection in dendritic cells and in vivo. J.Virol. 2012;87(2):1242–1251. doi: 10.1128/JVI.01213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Liu T, Shan Y, Li K, Garofalo RP, Casola A. Human metapneumovirus glycoprotein G inhibits innate immune responses. PLoS.Pathog. 2008;4:e1000077. doi: 10.1371/journal.ppat.1000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Sinha M, Liu T, Hong C, Luxon BA, Garofalo RP, Casola A. Identification of human metapneumovirus-induced gene networks in airway epithelial cells by microarray analysis. Virology. 2008;374:114–127. doi: 10.1016/j.virol.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B. TNF, immunity and inflammatory disease: Lessons of the past decade. J.Invest.Med. 1995;43:227–235. [PubMed] [Google Scholar]
- Bhoj VG, Sun Q, Bhoj EJ, Somers C, Chen X, Torres JP, Mejias A, Gomez AM, Jafri H, Ramilo O, Chen ZJ. MAVS and MyD88 are essential for innate immunity but not cytotoxic T lymphocyte response against respiratory syncytial virus. Proc.Natl.Acad.Sci.U.S.A. 2008;105:14046–14051. doi: 10.1073/pnas.0804717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro SM, Guerrero-Plata A, Suarez-Real G, Adegboyega PA, Colasurdo GN, Khan AM, Garofalo RP, Casola A. Antioxidant Treatment Ameliorates Respiratory Syncytial Virus-induced Disease and Lung Inflammation. Am.J.Respir.Crit Care Med. 2006;174:1361–1369. doi: 10.1164/rccm.200603-319OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty K, Zhou Z, Wakamatsu N, Guerrero-Plata A. Interleukin-12p40 modulates human metapneumovirus-induced pulmonary disease in an acute mouse model of infection. PLoS.ONE. 2012;7:e37173. doi: 10.1371/journal.pone.0037173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JE, Jr, Williams JV. Immunology of viral respiratory tract infection in infancy. Paediatr.Respir.Rev. 2003;4:112–119. doi: 10.1016/s1526-0542(03)00033-2. [DOI] [PubMed] [Google Scholar]
- Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- Goutagny N, Jiang Z, Tian J, Parroche P, Schickli J, Monks BG, Ulbrandt N, Ji H, Kiener PA, Coyle AJ, Fitzgerald KA. Cell type-specific recognition of human metapneumoviruses (HMPVs) by retinoic acid-inducible gene I (RIG-I) and TLR7 and viral interference of RIG-I ligand recognition by HMPV-B1 phosphoprotein. J.Immunol. 2010;184:1168–1179. doi: 10.4049/jimmunol.0902750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J Virol. 2005;79:10190–10199. doi: 10.1128/JVI.79.16.10190-10199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Garofalo RP. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. J Virol. 2005;79:14992–14997. doi: 10.1128/JVI.79.23.14992-14997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Casola A, Suarez G, Yu X, Spetch L, Peeples ME, Garofalo RP. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am.J Respir.Cell Mol.Biol. 2006;34:320–329. doi: 10.1165/rcmb.2005-0287OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Plata A, Kolli D, Hong C, Casola A, Garofalo RP. Subversion of pulmonary dendritic cell function by paramyxovirus infections. J.Immunol. 2009;182:3072–3083. doi: 10.4049/jimmunol.0802262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin ME, Prince GA, Gomez AM, Kinkead R, Boivin G. Human metapneumovirus infection induces long-term pulmonary inflammation associated with airway obstruction and hyperresponsiveness in mice. J.Infect.Dis. 2006;193:1634–1642. doi: 10.1086/504262. [DOI] [PubMed] [Google Scholar]
- Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am.J Respir.Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- Hartmann E, Graefe H, Hopert A, Pries R, Rothenfusser S, Poeck H, Mack B, Endres S, Hartmann G, Wollenberg B. Analysis of plasmacytoid and myeloid dendritic cells in nasal epithelium. Clin.Vaccine Immunol. 2006;13:1278–1286. doi: 10.1128/CVI.00172-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Graham BS, Ho SB, Adler KB, Collins RD, Olson SJ, Zhou W, Suzutani T, Jones PW, Goleniewska K, O'Neal JF, Peebles RS., Jr Respiratory syncytial virus in allergic lung inflammation increases Muc5ac and gob-5. Am.J.Respir.Crit Care Med. 2004;170:306–312. doi: 10.1164/rccm.200301-030OC. [DOI] [PubMed] [Google Scholar]
- Herd KA, Mahalingam S, Mackay IM, Nissen M, Sloots TP, Tindle RW. Cytotoxic T-lymphocyte epitope vaccination protects against human metapneumovirus infection and disease in mice. J.Virol. 2006;80:2034–2044. doi: 10.1128/JVI.80.4.2034-2044.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnardi I, Ng YS, Srdanovic I, Motaghedi R, Rudchenko S, von BH, Zhang SY, Puel A, Jouanguy E, Picard C, Garty BZ, Camcioglu Y, Doffinger R, Kumararatne D, Davies G, Gallin JI, Haraguchi S, Day NK, Casanova JL, Meffre E. IRAK-4-and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junping Ren QWDKDJPC-TKTZJCKLTGWaXB. Human metapneumovirus M2-2 protein inhibits the innate cellular signaling by targeting MAVS. 2012 doi: 10.1128/JVI.01248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Toll-like receptor downstream signaling. Arthritis Res.Ther. 2005;7:12–19. doi: 10.1186/ar1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat.Immunol. 2006a;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death.Differ. 2006b;13(5):816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Kolli D, Bao X, Liu T, Hong C, Wang T, Garofalo RP, Casola A. Human metapneumovirus glycoprotein G inhibits TLR4-dependent signaling in monocyte-derived dendritic cells. J.Immunol. 2011;187:47–54. doi: 10.4049/jimmunol.1002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolli D, Bataki EL, Spetch L, Guerrero-Plata A, Jewell AM, Piedra PA, Milligan GN, Garofalo RP, Casola A. T lymphocytes contribute to antiviral immunity and pathogenesis in experimental human metapneumovirus infection. J Virol. 2008;82:8560–8569. doi: 10.1128/JVI.00699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Gottwein JM, Scheel TK, Jensen TB, Bukh J. MicroRNA-122 antagonism against hepatitis C virus genotypes 1–6 and reduced efficacy by host RNA insertion or mutations in the HCV 5' UTR. Proc.Natl.Acad.Sci.U.S.A. 2011;108:4991–4996. doi: 10.1073/pnas.1016606108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Bao X, Liu T, Lai S, Li K, Garofalo RP, Casola A. Role of retinoic acid inducible gene-I in human metapneumovirus-induced cellular signalling. J.Gen.Virol. 2008;89:1978–1986. doi: 10.1099/vir.0.2008/000778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat.Rev.Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic Acid-inducible gene I mediates early antiviral response and toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J.Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J.Virol. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcillo EJ, Cortijo J. Mucus and MUC in asthma. Curr.Opin.Pulm.Med. 2006;12:1–6. doi: 10.1097/01.mcp.0000198064.27586.37. [DOI] [PubMed] [Google Scholar]
- Picard C, von BH, Ghandil P, Chrabieh M, Levy O, Arkwright PD, McDonald D, Geha RS, Takada H, Krause JC, Creech CB, Ku CL, Ehl S, Marodi L, Al-Muhsen S, Al-Hajjar S, Al-Ghonaium A, Day-Good NK, Holland SM, Gallin JI, Chapel H, Speert DP, Rodriguez-Gallego C, Colino E, Garty BZ, Roifman C, Hara T, Yoshikawa H, Nonoyama S, Domachowske J, Issekutz AC, Tang M, Smart J, Zitnik SE, Hoarau C, Kumararatne DS, Thrasher AJ, Davies EG, Bethune C, Sirvent N, de RD, Camcioglu Y, Vasconcelos J, Guedes M, Vitor AB, Rodrigo C, Almazan F, Mendez M, Arostegui JI, Alsina L, Fortuny C, Reichenbach J, Verbsky JW, Bossuyt X, Doffinger R, Abel L, Puel A, Casanova JL. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 2010;89:403–425. doi: 10.1097/MD.0b013e3181fd8ec3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd BD, Schaller MA, Smit JJ, Kunkel SL, Neupane R, Kelley L, Berlin AA, Lukacs NW. MyD88-mediated instructive signals in dendritic cells regulate pulmonary immune responses during respiratory virus infection. J.Immunol. 2007;178:5820–5827. doi: 10.4049/jimmunol.178.9.5820. [DOI] [PubMed] [Google Scholar]
- Rutigliano JA, Rock MT, Johnson AK, Crowe JE, Jr, Graham BS. Identification of an H-2D(b)-restricted CD8+ cytotoxic T lymphocyte epitope in the matrix protein of respiratory syncytial virus. Virology. 2005;337:335–343. doi: 10.1016/j.virol.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Skrzeczynska-Moncznik J, Wawro K, Stefanska A, Oleszycka E, Kulig P, Zabel BA, Sulkowski M, Kapinska-Mrowiecka M, Czubak-Macugowska M, Butcher EC, Cichy J. Potential role of chemerin in recruitment of plasmacytoid dendritic cells to diseased skin. Biochem.Biophys.Res.Commun. 2009;380:323–327. doi: 10.1016/j.bbrc.2009.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KS, Kim HJ, Kim K, Lee JG, Yoon JH. Regulator of G-protein signaling 4 suppresses LPS-induced MUC5AC overproduction in the airway. Am.J.Respir.Cell Mol.Biol. 2009;41:40–49. doi: 10.1165/rcmb.2008-0280OC. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S. Toll-like receptors in innate immunity. Int.Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat.Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schaik SM, Enhorning G, Vargas I, Welliver RC. Respiratory syncytial virus affects pulmonary function in BALB/c mice. J Infect Dis. 1998;177:269–276. doi: 10.1086/514208. [DOI] [PubMed] [Google Scholar]
- Vicente D, Cilla G, Montes M, Perez-Trallero E. Human metapneumovirus and community-acquired respiratory illness in children. Emerg.Infect Dis. 2003;9:602–603. doi: 10.3201/eid0905.020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von BH, Picard C, Puel A, Casanova JL. Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans. Eur.J.Immunol. 2012;42:3126–3135. doi: 10.1002/eji.201242683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am.J.Respir.Cell Mol.Biol. 2006;34:661–665. doi: 10.1165/rcmb.2006-0035SF. [DOI] [PubMed] [Google Scholar]
- Wang S, Welte T, McGargill M, Town T, Thompson J, Anderson JF, Flavell RA, Fikrig E, Hedrick SM, Wang T. Drak2 contributes to West Nile virus entry into the brain and lethal encephalitis. J.Immunol. 2008;181:2084–2091. doi: 10.4049/jimmunol.181.3.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SZ, Bao YX, Rosenberger CL, Tesfaigzi Y, Stark JM, Harrod KS. IL-12p40 and IL-18 modulate inflammatory and immune responses to respiratory syncytial virus infection. J Immunol. 2004;173:4040–4049. doi: 10.4049/jimmunol.173.6.4040. [DOI] [PubMed] [Google Scholar]
- Williams JV, Wang CK, Yang CF, Tollefson SJ, House FS, Heck JM, Chu M, Brown JB, Lintao LD, Quinto JD, Chu D, Spaete RR, Edwards KM, Wright PF, Crowe JE., Jr The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J.Infect.Dis. 2006;193:387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LL, Warren MK, Rose WL, Gong W, Wang JM. Human recombinant monocyte chemotactic protein and other C-C chemokines bind and induce directional migration of dendritic cells in vitro. J.Leukoc.Biol. 1996;60:365–371. doi: 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.