Abstract
Introduction:
An inability to tolerate distress is a significant predictor of early smoking lapse following a cessation attempt. We conducted a preliminary randomized controlled trial to compare a distress tolerance (DT) treatment that incorporated elements of exposure-based therapies and Acceptance and Commitment Therapy to standard smoking cessation treatment (ST).
Methods:
Smokers with a history of early lapse in prior quit attempts received either DT (N = 27; 9 2-hr group and 6 50-min individual sessions) or ST (N = 22; 6 90-min group and 1 20-min individual session), plus 8 weeks of transdermal nicotine patch.
Results:
At the end of behavioral treatment, odds of abstinence among participants receiving DT were 6.46 times greater than among participants receiving ST (66.7% vs. 31.8%), equivalent to a medium- to large-effect size. Odds of abstinence for DT were still 1.73 times greater at 8 weeks, corresponding to a small- to medium-effect size, although neither this difference nor those at 13 and 26 weeks were statistically significant. Furthermore, of those who lapsed to smoking during the first week postquit, DT participants had more than 4 times greater odds of abstinence than ST participants at the end of treatment. Relative to ST, DT participants also reported a larger decrease in experiential avoidance, a hypothesized DT treatment mediator, prior to quit day. The trajectory of negative mood and withdrawal symptoms in DT differed from ST and was largely consistent with hypotheses.
Conclusions:
Reasons for the decrease in abstinence in DT after treatment discontinuation and suggestions for future research are discussed.
INTRODUCTION
Distress Tolerance and Early Smoking Lapse
Despite the structured planning and preparation prior to quit date, many participants in smoking cessation programs are unable to sustain their quit attempts for even a matter of days. Anecdotal evidence and emerging findings suggest that this is a common experience and that these “early lapsers” are at substantial risk of subsequent relapse. Shiffman et al. (2006) have proposed several “milestones” in the process of successful smoking cessation, with the first two being initial abstinence (24hr without smoking) and avoiding a lapse (one instance of smoking after initial abstinence). Recent investigations of these milestones reveal that many smokers are unable to sustain their quit attempts for even a matter of days. In one large study of 1,429 smokers, 44% failed to quit for seven consecutive days, including 12% who never achieved initial abstinence (i.e., 24-hr abstinence) (Japuntich et al., 2011). Other studies have reported similar outcomes; for example, in a study of over 3,000 smokers who received telephone-assisted smoking cessation treatment (Zhu et al., 1996), approximately 50% were smoking within the first week postquit.
These “early lapsers” are at substantial risk of subsequent relapse, generally defined as smoking for 7 consecutive days. By this definition, over 60% of lapsers relapse, with most experiencing their second lapse on average within 3–4 days of the first (Shiffman et al., 1996) and 50% of relapses occurring within 2 weeks. Therefore, it is crucial to develop novel treatment strategies for smokers with a history of repeated early lapses.
Over the past decade, researchers have highlighted the central role of response to negative affect in smoking lapse and subsequent relapse. Relatedly, recent studies have shown that it may not be primarily the severity or intensity of distress that predicts smoking lapses, but rather the degree to which an individual is able to tolerate discomfort (i.e., distress tolerance [DT], Brown, Lejuez, Kahler, & Strong, 2002; Brown et al., 2009) and to exhibit task persistence (Brandon et al., 2003; Quinn, Brandon, & Copeland, 1996). For example, Brown et al. (2002) found that current smokers who failed to sustain any previous quit attempt for more than 24hr (immediate relapsers) compared with current smokers who had quit smoking in the past for a period of at least 3 months (delayed relapsers) had higher levels of depressive symptoms, a greater tendency to react to stress with negative affect and shorter latency to termination of physical and psychological challenge tasks. Data from a subsequent prospective study confirmed that some combination of psychological and physical DT, as indexed by limited persistence on novel laboratory challenge tasks, may underlie smoking lapse and have important implications for smoking cessation treatment (tracing figures in a mirror, Brandon et al., 2003; breath-holding and breathing carbon-monoxide-rich air, Brown et al., 2009).
Two paradigms for managing DT come from behavioral exposure therapy (e.g., Barlow, Craske, Cerny, & Klosko, 1989) and Acceptance and Commitment Therapy (ACT) (Hayes, Strosahl, & Wilson, 1999). The current study describes the outcomes from a behavioral therapy development project that incorporated elements of exposure and ACT to increase DT in this high-risk population of smokers with a history of early smoking lapse. A more detailed description of this treatment and preliminary findings in a small, uncontrolled sample of early lapse smokers is available elsewhere (Brown et al., 2008).
Behavioral Exposure and ACT for Smoking Cessation
Recent negative reinforcement models of smoking (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Parrott, 1999) are similar to well-established learning theory accounts of anxiety disorders that describe anxiety as being maintained by avoidance/escape behaviors (Mowrer, 1960). In the treatment of anxiety disorders, researchers have consistently found that confrontation of previously avoided distress in the absence of avoidance/escape behavior will lead to greater response flexibility and an eventual decrease in distress (Meuret, Wolitzky-Taylor, Twohig, & Craske, 2012). Extending these findings from the anxiety literature, a behavioral smoking cessation intervention that addresses DT should have at its core the systematic and repeated exposure to increasingly lengthy periods of smoking abstinence. Therefore, we would predict that relative to smokers who do not engage in such exposure prior to quitting, smokers who are exposed to nicotine withdrawal and negative affect during increasing periods of abstinence and who do not engage in the avoidance/escape behavior will experience more severe withdrawal and negative affect prior to quit date, but they will report an eventual decrease in withdrawal and negative affect after quitting.
Accumulating evidence suggests that in order for an exposure treatment to be maximally effective, individuals must fully engage in exposure without attempts to use distraction or engage in avoidance strategies (Craske, Street, & Barlow, 1989; Grayson, Foa, & Steketee, 1982). Thus, we proposed that prospective quitters needed to demonstrate a willingness to remain in this uncomfortable state as they work toward their desired goal of quitting smoking. To this end, ACT (Hayes et al., 1999) strategies were incorporated into the treatment.
ACT places a central focus on acceptance, defined as the behavior of approaching psychologically aversive or troubling internal stimuli while behaving adaptively (Gifford, 1994; Gifford & Hayes, 1997). Distress intolerant individuals may be particularly likely to benefit from an ACT-based approach that teaches them how to accept uncomfortable symptoms and sensations while committing to achieving their valued goal of abstinence from smoking. Previous studies provide support for the application of ACT to smoking cessation in general populations of adult smokers (Bricker, Mann, Marek, Liu, & Peterson, 2010; Gifford et al., 2004; Hernández-López, Luciano, Bricker, Roales-Nieto, & Montesinos, 2009). However, only one previous study compared acceptance-based smoking cessation treatment with a standard cognitive-behavioral treatment (Hernández-López et al., 2009), and no previous studies with the exception of our own small uncontrolled study (Brown et al., 2008) have focused specifically on the treatment of smokers with a history of early smoking lapse.
This present study evaluated the results of a preliminary randomized control trial comparing this novel DT treatment to standard smoking cessation treatment, in combination with 8 weeks of transdermal nicotine patch. We selected a sample of early lapse smokers, defined as regular smokers with a history of at least one serious quit attempt in the past 10 years, with no attempt lasting longer than 72 hours. We expected that early lapse smokers assigned to the DT treatment would have higher point-prevalence abstinence rates at short- and long-term follow-up compared with those in the standard treatment (ST). We further predicted that abstinence would be mediated by decreases in both general and smoking-specific experiential avoidance, defined as an unwillingness to remain in contact with uncomfortable bodily sensations, emotions, and thoughts, and involving efforts to control or suppress these experiences. Additionally, we predicted that, compared with those in ST, smokers in DT would experience greater negative affect and withdrawal symptoms at the group sessions preceding quit day and less negative affect and withdrawal after quit day. In line with previous research, we expected that abstinence would be predicted by negative affect and withdrawal experienced on quit day.
METHODS
Participants
Adult smokers were recruited through newspaper and radio advertisements targeting smokers who had “previous difficulty quitting for even short periods of time.” Inclusion criteria were age between 18 and 65 years, smoking at least 15 cigarettes per day for at least the past 3 years, motivated to quit smoking in the next month, and within the past 10 years made at least one serious quit attempt but never able to remain abstinent for more than 72hr. Participants were excluded for current Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV Axis I disorder; psychoactive substance abuse or dependence (excluding nicotine dependence) within the past 6 months; current use of psychotropic medication, a history of a significant medical condition (e.g., cardiovascular, neurologic, gastrointestinal, other systemic illness) or deemed as currently unhealthy in the context of a complete physical examination, pregnancy, or breast feeding; and current use of pharmacotherapy for smoking cessation or other tobacco products. Those who met preliminary criteria during an initial phone screening were scheduled for a more comprehensive baseline assessment, which included informed consent, the Structured Clinical Interview for DSM-IV–Non-Patient Version (SCID-NP) (First, Spitzer, Gibbon, & Williams, 2002), and completion of self-report measures. We conducted 1,047 phone screens over a 12-month period. Of 118 potential participants who were scheduled for a baseline assessment, 59 no showed or did not complete baseline assessments, and 10 were ruled out based on SCID-NP results. Thus, there were 49 participants in the clinical trial. The CONSORT flow diagram is shown in Figure 1. The Institutional Review Board at Butler Hospital provided ethical approval for this study.
Figure 1.
CONSORT flow diagram.
Treatments
All participants received 8 weeks of nicotine replacement therapy in the form of the nicotine patch (Nicoderm CQ) beginning on quit day, including 4 weeks of the 21mg patch, 2 weeks of 14mg, and 2 weeks of 7mg. The anticipated number and size of the behavioral treatment groups were determined in advance (i.e., three groups for each treatment condition with no more than 10 group members per group), and each treatment assignment was randomly selected from the fixed pool of possible assignments. Detailed therapist manuals were used to ensure standardized delivery of content in both conditions. Doctoral-level psychologists or trainees (psychology interns/postdoctoral fellows) delivered the treatment. The senior author (RAB) and second author (KMPR) trained the therapists and conducted weekly group supervision sessions to ensure standardization of protocol delivery.
Standard Smoking Cessation Treatment
The ST protocol used in this study was based upon a standard behavioral protocol that has been published previously (Brown, 2003) and has yielded positive outcomes in controlled trials (Brown et al., 2001). In brief, the ST components included self-monitoring, identifying triggers, developing self-management strategies for coping with triggers (e.g., avoid, alter, use a substitute), and relapse prevention skills (e.g., identifying and planning for high-risk situations). ST was delivered over a 6-week period and included six 90-min group sessions and one 20-min phone session with quit week occurring at the beginning of week 3.
DT Treatment
A description of the specific DT treatment components has been detailed elsewhere (Brown et al., 2008). DT elements were drawn from exposure-based and acceptance-based (ACT) (Hayes et al., 1999) treatment approaches. The treatment was comprised of six 50-min individual sessions and nine 2-hr group sessions over an 8-week period, with quit day occurring at the beginning of week 5. During these treatment sessions, participants engaged in exercises aimed at increasing their tolerance of distress while maintaining a focus on the valued life goals associated with quitting smoking. Examples of strategies emphasized in this treatment included nicotine fading (Foxx & Brown, 1979), scheduled abstinence, values clarification, acceptance and defusion exercises, and self-management skills.
Rationale for Choice of ST Comparison Condition
We considered comparing DT with a comparison condition that would equate for contact time, which would provide a stringent comparison for the DT treatment and would allow for any treatment effects to be clearly attributed to the differing treatment content. However, given the developmental nature of this project and the limited power to detect significant differences (see Power Analysis below), we decided that this was too restrictive and was not consistent with the developmental goals of this project. Thus, we decided to use a comparable comparison that did not fully equate for contact time, with the expectation that in a larger randomized controlled trial, we would fully equate for contact time. The majority of the additional treatment contact in the DT treatment was specifically devoted to delivering training in ACT/DT skills, with both “front-loaded” sessions prior to quit date and one-on-one treatment sessions intended to help individualize the learning of these skills.
Measures
Assessments occurred at baseline, prior to each group treatment session, and at 8-, 13-, and 26-week follow-up (postquit date), with 96%, 96%, and 92% completion rates, respectively. Participants were paid $25 for completing the end of treatment, 8-, and 13-week assessments and $50 for completing the 26-week assessment. Additionally, they received $20 for providing a saliva sample for cotinine analysis to verify abstinence at the 13- and 26-week assessments.
Smoking Status
Self-reports of smoking during the past 7 days (7-day point prevalence) were collected from participants at each group treatment session and at the 8-, 13-, and 26-week follow-ups. Participant self-reports of abstinence were verified by expired carbon monoxide (CO, 5 ppm or less) at 8, 13, and 26 weeks and by cotinine verification (cotinine, 10ng/ml or less) at 13 and 26 weeks. When CO was unavailable for an assessment (n = 6 at week 8, n = 12 at week 13, n = 9 at week 26), abstinence was verified by informants identified by participants prior to quitting when possible. Unverified reports of abstinence were considered to be smoking.
Smoking History and Pattern
Smoking history and pattern were assessed with a standard Smoking History Questionnaire (Brown et al., 2002). The Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) was used as a continuous measure of nicotine dependence.
Mediating Processes: Experiential Avoidance
General.
The Acceptance and Action Questionnaire (AAQ; Cronbach’s α = .70; Hayes et al., 2004) is a 9-item questionnaire designed to measure the tendency to engage in experiential avoidance of a general nature. Each item is rated on a 7-point Likert scale (1 = never true to 7 = always true). Sample items include “I’m not afraid of my feelings” and “When I feel depressed or anxious, I am unable to take care of my responsibilities.” Overall, high scores indicate more experiential avoidance, and low scores indicate greater psychological acceptance/willingness.
Smoking-Specific.
The Avoidance and Inflexibility Scale (AIS; Cronbach’s α = .93) (Gifford, Antonuccio, Kohlenberg, Hayes, & Piasecki, 2002) is a 13-item measure designed to assess the likelihood that smoking-related internal experiences (thoughts of smoking; feelings associated with craving to smoke such as stress, boredom, or enjoyment; and bodily sensations such as physical withdrawal symptoms) will lead one to smoke and the degree to which one believes that reduction in the frequency and intensity of these internal experiences (i.e., avoidance of them) is necessary in order not to smoke. Responses are given on Likert scales ranging from 1 to 5.
Weekly Measures During Group Treatment: Negative Mood and Withdrawal Symptoms
The tension, depression, and anger subscales of the Profile of Mood States (POMS) (McNair, Lorr, & Droppleman, 1971) were summed to create a total negative mood score. Withdrawal symptoms were assessed with items from the Minnesota Nicotine Withdrawal Scale (Hughes & Hatsukami, 1986; Piasecki et al., 2000), which were divided into subscales of affect (irritability/frustration/anger, anxiety/nervousness, sadness or depression, difficulty concentrating), physical symptoms (restlessness, appetite increase), craving (single item), and sleep disturbance (trouble waking up, trouble falling asleep, dreaming more than usual, disrupted/waking up frequently).
Treatment Integrity
For the DT condition, adherence was rated using a modified version of the ACT Tape Rating Scale (Gifford & Hayes, 1998) that was developed to map closely onto the DT treatment manual. Specific therapist behaviors were considered “prescribed” and “proscribed” (Waltz, Addis, Koerner, & Jacobson, 1993), and raters assessed the presence or absence of each of these behaviors. A separate ST treatment rating scale was developed in a similar manner. All treatment sessions in both conditions were videotaped, and approximately 40% of the ST and DT tapes were randomly selected for rating by two of five possible independent raters. Raters were research assistants not otherwise associated with the treatment delivery, who received approximately 10hr of adherence coding training plus weekly supervision. Kappa coefficients were computed to ascertain reliability estimates. If there was disagreement in coding among raters, we conservatively assessed the behavior in the direction of “nonadherence.”
Power Analysis
Given the developmental nature of this project, our main concern in determining sample size was to obtain reasonable estimates of effect size, rather than power for certain effect sizes at certain p values. Effect size estimates are presented in odds ratios (ORs) for smoking abstinence. Chinn (2000) suggested that ORs of 1.44, 2.47, and 4.25 are equivalent to Cohen’s d values of 0.2 (small), 0.5 (medium), and 0.8 (large), respectively (Cohen, 1992). Group means on continuous variables typically begin to stabilize at about 15 participants. For our dichotomous variable of point-prevalence abstinence, a sample size of 50 (25 in each condition) should provide relatively stable group proportions for effect size estimates. We estimated that a sample size of 50 would allow us to evaluate the potential of the DT intervention within the budgetary constraints of a developmental project, although we recognize that only large or very large effects (Cohen’s d > 0.80) with power of .80 using an alpha of .05 will attain statistical significance with a sample of this size (Cohen, 1988).
Data Analysis
Our primary aim was to examine if DT for early lapsers led to higher abstinence rates during behavioral treatment and improved long-term smoking cessation outcomes at 8, 13, and 26 weeks after quit day. Tests of the effects of DT treatment on verified 7-day point-prevalence abstinence during behavioral treatment (weekly assessments from quit date through 4 weeks after quit date) and during the long-term follow-up period (8-, 13-, and 26-week assessments) were conducted using generalized estimating equations (GEE). GEE is a method of repeated measures analyses for categorical outcomes, which allows for appropriate modeling of abstinence observations across time. All participants randomized to treatment were included in an “intent-to-treat” analysis. All analyses assumed α = .05; however, as noted previously, effect sizes were of greater interest than absolute significance levels given the small sample size.
To explore potential mechanisms of change operating in the DT treatment, we conducted a series of successive regression analyses relating (a) DT treatment to changes in experiential avoidance (both general and smoking-specific) prior to quit day and (b) changes in proposed mediators prior to quit day and biochemically verified abstinence during each of the 4 weeks of behavioral treatment after quit day, controlling for treatment. Regression analyses were also used to compare levels of negative mood (POMS) and the four subscales of withdrawal symptoms in the weeks prior to quit day and on quit day in DT versus ST. Treatment differences in negative mood and withdrawal symptoms following quit day were examined using GEE, controlling for baseline levels of corresponding variable, time (weeks) from quit day, and planned covariates of age and level of nicotine dependence. Negative mood and withdrawal symptoms assessed at quit day, 1, 2, and 4 weeks (end of group treatment) after quitting were included in the analyses (the ST group did not complete these assessments at week 3).
RESULTS
Preliminary Analyses
Treatment Assignment
In the final sample of 49 participants, 27 received DT treatment and 22 received ST treatment. The DT and ST groups did not differ on any characteristics prior to randomization (see Table 1). Participants mean age was 47.68 (SD = 10.31) years, 49% were females, 32.7% had a high-school education or less, 4.1% were Hispanics/Latinos, and 90% were Caucasians. The participants had a moderate level of nicotine dependence (mean FTND = 6.3; SD = 1.73, mean cigarettes per day = 21.65; SD = 8.33) and entered treatment with a mild level of depressive symptoms (mean CES-D = 9.32; SD = 7.70). Of the 49 participants, 14 (28.6%) reported prior major depressive episode(s) using DSM-IV criteria, 50% (n = 7) of whom endorsed recurrent (more than one) major depressive episodes.
Table 1.
Demographic and Smoking Characteristics for Final Sample
| Distress tolerance treatment | Standard treatment | |
|---|---|---|
| N = 27 | N = 22 | |
| n (%) | n (%) | |
| Female | 12 (44.4) | 12 (54.5%) |
| Race/ethnicity | ||
| Hispanic | 2 (7.4) | 0 (0.0%) |
| White | 24 (88.9) | 20 (90.9%) |
| African American | 3 (11.1) | 2 (9.1%) |
| Education | ||
| <High-school graduate | 2 (7.4) | 4 (18.2%) |
| High-school graduate | 4 (14.8) | 6 (27.3%) |
| Some college | 14 (51.9) | 9 (40.9 %) |
| College degree | 3 (11.1) | 2 (9.1%) |
| >Master’s degree | 4 (14.8) | 1 (4.5%) |
| Marital status | ||
| Married | 15 (55.6) | 8 (36.4%) |
| Engaged | 0 (0.0) | 1 (4.5 %) |
| Divorced | 8 (29.6) | 6 (27.3%) |
| Separated | 0 (0.0) | 1 (4.5%) |
| Never married | 2 (7.4) | 5 (30.8%) |
| Living together | 1 (3.7) | 0 (0.0%) |
| Widowed | 1 (3.7) | 1 (4.5%) |
| Employment | ||
| Employed | 23 (85.2) | 17 (77.3%) |
| Depression history | ||
| No depressive episode | 18 (66.7) | 17 (77.3) |
| Single depressive episode | 3 (11.1) | 4 (18.2) |
| Recurrent depressive episodes | 6 (22.2) | 1 (4.5) |
| M (SD) | M (SD) | |
| Age | 47.19 (11.4) | 48.30 (9.01) |
| Fagerström Test for Nicotine Dependence | 6.33 (1.52) | 6.27 (2.00) |
| Average cigarettes per day | 22 (7.26) | 21 (9.65) |
Treatment Integrity
Treatment integrity ratings indicated that 99.9% of prescribed elements were present in the DT tapes that were rated, and 100% of the prescribed behaviors were present in the ST tapes. There were no proscribed behaviors observed in either ST or DT session tapes. Thus, there was good treatment integrity in both treatment conditions. The average kappa coefficient for raters coding the treatment tapes was .99 for the DT treatment and 1.0 for the ST, indicating excellent agreement.
Smoking Outcomes During Behavioral Treatment
The GEE models first examined age, gender, and years of education as potential covariates in addition to planned inclusion of level of nicotine dependence and the linear effect of time. Of the proposed covariates, age (adjusted odds ratio [AOR] = 1.04, 95% CI = 0.99–1.10, p = .11) alone was included along with level of dependence (AOR = 0.81, 95% CI = 0.62–1.07, p = .14) and the linear effect of time as a primary set of covariates, given that gender and years of education showed little relationship with outcome (ps > .5). After controlling for the primary set of covariates, changes in verified abstinence rates at 1, 2, 3, and 4 weeks after quit day were significantly different for smokers in DT (vs. ST) as evidenced by a significant treatment by time interaction (AOR = 1.76, 95% CI = 1.24–2.50, p = .002). At the end of behavioral treatment (4 weeks after quit day), smokers in DT were significantly more likely to be abstinent than those in ST (66.7% vs. 31.8%, respectively) (AOR = 6.46, 95% CI = 1.58–26.50, p = .001), which is equivalent to a large effect size of d = 1.03 (Chinn, 2000). However, at the end of the nicotine patch treatment (8-week follow-up), abstinence rates among smokers in DT and ST were 40.7% and 31.8%, respectively, a difference that was no longer significantly different (AOR = 1.73, 95% CI = 0.46–6.48), reflecting a small to medium effect size of d = .30 (see Figure 2).
Figure 2.
Abstinence rates (7-day point prevalence) for distress tolerance (DT) and standard treatment (ST) conditions. At week 4 (end of group treatment), the abstinence rate for DT (66.7%) was significantly higher than ST (40.9%), p = .049, d = .71. Verified 7-day point-prevalence abstinence rates at 8, 13, and 26 weeks after quit day did not significantly differ in DT versus ST and were 40.7%, 22.2%, and 14.8%, and 31.8%, 27.3%, and 9.1%, respectively.
Long-Term Smoking Outcomes
We also evaluated 7-day point-prevalence abstinence rates at 4 (end of group treatment), 8 (end of patch treatment), 13, and 26 weeks postquit assessments. In GEE analysis with the same covariates, the main effect of treatment condition was not significant statistically (AOR = 1.83, 95% CI = 0.46–7.34, p = .39). In a next step, all four two-way interactions of covariates and treatment condition were entered as a block after all other terms. In this model, there were no significant interactions between treatment condition and time or any of the other covariates (ps > .10). Verified 7-day point-prevalence abstinence rates at each assessment point for the DT and ST groups are shown in Figure 2.
Recovery From Lapses
Secondary analyses were conducted to examine group differences in rates of recovery from early lapse. Of those who lapsed during the first week after quit date (51.0% overall), participants in the DT condition were over five times more likely (OR = 5.55) than participants in the ST condition to be abstinent at the end of group treatment (4 weeks postquit date), reflecting a large effect size (d = .95). Of those who were fully abstinent for the first week (49.0% overall), 100% of participants in the DT condition, and 54.6% of participants in the ST condition were abstinent at the end of group treatment.
Mediating Processes—Decreasing Experiential Avoidance
In order to evaluate proposed treatment mechanisms, we used a sequential process to evaluate the degree to which smokers in DT relative to ST had larger decreases in experiential avoidance (both general and smoking-specific) from baseline to quit date. We examined the AAQ as an index of general experiential avoidance and the AIS as an index of smoking-specific experiential avoidance. Given that the DT treatment was hypothesized to change experiential avoidance prior to a quit attempt, we examined prequit experiential avoidance as a mediator. We did not examine postquit experiential avoidance as a mediator because it would be confounded with smoking outcome. In regression analyses, there was no significant difference in changes in AAQ (B = 1.61, SE = 1.97, p = .42) scores for smokers in DT and ST treatments. However, there was a significant difference in AIS scores (B = −5.16, SE = 2.46, p = .04), with smokers in the DT treatment on average having a larger decrease in levels of smoking-specific experiential avoidance than smokers in ST.
We used logistic regression models to examine whether changes in experiential avoidance were related to successful cessation at the end of behavioral treatment. Logistic models controlled for the standard covariates, treatment condition, and then in successive models included either the AAQ or the AIS along with the corresponding measure assessed at baseline. There was a marginal relationship between changes in AAQ scores prior to cessation and the odds of abstinence (B = −0.17, SE = 0.09, p = .059). Decreases in general experiential avoidance prior to cessation were marginally associated with greater rates of abstinence at the end of treatment. There was no significant relationship between changes in the AIS scores prior to cessation and the odds of abstinence at the end of treatment (B = −0.02, SE = 0.07, p = .80). Mediation requires that treatment affects the mediator and that the mediator is related to the outcome when controlling for treatment. Full mediation analyses were not conducted since AAQ was not changed, and AIS did not relate to outcome.
Negative Mood and Withdrawal Symptoms Prior to and on Quit Day
Consistent with our prediction of the effect of our exposure-based intervention, regression analyses showed that smokers in DT, relative to ST, reported a significantly greater increase in negative mood (POMS) immediately prior to the group session that occurred 2 weeks before quit day (i.e., directly after they engaged in prescribed exposure exercises) (B = 7.75, SE = 2.40, p = .003), as well as immediately prior to the group session that occurred the week before quit day (B = 17.09, SE = 5.22, p = .002), when controlling for baseline levels of negative mood. In support of the effectiveness of these exercises, participants in DT reported significantly lower levels of negative mood on quit day relative to ST, when controlling for negative mood reported at the week prior to quit day, (B = −8.73, SE = 3.65, p = .02) (see Figure 3).
Figure 3.
Weekly negative mood scores for distress tolerance and standard treatment (ST) conditions. POMS = Profile of Mood States. Negative mood was not assessed at week 3 for the ST condition and therefore not included in this figure.
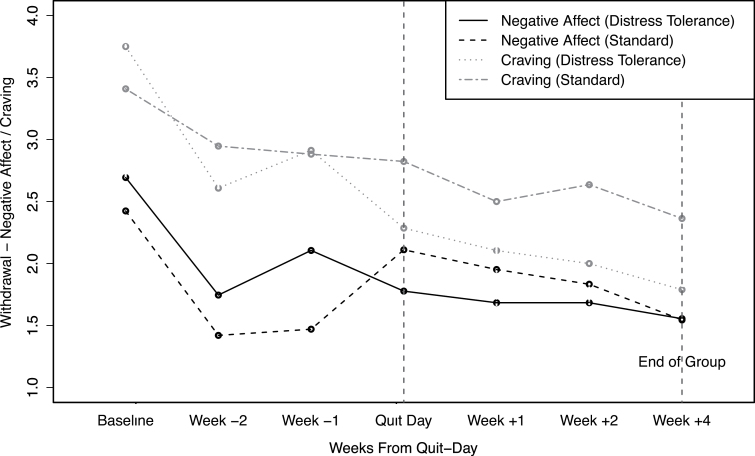
No differences in any of the four withdrawal subscale scores (affect, physical symptoms, craving, sleep) across conditions at baseline was observed (ps > .05). When controlling for levels of the corresponding withdrawal subscale at baseline, DT participants reported significantly higher affect (B = 0.44, SE = 0.21, p = .047) and physical symptoms (B = 0.51, SE = 0.22, p = .026) in the week prior to quit day, but not on quit day (ps > .05), compared with ST. Craving scores did not increase significantly prior to quit day in either condition (ps > .2); however, consistent with our hypothesis, DT participants had lower craving on quit day than did those receiving ST when controlling for craving the week before quit day (B = −0.54, SE = 0.25, p = .04) (see Figure 4). There were no differences across conditions in levels of sleep disturbance during the weeks prior to or on quit day (ps > .05).
Figure 4.
Weekly affect withdrawal and craving scores for distress tolerance and standard treatment (ST) conditions. Withdrawal symptoms, including affect and craving, were not assessed at week 3 for the ST condition and therefore not included in this figure.
Negative Mood and Withdrawal Symptoms After Quit Day
As expected, GEE models revealed that participants in the DT treatment, relative to ST, maintained significantly lower levels of craving (B = −0.63, SE = .14, z =19.22, p < .01) and affect (withdrawal) (B = −0.26, SE = .13, z = 3.97, p = .046) through the end of group treatment following quit day, but no difference in negative mood (POMS), physical symptoms, or sleep disturbance across conditions was observed (ps > .05) (see Figures 3 and 4).
DISCUSSION
Overall, the findings from this preliminary randomized control trial of a novel DT treatment for smokers with a history of early lapse were encouraging. At the completion of behavioral treatment (4 weeks after quit date), the odds of verified 7-day point-prevalence abstinence were 6.46 times greater among those receiving DT (66.7% abstinent) than among those receiving ST (31.8% abstinent) participants, equivalent to a medium to large effect size. This difference was both statistically and clinically significant and particularly noteworthy given that all study participants had been unable to quit smoking for more than 72hr in the past 10 years.
Furthermore, participants who received the DT treatment showed a tendency to recover from early smoking lapses to regain abstinence: a pattern not characteristically seen in smoking cessation treatment outcome research. Although few studies have examined this directly, treatment outcome findings suggest that many smokers who lapse during treatment drop out of treatment shortly thereafter (Borrelli et al., 2002; Patterson et al., 2003; Shiffman et al., 2006). Yet, the current findings suggest that the participants receiving the DT treatment in this study continued to stay in treatment despite smoking lapses and were successful in their efforts at persisting in the face of smoking lapse and regaining abstinence status by the end of the behavioral treatment sessions. Of those who lapsed to smoking during the first week after quit date, DT participants were over four times more likely than ST participants to be abstinent at the end of treatment, which corresponds to a large effect size. A similar pattern has been shown in previous ACT-based smoking cessation research studies. For example, both Gifford et al. (2004) and Gifford et al. (2011) showed higher quit rates at 1-year follow-up versus 6 months because participants who lapsed regained abstinence.
Finally, the DT treatment produced some evidence that the exposure and ACT components resulted in hypothesized changes in participants’ reactions to the discomfort of quitting smoking. Although there was no evidence of changes in general tendency toward experiential avoidance as measured by the AAQ, DT participants did report a decrease in smoking-specific experiential avoidance (i.e., avoidance of smoking and withdrawal-related thoughts, feelings, and bodily sensations) prior to quit day. This finding likely reflects the fact that the primary emphasis of the DT intervention was on reducing attempts to avoid smoking-specific rather than general distress. Furthermore, consistent with the rationale for the exposure-based DT intervention, participants in DT, relative to ST, reported a significantly greater increase in negative mood prior to each group session in the 2 weeks before quit day. We expected to observe this increase because during these weeks, DT participants were prescribed exposure exercises to engage in just prior to arriving at group (e.g., refraining for smoking for increasingly longer periods of time). In support of the effectiveness of these exercises, participants in DT also reported significantly lower levels of negative mood and craving on quit day relative to ST participants. We believe these exposure-based procedures hold significant promise given the strong relationship between levels of negative mood reactions on quit day and early lapse (Kenford et al., 2002; McCarthy, Piasecki, Fiore, & Baker, 2006; Piasecki, Kenford, Smith, Fiore, & Baker, 1997; Piasecki et al., 2000). We hypothesize that the change in reactions to the discomfort of quitting is one mechanism for the effect of DT in improving cessation outcomes.
The odds of abstinence were still 1.73 times greater in favor of DT at the 8-week follow-up (end of nicotine patch treatment), roughly corresponding to a small to medium effect size. However this difference was not maintained once both behavioral treatment and patch had been discontinued (13 and 26 weeks). The large decreases in abstinence that occurred in DT between 4 weeks (end of behavioral treatment) and 8 weeks (end of patch) postquit and between 8 weeks and 13 weeks postquit likely reflect the sudden discontinuation of first the intensive behavioral treatment and then the pharmacological treatment. This early lapsing population may require an even more extended active treatment phase that is reduced in intensity more gradually to succeed in maintaining long-term abstinence. Support for extended treatments in increasing long-term abstinence rates is beginning to accumulate; for example, Hall and colleagues have conducted several studies indicating that extending pharmacological and behavioral treatment for a full year results in significantly higher long-term abstinence rates (40% or more) than typically observed in smoking cessation studies (Hall et al., 2011; Hall et al., 2009; Hall, Humfleet, Reus, Muñoz, & Cullen, 2004).
Limitations
The current study had several limitations that merit discussion. With respect to the participants, smokers with current psychiatric and other substance-use comorbidities were excluded. Relative to the general population, smoking prevalence among individuals with these conditions is substantially higher (CDC, 2013; Lasser et al., 2000), and results of treatment studies involving these populations have generally produced low abstinence rates (Prochaska, Delucchi, & Hall, 2004). Future research is needed to evaluate the efficacy of the DT approach in these populations; it is expected that a DT approach may be of value as it directly targets emotional vulnerabilities that are at the core of many psychiatric and substance-use disorders. With respect to methodology, the sample size was small, and although we were able to obtain stable estimates of effect sizes, our power to detect significant group differences in abstinence rates and relationships between hypothesized mediators and outcomes was limited. Although the possibility of group effects on smoking outcome exists, the small sample size did not allow us to construct a stable-model treating groups as a level of analysis. Also, the total contact time was greater in DT than in ST; therefore, some of the differences observed between the treatments could be attributable to this additional time rather than differences in treatment content. Future studies are needed to compare DT with a ST that equates for contact time.
With respect to treatment content, combining DT treatment with nicotine patch may have conveyed an inconsistent message and reduced participants’ opportunities for exposure and practice of DT skills, as the purpose of the nicotine patch is to decrease the discomfort and intensity of nicotine withdrawal and cravings. In line with this possibility, previous smoking cessation studies have found somewhat higher quit rates for ACT alone than for ACT when combined with pharmacotherapy (e.g., compare Gifford et al., 2004 with Gifford et al., 2011). About half of individuals who were scheduled for baseline assessments did not show up and were never randomized to a treatment; therefore, the final sample may have been especially motivated and committed to quitting smoking relative to the population of smokers with a history of early lapse. Finally, given that all participants had a history of early lapse, results may not generalize to the general population of treatment-seeking smokers, many of whom have a history of longer duration quit attempts. Future research is needed to determine whether the DT approach is especially well suited for smokers with a history of early lapse or whether it could also benefit a more general population of smokers.
CONCLUSIONS
In summary, we conclude that this novel DT treatment had significant clinical impact on these early lapse smokers during their treatment participation. Future research is needed to develop and evaluate innovative methods to assist early lapse smokers to extend and maintain these treatment gains over extended time periods. If such methods can be developed and longer term successful outcomes achieved, DT treatment would hold the promise of becoming an important clinical smoking cessation resource for this treatment-resistant subpopulation. In addition, larger studies are necessary to determine the stability of the effect size estimates observed in the current study and whether these findings are applicable to broader populations of smokers.
FUNDING
National Institute on Drug Abuse (DA017332) to R.A.B.
DECLARATION OF INTERESTS
None declared.
REFERENCES
- Baker T. B., Piper M. E., McCarthy D. E., Majeskie M. R., Fiore M. C. (2004). Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review, 111, 33–51.10.1037/ 0033-295X.111.1.33 [DOI] [PubMed] [Google Scholar]
- Barlow D. H., Craske M. G., Cerny J. A., Klosko J. S. (1989). Behavioral treatment of panic disorder. Behavior Therapy, 20, 261–282.10.1016/S0005-7894(89)80073–5 [Google Scholar]
- Borrelli B., Hogan J. W., Bock B., Pinto B., Roberts M., Marcus B. (2002). Predictors of quitting and dropout among women in a clinic-based smoking cessation program. Psychology of Addictive Behaviors, 16, 22–27.10.1037/0893-164X.16.1.22 [DOI] [PubMed] [Google Scholar]
- Brandon T. H., Herzog T. A., Juliano L. M., Irvin J. E., Lazev A. B., Simmons V. N. (2003). Pretreatment task persistence predicts smoking cessation outcome. Journal of Abnormal Psychology, 112, 448–456.10.1037/0021-843X.112.3.448 [DOI] [PubMed] [Google Scholar]
- Bricker J. B., Mann S. L., Marek P. M., Liu J., Peterson A. V. (2010). Telephone-delivered Acceptance and Commitment Therapy for adult smoking cessation: A feasibility study. Nicotine & Tobacco Research, 12, 454–458.10.1093/ ntr/ntq002 [DOI] [PubMed] [Google Scholar]
- Brown R. A. (2003). Intensive behavioral treatment. In Abrams D. B., Niaura R. S., Brown R. A., Emmons K. M., Goldstein M. G., Monti P. M. (Eds.), The tobacco dependence treatment handbook: A guide to best practices (pp. 118–177). New York: Guilford Press [Google Scholar]
- Brown R. A., Kahler C. W., Niaura R., Abrams D. B., Sales S. D., Ramsey S. E, … Miller I. W. (2001). Cognitive-behavioral treatment for depression in smoking cessation. Journal of Consulting and Clinical Psychology, 69, 471–480.10.1037/0022-006X.69.3.471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. A., Lejuez C. W., Kahler C. W., Strong D. R. (2002). Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology, 111, 180–185.10.1037/0893-164X.19.2.208 [PubMed] [Google Scholar]
- Brown R. A., Lejuez C. W., Strong D. R., Kahler C. W., Zvolensky M. J., Carpenter L. L, … Price L. H. (2009). A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine & Tobacco Research, 11, 493–502.10.1093/ntr/ntp041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. A., Palm K. M., Strong D. R., Lejuez C. W., Kahler C. W., Zvolensky M. J, … Gifford E. V. (2008). Distress tolerance treatment for early-lapse smokers: Rationale, program description, and preliminary findings. Behavior Modification, 32, 302–332.10.1177/0145445507309024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2013). Vital signs: Current cigarette smoking among adults aged ≥ 18 years with mental illness—United States, 2009–2011. Morbidity and Mortality Weekly Report, 62, 81–87 [PMC free article] [PubMed] [Google Scholar]
- Chinn S. (2000). A simple method for converting an odds ratio to effect size for use in meta-analysis. Statistics in Medicine, 19, 3127–3131.10.1002/1097-0258(20001130)19 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum [Google Scholar]
- Cohen J. (1992). A power primer. Psychological Bulletin, 112, 155–159.10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Craske M. G., Street L., Barlow D. H. (1989). Instructions to focus upon or distract from internal cues during exposure treatment of agoraphobic avoidance. Behaviour research and therapy, 27, 663–672.10.1016/0005-7967(89)90150–2 [DOI] [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M., Williams J. B. W. (2002). Structured clinical interview for DSM-IV-TR Axis I disorders, research version, non-patient edition (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute [Google Scholar]
- Foxx R. M., Brown R. A. (1979). Nicotine fading and self-monitoring for cigarette abstinence or controlled smoking. Journal of Applied Behavior Analysis, 12, 111–125.10.1901/jaba.1979.12-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford E. V. (1994). Setting a course for behavior change: The verbal context of acceptance. In Hayes S. C., Jacobson N. S., Follette V. M., Dougher M. J. (Eds.), Acceptance and change: Content and context in psychotherapy (pp. 218–222). Reno, NV: Context Press [Google Scholar]
- Gifford E. V., Antonuccio D. O., Kohlenberg B. S., Hayes S. C., Piasecki M. M. (2002). Combining Bupropion SR with acceptance and commitment-based behavioral therapy for smoking cessation: Preliminary results from a randomized controlled trial. Paper presented at the Association for Advancement of Behavioral Therapy, Reno, NV: [Google Scholar]
- Gifford E. V., Hayes S. C. (1997). Discrimination training and the function of acceptance. Paper presented at the meeting of the Association for Behavior Analysis, Chicago, IL: [Google Scholar]
- Gifford E. V., Hayes S. C. (1998). ACT tape rating scale (Unpublished manuscript)
- Gifford E. V., Kohlenberg B. S., Hayes S. C., Antonuccio D. O., Piasecki M. M., Rasmussen-Hall M. L., Palm K. M. (2004). Acceptance-based treatment for smoking cessation. Behavior Therapy, 35, 689–705.10.1016/S0005-7894(04)80015–7 [DOI] [PubMed] [Google Scholar]
- Gifford E. V., Kohlenberg B. S., Hayes S. C., Pierson H. M., Piasecki M. P., Antonuccio D. O., Palm K. M. (2011). Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior Therapy, 42, 700–715.10.1016/j.beth.2011.03.002 [DOI] [PubMed] [Google Scholar]
- Grayson J. B., Foa E. B., Steketee G. (1982). Habituation during exposure treatment: Distraction vs attention-focusing. Behaviour Research and Therapy, 20, 323–328.10.1016/0005-7967(82)90091–2 [DOI] [PubMed] [Google Scholar]
- Hall S. M., Humfleet G. L., Muñoz R. F., Reus V. I., Prochaska J. J., Robbins J. A. (2011). Using extended cognitive behavioral treatment and medication to treat dependent smokers. American Journal of Public Health, 101, 2349–2356.10.2105/AJPH.2010.300084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. M., Humfleet G. L., Muñoz R. F., Reus V. I., Robbins J. A., Prochaska J. J. (2009). Extended treatment of older cigarette smokers. Addiction, 104, 1043–1052.10.1111/j.1360-0443.2009.02548.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S. M., Humfleet G. L., Reus V. I., Muñoz R. F., Cullen J. (2004). Extended nortriptyline and psychological treatment for cigarette smoking. American Journal of Psychiatry, 161, 2100–2107.10.1176/appi.ajp.161.11.2100 [DOI] [PubMed] [Google Scholar]
- Hayes S. C. (2004). Acceptance and commitment therapy and the new behavior therapies: Mindfulness, acceptance, and relationship. In Hayes S. C., Follette V. M., Linehan M. M. (Eds.), Mindfulness and acceptance. New York: The Guilford Press [Google Scholar]
- Hayes S. C., Strosahl K. D., Wilson K. G. (1999). Acceptance and commitment therapy: An experiential approach to behavior change. New York: The Guilford Press [Google Scholar]
- Hayes S. C., Strosahl K. D., Wilson K. G., Bissett R. T., Pistorello J., Toarmino D, … McCurry S. M. (2004). Measuring experiential avoidance: A preliminary test of a working model. The Psychological Record, 54, 553–578 [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127.10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hernández-López M., Luciano M. C., Bricker J. B., Roales-Nieto J. G., Montesinos F. (2009). Acceptance and commitment therapy for smoking cessation: A preliminary study of its effectiveness in comparison with cognitive behavioral therapy. Psychology of Addictive Behaviors, 23, 723–730.10.1037/a0017632 [DOI] [PubMed] [Google Scholar]
- Hughes J. R., Hatsukami D. (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43, 289–294.10.1001/archpsyc.1986.01800030107013 [DOI] [PubMed] [Google Scholar]
- Japuntich S. J., Leventhal A. M., Piper M. E., Bolt D. M., Roberts L. J., Fiore M. C., Baker T. B. (2011). Smoker characteristics and smoking-cessation milestones. American Journal of Preventive Medicine, 40, 286–294.10.1016/j.amepre.2010.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenford S. L., Smith S. S., Wetter D. W., Jorenby D. E., Fiore M. C., Baker T. B. (2002). Predicting relapse back to smoking: Contrasting affective and physical models of dependence. Journal of Consulting and Clinical Psychology, 70, 216–227.10.1037/0022-006X.70.1.216 [PubMed] [Google Scholar]
- Lasser K., Boyd J. W., Woolhandler S., Himmelstein D. U., McCormick D., Bor D. H. (2000). Smoking and mental illness: A population-based prevalence study. JAMA, 284, 2606–2610.10.1001/jama.284.20.2606 [DOI] [PubMed] [Google Scholar]
- McCarthy D. E., Piasecki T. M., Fiore M. C., Baker T. B. (2006). Life before and after quitting smoking: An electronic diary study. Journal of Abnormal Psychology, 115, 454–466.10.1037/0021-843X.115.3.454 [DOI] [PubMed] [Google Scholar]
- McNair D. M., Lorr M., Droppleman L. F. (1971). EITS manual for the profile of mood states. San Diego, CA: Educational and Industrial Testing Service [Google Scholar]
- Meuret A. E., Wolitzky-Taylor K. B., Twohig M. P., Craske M. G. (2012). Coping skills and exposure therapy in panic disorder and agoraphobia: Latest advances and future directions. Behavior Therapy, 43, 271–284.10.1016/j.beth.2011.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowrer O. H. (1960). Learning theory and behavior. New York: Wiley [Google Scholar]
- Parrott A. C. (1999). Does cigarette smoking cause stress? The American Psychologist, 54, 817–820.10.1037/0003-066X.54.10.817 [DOI] [PubMed] [Google Scholar]
- Patterson F., Jepson C., Kaufmann V., Rukstalis M., Audrain-McGovern J., Kucharski S., Lerman C. (2003). Predictors of attendance in a randomized clinical trial of nicotine replacement therapy with behavioral counseling. Drug and Alcohol Dependence, 72, 123–131.10.1016/S0376-8716(03)00194-7 [DOI] [PubMed] [Google Scholar]
- Piasecki T. M., Kenford S. L., Smith S. S., Fiore M. C., Baker T. B. (1997). Listening to nicotine: Negative affect and the smoking withdrawal conundrum. Psychological Science, 8, 184–189.10.1111/j.1467–9280.1997.tb00409.x [Google Scholar]
- Piasecki T. M., Niaura R., Shadel W. G., Abrams D., Goldstein M., Fiore M. C., Baker T. B. (2000). Smoking withdrawal dynamics in unaided quitters. Journal of Abnormal Psychology, 109, 74–86.10.1037/0021-843X.109.1.74 [DOI] [PubMed] [Google Scholar]
- Prochaska J. J., Delucchi K., Hall S. M. (2004). A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. Journal of Consulting and Clinical Psychology, 72, 1144–1156.10.1037/0022-006X.72.6.1144 [DOI] [PubMed] [Google Scholar]
- Quinn E. P., Brandon T. H., Copeland A. L. (1996). Is task persistence related to smoking and sustance use? Applying learned industriousness theory to addictive behaviors. Experimental and Clinical Psychopharmacology, 4, 186–190.10.1037/1064-1297.4.2.186 [Google Scholar]
- Shiffman S., Hickcox M., Paty J. A., Gnys M., Kassel J. D., Richards T. J. (1996). Progression from a smoking lapse to relapse: Prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. Journal of Consulting and Clinical Psychology, 64, 993–1002 [DOI] [PubMed] [Google Scholar]
- Shiffman S., Scharf D. M., Shadel W. G., Gwaltney C. J., Dang Q., Paton S. M., Clark D. B. (2006). Analyzing milestones in smoking cessation: Illustration in a nicotine patch trial in adult smokers. Journal of Consulting and Clinical Psychology, 74, 276–285.10.1037/0022-006X.74.2.276 [DOI] [PubMed] [Google Scholar]
- Waltz J., Addis M. E., Koerner K., Jacobson N. S. (1993). Testing the integrity of a psychotherapy protocol: Assessment of adherence and competence. Journal of Consulting and Clinical Psychology, 61, 620–630.10.1037/0022-006X.61.4.620 [DOI] [PubMed] [Google Scholar]
- Zhu S. H., Stretch V., Balabanis M., Rosbrook B., Sadler G., Pierce J. P. (1996). Telephone counseling for smoking cessation: Effects of single-session and multiple-session interventions. Journal of Consulting and Clinical Psychology, 64, 202–211 [DOI] [PubMed] [Google Scholar]