Abstract
Introduction:
Our aim was to understand the strength of association between parental smoking and child environmental tobacco smoke (ETS) exposure in order to inform the development of future tobacco control policies. ETS was measured using child cotinine levels below the active smoking threshold.
Methods:
Participants were drawn from the Avon Longitudinal Study of Parents and Children and included 3,128 participants at age 7 years and 1,868 participants at age 15 years. The primary outcome was cotinine levels of nonsmoking children, to investigate the relationship between maternal smoking and child cotinine levels. The secondary outcome was cotinine levels of all individuals to investigate the relationship between child smoking and child cotinine levels. Maternal and child smoking behavior was assessed by self-report questionnaire. We adjusted for several sociodemographic variables.
Results:
We found an association between maternal smoking and child cotinine at age 7 years (mean cotinine = 1.16ng/ml serum, ratio of geometric means = 3.94, 95% CI = 2.86–5.42) and at age 15 years (mean cotinine = 0.94ng/ml serum, ratio of geometric means = 5.26, 95% CI = 3.06–9.03), after adjustment for potential confounders.
Conclusions:
The magnitude of this association for children whose mothers were heavy smokers was comparable with the quantity of half the levels of cotinine observed among children who were irregular (i.e., nonweekly) active smokers, and it was greater than five times higher than that seen in nonsmoking children whose mothers didn’t smoke. This provides further evidence for the importance of public health interventions to reduce smoking exposure in the home.
INTRODUCTION
Environmental tobacco smoke (ETS) exposure (sometimes described as secondhand smoke exposure or “passive smoking”) is acknowledged to have detrimental health consequences, such as elevated risk for cardiovascular disease, lung cancer, and respiratory disease (Royal College of Physicians, 2010) and has been shown to be positively associated with smoking initiation in adolescents (Voorhees et al., 2011). As a result, tobacco control efforts have increasingly focused on limiting exposure to ETS, for example through the introduction of smoking bans in public places and workplaces. Despite this, there is reluctance to regulate cigarette smoking in certain areas such as private homes and cars (Mills, White, Pierce, & Messer, 2011; Semple et al., 2012). For children, parental smoking remains the major determinant of ETS exposure, with maternal smoking having been reported to play a more important role than paternal smoking (Jarvis et al., 1985). Understanding the strength of association between parental smoking and child ETS exposure will inform the development of future tobacco control policies.
The majority of studies of the association between parental smoking and ETS exposure in children have focused on children at an age when active smoking is a possibility (i.e., early adolescence onwards; Heron, Hickman, Macleod, & Munafo, 2011; Sims et al., 2010). This raises the potential problem of misclassification in such studies, where child reports of active smoking may be particularly inaccurate (Dolcini, Adler, Lee, & Bauman, 2003). Unfortunately, using cotinine levels to validate smoking status in children and young adolescents can be imprecise, when light, irregular smoking is common (Benowitz, 1996). Therefore, in order to more accurately assess the association between parental smoking and child ETS exposure, data on children collected at an age before active smoking is common are required. By comparing estimates at two ages, where active smoking is and is not likely, respectively, we can make indirect comparisons to assess the extent to which we have adequately adjusted for potential biases in measurement. The use of cotinine as an assessment for ETS exposure, which is the primary metabolite of nicotine, can further improve the precision of these comparisons. Earlier studies may have underestimated the health consequences of ETS exposure through the use of self-report measures of exposure given that adults are likely to underreport their active smoking in the presence of children (Jefferis et al., 2010; Whincup et al., 2004).
In this study, we sought to explore the association between maternal smoking behavior and child cotinine levels within nonsmokers, adjusting for other potential confounders. We also investigated the association child smoking has on cotinine levels of all participants. We used data from a birth cohort study based in the United Kingdom, where cotinine levels in the child were assessed when the child was aged 7 and 15 years. This allowed a comparison of the impact of maternal smoking behavior on child cotinine levels at an age when child smoking was unlikely (7 years) and at an age where it was more likely (15 years).
METHODS
Avon Longitudinal Study of Parents and Children
The sample for this study was drawn from the Avon Longitudinal Study of Parents and Children (ALSPAC) (Boyd et al., 2012), an on-going population-based cohort study in the South-West of England. Recruitment to ALSPAC began in 1990–1992. The complete case sample comprised 1,353 participants with data recorded at age 7 and 15 years. Since the age of 7 years, the ALSPAC study children have been invited to regular clinics for a variety of assessments. All aspects of the study were reviewed and approved by the ALSPAC Law and Ethics Committee, which is registered as an Institutional Review Board. Approval was also obtained from the National Health Service Local Research Ethics Committees.
Measures
The primary outcome in this study was child cotinine level at the approximate ages of 7 and 15 years for nonsmokers. The secondary outcome was child cotinine levels for all participants (smokers and nonsmokers) for 15 year olds. Cotinine was assayed from ethylenediaminetetraacetic acid serum plasma samples taken in a clinical assessment at 7 and 15 years. Plasma samples were stored at −80 °C and allowed to thaw at room temperature before use. Cotinine was measured using the Cozart Cotinine Enzyme Immunoassay (Concateno UK, Abingdon) serum kit (M155B1). All samples, calibrators, and controls were brought to room temperature before use and were run in duplicate. Where required, samples were diluted using cotinine-free serum (fetal calf serum). Absorbance was measured spectrophotometrically at a wavelength of 450nm. The lowest calibrator used was 0.5ng/ml serum, and values below this were treated as undetectable/null (0ng/ml serum). Cotinine concentrations are expressed as nanogram per milliliter of serum. The sample was restricted to nonsmokers by only using values below the cutoff of 9.5ng/ml (Jarvis, Fidler, Mindell, Feyerabend, & West, 2008) serum at ages 7 and 15 and for individuals who reported themselves to be nondaily smokers (at age 15).
Maternal smoking behavior was assessed at the same timepoints by self-report questionnaire (does not smoke, smokes fewer than 10 cigarettes/day, smokes 10 or more cigarettes/day). Child smoking behavior at age 15 years was also assessed by self-report questionnaire, which again measured heaviness of smoking but at a weekly cutoff due to the low number of daily smokers in this age group (does not smoke, smokes but not weekly, smokes weekly). Child smoking behavior at age 7 years was not measured.
Covariates included demographic variables collected pre-birth, which comprised sex, housing tenure (coded as owned/mortgaged, privately rented, subsidized housing rented from council/housing association), crowding status (coded as the ratio of number of residents to number of rooms in house), maternal educational attainment (coded as education up to 16 years and education up to 18 years or older), and family position (coded as whether study child is first/second/third child or greater), which have been shown to be associated with smoking behavior (Bard & Rodgers, 2003).
Statistical Analysis
Univariable linear regression was used to assess the relationship of sociodemographic variables and maternal smoking with nonsmoking child cotinine levels at age 7 and 15 years, which were natural log transformed due to nonnormality. Sociodemographic variables potentially associated with ETS exposure included sex, measures of deprivation (housing tenure, crowding status), and maternal educational attainment. Measures of smoking for the mother’s partner were incomplete, and because these were highly correlated with maternal smoking at both ages 7 and 15 (p < .001, r 2 = 0.07) these were not included, given their lack of contribution to the model and in order to reduce the extent of missing data. Multivariable linear regression was used to assess the relationship of maternal smoking with child cotinine levels at age 7 and 15 years for nonsmokers, with adjustment for sociodemographic variables. Multivariable linear regression was also used to assess the relationship of child smoking on cotinine levels at age 15 for all individuals (smokers and nonsmokers). In addition, the analysis of child smoking was adjusted for maternal smoking at age 15 years. Effect estimates for maternal and child smoking are presented as the ratio of geometric means following back transformation by exponentiation of log scale results. Analyses were conducted using Stata version 12 (StataCorp, 2011).
RESULTS
Sample Derivation and Description
Cotinine was measured on 5,641 children at 7 years of age (mean age = 7.54 years, SE = 0.05; mean cotinine = 1.21ng/ml serum, SE = 0.02), and 3,202 children at 15 years of age (mean age = 15.41 years, SE = 0.07; mean cotinine = 0.97ng/ml serum, SE = 0.02; Table 1). This was the main restriction for the univariable analysis, along with availability of data on each risk factor considered (sex, housing tenure, maternal education, crowding index, parity, mother smoking, and child smoking). For the multivariable analysis, we included participants on whom complete data were available at each age, which included 3,128 children at age 7 years and 1,868 children at age 15 years for the mother smoking model, and 2,015 individuals at age 15 for the child smoking model. The range of cotinine levels at 7 is 0–9.42ng/ml serum.
Table 1.
Descriptive Characteristics of Participants at Age 7 and 15 Years
| Age 7 | Age 15 | |||||
|---|---|---|---|---|---|---|
| n | Mean | SE | n | Mean | SE | |
| Total samplea | 5,641 | 1.21 | 0.02 | 3,202 | 0.97 | 0.02 |
| Complete case (nonsmokers)b | 3,128 | 1.16 | 0.02 | 1,868 | 0.94 | 0.03 |
| Complete case (all)c | – | – | – | 2,015 | 5.76 | 0.59 |
| Sex | ||||||
| Male | 2,906 | 1.19 | 0.02 | 1,584 | 1.02 | 0.03 |
| Female | 2,743 | 1.25 | 0.03 | 1,637 | 0.95 | 0.03 |
| Housing tenure | ||||||
| Owned | 4,480 | 1.13 | 0.02 | 1,807 | 0.93 | 0.02 |
| Private rent | 159 | 1.31 | 0.09 | 67 | 1.06 | 0.12 |
| Subsidized rent | 489 | 1.88 | 0.09 | 130 | 0.88 | 0.16 |
| Crowding index | ||||||
| <1 | 384 | 1.53 | 0.08 | 153 | 1.24 | 0.15 |
| 1–1.5 | 2,254 | 1.21 | 0.03 | 1081 | 0.97 | 0.04 |
| >1.5 | 2,002 | 1.15 | 0.02 | 922 | 0.92 | 0.04 |
| Maternal education | ||||||
| Up to 16 years | 3,099 | 1.27 | 0.02 | 1,751 | 1.03 | 0.03 |
| 18+ years | 1,573 | 1.03 | 0.02 | 1,110 | 0.85 | 0.03 |
| Number of siblings | ||||||
| 0 | 1,725 | 1.16 | 0.03 | 1,008 | 0.93 | 0.03 |
| 1 | 1,777 | 1.18 | 0.03 | 770 | 0.98 | 0.05 |
| 2 | 968 | 1.25 | 0.04 | 248 | 0.98 | 0.10 |
| 3 | 475 | 1.34 | 0.07 | 70 | 1.12 | 0.20 |
| 4+ | 598 | 1.35 | 0.06 | 60 | 1.14 | 0.17 |
| Mother smokes | ||||||
| 0 | 3,341 | 1.07 | 0.01 | 2,313 | 0.92 | 0.02 |
| <10 | 275 | 1.38 | 0.08 | 157 | 1.10 | 0.11 |
| 10+ | 432 | 2.17 | 0.09 | 235 | 1.56 | 0.13 |
Note. Cotinine levels (ng/ml serum) at 7 and 15 years are shown for: a the total number of nonsmoking individuals who have had cotinine measured (i.e., maximum number for the univariable analysis); bthe number of complete case nonsmoking individuals (i.e., who self-report as nonsmokers and are below the cutoff of 9.5ng/ml serum); and, cthe number of individuals on whom complete case data are available (i.e., also including smokers). Cotinine levels are also shown at each level of the predictors used. The geometric means are shown above.
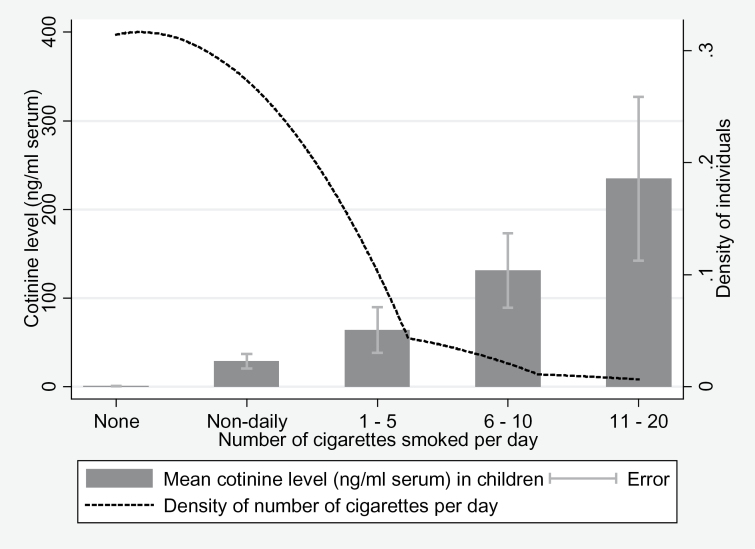
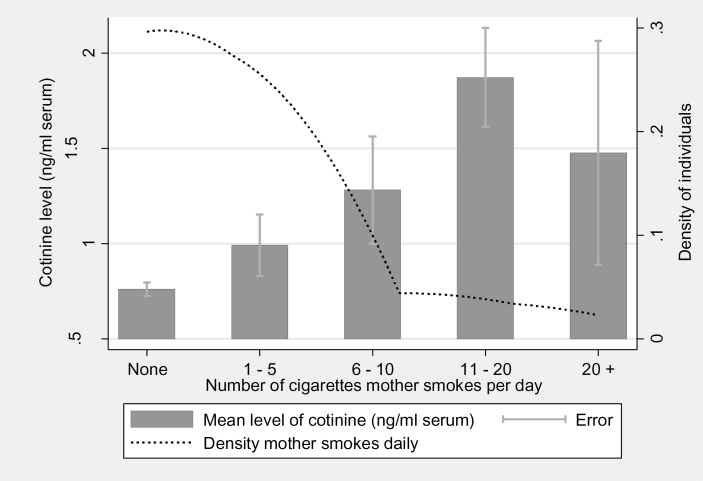
Child cotinine level at age 15 was associated with child smoking behavior at age 15 as expected (Figure 1). Child cotinine level at age 15 was also associated with maternal smoking behavior measured at the same timepoint, consistent with maternal smoking being a major source of ETS exposure (Figure 2). Univariable analysis indicated that maternal smoking was strongly associated with child cotinine levels (Table 2). Complete case data at age 15 years were used to confirm the results were representative in Table 2 of our principal study sample in Table 3 (Supplementary Table S1). The range of cotinine levels at age 15 is 0–9.31ng/ml serum. The findings were not substantially altered if we restrict the analysis to complete cases, 1,353 individuals who attended both assessment clinics at both ages 7 and 15 (Supplementary Table S2).
Figure 1.
Association of child cotinine level with child smoking behavior at age 15. The bar chart shows the means of child’s cotinine level (ng/ml serum) by number of cigarettes smoked per day (none, nondaily, 1–5, 6–10, 11–20) at age 15 years. A kernel density plot has been superimposed over this graph to show the density of individuals by the number of cigarettes smoked per day. This graph shows an increasing mean of cotinine level as the number of cigarettes smoked per day increases. The density of the population decreases as the number of cigarettes smoked per day increases.
Figure 2.
Association of child cotinine level with maternal smoking behavior at age 15. The bar chart shows the means of child’s cotinine level (ng/ml serum) at age 15 years within nonsmokers by number of cigarettes the mother smokes per day (none, 1–5, 6–10, 11–20, more than 20) measured at the same timepoint. A kernel density plot has been superimposed over this graph to show the density of children by the number of cigarettes the mother smokes per day. This graph shows an increasing mean of cotinine level as the number of cigarettes the mother smokes per day increases although this pattern reverses in very heavy smokers (20+ cigarettes/day). The density of the population decreases as the number of cigarettes smoked per day increases.
Table 2.
Univariable Analysis of Child Cotinine Levels at 7 and 15 Years for Nonsmokers and a Univariable Analysis of Child Smoking With Child Cotinine Levels at 15
| Predictor | 7 Years | 15 Years | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Ratio of geometric means | 95% CI | p value | n | Ratio of geometric means | 95% CI | p value | |
| Child sex | ||||||||
| Male | 2,904 | – | – | – | 1,574 | – | – | – |
| Female | 2,737 | 1.09 | 0.96, 1.26 | .186 | 1,628 | 0.73 | 0.59, 0.91 | .005 |
| Housing tenure | ||||||||
| Owned | 4,477 | – | – | – | 1,800 | – | – | – |
| Private rent | 159 | 1.60 | 1.07, 2.41 | .023 | 67 | 1.32 | 0.62, 2.80 | .471 |
| Subsidized rent | 487 | 3.56 | 2.80, 4.53 | <.001 | 128 | 2.58 | 1.49, 4.53 | .001 |
| Crowding Index | ||||||||
| <1 | 384 | – | – | – | 150 | – | – | – |
| 1– 1.5 | 2,250 | 0.48 | 0.36, 0.63 | <.001 | 1,075 | 0.65 | 0.38, 1.09 | .105 |
| >1.5 | 2,000 | 0.40 | 0.30, 0.53 | <.001 | 918 | 0.90 | 0.53, 1.54 | .705 |
| Maternal education | ||||||||
| Up to 16 years | 3,093 | – | – | – | 1,741 | – | – | – |
| 18+ years | 1,573 | 0.59 | 0.50, 0.68 | <.001 | 1,103 | 0.64 | 0.51, 0.81 | <.001 |
| Number of siblings | ||||||||
| 0 | 1,724 | – | – | – | 1,007 | – | – | – |
| 1 | 1,776 | 1.07 | 0.90, 1.27 | .439 | 765 | 1.03 | 0.77, 1.38 | .848 |
| 2 | 967 | 1.20 | 0.98, 1.46 | .086 | 243 | 0.58 | 0.38, 0.89 | .012 |
| 3 | 473 | 1.45 | 1.12, 1.90 | .005 | 68 | 1.17 | 0.55, 2.48 | .683 |
| 4+ | 595 | 1.32 | 1.04, 1.68 | .022 | 60 | 2.03 | 0.90, 4.53 | .084 |
| Mother smokes | ||||||||
| 0 | 3,341 | – | – | – | 2,301 | – | – | – |
| <10 | 273 | 1.95 | 1.43, 2.69 | <.001 | 156 | 1.73 | 1.05, 2.86 | .031 |
| 10+ | 431 | 5.10 | 3.94, 6.55 | <.001 | 230 | 5.10 | 3.35, 7.69 | <.001 |
| Child smokes | ||||||||
| No | – | – | – | – | 2,827 | – | – | – |
| Nonweekly | – | – | – | – | 395 | 2.30 | 2.14, 2.46 | <.001 |
| Weekly | – | – | – | – | 219 | 33.78 | 30.88, 37.34 | <.001 |
Note. CI = confidence interval.
Predictors comprised child sex, housing tenure (owned/mortgaged, privately rented, subsidized housing rented from council/housing association), maternal education, crowding index (ratio of number of residents to number of rooms in house), maternal education (education up to 16 years and education up to 18 years or older), parity (whether study child is first/second/third child or greater), and mother’s smoking status (does not smoke, smokes fewer than 10 cigarettes/day, smokes 10 or more cigarettes/day). All univariable analyses use cotinine levels within nonsmoking children, except the child smoking model, which includes smoking and nonsmoking participants.
Table 3.
Multivariable Analysis of Maternal Smoking on Child Cotinine Levels at 7 and 15 Years for Nonsmokers, and a Multivariable Analysis of Child Smoking on Child Cotinine Levels at 15 on All Individuals
| Predictor | 7 Years (n = 3,128) | 15 Years | ||||
|---|---|---|---|---|---|---|
| Ratio of geometric means | 95% CI | p value | Ratio of geometric means | 95% CI | p value | |
| Mother smokes | ||||||
| 0 | – | – | – | – | – | – |
| <10 | 1.58 | 1.08, 2.32 | .017 | 1.68 | 0.84, 2.89 | .106 |
| 10+ | 3.94 | 2.86, 5.42 | <.001 | 5.26 | 3.06, 9.03 | <.001 |
| Child smokes | ||||||
| No | – | – | – | – | – | – |
| Nonweekly | – | – | – | 1.90 | 1.75, 2.05 | <.001 |
| Weekly | – | – | – | 27.11 | 24.05, 30.57 | <.001 |
Note. CI = confidence interval.
Covariates included in the model comprised child sex, housing tenure (owned/mortgaged, privately rented, subsidized housing rented from council/housing association), maternal education, crowding index (ratio of number of residents to number of rooms in house), maternal education (education up to 16 years and education up to 18 years or older), parity (whether study child is first/second/third child or greater), and mother’s smoking status (does not smoke, smokes fewer than 10 cigarettes/day, smokes 10 or more cigarettes/day). The mother smoking model included individuals below the cutoff for active smoking (9.5ng/ml serum) and who self-report as nonsmokers (for 15 only). The child smoking model included all individuals; in addition, the model for child smoking included the mother’s smoking status. Maternal smoking at 15 years old, N =1,868; child smoking at 15 years, N = 2,015.
Environmental Tobacco Smoke Exposure at Age 7
Univariable analyses indicated that maternal smoking was associated with child cotinine levels (<10 cigarettes/day vs. nonsmoking mothers: ratio of geometric means [RGM] = 1.95, 95% confidence interval [CI] = 1.43–2.69, p < .001; 10+ cigarettes/day vs. nonsmoking mothers: RGM = 5.10, 95% CI = 3.94–6.55, p < .001) (Table 2). These results did not change substantially when restricted to the sample on which complete data were available (Supplementary Table S1). In multivariable analysis, this association remained although it was attenuated (<10 cigarettes/day vs. nonsmoking mothers: RGM = 1.58, 95% CI = 1.08–2.32, p = .017; 10+ cigarettes/day vs. nonsmoking mothers: RGM = 3.94, 95% CI = 2.86–5.42, p < .001) (Table 3). For individuals who attend assessment clinics at both ages 7 and 15, the RGM (Supplementary Table S2) was slightly higher although consistent with the findings presented in Table 3.
Environmental Tobacco Smoke Exposure at Age 15
Univariable analyses indicated that maternal smoking was associated with child cotinine levels (<10 cigarettes/day vs. nonsmoking mothers: RGM = 1.73, 95% CI = 1.05–2.86, p = .03; 10+ cigarettes/day vs. nonsmoking mothers: RGM = 5.10, 95% CI = 3.35–7.69, p < .001) (Table 2). These results did not change substantially when restricted to the sample on which complete data were available (Supplementary Table S1). In the multivariable analysis, which comprised the association between mother smoking and child cotinine levels at age 7 and 15 years controlling for covariates, the association of maternal smoking with child cotinine levels remained for heavy smoking mothers (10+ cigarettes/day), there was a slight attenuation for lighter smoking mothers (<10 cigarettes/day) but none for heavy smoking mothers; (<10 cigarettes/day vs. nonsmoking mothers: RGM = 1.68, 95% CI = 0.84–2.89, p = .164; 10+ cigarettes/day vs. nonsmoking mothers: RGM = 5.26, 95% CI = 3.06–9.03, p < .001) (Table 3). For individuals who attended assessment clinics at both ages 7 and 15, the RGM (Supplementary Table S2) was similar to that for the complete sample (Table 3).
Tobacco Smoke Exposure at Age 15
Child active smoking which uses cotinine levels of both smokers and nonsmokers, in order to quantify the effect of child smoking on cotinine levels, is strongly associated with child cotinine levels in the univariable analysis (nonweekly smoking vs. nonsmoking children: RGM = 2.30, 95% CI = 2.14–2.46, p < .001; weekly smoking vs. nonsmoking children: RGM = 33.78, 95% CI = 30.88–37.34, p < .001). These results did not substantially change when restricted to the sample on which complete data were available (Supplementary Table S1)
In the multivariable analysis, child smoking remained strongly associated with child cotinine levels (nonweekly smoking vs. nonsmoking children: RGM = 1.90, 95% CI = 1.75–2.05, p < .001; weekly smoking vs. nonsmoking children: RGM = 27.11, 95% CI = 24.05–30.57, p < .001) (Table 3).
DISCUSSION
We found a consistent association between maternal smoking and child cotinine levels at age 7 and 15 years in a large, representative sample of UK children and further provided a precise quantification of ETS exposure in these children through the use of cotinine as a biomarker. At age 7 years, exposure to ETS is likely to be predominantly from either the mother or partner (or both) (Sims et al., 2010). At this age, few children will be smoking themselves. Maternal smoking is likely to be the strongest influence on ETS exposure due to the likelihood of spending more time with the mother. At age 15 years, exposure to ETS is likely to be from either the mother or partner (or both), and peers who smoke, and many more children will be smoking themselves. It is possible that maternal smoking may have a weaker direct influence on ETS exposure at older age-groups. However, maternal smoking may also be correlated with exposure and “time with peers,” and thus could amplify the cotinine association at age 15. Despite this difference in the nature of the sources of nicotine (and therefore cotinine) exposure, the results from our multivariable analyses are consistent, indicating clear associations between maternal smoking and child cotinine levels at both ages. Most importantly, the magnitude of cotinine levels associated with heavy maternal smoking at 15 (10 or more cigarettes/day) in the multivariable analysis is comparable with 5.26 times the level of child with a nonsmoking mother, or nearly half the quantity of cotinine of an infrequent active smoker (heavy smoking mother, child cotinine level: 4.94ng/ml serum vs. infrequent active smoking levels: 10.93ng/ml serum). Child mean cotinine level at age 15 within nonsmokers increases with number of cigarettes the mother smokes, which is displayed in our figure (Figure 2). This shows a positive association between the two even at age 15. We also see a positive association between cotinine levels and the number of cigarettes smoked by child active smokers (Figure 1), with an increasing difference of cotinine levels measured between categories.
Although the harmful effects of ETS exposure are now well known (Gehrman & Hovell, 2003; Jaakkola & Jaakkola, 2002), this study provides further evidence of the magnitude of this exposure. Our data indicate that maternal smoking of 10 or more cigarettes a day is associated with high child cotinine levels and enables an indirect comparison with active smoking. However, this comparison should be interpreted with caution; ingested nicotine will not be as harmful as inhaled nicotine (e.g., no airway irritation). Given this, our study still provides a clear public health message and reinforces the importance of smoking in the home as a target for intervention, even in families of adolescent children where the relative impact of smoking in the home might be expected to be less than for younger children. Although the effects of ETS exposure, including the effects of smoking in the home on other family members including offspring, are used in public health campaigns, direct feedback of levels of exposure might promote behavior change. This is shown in the recent REFRESH study (Wilson et al., 2012). While cotinine levels are not amenable to this form of intervention, proxy measures of exposure such as air quality in the home might be a suitable alternative. This will require further study.
There are a number of limitations to this study, which should be considered when interpreting these results. First, immunoassay of cotinine has been shown to not be as precise as gas chromatography-mass spectrometric quantitative method for cotinine extraction. We were unable to include measures of partner smoking in our analysis because this would have reduced our available sample size considerably due to missing data. Second, and for the same reason, we were unable to include measures of peer smoking in our model. However, the stability of our estimates for the effects of maternal smoking at age 7 (when few children smoke) and 15 years (when many more are likely to do so) raises confidence in our results and suggests that the primary source of ETS exposure is from smoking in the home. Third, the maternal smoking question only takes into account the number of cigarettes the mother smokes per day, and does not indicate where these cigarettes are smoked. This could have a profound impact on ETS if, for example, the mother does not smoke inside the house or smokes in the car with the child present (Kalkbrenner et al., 2010). We were unable to consider other potential sources of nicotine, which the child may have come in to contact with, such as nicotine replacement therapy. However, although nicotine replacement therapy is available over the counter in the United Kingdom, we feel that it is unlikely that this would be a major source of nicotine exposure in children at age 15. There is a risk to toddlers by ingesting nicotine laden dust, “thirdhand smoke hazards” are an unappreciated health hazard as nicotine can react with ambient nitros oxide to form carcinogenic nitrosamines (Sleiman et al., 2010). It is unlikely that at 7 years old children are still showing behaviors of toddlers that might increase exposure (e.g., crawling). Unfortunately, these data are not available in ALSPAC. Similarly, we were unable to quantify the effects of peer smoking on ETS exposure. We should also consider the effects of the initiation of smoke-free legislation in England on the July 1, 2007. Sims et al. (2012) found post-legislative geometric means of cotinine fell by 27%, due to the reduced exposure to ETS in communal places such as pubs, bus stops, and restaurants. However, we did not observe this level of reduction at 15 years; which may be due to the lack of reduction of smoking in the home especially in households with heavy smoking mothers. Finally, variation in nicotine metabolism at different ages could be a potential explanation for the different levels of cotinine although evidence in support for such a hypothesis currently is weak (Dempsey et al., 2012).
In conclusion, we found clear evidence for association of maternal smoking with child ETS exposure, assessed using cotinine as a biomarker of nicotine exposure. In our multivariable analysis, the magnitude of this association for children with heavy smoking mothers was comparable with half the exposure observed among children who were irregular (i.e., nonweekly) active smokers. The majority of mothers agree that ETS exposure is a risk to the health of their children, but may erroneously believe that restrictions they have in place to minimize the child’s exposure are sufficient (Mills et al., 2012). Quantifying the magnitude of the exposure conferred by heavy smoking may serve to reinforce this important public health message and encourage either cessation in the mother or the enforcement of smoking restrictions in the home to reduce exposure.
SUPPLEMENTARY MATERIAL
Supplementary Tables S1 and S2 can be found online at http://www.ntr.oxfordjournals.org
FUNDING
MRM is a member of the UK Centre for Tobacco Control Studies, a UK Clinical Research Collaboration Public Health Research: Centre of Excellence. NJT and MRM work in the UK Medical Research Council and University of Bristol funded Integrative Epidemiology Unit. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration, are gratefully acknowledged. This research was funded in part by the Wellcome Trust (086684). NJT supported by Medical Research Council centre funding (MRC Centre for Causal Analyses in Translational Epidemiology) (Grant ref: 90600705). The UK Medical Research Council and the Wellcome Trust (Grant ref: 092731) and the University of Bristol provide core support for ALSPAC. This publication is the work of Alexander Stiby, Marcus Munafò, Nicholas Timpson, John Macleod, and Matthew Hickman will serve as guarantors for the contents of this article. AIS is supported by a Wellcome Trust 4-year PhD studentship in molecular, genetic, and lifecourse epidemiology (WT083431MA).
DECLARATION OF INTERESTS
None declared.
Supplementary Material
ACKNOWLEDGMENTS
We are extremely grateful to all the families who took part in the study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
REFERENCES
- Bard D. E., Rodgers J. L. (2003). Sibling influence on smoking behavior: A within-family look at explanations for a birth-order effect. Journal of Applied Social Psychology, 33, 1773–1795 doi:10.1111/j.1559–1816.2003.tb02080.x [Google Scholar]
- Benowitz N. (1996). Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiologic Reviews, 18, 188–204 doi:10.1093/oxfordjournals.epirev.a017925 [DOI] [PubMed] [Google Scholar]
- Boyd A., Golding J., Macleod J., Lawlor D. A., Fraser A., Henderson J. … Davey Smith G. (2012). Cohort profile: The ‘Children of the 90s’—the index offspring of the Avon Longitudinal Study of Parents and Children. International Journal of Epidemiology, 42, 111–127 doi:dys06410.1093/ije/dys064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey D. A., Meyers M. J., Oh S. S., Nguyen E. A., Fuentes-Afflick E., Wu A. H. … Benowitz N. L. (2012). Determination of tobacco smoke exposure by plasma cotinine levels in infants and children attending urban public hospital clinics. Archives of Pediatrics & Adolescent Medicine, Archpediatrics. doi:2012.2170 v2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcini M. M., Adler N. E., Lee P., Bauman K. E. (2003). An assessment of the validity of adolescent self-reported smoking using three biological indicators. Nicotine and Tobacco Results, 5, 473–483 doi:FEADEAKUJ1PQJ54A [PubMed] [Google Scholar]
- Gehrman C. A., Hovell M. F. (2003). Protecting children from environmental tobacco smoke (ETS) exposure: A critical review. Nicotine & Tobacco Research, 5, 289–301 doi:10.1080/1462220031000094231 [DOI] [PubMed] [Google Scholar]
- Heron J., Hickman M., Macleod J., Munafo M. R. (2011). Characterizing patterns of smoking initiation in adolescence: Comparison of methods for dealing with missing data [Research Support, Non-U.S. Gov’t]. Nicotine & Tobacco Research, 13, 1266–1275 doi:10.1093/ntr/ntr161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola J. J., Jaakkola M. S. (2002). Effects of environmental tobacco smoke on the respiratory health of children. Scandinavian Journal of Work, Environment & Health, 28(Suppl. 2), 71–83 Received from www.jstor.org/stable/40967256 [PubMed] [Google Scholar]
- Jarvis M. J., Fidler J., Mindell J., Feyerabend C., West R. (2008). Assessing smoking status in children, adolescents and adults: Cotinine cut-points revisited. Addiction, 103, 1553–1561 doi:10.1111/j.1360-0443.2008.02297.x [DOI] [PubMed] [Google Scholar]
- Jarvis M. J., Russell M. A., Feyerabend C., Eiser J. R., Morgan M., Gammage P., Gray E. M. (1985). Passive exposure to tobacco smoke: Saliva cotinine concentrations in a representative population sample of non-smoking schoolchildren. British Medical Journal (Clinical Research Edition), 291, 927–929 doi:10.1136/bmj.291.6500.927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis B. J., Lawlor D. A., Ebrahim S., Wannamethee S. G., Feyerabend C., Doig M. … Whincup P. H. (2010). Cotinine-assessed second-hand smoke exposure and risk of cardiovascular disease in older adults. Heart, 96, 854–859 doi:96/11/85410.1136/hrt.2009.191148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner A. E., Hornung R. W., Bernert J. T., Hammond S. K., Braun J. M., Lanphear B. P. (2010). Determinants of serum cotinine and hair cotinine as biomarkers of childhood secondhand smoke exposure. Journal of Exposure Science and Environmental Epidemiology, 20, 615–624 doi:10.1038/jes.2010.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A. L., White M. M., Pierce J. P., Messer K. (2011). Home smoking bans among U.S. households with children and smokers. Opportunities for intervention. American Journal of Preventive Medicine, 41, 559–565 doi:10.1016/j.amepre.2011.08.016 [DOI] [PubMed] [Google Scholar]
- Mills L. M., Semple S. E., Wilson I. S., Maccalman L., Amos A., Ritchie D. … Turner S. W. (2012). Factors influencing exposure to secondhand smoke in preschool children living with smoking mothers. Nicotine & Tobacco Research, 14, 1435–1444 doi:nts07410.1093/ntr/nts074 [DOI] [PubMed] [Google Scholar]
- Royal College of Physicians (2010). Passive smoking and children. London: Author [Google Scholar]
- Semple S., Apsley A., Galea K. S., Maccalman L., Friel B., Snelgrove V. (2012). Secondhand smoke in cars: Assessing children’s potential exposure during typical journey conditions. Tobacco Control, 21, 578–583 doi:10.1136/tobaccocontrol-2011–050197 [DOI] [PubMed] [Google Scholar]
- Sims M., Mindell J.S., Jarvis M.J., Feyerabend C., Wardle H., Gilmore A. (2012). Did smokefree legislation in England reduce exposure to secondhand smoke among nonsmoking adults? Cotinine analysis from the Health Survey for England. Environmental health perspectives, 120(3), 425–430 doi:10.1289/ehp.1103680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims M., Tomkins S., Judge K., Taylor G., Jarvis M. J., Gilmore A. (2010). Trends in and predictors of second-hand smoke exposure indexed by cotinine in children in England from 1996 to 2006. Addiction, 105, 543–553 doi:10.1111/j.1360-0443.2009.02805.x [DOI] [PubMed] [Google Scholar]
- Sleiman M., Gundel L. A., Pankow J. F., Jacob P., III, Singer B. C., Destaillats H. (2010). Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proceedings of the National Academy of Sciences, 107, 6576–6581 doi:10.1073/pnas.0912820107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp (2011). Stata Statistical Software: Release 12. College Station, TX: StataCorp LP [Google Scholar]
- Voorhees C. C., Ye C., Carter-Pokras O., MacPherson L., Kanamori M., Zhang G. … Fiedler R. (2011). Peers, tobacco advertising, and secondhand smoke exposure influences smoking initiation in diverse adolescents. American Journal of Health Promotion, 25, e1–11 doi:10.4278/ajhp.090604-QUAN-180 [DOI] [PubMed] [Google Scholar]
- Whincup P. H., Gilg J. A., Emberson J. R., Jarvis M. J., Feyerabend C., Bryant A. … Cook D. G. (2004). Passive smoking and risk of coronary heart disease and stroke: Prospective study with cotinine measurement. British Medical Journal, 329, 200–205 doi:10.1136/bmj.38146.427188.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I., Semple S., Mills L. M., Ritchie D., Shaw A., O’Donnell R. … Amos A. (2012). REFRESH—reducing families’ exposure to secondhand smoke in the home: A feasibility study. Tobacco Control, 27, 85–92 doi:10.1136/tobaccocontrol-2011-050212 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.