Abstract
Background:
Neurofeedback delivered via real-time functional magnetic resonance imaging (rtfMRI) is a promising therapeutic technique being explored to facilitate self-regulation of craving in nicotine-dependent cigarette smokers. The current study examined the role of nicotine-dependence severity and the efficacy of multiple visits of neurofeedback from a single region of interest (ROI) in the anterior cingulate cortex (ACC) on craving reduction.
Methods:
Nine nicotine-dependent cigarette smokers participated in three rtfMRI visits that examined cue-induced craving and brain activation. Severity of nicotine dependence was assessed with the Fagerström Test for Nicotine Dependence. When viewing smoking-related images with instructions to “crave,” patient-tailored ROIs were generated in the vicinity of the ACC. Activity levels from the ROI were fed back while participants viewed smoking cues with the instruction to reduce craving.
Results:
Neurofeedback from a single ROI in the ACC led to consistent decreases in self-reported craving and activation in the ACC across the three visits. Dependence severity predicted response to neurofeedback at Visit 3.
Conclusions:
This study builds upon previous rtfMRI studies on the regulation of nicotine craving in demonstrating that feedback from the ACC can reduce activation to smoking cues across three separate visits. Individuals with lower nicotine-dependence severity were more successful in reducing ACC activation over time. These data highlight the need to consider dependence severity in developing more individualized neurofeedback methods.
INTRODUCTION
Cigarette craving is a key factor contributing to relapse among smokers attempting abstinence (Allen, Bade, Hatsukami, & Center, 2008; Ferguson & Shiffman, 2009; Killen & Fortmann, 1997), and therapies that help smokers control their craving may facilitate smoking cessation. Neurofeedback, delivered via real-time functional magnetic resonance imaging (rtfMRI), can facilitate self-regulation of internal states by providing feedback from localized regions of interest (ROIs) to individuals while performing a task (Hartwell et al., 2013; Weiskopf, 2012). In recent years, rtfMRI feedback has demonstrated therapeutic potential by facilitating modulation of brain activation associated with pain, depression, and Parkinson’s disease (deCharms et al., 2005; Linden et al., 2012; Subramanian et al., 2011).
In a recent rtfMRI study from our group, nicotine-dependent smokers used neurofeedback from the anterior cingulate cortex (ACC), a key craving region, to reduce craving-related brain activation and self-reported craving in one visit (Li et al., 2013). Another study found that feedback from a craving-related ROI in the ACC was used more effectively than a resistance-related ROI in the medial prefrontal cortex over multiple sessions of simultaneous feedback (Hanlon et al., 2013). Although these studies attest to the therapeutic potential of neurofeedback, an important question is whether multiple sessions of neurofeedback from the ACC alone can improve modulation of brain activity. Additionally, individual factors that may impact neurofeedback response in smokers have not been explored. Dependence severity, for example, is associated with craving and smoking cessation outcomes (Breslau & Johnson, 2000; Watson, Carpenter, Saladin, Gray, & Upadhyaya, 2010) and has been linked with ACC activation during exposure to smoking cues (McClernon, Kozink, & Rose, 2008; Smolka et al., 2006). Thus, severity of nicotine dependence may influence response to neurofeedback. The purpose of the current study was to determine (a) the efficacy of multiple ACC neurofeedback sessions, and (b) the impact of dependence severity on the effect of multiple rtfMRI visits on craving-related brain activation.
METHOD
Participants
Participants (N = 9; 8 males) were right-handed, nicotine-dependent (≥10 cigarettes a day) smokers. Participants reported having motivation to quit but were not undergoing treatment. Exclusion criteria included the use of tobacco products other than cigarettes, current use of nicotine replacement therapy, bupropion, or varenicline, medical conditions or medications that could affect brain function, pregnancy, non-nicotine substance dependence or abuse, and history of any major psychiatric disorder. Additional fMRI-related exclusionary criteria included previous brain injury, nonremovable metal implants or objects, and claustrophobia.
Participants between 18 and 60 years old were recruited through local flyers, newspapers, and Internet advertisements. Study procedures were approved by the Medical University of South Carolina Institutional Review Board.
Measures
During initial assessment, the Mini-International Neuropsy chiatric Interview (Sheehan et al., 1998) and a physical examination were completed. Exhaled carbon monoxide level (≥10 p.p.m.) was measured with a MicroSmokelyzer (Bedfont Scientific Ltd.) to assess recency of smoking. Dependence was assessed at the first scanning visit using the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
Scanning Procedures
Participants were instructed not to smoke for 2hr before each of three separate scanning visits. Each visit consisted of four 10-min scanning runs: an ROI isolation run (Run 1) and three neurofeedback runs (Runs 2–4) (Supplementary Figure S1). Each run was composed of a smoking cue-reactivity task used previously (e.g., Hartwell et al., 2011; Li et al., 2013). Stimuli were presented with EPrime 2.0 software (Psychology Software Tools) via a mirrored projector. Runs contained three block types: “smoke” (smoking related) and “neutral” (matched objects) images, and “rest” (crosshair). Blocks contained five randomly ordered pictures displayed for 4.4 s each. Images were repeated across runs and visits. During Run 1, individuals were instructed to “crave” when viewing the “smoke” images and a three-slice craving-related ROI was selected approximate to the ACC (t-value threshold = 3, cluster size threshold = 4). Feedback from this ROI was provided in the subsequent runs. Neurofeedback runs (Runs 2–4) began with a craving baseline period followed by five craving-reduction blocks. It was explained to participants that the thermometer bar would reflect the level of craving-related brain activity. Verbal instructions were given to reduce the urge to smoke and reduce the thermometer bar when viewing the “smoke” images, and a screen with instruction to reduce craving was presented before each “smoke” block. Specific instructions were not given for neutral and rest blocks. Stimulus blocks were followed by screens displaying a 5-point self-rating of craving followed by the neurofeedback display (thermometer; Supplementary Figure S1).
Prior to each run, participants handled and smelled a preferred brand cigarette. Verbal craving ratings (1 = low to 10 = high) were acquired before and after each run.
Image Acquisition
Scanning was performed with a Siemens 3-Tesla TIM Trio using a standard multislice single-shot gradient echo-planar imaging sequence (repetition time = 2.2 s, echo time = 35ms, 64×64 matrix, parallel imaging factor of 2, 3×3 × 3mm voxels, 271 volumes, 36 slices).
Real-Time Data Processing
Real-time in-scan processing was performed using Turbo-Brain Voyager (TBV) 2.0 software (Maastricht, The Netherlands), using a fast connection between the MRI scanner and the analysis/display computer (Supplementary Figure S2). Real-time preprocessing included motion correction and spatial smoothing using an 8mm3 Gaussian kernel.
The following settings were used for generating neurofeedback: average values to calculate feedback value = 6 timepoints, maximum percent signal change (PSC) displayed in feedback bar = 2.5, general linear model baseline enabled for stable baseline estimation, and dynamic ROI enabled (using best voxel selection of top 33%). The resultant signal estimate for each incoming functional imaging volume within the ROI was displayed to the participant. The initial thermometer value reflected the difference between response to “smoke” images during neurofeedback relative to craving baseline ([feedback–crave]/crave × 100). Response during “rest” blocks previous to each feedback was incorporated in subsequent calculations.
Offline ROI Analysis
Off-line fMRI data analysis was performed using SPM8 (Wellcome Trust) in Matlab 7.3 (Mathworks Inc.). Preprocessing steps included realignment, normalization to Montreal Neurological Institute space with a resolution of 3mm3 voxels, and smoothing with an isotropic 8mm3 Gaussian kernel. Time-series of each individual’s neurofeedback ROI across each run was extracted from spatially smoothed data using Marsbar (Brett, Anton, Valabregue, & Poline, 2002). Data were averaged across blocks. PSC from rest was calculated for neurofeedback ([feedback smoke−rest]/rest × 100) during Runs 2–4 and crave ([smoke−rest]/rest × 100) during Run 1.
To examine the specificity of the neurofeedback response to smoking images, PSC was also calculated for neutral images. To examine the specificity of the neurofeedback response in the ROI, time-series were extracted from a lateral region of the inferior frontal gyrus (IFG; BA 47), a region involved in regulatory processes but not used for feedback.
Statistical analyses examined the (a) effect of multiple neurofeedback runs on PSC and postfeedback craving, (b) effect of neurofeedback on PSC and craving reduction during multiple visits, and (c) effect of dependence severity on craving-related brain activation. Two 3×3 repeated-measures analyses of variance (RM-ANOVA) assessed the effect of the three runs across the three visits on neurofeedback PSC scores and postfeedback verbal craving ratings. Two 2×3 RM-ANOVAs assessed the effect of visit and condition on (a) PSC (crave PSC, neurofeedback PSC) and (b) craving report (prefeedback, postfeedback). In addition, 2×3 RM-ANOVAS examined the effect of visit and condition on PSC to neutral images and PSC to smoking images in the IFG. The role of dependence severity (FTND score) on reductions in craving-related activation (neurofeedback PSC) at each visit was explored with linear regressions.
RESULTS
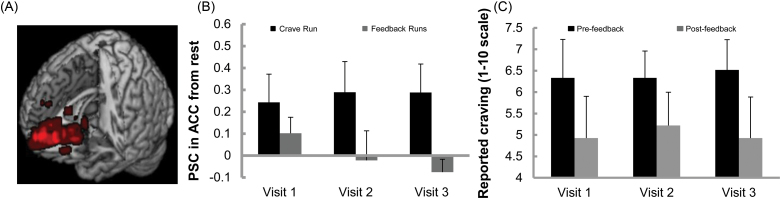
Mean FTND score was 3.67 (SD = 1.73), and mean exhaled CO levels were 15.44 p.p.m. (SD = 7.14). Participants (mean age = 32.7, SD = 13.01) reported smoking an average of 13.44 cigarettes a day (SD = 4.30). Participants completed the visits with an average of 5.33 days between Visits 1 and 2 (SD = 4.09) and 4.33 days between Visits 2 and 3 (SD = 2.92). Individual craving-related ROIs selected during Run 1 and used for rtfMRI feedback were approximate to the ACC (Figure 1a).
Figure 1.
Location of region of interests (ROIs) and response to feedback across visits. (A) The individual craving-related ROIs isolated for feedback during Run 1. The ROIs customized for each participant at each visit are plotted in Montreal Neurological Institute space (N = 27). Brighter color = greater ROI overlap. (B) Main effect of condition in repeated-measures analyses of variance (RM-ANOVA) showing that PSC in the ACC was significantly lower during feedback runs (M = .00) compared with the crave run (M = .27), p = .004. Means for all three visits shown. (C) Main effect of condition in RM-ANOVA showing that craving reports were significantly lower after feedback (M = 5.03) compared with before feedback (M = 6.40), p = .024. Means for all three visits shown.
Multiple Feedback Runs
Analysis revealed no effect of feedback run number or an interaction between run and visit on PSC in the ACC (p = .551, .729) or craving (p = .398, .217). Thus, the three runs were averaged within visits.
Effect of Neurofeedback Across Visits
One outlier during the Visit 3 “crave” run (PSC > 2 SD above mean) was excluded from the PSC analysis to reduce bias in the means. Analysis showed a significant effect of condition on PSC, F(1, 7) = 18.27, p =.004 (Figure 1b). PSC in the ACC was lower during neurofeedback (M = .00) compared with the crave run (M = .27). There was also a significant effect of condition on craving, F(1, 8) = 7.73, p =.024 (Figure 1c). Craving reports were lower after feedback (M =5.03) compared with before feedback (M = 6.40). There were no effects of visit number or interactions. Although greater decreases in PSC tended to be associated with lower craving, they were not significantly correlated (rho = .45, p =.224).
Examination of Controls
PSC during the neutral blocks did not differ between crave (M =.12) and neurofeedback (M = .07) runs (p = .403). PSC in the IFG also did not differ between crave (M = .21) and neurofeedback (.18) runs (p = .661). There were no effects of visit number or interactions.
Role of Dependence Severity
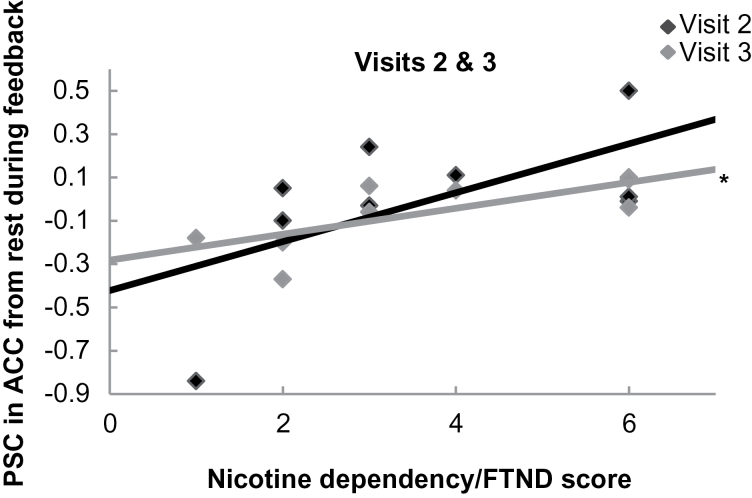
FTND score did not predict neurofeedback PSC at Visit 1, b = −.46, t(7) = −1.38, p = .211. However, FTND score trended toward predicting neurofeedback PSC at Visit 2, b = .61, t(7) = 2.04, p = .081, and significantly predicted PSC at Visit 3, b = .72, t(7) = 2.75, p = .028, R 2 = .52 (Figure 2).
Figure 2.
Relationship between nicotine dependency and anterior cingulate cortex (ACC) activation at neurofeedback Visits 2 and 3. Nicotine dependence as measured by the Fagerström Test for Nicotine Dependence (FTND) did not predict modulation of the neurofeedback region of interest in response to smoking cues at the first visit. At Visit 2, a positive trend began to emerge and at Visit 3, FTND did predict neural response to feedback (*p = .03), suggesting that participants with lower dependence were better able to use the feedback with time. Lower percent signal change (PSC) values indicate greater decreases in crave-related brain activation.
DISCUSSION
This preliminary study supports and expands previous research demonstrating that low-to-moderate nicotine-dependent smokers can use neurofeedback from the ACC to decrease craving-related activation (Hanlon et al., 2013; Li et al., 2013). The current study shows that feedback from a single ROI in the ACC results in consistent decreases in ACC activity and craving across three visits, and that successful use of rtfMRI over time may be related to severity of nicotine dependence.
Reductions in ACC activity and craving were evident at the first visit and consistent across visits and runs, suggesting that effective regulatory strategies can be learned quickly with rtfMRI feedback. The ability of participants to modulate ACC activity using neurofeedback from the first visit is consistent with previous studies of other patient populations (e.g., Caria et al., 2007; Hamilton, Glover, Hsu, Johnson, & Gotlib, 2011). The changes in activity in the ACC were not found in response to neutral cues, and neurofeedback did not result in craving-related modulation of the IFG. Without a control condition, however, the reduced response to smoking cues during feedback conditions must be accepted with caution.
Although there was no change in the effectiveness of neurofeedback over time across the whole sample, exploratory analyses found that severity of nicotine dependence affected the response to feedback over visits. Thus, it appears that neurofeedback may be more effective at reducing craving-related activation over time in people with lower nicotine dependence, whereas those smokers with higher dependence ratings fail to improve. This is consistent with research showing that nicotine dependence affects treatment and cessation outcomes (e.g., Fagerström & Hughes, 2008; Hyland et al., 2004). Highly addicted smokers motivated to quit are often only able to quit smoking for a few hours (Shiffman et al., 2006), suggesting greater vulnerability to craving and/or withdrawal symptoms. Effective modulation of cue-elicited craving is crucial to successful smoking cessation, as most first-line pharmacotherapies are minimally effective at attenuating the cue-elicited craving that frequently leads to smoking lapses (Ferguson & Shiffman, 2009; Tiffany, Cox, & Elash, 2000). It is possible that an alternative feedback paradigm could aid highly dependent individuals in controlling craving. For instance, shorter training periods might reduce the withdrawal and frustration associated with the relatively long scanner times; also, training of specific strategies to manage craving might be beneficial.
These preliminary findings must be interpreted with caution considering the significant limitations including the small sample of mostly men. Importantly, due to the lack of a control group, it cannot be ruled out that the craving reduction was due to the instructions to reduce craving alone. In addition, the average FTND score of smokers was low to moderate, which limits the generalizability to highly addicted smokers. Ongoing studies by our group involve a larger, more diverse sample, and a relevant control. However, the current findings support the continued investigation of rtfMRI as a research and therapeutic tool in addiction.
SUPPLEMENTARY MATERIAL
Supplementary Figures S1 and S2 can be found online at http://www.ntr.oxfordjournals.org.
FUNDING
This work was supported by the National Institute on Drug Abuse at the National Institutes of Health (R33DA026085 to KTB and MSG). Effort was supported by the National Institutes of Health (T32DA02788 to MC and K01DA0267756 to CAH).
DECLARATION OF INTERESTS
None declared.
Supplementary Material
REFERENCES
- Allen S. S., Bade T., Hatsukami D., Center B. (2008). Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine & Tobacco Research, 10, 35–45.10.1080/14622200701705076 [DOI] [PubMed] [Google Scholar]
- Breslau N., Johnson E. O. (2000). Predicting smoking cessation and major depression in nicotine-dependent smokers. American Journal of Public Health, 90, 1122–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J., Valabregue R., Poline J. (2002). Region of interest analysis using an SPM toolbox. Abstract Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan Available on CD-ROM in NeuroImage, Vol 16 [Google Scholar]
- Caria A., Veit R., Sitaram R., Lotze M., Weiskopf N., Grodd W., Birbaumer N. (2007). Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage, 35, 1238–1246.10.1016/j.neuroimage.2007.01.018 [DOI] [PubMed] [Google Scholar]
- deCharms R. C., Maeda F., Glover G. H., Ludlow D., Pauly J. M., Soneji D. ,… Mackey S. C. (2005). Control over brain activation and pain learned by using real-time functional MRI. Proceedings of the National Academy of Sciences of the United States of America, 102, 18626–18631.10.1073/pnas.0505210102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström K., Hughes J. (2008). Varenicline in the treatment of tobacco dependence. Neuropsychiatric Disease and Treatment, 4, 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. G., Shiffman S. (2009). The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment, 36, 235–243.10.1016/j.jsat.2008.06.005 [DOI] [PubMed] [Google Scholar]
- Hamilton J. P., Glover G. H., Hsu J. J., Johnson R. F., Gotlib I. H. (2011). Modulation of subgenual anterior cingulate cortex activity with real-time neurofeedback. Human Brain Mapping, 32, 22–31.10.1002/hbm.20997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon C. A., Hartwell K. J., Canterberry M., Li X., Owens M., Lematty T. ,… George M. S. (2013). Reduction of cue-induced craving through realtime neurofeedback in nicotine users: The role of region of interest selection and multiple visits. Psychiatry Research, 213, 79–81.10.1016/j.pscychresns.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell K. J., Johnson K. A., Li X., Myrick H., LeMatty T., George M. S., Brady K. T. (2011). Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addiction Biology, 16, 654–666.10.1111/j.1369-1600.2011.00340.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell K. J., Prisciandaro J. J., Borckardt J., Li X., George M. S., Brady K. T. (2013). Real-time fMRI in the treatment of nicotine dependence: A conceptual review and pilot studies. Psychology of Addictive Behaviors, 27, 501–509.10.1037/a0028215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. (1991). The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction, 86, 1119–1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hyland A., Li Q., Bauer J. E., Giovino G. A., Steger C., Cummings K. M. (2004). Predictors of cessation in a cohort of current and former smokers followed over 13 years. Nicotine & Tobacco Research, 6(Suppl. 3),S363–S369. 10.1080/14622200412331320761 [DOI] [PubMed] [Google Scholar]
- Killen J. D., Fortmann S. P. (1997). Craving is associated with smoking relapse: Findings from three prospective studies. Experimental and Clinical Psychopharmacology, 5, 137–142. 10.1037/1064-1297.5.2.137 [DOI] [PubMed] [Google Scholar]
- Li X., Hartwell K. J., Borckardt J., Prisciandaro J. J., Saladin M. E., Morgan P. S. ,… George M. S. (2013). Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: A preliminary real-time fMRI study. Addiction Biology, 18, 739–748.10.1111/j.1369-1600.2012.00449.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden D. E., Habes I., Johnston S. J., Linden S., Tatineni R., Subramanian L. ,… Goebel R. (2012). Real-time self-regulation of emotion networks in patients with depression. PLoS One, 7, e38115.10.1371/journal.pone.0038115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon F. J., Kozink R. V., Rose J. E. (2008). Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology, 33, 2148–2157.10.1038/sj.npp.1301618 [DOI] [PubMed] [Google Scholar]
- Sheehan D. V., Lecrubier Y., Sheehan K. H., Amorim P., Janavs J., Weiller E. ,… Dunbar G. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of Clinical Psychiatry, 59(Suppl 20),22–33; quiz 34 [PubMed] [Google Scholar]
- Shiffman S., Scharf D. M., Shadel W. G., Gwaltney C. J., Dang Q., Paton S. M., Clark D. B. (2006). Analyzing milestones in smoking cessation: Illustration in a nicotine patch trial in adult smokers. Journal of Consulting and Clinical Psychology, 74, 276–285.10.1037/ 0022-006X.74.2.276 [DOI] [PubMed] [Google Scholar]
- Smolka M. N., Bühler M., Klein S., Zimmermann U., Mann K., Heinz A., Braus D. (2006). Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology, 184, 577–588.10.1007/s00213- 005-0080-x [DOI] [PubMed] [Google Scholar]
- Subramanian L., Hindle J. V., Johnston S., Roberts M. V., Husain M., Goebel R., Linden D. (2011). Real-time functional magnetic resonance imaging neurofeedback for treatment of Parkinson’s disease. The Journal of Neuroscience, 31, 16309–16317.10.1523/JNEUROSCI.3498-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany S. T., Cox L. S., Elash C. A. (2000). Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. Journal of Consulting and Clinical Psychology, 68, 233–240. 10.1037/0022- 006X.68.2.23 [DOI] [PubMed] [Google Scholar]
- Watson N. L., Carpenter M. J., Saladin M. E., Gray K. M., Upadhyaya H. P. (2010). Evidence for greater cue reactivity among low-dependent vs. high-dependent smokers. Addictive Behaviors, 35, 673–677.10.1016/j.addbeh.2010.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N. (2012). Real-time fMRI and its application to neurofeedback. Neuroimage, 62, 682–692.10.1016/ j.neuroimage.2011.10.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.