Abstract
Objectives
The phase 3 trial, RESPOND-2, demonstrated that the addition of boceprevir (BOC) to peginterferon-ribavirin (PR) resulted in significantly higher rates of sustained virologic response (SVR) in previously treated patients with chronic hepatitis C virus (HCV) genotype-1 infection as compared with PR alone. We evaluated the cost-effectiveness of treatment with boceprevir in previously treated chronic hepatitis C patients in the United States utilizing treatment related data from RESPOND-2 and PROVIDE.
Methods
We developed a Markov cohort model to project the burden of HCV disease, lifetime costs and quality-adjusted life years (QALY) associated with PR and two BOC-based therapies—response-guided therapy (BOC/RGT) and fixed-duration therapy for 48 weeks (BOC/PR48). We estimated treatment related inputs (efficacy, adverse events, and discontinuations) from clinical trials and obtained disease progression rates, costs and quality-of-life data from published studies. We estimated the incremental cost-effectiveness ratio (ICER) for BOC-based regimens as studied in RESPOND-2, as well as by patient’s prior response to treatment and IL-28B genotype.
Results
Boceprevir-based regimens were projected to reduce the lifetime incidence of liver-related complications by 43–53% in comparison with treatment with PR. The ICER of BOC/RGT in comparison with PR was $30,200, and the ICER of BOC/PR48 in comparison with BOC/RGT was $91,500. At $50,000 willingness-to-pay, the probabilities BOC/RGT and BOC/PR48 being the preferred option were 0.74 and 0.25, respectively.
Conclusions
In patients previously treated for chronic HCV genotype-1 infection, boceprevir was projected to increase QALYs and reduce the lifetime incidence of liver complications. In addition, boceprevir-based therapies were projected to be cost-effective in comparison with PR alone at commonly used willingness-to-pay thresholds.
Keywords: protease inhibitor, Hepatitis C, Markov model, decision analytic
Chronic infection with hepatitis C virus (HCV) is a major public health problem, with more than 170 million people infected worldwide [1,2]. In the United States, chronic HCV infection is a leading cause of chronic liver diseases and hepatocellular carcinoma (HCC), and is the most common indication for liver transplantation [1]. In 2007, there were 15,000 deaths related to HCV infection in the United States, surpassing the nearly 13,000 deaths caused by HIV infection [3].
Of the six HCV genotypes, genotype 1 is the most prevalent in the United States and accounts for at least 70% of all chronic infections, followed by genotypes 2 and 3 (14% and 8%, respectively) [4]. HCV genotype 1 is also the most difficult to treat with a combination of peginterferon-ribavirin (PR) – less than 50% of treated patients achieve a sustained virologic response (SVR), which is the primary goal of the treatment. Response rates are even lower in non-responders (15.6%, C.I.:12.4%–19.4%) to previous PR therapy who are retreated with PR [5].
The launch of two protease inhibitors (PIs), boceprevir and telaprevir in 2011 represents a major advance in the treatment of chronic HCV with significant improvements in SVR rates [6–9]. The Serine Protease Inhibitor Boceprevir and PegIntron/Rebetol-2 (RESPOND-2) trial, an international, randomized, multicenter, double-blind study, demonstrated that boceprevir, when added to PR, leads to high SVR rates in genotype 1 patients who failed prior treatment with PR therapy [8]. The SVR rates were significantly higher in the two boceprevir-containing regimens (59% and 66%) than in the control regimen of PR alone (21%, P<0.001). RESPOND-2 did not include null-responders. PROVIDE, a single-arm trial, however, evaluated the effectiveness of boceprevir in prior null responders and reported significantly higher SVR rates of 39% in comparison with a low historic rate of 16% using PR [10]. Similarly, the pivotal trial of telaprevir, REALIZE, evaluated the addition of telaprevir to PR in patients with HCV genotype 1 infection who had no response or a partial response to previous therapy or who had a relapse after an initial response [7]. The SVR rates were significantly higher (33–83%) with telaprevir-based regimens than in the control groups.
By substantially increasing the SVR rates, the use of PIs is expected to influence the course of the disease by reducing the incidence of liver-related complications and deaths. Since the treatment cost of PI-based triple therapy is substantially higher compared to PR therapy, it is not clear if the PI-containing regimens provide sufficient value in patients who failed prior treatment with PR. The main objective of our study was to evaluate the cost-effectiveness of boceprevir-based regimens as studied in RESPOND-2 and PROVIDE in comparison with PR alone in previously treated patients with chronic HCV genotype 1 infection. In addition, we also evaluated the cost-effectiveness of telaprevir in previously treated patients using data from REALIZE.
METHODS
We created a multi-cohort Markov model that simulated each cohort through the trial design of RESPOND-2, and projected health-related outcomes (costs and benefits) beyond the time-period of the trial using the natural history of progression of HCV disease. Each cohort was determined by the following risk factors or demographic characteristics: age (mean age), sex (male/female), and baseline fibrosis score (F0–F4). The patient characteristics were based on the patients enrolled in the RESPOND-2 (Table 1). A total of 10 different patient profiles from RESPOND-2 defined the cohorts explored in our model.
Table 1.
Baseline patient characteristics from RESPOND-2
| Characteristics | N = 403 |
|---|---|
| Gender – no. (%) | |
| Male | 268 (67) |
| Female | 135 (33) |
| Age – years | |
| Mean | 52.7 |
| Standard Deviation | 7.7 |
| Range | 26–74 |
| Race – no. (%) | |
| Black | 49 (12) |
| Non-Black | 354 (88) |
| Prior Treatment Experience – no. (%)* | |
| Non-Responders | 144 (36) |
| Relapsers | 259 (64) |
| Baseline METAVIR Score – no. (%)† | |
| F0 – no fibrosis | 18 (4) |
| F1 – portal fibrosis without septa | 200 (50) |
| F2 – portal fibrosis with few septa | 79 (20) |
| F3 – numerous septa without cirrhosis | 29 (7) |
| F4 – cirrhosis | 49 (12) |
| Missing‡ | 28 (7) |
Prior nonresponders had a decrease in plasma HCV-RNA of at least 2-log10 by week 12 of prior therapy but with detectable HCV-RNA throughout the course of therapy. Prior relapsers had undetectable HCV-RNA at end of prior therapy without subsequent attainment of a sustained virologic response.
A central pathologist determined fibrosis score. Twenty-eight patients had missing data.
Patients with missing METAVIR score were not included in the model.
Treatment regimens based on RESPOND-2 Study design
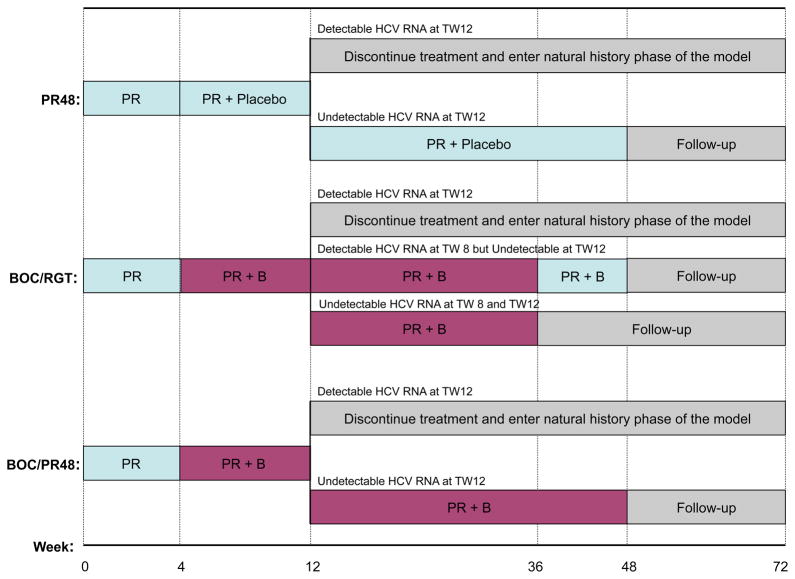
The trial randomized 403 patients in a 1:2:2 ratio to one of three treatment groups (Fig. 1). The first group received PR for 48 weeks (abbreviated as PR48). The second group received response-guided-therapy (RGT), starting with 4-weeks of PR, followed by boceprevir plus PR for 32 weeks (abbreviated as BOC/RGT). Those with undetectable HCV-RNA at weeks 8 and 12 completed therapy at week 36, whereas those with detectable HCV-RNA at week 8 (but undetectable at week 12) received PR for an additional 12 weeks. The third group received the 4-weeks PR followed by boceprevir plus PR for 44 weeks (abbreviated as BOC/PR48). In all three groups, patients who failed to achieve undetectable HCV-RNA at week 12 were discontinued and entered follow-up, regardless of their previous HCV-RNA measurements. At the end of the treatment, patients were followed up to week 72.
Figure 1.
Strategies based on RESPOND-2 trial for treatment-experienced patients.
Model Structure
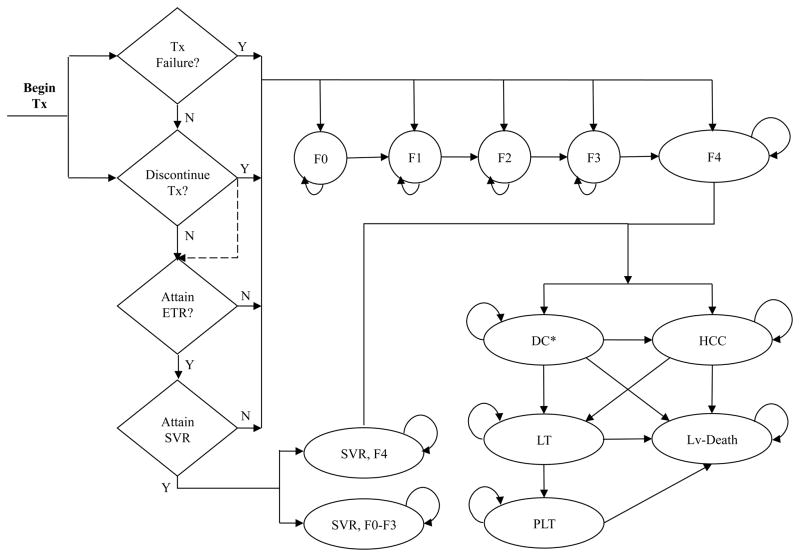
We divided the model in two parts: the first part simulated the treatment strategies and the second part modeled the natural history of the hepatitis C disease (Fig. 2). The treatment and follow-up period were modeled using a weekly cycle to allow for early discontinuations, whereas the natural-history part used a cycle length of one year.
Figure 2.
State-transition diagram for chronic hepatitis C and liver disease model. The model consists of two components: treatment and natural history. If patients discontinue treatment, or fail to achieve an end-of-treatment response (ETR) or a sustained virologic response (SVR), they enter the natural history component of the model, which consists of 14 health states. These include fibrosis states (F0–F4); decompensated cirrhosis (first year [DC1] and subsequent years [DC+]); hepatocellular carcinoma (HCC); liver transplant (first year [LT] and subsequent years [PLT]; liver-related death (Lv-Death); death from all other causes (not shown here); and SVR status states stratified by fibrosis stage (“SVR, F0–F3” and “SVR, F4”).
Y= Yes; N=No.
*For clarity, two decompensated states--DC1 and DC+ are shown as one state, i.e. DC.
During the treatment phase, patients entered the model with chronic HCV and began antiviral drug therapy. At each cycle, a patient could discontinue treatment for medical or non-medical reasons, fail to pass a futility rule, or continue treatment. Patients could develop anemia during treatment, which was managed by erythropoietin (EPO) or ribavirin dose reduction. At the end of treatment, patients who failed to pass a futility rule or had detectable HCV-RNA were considered treatment failures and returned to the chronic HCV health states. Patients who had undetectable HCV-RNA at the end of treatment (i.e. end-of-treatment response [ETR]) were followed for 24 weeks. After 24 weeks, if the patient still had undetectable HCV-RNA, the patient had achieved SVR; otherwise, he/she was considered to be a treatment relapse.
The second component of the model simulated the natural history of chronic HCV disease. The model was designed to be consistent with the current understanding of the biology of chronic HCV-related liver disease and its treatment and is similar to the other published health economic models of HCV disease [11–14]. Our state-transition model consists of 14 health states (Fig. 2). States capturing the severity of chronic HCV infection are described by the degree of fibrosis using the METAVIR scoring system: no fibrosis (F0), portal fibrosis without septa (F1), portal fibrosis with few septa (F2), numerous septa without fibrosis (F3), and cirrhosis (F4). In addition, the model includes states that define advanced liver diseases, liver transplant, SVR (cirrhotic and non-cirrhotic at baseline) and death. The model was developed using Microsoft Excel (Microsoft Corp., Redmond, WA).
The progressive disease model assumed that a person with a given fibrosis score may progress to more severe stages of liver disease or may remain in that health state. In the absence of successful treatment, regression to less severe health states was not permitted. After a successful treatment, however, a person can achieve SVR, which was considered a cure for HCV in non-cirrhotic patients. We assumed that a cured person who started treatment in health states F0–F3 would not become symptomatic again. On the other hand, cirrhotic patients continued to face some risk of liver disease (decompensated cirrhosis [DC] and HCC) even if they achieved SVR [15]. For this purpose, we stratified SVR state by patient’s baseline fibrosis stage before treatment (“SVR, F0-F3” and “SVR, F4”).
Patients who return to the chronic HCV health states can develop serious liver disease. Patients with compensated cirrhosis are at risk for developing DC and HCC. Although there are different modes of decompensation (i.e., ascites, variceal hemorrhage, and encephalopathy), we modeled them as one health state instead of different health states because these decompensation modes are not mutually exclusive. If a patient developed DC and/or HCC then the patient could receive a liver transplant. To account for different mortality rates of DC during the first year and subsequent years, DC state was divided into two states: first year (DC1) and subsequent years (DC+). Similarly, the liver-transplant health state was divided into two —“Liver Transplant” and “Post-Liver Transplant”. Patient in DC, HCC and liver transplant were subjected to excess mortality compared with the general population; whereas, all other patients faced the same mortality risk as the general population.
Assumptions
We assumed that there is no progression of disease while patients are on treatment. This assumption will only have a minimal or no impact on the results because HCV is a slow progressing disease that can take 20–30 years to reach cirrhosis from no-fibrosis state whereas the treatment period lasts at most 48 weeks. In addition, only a fraction of patients in whom treatment will eventually fail continue beyond week 12. We did not model the possibility of remission from health states F0 and F1 because the likelihood of a chronically infected persons spontaneously clearing HCV is very small [14]. As was done in many previous models, we assumed that all patients continue to progress when not treated. Some studies, however, suggest that a proportion of patients in F0 state will not progress even if untreated [11,13]. This assumption biases the base case analysis in favor of the use of triple therapy. The importance of this assumption was tested in the sensitivity analysis. Patients who received a liver transplant were not explicitly modeled for the risk of reactivation and progression to liver disease. The post-liver-transplant state indirectly took into account the mortality, however, quality-of-life and cost of re-infection after the liver-transplant. We also assumed no long-term benefits of treatment for patients who relapsed or did not respond. This assumption leads to underestimation of the benefits both dual and triple therapy. Because the proportion of patients who relapsed or did not respond was higher with dual therapy, this assumption also biases the analysis against the use of dual therapy. Our model only included the currently approved and available treatments, and did not include any treatment that would be available in the future.
Treatment-related inputs
We used RESPOND-2 data to estimate all treatment-related input parameters (Table 2). Specifically, we estimated efficacy rates, treatment-failure rates, probability and duration of anemia, and duration of EPO use for management of anemia associated with each treatment strategy.
Table 2.
Treatment-related outcomes of patients enrolled in RESPOND-2
| Treatment Characteristics | PR48 (n=80) | BOC/RGT (n=162) | BOC/PR48 (n=161) |
|---|---|---|---|
| Experienced anemia – no. (%) | 16 (20)* | 70 (43) | 75 (47) |
| Erythropoietin (EPO) use – no. (%) | 17 (21) | 66 (41) | 74 (46) |
| Mean duration of anemia (days) | 97.4 | 122.1 | 150.6 |
| Mean duration of EPO use (days) | 64.6 | 135.0 | 130.2 |
| Discontinued before TW12 - n/m (%) | 5/80 (6) | 13/162 (8) | 4/161 (2.5) |
| Discontinued due to treatment failure at TW12 - n/m (%)† | 49/75 (65) | 36/149 (24) | 29/157 (18) |
| Discontinued after TW12 - n/m (%)† | 3/26 (12) | 7/113 (6) | 23/128 (18) |
| Assigned 36 weeks therapy - n/m (%)† | NA | 66/106 (62) | NA |
| Sustained virologic response (SVR) – no. (%) | 17 (21) | 95 (59) | 107 (66) |
All patients receiving EPO were assumed as anemic by the model. Since more patients received EPO than who experienced anemia in PR48, the number of patients who experienced anemia in PR48 were assumed to be 17 in the model.
Conditional on the proportion of subjects reaching this week in the trial (as needed by the model). The denominator was determined by the number of patients in the trial at the given week.
NA = not applicable; PR48= peginterferon-ribavirin regimen; BOC/RGT = Response Guided Therapy; BOC/PR48 peginterferon–ribavirin-boceprevir regimen
Epidemiological Inputs
The model required epidemiological inputs that describe the rate of HCV progression, the probability of receiving a liver transplant, and both all-cause and liver-related mortality rates (Table 3). The progression rates determined the amount of time patients spent in each health state, the likelihood of developing serious complications associated with liver disease and the probability of requiring a liver transplant.
Table 3.
Clinical, cost, and quality of life inputs, and SVR rates: baseline values, ranges and parameters for distributions used in deterministic and probabilistic sensitivity analyses
| Input | Base Case | Range | Distribution | Parameter 1* | Parameter 2† |
|---|---|---|---|---|---|
| Transition probabilities (annual) | |||||
| F0 to F1 (16) | 0.117 | 0.104–0.130 | Beta | 274.98 | 2,075.30 |
| F1 to F2 (16) | 0.085 | 0.075–0.096 | Beta | 210.06 | 2,261.18 |
| F2 to F3 (16) | 0.120 | 0.109–0.133 | Beta | 288.05 | 2,112.38 |
| F3 to F4 (16) | 0.116 | 0.104–0.129 | Beta | 270.61 | 2,062.22 |
| Cirrhosis to DC (18–22) | 0.029 | 0.010–0.039 | Beta | 16.67 | 558.01 |
| Cirrhosis to HCC (18–26) | 0.028 | 0.010–0.079 | Beta | 22.97 | 791.67 |
| SVR after cirrhosis to DC (15) | 0.008 | 0.002–0.036 | Beta | 6,348.80 | 787,251.20 |
| SVR after cirrhosis to HCC (15) | 0.005 | 0.002–0.013 | Beta | 2,487.50 | 495,012.50 |
| DC to HCC (27) | 0.068 | 0.030–0.083 | Beta | 10.88 | 149.15 |
| DC to transplantation (30, 31) | 0.023 | 0.010–0.062 | Beta | 1.31 | 55.44 |
| DC (first year) to death from liver disease (27) | 0.182 | 0.065–0.190 | Beta | 68.42 | 307.52 |
| DC (subsequent year) to death from liver disease (27) | 0.112 | 0.065–0.190 | Beta | 28.13 | 223.02 |
| HCC to transplantation (32, 49) | 0.040 | 0.000–0.140 | Beta | 3.88 | 93.09 |
| HCC to death from liver disease (19) | 0.427 | 0.330–0.860 | Beta | 263.82 | 354.02 |
| Liver transplantation (first year) to death from liver disease (28) | 0.116 | 0.060–0.420 | Beta | 30.04 | 228.91 |
| Post-Liver transplantation to death from liver disease (28) | 0.044 | 0.024–0.110 | Beta | 4.67 | 101.55 |
| Drug therapy-related costs (weekly) | |||||
| Peginterferon alpha-2b (37) | 588 | ||||
| Ribavirin (37) | 309 | ||||
| Boceprevir (37) | 1,100 | ||||
| Erythropoietin (40,000 IU/ml) (37) | 875 | ||||
| Monitoring Costs (50) | 64 | ||||
| Health state costs (annual) | |||||
| F0, F1 (33, 36) | 678 | ±25% | Gamma | 61.47 | 11.03 |
| F2 (33, 36) | 687 | ±25% | Gamma | 61.47 | 11.17 |
| F3 (33, 36) | 1,394 | ±25% | Gamma | 61.47 | 22.67 |
| Compensated Cirrhosis (33) | 1,626 | ±25% | Gamma | 61.47 | 26.46 |
| DC (33) | 18,064 | ±25% | Gamma | 61.47 | 293.89 |
| HCC (33) | 33,218 | ±25% | Gamma | 61.47 | 540.44 |
| Liver transplant (first year) (33) | 95,971 | ±25% | Gamma | 61.47 | 1,561.38 |
| Post Liver transplant (33) | 25,208 | ±25% | Gamma | 61.47 | 410.11 |
| Health state quality of life weights | |||||
| US population norms, men (39) | |||||
| 20–29 | 0.928 | 0.922–0.934 | Beta | 6,616.65 | 513.36 |
| 30–39 | 0.918 | 0.912–0.925 | Beta | 7,374.10 | 658.69 |
| 40–49 | 0.887 | 0.880–0.894 | Beta | 6,970.14 | 887.97 |
| 50–59 | 0.861 | 0.853–0.870 | Beta | 6,185.19 | 998.54 |
| 60–69 | 0.840 | 0.827–0.852 | Beta | 2,566.28 | 488.82 |
| 70–79 | 0.802 | 0.788–0.816 | Beta | 2,496.15 | 616.26 |
| 80–89 | 0.782 | 0.757–0.807 | Beta | 819.41 | 228.43 |
| US population norms, women (39) | |||||
| 20–29 | 0.913 | 0.905–0.920 | Beta | 4,353.04 | 414.80 |
| 30–39 | 0.893 | 0.886–0.900 | Beta | 6,689.64 | 801.56 |
| 40–49 | 0.863 | 0.855–0.871 | Beta | 6,124.55 | 972.26 |
| 50–59 | 0.837 | 0.829–0.846 | Beta | 6,854.42 | 1,334.85 |
| 60–69 | 0.811 | 0.800–0.822 | Beta | 3,946.67 | 919.75 |
| 70–79 | 0.771 | 0.758–0.784 | Beta | 3,094.35 | 919.07 |
| 80–89 | 0.724 | 0.701–0.747 | Beta | 1,050.61 | 400.51 |
| Drug therapy-related multiplier (12) | 0.90 | 0.84–0.96 | Beta | 86.44 | 9.60 |
| Anemia multiplier (51) | 0.83 | 0.75–0.97 | Beta | 70.30 | 14.40 |
| F0, F1 (38) | 0.93 | 0.84–1.00 | Beta | 47.47 | 3.57 |
| F2, F3 (38) | 0.93 | 0.84–1.00 | Beta | 47.47 | 3.57 |
| Compensated Cirrhosis (38) | 0.90 | 0.81–1.00 | Beta | 31.12 | 3.46 |
| DC (38) | 0.80 | 0.57–1.00 | Beta | 12.29 | 3.07 |
| HCC (38) | 0.79 | 0.54–1.00 | Beta | 11.42 | 3.03 |
| First-year, Post Liver transplant (38) | 0.84 | 0.77–0.93 | Beta | 53.54 | 10.20 |
| Post SVR | 1.00 | 0.92–1.0 | Beta | 6,368.04 | 15.96 |
| SVR rates | |||||
| PR48 (8) | 0.21 | 0.13–0.30 | Beta | 19.56 | 72.50 |
| BOC/RGT (8) | 0.59 | 0.51–0.66 | Beta | 92.87 | 65.50 |
| BOC/PR48 (8) | 0.66 | 0.59–0.74 | Beta | 108.65 | 54.83 |
Parameter 1 corresponds to α parameter for beta distribution and k (shape) parameter for gamma distribution
Parameter 2 corresponds to β parameter for beta distribution and θ (scale) parameter for gamma distribution
DC = Decompensated Cirrhosis; HCC= hepatocellular carcinoma; SVR = sustained virologic response; PR48= peginterferon-ribavirin regimen; BOC/RGT = Response Guided Therapy; BOC/PR48 peginterferon–ribavirin-boceprevir regimen
We used the progression rates of fibrosis stages from Thein et al. [16], a recent study that provides a systematic review and meta-analysis of published progression rates from 111 studies of individuals with chronic HCV infection. They provided stage specific progression rates by fibrosis-level. These estimates also adjust for biases attributable to study design and selection factors associated with study population and clinical characteristics as shown in earlier studies [17].
We estimated the likelihood of cirrhosis advancing to DC from a pooled analysis of five studies [18–22], and cirrhosis advancing to HCC from a pooled analysis of nine studies [18–26]. The baseline likelihood of developing HCC from DC and annual mortality associated with DC were estimated from a study by Planas et al. [27] that followed 200 patients with DC for a mean period of 32 months. The patients developing DC or HCC were eligible to receive a liver-transplant. The mortality associated with liver transplant was estimated from a recently published study [28], which was not specific to HCV patients; however, this was tested by sensitivity analysis. The age- and gender-specific all-cause mortality rates were taken from the US life tables [29].
Probability of receiving a liver transplant
Most of the previously published US based cost-effectiveness models used the probability of receiving a liver transplant from DC estimated using data from 1987 and 1997 [14]. The liver transplant practice and prevalence of DC in the US population, however, has changed since then. For example, according to the analysis of the Scientific Registry of Liver Transplant Recipients (SRTR) data, from 1999 to 2007, the number of recipients with HCV increased to a peak of 2,481 in 2006 and remained relatively afterwards [30]. Also, HCV-related DC became more prevalent after 1995 [31]. Using the approach of Bennett et al. and most recent data, we estimated the annual probability of a patient with DC receiving a liver transplant equal to 2.33% (i.e. 2400/103117). Our estimate is lower than that of Bennett et al (3.1%) primarily because of a substantial increase in the prevalence of DC since then. Finally, we estimated the annual probability of an HCC patient receiving a liver transplant to be 4.0% from a study by Lang et al.[32].
Treatment Costs
The model was developed from the payer perspective. We estimated the baseline health-state specific annual costs from a study by McAdam-Marx et al. [33] that conducted a retrospective, matched cohort study of 34,597 HCV patients enrolled in a large managed care claims database. We subtracted pharmacy-related costs from HCV states without cirrhosis (F0–F3) and compensated cirrhosis (F4), which were primarily due to antiviral therapy. We also adjusted the inpatient hospitalization costs by using the national hospital cost-to-charge ratio of 0.329, which was estimated by taking the weighted average of state-wide operating cost-to-charge ratio [34] and the number of hospital discharges in each state [35]. McAdam-Marx et al. only provided the combined cost associated with health states F0–F3. To estimate the cost associated with each fibrosis stage, we used the proportion of cost spent in each health state – mild (F0, F1) to moderate (F2) to severe (F3) chronic HCV, from another study [36].
The total treatment costs for patients on antiviral therapy were based on the weekly drug costs and monitoring costs. We assumed the drug costs to be equal to the wholesale acquisition cost (WAC) as listed by First DataBank [37]. The price of pegylated interferon alfa-2b was $587.51 per week. Using the price of the generic version of ribavirin equal to $8.83 per 200 mg capsule at a daily dose of 1000 mg and the mean patient body weight of approximately 80 kg, we estimated the weekly cost of ribavirin at $309.05. The weekly cost of boceprevir was $1,100. The cost of treating anemia was estimated using the percentage of patients who used EPO (at a weekly cost of $875) and the mean duration of EPO in the trial. We added a weekly monitoring cost of $64, which included physician visits, blood counts, liver function tests and HCV quantitative PCR tests. We did not include any indirect costs (e.g., lost productivity) in the model. An annual discount rate of 3% was applied to future costs accrued.
Utility weights
All treatment and health-state specific utility weights were estimated from a previously published study using EQ-5D instrument [12,38], and adjusted to the US population norm [39]. Quality of life of patients who achieved SVR was assumed to be equivalent to that of the general population [38]. Future QALYs were discounted at 3% per year.
Outcomes
Our model provided the average total costs and QALYs associated with each treatment strategy, and the incremental cost-effectiveness ratios (ICERs) per additional QALY of boceprevir-based regimens — BOC/PR48 and BOC/RGT — compared incrementally with PR. In addition, we projected the incidence of advanced liver-related complication (DC and HCC), liver-transplants and liver-related deaths with the three treatment strategies. A half-cycle correction was performed when calculating all outcomes. Finally, we performed 1-way and probabilistic sensitivity analysis (PSA) to measure uncertainty in the outcomes because of uncertainty in the efficacy, epidemiological, quality of life (QOL), discount rates, and cost inputs.
RESULTS
We cross-validated our model by comparing the natural history of HCV infection with previously published models. For that purpose, we projected the 20-year cumulative probability of developing cirrhosis in a 44-year old untreated patient with F0 and F1 stage equal to 17.2% and 35.5%, respectively. Siebert et al. [12] projected the 20-year probability of cirrhosis in a 44-year old mild chronic HCV patient equal to 27%, and Bennett et al. [14] projected the corresponding probability in a 35-year old mild chronic HCV patient equal to 28%. Assuming 35% patients in mild HCV having F0 stage and 65% F1 stage in 2010 [31], our model predicted the 20-year cirrhosis probability of 29.1% in 44-year old patient, which is comparable to the reported values. Salomon et al. [11] projected 30-year cumulative probability from F0 to cirrhosis and F2 to cirrhosis equal to 20% and 65%, respectively. The corresponding probabilities from our model were higher at 38.2% and 79%.
We also compared our results with a recently published multicenter follow-up study of patients with advanced fibrosis by van der Meer et al. [40]. In patients who failed to achieve SVR, the study reported the 10-year cumulative incidence rates of DC, HCC, and combined LRD and LT equal to 29.9% (CI: 24.3–35.5%), 21.8% (95% CI: 16.6–27.0%) and 27.4% (95% CI: 22.0–32.8%), respectively. The corresponding values predicted by our model were 17.0%, 18.7%, and 23.7%, respectively. The predicted incidence of HCC and LRD plus LT in this group were within the reported confidence limits, however the incidence of DC was lower than the reported values. In patients who achieved SVR, the study reported the 10-year cumulative incidence rates of DC, HCC, and combined LRD and LT equal to 2.1% (95% C.I.: 0–4.5%), 5.1% (95% C.I.: 1.3–8.9%), and 1.9% (95% C.I.: 0–4.1%), respectively. The corresponding values predicted by our model were 5.2%, 3.9%, and 5.6% respectively. The predicted incidence of HCC in this group was within the reported confidence limits; however the incidence of DC and LRD plus LT were higher than the reported values.
Base case analysis
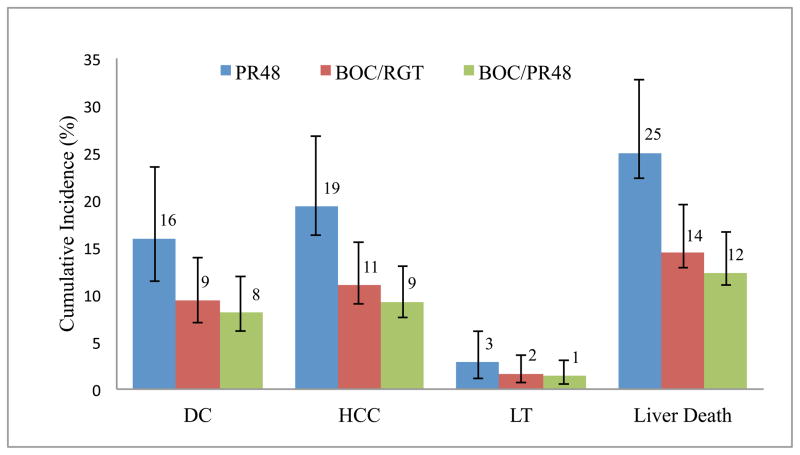
Treatment with PR therapy would result in a 16.0% likelihood of DC compared with 9.4% (relative reduction [RR] of 41.1%) with BOC/RGT and 8.1% (RR of 49.4%) with BOC/PR48 (Fig. 3). Similarly, the likelihood of HCC, LT and liver-related death were projected to reduce 41.1–52.0% with boceprevir-based regimens in comparison with PR48. The total projected life years associated with PR48, BOC/RGT and BOC/PR48 were 24.74, 26.07 and 26.34, respectively, and the corresponding discounted QALYs were 12.79, 13.64 and 13.80, respectively (Table 4). The total expected discounted lifetime costs of PR48, BOC/RGT and BOC/PR48 were $53,500, $79,000 and $94,500, respectively. The average boceprevir cost account for 33% and 39% of total HCV-associated cost in BOC/RGT and BOC/PR48, respectively (Table 4). The ICER of BOC/RGT in comparison with PR48 was $30,200 per QALY, and the ICER of BOC/PR48 in comparison with BOC/RGT was $91,500 per QALY.
Figure 3.
Cumulative incidence of liver-related complications with PR48, BOC/RGT and BOC/PR48 treatment strategies; PR48= peginterferon-ribavirin regimen; BOC/RGT = Response Guided Therapy; BOC/PR48 peginterferon–ribavirin-boceprevir regimen; DC = decompensated cirrhosis; HCC = hepatocellular carcinoma; LT = liver-transplant Error bars were estimated using 10,000 Monte Carlo simulation runs.
Table 4.
Life expectancy, breakdown of total discounted expected costs and QALYs, and ICER associated with each treatment strategy.
| Category | Cost ($) | QALYs* | ||||
|---|---|---|---|---|---|---|
| PR48 | BOC/RGT | BOC/PR48 | PR48 | BOC/RGT | BOC/PR48 | |
| Life expectancy | NA | NA | NA | 24.74 | 26.07 | 26.34 |
| Drug† | 19,948 | 52,787 | 69,776 | NA | NA | NA |
| Anemia | 1,716 | 6,875 | 7,480 | −0.01 | −0.02 | −0.02 |
| Monitoring | 1,423 | 1,929 | 2,376 | NA | NA | NA |
| SVR | 0 | 0 | 0 | 2.90 | 7.99 | 9.06 |
| F0–F3 | 7954 | 4560 | 3843 | 7.06 | 4.1 | 3.47 |
| F4 | 5,376 | 2,928 | 2,428 | 2.41 | 1.31 | 1.09 |
| DC | 5,933 | 3,560 | 3,078 | 0.21 | 0.13 | 0.11 |
| HCC | 7,096 | 4,070 | 3,453 | 0.14 | 0.08 | 0.07 |
| Liver transplant | 4,028 | 2,376 | 2,041 | 0.08 | 0.05 | 0.04 |
| Total | 53,474 | 79,085 | 94,475 | 12.79 | 13.64 | 13.8 |
| ICER ($/QALY) | -- | 30,241 | 91,506 | |||
QALYs were rounded to 2 decimal places for reporting in the table, but ICERs were estimated using exact QALYs from the model
Drug cost includes dual therapy cost for PR48 and triple therapy cost for BOC/RGT and BOC/PR48
PR48= peginterferon-ribavirin regimen; BOC/RGT = Response Guided Therapy; BOC/PR48 peginterferon–ribavirin-boceprevir regimen; DC = decompensated cirrhosis; HCC = hepatocellular carcinoma; QALYs = quality-adjusted life years; ICER = incremental cost-effectiveness ratio.
Sensitivity analysis
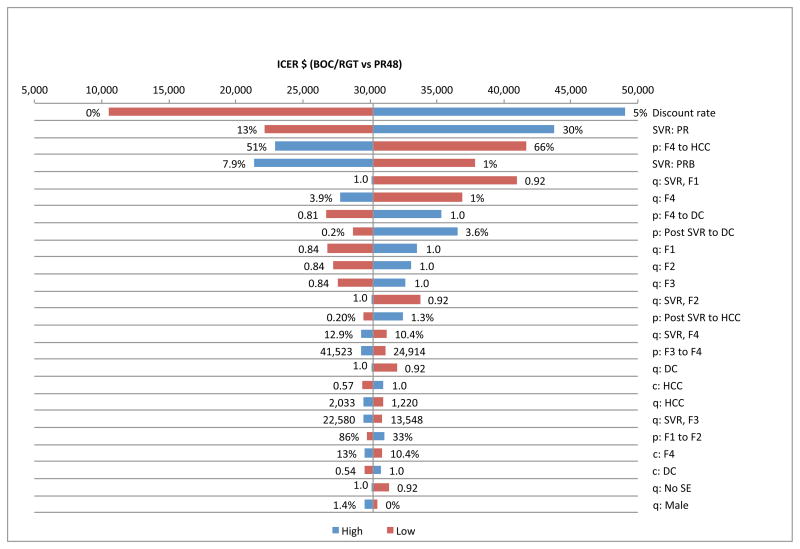
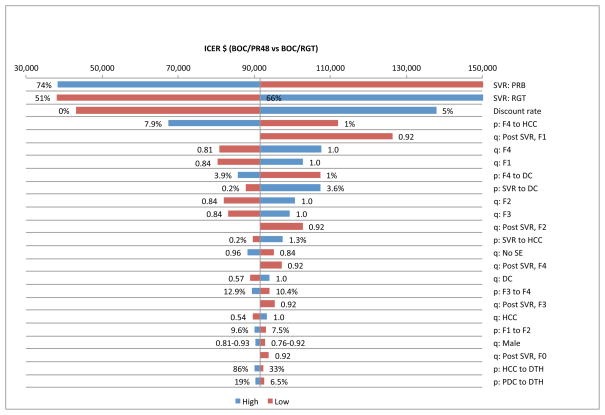
We performed one-way sensitivity analysis on efficacy, transition probabilities, QOL weights, discount rates, and treatment-related costs, and identified the top 25 variables that had the biggest impact on ICERs by plotting the tornado diagrams (Fig. 4 & Fig. 5). We found that ICERs were most sensitive to the SVR rates, discount rate, probability of DC or HCC in cirrhotic patients, probability of DC after achieving SVR and quality of life weights associated with fibrosis stages, F0–F4.
Figure 4.
Tornado diagram showing 25 most sensitive parameters in BOC/RGT p = transition probability, q: quality of life weight, c = cost
Figure 5.
Tornado diagram showing 25 most sensitive parameters in BOC/PR48 p = transition probability, q: quality of life weight, c = cost
We also a scenario where 24% of the F0 patients will not progress even without treatment, and found similar results as with the base case analysis. Next, we performed sensitivity analysis by including hazard ratio for sex-, race-, and age-specific mortality (white male: 2.56; white female: 1.90; black male: 2.75; and black female: 2.48) from non-liver causes in patients with chronic HCV [13]. Using proportion of blacks and whites from RESPOND-2, we estimated the weighted hazard ratio for males as 2.58 and females as 1.97. We linearly decreased the hazard ratio from age 70 onwards to 1.0 by age 100 to avoid overestimation of mortality in older patients. The cost of PR48, BOC/RGT and BOC/PR48 went down to $50,000, $74,600 and $90,568 respectively. The corresponding QALYs went down to 10.63, 11.22 and 11.34, respectively. The ICER of BOC/RGT in comparison was $48,900 per QALY with PR48, and the ICER of BOC/PR48 in comparison with BOC/RGT was $138,100 per QALY.
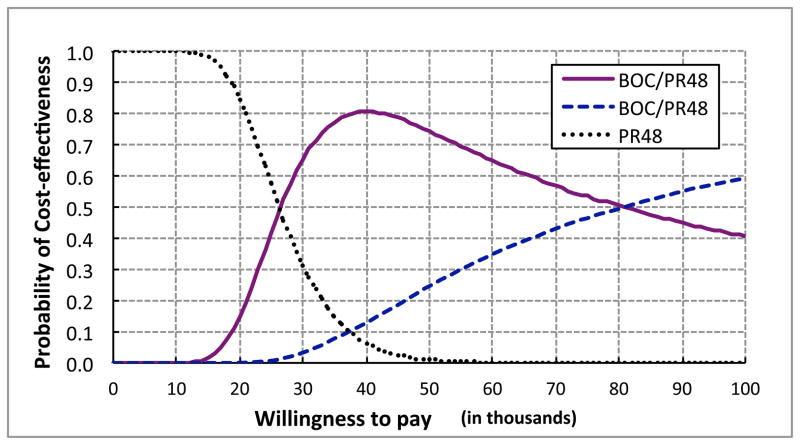
Next, we performed PSA on the parameters defined in Table 3. Using 10,000 Mote Carlo simulations, the total mean QALYs associated with PR48, BOC/RGT and BOC/PR48 were 13.48, 14.48 and 14.61, respectively, and the corresponding total expected cost were $55,785, $80,398 and $95,570, respectively. At $50,000 willingness-to-pay (WTP), the probabilities BOC/RGT and BOC/PR48 being the preferred option were 0.74 and 0.25, respectively (Fig. 6). At $100,000 WTP, the corresponding probabilities were 0.41 and 0.59.
Figure 6.
Cost-effectiveness acceptability curve. PR48= peginterferon-ribavirin regimen; BOC/RGT = Response Guided Therapy; BOC/PR48 peginterferon–ribavirin-boceprevir regimen.
Subgroup analysis: Prior treatment response
Efficacy of re-treatment in patients who failed to achieve SVR earlier depends upon patient’s response to prior treatment [8]; hence the cost-effectiveness of re-treatment may vary by patients’ prior treatment response. We performed cost-effectiveness analysis in the following three subgroups: 1) Prior relapsers, i.e., patients with undetectable HCV RNA level at the end of treatment but failed to achieve SVR; 2) Partial responders, i.e., patients whose HCV RNA level decreased by at least 2 log10 at week 12 but remained detectable during the therapy period); and 3) Null-responders, i.e., patients whose HCV RNA level decreased by less than 2 log10 at week 12.
The treatment regimens and efficacy data of prior relapsers and partial responders were based on RESPOND-2 study (Table S1). Since the null-responders were not included in RESPOND-2, we used data from an ongoing PROVIDE study to evaluate the cost-effectiveness in this subgroup [10]. For the comparator strategy of treatment with peginterferon-ribavirin, we estimated model parameters of null responders from an earlier study [41]. The treatment regimen of null-responders was defined as follow: initiate with a lead-in period with PR alone for 4 weeks, followed by BOC+PR for 44 weeks (Fig. S2]. In all patients, the treatment was stopped if they either had HCV-RNA greater than or equal to 100 IU/mL at TW 12, or detectable at TW 24. Table S2 summarizes treatment related data of null-responders used in our model.
In prior relapsers, the ICER of BOC/RGT in comparison with PR48 was $29,000, and the ICER of BOC/PR48 compared with BOC/RGT was $134,300 (Table 5). In partial responders, the corresponding ICERs were $33,600 and $58,200 per additional QALY. Finally, in null responders, the ICER of boceprevir-based regimen in comparison with PR48 was $33,300 per additional QALY. The cost-effectiveness results in the three subgroups were comparable, and the boceprevir-based triple therapy was cost-effective (using $100,000 WTP) irrespective of prior treatment response to therapy. Since RESPOND-2 was neither designed nor powered to detect differences in the outcomes by previous treatment history, caution should be taken in interpreting the cost-effectiveness of boceprevir in these subgroups.
Table 5.
Subgroup and scenario analysis of total discounted expected costs and QALYs, and ICER associated with each treatment strategy.
| Treatment Strategy | Cost ($) | QALYs | ICER ($/QALY) | Prob. of CE at $50K | Prob. of CE at $100K |
|---|---|---|---|---|---|
|
Subgroup analysis by prior treatment response
| |||||
| Prior relapser | |||||
| PR48 | 54,413 | 12.98 | -- | 0.022 | 0 |
| BOC/RGT | 80,208 | 13.87 | 29,017 | 0.830 | 0.556 |
| BOC/PR48 | 97,225 | 13.99 | 134,363 | 0.148 | 0.444 |
| Partial responder | |||||
| PR48 | 51,897 | 12.46 | -- | 0.062 | 0 |
| BOC/RGT | 77,716 | 13.23 | 33,613 | 0.510 | 0.313 |
| BOC/PR48 | 91,846 | 13.47 | 58.177 | 0.428 | 0.687 |
| Null responder | |||||
| PR48 | 51,149 | 12.30 | -- | 0.059 | 0.001 |
| BOC/PR48 | 80,487 | 13.18 | 33,255 | 0.941 | 0.999 |
|
| |||||
|
Subgroup analysis by IL-28B rs12979860 genotype
| |||||
| IL-28B genotype CC | |||||
| PR48 | 45,225 | 13.35 | -- | 0.259 | 0.080 |
| BOC/RGT | 71,372 | 14.08 | 35,444 | 0.672 | 0.717 |
| BOC/PR48 | 90,893 | 14.05 | Dominated* | 0.069 | 0.203 |
| IL-28B genotype CT | |||||
| PR48 | 54,803 | 12.70 | -- | 0.005 | 0 |
| BOC/RGT | 79,468 | 13.69 | 24,832 | 0.539 | 0.291 |
| BOC/PR48 | 92,399 | 13.94 | 51,393 | 0.456 | 0.709 |
| IL-28B genotype TT | |||||
| PR48 | 43,951 | 13.43 | 0.680 | 0.401 | |
| BOC/RGT | 82,746 | 13.54 | Dominated† | 0.138 | 0.152 |
| BOC/PR48 | 92,566 | 13.93 | 97,077 | 0.182 | 0.447 |
|
| |||||
|
Scenario analysis: FDA approved regimens
| |||||
| All previously treated | |||||
| PR48-FDA | 55,603 | 12.73 | -- | 0 | 0 |
| BOC/PR-FDA | 84,011 | 13.83 | 25,747 | 1 | 1 |
| Non-cirrhotic patients (METAVIR F0–F3) | |||||
| PR48-FDA | 51,125 | 13.26 | -- | 0.065 | 0 |
| BOC/PR-FDA | 78,947 | 14.05 | 35,285 | 0.935 | 1 |
| Cirrhotic patients (METAVIR F4) | |||||
| PR48-FDA | 85,394 | 9.18 | -- | 0 | 0 |
| BOC/PR-FDA | 117,701 | 12.37 | 10,102 | 1 | 1 |
|
| |||||
|
Scenario analysis: management of anemia with ribavirin dose reduction
| |||||
| PR48 | 51,759 | 12.79 | -- | 0.001 | 0 |
| BOC/RGT | 72,209 | 13.64 | 24,149 | 0.729 | 0.406 |
| BOC/PR48 | 86,995 | 13.80 | 87,906 | 0.270 | 0.594 |
|
| |||||
|
Scenario analysis: telaprevir versus peginterferon-ribavirin
| |||||
| All previously treated | |||||
| REALIZE-PR48 | 71,299 | 12.32 | -- | 0 | 0 |
| TEL12PR48 | 108,795 | 13.85 | 24,431 | 1 | 1 |
| Prior relapser | |||||
| REALIZE-PR48 | 76,040 | 12.55 | -- | 0 | 0 |
| TEL12PR48 | 103,595 | 14.47 | 14,397 | 1 | 1 |
| Partial responder | |||||
| REALIZE-PR48 | 63,417 | 12.23 | -- | 0 | 0.001 |
| TEL12PR48 | 107,135 | 13.67 | 30,322 | 1 | 0.999 |
| Null responder | |||||
| REALIZE-PR48 | 67,516 | 11.94 | -- | 0.942 | 0 |
| TEL12PR48 | 120,214 | 12.73 | 66,779 | 0.058 | 1 |
BOC/PR48 was dominated because it had lower QALYs but higher cost than BOC/RGT
BOC/RGT was weakly dominated because it had lower QALYs but higher ICER than BOC/PR48 Prob. = Probability, CE = cost-effectiveness; PR48= peginterferon-ribavirin regimen as in RESPOND-2 trial; BOC/RGT = Response guided therapy as in RESPOND-2 trial; BOC/PR48 peginterferon–ribavirin-boceprevir regimen as in RESPOND-2 trial; PR48-FDA = peginterferon-ribavirin regimen as in the FDA approved label; BOC/PR-FDA = boceprevir-based triple therapy as in the FDA approved label; REALIZE-PR48 = peginterferon-ribavirin regimen as in REALIZE trial; TEL12PR48 = telaprevir-based triple therapy as in REALIZE trial.
Subgroup analysis: IL28B genotype
Response to interferon-based therapies is known to depend upon interleukin (IL)-28B polymorphism [42]. Data from RESPOND-2 study showed that single nucleotide polymorphism (SNP) at IL-28B rs12979860 is strongly associated with response to triple therapy [43], with CC genotype having a more favorable treatment response and non-CC genotypes. Therefore, we evaluated the cost-effectiveness of triple therapy by patient’s IL-28B genotype. We used efficacy as the main input of our subgroup analysis by IL28B genotypes. It should be noted that the RESPOND-2 trial was neither designed nor powered to assess the impact of IL28B genotype on SVR. Also, approximately one third of patients in RESPOND-2 did not consent to genomic testing. For these reasons, we did not include race (and other treatment-specific parameters) into our analysis by IL28B genotype. Because of the relatively low cost, we also did not include the cost of a one-time genotype IL28B test. Table S3 summarizes the data available from RESPOND-2 that was used in our model. Since discontinuation rates and treatment failure rates were not available by IL-28B genotype, we used the corresponding rates as estimated in base case analysis.
In IL-28B CC patients, the ICER of BOC/RGT in comparison with PR48 was $35,400 per additional QALY, and BOC/PR48 was dominated (Table 5). In IL-28B CT patients, the ICERs of BOC/RGT and BOC/PR48 were $24,800 and $51,400 per additional QALY, respectively. Finally, in IL-28B TT patients, BOC/RGT was ruled out using extended or weak dominance principle (because BOC/RGT had lower QALYs but higher ICER than BOC/PR48), resulting in BOC/PR48’s ICER of $97,000 per additional QALY in comparison with PR48. Using $100,000 WTP, boceprevir-based RGT was cost-effective in patients with genotype CC and genotype CT. GT, however, was weakly dominated in genotype TT patients; instead the 48-week fixed treatment arm was cost-effective in genotype TT. RESPOND-2 was neither designed nor powered to detect differences in the outcomes by IL-28B genotype; therefore, caution should be taken in interpreting the cost-effectiveness of boceprevir in these subgroups.
Scenario analysis: FDA approved regimens
The FDA recommendations and the American Association for the Study of Liver diseases (AASLD) treatment guidelines for the use of boceprevir are different than those studied in RESPOND-2; therefore, our model also simulated the recommended treatment design [44]. The FDA recommends BOC/RGT in non-cirrhotic treatment-experienced patients who are prior relapsers or partial responders, and fixed duration therapy of 48 weeks in null-responders and patients with cirrhosis (Supplementary Material, Figure S1–S2 at: XXX). In addition, the boceprevir label recommended a different stopping rule than that applied in RESPOND-2 trial. We performed post-hoc analysis to estimate label-related model parameters (details are provided in Supplemental Materials Table S4 at: XXX).
In patients without cirrhosis, the ICER of PR+BOC in comparison with PR was $35,300/QALY (Table 5). In patients with cirrhosis, the ICER of PR+BOC in comparison with PR was $10,100 per additional QALY. In comparison with the trial based analysis, the label-based analysis shows a more favorable cost-effectiveness of treatment with triple therapy. In addition, the treatment with triple therapy provides more benefits per dollar spent in cirrhotic patients than in non-cirrhotic patients.
Scenario analysis: management of anemia with ribavirin dose reduction
Though erythropoietin (EPO) was used to manage anemia in RESPOND-2, a recent study showed that SVR rates in patients managed with ribavirin dose reduction alone were comparable to those in patients managed with EPO [45]. Our base case analysis assumed management of anemia with EPO use as observed in the trial; however, we also analyzed a scenario where all anemic patients would be managed with ribavirin dose reduction only. For this scenario, the ICER of BOC/RGT in comparison with PR48 and BOC/PR48 in comparison with BOC/RGT went down to $24,100 and $87,900, respectively (Table 5).
Telaprevir-based analysis
In addition to boceprevir, FDA also approved another protease inhibitor, telaprevir for chronic hepatitis C treatment in previously treated patients [7]. Since no head-to-head trial compares the effectiveness of telaprevir with boceprevir, and the baseline patient characteristics, adverse event profiles, and futility rules of RESPOND-2 and REALIZE were different; direct comparison of the cost-effectiveness of two drugs was not feasible. Therefore, we only evaluated the cost-effectiveness of telaprevir in previously treated patients in comparison with peginterferon-ribavirin using data from REALIZE study [7].
As in the case of boceprevir, the model was divided into two parts: the first part simulated the treatment strategies and the second part modeled the natural history of the hepatitis C disease. We simulated treatment with peginterferon plus ribavirin for 48 weeks (REALIZE-PR48) and the FDA approved treatment arm using telaprevir for 12 weeks and peginterferon plus ribavirin for a total of 48 weeks without any lead-in (T12PR48). The treatment was discontinued if patients had less than a 2 log10 decrease in HCV RNA at week 12 (futility rule). All treatment related parameters are summarized in Appendix Table S5. Any patient who either failed to achieve SVR or a cirrhotic patient (even if he/she achieved SVR) continued to the natural history part of the model.
Using the telaprevir price of $4,400 per week, the ICER of T12PR48 in comparison with REALIZE-PR48 was $24,400/QALY (Table 5). The cost-effectiveness of telaprevir compared with PR was more favorable in prior relapsers (ICER = $14,400/QALY) and partial responders (ICER = $30,300/QALY) than in null responders (ICER = $66,800/QALY).
DISCUSSION
A significant increase in SVR rates was observed in patients treated with boceprevir-based therapies over PR therapy alone in RESPOND-2 and PROVIDE [8]. It is not clear, however, if the boceprevir-containing regimens provide sufficient value in previously treated patients given the high cost of triple therapy. We developed a Markov-cohort model to project the lifetime clinical burden of HCV, total cost and cost-effectiveness of boceprevir-based regimens studied in RESPOND-2 and PROVIDE. We also estimated the cost-effectiveness of boceprevir regimens as per FDA recommendations and AASLD guidelines, and telaprevir-based regimens as studied in REALIZE trial.
Boceprevir-based regimens were projected to reduce the incidence of liver-related complications (DC and HCC), mortality and liver-transplants by 43%–53% in comparison with treatment with PR alone. At a price of $1,100 per week only response-guided therapy was cost-effective at $50,000/QALY willingness-to-pay, whereas both response-guided and fixed duration therapy for 48 weeks were cost-effective at $100,000/QALY willingness-to-pay. In addition, boceprevir-based regimens as approved by the FDA were also cost-effective in previously treated HCV genotype 1 non-cirrhotic as well as cirrhotic patients.
We performed subgroup analysis by prior treatment response and patient’s IL-28B genotype. Our results show that both boceprevir-based therapies were cost-effective irrespective of patient’s prior response to treatment; i.e. prior relapsers, partial responders, and null responders. Second, the boceprevir-based RGT was found to be cost-effective in patients with IL-28B genotype CC and CT at $50,000 WTP; whereas, in IL-28B genotype TT patients, boceprevir-based RGT therapy was weakly dominated by fixed-duration boceprevir-based therapy, which was cost-effective only at $100,000 WTP. Since RESPOND-2 was neither powered nor designed to detect differences by sub-groups, caution should be taken in interpreting the cost-effectiveness results in these subgroups.
We also evaluated the cost-effectiveness of telaprevir-based triple therapy in comparison with peginterferon-ribavirin using REALIZE trial results. At a price of $4,400 per week, telaprevir-based therapy was cost-effective in genotype 1 patients who are prior relapsers and partial responders (using $50,000 WTP). In null-responders, however, telaprevir-based therapy was cost-effective only at the WTP threshold of $100,000. We did not perform a direct comparison of cost-effectiveness of telaprevir with boceprevir because no head-to-head trial compares the effectiveness of these two drugs.
Our model was extensively validated against a recently published clinical study as well as with other modeling studies. The predicted progression to advanced HCV diseases in patients who failed to achieve SVR was lower and in patients who achieved SVR was higher in comparison to those reported in van der Meer et al. [40]. This trend may have resulted in an over-estimation of ICERs of boceprevir-based regimens. In comparison with the modeling study of Salomon et al., our model projected faster progression of fibrosis in untreated patients [11]. The difference could be attributed to the exclusion of non-progressing F0 patients, different natural history parameters, and assumption of no higher all-cause mortality in the base model. On the other hand, our model’s fibrosis progression rates were similar to those reported in Bennett et al. [14] and Siebert et al. [12].
A recently published study evaluated the cost-effectiveness of boceprevir and telaprevir in patients who failed prior treatment in Europe and found very similar results [46]. To our knowledge, no previous study has evaluated the cost-effectiveness of treatment with PIs in patients who failed prior treatment in the US. Several studies have evaluated the cost-effectiveness of PIs in treatment naïve patients [13,47,48]. We also made several updates in the model structure and inputs, in comparison with previously published models on hepatitis C. First, our model included two components — treatment phase and natural history phase, and included early discontinuations and management of anemia. Second, we estimated the probability of receiving a liver-transplant that takes into account the changes in the practice over the last two decades. Third, we estimated the probability of HCC after cirrhosis or DC using a pooled analysis of several studies. Fourth, unlike most previous models, we allowed for a progression of disease in cirrhotic patients even after they attained SVR. Finally, we estimated health-state related costs using a recent data and appropriate cohort of hepatitis C patients.
Sensitivity analysis showed that the ICERs were most sensitive to the joint discount rates for costs and QALYs. We also found that ICERs were sensitive to the probability of development of HCC or DC in cirrhotic patients, and quality-of-life weights associated with fibrosis stages. This underscores a need for a better understanding of the natural history of end-stage liver diseases and quality of life of HCV patients. When we considered a higher mortality due to non-liver causes in HCV patients, only response-guided therapy was cost-effective in comparison with PR-based therapy at $100,000 WTP. Finally, PSA showed that boceprevir-based regimens were cost-effective with a very high probability at commonly used willingness-to-pay thresholds. In general, our conclusions were robust to a wide range of input parameters.
Our study has several limitations. First, we did not model the possibility of re-infection after an F3 patient achieved SVR and assumed that DC and HCC are mutually exclusive; whereas, this may not be the case in real-life. This may have underestimated the ICERs of boceprevir-based therapies. Second, our model cannot be applied to special populations such as HIV-HCV or HBV-HCV co-infected patients because RESPOND-2 only enrolled patients without such co-infections. Third, we assumed that there is no progression of disease while patients are on treatment, which may have some impact (albeit small) on our results. Fourth, our model was based on trial data whereas treatment related parameters like SVR rates, discontinuations and treatment-completion rates may be different in practice, and influence the cost-effectiveness of boceprevir-based regimens. Fifth, we did not use higher all-cause mortality in HCV patients in our base case, which resulted in an underestimation of ICERs of boceprevir-based therapies. Sixth, our probabilistic sensitivity analysis assumed independence of all variables; however, costs and quality of life weights are correlated, which may potentially bias our results. Finally, though Il28B guided therapy may be valuable in treatment-experienced patients, considering the above factors and lack of reliable data on IL28B in treatment-experienced patients, we did not perform IL28B genotype guided analysis.
In summary, protease inhibitors were projected to substantially reduce the burden of liver-related complications such as decompensated cirrhosis, hepatocellular carcinoma, liver-related mortality and liver-transplants in previously treated HCV genotype 1 patients. In addition, first generation protease inhibitors were projected to be cost-effective in comparison with treatment with peginterferon and ribavirin in previously treated patients at $100,000 willingness-to-pay.
Supplementary Material
Acknowledgments
Source of financial support: Dr. Chhatwal’s effort was in parts supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number KL2TR000146. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was sponsored in part by Merck Sharp &Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ.
We thank Drs. John R. Cook (Merck) and Erik Dasbach (Merck) for providing programming support, helpful discussions, and reviewing the model; Jane Liao (Merck) for providing programming support to determine the model inputs; and Dr. Heather L. Sings (Merck) for medical-writing support.
Footnotes
All authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and report the following. Dr. Chhatwal is a former employee of Merck Sharp &Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ and has received consulting fees. Drs. Ferrante, El Khoury, Burroughs and Elbasha are current employees of Merck and hold stock and/or stock options. Dr. Brass is a former employee of Merck and holds stock and/or stock options. Dr. Bacon has received consultancy fees from Gilead, Three Rivers Pharmaceuticals, Valeant, Vertex, and Human Genome Sciences; has grants/grants pending from Roche, Gilead, Bristol Myers Squibb, Three Rivers Pharmaceuticals, Valeant, Vertex, Human Genome Sciences, Wyeth, and Romark Laboratories; payment for lectures including service on speakers bureaus for Three Rivers Pharmaceuticals, Gilead, and Merck; and served on Data and Safety Monitoring Boards for Novartis, ISIS, Vertex and Gilead. Dr Esteban is a member of the speaker’s bureau or is an advisor of Merck, Gilead, Novartis, Bristol-Myers Squibb and GlaxoSmithKline.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hepatitis C fact sheet. Geneva: World Health Organization; [Accessed December 8, 2012]. ( http://www.who.int/mediacentre/factsheets/fs164/en.) [Google Scholar]
- 3.Ly KN, Xing J, Klevens RM, et al. The Increasing Burden of Mortality From Viral Hepatitis in the United States Between 1999 and 2007. Ann Intern Med. 2012;156:271–8. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 4.Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol. 2007;42:513–21. doi: 10.1007/s00535-007-2064-6. [DOI] [PubMed] [Google Scholar]
- 5.Camm C, Cabibbo G, Bronte F, et al. Retreatment with pegylated interferon plus ribavirin of chronic hepatitis C non-responders to interferon plus ribavirin: a meta-analysis. J Hepatol. 2009;51:675–81. doi: 10.1016/j.jhep.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–16. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- 7.Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–28. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 8.Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–17. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poordad F, McCone J, Jr, Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronowicki J, Davis M, Flamm S, et al. Sustained Virologic Response (SVR) in Prior PegInterferon/Ribavirin (PR) Treatment Failures After Retreatment with Boceprevir (BOC)+ PR: PROVIDE Study Interim Results. J Hepatol. 2012;56(Suppl):S6. [Google Scholar]
- 11.Salomon J, Weinstein M, Hammitt J, Goldie S. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. JAMA. 2003;290:228. doi: 10.1001/jama.290.2.228. [DOI] [PubMed] [Google Scholar]
- 12.Siebert U, Sroczynski G, Rossol S, et al. Cost effectiveness of peginterferon-2b plus ribavirin versus interferon-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52:425. doi: 10.1136/gut.52.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Cipriano LE, Holodniy M, et al. New Protease Inhibitors for the Treatment of Chronic Hepatitis C. Ann Intern Med. 2012;156:279–90. doi: 10.1059/0003-4819-156-4-201202210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett W, Inoue Y, Beck J, et al. Estimates of the cost-effectiveness of a single course of interferon- 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127:855. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso AC, Moucari R, Figueiredo-Mendes C, et al. Impact of peginterferon and ribavirin therapy on hepatocellular carcinoma: incidence and survival in hepatitis C patients with advanced fibrosis. J Hepatol. 2010;52:652–7. doi: 10.1016/j.jhep.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Thein H, Yi Q, Dore G, Krahn M. Estimation of stage specific fibrosis progression rates in chronic hepatitis C virus infection: A meta analysis and meta regression. Hepatology. 2008;48:418–31. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 17.Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–16. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 18.Benvegnu L, Noventa F, Bernardinello E, et al. Evidence for an association between the aetiology of cirrhosis and pattern of hepatocellular carcinoma development. Gut. 2001;48:110. doi: 10.1136/gut.48.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterol. 1997;112:463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 20.Gentilini P, Laffi G, La Villa G, et al. Long course and prognostic factors of virus-induced cirrhosis of the liver. Am J Gastroenterol. 1997;92:66–72. [PubMed] [Google Scholar]
- 21.Sangiovanni A, Prati GM, Fasani P, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17 year cohort study of 214 patients. Hepatology. 2006;43:1303–10. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 22.Serfaty L, Aumaître H, Chazouillères O, et al. Determinants of outcome of compensated hepatitis C virus-related cirrhosis. Hepatology. 1998;27:1435–40. doi: 10.1002/hep.510270535. [DOI] [PubMed] [Google Scholar]
- 23.Bruno S, Silini E, Crosignani A, et al. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: a prospective study. Hepatology. 1997;25:754–8. doi: 10.1002/hep.510250344. [DOI] [PubMed] [Google Scholar]
- 24.Tateyama M, Yatsuhashi H, Taura N, et al. Alpha-fetoprotein above normal levels as a risk factor for the development of hepatocellular carcinoma in patients infected with hepatitis C virus. J Gastroenterol. 2011:1–9. doi: 10.1007/s00535-010-0293-6. [DOI] [PubMed] [Google Scholar]
- 25.Tsukuma H, Hiyama T, Tanaka S, et al. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida H, Shiratori Y, Moriyama M, et al. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan. Ann Intern Med. 1999;131:174. doi: 10.7326/0003-4819-131-3-199908030-00003. [DOI] [PubMed] [Google Scholar]
- 27.Planas R, Ballesté B, Antonio Álvarez M, et al. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J Hepatology. 2004;40:823–30. doi: 10.1016/j.jhep.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe R, Roys E, Merion R. Trends in Organ Donation and Transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(4p2):961–72. doi: 10.1111/j.1600-6143.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 29.Arias E. United states life tables, 2006. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. Nat Vit Stat Sys. 2010;58:1. [PubMed] [Google Scholar]
- 30.Thuluvath P, Guidinger M, Fung J, et al. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1003–19. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 31.Davis G, Alter M, El-Serag H, Poynard T, Jennings L. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterol. 2010;138:513–21. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 32.Lang K, Danchenko N, Gondek K, et al. The burden of illness associated with hepatocellular carcinoma in the United States. J Hepatol. 2009;50:89–99. doi: 10.1016/j.jhep.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 33.McAdam-Marx C, McGarry LJ, Hane CA, et al. All-Cause and Incremental Per Patient Per Year Cost Associated with Chronic Hepatitis C Virus and Associated Liver Complications in the United States: A Managed Care Perspective. J Manag Care Pharm. 2011;17:531–46. doi: 10.18553/jmcp.2011.17.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Medicare & Medicaid Services (CMS), HHS. Medicare program; changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. Fed Regist. 2007;72:47129–8175. [PubMed] [Google Scholar]
- 35.American Hospital Directory, Hospital Statistics by State. [Accessed July 12, 2012]; Available at: ( http://www.ahd.com/state_statistics.html)
- 36.Davis KL, Mitra D, Medjedovic J, et al. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol. 2011;45:e17. doi: 10.1097/MCG.0b013e3181e12c09. [DOI] [PubMed] [Google Scholar]
- 37.First DataBank, Inc. [Accessed July 12, 2012];Drug databases. http://www.firstdatabank.com/Support/drug-pricing-policy.aspx.
- 38.Chong CAKY, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630–8. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 39.Hanmer J, Lawrence WF, Anderson JP, et al. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26:391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 40.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 41.Poynard T, Colombo M, Bruix J, et al. Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterol. 2009;136:1618–28. e2. doi: 10.1053/j.gastro.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 42.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 43.Poordad F, Bronowicki JP, Gordon SC, et al. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterol. 2012;143:608–18. e1-5. doi: 10.1053/j.gastro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Ghany MG, Nelson DR, Strader DB, et al. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011 doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sulkowski M, Poordad F, Manns MP, et al. Anemia during treatment with peginterferon alfa-2b/ribavirin with or without boceprevir is associated with higher SVR rates: analysis of previously untreated and previous-treatment-failure patients. Gastroenterol. 2011;140(Suppl):S-941–S-942. [Google Scholar]
- 46.Cammà C, Petta S, Cabibbo G, et al. Cost-effectiveness of boceprevir or telaprevir for previously treated patients with genotype 1 chronic hepatitis C. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.05.019. (in press) [DOI] [PubMed] [Google Scholar]
- 47.Ferrante SA, Chhatwal J, Brass CA, et al. Boceprevir for previously untreated patients with chronic hepatitis C Genotype 1 infection: a US-based cost-effectiveness modeling study. BMC Infectious Dis. 2013;13:190. doi: 10.1186/1471-2334-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cammà C, Petta S, Enea M, et al. Cost-effectiveness of boceprevir or telaprevir for untreated patients with genotype 1 chronic hepatitis C. Hepatology. 2012;56:850–60. doi: 10.1002/hep.25734. [DOI] [PubMed] [Google Scholar]
- 49.Saab S, Hunt DR, Stone MA, et al. Timing of hepatitis C antiviral therapy in patients with advanced liver disease: A decision analysis model. Liver Transplant. 2010;16:748–59. doi: 10.1002/lt.22072. [DOI] [PubMed] [Google Scholar]
- 50.Younossi Z, Singer M, McHutchison J, Shermock K. Cost effectiveness of interferon 2b combined with ribavirin for the treatment of chronic hepatitis C. Hepatology. 1999;30:1318–24. doi: 10.1002/hep.510300518. [DOI] [PubMed] [Google Scholar]
- 51.Wilson J, Yao G, Raftery J, et al. A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technol Assess. 2007;11:1–202. doi: 10.3310/hta11130. [DOI] [PubMed] [Google Scholar]
- 52.Poynard T, Colombo M, Bruix J, et al. Peginterferon alfa-2b and ribavirin: effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterol. 2009;136:1618–28. e2. doi: 10.1053/j.gastro.2009.01.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.