Abstract
The substituted 4-phenylpiperazine D3 dopamine receptor selective antagonist PG01037 ((E)-N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)but-2-enyl)-4-(pyridin-2-yl)benzamide) was reported to attenuate L-dopa associated abnormal involuntary movements (AIMs) in unilaterally lesioned rats, a model of L-dopa-dependent dyskinesia in patients with Parkinson’s Disease (Kumar et al., 2009a). We now report that PG01042 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-3-yl)benzamide), which is a D3 dopamine receptor selective agonist for adenylyl cyclase inhibition and a partial agonist for mitogenesis, is also capable of attenuating AIMs scores. The intrinsic activity of PG01037 and PG01042 were determined using a) a forskolin-dependent adenylyl cyclase inhibition assay and b) an assay for agonist-associated mitogenesis. It was observed that the in vivo efficacy of PG01042 increased when administered by intraperitoneal (i.p.) injection simultaneously with L-dopa/benserazide (8 mg/kg each), as compared to a 60 minute or 30 minute pretreatment. PG01042 was found to attenuate AIM scores in these animals in a dose dependent manner. While PG01042 did not effectively inhibit SKF 81297-dependent AIMs, it inhibited apomorphine-dependent AIM scores. Rotarod studies indicate that PG01042 at a dose of 10 mg/kg did not adversely affect motor coordination of the unilaterally lesioned rats. Evaluation of lesioned rats using a cylinder test behavioral paradigm indicated that PG01042 did not dramatically attenuate the beneficial effects of L-dopa. These studies and previously published studies suggest that both D3 dopamine receptor selective antagonists, partial agonists and agonists, as defined by an adenylyl cyclase inhibition assay and a mitogenic assay, are pharmacotherapeutic candidates for the treatment of L-dopa-associated dyskinesia in patients with Parkinson’s Disease.
Keywords: Parkinson’s Disease, dyskinesia, L-dopa, dopamine receptors, D3 dopamine receptors
Introduction
There are three dopaminergic pathways in the brain; the nigrostriatal pathway, the mesocorticolimbic pathway and the tuberoinfundibular pathways. These pathways are involved in movement coordination, cognition, emotion, affect, memory, reward mechanism and the regulation of prolactin secretion by the pituitary. Progressive neurodegeneration of the midbrain dopamine neurons of the nigrostriatal pathway is known to lead to the development of Parkinson’s Disease (PD), a neurological disorder characterized by resting tremor, rigidity, bradykinesia and postural instability. Levadopa (L-dopa) is used therapeutically in patients with PD to increase dopamine levels in the CNS. Given the complexity of the dopaminergic pathways, it is not surprising that there is a variety of motor and nonmotor side effects associated with L-dopa administration including, hypotension, nausea, gastrointestinal bleeding, confusion, anxiety, increased libido, vivid dreams, visual and/or auditory hallucinations, altered sleep patterns, compulsive behaviors such as gambling and amphetamine-like psychosis. In addition, chronic administration of L-dopa can also lead to abnormal involuntary movements, often referred to as L-dopa induced dyskinesia (LID).
In PD, the progressive death of dopaminergic neurons in the substantia nigra (SN) pars compacta upsets the equilibrium between the direct and indirect pathways leading to hyperactivity of the globus pallidus (GP), which normally acts as a brake for supplementary motor areas by inhibiting the motor thalamus. Excessive inhibition leads to parkinsonian syndrome. Dopamine and dopamine receptors are thought to play a pivotal role in the induction of parkinsonian symptoms because the direct pathway is activated by D1-like dopamine receptors and D2-like receptor activations inhibits the indirect pathway (Bezard et al., 2001). The neuronal basis for the development of LID in PD patients remains unclear (Fabbrini et al., 2007). LID appears to be associated with a sequence of events that include a) pulsatile stimulation of dopamine receptors, b) changes in neuronal protein and gene expression and c) abnormalities in nondopamine transmitter systems, including the 5-HT receptor systems. These events culminate in alterations in the firing patterns between the basal ganglia and the cortex.
Molecular genetic studies have identified two major types or categories of dopamine receptors, the D1-like (D1 and D5 receptor subtypes) and D2-like (D2, D3 and D4 receptor subtypes) receptors based upon structural and pharmacological similarities. D1-like receptors are structurally similar and positively linked to the activation of adenylyl cyclase via coupling to the Gs/Golf class of G proteins (Hervé et al., 2001). Conversely, stimulation of the D2-like receptors results in coupling with the Gi/Go class of G proteins, leading to the inhibition of adenylyl cyclase activity (Sibley et al., 1993; Sealfon and Olanow, 2000; Vallone et al., 2000). In addition to the inhibition of adenylyl cyclase activity, agonist activation of D2-like receptor can lead to mitogenic activation, phospholipase D (PLD) stimulation, increased activity of G protein-regulated inwardly rectifying potassium channels (GIRK), MAP kinase activation and the activation of the Na+/H+ exchanger (Huff, 1996; Kuzhikandathil et al., 1998; Senogles, 2003; Neve et al., 2004). The discovery of multiple dopamine receptor subtypes and multiple signaling pathways necessitates a re-evaluation of the role of dopamine receptor subtype selective agonists, partial agonists and antagonists as therapeutic agents for the treatment and management of neurodegenerative disorders (Hermanowicz, 2007; Luedtke and Mach, 2003; Newman et al., 2005).
This study focuses on the evaluation of D3 dopamine receptor selective compounds as potential antidyskinetic pharmacotherapeutic agents. Although the D2 and D3 dopamine receptor subtypes have only 46% amino acid homology, the transmembrane spanning (TMS) regions of the D2 and D3 receptors, which are thought to construct the ligand binding site, share 78% homology (Sokoloff et al., 1990). The D2 and D3 receptors differ in their a) neuroanatomical localization, b) levels of receptor expression, c) efficacy in response to agonist stimulation and d) regulation and desensitization (Joyce, 2001; Kuzhikandathil et al., 2004). Because of the high degree of homology between D2 and D3 receptor binding sites, it has been difficult to obtain compounds that can bind selectively to either the D2 or the D3 dopamine receptor subtypes (Luedtke and Mach, 2003; Newman et al., 2005). However, recently D2 or D3 dopamine receptor selective agonists, partial agonists and antagonists have been developed (Grundt et al., 2005, Chu et al., 2005; Vangveravong et al., 2006; Grundt et al., 2007; Chen et al., 2008; Newman et al., 2009). These compounds will undoubtedly prove to be useful pharmacologic tools to dissect the role of these two D2-like receptor subtypes not only as potential antidyskinetic agents, but also in a variety of experimental physiological and behavioral situations, including the reinforcing and toxic properties of psychomotor stimulants such as cocaine and methamphetamine, socialization, memory, fine motor skills, neuropsychiatric symptoms and the regulation of interneuronal activity in the basal ganglia (Canales and Iversen, 2000; Gendreau et al., 2000).
While it is generally accepted that the highest levels of D3 dopamine receptor subtype expression is in the limbic areas, the neuroanatomical distribution of D3 receptor mRNA expression in the human brain using in situ hybridization indicates a heterogeneous expression of D3 receptor mRNA throughout the human brain. The most abundant D3 mRNA expression levels are found in the Islands of Calleja and within the ventral striatum/nucleus accumbens region. However, high levels are also evident within the dentate gyrus and striate cortex. Low to moderate D3 mRNA expression levels were found in cortical regions, caudate nucleus, putamen, anterior and medial thalamic nucleus, mammillary body, amygdala (AMG), hippocampal CA region, lateral geniculate body, SN pars compacta, locus coeruleus and raphe nuclei (Bouthenet et al., 1991). Thus, the D3 dopamine receptor has been hypothesized to play a role in pyramidal motor functions (Suzuki et al., 1998).
In addition, there is also evidence supporting the hypothesis that dopamine can act as an extrasynaptic modulator (Dumartin et al., 1998; Garris and Wightman, 1995; Paspalas and Goldman-Rakic, 2004; Sesack, 2002). The observation of infrequent synaptic convergence between prefrontal cortex (PFC) and dopaminergic terminals in the AMG suggests that dopamine modulates PFC inputs primarily through an extrasynaptic mechanism. In situ hybridization and immunohistochemical neuroanatomical studies have indicated the presence of D1-like and D2-like receptors in the AMG (Fremeau et al., 1991; Fuxe et al., 2003; Maltais et al., 2000; Meador-Woodruff et al., 1991; Pickel et al., 2006; Weiner et al., 1991). Neuronal projections from the PFC to the AMG regulate affective behaviors which can be modulated by dopamine receptor subtype selective compounds. AMG dysfunction has been implicated in the formation of phobic and anxiety disorders (Davidson, 2002; Davis and Shi, 2000; LeDoux, 2000), as well as patients with affective disorders and schizophrenia (Drevets, 2003; Grace and Rosenkranz, 2002; Rauch et al., 2003).
In this report we have focused on the in vivo properties of D3 receptor selective compound, PG01042, which is a member of the class of 2,3-chloro substituted 4-phenylpiperazine compounds. We compared the D2-like receptor in vitro binding selectivity and intrinsic activity of PG01042 with our prototypic D3 receptor selective antagonist PG01037 at D2 and D3 receptors using an adenylyl cyclase inhibition assay and a mitogenic assay. We then examined the effects of PG01042 in a rat model of human LID and compared the results to those previously obtained for PG01037, specifically the ability to attenuate L-dopa dependent abnormal involuntary movements (AIMs) for potential antidyskinetic activity.
Methods and Materials
Animals
This study was performed using male Sprague Dawley rats that were unilaterally lesioned in the medial forebrain bundle (MFB) by the commercial vendor Charles Rivers. This procedure involves the injection of 6-OHDA into the MFB. Approximately seven days after surgery an amphetamine challenge was performed to test the validity of the lesion. Upon arrival to our animal facility, the lesioned animals were housed under a 12-hour light: 12-hour dark cycle with free access to tap water. The lesioned animals food was restricted to maintain their weights at approximately 350 grams. Lesioned animals were acclimated for at least one week before the L-dopa/benserazide injections were initiated. The treatment of the lesioned animals and the experimental procedures have been approved by an institutional animal welfare committee. Animal care and housing were in adherence with the conditions set forth in the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996).
Test drugs and treatment regimens
One week after their arrival the lesioned rats, were screened behaviorally using an amphetamine-induced rotation test to verify the authenticity of the lesion. The amphetamine test was used to assess the authenticity of the lesioning. In unilaterally lesioned animals an indirect dopaminergic agonist, in this case amphetamine, produces a release of dopamine from the intact nigrostriatal terminals causing lesioned rat to rotate away from the lesioned side (contraversive rotation), where the striatal dopamine receptors are supersensitized. This type of drug induced rotation is commonly used to assess the extent of the unilateral dopamine denervation (Cenci and Lundblad, 2005).
Subsequently, 8 mg/kg L-dopa with 8 mg/kg benserazide was administered to each rat as a daily i.p. injection (injection volume approximately 0.3 ml using a 26 gauge, 1/2 inch needle (Becton Dickinson & Co.)) for 21 consecutive days to induce the development of dyskinetic-like movements. Benserazide was included because it is a peripherally-acting aromatic L-amino acid decarboxylase inhibitor which prevents the peripheral metabolism of L-dopa.
The L-dopa/benserazide solutions were dissolved in sterile saline (9 g NaCl/liter). The highest concentration of PG01042 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-3-yl)benzamide HCl; prepared as reported in Grundt et al., 2005) that was prepared was 10 mg/ml in sterile water containing 10% dimethylsulfoxide (DMSO: Sigma). Heating the drug solution with hot tap water (55–60 °C) and vigorous vortexing was required to keep the drug in solution at that concentration. After the drug solution was loaded into the syringe, a heated DELTAPHASE ISOTHERMAL PAD (Braintree Scientific) was used to transport the drug to the animal facility. The usual injection volume was approximately 0.3 ml, therefore the amount of DMSO injected per animal was approximately 100-fold less than the oral acute LD50 value for rat (14500 mg/kg) (ScienceLab.com).
In the drug testing experiments, the compound was evaluated using an observer blind, randomized design, whereby half of the lesioned rats were administered test drug or vehicle followed by L-dopa (8 mg/kg combined with 8 mg/kg benserazide). The test compound was administered prior to L-dopa at various time intervals. Buspirone (N-[4-[4-(2-pyrimidinyl)-1- piperazinyl]butyl]-8-azaspiro[4.5]decane-7,9-dione hydrochloride, Sigma–Aldrich) was administered i.p., generally 60 min prior to L-dopa administration. These experiments were designed such that any given animal had >5 days between the administration of test drug and no animal received test drug more than twice a week. Throughout the course of these studies each animal received a minimum of two doses of L-dopa/benserazide (8 mg/kg each) per week to maintain the involuntary movement behaviors. The studies described in this manuscript were performed using a set of 24 animals.
AIMs Ratings
AIMs ratings were performed by an investigator who was unaware of the pharmacological intervention, as previously described (Kumar et al., 2009a). After injection of the L-dopa, the severity of the AIMs was quantified using lesioned rats that were observed individually in their home cages at 20 minute intervals, starting 10 minutes after the injection of L-dopa, until the AIMs subsided (approximately 2 hours). The observer scored < 6 animal concurrently for a 5 minute interval. AIMs were scored in four categories, as discussed by Dekundy and colleagues (2007): a) axial AIMs, including dystonic or choreiform torsion of the trunk and neck towards the side contralateral to the lesion; b) limb AIMs, including jerky and/or dystonic movements of the forelimb contralateral to the lesion; c) orolingual AIMs, including twitching of orofacial muscles with empty masticatory movements and protrusion of the tongue towards the side contralateral to the lesion and d) locomotive AIMs, including increased locomotion with contralateral side bias.
Each of the four categories were scored on a severity scale from 0 to 4, where 0 = absent, 1 = present during less than half of the observation time, 2 = present for more than half of the observation time, 3 = present all the time but suppressible by external stimuli, and 4 = present all the time and not suppressible by external stimuli. For these experiments the external stimuli that was used was the movement of the animal’s cage forward and then back 1 to 2 inches, within 1–2 seconds, in a smooth motion. Generally, the summation of AIMs scores included a) axial, limb and orolingual AIMs or b) axial, limb, orolingual and locomotor AIMs over the total observation period.
Rotarod Test
The rotarod test was performed to assess the effect of PG01042 on motor performance and coordination (Lundblad et al., 2002; Dekundy et al., 2007). The lesioned animals were fed approximately 1 hour prior to testing. Unilaterally lesioned rats were placed on a gradually accelerating rotarod apparatus set to 0 to 40 rpms/minute over 90 seconds. The first three days were training sessions and animals were not injected with test drug or vehicle. Two training sessions were performed per day, one session in the morning and one in the afternoon (Dekundy et al., 2007; Cenci et al., 2005). On the fourth and sixth days rats were administered 10 mg/kg PG01042 i.p. and then evaluated at 30 and 60 minutes post drug administration. On the fifth day rats were tested after administration with a similar volume of vehicle. During the training and testing sessions the rats were tapped on their tails to maintain attention and focus (Cenci et al., 2005). The data from the rotarod experiments was expressed as the mean number of seconds that animal was able to remain on the rod before falling (latency to fall).
Cylinder Test
The cylinder test was used to assess the spontaneous and independent use of each of the rat’s forelimbs in the context of an instinctive rearing behavior, with the rat standing on its hind legs and leaning on the enclosing walls (Schallert, 2000). Rats were placed individually in open ended Plexiglas cylinder (21 × 34 cm) without habituation in a dimly lighted room. The investigator scored the lesioned animals on the basis of the first forelimb to make contact (left, right or both) with the enclosing Plexiglas walls. Asymmetrical forelimb usage was calculated as the percentage of total performance, within two five minute intervals, one beginning 12 minutes post injection and the next beginning 20 minutes post injection.
Statistics
SYSTAT 12 software (San Jose, CA) was used for statistical analysis of the AIMs scores. ANOVA was used to determine F values, degrees of freedom and the level of significant difference between the test drug treated and vehicle treated groups. In the figures, the data points are generally presented as the mean values ± S.E.M. that was obtained for each treatment group and data is often expressed as a normalized (to 100) value. For the rotarod experiment an ANOVA analysis with repeated measures was performed. A probability value less than or equal to 0.05 (p < 0.05) was the minimum value considered to indicate significance.
Results
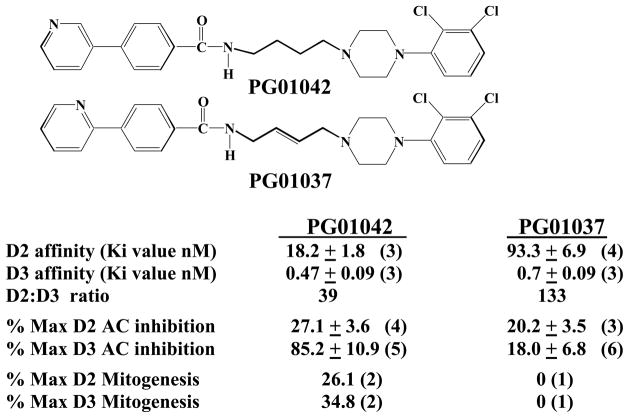
In Figure 1 the structure and pharmacological profile of the 2,3-dichloro substituted phenylpiperazine PG01042 is compared to PG01037 (Grundt et al., 2005). PG01042 binds with high affinity to the D3 dopamine receptor, with 39-fold selectivity compared to the D2 dopamine receptor subtype. Our studies on the inhibition of forskolin-dependent stimulation of adenylyl cyclase in transfected HEK cells indicate that PG01042 is an agonist at D3 dopamine receptors and a weak partial agonist at D2 receptors. The results of mitogenic assays using transfected CHO cells indicate that PG01042 is a partial agonist at both D2 and D3 dopamine receptor subtypes (Grundt et al., 2005). Based upon these results, in this communication we refer to PG01042 as an agonist/partial agonist.
Figure 1. Structure and Pharmacologic Profiles of PG01037 and PG01042.
The chemical structure and summary of our current available information on the pharmacological properties of PG01042 is shown. Mean values for the affinity measurements (Ki values) are expressed as nMolar. For the cyclase inhibition assay the intrinsic activity was evaluated using the test ligand and the full D2-like dopamine receptor agonist quinpirole at concentrations approximately 10x the Ki values (PG01042, 200 nM for D2 and 10 nM for D3; PG01037, 1000 nM for D2 and 10 nM for D3; quinpirole, 1000 nM for D2 and 100 nM for D3). The maximum inhibition for quinpirole was 89.3 ± 1.1 (5) for human D2 receptors and 35.0 ± 3.6 (5) for D3 receptors. The percent maximum mitogenesis is based upon dose response curves (one concentration per decade) using a maximum of 10−5 M test drug performed by the Division of Treatment Research and Development (DTRD) of NIDA. The numerical values are the mean ± the S.E.M. and the number in the parentheses is the number of independent experiments (n). The synthetic methods for these two compounds was previously reported (Grundt et al., 2005).
In contrast, PG01037 binds to the human D3 dopamine receptor with >130-fold selectivity compared to the human D2 dopamine receptor subtype. Based upon adenylyl cyclase studies we characterize this compound as a) an antagonist at D3 dopamine receptors and b) a weak partial agonist at D2 receptors. PG01037 is an antagonist at both D2 and D3 dopamine receptor subtypes in both the cyclase and mitogenic assays (Grundt et al., 2005). In our previous publication we reported that PG01037 administration attenuated L-dopa associated abnormal involuntary movements (AIMs) in unilaterally lesioned male Sprague Dawley rats, which is a model of L-dopa-dependent dyskinesia in patients with Parkinson’s Disease (Kumar et al., 2009a).
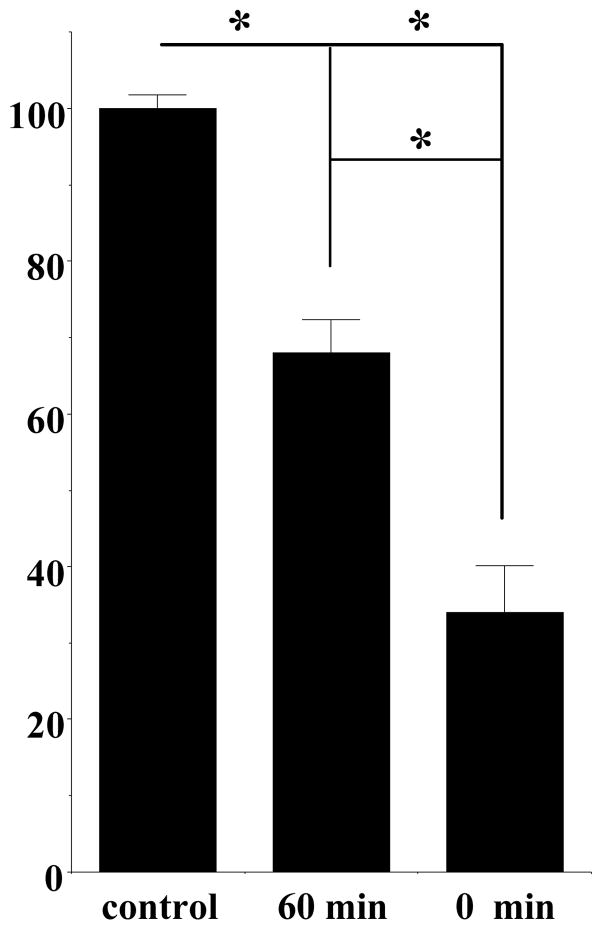
In this study we examine the effect of PG01042 in this same animal model to further investigate the in vivo mechanism of action of this class of compounds. We began our studies by determining if our novel D3 dopamine receptor selective agonist/partial agonist, PG01042, would attenuate, potentiate or possibly have no effect on L-dopa dependent AIMs in our lesioned rats. We investigated efficacy and stability of PG01042 by examining the effect of the pre-administration time of test drug on the attenuation of AIMs. For this comparison, PG01042 (10 mg/kg) was administered by i.p. injection at 60 and 0 minutes prior to L-dopa administration (8 mg/kg). At 0 minute pretreatment, we found that PG01042 produced a greater attenuation of the L-dopa-dependent AIMs scores, than at 60 minutes pretreatment (Figure 3). When PG01042 and L-dopa were administered simultaneously we achieved a mean reduction in total AIMs scores of 71.1 ± 5.9% and a 68.1 ± 6.4% reduction in the mean AIMs minus locomotion score. This effect is similar to that we reported for our D3 dopamine receptor selective antagonist PG01037 (Kumar et al., 2009a). It is noteworthy that although PG01042 is a D3 receptor agonist/partial agonist in our in vitro functional assays, it did not precipitate any AIMs in the lesioned animals during the 60 pretreatment period in any of the animals that were tested.
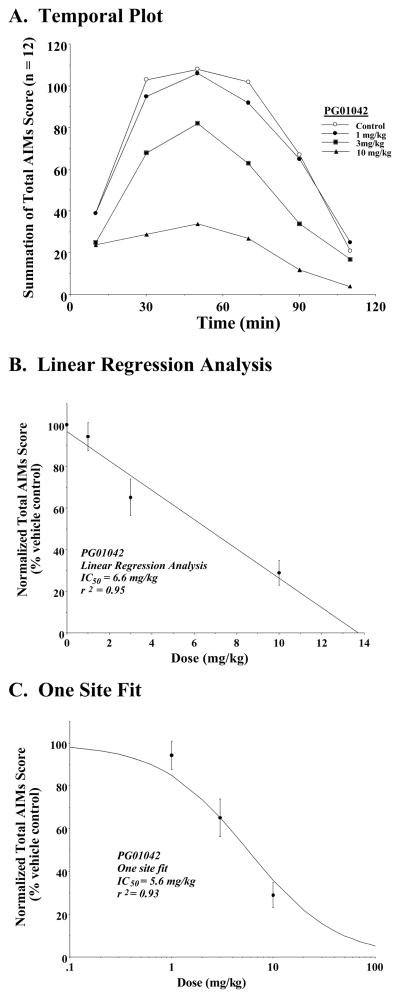
Figure 3. Dose Response Curve for the Attenuation of Total AIMs Score by PG01042.
Unilaterally lesioned rats were injected (i.p.) with varying doses of PG01042 (0 to 10 mg/kg) followed immediately with a constant dose of L-dopa and benserazide (8 mg/kg each). The normalized mean total AIMs score ± S.E.M. (n = 12; normalized to 100) relative to vehicle controls as a function the dose of PG01042 is shown. A. The variation in the total AIMs score as a function of time (temporal plot) is shown using zero time pretreatment with 10 mg/kg PG01042 (▲), 3 mg/kg PG01042 (■), 1 mg/kg PG01042 (●) and for the vehicle control (❍). Unilaterally lesioned animals were also injected i.p. with 8 mg/kg L-dopa and benserazide. Each point is the summation of total AIMs scores for a total of 12 animals at each observation time point.
The calculated IC50 value for this data was obtained using either a (B) linear regression analysis which included a data point at a dose of 0 mg/kg which is essentially equal to the vehicle control (IC50 = 6.6 mg/kg) or (C) a one site fit based upon the law of mass action in which the curve is constrained to values of 0 (infinitely high dose) and 100% (vehicle control) (IC50 = 5.6 mg/kg). For a) 1 mg/kg PG01042, this represents a mean 8.4 ± 7.3% reduction, b) 3 mg/kg PG01042, this represents a mean 25.2 ± 4.4 percent reduction c) 10 mg/kg PG01042, this represents a mean 70.5 ± 6.1% reduction, in the total AIMs score over the observation time.
It was previously suggested that locomotive AIMs component may not provide a specific measure of dyskinesia (Dekundy et al., 2007), but rather a correlate of contralateral turning behavior in rodents with unilateral 6-OHDA lesions. In our previous study with PG01037 we found that a) the percent decreases in total AIM scores and AIM scores minus the locomotion component compared to vehicle control values were essentially the same and b) there was no preferential reduction in any of the four components of AIM scoring system. Similar results were found for PG01042 (Table I). For 0 minute pretreatment and at a dose of 10 mg/kg, PG01042 reduced both the total AIM and the AIM minus locomotion scores by approximately 70%. The percent reduction in the locomotor, axial twisting and orolingual scores were similar, ranging from 76.5% to 80.4%. The reduction in forelimb movement was found to be slightly less at 56%. A one-way ANOVA analysis indicates that there is no significant difference (F(5,66) = 2.01, p > 0.05, n = 12) in the percent reduction of the a) total AIM score, b) AIM-locomotion score, c) locomotor score, axial twisting score, d) orolingual score and the e) forelimb movement score. Since no preferential deduction in any of the AIM components was observed, the subsequent results obtained with PG01042 are reported as total AIMs scores.
Table I.
Effect of PG01042 on Combined and Individual AIM scores.
| AIM component | Percent Reduction |
|---|---|
| Total AIM Score | 70.5 ± 4.5 |
| AIM – Locomotion | 68.5 ± 2.8 |
| Locomotion | 80.4 ± 9.5 |
| Axial twisting | 78.5 ± 4.6 |
| Orolingual | 76.5 ± 10.8 |
| Forelimb movement | 56.0 ± 3.1 |
PG01042 was administered at a dose of 10 mg/kg by i.p. injection at the same time that the L-dopa and benserazide (8 mg/kg each) was administered (0 min pretreatment). The values are the mean ± S.E.M. scores that have been normalized to the control values (n = 12). Results of one-way ANOVA suggest no preferential deduction in any of the AIM components (F(5,66) = 2.01, p > 0.05, n = 12).
A concentration dependent analysis was performed to determine the effect on L-dopa dependent AIMs when varying doses of PG01042 (1 to 10 mg/kg) were administered simultaneously (0 min pretreatment) with L-dopa (Figure 3). The dose dependent data for PG01042 is plotted as a function of time after the administration of PG01042 and L-dopa (Figure 3A). When this data was fit to a straight line, an IC50 = 6.6 mg/kg values was obtained (Figure 3B). If it was assumed that at very high doses of PG01042 the AIMs scores would approach zero, the data fit well to a reversible, one site fit model with an IC50 value of 5.6 mg/kg with a nonlinear regression coefficient (r2) equal to 0.98 (Figure 3C). This is similar to what we have reported previously for the antagonist PG01037 (IC50 = 7.4 with r2 = 0.98) (Kumar et al., 2009a).
PG01042, PG01037 and buspirone were administered in combination to determine if they might act in vivo in an additive, synergistic or antagonist manner. The test combinations were: a) PG01042 at 3 mg/kg (i.p., control), b) PG01037 at 3 mg/kg (i.p., control), c) buspirone at 1 mg/kg (i.p., control), d) PG01042 at 3 mg/kg and PG01037 at 3 mg/kg (both i.p.), e) PG01042 at 3 mg/kg and buspirone at 1 mg/kg (both i.p.) and f) PG01037 at 3 mg/kg and buspirone at 1 mg/kg (both i.p.) (Table II). Although the in vitro cyclase analysis indicated that PG01042 is an agonist/partial agonist at D3 receptors and PG01037 is an antagonist at D3 receptors, when these compounds were administered in combination there was an essentially additive reduction in total AIM scores. The combinations of buspirone plus PG01037 or buspirone plus PG01042 also appeared to be pharmacologically additive.
Table II.
Combination Effects of PG01042 and PG01037.
| Drug regimen | %Reduction |
|---|---|
| PG01042 | 35.1 ± 9.1 |
| PG01037 | 21.6 ± 4.4 |
| PG01042 and PG01037 | 44.6 ± 6.9 |
| buspirone | 34.8 ± 6.2 |
| PG01042 and buspirone | 50.5 ± 7.0 |
| PG01037 and buspirone | 65.2 ± 7.9 |
The PG01042 and PG01037 were administered alone or concurrently to evaluate their effect on AIM scores. The test doses were a) PG01042 at 3 mg/kg, b) PG01037 at 3 mg/kg or c) buspirone at 1 mg/kg. The mean values ± S.E.M. for the normalized reduction (percent reduction) of total AIMs values is shown for n = 12. Test compounds were administered by i.p. injection with a constant dose of L-dopa (8 mg/kg) and benserazide (8 mg/kg).
The involvement of the serotonergic system in L-dopa induced AIMs has been studied in unilaterally lesioned rats. Results from Carta and colleagues (2006) suggest that dopamine, may act as a false transmitter in serotonergic neurons, and interfere with transmitter release of L-dopa derived dopamine reducing the L-dopa-induced AIMs. Serotonin neurons have been shown to a) convert L-dopa to dopamine, and b) store and release dopamine in an activity dependent manner (Carta et al., 2007). The authors proposed that this dysregulation of dopamine synthesized and released from serotonergic terminals might trigger involuntary movements in unilaterally lesioned rats, while having little to no effect on similar involuntary movements induced by a) L-dopa-derived dopamine from dopaminergic terminals or b) dopaminergic agonists.
Substituted phenyl piperazines have been reported to bind to 5-HT1A receptors, as well as D2-like receptors. However, for a compound to be active in this model (i.e., antiAIMs activity) it would have to be an agonist at 5-HT1A receptors. PG01042 acts as a weak partial agonist at D2 and partial or nearly full agonist at D3 dopamine receptors in vitro (Figure 1). However, we currently have no information on the intrinsic activity of PG01042 at 5-HT1A receptors. The following experiments were performed to investigate the possibility that PG01042 might act in vivo via 5-HT1A receptors.
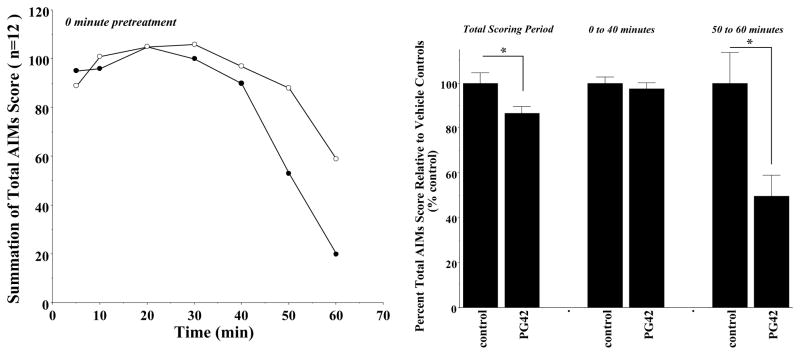
First, apomorphine is a D1/D2 dopamine receptor agonist that acts directly on dopamine receptors. Apomorphine’s action in vivo is independent of any extrinsic L-dopa derived dopamine synthesis or release. We evaluated the ability of PG01042 to attenuate AIMs score when animals were administered the dopaminergic agonist apomorphine. PG01042 (10 mg/kg) was administered simultaneously with apomorphine (0.05 mg/kg s.c.). For these studies we found that there was only a mean 14.5 ± 3.0 (S.E.M.) percent reduction in the total AIMs score over the observation time (Figure 4). However, we also observed that there was a temporal dependence for that attenuation. The attenuation of the apomorphine-dependent involuntary movements was minimal in the beginning of the session (at the 5 minute post injection time point through 40 minutes post injection) and appeared to increase with time with the most significant attenuation occurring at the end of the scoring period (after 40 minutes post injection). The temporal dependence of the attenuation of apomorphine-dependent AIMs scores is shown in Figure 4. The significant attenuation of apomorphine induced AIMs, supports the idea the PG01042 is acting via a dopaminergic mechanism.
Figure 4. Effect of Zero Minute Pretreatment of PG01042 on the Temporal Expression of Apomorphine-Dependent AIMs in Lesioned Rats.
Unilaterally lesioned rats were injected (i.p.) with vehicle or PG01042 (10 mg/kg) followed immediately with apomorphine (0.05 mg/kg, s.c.). (Left panel) The temporal plots of the summation of total AIM scores is shown as a function of time for animals (n = 12) administered either the vehicle control (❍) or PG01042 (●). (Right panel) The percent of the mean total AIMs score ± S.E.M. (n = 12) relative to vehicle controls (control) as a function the dose of PG01042 (PG42) is shown. The mean value ± S.E.M. for the normalized total AIMs values as a function of PG01042 administration are shown for a) 0 to 60 minutes, b) 0 to 40 minutes and b) 50 to 60 minutes. A significant reduction in total AIM scores for the 0 to 60 minute time period (F(1,22) = 4.4, p < 0.05) and for the 50 to 60 minute time period (F(1,22) = 9.7, p < 0.05) is designated with an asterisk (*).
Second, studies by Delfino and colleagues reported that AIMs could be induced in unilaterally lesioned rats using the D1/D5 dopamine receptor selective agonist SKF 81297. Sensitization of striatal neurons to D1 and/or combined D1 and D2 receptor stimulation contribute to drug-induced dyskinesia (Delfino et al., 2007). Dopamine receptor agonists exert their effects by directly activating dopamine receptors, bypassing the presynaptic synthesis of dopamine and the degenerating nigrostriatal dopamine system. We evaluated the effect of 10 mg/kg of PG01042 administered by i.p. injection simultaneously with SKF 81297 (0.1 mg/kg, s.c.). This dose and route of administration of SKF 81297 was based upon previously published studies (Kumar et al., 2009a). In this experiment there was no significant reduction of SKF 81297-dependent involuntary movements (F(1,22) = 0.72, p > 0.05) (Figure 5), which is consistent with previously reported studies using PG01037 (Kumar et al., 2009a). In addition, although we were administering both a D1-like and a D3 dopamine receptor agonist we did not observe an exacerbation of the AIMs in these animals.
Figure 5. Effect of Zero Minute Pretreatment of PG01042 on the Temporal Expression of SKF 81297-Dependent AIMs in Lesioned Rats.
Unilaterally lesioned rats were injected (i.p.) with PG01042 (●, 10 mg/kg) followed immediately with a constant dose of SKF 81297 (0.05 mg/kg, s.c.). The percent of the mean total AIMs score ± S.E.M. (n = 12) relative to vehicle control (○) as a function of the dose of PG01042 is shown. The mean reduction ± S.E.M. for the normalized total AIMs values using PG01042 was 10 mg/kg, 8.0 ± 6.8% reduction but significance was not achieved (F(1,22) = 0.72, p = 0.40).
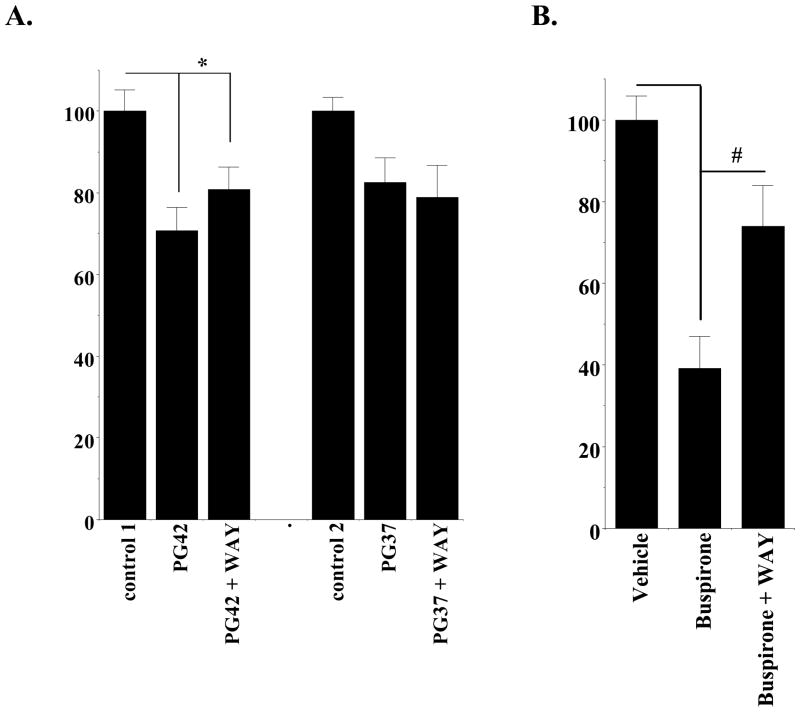
Third, recent studies have shown that 5-HT1A agonist in vivo activity could be attenuated by the co-administration of a 5-HT1A receptor selective antagonist WAY 100635 (Carta et al., 2007). We performed a similar experiment to determine if WAY 100635 could inhibit the attenuation effect of PG01042 or PG01037 on AIMs scores. In this experiment we found that at a dose of 3 mg/kg PG01042 did significantly attenuate the total AIM scores (F(1, 22) = 14.4, p < 0.005, n = 12). However, at a dose of 3 mg/kg PG01042, WAY 100635 (1 mg/kg) did not significantly (F(1, 22) = 1.5, p > 0.05, n = 12) inhibit the ability of PG01042 to attenuate total AIMs scores (Figure 6Aleft). Consistent with our previous studies, we also found that at a dose of 3 mg/kg PG01037, WAY 100635 (1 mg/kg) did not significantly (F(1,18) = 3.67, p > 0.5, n = 10) inhibit the ability of PG01037 to attenuate total AIMs scores (Figure 6Aright). As a positive control we tested the ability of WAY 100635 (1 mg/kg) to inhibit the activity of the 5-HT1A agonist buspirone and found that WAY 100635 significantly (F(1,18) = 15.4, p < 0.001, n = 10) reduced the ability of buspirone (4 mg/kg) to attenuate total AIMs scores in our unilaterally lesioned animals (Figure 6B).
Figure 6. Effect of the 5-HT1A Antagonist WAY 100635 on the Activity of PG01042, PG01037 and Buspirone.
Unilaterally lesioned animals were used to test the effect of the 5-HT1A antagonist WAY 100635 on the ability of either PG01042 (A left) or PG01037 (A right) to attenuate L-dopa-dependent abnormal involuntary movements. L-dopa was administered at a dose of 8 mg/kg by i.p. injection. PG01042 or PG01037 and L-dopa were administered simultaneously. WAY 100635 was always administered 10 minutes prior to the administration of test compounds (PG01042 or PG01037). Data presented is total AIMs score normalized to 100 ± S.E.M. The conditions of the experiment are as follows: a) L-dopa in the absence of any test compound (control 1); b) L-dopa in the presence of PG01042 (3 mg/kg); c) L-dopa in the presence of both PG01042 and WAY 100635 (1 mg/kg); d) L-dopa in the absence of any test compound (control 2); e) L-dopa in the presence of PG01037 (3 mg/kg); and f) L-dopa in the presence of both PG01037 (3 mg/kg) and WAY 100635 (1 mg/kg). The administration of PG01042 or PG01037 with WAY 100635 resulted in a significant reduction in total AIM score compared to the vehicle control (F(1,22) = 14.4, p < 0.05 and F(1, 22) = 6.4, p < 0.05, respectively with n = 12). While the administration of WAY 100635 (1 mg/kg) resulted in a 10% reduction in the attenuation of total AIMs scores by PG01042, this difference did not achieve significance (F(1, 22) = 1.5, p > 0.05). In addition, the administration of WAY 100635 (1 mg/kg) resulted in no reduction in the attenuation of total AIMs score by PG01037 (3 mg/kg) (F(1, 18) = 0.008, p > 0.5). In Figure 6A the asterisk (*) denotes significant difference between vehicle control and test drugs.
(B) Unilaterally lesioned animals were also used to test the effect of WAY 100635 on the ability of buspirone to attenuate L-dopa-dependent abnormal involuntary movements. L-dopa was administered at a dose of 8 mg/kg by i.p. injection. Buspirone was administered 30 minutes prior to administration of L-dopa. WAY 100635 was administered 10 minutes prior to the administration of test compounds (40 minutes prior to L-dopa administration). Data is presented as the total AIMs score normalized to 100 ± the normalized S.E.M. The conditions of the experiment are as follows: a) L-dopa in the absence of any test compound (vehicle control); b) L-dopa in the presence of buspirone (4 mg/kg); and c) L-dopa in the presence of both buspirone (4 mg/kg) and WAY 100635 (1 mg/kg). The administration of buspirone resulted in a significant reduction in total AIM scores compared to vehicle control (F(1,18) = 55.4, p < 0.001, n = 10). The administration of WAY 100635 (1 mg/kg) resulted in a 35% reduction in a significant attenuation of total AIMs scores by buspirone (4 mg/kg) (F(1,18) = 15.4, p < 0.01, n = 10). In Figure 6B the asterisk (*) denotes significant difference (p < 0.001) between the effects of buspirone versus vehicle control. The number symbol (#) denotes a significant difference (p < 0.005) between the effects of buspirone in the absence and presence of WAY 100635.
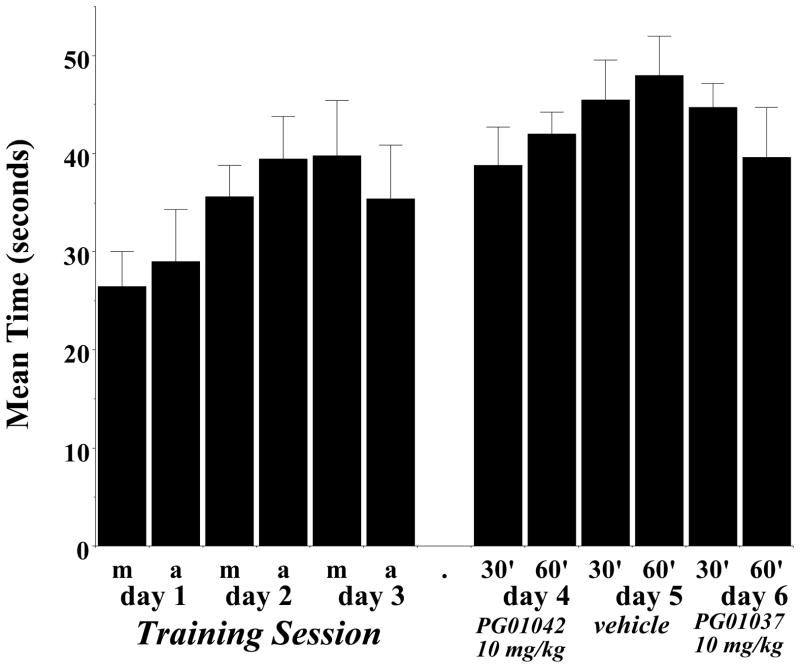
At this point our studies indicated that despite their differences in the in vitro intrinsic efficacy at D3 receptors, the antagonist PG01037 and our agonist/partial agonist PG01042 exert similar effects in our unilaterally lesioned animals. To further evaluate PG01042 as a potential antidyskinetic agent we investigated whether or not PG01042 might inhibit motor skills or the beneficial effects of L-dopa administration in these animals. First, during visual observations for AIMs scoring, there was nothing indicating that PG01042 administration interferes with motor coordination or acts like a muscle relaxant. However, to further investigate PG01042 for possible side effects we performed a rotarod test. The first three days were training sessions and animals were not injected with test drug or vehicle. Two training sessions were performed per day, one session in the morning and one in the afternoon (Dekundy et al., 2007; Cenci et al., 2005). On the fourth and sixth days lesioned rats were administered 10 mg/kg PG01042 i.p. and then evaluated at 30 and 60 minutes post drug administration. On the fifth day rats were tested after administration with a similar volume of vehicle. We did not find any significant difference between performance of rats treated with PG01042 and vehicle control (Figure 7).
Figure 7. Effect of PG01042 on Rotarod Performance.
A rotarod apparatus was used to assess the effect of PG01042 on motor coordination and agility of unilaterally lesioned rats in the presence or absence of test compound. The data is presented as the mean amount of time the animal remained on the rotarod before falling (Latency to Fall; in seconds) ± S.E.M. The acceleration conditions for the rotarod test was 0 to 44 rpms in 90 seconds. This experiment was performed on 6 consecutive days. Days 1 through 3 were training sessions that were conducted in the morning and afternoon (Training day 1–3, m and a, respectively). After the training session was completed, lesioned rats were administered PG01042 (i.p.) at a dose of 10 mg/kg for the inhibition of total AIMs score and evaluated at 30 minutes (30′) and 60 minutes (60′) post drug administration on days 4 and 6. On day 5 the vehicle was administered and animals were evaluated at 30 minutes (30′) or 60 minutes (60′) post vehicle administration. An ANOVA with repeated measures was used for the statistical comparison of the effect of the mean values of test drug (day 4 and day 6) to its vehicle control (day 5) indicated no statistical significant difference in latency to fall (p ≥ 0.10).
Second, to investigate for possible inhibition of the beneficial effects of L-dopa by PG01042, a cylinder test was performed to measure forelimb usage preference. The cylinder test assesses the independent use of each forelimb in the context of the rat’s natural rearing/exploratory behavior. It was observed that early in (10 to 12 minutes post injection) scoring the lesioned animals for AIMs it appeared that animals treated with PG01042 and L-dopa began to use their compromised front forelimb (contralateral to the lesion) to touch the side of the cage during exploratory rearing as frequently as L-dopa treated animals. At 12 minutes post injection, the lesioned animals injected with both L-dopa and PG01042 used their right (compromised) forelimb 70.1 ± 2.3% compared with the 3.0 ± 4.9% found with the administration of vehicle only. The lesioned animals also increased their use of the right forelimb to 87.8 ± 2.4% of the time after 12 minutes of administration of L-dopa and vehicle control. At 20 minutes post injection, we found that lesioned rats used right paw 2.9 ± 1.9% of the time when administered saline. However, lesioned animals administered both PG01042 and L-dopa used the right paw 85.0 ± 4.6 % of the time, 20 minutes post-injection. We could not perform the cylinder test at the 20 minute time period when rats were treated with L-dopa and vehicle because of the presence of severe dyskinesia. No significant difference was observed between L-dopa ± vehicle and L-dopa ± PG01042 treated animals at 12 minute post injection in the use their right fore paw (Figure 8) (F(1,12) = 3.85, p > 0.05, n = 7).
Figure 8. Effects of PG01042 on the Cylinder Test.
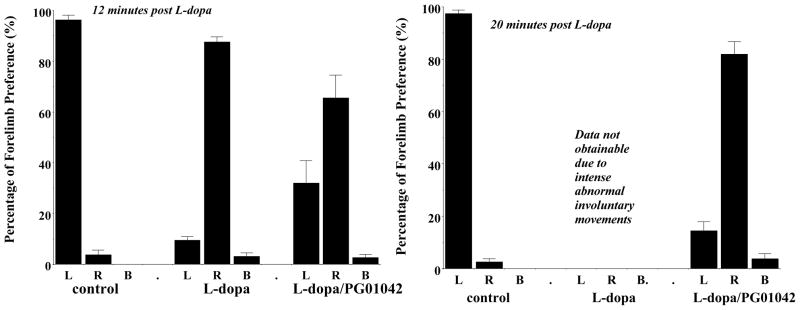
The data shown is the mean percent preferential usage of either the left (L), right (R) or both (B) forelimbs ± S.E.M. For this experiment the right forelimb is the compromised limb, contralateral to the lesion. Unilaterally lesioned animals were administered either a) saline (control), b) L-dopa and benserazide (L-dopa) at a dose of 8 mg/kg each, c) L-dopa and benserazide with PG01042 (L-dopa/PG01042) at a dose of 10 mg/kg. Animals were evaluated at 12 minutes (left) and (b) 20 minutes (right) post injection. (Left) At the 12 minute time point, animals were evaluated for a total of 5 minutes then returned to their home cage for 3 minutes. At the 12 minute time point there was a significant increase in the usage of the right forelimb following that administration of L-dopa (F(1,12) = 696, p < 0.001, n = 7) or L-dopa with PG01042 (F(1,12) = 36.7, p < 0.001, n = 7), compared to vehicle control animals. In addition, there was a marginal attenuation of right forelimb usage by PG01042 (F(1,12) = 3.85, p > 0.5). (Right) At the 20 minute time point, animals were further evaluated until >13 touches/rat were observed. After administration of L-dopa + vehicle and L-dopa + PG01042 contralateral forelimb usage improved significantly from 2.8% (in presence of saline and vehicle) to 82% (mean percentage use of right paw ± S.E.M.) (F(1,14) = 253, p < 0.001). At 20 minutes post L-dopa/benserazide administration data on forelimb usage (in the absence of PG01042) was not obtainable due to the intensity of the L-dopa-dependent AIMs.
Discussion
Rodent models of LID provide a cost-effective tool for pathophysiological investigations and drug-screening experiments. This unilateral lesion rat model of human LID has been characterized and validated in a number of previous publications (Ungerstedt, 1976; Shi et al., 2004; Cenci, 2005; Cenci et al., 2002). The present study uses this model to examine the motor effects of PG01042, a D3 dopamine receptor selective agonist/partial agonist, in the rat model of human LID. In addition, we compare the data for PG01042 to previously reported effects of a structurally similar D3 receptor antagonist, PG01037 (Figure 1) (Kumar et al., 2009a).
We investigated the in vivo efficacy and stability of PG01042 by comparing the pre-administration time of test drug and the attenuation of AIMs. These studies revealed that the level of attenuation was increased if PG01042 was administered simultaneously with L-dopa rather than using a pretreatment regime. Using a zero minute pretreatment, we performed a dose response curve for the attenuation of the L-dopa induced AIMs by PG01042. The IC50 value calculated for PG01042 was very similar to the value calculated for PG01037 (Kumar et al., 2009a) and no overt behavioral changes in animal behavior or locomotor activity was observed when PG01042 was administered alone. These data also indicate that despite its moderately high affinity for dopamine D2 receptors (Ki = 18.2 nM, Figure 1), and perhaps due to its partial agonist profile at D2 receptors, in this animal model no adverse motor behaviors were elicited at the behaviorally active doses of PG01042.
Since the agonist/partial agonist PG01042 and antagonist PG01037 were both able to inhibit dyskinesia, we injected PG01042 (3 mg/kg) and PG01037 (3 mg/kg) simultaneously to determine if they acted in vivo in an antagonist, additive or synergistic manner. Co-administration indicated that these compounds acted in a less then additive manner, suggesting that in this in vivo protocol they may be acting with a similar mechanism of action.
During the course of our studies it was reported that the serotonin system may play an important role in the observed L-dopa-dependent AIMs observed in this unilaterally lesioned rat model of LID. Serotonin neurons have been shown to convert L-dopa to dopamine, and store and release dopamine in an activity dependent manner. Results from Carta and colleagues suggest that dopamine, may act as a false transmitter in serotonergic neurons, and interfering with transmitter release of L-dopa derived dopamine may reduce the L-dopa-induced AIMs (Carta et al., 2007). In previous studies we reported that phenyl substituted piperazine compounds, such as PG01037, can bind to the 5-HT1A receptor subtype (Grundt et al., 2007; Chu et al., 2005). Therefore we considered the possibility that PG01042 might be acting via its binding to 5-HT1A receptors rather than at a dopamine receptor subtype to attenuate the AIMs scores. For PG01042, or possibly PG01037, to be active at 5-HT1A receptors in this in vivo model it would have to be an agonist/partial agonist at 5-HT1A receptors.
To investigate whether PG01042 might be attenuating AIMs via its interaction at serotonergic, rather than at dopaminergic, receptor systems we evaluated its ability to inhibit the activity of a dopamine receptor selective agonist. Administration of the D1-like/D2-like dopaminergic agonist apomorphine (0.05 mg/kg s.c.) elicited involuntary movements in our lesioned animals that were qualitatively (i.e., intensity) similar to what we observed following the administration of L-dopa/benserazide. However, the time to onset and duration of the involuntary movements were diminished by at least 50% (Kumar et al., 2009a). Therefore, we scored the animals at shorter time points. When animals were administered apomorphine and PG01042 simultaneously changes in AIMs scores were found. The percent attenuation of the AIMs minus locomotor scores over the whole time period was approximately 15%. However, examination of the temporal plots suggest that the magnitude of change was greater at the 45 minute and 55 minute time points compared to the earlier time points, suggesting that the pharmacokinetic parameters of the two drugs may be influencing the effectiveness of PG01042. Nonetheless, this observation does suggest that PG01042 blocks a dopaminergic-based induction of the involuntary movements observed in lesioned animals.
There have been several studies that provide evidence for a possible “cross-talk” between the D1-like and D3 dopamine receptors (Schwartz et al., 1998; Tillerson et al., 2002; Marcellino et al., 2008). Recent fMRI studies suggest a dysregulation of dopamine D1 and D3 receptor function in dyskinesia in rodent and primate models (Sánchez-Pernaute et al., 2007). However, our studies indicate a lack of attenuation of AIM scores when using a D1-like dopamine receptor selective agonist, suggesting that PG01042 acts primarily by blocking D3 dopamine receptor activation. Again, this is similar to results obtained using PG01037.
Further pharmacological evidence that the in vivo activity of PG01042 is not mediated via binding to a 5-HT1A receptor comes from our studies on the effect of the 5-HT1A selective antagonist WAY 100635. WAY 100635 has been used as a selective serotonin 5-HT1A receptor antagonist in both in vitro and in vivo experiments (Cliffe, 2000). We found that while WAY 100635 had little to no effect on the beneficial effects of PG01042 and PG01037, it was an effective inhibitor of buspirone. This provides further evidence that the in vivo activity of PG01042 and PG01037 are not acting via its binding to the 5-HT1A receptor (Chemel et al., 2006; Martel et al., 2007).
Finally, the usefulness of PG01042, or any other D3 dopamine receptor selective compound as pharmacotherapeutic agent would be diminished if it interfered with the beneficial effects of L-dopa. Therefore, a rotarod apparatus and the cylinder test (Schallert et al., 2000) was used to test for possible motor side effects associated with PG01042 administration. The rotarod test did not show any evidence of deleterious side effects on the motor coordination or agility of rats administered PG01042 compared to animals treated with vehicle. Again, these studies suggest that D2-receptor mediated motor side effects are not likely going to interfere with or confound the potential therapeutic usefulness of these agents.
During the course of our studies, we had observed that at 10 to 12 minutes post L-dopa/benserazide administration the animals in their home cages were using their contralateral (compromised) forelimb more readily. This was verified experimentally using the cylinder test where there was an almost complete reversal of forelimb usage at 12 minutes post L-dopa administration. However, this was unique to this set of animals. Normal unlesioned animals will use their forelimbs unpreferentially in a rearing test. In subsequent studies we have not observed such a dramatic reversal of forelimb preferential placement. However, for this study, perhaps it was a fortuitous observation. The reversal of forelimb preference was not altered by PG01042 administration (10 mg/kg). At 20 minutes post L-dopa administration the control animals exhibited intense AIMs, which prevented rearing. However, in the presence of PG01042 the involuntary movements of the animals were diminished and they were still able to rear in the cylinder. At that time point animals had received both L-dopa and test drug retained preferential usage of the compromised (right, contralateral) forelimb.
In summary, we have used the unilateral lesion rat model of L-dopa involuntary movements to evaluate the antidyskinetic potential of a D3 dopamine receptor selective agonist/partial agonist PG01042, which binds with a >39-fold selectivity to the D3 receptor compared to the D2 receptor subtype. We found that the acute administration of PG01042 can attenuate the total AIMs, and AIMs minus locomotor scores used to quantitate the intensity and duration of the involuntary movements associated with the L-dopa administration in this rat model of human LID. The effectiveness of this attenuation was found to be dependent upon both the dose and the time of administration of PG01042, relative to the administration of L-dopa. No overt behavioral side effects were observed in the rats during the treatment time when a 10 mg/kg dose of PG01042 was administered. Based upon our in vivo studies with dopaminergic and serotonergic selective compounds it appears unlikely that the in vivo activity of PG01042 is mediated by the activation of the 5-HT1A receptor. The results from studies using a rotarod apparatus suggest that PG01042 does not compromise the motor skills, muscle coordination or agility of lesioned animals. The results from a cylinder test are consistent with a lack of interference with the beneficial effects of L-dopa, although this potentially deleterious side effect will have to be evaluated in more detail.
It is both provocative and perplexing that both our agonist/partial agonist PG01042 and our antagonist PG01037 appear to have similar in vivo pharmacological properties in this animal model of L-dopa-dependent dyskinesia. However, similar results have been recently reported using a panel of D3 receptor selective compounds of varying intrinsic efficacy that were synthesized by Mach and associates (Kumar et al., 2009b). In that study it was reported that a D3 receptor antagonist, two partial agonists and an agonist each attenuated AIM scores in this animal model with IC50 values comparable to those we report for PG01037 and PG01042.
It is known that D2-like dopamine receptors are linked to several different second messenger systems besides the inhibition of adenylyl cyclase (Chio et al., 1994; MacKenzie et al., 1994; Asghari et al., 1995; Watts & Neve, 1997; Robinson and Caron, 1997; Pilon et al., 1994; Griffon et al., 1997; Schwartz et al., 1998; Kuzhikandathil & Oxford, 2000; Kuzhikandathil et al., 1998; Kuzhikandathil et al., 2004; Everett and Senogles, 2004; Senogles, 2003; Senogles, 2000). A revision of classic receptor theory, termed functional selectivity (Gay et al., 2004; Kenakin, 2002; Clarke, 2005), proposes that ligands can have differing efficacy and/or potency for different effector pathways. Therefore, a compound, such as PG01042, that might be classified as essentially a full agonist at the D3 receptor subtype for adenylyl cyclase inhibition, while being classified as a partial agonist when evaluated for mitogenesis (Figure 1).
One possible reason that both PG01037 and PG01042 are both capable of attenuating the AIM scores in the unilaterally 6-OHDA lesioned animals is that their intrinsic efficacy, as defined by adenylyl cyclase activity, does not reflect (not predictive) of in vivo efficacy for this experimental behavioral system. The ability to modulate adenylyl cyclase activity may not be pivotal in this behavioral paradigm. As with the compounds synthesized by Mach and associates (Chu et al., 2006; Kumar et al., 2009), the in vivo co-administration of PG01042 and PG01037 was found to be additive in the attenuation of the AIMs score, rather than being competitive or synergistic. This result suggests a common mechanism of action for the two compounds in this behavioral paradigm, rather than a fundamental difference in intrinsic efficacy.
While we propose that binding to the D3 dopamine receptor is the likely in vivo mechanism of action for PG01037 and PG01042, the precise site of action is, at this point, unclear. There are three possible neuroanatomical sites of action. First, recently published data using the D3 receptor selective antagonist radioligand 3H-WC 10 indicate a higher level of binding sites in the dorsal striatum than previously found using the tritium labeled D3 receptor agonist 7-0H-DPAT (Xu et al., 2009; Xu et al, 2010). In addition, it has been reported that in D3 receptor knock-out mice there was only a 50% reduction in ligand binding in the striatum of knock-out compared to wild type mice, suggesting that D2 and D3 receptors are coexpressed with similar densities in the striatum (Yaroslavsky et al., 2006). Therefore, previously determined D3 receptor expression levels, and their importance, in motor areas of the brain, may have been underestimated.
Second, studies by Barik and Beaurepaire (1996) in normal Sprague-Dawley rats suggest that D3 receptor expressed in lobules 9 and 10 of the cerebellum may play a role in the regulation of locomotor activity. When nafadotride, a D3 receptor selective antagonist, was microinjected into the lobules 9 and 10 of the cerebellum, a dose-dependent alteration of locomotor activity was observed. At low doses (10 to 100 ng) stimulation of locomotor activity was observed, while inhibition was observed at higher (10 μg) doses. Similar, but stronger, effects are found when this drug was microinjected into the nucleus accumbens. Interestingly, microinjection of a high dose (10 μg) of both the D2 receptor antagonist haloperidol and the dopaminergic agonist apomorphine into the cerebellum decreased locomotor activity. It is possible that in a compromised dopaminergic system the cerebellar D3 receptor-mediated effects on locomotor activity may become more pronounced.
Third, as mentioned in the Introduction Section, there is evidence to suggest a) that dopamine may act not only as classic synaptic receptor but also as an extrasynaptic modulator. Studies by Robinson and Caron (1997) reported that despite a relative inability to promote Gi protein activation in transfected cells, the D3 receptor is capable of inhibition of AC activity (75% inhibition) if it is co-expressed with adenylyl cyclase isoform V (ACV). ACV appears to be expressed preferentially in dopaminergically innervated brain regions, suggesting that co-expression of these two components in vivo might be required for linkage of D3 receptors to AC activity (Mons and Cooper, 1994). The heterogeneous network of D3 receptors expression of low levels throughout the brain (Bouthenet et al., 1991) that act as modulators of neuronal activity without being coupled to adenylyl cyclase activity, would represent an alternative possible site of action for our antagonist PG01037 and our agonist/partial agonist PG01042.
In conclusion, the delayed onset of LID in PD patients suggests that some form of L-dopa-dependent re-organization of the dopaminergic network has occurred with time. The contribution of the D3 dopamine receptor network in fine motor skill performance may become more pronounced than that found in a normal individual when compared to a CNS system a) compromised by the degeneration of the dopamine neurons of the nigrostriatal pathway and b) chronically bombarded with a dopamine precursor. The onset of LID is likely complex and multifactorial, involving both dopaminergic and nondopaminergic pathways. However, the studies detailed in this report provide further evidence that D3 dopamine receptor subtype represents viable pharmacotherapeutic target for the management of L-dopa-associated dyskinesias (Kumar et al., 2009a; Kumar et al., 2009b).
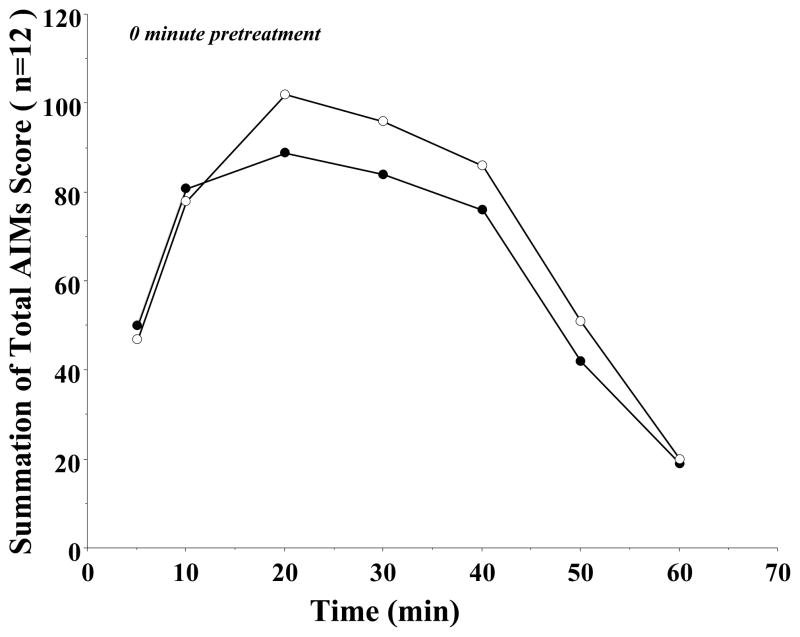
Figure 2. The Effect of Varying the Pretreatment Time of PG01042 on the Mean L-Dopa Dependent AIMs Scores.
The effect of varying the pretreatment time for PG01042 (10 mg/kg) prior to L-dopa administration (8 mg/kg L-dopa and benserazide) on the total AIMs score is shown. Values are expressed as the percent total AIMs relative to vehicle control values. The bar graph corresponds to the following values for the mean ± S.E.M. normalized total AIMs scores: a) 60- minutes pretreatment, 68.0 ± 8.6% and b) 0-minutes pretreatment, 29.4 ± 6.1% with n = 12 in both cases. The asterisk (*) denotes significant difference (defined as p ≤ 0.05) between the effects of a) PG01042 (10 mg/kg) 60 minute pretreatment versus vehicle control (F(1,18) = 10.1, p < 0.05 for n = 10), b) PG01042 (10 mg/kg) 0 minute pretreatment versus vehicle control (F(1, 22) = 86.6, p < 0.001, n = 12) and c) PG01042 (10 mg/kg) 60 minute pretreatment versus PG01042 (10 mg/kg) 0 minute pretreatment (F(1, 20) – 13.2, p < 0.002, n = 10).
Acknowledgments
This research was supported by a Community Fast Track 2006 from the Michael J. Fox Foundation for Parkinson’s Research, a R-01 DA13584-03S1 (RRL) and the NIDA-IRP (AHN and PG). The authors would like to thank Drs. Eunson Jung and Nathalie Sumien for their assistance with the statistical analysis and helpful discussions. The authors also thank the NIDA Addiction Treatment Discovery Program for contract resources used to conduct the D2 and D3 receptor based mitogenic assays. These were contract N01DA-1-8816 to SRI International (PI Larry Toll) and interagency agreement IAGY1 DA 5007-05 to the Portland VA Medical Center (PI Aaron Janowsky).
Abbreviations
- 6-OHDA
6-hydroxydopamine
- AIM
abnormal involuntary movement
- AMG
amygdala
- DMSO
dimethylsulfoxide
- GIRK
G protein-regulated inward rectifying potassium channels
- GP
globus pallidus
- i.p
intraperitoneal
- LID
L-dopa-induced dyskinesia
- MFB
medial forebrain bundle
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD
Parkinson’s Disease
- PFC
prefrontal cortex
- PG01037
(E)-N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)but-2-enyl)-4-(pyridin-2-yl)benzamide)
- PG01042
N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-3-yl)benzamide
- PLD
phospholipase D
- s.c
subcutaneous
- SN
substantia nigra
- TMS
transmembrane spanning
References
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HHM. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. Journal Neurochemistry. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Barik S, de Beaurepaire R. Evidence for a functional role of the dopamine D3 receptors in the cerebellum. Brain Research. 1996;737:347–350. doi: 10.1016/0006-8993(96)00964-x. [DOI] [PubMed] [Google Scholar]
- Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskineia: Potential for new therapies. Nature Reviews: Neuroscience. 2001;2:577–588. doi: 10.1038/35086062. [DOI] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Research. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Canales JJ, Iversen SD. Psychomotor-activating effects mediated by dopamine D(2) and D(3) receptors in the nucleus accumbens. Pharmacology Biochemistry & Behavior. 2000;67:161–168. doi: 10.1016/s0091-3057(00)00311-7. [DOI] [PubMed] [Google Scholar]
- Carta M, Lindgren HS, Lundblad M, Stancampiano R, Fadda F, Cenci MA. Role of striatal L-DOPA in the production of dyskinesia in 6-hydroxydopamine lesioned rats. Journal Neurochemistry. 2006;96:1718–1727. doi: 10.1111/j.1471-4159.2006.03696.x. [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nature Reviews: Neuroscience. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. Utility of 6-hydroxydopamine lesioned rats in the preclinical screening of novel treatments for Parkinson Disease. Animal Methods of Movement Disorders. 2005;Chapter B7:193–208. [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology (Berlin) 2006;188:244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- Chen J, Collins GT, Zhang J, Yang CY, Levant B, Woods J, Wang S. Design, synthesis, and evaluation of potent and selective ligands for the dopamine 3 (D3) receptor with a novel in vivo behavioral profile. Journal Medicinal Chemistry. 2008;51:5905–5908. doi: 10.1021/jm800471h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio CL, Lajiness ME, Huff RH. Activation of heterologously expressed D3 dopamine receptors: Comparison with D2 dopamine receptors. Molecular Pharmacology. 1994;45:51–60. [PubMed] [Google Scholar]
- Chu W, Tu Z, McElveen E, Xu J, Taylor M, Luedtke RR, Mach RH. Synthesis and in vitro binding of N-phenyl piperazine analogs as potential dopamine D3 receptor ligands. Bioorganic & Medicinal Chemistry. 2005;13:77–87. doi: 10.1016/j.bmc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- Clarke WP. What’s for lunch at the conformational cafeteria? Molecular Pharmacology. 2005;67:1819–1821. doi: 10.1124/mol.105.013060. [DOI] [PubMed] [Google Scholar]
- Cliffe IA. A retrospect on the discovery of WAY-100635 and the prospect for improved 5-HT(1A) receptor PET radioligands. Nuclear Medicine & Biology. 2000;27:441–447. doi: 10.1016/s0969-8051(00)00109-8. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Anxiety and affective style: Role of prefrontal cortex and amygdala. Biological Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The amygdala. Current Biology. 2000;10:R131. doi: 10.1016/s0960-9822(00)00345-6. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behavioral Brain Research. 2007;179:76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Delfino M, Kalisch R, Czisch M, Larramendy C, Ricatti J, Taravini IR, Trenkwalder C, Murer MG, Auer DP, Gershanik OS. Mapping the effects of three dopamine agonists with different dyskinetogenic potential and receptor selectivity using pharmacological functional magnetic resonance imaging. Neuropsychopharmacology. 2007;32:1911–1921. doi: 10.1038/sj.npp.1301329. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Annuals New York Academy Science. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- Dumartin B, Caillé I, Gonon F, Bloch B. Internalization of D1 dopamine receptor in striatal neurons in vivo as evidence of activation by dopamine agonists. Journal Neuroscience. 1998;18:1650–1661. doi: 10.1523/JNEUROSCI.18-05-01650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett PB, Senogles SE. D3 dopamine receptor activates phospholipase D through a pertussis toxin-insensitive pathway. Neuroscience Letters. 2004;371:34–39. doi: 10.1016/j.neulet.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Brotchie JB, Grandas F, Nomoto M, Christopher G, Goetz CG. Levodopa-induced dyskinesias. Movement Disorders. 2007;22:1379–1389. doi: 10.1002/mds.21475. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Duncan GE, Fornaretto MG, Dearry A, Gingrich JA, Breese GR, Caron MG. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proceeding National Academy Science USA. 1991;88:3772–3776. doi: 10.1073/pnas.88.9.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, Tinner-Staines B, Staines W, Rosin D, Terasmaa A, Popoli P, Leo G, Vergoni V, Lluis C, Ciruela F, Franco R, Ferré S. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology. 2003;9:S19–23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Distinct pharmacological regulation of evoked dopamine efflux in the amygdala and striatum of the rat in vivo. Synapse. 1995;20:269–279. doi: 10.1002/syn.890200311. [DOI] [PubMed] [Google Scholar]
- Gay EA, Urban JD, Nichols DE, Oxford GS, Mailman RB. Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: Evidence for induction of ligand-specific receptor states. Molecular Pharmacology. 2004;66:97–105. doi: 10.1124/mol.66.1.97. [DOI] [PubMed] [Google Scholar]
- Gendreau PL, Petitto JM, Petrova A, Gariépy J, Lewis MH. D(3) and D(2) dopamine receptor agonists differentially modulate isolation-induced social-emotional reactivity in mice. Behavioral Brain Research. 2000;114:107–117. doi: 10.1016/s0166-4328(00)00193-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Rosenkranz JA. Regulation of conditioned responses of basolateral amygdala neurons. Physiology & Behavior. 2002;77:489–493. doi: 10.1016/s0031-9384(02)00909-5. [DOI] [PubMed] [Google Scholar]
- Griffon N, Pilon C, Sautel F, Schwartz JC, Sokoloff P. Two intracellular signaling pathways for the dopamine D3 receptor: opposite and synergistic interactions with cyclic AMP. Journal Neurochemistry. 1997;6:1–9. doi: 10.1046/j.1471-4159.1997.68010001.x. [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Hauck-Newman A. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. Journal Medicinal Chemistry. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi J-K, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic analogues of N-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: Potential substance abuse therapeutic agents. Journal Medicinal Chemistry. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- Hermanowicz N. Drug therapy for Parkinson’s disease. Seminars in Neurology. 2007;27:97–105. doi: 10.1055/s-2007-971177. [DOI] [PubMed] [Google Scholar]
- Hervé D, Le Moine C, Corvol JC, Belluscio L, Ledent C, Fienberg AA, Jaber M, Studler JM, Girault JA. Galpha(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. Journal Neuroscience. 2001;21:4390–4399. doi: 10.1523/JNEUROSCI.21-12-04390.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff RM. Signal transduction pathways modulated by the D2 subfamily of dopamine receptors. Cellular Signalling. 1996;8:453–459. doi: 10.1016/s0898-6568(96)00074-5. [DOI] [PubMed] [Google Scholar]
- Joyce JN. Dopamine D3 receptor as a therapeutic target for antipsychotic and antiparkinsonian drugs. Pharmacology & Therapeutics. 2001;90:231–159. doi: 10.1016/s0163-7258(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Efficacy at G-protein-coupled receptors. Nature Review Drug Discovery. 2002;1:103–110. doi: 10.1038/nrd722. [DOI] [PubMed] [Google Scholar]
- Kumar R, Riddle LR, Griffin SA, Chu W, Vangvervong S, Neissewander J, Mach RH, Luedtke RR. Evaluation of D2 and D3 dopamine receptor selective compounds on L-dopa dependent abnormal involuntary movements in rats. Neuropharmacology. 2009b;56:956–969. doi: 10.1016/j.neuropharm.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Riddle LR, Griffin SA, Grundt P, Newman AH, Luedtke RR. Evaluation of the D3 dopamine receptor selective antagonist PG01037 on L-dopa dependent abnormal involuntary movements in rats. Neuropharmacology. 2009a;56:944–955. doi: 10.1016/j.neuropharm.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Oxford GS. Dominant-negative mutants identify a role for GIRK channels in D3 dopamine receptor-mediated regulation of spontaneous secretory activity. Journal General Physiology. 2000;115:697–706. doi: 10.1085/jgp.115.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Westrich L, Bakhos S, Pasuit J. Identification and characterization of novel properties of the human D3 dopamine receptor. Molecular & Cellular Neuroscience. 2004;26:144–155. doi: 10.1016/j.mcn.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Yu W, Oxford GS. Human dopamine D3 and D2L receptors couple to inward rectifier potassium channels in mammalian cell lines. Molecular & Cellular Neuroscience. 1998;12:390–402. doi: 10.1006/mcne.1998.0722. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Luedtke RR, Mach RH. Progress in developing D3 dopamine receptor ligands as potential therapeutic agents for neurological and neuropsychiatric disorders. Current Pharmaceutical Design. 2003;9:643–671. doi: 10.2174/1381612033391199. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Anderson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioral measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. European Journal Neuroscience. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- MacKenzie RGD, VanLeeuwen D, Pugsley TA, Shih YH, Demattos S, Tang L, Todd RD, O’Mally KL. Characterization of the human dopamine D3 receptor expressed in transfected cell lines. European Journal Pharmacology. 1994;266:79–85. doi: 10.1016/0922-4106(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Maltais S, Cté S, Drolet G, Falardeau P. Cellular colocalization of dopamine D1 mRNA and D2 receptor in rat brain using a D2 dopamine receptor specific polyclonal antibody. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2000;24:1127–1149. doi: 10.1016/s0278-5846(00)00125-1. [DOI] [PubMed] [Google Scholar]
- Marcellino D, Ferré S, Casadó V, Cortés A, Le Foll B, Mazzola C, Drago F, Saur O, Stark H, Soriano A, Barnes C, Goldberg SR, Lluis C, Fuxe K, Franco R. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. Journal Biological Chemistry. 2008;283:26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel JC, Leduc N, Ormière AM, Faucillon V, Danty N, Culie C, Cussac D, Newman-Tancredi A. WAY-100635 has high selectivity for serotonin 5-HT1A versus dopamine D4 receptors. European Journal of Pharmacology. 2007;574:15–19. doi: 10.1016/j.ejphar.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Healy DJ, Kuehn R, Zhou QY, Bunzow JR, Akil H, Civelli O, Watson SJ., Jr Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology. 1991;5:231–242. [PubMed] [Google Scholar]
- Mons N, Cooper DMF. Selective expression of one Ca2+ inhibitable adenylyl cyclase in dopaminergically innervated rat brain regions. Molecular Brain Research. 1994;22:236–244. doi: 10.1016/0169-328x(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. Journal Receptor Signal Transduction Research. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Cyriac G, Deschamps JR, Taylor M, Kumar R, Ho D, Luedtke RR. N-(4-(4-(2,3-Dichloro- or 2-methoxyphenyl)piperazin-1-yl)-butyl)-heterobiarylcarboxamides with functionalized linking chains as high affinity and enantioselective D3 receptor antagonists. Journal Medicinal Chemistry. 2009;52:2559–2570. doi: 10.1021/jm900095y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. Journal Medicinal Chemistry. 2005;48:3663–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS. Microdomains for dopamine volume neurotransmission in primate prefrontal cortex. Journal Neuroscience. 2004;24:5292–5300. doi: 10.1523/JNEUROSCI.0195-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Kearn CS, Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. Journal Computational Neurology. 2006;495:299–313. doi: 10.1002/cne.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon C, Lévesque D, Dimitriadou V, Griffon N, Martres MP, Schwartz JC, Sokoloff P. Functional coupling of the human dopamine D3 receptor in a transfected NG 108-15 neuroblastoma-glioma hybrid cell line. European Journal Pharmacology. 1994;268:129–139. doi: 10.1016/0922-4106(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Annals New York Academy Science. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Robinson SW, Caron MG. Selective inhibition of adenylyl cyclase type V by the dopamine D3 receptor. Journal Pharmacology Experimental Therapeutics. 1997;52:508–514. doi: 10.1124/mol.52.3.508. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pernaute R, Jenkins BG, Choi JK, Iris Chen YC, Isacson O. In vivo evidence of D(3) dopamine receptor sensitization in parkinsonian primates and rodents with l-DOPA-induced dyskinesias. Neurobiology Disease. 2007;27:220–227. doi: 10.1016/j.nbd.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical abalation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Ridray S, Bordet R, Diaz J, Sokoloff P. D1/D3 receptor relationships in brain coexpression, coactivation, and coregulation. Advances in Pharmacology. 1998;42:408–411. doi: 10.1016/s1054-3589(08)60775-9. [DOI] [PubMed] [Google Scholar]
- Sealfon SC, Olanow CW. Dopamine receptors: from structure to behavior. Trends Neuroscience. 2000;23:S34–40. doi: 10.1016/s1471-1931(00)00025-2. [DOI] [PubMed] [Google Scholar]
- Senogles SE. The D2s dopamine receptor stimulates phospholipase D activity: a novel signaling pathway for dopamine. Molecular Pharmacology. 2000;58:455–462. doi: 10.1124/mol.58.2.455. [DOI] [PubMed] [Google Scholar]
- Senogles SE. D2s dopamine receptor mediates phospholipase D and antiproliferation. Molecular & Cellular Endocrinology. 2003;209:61–69. doi: 10.1016/j.mce.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiology & Behavior. 2002;77:513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- Shi LH, Woodward DJ, Luo F, Anstrom K, Schallert T, Chang JY. High-frequency stimulation of the subthalamic nucleus reverses limb-use asymmetry in rats with unilateral 6-hydroxydopamine lesions. Brain Research. 2004;1013:98–106. doi: 10.1016/j.brainres.2004.03.053. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ, Jr, Shen Y. Molecular neurobiology of dopaminergic receptors. International Review Neurobiology. 1993;35:391–415. doi: 10.1016/s0074-7742(08)60573-5. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hurd YL, Sokoloff P, Schwartz JC, Sedvall G. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Research. 1998;779:58–74. doi: 10.1016/s0006-8993(97)01078-0. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced nonuse in unilateral parkinsonian rats exacerbates injury. Journal Neuroscience. 2002;22:6790–6799. doi: 10.1523/JNEUROSCI.22-15-06790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxydopamine-induced degeneration of the nigrostriatal dopamine pathway: the turning syndrome. Pharmacological & Therapeutics Part B: General & Systematic Pharmacology. 1976;1976:37–40. doi: 10.1016/0306-039x(76)90016-7. [DOI] [PubMed] [Google Scholar]
- Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neuroscience Biobehavioral Review. 2000;24:125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Vangveravong S, McElveen E, Taylor M, Griffin SA, Luedtke RR, Mach RH. Identification and pharmacologic characterization of D2 dopamine receptor selective antagonists. Bioorganic & Medicinal Chemistry. 2006;14:815–825. doi: 10.1016/j.bmc.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Watts VJ, Neve KA. Activation of type II adenylate cyclase by D2 and D4 but not D3 dopamine receptors. Molecular Pharmacology. 1997;52:181–186. doi: 10.1124/mol.52.2.181. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Sunahara RK, Niznik HB, O’Dowd BF, Seeman P, Brann MR. D1 and D2 dopamine receptor mRNA in rat brain. Proceeding National Academy Science USA. 1991;88:1859–1863. doi: 10.1073/pnas.88.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chu W, Vangveravong S, Jones LA, Lewis J, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH. [3H]4-(Dimethylamino)-N-[4-(4-(2-methoxyphenyl)piperazin- 1-yl)butyl]benzamide, a selective radioligand for dopamine D3 receptors. I. In vitro characterization. Synapse. 2009;63:717–728. doi: 10.1002/syn.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Hassanzadeh B, Chu W, Tu Z, Jones LA, Luedtke RR, Perlmutter JS, Mintun MA, Mach RH. [3H]4-(Dimethylamino)-N-[4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl]benzamide, a selective radioligand for dopamine D3 receptors. II. Quantitative analysis of dopamine D3 and D2 receptor density ratio in the caudate-putamen. Synapse. 2010;64:449–459. doi: 10.1002/syn.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Colletti M, Jiao X, Tejani-Butt S. Strain differences in the distribution of dopamine (DA-2 and DA-3) receptor sites in rat brain. Life Sciences. 2006;79:772–776. doi: 10.1016/j.lfs.2006.02.030. [DOI] [PubMed] [Google Scholar]