Abstract
The D3 dopamine receptor selective antagonist PG01037 has been evaluated for the ability to attenuate L-dopa associated abnormal involuntary movements (AIMs) in unilaterally lesioned male Sprague Dawley rats, which is a model of L-dopa-dependent dyskinesia in patients with Parkinson’s Disease. The intrinsic activity of PG01037 was determined using a) a forskolin-dependent adenylyl cyclase inhibition assay with transfected HEK 293 cells expressing either the human D2Long or D3 dopamine receptor subtype and b) an assay for agonist-associated mitogenesis. For the initial experiments, the 5-HT1A receptor selective partial agonist buspirone was used as a positive control to verify our ability to quantitate changes in total AIMs and AIMs minus locomotor scores. Subcutaneous (s.c.) administration of PG01037 was found to have minimal effect on AIMs score. However, it was observed that the in vivo efficacy of PG01037 increased when administered by intraperitoneal (i.p.) injection 15 minutes after L-dopa/benserazide administration, as compared to a 60 minute, 30 minute or 0 minute pretreatment. It was also found that i.p. administration of PG01037 could inhibit involuntary movements after they had achieved maximum intensity. PG01037 was found to attenuate AIM scores in these animals in a dose dependent manner with IC50 value equal to a) 7.4 mg/kg following L-dopa/benserazide administration (8 mg/kg each, i.p.) and b) 18.4 mg/kg following the administration of apomorphine (0.05 mg/kg, s.c.). However, PG01037 did not effectively inhibit SKF 81297-dependent abnormal involuntary movements. Rotarod studies indicate that PG01037 at a dose of 10 mg/kg did not adversely affect motor coordination of the unilaterally lesioned rats. Evaluation of lesioned rats using a cylinder test behavioral paradigm indicated that PG01037 did not dramatically attenuate the beneficial effects of L-dopa. These studies suggest that D3 dopamine receptor selective antagonists are potential pharmacotherapeutic candidates for the treatment of L-dopa-associated dyskinesia in patients with Parkinson’s Disease.
Keywords: Parkinson’s Disease, dyskinesia, L-dopa, dopamine receptors, D3 dopamine receptors
Introduction
Parkinson’s Disease (PD) is a progressive, neurodegenerative disease of the midbrain dopaminergic neurons that innervate the striatum and is characterized by resting tremor, rigidity, bradykinesia and postural instability. Although the risk of PD increases with age, 15% of PD patients are diagnosed before the age of 40 with early onset PD.
L-dopa therapy for PD is associated with both motor (wear-off phenomena, rigidity, akinesia and dyskinesia) and non-motor (sweating, tachycardia, dizziness, dyspnea, pain, restless leg syndrome, anxiety, panic attacks, depression, confusion, reduced alertness, psychosis and/or dementia) side effects. Involuntary movements, known as L-dopa-induced dyskinesia (LID), includes involuntary movements (chorea) and sustained involuntary muscle contractions (dystonia) that occur after prolonged treatment with L-dopa in PD patients (Blanchet et al., 1996). The prevalence of LID in PD patients increases with L-dopa treatment duration and once LID develops its severity generally increases (Deumens et al., 2002; Fabbrini et al., 2007).
LID is likely caused by regulatory changes in the basal ganglia circuitry following both dopamine depletion and L-dopa treatment. The motor fluctuations of LID may be associated with an up-regulation or increased sensitization of D3 dopamine, N-methyl-D-aspartate (NMDA) and/or amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors following chronic stimulation of striatal dopaminergic receptors (Chase et al., 2000; Calon et al., 2003a; Calon et al., 2003b; Cenci 2007).
A model of LID has been described for the abnormal involuntary movements (AIMs) found in rats with unilateral 6-hydroxydopamine (6-OHDA) lesions (Cenci et al., 1998; Cenci and Lundblad, 2005). Following chronic administration of L-dopa, rats exhibit abnormal movements and postures reminiscent of LID in primates. The severity of rat AIMs can be quantified using a rating scale analogous to that used for clinical studies. L-dopa-induced axial, limb and orolingual AIM scores can be reduced by the administration of antidyskinetic drugs that have been used in PD patients and/or nonhuman primates, including clozapine (8 mg/kg) and amantadine (40 mg/kg) (Carta et al., 2006; Lundblad et al., 2002). Validation of the rat dyskinetic model as an authentic model for identifying and evaluating potential antidyskinetic compounds has been provided by the studies of Dekundy and colleagues (2007).
The discovery of multiple subtypes of D1-like (D1a and D1b) and D2-like (D2, D3 and D4) dopamine receptor subtypes has necessitated a re-evaluation of the role of dopamine receptor antagonists as therapeutic agents in the treatment and management of neurodegenerative disorders (Hermanowicz, 2007, Luedtke and Mach 2003). Previous studies have suggested a possible role for the dopamine D3 receptor subtype in LID. In monkeys rendered Parkinsonian with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), administration of a putative D3 receptor partial agonist BP 897 was found to attenuate LID (Bezard et al., 2003). However, in a subsequent study using a different primate model, the same compound significantly reduced LIDs but also attenuated the antiparkinsonian effect of L-dopa (Hsu et al., 2004). MPTP treatment of monkeys led to a >50% decrease in D3 receptor expression in the basal ganglia. Following L-dopa treatment, there was a a) normalization of D3 receptor expression in animals without LID and b) >50% increase in D3 receptor expression above normal levels in animals with LID (Guigoni et al., 2005). A similar L-dopa-dependent increase in D3 receptor expression in the caudate putamen, accompanied by increased responsiveness to L-dopa, was reported for rats with nigrostriatal lesions (Bordet et al., 1997). Results from studies using tissue from a non-human primate brain bank have shown a strong correlation between LID and D3 receptor subtype expression, while no correlation was found for D1 or D2 dopamine receptor subtype expression (Guigoni et al., 2005).
While it is generally accepted that the highest levels of D3 dopamine receptor subtype expression are in the limbic areas, the neuroanatomical distribution of D3 receptor mRNA expression of the human brain using in situ hybridization indicates a heterogeneous expression of D3 receptor mRNA throughout the human brain. As expected, the most abundant D3 mRNA expression levels were found in the Islands of Calleja and within the ventral striatum/nucleus accumbens region. However, high levels were also evident within the dentate gyrus and striate cortex. Low to moderate D3 mRNA expression levels were found in cortical regions, caudate nucleus, putamen, anterior and medial thalamic nucleus, mammillary body, amygdala, hippocampal CA region, lateral geniculate body, substantia nigra pars compacta, locus coeruleus and raphe nuclei. Thus the D3 dopamine receptor might contribute to both a) limbic-related functions and b) motor and sensory processing in the human brain (Suzuki et al., 1998) and may play a role in pyramidal motor functions.
Because of the high degree of homology between the binding sites of the D2 and D3 dopamine receptor subtypes, it has been a difficult to develop authentic D2 or D3 receptor subtype selective compounds. We have recently synthesized and pharmacologically characterized panels of 2,3-dichloro substituted phenyl-piperazine compounds with high affinity (dissociation constants in the nM range) and >100-fold binding selectivity at the D3 dopamine receptor compared to the D2 receptor subtype, while binding with low affinity at D4 receptors (Grundt et al., 2005; Grundt et al., 2007). The lead compound PG01037 (N-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl-benzamide; Grundt et al., 2005) has been determined to be a selective D3 receptor antagonist in vivo (Collins et al., 2005; 2007; 2008) and using fMRI to assess cerebral blood flow (Grundt et al., 2007).
In this communication we report our initial finding on the ability of our PG01037 to attenuate AIMs in lesioned rats. Our results suggest that the D3 receptor may have a role for the development of L-dopa-dependent dyskinesias and that D3 receptor selective compounds may be candidates for development of pharmacotherapeutic agents for the treatment of human LID.
Methods and Materials
Animals
This study was performed using male Sprague Dawley rats that were unilaterally lesioned in the medial forebrain bundle (MFB) by a commercial vendor (Charles River). Approximately 7 days after surgery an amphetamine challenge was performed to verify the authenticity of the lesion. Upon their arrival, the animals were housed under a 12 hr light:12hr dark cycle with free access to tap water. The animals’ food was restricted to maintain their weights at approximately 350 (minimum of 320 to a maximum of 370) grams. Animals were acclimated for at least one week before the L-dopa/benserazide injections were initiated. The treatment of the animals and the experimental procedures were approved by an institutional animal welfare committee. Animal care and housing were in adherence with the conditions set forth in the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996).
Test drugs and treatment regimens
One week after arrival, the lesioned rats were screened behaviorally using an amphetamine-induced rotation test and all animals exhibited >6 full body turns/minute toward the side of the dopamine deficiency. Subsequently, 8 mg/kg L-dopa with 8 mg/kg benserazide was administered to each rat as a daily intraperitoneal (i.p.) injection (injection volume 0.3 ml using a 26 gauge, 1/2 inch needle (Becton Dickinson & Co.)) for 21 consecutive days to induce the development of dyskinetic-like movements. The L-dopa/benserazide solutions were dissolved in sterile saline (9 g NaCl/liter). All of the lesioned animals exhibited l-dopa-dependent involuntary movements within one week of the first L-dopa/benserazide injection.
PG01037 (N-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl-benzamide) was synthesized as the HCl salt, (Grundt et al., 2005). The highest concentration of PG01037 that was prepared was 10 mg/ml in sterile saline containing 5% dimethylsulfoxide (DMSO: Sigma). Heating the drug solution with hot tap water (55-60 °C) and vigorous vortexing was required to keep the drug in solution at that concentration. After the drug solution was loaded into the syringe, a heated Deltaphase Isothermal Pad (Braintree Scientific) was used to transport the drug to the animal facility. During the course of these studies we realized that PG01037 was readily soluble in a solution containing sterile distilled water and 5% DMSO at room temperature. The usual injection volume was 0.3 ml, therefore, the amount of DMSO injected per animal was 100-fold less than the oral acute LD50 value for rat (14500 mg/kg) (ScienceLab.com).
In the drug testing experiments, the compound was evaluated using an observer blind, randomized design, whereby half of the rats were administered either test drug or vehicle followed by L-dopa (8 mg/kg combined with 8 mg/kg benserazide). The test compound was administered prior to L-dopa at various time intervals. Buspirone (N-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-8-azaspiro[4.5]decane-7,9-dione hydrochloride, Sigma–Aldrich) was also administered i.p. at varying doses and times prior to L-dopa administration. The experiments were designed such that any given animal had >5 days between the administration of a test drug. Throughout these studies each animal received a minimum of two doses of L-dopa/benserazide (8 mg/kg each) per week to maintain the involuntary movement behaviors.
AIMs ratings
Abnormal involuntary movement (AIM) ratings were performed by an investigator who was unaware of the pharmacological intervention. After injection of the L-dopa, the severity of the AIMs was quantified using rats that were observed individually in their home cages at 20 minute intervals, starting 10 minutes after the injection of L-dopa, until the AIMs subside (approximately 2 hours). The observer scored ≤ 6 animals concurrently for a 5 minute interval. AIMs were scored in four categories, as discussed by Dekundy and colleagues (2007): a) axial AIMs, including dystonic or choreiform torsion of the trunk and neck towards the side contralateral to the lesion; b) limb AIMs, including jerky and/or dystonic movements of the forelimb contralateral to the lesion; c) orolingual AIMs, including twitching of orofacial muscles with empty masticatory movements and protrusion of the tongue towards the side contralateral to the lesion and d) locomotive AIMs, including increased locomotion with contralateral side bias. Each of the four categories were scored on a severity scale from 0 to 4, where 0 = absent, 1 = present during less than half of the observation time, 2 = present for more than half of the observation time, 3 = present all the time but suppressible by external stimuli, and 4 = present all the time and not suppressible by external stimuli. The external stimuli that we used was the movement of the animal’s cage forward and then back 1 to 2 inches, within 1-2 seconds, in a smooth motion. Generally, the summation of AIM scores included a) axial, limb and orolingual AIMs or a) axial, limb, orolingual and locomotor AIMs over the total observation period.
Rotarod Test
The rotarod test was used to assess the effect of PG01037 on motor performance and coordination (Lundblad et al., 2003; Dekundy et al., 2007). Animals were acclimated to the rotarod apparatus (AccuScan Instruments Inc., Columbus, OH) once per week in the two weeks prior to training and testing. The animals were fed approximately 1 hour prior to testing. Unilaterally lesioned rats were placed on a gradually accelerating rotarod apparatus set at 0 to 40 rpm/minute over 90 seconds. The first three days were training sessions and animals were not injected with test drug or vehicle. Two training sessions were performed per day, one session was conducted in the morning and one in the afternoon. At the end of the training sessions animals were given a shorter motivational session, where the rod speed was reduced to a setting of 0 to 14 rpm/minute over 30 seconds (Dekundy et al., 2007; Cenci et al., 2005). On the fourth and sixth days rats were administered 10 mg/kg PG01037 i.p. and then evaluated at 30 and 60 minutes post drug administration. On the fifth day rats were tested after administration with a similar volume of vehicle. During the training and testing sessions we tapped the animals tails to maintain attention and focus (Cenci et al., 2005). The data from the rotarod experiments are expressed as the mean number of seconds that the animal was able to remain on the rod before falling (latency to fall).
Cylinder Test
The cylinder test was used to assess the spontaneous and independent use of each of the rat’s forelimb in the context of an instinctive rearing behavior, with the rat standing on its hind legs and leaning on the enclosing walls (Schallert and Tillerson, 2000). Rats were placed individually in an open ended Plexiglas cylinder (21× 34 cm) without habituation in a dimly lighted room. The investigator scored the animals on the basis of the first forelimb to make contact (left, right or both) with the enclosing Plexiglas walls. Asymmetrical forelimb usage was calculated as the percentage of total performance, with a minimum of twenty wall contacts scored.
Statistical Analysis
SPSS 16 software was used for statistical analysis of the AIM scores. One-way analysis of variance (ANOVA) was used to determine the level of significant difference between the test drug treated and vehicle treated groups. The null hypothesis was rejected when p ≤ 0.05. A post-hoc test was used with LSD (least significant difference for mean separation) correction for multiple comparisons. In figures the data points are generally presented as the mean values ± S.E.M. that was obtained for each treatment group and data are generally expressed as normalized (to 100) value.
Results
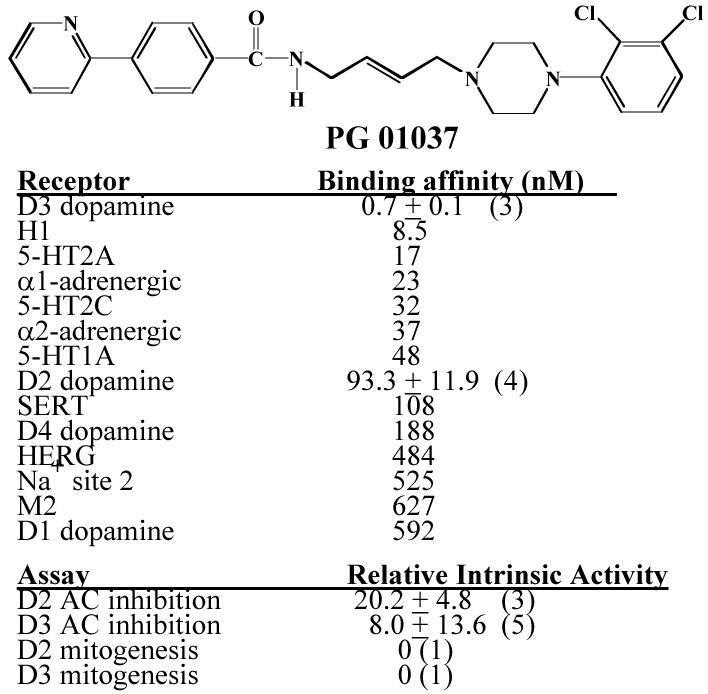
Figure 1 shows the structure and our current pharmacological profile of PG01037. PG01037 binds to the human D3 dopamine receptor with >100-fold selectivity compared to the human D2 dopamine receptor subtype. Based upon adenylyl cyclase studies we characterize this compound as a) an antagonist at D3 dopamine receptors and b) a weak partial agonist at D2 receptors. Based upon the results of mitogenic assays PG01037 is an antagonist at both D2 and D3 dopamine receptor subtypes (Grundt et al., 2005). PG01037 acts like a D3 receptor selective antagonist in vivo using fMRI techniques to measure changes in cerebral blood volume of the rat (rCBV) (Grundt et al., 2007).
Figure 1. Structure and pharmacologic profile of PG01037.
The chemical structure and summary of our current available information on the pharmacological properties of PG01037 is shown. Mean values for the affinity measurements (Ki values) are expressed as nM. Mean values ± S.E.M. are provided for the human D2 and D3 dopamine receptor with n ≥ 3. The remaining affinity values were obtained from Novascreen through the NIDA Contract N01-DA-8-8839. PG01037 was tested at a concentration of 10 μM in 61 binding assays. For the assays in which PG01037 inhibited binding >50%, mean Ki values were obtained (n=2).
Ki values for the binding to the low affinity state of human D2 and D3 dopamine receptors were obtained using competitive radioligand binding experiments with 125I-IABN as the radiotracer (Luedtke et al., 2000). Maximal intrinsic activity was achieved using the test ligand and the full D2-like dopamine receptor agonist quinpirole at concentrations approximately 10× the Ki values (PG01037, 1000 nM for D2 and 10 nM for D3; quinpirole, 1000 nM for D2 and 100 nM for D3). The percent maximum mitogenesis is based upon dose response curves (one concentration per decade) using a maximum of 10−5 M test drug performed by the Division of Treatment Research and Development (DTRD) of NIDA. The numerical values are the mean ± the S.E.M. and the number in the parentheses is the number of independent experiments (n).
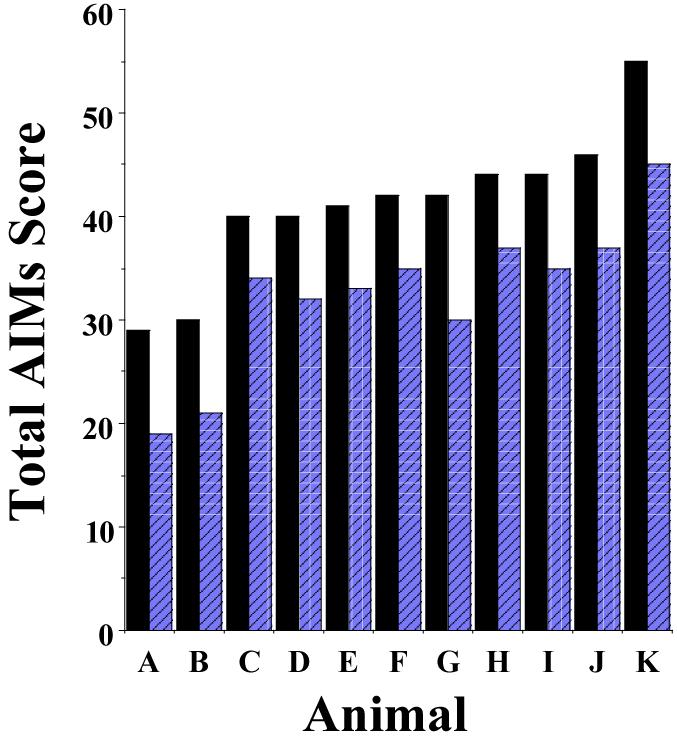
During the consecutive 21 day administration of L-dopa with benserazide all animals exhibited some form of AIMs by the fourth day of L-dopa administration. We evaluated the animals on day 20 of the 21 day regimen and found that the majority of animals provide similar total AIMs and AIMs minus locomotor scores, with an overall variability of about 15 to 20% (Figure 2).
Figure 2. AIM scores on day 20 of the 21 day dyskinetic induction protocol.
The bar graph shows the total AIMs score (closed bars) and the AIMs minus locomotion scores (hatched bars) for 11 rats (A-K) on day 20 of the 21 day dyskinetic induction protocol. Rats received daily doses of L-dopa and benserazide at 8 mg/kg each. AIM scores were determined using the scoring protocol described by Dekundy and co-workers (2007). Scores are plotted in the increasing order of the total AIMs score for each animal. The mean and S.E.M. values for the total AIMs score are 41.2 ± 6.8 and for the AIMs minus the locomotor component 32.5 ± 7.0, which is 16.5% and 21.5% variability, respectively.
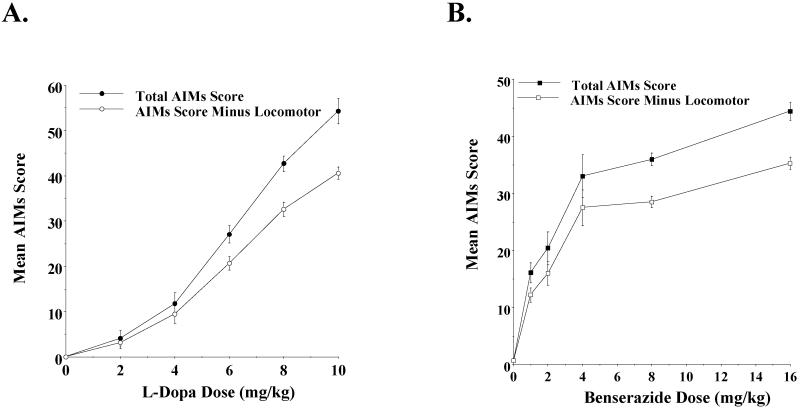
After the consecutive 21 day administration of L-dopa (8 mg/kg/day) and benserazide (8 mg/kg/day) and prior to the administration of any test compounds, we performed a dose response experiment to measure the AIMs score for each of the animals using increasing doses of L-dopa (0-10 mg/kg) with a constant dose of benserazide (8 mg/kg) to verify a) that the AIM scores were dependent upon the administration of L-dopa and b) our ability to quantitate differences in those scores (Figure 3A). We observed a dose dependent response over a range of 0 to 10 mg/kg of L-dopa for both the total AIMs score and the AIMs score minus the locomotor component. For the majority of animals the AIM scores increased at 10 mg/kg compared to 8 mg/kg, while for other animals the AIMs appeared to plateau. A dose response curve was also performed using increasing concentrations of benserazide with a constant amount of L-dopa (8 mg/kg) (Figure 3B). A dose of 8 mg/kg of both L-dopa and benserazide was selected for all further studies because it allowed us to determine if our test drug would attenuate or exacerbate AIM scores.
Figure 3. Dose response curve for the rat AIM scores with varying doses of L-dopa or benserazide.
After the 21-day induction and stabilization of L-dopa-dependent involuntary movement was completed, a dose dependent evaluation of AIM scores was performed using the scoring protocol described by Dekundy and co-workers (2007). The left panel (A) shows the mean total AIM scores (●) ± S.E.M. and the total AIMs score minus the locomotor component (○) ± S.E.M. for varying dose of L-dopa. Mean values are for n = 11 animals. L-dopa (0 to 10 mg/kg) was administered by i.p. injection with a constant amount of benserazide (8 mg/kg). The right panel (B) shows the mean total AIM scores (■) ± S.E.M. and the total AIMs score minus the locomotor component (○) ± S.E.M. for varying dose of benserazide. Mean values are for n = 12 animals. Benserazide (0 to 16 mg/kg) was administered by i.p. injection with a constant amount of L-dopa (8 mg/kg).
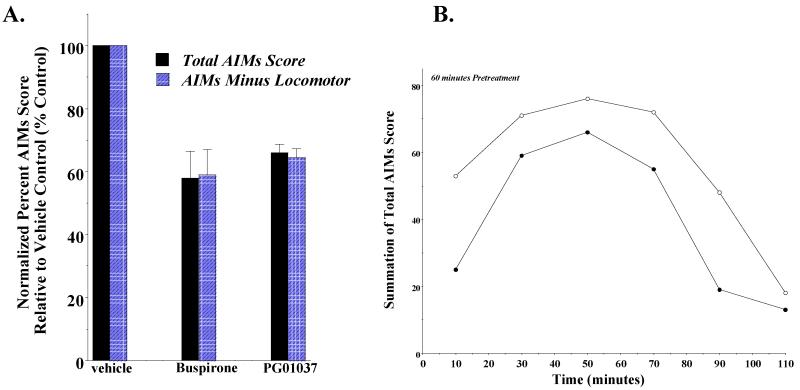
To further validate our ability to quantitate the attenuation of AIM scores in these animals we examined the ability of the 5-HT1A partial agonist buspirone to attenuate the L-dopa dependent AIM scores of our animals (Figure 4). Dekundy and colleagues (2007) reported that in unilaterally lesioned rats the administration of buspirone (4 mg/kg) 30 minutes prior to L-dopa/benserazide (6 mg/kg and 12-15 mg/kg) administration led to an 83% decrease in axial, limb and orolingual AIMs score, without a decrease in L-dopa induced locomotor scores. We found that for this group of animals the administration of buspirone (4 mg/kg) by i.p. injection 60 minutes prior to L-dopa/benserazide (8 mg/kg each) resulted in a 42.1 ± 8.5% reduction in the total AIMs score and a 41.0 ± 8.1% reduction in AIMs score minus the locomotor component. Although the magnitude of the observed effect was less than the reported values, it demonstrated our ability to quantitate attenuation of AIMs in these animals.
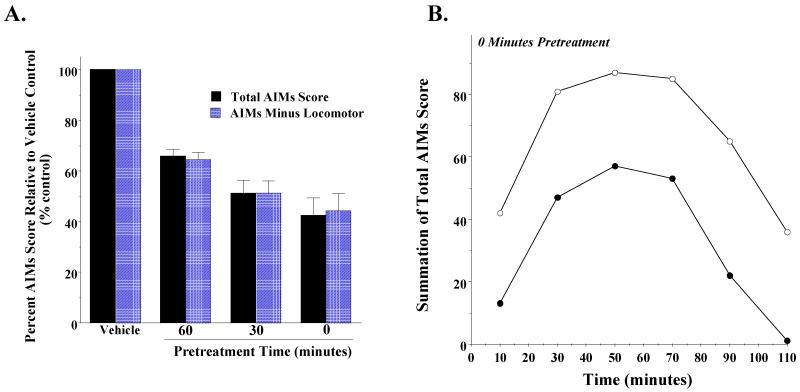
Figure 4. Effect of administration of PG01037 or buspirone 60 minutes prior to L-dopa administration on rat AIMs score.
(A) A comparison was made of the ability of PG01037 (10 mg/kg; n = 9) and buspirone (4 mg/kg; n = 11) to attenuate L-dopa-dependent AIMs in rats. Both drugs were administered i.p. 60 minutes prior to the L-dopa (8 mg/kg). This graph shows the mean total AIM scores (solid bars) ± S.E.M. and the total AIMs score minus the locomotor component (hatched bars) ± S.E.M. for PG01037 (65.9 ± 2.7 and 64.7 ± 2.9) and buspirone (57.9 ± 8.5 and 58.9 ± 8.1), respectively. Under these conditions both drugs statistically decreased the abnormal involuntary movements (p < 0.0001). (B) The variation in the total AIMs minus the locomotor component score as a function of time is shown using a 60 minute pretreatment time with 10 mg/kg PG01037 (●) or vehicle control (○). Each point is the summation of total AIMs minus the locomotor scores for 11 animals at each observation time point. This represents a mean 34.1 ± 8.0 (S.E.M.) percent reduction in the AIMs minus locomotor score over the observation time. Plots of the summation of the total AIMs score are essentially identical in shape with a 35.5 ± 8.4 percent reduction in the mean score.
We then proceeded to determine if PG01037 could attenuate L-dopa dependent involuntary movements in our lesioned rats. We began by administering PG01037 i.p. at a dose of 10 mg/kg 60 minutes prior to L-dopa (8 mg/kg) administration (Collins et al., 2005). A 34.1% reduction in the mean of the total AIMs score and a 35.6% reduction in the mean AIMs minus locomotion score was observed (Figure 4A).
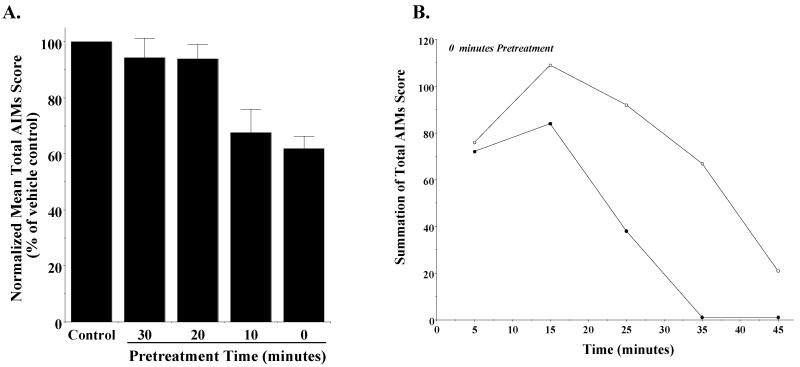
When we looked at the temporal effects of PG01037 administration on our scores, it appeared that the greatest decrease in AIM scores occurred at the earliest time point (15 minutes post L-dopa administration) (Figure 4B). Based upon this observation we investigated the effect pre-administration time of PG01037 on the attenuation of involuntary movements. A comparison of the effect of PG01037 (10 mg/kg) was made when administered by i.p. injection at 60, 30 and 0 minutes prior to L-dopa administration (8 mg/kg). Decreasing the pre-administration time for PG01037 increased the mean percent attenuation of the L-dopa dependent AIM scores (Figure 5A). When PG01037 and L-dopa were administered simultaneously a mean reduction in total AIM scores of 57.6 ± 7.0 and 48.7 ± 5.7 percent was achieved (Figure 5B).
Figure 5. The effect of varying the pre-administration time of PG01037 on the mean L-dopa-dependent AIM scores.
(A) The effect of varying the pretreatment time for PG01037 (10 mg/kg) prior to L-dopa administration (8 mg/kg L-dopa and benserazide) on both the total AIMs score (solid bars) and the AIMs score minus the locomotor component (hatched bars) is shown. Values are expressed as the percent of the corresponding vehicle control values. The bar graph corresponds to the following values for the mean ± S.E.M. normalized total AIM scores and the AIM scores minus the locomotor component, respectively: a) 60 minutes pretreatment, 65.9% ± 2.7 and 64.4% ± 2.9 with n = 9, b) 30 minutes pretreatment, 51.3% ± 5.7 and 51.2% ± 4.8 with n = 8 and c) 0 minutes pretreatment, 42.4% ± 7.0 and 44.2% ± 7.0 with n = 10. PG01037 statistically decreased the abnormal involuntary movements (p < 0.0001) at all time points. (B) The variation in the total AIMs score as a function of time is shown using zero time pretreatment with 10 mg/kg PG01037 (●) or vehicle control (○). Animals were also injected (i.p.) with 8 mg/kg L-dopa and benserazide. Each point is the summation of total AIM scores for 11 animals at each observation time point. This represents a mean 59.0 ± 21.8 (S.E.M.) percent reduction in the total AIMs score over the observation time. Plots of the summation of the AIMs score minus the locomotor component are essentially identical in shape with a 56.9 ± 22.3 percent reduction in the AIMs minus locomotor component.
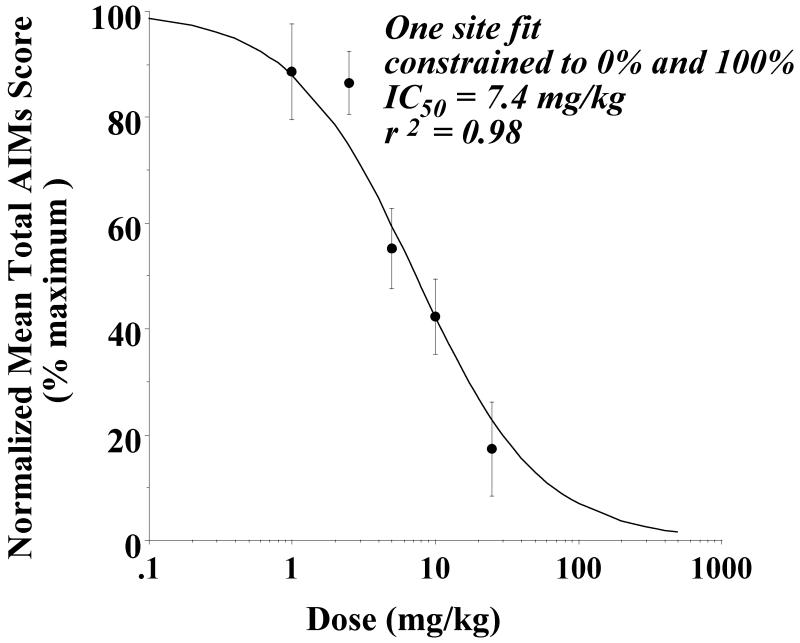
A concentration dependence analysis was then performed to determine the effect of the doses of PG01037 (1 to 25 mg/kg) on AIM scores (Figure 6) when the compound was administered simultaneously (0 minute pretreatment) with the L-dopa. If we assumed that at very high doses of PG01037 the rat AIM scores would approach zero (which may or may not be a valid assumption), the data fit reasonably well to a reversible, one site fit model with an IC50 value of 7.4 mg/kg and a nonlinear regression coefficient (r2) equal to 0.98.
Figure 6. Concentration dependence analysis to determine the effect of doses of PG01037 on AIM scores.
Unilaterally lesioned rats were injected (i.p.) with varying doses of PG01037 (0 to 25 mg/kg) followed immediately with a constant dose of L-dopa and benserazide (8 mg/kg each). The percent of the mean total AIMs score ± S.E.M. (n ≥ 8) relative to vehicle controls as a function of the dose of PG01037 is shown. The mean values ± S.E.M. for the normalized total AIMs values as a function of PG01037 concentrations are as follows: a) 1 mg/kg, 88.6 ± 9.1, b) 2.5 mg/kg, 86.6 ± 5.9, c) 5 mg/kg, 55.2 ± 7.6, d) 10 mg/kg, 42.4 ± 7.1 and e) 25 mg/kg, 17.3 ± 8.9. A graph of the percent change in the AIMs score minus the locomotor component as a function of the dose of PG01037 is essentially identical to the one shown in this figure. At doses of 1 and 2.5 mg/kg there is not a significant attenuation of the total AIMs score. However, at doses of 5, 10 and 25 mg/kg there was a significant reduction (p < 0.0001) of total AIM scores relative to the vehicle controls. The calculated IC50 value was found to be 7.4 mg/kg using a reversible one site fit model constraining the maximum and minimum mean values to 100% (no test drug) and 0%, respectively.
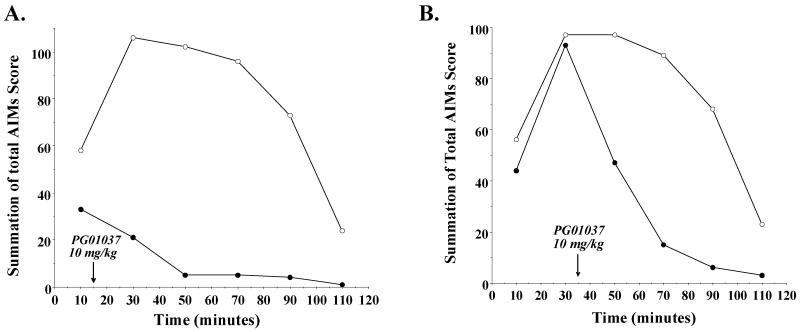
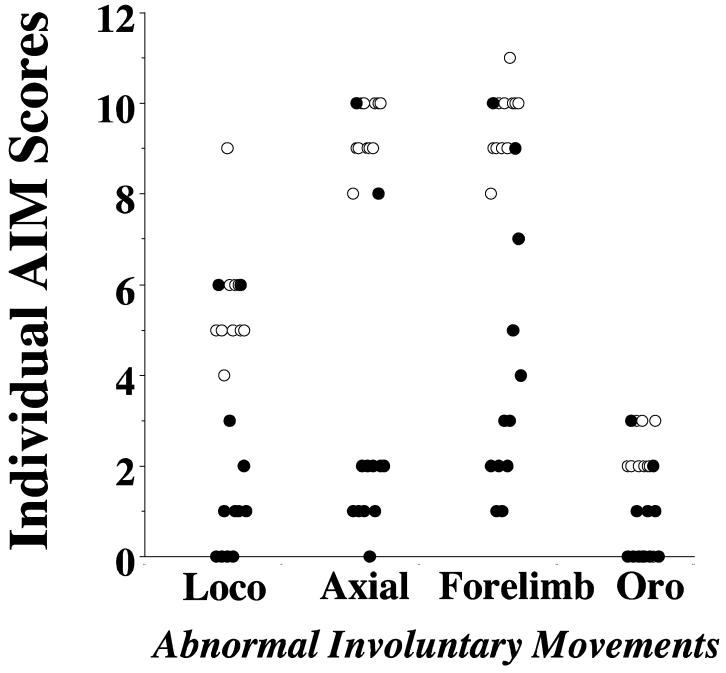
We then asked if PG01037 could modulate the intensity/duration of L-dopa dependent involuntary movement once they had begun. At either 15 or 35 minutes post-L-dopa injection time, half of the animals were administered 10 mg/kg PG01037 and the remaining animals received an equal volume of vehicle. We continued to score the animals for involuntary movements at 20 minute intervals (30, 50, 70, 90 and 100 minutes post L-dopa administration). Figure 7 shows the results of those post-L-dopa administration experiments. At time points post L-dopa injection and prior to the administration of test compound the intensity of the involuntary movements for both groups of animals was essentially identical. For both 15 minute (Figure 7A) and 35 minute (Figure 7B) post L-dopa administration protocols we found an attenuation of the AIM scores. If we compared the reduction in total AIMs score over the total time course for both pre- and post-administration of PG01037, the 15 minute post-administration of PG01037 produced the maximum effect: a) 34.1% ± 2.7 reduction for 60 minute pre-administration, b) 48.7% ± 5.1 reduction for 30 minute pre-administration, c) 57.6% ± 7.0 reduction for 0 minute pre-administration, d) 86.5% ± 3.9 reduction for 15 minute post-administration and e) 47.6 ± 8.2 for 35 minute post-administration. We then examined each of the components of the total AIMs score to determine if the 35 minute post-administration of L-dopa attenuated each component in a similar manner or if one component was preferentially affected (Figure 8). We found the following percent reduction in each score: a) locomotor, 76.5%, b) axial/torso movements, 77.9%, c) forelimb movement, 74.2% and d) orolingual, 69.4%. Thus, although each of the four components of the AIMs scoring system are not weighted the same for the calculation of the total AIMs score, there did not appear to be any preferential attenuation of the components.
Figure 7. Effect of post treatment of PG01037 on the temporal expression of L-dopa dependent AIMs in lesioned rats.
(A) The variation in the total AIMs score as a function of time is shown using 10 mg/kg PG01037 (●) or vehicle control (○) fifteen minutes after L-dopa/benserazide (8 mg/kg each) administration. Each point is the summation of total AIM scores for 11 animals at each observation time point. This represents a mean 86.5 ± 3.9 (S.E.M.) percent reduction in the total AIMs score over the total observation time. The time (minutes) denotes that amount of time following the administration of the L-dopa/benserazide and the arrow marks the time of the administration of PG01037. Plots of the summation of the AIMs score minus the locomotor component are essentially identical in shape. (B) The variation in the total AIMs score as a function of time is shown using 10 mg/kg PG01037 (●) or vehicle control (○) thirty five minutes after L-dopa/benserazide (8 mg/kg each) administration. Each point is the summation of total AIM scores for 11 animals at each observation time point. This represents a mean 47.6 ± 8.2 (S.E.M.) percent reduction in the total AIMs score over the total observation time. The time (minutes) denotes the amount of time following the administration of the L-dopa/benserazide and the arrow marks that time of the administration of PG01037. Plots of the summation of the AIMs score minus the locomotor component are essentially identical in shape.
Figure 8. Effect of thirty five minute post-treatment of PG01037 on the L-dopa dependent individual AIMs in lesioned rats.
A scatter gram of the summation of locomotor activity (Loco), axial dystonic movements (Axial), forelimb movement (Forelimb) and orolingual movements (Oro) for n = 12 animals is shown. Open circles (○) designate vehicle control values and solid circles (●) designate animals administered PG01037 35 minutes after the L-dopa administration and then scored at 50, 70, 90 and 100 minutes post L-dopa treatment. There was a 66.6%, 69.5%, 61.9% and 55.0% reduction in the locomotor, axial, forelimb and orolingual movements, respectively. The overall mean reduction in total AIMs score was 64.5 ± 35.1 (S.E.M.).
At this point we asked if the route of PG01037 administration was an important variable for attenuating L-dopa dependent AIMs in our unilaterally lesioned rats. When we compared the magnitude of attenuation of AIMs score by PG01037 (10 mg/kg) that was administered by either i.p. or subcutaneous (s.c.) injection, we found that s.c. administration resulted in only a marginal, non-statistically significant, reduction in AIM scores (data not shown). Therefore, no further studies were performed using s.c. administration.
To investigate the mechanism of action of PG01037, we first asked if PG01037 could attenuate AIM scores when animals were administered the nonselective dopaminergic (D1-like and D2-like dopamine receptor) agonist apomorphine. In initial experiments we found that 0.05 mg/kg apomorphine administered s.c. produced involuntary movements (Shi et al., 2004) qualitatively similar to those observed using L-dopa/benserazide. Although the intensity of the involuntary movements were similar, the onset was more rapid (1-2 minutes versus 10 minutes) and the duration was less (1 hour versus 2 hours). Therefore, for apomorphine-dependent AIMs we scored animals at 10 minute intervals post-apomorphine administration.
If we injected PG01037 immediately prior to apomorphine administration (0 minutes pre-administration), we found that there was a mean 38.1 ± 4.4 (S.E.M.) percent reduction in the total AIMs score and 38.8 ± 4.1 percent reduction in the AIMs minus locomotor component over the observation time (Figure 9A). The attenuation of the apomorphine-dependent involuntary movements was minimal in the beginning of the session (at the 5 minute post-injection time point) and appeared to increase with time (up to 35 minutes) (Figure 9B). Based upon this result we performed experiments in which PG01037 (10 mg/kg) was administered 30, 20 and 10 minutes prior to apomorphine (0.05 mg/kg) administration (s.c.). When a 30 or 20 minute pretreatment time was used, no attenuation of the apomorphine-dependent AIM scores was observed. When a 10 minute pretreatment time was used, the percent attenuation of the AIM scores was approximately the same for a 0 minute pre-administration time (Figure 9A). Therefore, although the pretreatment time clearly affected the percent attenuation of AIM scores, at a dose of 10 mg/kg of PG01037 we were unable to increase the percent attenuation beyond our original values (using 0 minutes pretreatment).
Figure 9. Effect of pretreatment times for PG01037 on apomorphine-dependent AIMs in lesioned rats.
(A) Unilaterally lesioned rats were treated with PG01037 (10 mg/kg) at varying times (30, 20, 10 and 0 minutes) prior to the administration of apomorphine (0.05 mg/kg, s.c.). The mean normalized values for the total AIM scores ± S.E.M. are shown relative to the values obtained from animals injected with vehicle. The number of animals (n) for each pretreatment time is as follows: a) 0 minutes, n = 12, b) 10 minutes, n = 6, c) 20 minutes, n = 5 and d) 30 minutes, n = 6. Similar plots for the total AIMs score are essentially identical to those shown in this figure. Although there was no significant reduction of the total AIMs score at the 30 and 20 minute pretreatment times, there was significant reduction when PG01037 was administered at 10 minutes (p < 0.01) and 0 minute (p < 0.0001) prior to the administration of L-dopa. The normalized mean total AIMs activity for animals administered PG01037 at zero minutes prior to apomorphine administration is 61.9 ± 4.4% of vehicle control values. (B) The variation in the total AIMs score as a function of time is shown using zero time pretreatment with 10 mg/kg PG01037 (●) or vehicle control (○). Animals were also injected (s.c.) with 0.05 mg/kg apomorphine. Each point is the summation of total AIM scores for a total of 12 animals at each observation time point. This represents a mean 38.1 ± 4.4 (S.E.M.) percent reduction in the total AIMs score over the observation time. Plots of the summation of the AIMs score minus the locomotor component are essentially identical in shape with a 38.8 ± 4.1 percent reduction in the AIMs minus locomotor component.
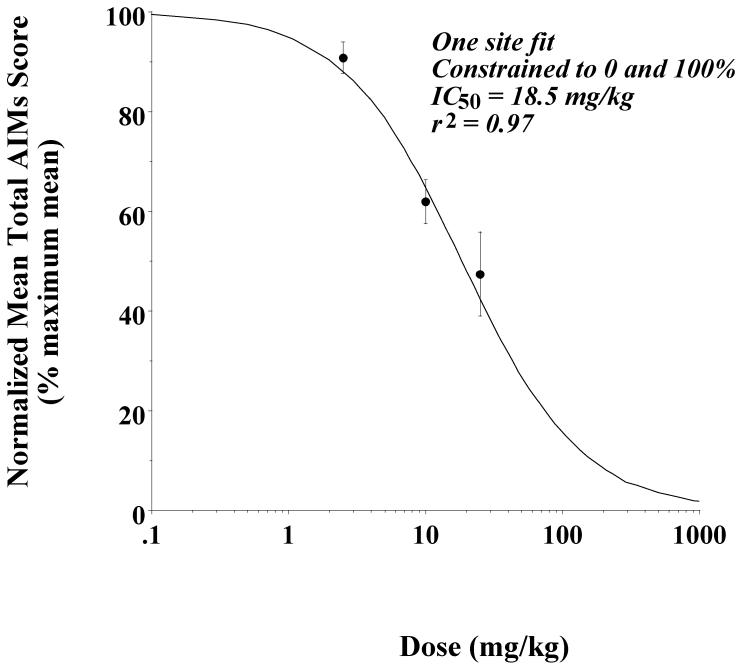
Based upon these results we performed a three point dose response evaluation of the ability of PG01037 (2.5 to 25 mg/kg) to inhibit apomorphine-dependent abnormal involuntary movements in our lesioned rats. The data fit reasonably well to a reversible, one site fit model with an IC50 value of 18.5 mg/kg and a nonlinear regression coefficient (r2) equal to 0.97 (Figure 10).
Figure 10. Concentration dependence analysis to determine the effect of doses of PG01037 on apomorphine-induced total AIM scores.
Unilaterally lesioned rats were injected (i.p.) with varying doses of PG01037 (0 to 25 mg/kg) followed immediately with a constant dose of apomorphine (0.05 mg/kg, s.c.). The percent of the mean total AIMs score ± S.E.M. (n ≥ 11) relative to vehicle controls as a function of the dose of PG01037 is shown. The mean values ± S.E.M. for the normalized total AIMs values as a function of PG01037 concentrations are as follows: a) 2.5 mg/kg, 90.8 ± 3.1, b) 10 mg/kg, 61.9 ± 4.4 and c) 25 mg/kg, 47.4 ± 8.4. The calculated IC50 value was found to be 18.5 mg/kg using a reversible one site fit model constraining the maximum and minimum mean values to 100% (no test drug) and 0%, respectively.
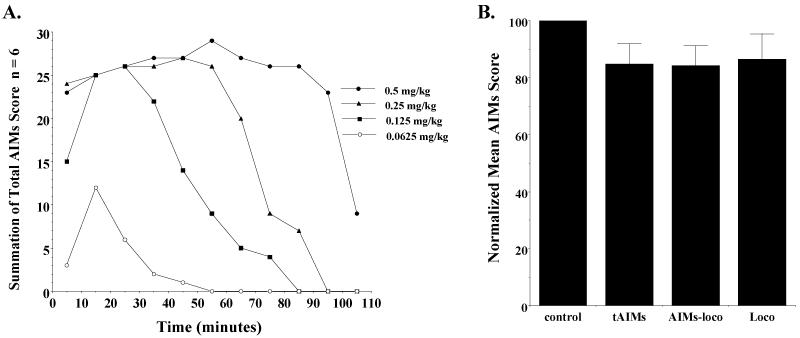
It has been reported that abnormal involuntary movements can be induced in unilaterally lesioned rats using the D1-like dopamine receptor selective agonist SKF 81297 (Delfino et al., 2007). Before undertaking an experiment to determine if PG01037 can attenuate SKF 81297 dependent abnormal involuntary movements in our unilaterally lesioned rats, we performed a dose response curve with SKF 81297 to determine the effect of dose on the intensity and duration of the involuntary movements. Qualitatively the abnormal movements observed using SKF 81297 were essentially identical to those that we had observed using L-dopa/benserazide. At doses of 0.5, 0.25 and 0.125 mg/kg SKF 81297 a maximum intensity of involuntary movements was achieved but the duration of the involuntary movements increased as a function of dose (Figure 11A). At a dose of 0.0625 mg/kg of SKF 81297 the animals did not appear to reach a maximum intensity of involuntary movement. A dose of 0.1 mg/kg of SKF 81297 was selected for further studies. We evaluated the effect of 10 mg/kg of PG01037 administered by i.p. injection at either a) 10 minutes prior to SKF 81297 administration (0.1 mg/kg, s.c.) or b) simultaneously with SKF 81297 (Figure 11B). In both experimental scenarios there was no significant reduction of SKF 81297 dependent involuntary movements.
Figure 11. SKF 81297 dependent induction of abnormal involuntary movements and the effect of PG01037 on SKF 81297 dependent abnormal involuntary movements in unilaterally lesioned animals.
(A) The temporal plots for the induction of abnormal involuntary movements in unilaterally lesioned rats is shown. Summation of total AIM scores (n = 6) were determined at 10 minute intervals starting at t = 5 minutes. SKF 81297 was administered s.c. at the following doses: a) 0.5 mg/kg (●), b) 0.25 mg/kg (▲), c) 0.125 mg/kg (■) and d) 0.0625 mg/kg (○). (B) The effect of PG01037 (10 mg/kg, i.p.) on SKF 81297 (0.1 mg/kg, s.c.) on the total AIMs (tAIMs), AIMs minus locomotor activity (AIMs-loco) and locomotor activity (Loco) scores is shown. PG01037 or vehicle was administered 10 minutes prior to the administration of SKF 81297. The data are the normalized mean values ± S.E.M. for n = 9 animals. The normalized mean scores for each is as follows: a) total AIMs, 84.7 ± 7.3; b) AIMs-locomotor, 84.2 ± 7.0; c) locomotor score, 86.4 ± 9.0. The significance level for each of these scores compared to the vehicle control (control) is p ≥ 0.09.
Studies by Carta and co-workers (2007) suggest that dysregulation of dopamine synthesized and released from serotonergic terminals may be a trigger for involuntary movements in the rat lesion model of Parkinson’s disease. In those studies they showed that agonists selective for the 5-HT1A (8-OH-DPAT) and 5-HT1B (CP-94253) receptor subtypes could effectively attenuate L-dopa dependent involuntary movements, but had little to no effect on similar involuntary movements induced by the nonselective dopaminergic agonist apomorphine (0.025 mg/kg, s.c.) in partial lesioned animals. These authors propose that in animals with complete dopamine lesions, dopamine release from spare serotonergic terminals acts as a false transmitter at the serotonergic synapse, leading to the dyskinetic behaviors observed after L-dopa administration.
PG01037 binds with >65-fold selectivity at D3 receptors compared to 5-HT1A receptors (Figure 1). Therefore, it is possible that the activity observed with PG01037 might be due to activity at serotonergic terminals rather than dopaminergic terminals. PG01037 acts as an antagonist at D2 and D3 dopamine receptors, however we currently have no information on the intrinsic activity of PG01037 at 5-HT1A receptors.
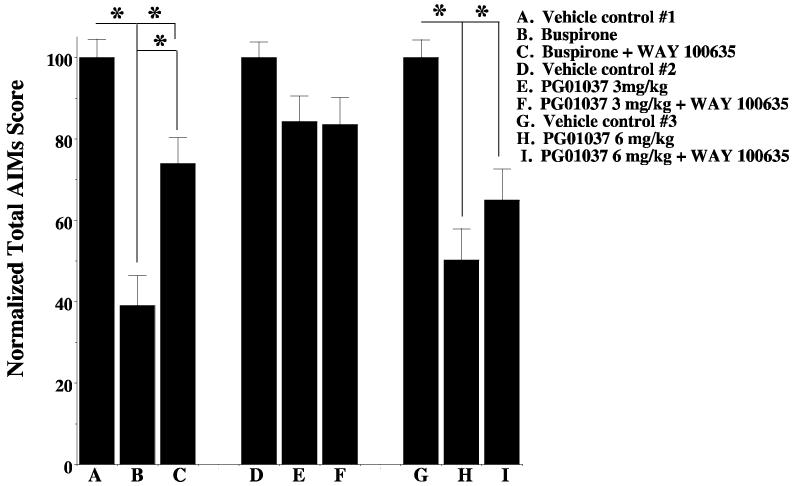
We evaluated the ability of WAY 100635, a 5HT1A receptor antagonist, to inhibit the attenuating effect of PG01037 on AIM scores. As a positive control we also tested the ability of WAY 100635 (1 mg/kg) to inhibit the activity of the 5 HT1A agonist buspirone and found that WAY 100635 significantly reduced the ability of buspirone (4 mg/kg) to attenuate total AIMs score in our unilaterally lesioned animals. However, at doses of 3 mg/kg or 6 mg/kg of PG01037, WAY 100635 (1 mg/kg) did not significantly (p > 0.05) inhibit the ability of PG01037 to attenuate total AIMs score (Figure 12).
Figure 12. Effect of the 5-HT1A antagonist WAY 100635 on the activity of PG01037.
Unilaterally lesioned animals were used to test the effect of the 5-HT1A antagonist WAY 100635 on the ability of either buspirone or PG01037 to attenuate L-dopa-dependent abnormal involuntary movements. L-Dopa was administered at a dose of 8 mg/kg by i.p. injection. PG01037 and L-dopa were always administered simultaneously. WAY 100635 was always administered 10 minutes prior to the administration of test compounds (PG01037 or buspirone). Buspirone was administered 30 minutes prior to the administration of L-dopa. Data are presented as the total AIMs score normalized to 100 ± the normalized S.E.M. The conditions of the experiment are as follows: A. L-dopa in the absence of any test compound (vehicle control #1); B. L-dopa in the presence of buspirone (4 mg/kg); C. L-dopa in the presence of both buspirone (4 mg/kg) and WAY 100635 (1 mg/kg); D. L-dopa in the absence of any test compound (vehicle control #2); E. L-dopa in the presence of 3 mg/kg PG01037; F. L-dopa in the presence of both PG01037 (3 mg/kg) and WAY 100634 (1 mg/kg); G. L-dopa in the absence of test compound (vehicle #3); H. L-dopa in the presence of PG01037 (6 mg/kg); I. L-dopa in the presence of both PG01037 (6 mg/kg) and WAY 100634 (1 mg/kg). The administration of WAY 100635 (1 mg/kg) resulted in a 35% reduction in the attenuation of total AIM scores by buspirone (4 mg/kg). The administration of WAY 100635 (1 mg/kg) resulted in no reduction in the attenuation of total AIM scores by PG01037 at a dose of 3 mg/kg. The administration of WAY 100635 (1 mg/kg) resulted in a 15% reduction in the attenuation of total AIM scores by PG01037 at a dose of 6 mg/kg. The asterisk (*) denotes significant difference (p < 0.05) between the effects of a) buspirone versus vehicle control (A vs. B) (p <0.0001) and buspirone with WAY 100635 with the vehicle control (A vs. C) (p < 0.01), b) buspirone in the absence and presence of WAY 100635 (B vs. C) (p = 0.001), c) PG01037 at 6 mg/kg and vehicle (G vs. H) (p < 0.0001) and d) PG01037 and WAY 100635 and the vehicle control (G vs. I) (p =0.001). There was no significant effect of WAY 100635 on PG01037 at either 3 or 6 mg/kg (p > 0.1).
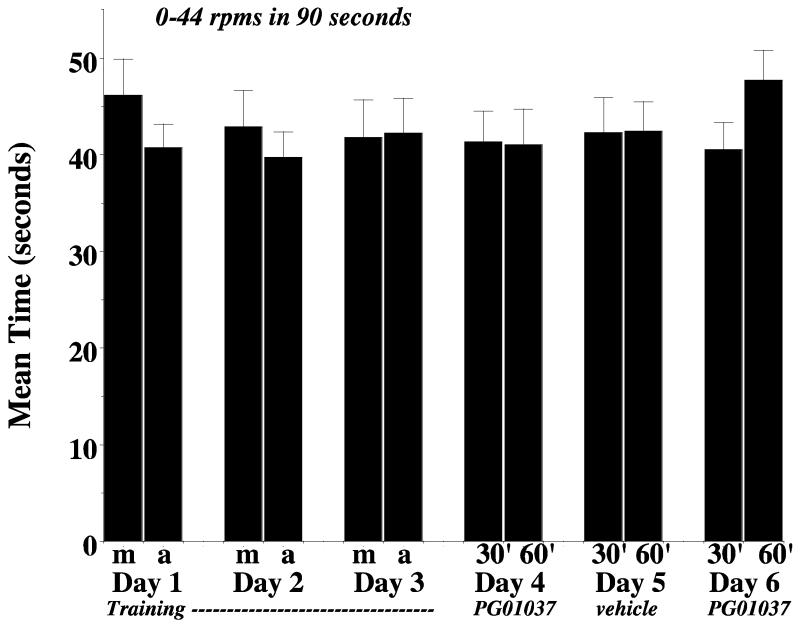
To further investigate the mechanism by which PG01037 was able to attenuate AIM scores, we performed a rotarod evaluation of our lesioned animals. We selected an acceleration rate that was used by Carta and co-workers (2006) to study the effects of compounds with known anti-dyskinetic properties. After six training sessions performed over the course of three days, lesioned animals were administered PG01037 (10 mg/kg, i.p.) and re-evaluated using the rotarod apparatus (day 4). On the following day (day 5) we again tested animals in the absence of drug and on day 6 animals were again administered PG01037 (Figure 13). No significant differences in the amount of time animals were able to stay on the rotarod apparatus in the presence or absence of PG01037 compared to the vehicle controls were observed.
Figure 13. Effect of PG01037 on rotarod performance.
The effect of PG01037 (10 mg/kg, i.p. injection) is shown for n = 12 animals. The data are presented as the mean values ± S.E.M. At the time of this experiment the lesioned animals were approximately 10 months old. Animals were acclimated to the rotarod apparatus once per week in the previous two weeks prior to training and testing. For this experiment the animals were fed approximately 1 hour prior to the morning session. The rotarod apparatus was accelerated from 0 to 44 rpm/minute in 90 seconds. For the training session (days 1 through 3), two test sessions were performed per day. One session was conducted in the morning (m) and one session was conducted in the afternoon (a). On days 1, 2 and 3 animals were not injected with test drug or vehicle. On days 4 and 6 rats were administered 10 mg/kg PG01037 i.p. and then evaluated at 30 and 60 minutes (30′ and 60′, respectively) post drug administration. On day 5 rats were tested after administration with a similar volume of vehicle (vehicle). No significant differences (p < 0.05) were found for a comparison of a) training session with vehicle controls, training session with test drug or b) matched vehicle controls with test drug.
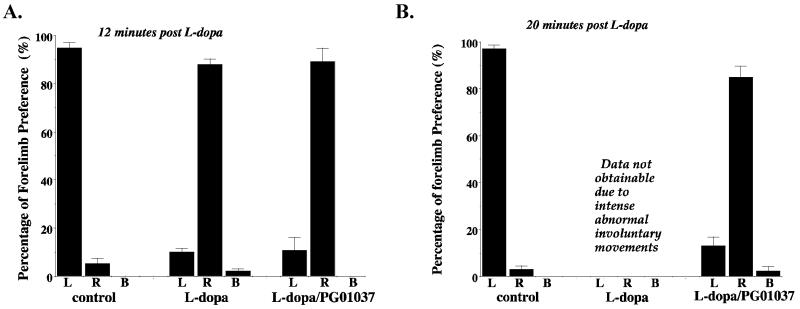
Finally, we attempted to evaluate the effect of PG01037 on the beneficial effects of L-dopa in unilaterally lesioned animals using a cylinder test developed by Schallert and coworkers (2000), which monitors preferential forelimb usage as the animal spontaneously rears in a cylinder. Untreated unilaterally lesioned rats preferentially used the ipsilateral (uncompromised) forelimb. We observed that 10 to 12 minutes after our animals were administered L-dopa/benserazide they begin to use the compromised (contralateral) forelimb to touch the inner wall of the cylinder. This is at a time point before intense AIMs are generally initiated, although this response appears to vary from animal to animal. If the animals were injected simultaneously with L-dopa/benserazide and PG01037 (10 mg/kg) they continued to predominantly use the right forelimb (Figure 14A). When we attempted to continue to measure forelimb preference in the cylinder test at 20 minutes after L-dopa/benserazide administration we found that it was impossible to score the vehicle injected animals because of the intensity of the AIMs. However, we were able to score the animals that were co-administered L-dopa/benserazide and PG01037 (10 mg/kg) at 20 minutes post injection and those animals continued to preferentially use the compromised forelimb (Figure 14B).
Figure 14. Cylinder Test for PG01037.
Unilaterally lesioned rats were tested for the percentage of forelimb preference (either right (R), left (L) or both (B) forelimbs) using the cylinder test. Animals were injected with either vehicle (control) or L-dopa/benserazide (L-dopa) (8 mg/kg each) in the absence or presence of PG01037 (L-dopa/PG01037). Animals were injected with L-dopa and test drug or vehicle simultaneously, then evaluated at 12 minutes (A) and 20 minutes (B) post injection. It was not possible to evaluate the animals at 20 minutes post drug administration when animals were given L-dopa/benserazide in the absence of PG01037 due to the intensity of the abnormal involuntary movements. Data are plotted as the mean values ± S.E.M. for seven animals (n = 7).
Discussion
Although L-dopa is the mainstay of pharmacotherapy for PD, one prominent side effect associated with long-term L-dopa therapy is a form of dyskinesia referred to as LID (Grandas et al., 1999; Fabbrini et al., 2007). This puts early onset PD patients at increased risk because of the extended duration of the course of treatment. Clinical observations suggest that the development of LID requires a) nigrostriatal dopaminergic denervation, b) intact postsynaptic basal ganglian neurons, and c) exogenous administration of L-dopa (Nutt 2001; Grandas et al., 1999). Similar observations have been made in primates with unilateral MPTP lesions (Clarke et al., 1989), where the development of LID is dependent upon the administration of high doses of L-dopa (Jenner 2000). Therefore, nigrostrial denervation accompanied by changes in receptor expression or possible sensitization to dopamine (Bordet et al., 1997) are likely pivotal in LID development.
For the studies described in this report a rat model of human LID was adopted in which the MFB of animals is lesioned unilaterally using the neurotoxin 6-OHDA (Ungerstedt 1968, 1976; Deumens et al., 2002; Cenci and Lundblad 2005). Lesioned animals exhibit asymmetric L-dopa-dependent abnormal involuntary postures and movements. Dekundy and co-workers (2007) further validated this model by evaluating the efficacy of clinically used anti-dyskinetic drugs. The overall profile of effects produced by these clinically used drugs was in agreement with results reported in nonhuman primate models of LID and/or in PD patients exhibiting LID. Therefore, they concluded that this rat model could be a valid indicator for screening/identifying compounds as candidate antidyskinetic drugs. We adopted this model for evaluating the antidyskinetic potential of our D3 dopamine receptor subtype selective antagonist PG01037.
Our first set of experiments was performed to a) verify the L-dopa dependence of the AIM scores, b) verify that we could distinguish and quantitate dose dependent differences in the intensity/duration of the involuntary movements, c) obtain estimates of the variability of the AIMs score in this set of animals and d) verify the reproducibility of our scoring criteria. Subsequently, we used buspirone as a reference antidyskinetic drug so that we could compare our data to previously published values (Dekundy et al., 2007).
The selection of a dose of 10 mg/kg for PG01037 in these studies was based upon several considerations. The first observations (Collins et al., 2005) were that the putative D3 selective agonist PD-128,907 induces a yawning behavior in male Sprague-Dawley rats, defined as a prolonged (˜1 s), wide opening of the mouth followed by a rapid closure. At a dose of 0.1 mg/kg, PD-128,907 induces a yawning rate of approximately 1 yawn/minute. The IC50 value of the inhibition of this yawning behavior for PG01037 was found to be 32 mg/kg when administered s.c. Second, we recently reported that at a dose of 2 mg/kg administered by i.v. injection, PG01037 causes an increase in rCBV in the rat, that appears to localize to regions of the brain known to express the D3 dopamine receptor subtype (Grundt et al., 2007).
When we compared the magnitude of attenuation of AIMs score by PG01037 (10 mg/kg) that was administered by either i.p. or s.c. injection, we found that s.c. administration resulted in only a marginal reduction in AIM scores. It should be noted that the s.c. route of administration of PG01037 was used in previously published behavioral studies in rats (Collins et al., 2005; 2007; 2008), but that higher doses (e.g. 32 mg/kg) were required for antagonism of D3-mediated effects.
Using a zero minute pretreatment regimen with i.p. injections, we performed a dose response curve for the attenuation of the L-dopa involuntary movements by PG01037. The data were fit to a simple sigmoidal one site fit model in which a) reversibility is assumed and b) maximum and minimum values of 100% and 0% inhibition are assumed. The “goodness-of-fit” of the data to a one site model suggest, but does not definitively prove, that there may be a single biological target responsible for the observed in vivo efficacy of the test compound. It is worth noting that even at the highest dose of PG01037 that was used (25 mg/kg), no overt behavioral changes in the animals were observed.
Having established that PG01037 could attenuate L-dopa dependent involuntary movements in lesioned rats if administered either prior to or simultaneously with the L-dopa/benserazide drug combination, we proceeded to ask if this compound could ameliorate the involuntary movements once they had been initiated. Post administration of PG01037 dramatically, rapidly and consistently attenuated AIM scores indicating that once started, the manifestation of L-dopa dependent AIMs was reversible.
During the course of our studies Carta and co-workers (2007) proposed that the dyskinetic movements in this lesioned animal model might be due to dopamine released from 5-HT1A and/or 5-HT1B serotonergic terminals. In previous studies we reported that phenyl substituted piperazine compounds, such as PG01037, can bind to the 5-HT1A receptor subtype (Grundt et al., 2007; Chu et al., 2005). Therefore, we considered the possibility that PG01037 might be acting via its binding to 5-HT1A receptors rather than at a dopamine receptor subtype to attenuate the AIM scores, even though PG01037 binds with >65-fold selectivity at D3 receptors compared to 5-HT1A receptors (Figure 1). For PG01037 to be active at 5-HT1A receptors in this in vivo model it would have to be an agonist/partial agonist at 5-HT1A receptors. Pharmacological evidence that PG01037’s in vivo activity is not mediated due to binding to a 5-HT1A receptor comes from our studies on the effect of the 5-HT1A selective antagonist WAY 100635. WAY-100635 has been extensively used as a selective serotonin 5-HT1A receptor antagonist in both in vitro and in vivo experiments (Cliffe, 2000). Even though recent findings suggest that WAY-100635 may be a dopamine D4 receptor agonist and have raised the question about the pharmacological selectivity of this drug for serotonin 5-HT1A receptors (Chemel et al., 2006; Martel et al., 2007), PG01037 binds with low affinity at D4 receptors (Ki =188 nM: Figure 1).
Our second strategy to determine if PG01037 is attenuating involuntary movements via its interaction at dopaminergic receptor systems would be to evaluate its ability to inhibit the activity of a dopamine receptor selective agonist. Apomorphine is a nonselective D1-like/D2-like dopamine receptor agonist that can elicit AIMs movements in these animals similar to those observed for L-dopa. When animals were administered apomorphine and PG01037 simultaneously significant changes in AIM scores were observed. However, the pharmacokinetic parameters of the two drugs may be influencing the effectiveness of PG01037. Nonetheless, this observation does suggest that PG01037 is interfering with a dopaminergic-based induction of the involuntary movements observed in these lesioned animals.
We have recently published the results of pharmacologic magnetic resonance imaging studies (phMRI) to determine if PG01037 could enter the brain and occupy dopamine D3 receptors in vivo (Grundt et al., 2007). At a dose of 1.0 or 2.0 mg/kg (i.v.) PG01037 increased regional cerebral blood volume (rCBV) in the Islets of Calleja and the nucleus accumbens shell, while eliciting much smaller changes in the caudate/putamen. These regional changes in blood flow is consistent with areas of the brain known to express the highest levels of dopamine D3 receptors. The increase in rCBV in these regions demonstrates that PG01037 acts as a D3 receptor selective antagonist in vivo.
In the same year Sánchez-Pernaute and co-workers (2007) used phMRI to examine the role of D3 receptor sensitization in the pathophysiology of dyskinesia. They examined the hemodynamic response of both unilaterally lesioned rats and bilaterally lesioned non-human primates to 7-OH-DPAT, a putative D3 receptor selective agonist, in naive, unilaterally lesioned control rats and lesioned rats chronically injected with L-dopa to exhibit involuntary movements. Administration of 7-OH-DPAT induced minor negative changes in rCBV in the basal ganglia in naive and lesioned rats, but an increase in rCBV was observed in the striatum of rats that exhibited involuntary movements following chronic L-dopa administration. Similar results were observed for MPTP-treated dyskinetic primates. This observed increase in rCBV is consistent with what is observed in normal animals using a D1-like dopamine receptor agonist.
D1 and D3 dopamine receptors have been reported to have both opposite and synergistic cell signaling effects (Schwartz et al., 1998a; Schwartz et al., 1998b). Sensitization of the D3 receptor inhibitory response to dopamine has been previously proposed to enhance D1 receptor signaling (Bordet et al., 1997; Bordet et al., 2000), which could lead to facilitate the activation of striatonigral pathway. Other studies have indicated that an unbalanced activation of the D1 pathways in dyskinetic patients and animals leads to increased sensitivity of D1 receptors. Disturbance of this D1/D3 receptor interplay due to changes in receptor subtype expression and/or sensitivity could play a role in the manifestation of involuntary movements. Several studies (Bezard et al., 2003; Guigoni et al., 2005; Bordet et al., 1997) have suggested there may be an increase in D3 receptor expression in animal models of L-dopa dependent involuntary movements. Recently Fiorentini and co workers (2008) provided evidence a) for the heterodimerization of the D1 and D3 dopamine receptor subtypes using coimmunoprecipitation and bioluminescence resonance energy transfer techniques and b) that the D1/D3 heterodimer has unique trafficking properties.
Based on these reports we examined the ability of PG01037 to attenuate AIM scores induced by the D1-like receptor selective agonist SKF 81297. However, PG01037 appeared to have no significant positive or negative effect on SKF 81297-induced AIM scores, even if we evaluated total AIM, AIM minus locomotor and locomotor scores independently (Figure 11). The ability of PG01037 to attenuate apomorphine-dependent, but not SKF 81297-induced, involuntary movements in this animal model suggests a mechanism of action of PG01037 involving the ability to compensate for a dysregulation of D2-like receptor based striatopallidal (indirect), rather than the striatonigral (direct) pathway (Cenci, 2007).
Finally, the clinical utility of using PG01037, or any other D3 dopamine receptor selective compound as an antidyskinetic would be diminished if it interfered with the beneficial effects of L-dopa. We used a rotarod apparatus and the cylinder test (Schallert et al., 2000), to investigate this possibility. Rotarod evaluation of our lesioned animals indicated that PG01037 does not alter the motor coordination or agility of the animals, compared to vehicle control values. The cylinder test evaluation suggests that there is only moderate effect of this compound to modify the beneficial effects of L-dopa in this behavioral paradigm.
In conclusion, although it is clear that there are fundamental differences between the expression, regulation, coupling and in vivo pharmacological selectivity of D3 dopamine receptors in rodents and primates, the studies described in this manuscript provide further evidence that D3 dopamine receptor subtype selective compounds represent viable pharmacotherapeutic candidates for the management of L-dopa-associated dyskinesias.
Acknowledgments
The authors would like to thank Dr. Steven Gold and Mr. Brian Potts for their insightful discussions on the AIMs scoring system. We would like to thank Dr. Eunson Jung, for her assistance with the rotarod experiments. This research was supported by a Community Fast Track 2006 Award from the Michael J. Fox Foundation for Parkinson’s Research and the National Institute on Drug Abuse-Intramural Research Program.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- AIM
abnormal involuntary movement
- AMPA
amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- DMSO
dimethylsulfoxide
- i.p.
intraperitoneal
- LID
L-dopa-induced dyskinesia
- MFB
medial forebrain bundle
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NMDA
N-methyl-D-aspartate
- PD
Parkinson’s Disease
- rCBV
rat cerebral blood volume
- s.c.
subcutaneous
- TMS
transmembrane spanning
References
- Bezard E, Brotchie JM, Gross CE. Pathophysiology of levadopa-induced dyskinesia: Potential for new therapies. Nature Reviews Neuroscience. 2001;2:577–588. doi: 10.1038/35086062. [DOI] [PubMed] [Google Scholar]
- Bézard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, Gross C, Sokoloff P. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nature Medicine. 2003;9:762–767. doi: 10.1038/nm875. [DOI] [PubMed] [Google Scholar]
- Blanchet PJ, Allard P, Gregoire L, Tardif F, Bédard PJ. Risk factors for peak dose dyskinesia in 100 levodopa-treated parkinsonian patients. Canadian J Neurological Science. 1996;23:189–193. doi: 10.1017/s031716710003849x. [DOI] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Carboni S, Diaz J, Sokoloff P, Schwartz JC. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc. Natl. Acad. Sci. USA. 1997;94:3363–3367. doi: 10.1073/pnas.94.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Schwartz JC, Sokoloff P. Involvement of the direct striatonigral pathway in levodopa-induced sensitization in 6-hydroxydopamine-lesioned rats. European J Neuroscience. 2000;12:2117–2123. doi: 10.1046/j.1460-9568.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- Calon F, Morissette M, Rajput AH, Hornykiewicz O, Bédard PJ, Di Paolo T. Changes of GABA receptors and dopamine turnover in the postmortem brains of parkinsonians with levodopa-induced motor complications. Movement Disorders. 2003a;18:241–253. doi: 10.1002/mds.10343. [DOI] [PubMed] [Google Scholar]
- Calon F, Rajput AH, Hornykiewicz O, Bédard PJ, Di Paolo T. Levodopa-induced motor complications are associated with alterations of glutamate receptors in Parkinson’s disease. Neurobiology of Disease. 2003b;14:404–416. doi: 10.1016/j.nbd.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Carta AR, Lucia F, Annalisa P, Silvia P, Nicola S, Nicoletta S, Micaela M. Behavioral and biochemical correlates of the dyskinetic potential of dopaminergic agonists in the 6-OHDA lesioned rat. Synapse. 2008;62:524–533. doi: 10.1002/syn.20527. [DOI] [PubMed] [Google Scholar]
- Carta M, Carlsson T, Kirik D, Björklund A. Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–1833. doi: 10.1093/brain/awm082. [DOI] [PubMed] [Google Scholar]
- Carta M, Lindgren HS, Lundblad M, Stancampiano R, Fadda F, Cenci MA. Role of striatal L-DOPA in the production of dyskinesia in 6-hydroxydopamine lesioned rats. J Neurochemistry. 2006;96:1718–1727. doi: 10.1111/j.1471-4159.2006.03696.x. [DOI] [PubMed] [Google Scholar]
- Cenci MA. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. TRENDS in Neurosciences 2007. 2007;30:236–243. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. Utility of 6-hydroxydopamine lesioned rats in the preclinical screening of novel treatments for Parkinson Disease. In: LeDoux M, editor. Animal Methods of Movement Disorders. Elsevier Academic Press; San Diego, CA: 2005. pp. 193–208. [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-dopa-induced dyskinesia in the ras associated with striatal overexpression of prodynorphin- and glutamtamic acid decarboxylaase mRNA. European J Neuroscience. 1998;10:2694–2106. [PubMed] [Google Scholar]
- Chase TN, Oh JD, Konitsiotis S. Antiparkinsonian and antidyskinetic activity of drugs targeting central glutamatergic mechanisms. J Neurology. 2000;247(Suppl 2):II36–42. doi: 10.1007/pl00007759. [DOI] [PubMed] [Google Scholar]
- Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology (Berl) 2006;188:244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- Chu W, Tu Z, McElveen E, Xu J, Taylor M, Luedtke RR, Mach RH. Synthesis and in vitro binding of N-phenyl piperazine analogs as potential dopamine D3 receptor ligands. Bioorganic & Medicinal Chemistry. 2005;13:77–87. doi: 10.1016/j.bmc.2004.09.054. [DOI] [PubMed] [Google Scholar]
- Clarke CE, Boyce S, Robertson RG, Sambrook MA, Crossman AR. Drug-induced dyskinesia in primates rendered hemiparkinsonian by intracarotid administration of MPTP. J Neurological Sciences. 1989;90:307–314. doi: 10.1016/0022-510x(89)90117-2. [DOI] [PubMed] [Google Scholar]
- Cliffe IA. A retrospect on the discovery of WAY-100635 and the prospect for improved 5-HT(1A) receptor PET radioligands. Nuclear Medicine & Biology. 2000;27:441–447. doi: 10.1016/s0969-8051(00)00109-8. [DOI] [PubMed] [Google Scholar]
- Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. Food restriction alters pramipexole-induced yawning, hypothermia, and locomotor activity in rats: Evidence for sensitization of dopamine D2 receptor-mediated effects. J Pharmacology Experimental Therapeutics. 2008;325:691–697. doi: 10.1124/jpet.107.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. In vivo D3 receptor selectivity of dopamine agonists and antagonists by analysis of yawning and hypothermia in rats. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacology Experimental Therapeutics. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotzias GC, Van Woert MH, Schiffer LM. Aromatic amino acids and modification of parkinsonism. New England J Medicine. 1967;276:374–379. doi: 10.1056/NEJM196702162760703. [DOI] [PubMed] [Google Scholar]
- Dekundy A, Lundblad M, Danysz W, Cenci MA. Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behavioral Brain Research. 2007;179:76–89. doi: 10.1016/j.bbr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Delfino M, Kalisch R, Czisch M, Larramendy C, Ricatti J, Taravini IR, Trenkwalder C, Murer MG, Auer DP, Gershanik OS. Mapping the effects of three dopamine agonists with different dyskinetogenic potential and receptor selectivity using pharmacological functional magnetic resonance imaging. Neuropsychopharmacology. 2007;32:1911–1921. doi: 10.1038/sj.npp.1301329. [DOI] [PubMed] [Google Scholar]
- Deumens R, Blokland A, Prickaerts J. Modeling Parkinson’s Disease in rats: An evaluation of 6-OHDA lesions of the nigrostraital pathway. Experimental Neurology. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Brotchoie JM, Grandas F, Nomoto M, Goeta CG. Levadopa-induced dyskinesia. Movement Disorders. 2007;22:1379–1389. doi: 10.1002/mds.21475. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Molecular Pharmacology. 2008;74:59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- Grandas F, Galiano ML, Tabernero C. Risk factors for levodopa-induced dyskinesias in Parkinson’s disease. J Neurology. 1999;246:1127–1133. doi: 10.1007/s004150050530. [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlson EE, Cao J, Bennett CJ, McElveen E, Taylor M, Luedtke RR, Newman AH. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides as selective probes with high affinity for the dopamine D3 receptor. J Medicinal Chemistry. 2005;48:839–848. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi J-K, Jenkins BG, Luedtke RR, Newman AH. Heterocyclic analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl}arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: Potential Substance Abuse Therapeutic Agents. J Medicinal Chemistry. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Aubert I, Li Q, Gurevich VV, Benovic JL, Ferry S, Mach U, Stark H, Leriche L, Håkansson K, Bioulac BH, Gross CE, Sokoloff P, Fisone G, Gurevich EV, Bloch B, Bezard E. Pathogenesis of levodopa-induced dyskinesia: focus on D1 and D3 dopamine receptors. Parkinsonism & Related Disorders. 2005;11(Suppl 1):S25–S29. doi: 10.1016/j.parkreldis.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Hermanowicz N. Drug therapy for Parkinson’s disease. Seminars in Neurology. 2007;27:97–105. doi: 10.1055/s-2007-971177. [DOI] [PubMed] [Google Scholar]
- Hsu A, Togasaki DM, Bezard E, Sokoloff P, Langston JW, Di Monte DA, Quik M. Effect of the D3 dopamine receptor partial agonist BP897 [N-[4-(4-(2-methoxyphenyl)piperazinyl)butyl]-2-naphthamide] on L-3,4-dihydroxyphenylalanine-induced dyskinesias and parkinsonism in squirrel monkeys. J Pharmacology Experimental Therapeutics. 2004;311:770–777. doi: 10.1124/jpet.104.071142. [DOI] [PubMed] [Google Scholar]
- Jenner P. Factors influencing the onset and persistence of dyskinesia in MPTP-treated primates. Annals of Neurology. 2000;47:S90–104. [PubMed] [Google Scholar]
- Luedtke RR, Mach RH. Progress in developing D3 dopamine receptor ligands as potential therapeutic agents for neurological and neuropsychiatric disorders. Current Pharmaceutical Design. 2003;9:643–671. doi: 10.2174/1381612033391199. [DOI] [PubMed] [Google Scholar]
- Luedtke RR, Freeman RA, Boundy VA, Martin MW, Mach RH. Characterization of 125I-IABN, a novel azabicyclononane benzamide selective for D2-like dopamine receptors. Synapse. 2000;38:438–449. doi: 10.1002/1098-2396(20001215)38:4<438::AID-SYN9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Anderson M, Winkler C, Kirik D, Wierup N, Cenci MA. Pharmacological validation of behavioral measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. European J Neuroscience. 2002;15:120–132. doi: 10.1046/j.0953-816x.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Vaudano E, Cenci MA. Cellular and behavioral effects of the adenosine A2a receptor antagonist KW-6002 in a rat model of l-DOPA-induced dyskinesia. J Neurochemistry. 2003;84:1398–1410. doi: 10.1046/j.1471-4159.2003.01632.x. [DOI] [PubMed] [Google Scholar]
- Martel JC, Leduc N, Ormière AM, Faucillon V, Danty N, Culie C, Cussac D, Newman-Tancredi A. WAY-100635 has high selectivity for serotonin 5-HT(1A) versus dopamine D(4) receptors. European J Pharmacology. 2007;574:15–19. doi: 10.1016/j.ejphar.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Nutt JG. Motor fluctuations and dyskinesia in Parkinson’s disease. Parkinsonism & Related Disorders. 2001;8:101–108. doi: 10.1016/s1353-8020(01)00024-4. [DOI] [PubMed] [Google Scholar]
- Sánchez-Pernaute R, Jenkins BG, Choi JK, Iris Chen YC, Isacson O. In vivo evidence of D(3) dopamine receptor sensitization in parkinsonian primates and rodents with l-dopa-induced dyskinesias. Neurobiology of Disease. 2007;27:220–227. doi: 10.1016/j.nbd.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: Optimizing behavioral assessment of outcome. In: Emerich DF, Dean RL III, Sandberg PR, editors. Central Nervous System Diseases: Innovative Models of CNS Diseases From Molecule to Therapy. Humana Press; New Jersey: 2000. pp. 131–151. PR. [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Diaz J, Bordet R, Griffon N, Perachon S, Pilon C, Ridray S, Sokoloff P. Functional implications of multiple dopamine receptor subtypes: the D1/D3 receptor coexistence. Brain Research Reviews. 1998a;26:236–242. doi: 10.1016/s0165-0173(97)00046-5. [DOI] [PubMed] [Google Scholar]
- Schwartz JC, Ridray S, Bordet R, Diaz J, Sokoloff P. D1/D3 receptor relationships in brain coexpression, coactivation, and coregulation. Advances in Pharmacology. 1998b;42:408–411. doi: 10.1016/s1054-3589(08)60775-9. [DOI] [PubMed] [Google Scholar]
- Shi LH, Woodward DJ, Luo F, Anstrom K, Schallert T, Chang JY. High-frequency stimulation of the subthalamic nucleus reverses limb-use asymmetry in rats with unilateral 6-hydroxydopamine lesions. Brain Research. 2004;1013:98–106. doi: 10.1016/j.brainres.2004.03.053. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hurd YL, Sokoloff P, Schwartz JC, Sedvall G. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Research. 1998;779:58–74. doi: 10.1016/s0006-8993(97)01078-0. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-hydroxydopamine-induced degeneration of the nigrostriatal dopamine pathway: the turning syndrome. Pharmacological Therapy [B] 1976;1976:37–40. doi: 10.1016/0306-039x(76)90016-7. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. European J Pharmacology. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]