Abstract
We investigated pregnancy-associated plasma protein-A (PAPP-A) in diabetic nephropathy. Normal human kidney showed specific staining for PAPP-A in glomeruli, and this staining was markedly increased in diabetic kidney. To assess possible contribution of PAPP-A in the development of diabetic nephropathy, we induced diabetes with streptozotocin in 14-month-old wild-type (WT) and PAPP-A knock-out (KO) mice. Renal histopathology was evaluated after four months of stable hyperglycemia. Kidneys from diabetic WT mice showed multiple abnormalities including thickening of Bowman’s capsule (100% of mice), increased glomerular size (80% of mice), tubule dilation (80% of mice), and mononuclear cell infiltration (90% of mice). Kidneys of age-matched non-diabetic WT mice had similar evidence of tubule dilation and mononuclear cell infiltration as diabetic WT mice indicating that these changes were predominantly age-related. However, thickened Bowman’s capsule and increased glomerular size appeared specific for the experimental diabetes. Kidneys from diabetic PAPP-A KO mice had significantly reduced or no evidence of changes in Bowman’s capsule thickening and glomerular size. There was also a shift to larger mesangial area and increased macrophage staining in diabetic WT compared to PAPP-A KO mice. In summary, elevated PAPP-A expression in glomeruli is associated with diabetic nephropathy in humans and absence of PAPP-A is associated with resistance to the development of indicators of diabetic nephropathy in mice. These data suggest PAPP-A as a potential therapeutic target for diabetic nephropathy.
Keywords: Pregnancy-associated plasma protein-A, kidney, diabetes, Bowman’s capsule
INTRODUCTION
Pregnancy-associated plasma protein-A (PAPP-A) was initially identified as one of four proteins found at high concentrations in the circulation of pregnant women, thus its name (Lin et al. 1974). Subsequently, PAPP-A was discovered to be a novel zinc metalloprotease expressed by a variety of cell types and tissues unrelated to pregnancy (Lawrence et al. 1999, Conover et al. 2006, Qin et al. 2006). In vitro and in vivo studies indicate that PAPP-A functions to enhance the growth-stimulating actions of local insulin-like growth factors (IGFs) through cleavage of inhibitory IGF binding proteins (Reviewed in Conover 2004). Although a benefit in many tissues in early life, PAPP-A (and IGFs) can have detrimental effects in later life with promotion of aging and age-related disease (Conover et al. 2004, Conover 2010). Indeed, PAPP-A knockout (KO) mice live 30–40% longer than their wild-type littermates with reduced incidence and severity of degenerative diseases of age, such as nephropathy (Conover et al. 2010). These histopathological data suggesting a role for PAPP-A in the aging kidney, the renal expression of multiple components of the IGF-I system (Vasylyeva and Ferry 2007), and the reported effects of IGF-I in promoting matrix production and proliferation of kidney cells (Horney et al. 1998, Lupia et al. 1999) prompted us to investigate the role of PAPP-A in the development of diabetic nephropathy, the leading cause of end stage renal disease world-wide (US Renal Data System Annual Report 2008). There were two aims to this study: (i) to assess PAPP-A expression in kidneys from normal and diabetic subjects by immunohistochemistry, and (ii) to determine the effect of PAPP-A gene deletion on the development of nephropathy in an experimentally-induced mouse model of type 2 diabetes. The latter was of particular interest based on a study by Swindell et al. (Swindell et al. 2010) looking at differential gene expression in wild-type versus PAPP-A KO mice that highlighted the kidney as a tissue in which moderated IGF signaling could have favorable effects.
MATERIALS AND METHODS
PAPP-A immunohistochemistry human kidney
Fresh-frozen normal and diabetic kidney sections were obtained from AMSBIO (Abingdon, UK). Sections were fixed in 100% methanol, rehydrated in phosphate-buffered saline (PBS) and probed for PAPP-A protein expression using recombinant anti-PAPP-A monoclonal antibody (Mikkelsen and Oxvig, unpublished) and detected with a FITC labeled secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). PAPP-A staining was visualized using a LSM 510 Confocal Laser Scanning Microscopes (Carl Zeiss MicroImaging, Oberkochen, Germany) at 40X magnification with DAPI used as a nuclear counterstain. Mouse IgG2α was used as isotype control.
Mice
Adapting the protocol of Wu et al. (2010), 14-month-old female PAPP-A KO and wild-type (WT) mice were injected with streptozotocin i.p (50 μg/g per injection) freshly dissolved in 137 mM NaCitrate, pH 4.5, every 2–3 days for a total of 5–7 injections in order to induce stable hyperglycemia. Mice were placed in clean cages and fasted for four hours prior to each injection. Body weight and blood glucose levels were taken prior to the initial streptozotocin injection and weekly for four months following, also after a four hour fast. Mice were considered diabetic when their blood glucose level was sustained at ≥250 mg/dL. There was less than 10% mortality, and dead or euthanized animals were excluded from further study. Mice were not treated with insulin at any time during the study. After four months of stable diabetes, body and kidney weights were recorded, blood was collected, and right and left kidneys were prepared for fixation. All procedures were approved by Mayo Clinic’s Institutional Animal Care and Use Committee.
Renal histology
The right kidney was cut in cross section and the left kidney was cut longitudinally, rinsed in PBS and fixed overnight in Bouin’s solution. Fixed tissues were flushed for 10 minutes with tap water and stored at 4C in PBS until shipping to the Animal Histology Core at Mayo Clinic, Scottsdale, AZ for sectioning and staining with hematoxylin-eosin. Kidney ections were qualitatively assessed by an American College of Veterinary Pathologists board-certified veterinary pathologist (R.J.M). A blinded histopathology report was based on standard descriptions and terminology (see legend to Table 2), and given one of four subjective grades of minimal, mild, moderate and marked. Sections of kidney were also stained with periodic acid-Shiff (PAS) to measure mesangial area within each glomerulus using Adobe PhotoShop software (Adobe Systems, Inc.). At least 300 glomeruli from three mice were assessed per group.
Table 2.
Diabetic kidney histology
| Diabetic | Non-Diabetic | ||
|---|---|---|---|
| WT (n = 10) | PAPP-A KO (n = 13) | WT (n = 12) | |
| Mononuclear cell infiltration | 90% | 77% | 100% |
| Tubule dilation | 80% | 0* | 75% |
| Tubule cytoplasmic changes | 10% | 0 | 8% |
| Thickened Bowman’s | 100% | <1%* | 7%‡ |
| Increased glomerular size | 80% | 23% | 17% |
| 2 or more abnormalities | 100% | 23% | 58% |
| Other abnormalities | 40% | 0 | 58% |
Numbers represent the percentage of mice with the indicated observations, assessed in a blinded fashion as described in Methods and Results. Fisher’s exact test was used to compare proportions.
Significant difference between 18-month-old Diabetic PAPP-A KO and Diabetic WT.
Significant difference between 18-month-old Non-diabetic and Diabetic WT.
Infiltrate, mononuclear cell: aggregates of primarily lymphocytes that were prominently noted around arterioles in the cortex, but also seen around larger vessels in the pelvis. The blood vessels within the aggregates of mononuclear cells were often prominent.
Cortex/medulla, tubules, dilation, increased: dilated tubules that often contained eosinophilic (proteinacious) filtrate.
Cortex, tubules, cytoplasmic alteration: characterized by small groups of tubules with subtle cytoplasmic granularity. The tubules stained lightly eosinophilic to lightly basophilic.
Thickened Bowman’s membrane: noticeable thickening of the membrane in several glomerular tufts.
Increased glomerular size: the change was characterized by an increase in mesangial size which was often associated with an increase in cellularity, mesangial matrix and/or prominent juxtaglomerular area.
Other:
Amyloid: small aggregates of homogeneous, eosinophilic material within an area of mononuclear cell infiltrate. Presumptive amyloid.
Metaplasia: small bone spicule in left kidney.
None of the changes in any of the tissue section were graded greater than mild and most were minimal in severity.
Immunohistochemistry
De-paraffinized sections of mouse kidney were stained for macrophages using F4/80 as primary antibody. Slides were rinsed with Tris-buffered saline and 0.01% Tween-20 for 3 minutes, treated with Peroxidase Blocking Reagent (Dako, Carpinteria, CA) for 5 minutes, rinsed with Wash Buffer (Dako), and treated with Rodent Block M, (Biocare Medical, Concord, CA) for 30 minutes. Slides were then incubated with primary antibody (F4/80 CI:A3-1, rat monoclonal, Serotec) for 60 minutes at room temperature at a concentration of 1:200 [diluted in Background Reducing Diluent (Dako)], followed by incubation with secondary antibody (Rat on Mouse Probe, Biocare, prediluted) for 15 minutes at room temperature. Slides were rinsed 3 times in Wash Buffer, incubated with tertiary reagent (Rat on Mouse HRP, Biocare) for 15 min at room temperature, rinsed in Wash Buffer, and then stained for visualization with diaminobenzidine (Biocare) for 5 minutes. Counterstaining with hematoxylin, followed by dehydration in increasing concentrations of ethyl alcohol and xylene was performed prior to cover slipping.
Blood chemistries
Blood urea nitrogen (BUN) measurements were performed using a Urea Nitrogen assay kit purchased from Pointe Scientific Inc. (Canton, MI). Serum IGF-I was measured using a mouse IGF-I ELISA kit generously provided by ImmunoDiagnostic Systems (Fountain Hills, AZ)
Statistical analysis
Fisher’s exact test was used to compare proportions of mice between groups. Two-tailed ANOVA and Tukey tests were used for multiple comparisons of group means. P < 0.05 was considered statistically significant.
RESULTS
PAPP-A expression in human kidney
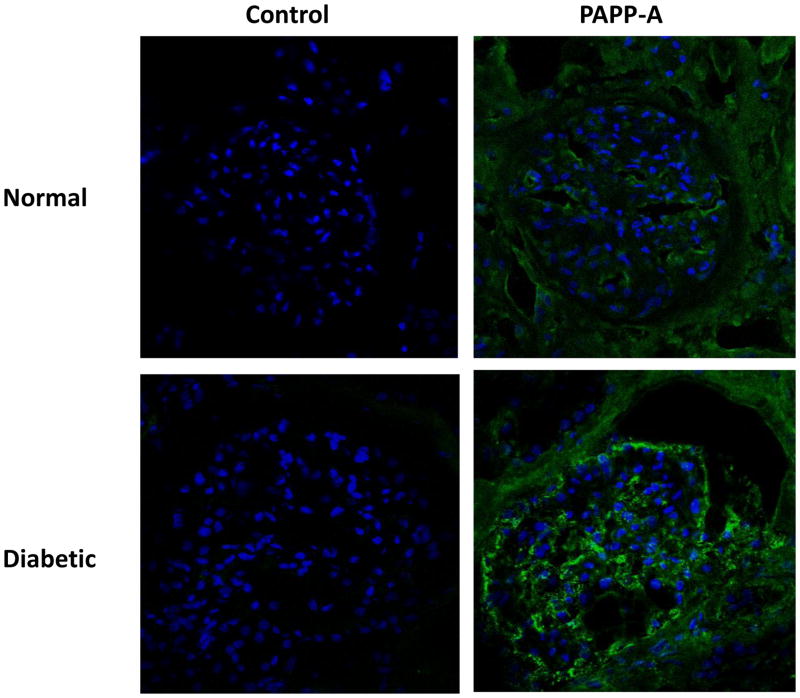
PAPP-A immunohistochemistry of sections from normal human kidney and kidney from a patient with diabetic nephropathy is presented in Figure 1. In normal kidney, there was specific staining for PAPP-A within and around the glomerulus, the structural unit responsible for filtration of the plasma that is eventually processed into urine. In comparison, diabetic kidney had manifestly more intense staining in the glomerulus as well as in association with the thickened Bowman’s capsule, the latter being a prominent feature of diabetic nephropathy.
Figure 1. PAPP-A expression in human kidney.
Sections of human kidney from a normal healthy subject and from a patient with diabetic nephropathy stained with primary antibody to PAPP-A or with isotype control, IgG2α.
Green – PAPP-A
Blue – nuclei
General characteristics of diabetic and non-diabetic mice
There was a significant difference in body weight between WT and PAPP-A KO mice, as reported previously (Conover et al. 2004), but there was no significant difference in body weight in the diabetic mice (WT and PAPP-A KO) when compared to their respective non-diabetic controls (Table 1). There was a significant difference in the kidney weight/body weight ratio between diabetic WT and PAPP-A KO mice. However, this was not caused by the diabetic phenotype, since a similar difference was seen in nondiabetic mice. There was no significant difference in the harvest blood glucose level between WT and PAPP-A KO mice, although levels were significantly elevated in the diabetic mice compared to non-diabetic mice as expected from this model. There was no significant difference in blood urea nitrogen levels between diabetic WT and PAPP-A KO mice, although slightly elevated in diabetic compared to non-diabetic mice. These characteristics suggest changes in kidney function with four months of hyperglycemia, although urine was not collected for measurement of creatinine or albumin. Furthermore, serum IGF-I levels were not significantly different between diabetic WT and PAPP-A KO mice or between diabetic and non-diabetic WT mice, confirming previous characterization of PAPP-A KO mice as a model of autocrine/paracrine versus endocrine regulation of IGF action (Conover et al. 2004).
Table 1.
General characteristics of diabetic and non-diabetic WT and PAPP-A KO mice
| Measurements | Diabetic | Non-Diabetic | ||
|---|---|---|---|---|
| WT (n = 10) | PAPP-A KO (n = 13) | WT (n = 8) | PAPP-KO (n = 6) | |
| Body weight (g) | 34.2 ± 2.05 | 22.0 ± 1.19* | 37.0 ± 3.17 | 24.4 ± 3.71* |
| Kidney/Body weight ratio (mg/g) | 10.0 ± 0.55 | 11.6 ± 0.13 | 10.5 ± 0.68 | 12.1 ± 1.68* |
| Blood glucose (mg/dL) | 277 ± 44 | 310 ± 28 | 130 ± 6.0† | 143 ± 4.5† |
| Blood urea nitrogen (mg/dL) | 22 ± 2.6 | 29 ± 1.5 | 19 ± 2.0 | 22 (n = 1) |
| Serum IGF-I (ng/mL) | 500 ± 40 | 475 ± 59 | 538 ± 66 | ND |
Data for 18-month-old diabetic and non-diabetic mice are presented as mean ± SEM with the number of mice for each group in parentheses. Analysis was by ANOVA and Tukey.
P < 0.05 PAPP-A KO vs WT.
P < 0.05 Diabetic vs non-diabetic.
ND – no data.
Renal histology
Diabetes induced by streptozotocin in aged mice results in glomerular, tubulointerstitial, and vascular kidney lesions with many features of human diabetic nephropathy (Wu et al. 2010, Fioretto and Mauer 2007). These are morphologically characterized by thickening of the Bowman’s capsule, glomerular hypertrophy with expansion of the extracellular matrix surrounding mesangial cells, and thickening of the basement membrane underlying proximal tubular epithelial cells.
WT diabetic mice developed thickening of Bowman’s capsule, tubular changes, and interstitial infiltrates (Fig. 2 and Table 2). The glomeruli were enlarged in 80% and the basement membrane of Bowman’s capsules was thickened in 100% of WT diabetic kidneys. Tubular dilation was noted in 80% of the kidneys and mononuclear infiltrates noted in 90%. Forty percent of the kidneys had additional abnormalities and all WT diabetic kidneys had two or more abnormalities. In contrast, diabetic PAPP-A KO kidneys showed little or no changes in glomerular size, Bowman’s capsule, and tubular dilation. However mononuclear cell infiltration was not different between diabetic WT and PAPP-A KO mice. To distinguish age-related versus hyperglycemia-induced effects on renal histology, we also examined 12 age-matched non-diabetic WT kidneys and saw little or no thickening of Bowman’s membrane or increase in glomerular size, although mononuclear cell infiltration and tubular dilation was similar to what was seen in diabetic WT kidneys (Table 2).
Figure 2. Renal histology of diabetic WT and PAPP-A KO mice.
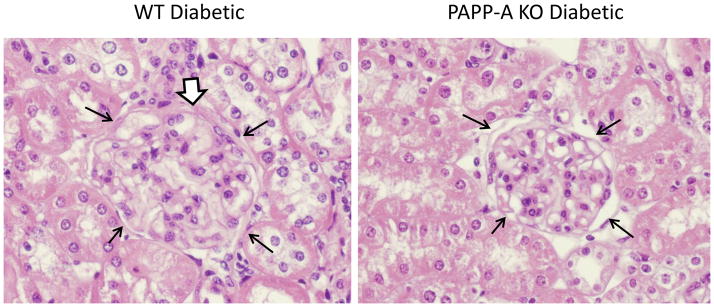
Representative H & E staining of kidney sections. Thin black arrows delineate a glomerulus. White-filled arrow indicates thickened Bowman’s capsule.
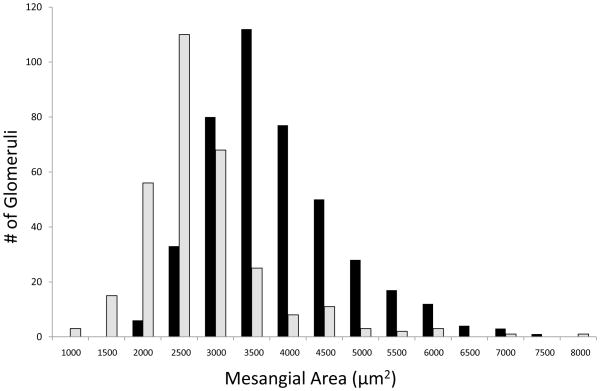
Expansion of mesangial matrix that is strongly PAS-positive is a hallmark pathological feature of diabetic nephropathy (Steffes 1989). Mesangial area was quantitated in PAS-stained diabetic kidneys and there was a significant shift to larger area in WT compared to PAPP-A KO mice (Fig. 3). Mean mesangial area in WT and PAPP-A KO was 3587 ± 79 μm2 and 2518 ± 132 μm2, respectively (P = 0.002). This was not just due to the overall larger body size of the WT mice because mesangial area in non-diabetic WT kidney was similar to what was seen in diabetic PAPP-A KO kidney (data not shown).
Figure 3. Mesangial area.
Kidney sections from diabetic WT (black bars) and PAPP-A KO (grey bars) mice were stained with PAS and mesangial area measured as described in Methods.
Macrophage immunohistochemistry
Immunostaining for F4/80, a classical macrophage-restricted surface glycoprotein, is specific for macrophages. Macrophages were prominent in kidneys of diabetic WT mice and appeared to be diminished in kidneys of diabetic PAPP-A KO mice (Fig. 4). Intensity of staining around the glomeruli was graded as 1, 2, or 3 (3 being the highest intensity). Non-diabetic control kidneys were all grade 1. Assessment of WT diabetic kidneys (N = 9) indicated 22% as grade 1, 22% as grade 2, and 56% as grade 3. In comparison, PAPP-A KO kidneys (N = 13) indicated 62% as grade 1, 38% as grade 2, and none as grade 3.
Figure 4. Macrophage immunohistochemistry of diabetic WT and PAPP-A KO kidney.
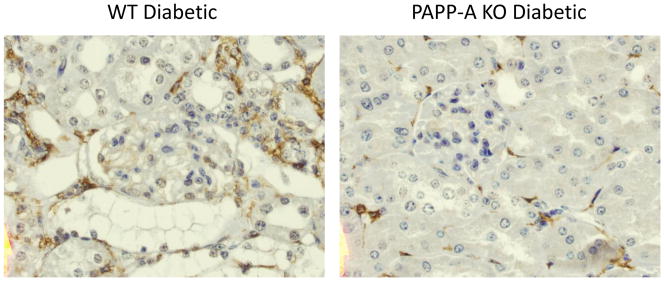
Macrophage staining for F4/80 (red brown) of kidney sections from WT diabetic (grade 3) and PAPP-A KO diabetic (grade 1).
DISCUSSION
Diabetic nephropathy is the leading cause of end-stage renal disease world-wide (US Renal Data System Annual Data Report 2008). Therefore, there is tremendous interest in identifying factors regulating the development of diabetic nephropathy with the intent to prevent or delay the associated morbidity and mortality. Based on previous findings that suggest a role for PAPP-A in age-related nephropathy (Conover et al. 2010), we investigated the role of PAPP-A in diabetic nephropathy. The two major and novel findings of this study were (i) PAPP-A expression is markedly elevated in glomeruli of human diabetic kidneys, and (ii) mice deficient in PAPP-A are resistant to the development of diabetic nephropathy.
Human diabetic nephropathy
It was strategic for the study of PAPP-A in diabetic nephropathy to ascertain where PAPP-A is expressed in the kidney and if there is site-specific regulation in diabetes. Although mouse kidney has been reported to have high levels of PAPP-A mRNA (Conover et al. 2004, Hourvitz et al. 2002), these studies were done in whole kidney and there were/are no antibodies available that recognize murine PAPP-A. Thus, our data are the first to demonstrate specific immunolocalization of PAPP-A to glomeruli. Furthermore, kidney of a patient with confirmed diabetic nephropathy had markedly elevated PAPP-A associated with the glomerulus as well as the surrounding Bowman’s capsule. This was of particular interest since the glomerulus is a major target of renal injury, especially in diabetes (Schlondorff 2008). It is not clear from immunohistochemistry which cells produce PAPP-A because it is a secreted protein that can associate with neighboring cells as well as the cells of origin (Conover et al 2007). In this way, PAPP-A can act as an autocrine/paracrine regulator of IGF action. A clinical study suggested prognostic value of circulating PAPP-A and increased all-cause mortality in type I diabetic patients with nephropathy (Astrup et al. 2007). However, it is not known whether this increase in circulating PAPP-A reflected changes in the kidney.
Mouse diabetic nephropathy
In order to investigate the physiological and pathophysiological role of PAPP-A in the kidney, we used WT and PAPP-A KO mice and induced stable hyperglycemia in these animals with a regimen of low dose streptozotocin injections, following the model developed by Wu et al. (Wu et al. 2010). In the original report, diabetes was induced in mice starting at 17 months, and these mice demonstrated severe nephropathy associated with elevated markers of oxidative stress, endoplasmic reticular stress, and macrophage-derived inflammation at 22 months (Wu et al. 2010). Induction of diabetes in younger mice (4-months-old) did not produce kidney lesions after a similar level and duration of hyperglycemia. We modified this model to start the steptozotocin injections in 14-month-old mice and assessed kidney histopathology after 4 months of stable hyperglycemia (approximately 18-months-old mice). We chose this younger age to minimize potential confounding effects of age-related cancers that become prominent, especially in WT mice, after 18 months (Conover et al. 2010). In our protocol, mice with visual signs of tumor at harvest would be removed from the study and there would be no further analyses; no animals were removed in this cohort due to tumors. Under this modified model, diabetic WT mice developed several features of nephropathy, i.e., thickening of Bowman’s capsule, and mesangial expansion, albeit not to the level of severity seen in the study by Wu et al. (Wu et al. 2010). They also had increased macrophage immunostaining especially around glomeruli. These were significantly reduced in diabetic PAPP-A KO mice. To confirm diabetes-induced versus age-related nephropathy, we also analyzed kidneys of non-diabetic WT and PAPP-A KO mice. There was no evidence of thickened Bowman’s capsule, increased glomerular size, or mesangial expansion. However, tubule dilation and mononuclear cell infiltration occurred to a similar extent. Inflammation and oxidative stress are important contributors to kidney aging. Diabetes accelerates aging phenotype in the kidney because of the additional hypeglycemia-induced stress (Wu et al. 2010). PAPP-A KO mice show enhanced resistance to inflammatory and oxidative stress (Conover 2010), which may explain, in part, the decrease in age-related kidney tubule dilation and relative resistance to the development of diabetic nephropathy
Proposed role of IGF-I and PAPP-A in diabetic nephropathy
The renal IGF system appears to be a major contributor to the development of diabetic nephropathy (Rabkin & Schaefer 2004, Vasylyeva & Ferry 2007, Bach 2012). IGF-I is synthesized and secreted by mesangial cells and has been shown to bind specific receptors on glomerular cells (Tack et al. 2002, Abrass et al. 1988). Furthermore, IGF-I synthesis and receptor expression are increased in mesangial cells from diabetic mice (Tack et al. 2002, Oemar et al. 1991) and experimental diabetes increases IGF-I receptor expression in the kidney (Werner et al. 1990). Exposing mesangial cells to IGF-I increases cell proliferation and matrix production while suppressing matrix degradation. Mesangial cell proliferation and matrix accumulation is mediated in part by increased mesangial cell sensitivity to IGF-I in the presence of high glucose (Horney et al. 1998). Thus, IGF-I action increases in diabetic kidney in autocrine/paracrine manner that promotes matrix production and mesangial cell proliferation. We propose PAPP-A as another regulatory component, enhancing local IGF-I bioavailability. PAPP-A expression has been shown to increase in response to tissue injury (Conover 2012). Moreover, PAPP-A expression is potently stimulated by macrophage-derived proinflammatory cytokines, tumor necrosis factor-α and interleukin-1β, in fibroblasts and vascular smooth muscle cells, and by transforming growth factor-β in human osteoblasts (Conover et al. 2006, Resch et al. 2004, Ortiz et al. 2003). These factors are also involved in injury response in kidney. Further in vitro studies are necessary to determine if there are similar effects on PAPP-A expression and IGF action in mesangial cells.
The studies herein advance our understanding of PAPP-A in the kidney and provide scientific rationale for targeting PAPP-A as a way to inhibit the development and/or progression of kidney pathology in diabetes and aging.
Acknowledgments
Jakob H. Mikkelsen: Grant, Ansh Labs; Patents, selective exosite inhibition of PAPP-A activity against IGFBP-4.
Claus Oxvig: Grant, Ansh Labs, Danish Research Council; Patents, Selective exosite inhibition of PAPP-A activity against IGFBP-4.
Cheryl A. Conover: Grants, NIH AG028141, HL074871, Ted Nash Long Life Foundation, Ansh Labs; Patents, Marker for Inflammatory Conditions (issued), Insulin-Like Growth Factor Binding Protein-04 Protease (issued), Transgenic mouse lacking PAPP-A Activity (issued), Treatment of Osteoporosis (issued); Royalties, Marker for Inflammatory Conditions.
FUNDING
This work was funded in part by a sponsored research grant from Ansh Labs (to C.A.C.).
The authors acknowledge the technical assistance of Laurie Bale, Jacquelyn Grell, Suban Chakraborty, Sally West and Anthony Croatt, all from the Mayo Clinic, Rochester, Minnesota.
Footnotes
DECLARATION OF INTEREST
Jessica R. Mader: No conflict of interest.
Zachary T. Resch: No conflict of interest.
Gary R. McLean: Employment, London Metropolitan University; travel/accommodations/meting expenses unrelated to activities listed, British Society of Immunology
Ronald J. Marler: No conflict of interest.
AUTHOR CONTRIBUTIONS
Jessica R. Mader: Designed and performed the mouse experiments, contributed to the draft manuscript, reviewed and edited revised versions of the manuscript.
Zachary T. Resch: Developed and performed PAPP-A immunohistochemistry, reviewed and edited manuscript.
Gary R. McLean: Provided Ig expression plasmids, reviewed manuscript.
Jakob H. Mikkelsen: Generated the PAPP-A monoclonal antibody, reviewed manuscript.
Claus Oxvig: Generated the PAPP-A monoclonal antibody, reviewed and edited manuscript.
Ronald J. Marler: Assessed histopathology and immunohistochemistry, reviewed and edited manuscript.
Cheryl A. Conover: Designed experiments, analyzed data, prepared tables and figures, drafted the manuscript, approved final version of manuscript.
References
- Abrass C, Raugi G, Gabourel L, Lovet DH. Insulin and insulin-like growth factor I binding to cultured rat glomerular mesangial cells. Endocrinology. 1988;123:2432–2439. doi: 10.1210/endo-123-5-2432. [DOI] [PubMed] [Google Scholar]
- Astrup AS, Tarnow L, Christiansen M, Hansen PR, Parving H-H, Rossing P. Pregnancy-associated plasma protein A in a large cohort of type 1 diabetic patients with and without diabetic nephropathy: a prospective follow-up study. Diabetes Medicine. 2007;12:1381–1385. doi: 10.1111/j.1464-5491.2007.02283.x. [DOI] [PubMed] [Google Scholar]
- Bach LA. The insulin-like growth factor system in kidney disease and hypertension. Current Opinion in Nephrology and Hypertension. 2012;21:86–91. doi: 10.1097/MNH.0b013e32834dc1a2. [DOI] [PubMed] [Google Scholar]
- Conover CA. PAPP-A: a new anti-aging target? Aging Cell. 2010;9:942–946. doi: 10.1111/j.1474-9726.2010.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends in Endocrinology and Metabolism. 2012;23:242–249. doi: 10.1016/j.tem.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Harrington SC, Resch ZT, Overgaard MT, Oxvig C. Cytokine stimulation of pregnancy-associated plasma protein A expression in human coronary artery smooth muscle cells: inhibition by resveratrol. American Journal of Physiology: Cell Physiology. 2006;290:C183–C188. doi: 10.1152/ajpcell.00199.2005. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RL. Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. Journals of Gerontology Series A, The Biological Sciences. 2010;65:590–599. doi: 10.1093/gerona/glq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Fuchtbauer E-M, Oxvig C, van Deursen J. Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development. 2004;131:1187–1194. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- Conover CA, Harrington SC, Bale LK, Oxvig C. Surface association of pregnancy-associated plasma protein-A accounts for its colocalization with activated macrophages. American Journal of Physiology: Heart and Circulatory Physiology. 292:H994–H1000. doi: 10.1152/ajpheart.00798.2006. 207. [DOI] [PubMed] [Google Scholar]
- Fioretto P, Mauer M. Histopathology of diabetic nephropathy. Seminars in Nephrology. 2007;27:195–207. doi: 10.1016/j.semnephrol.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horney MJ, Shirley DW, Kurtz DT, Rosenzweig SA. Elevated glucose increases mesangial cell sensitivity to insulin-like growth factor I. American Journal of Physiology: Renal Physiology. 1998;43:F1045–F1053. doi: 10.1152/ajprenal.1998.274.6.F1045. [DOI] [PubMed] [Google Scholar]
- Hourvitz A, Kuwahara A, Hennebold JD, Tavares AB, Negishi H, Lee TH, Erickson GF, Adashi EY. The regulated expression of the pregnancy-associated plasma protein-A in the rodent ovary: a proposed role in the development of dominant follicles and of corpora lutea. Endocrinology. 2002;143:1833–1844. doi: 10.1210/endo.143.5.8769. [DOI] [PubMed] [Google Scholar]
- Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR, III, Conover CA. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proceedings of the National Academy of Sciences, USA. 1999;96:3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TM, Galbert SP, Kiefer D, Spellacy WN, Gall S. Characterization of four human pregnancy-associated plasma proteins. American Journal of Obstetrics and Gynecology. 1974;118:223–236. doi: 10.1016/0002-9378(74)90553-5. [DOI] [PubMed] [Google Scholar]
- Lupia E, Elliot SJ, Lenz O, Zheng F, Hattori M, Striker GE, Striker LJ. IGF-1 decreases collagen degradation in diabetic NOD mesangial cells: implications for diabetic nephropathy. Diabetes. 1999;48:1638–1644. doi: 10.2337/diabetes.48.8.1638. [DOI] [PubMed] [Google Scholar]
- Oemar BS, Foellmer HG, Hodgdon-Anandant L, Rosenzweig SA. Regulation of insulin-like growth factor I receptors in diabetic mesangial cells. Journal of Biological Chemistry. 1991;266:2369–2373. [PubMed] [Google Scholar]
- Ortiz CO, Chen B-K, Bale LK, Overgaard MT, Oxvig C, Conover CA. Transforming growth factor-β regulation of the insulin-like growth factor binding protein-4 protease system in cultured human osteoblasts. Journal of Bone and Mineral Research. 2003;18:1066–1072. doi: 10.1359/jbmr.2003.18.6.1066. [DOI] [PubMed] [Google Scholar]
- Qin X, Wergedal JE, Rehage M, Tran K, Newton J, Lam P, Baylink DJ, Mohan S. Pregnancy-associated plasma protein-A increases osteoblast proliferation in vitro and bone formation in vivo. Endocrinology. 2006;147:5653–5661. doi: 10.1210/en.2006-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin R, Schaefer F. New concepts: growth hormone, insulin-like growth factor-I and the kidney. Growth Hormone and IGF Research. 2004;14:270–276. doi: 10.1016/j.ghir.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Resch ZT, Chen B-K, Bale LK, Oxvig C, Overgaard MT, Conover CA. Pregnancy-associated plasma protein A gene expression as a target of inflammatory cytokines. Endocrinology. 2004;145:1124–1129. doi: 10.1210/en.2003-1313. [DOI] [PubMed] [Google Scholar]
- Schlondorff DO. Overview of factors contributing to the pathophysiology of progressive renal disease. Kidney International. 2008;74:860–866. doi: 10.1038/ki.2008.351. [DOI] [PubMed] [Google Scholar]
- Steffes MW, Osterby R, Chavers B, Mayer SM. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes. 1989;38:1077–1081. doi: 10.2337/diab.38.9.1077. [DOI] [PubMed] [Google Scholar]
- Swindell WR, Masternak MM, Bartke A. In vivo analysis of gene expression in long-lived mice lacking the pregnancy-associated plasma protein A (PAPPA) gene. Experimental Gerontology. 2010;45:366–374. doi: 10.1016/j.exger.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack I, Elliot SJ, Potier M, Rivera A, Striker GE, Striker LJ. Autocrine activation of the IGF-I signaling pathway in mesangial cells isolated from diabetic NOD mice. Diabetes. 51:182–188. doi: 10.2337/diabetes.51.1.182. [DOI] [PubMed] [Google Scholar]
- US Renal Data System. USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; [Google Scholar]
- Vasyleva TL, Ferry RJ., Jr Novel roles of the IGF-IGFBP axis in etiopathophysiology of diabetic nephropathy. Diabetes Research and Clinical Practice. 2007;76:177–186. doi: 10.1016/j.diabres.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H, Shen-Orr Z, Stannard B, Burguera B, Roberts CT, Jr, LeRoith D. Experimental diabetes increases insulin-like growth factor I and II receptor concentration and gene expression in kidney. Diabetes. 1990;39:1490–1497. doi: 10.2337/diab.39.12.1490. [DOI] [PubMed] [Google Scholar]
- Wu J, Zhang R, Torreggiani M, Ting A Xiong H, Striker GE, Vlassara H, Zheng F. Induction of diabetes in Aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic regiculum stress, and inflammation. American Journal of Pathology. 2010;176:2163–2176. doi: 10.2353/ajpath.2010.090386. [DOI] [PMC free article] [PubMed] [Google Scholar]