Summary
Complement is an important component of the innate immune system that is crucial for defense from microbial infections and for clearance of immune complexes and injured cells. In normal conditions complement is tightly controlled by a number of fluid-phase and cell surface proteins to avoid injury to autologous tissues. When complement is hyperactivated, as occurs in autoimmune diseases or in subjects with dysfunctional regulatory proteins, it drives a severe inflammatory response in numerous organs. The kidney appears to be particularly vulnerable to complement-mediated inflammatory injury. Injury may derive from deposition of circulating active complement fragments in glomeruli, but complement locally produced and activated in the kidney also may have a role. Many kidney disorders have been linked to abnormal complement activation, including immune-complex–mediated glomerulonephritis and rare genetic kidney diseases, but also tubulointerstitial injury associated with progressive proteinuric diseases or ischemia-reperfusion.
Keywords: Complement, kidney, complement regulators, innate immunity, adaptive immunity, kidney diseases
The complement system is an important component of the innate immunity that functions primarily as a first-line host defense against pathogenetic infections.1, 2 More than 30 components and regulators have been identified that are widely distributed in the circulation and in tissues, where they are synthesized and secreted by a number of cells under various stimuli, including cytokines and hormones.
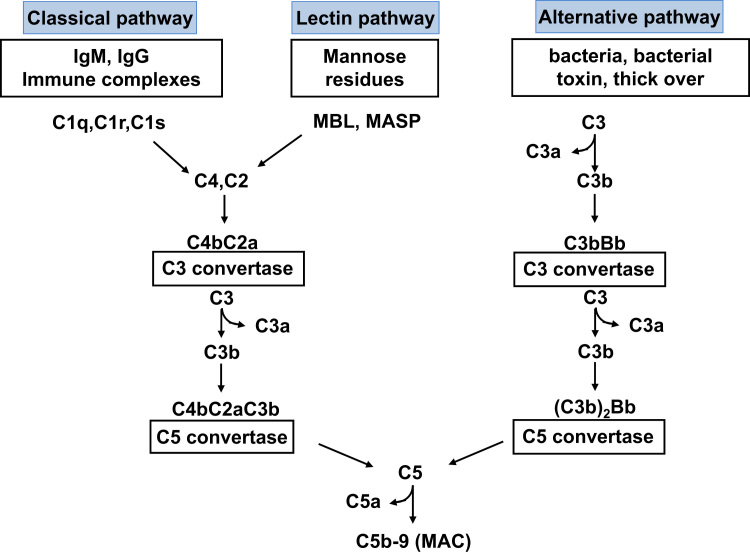
Despite the lack of specificity that characterizes the components of the acquired immune system, complement recognizes selectively foreign pathogens and damaged self cells, using the recognition molecules of the classic, lectin, and alternative pathways (Fig. 1).
Figure 1.
Schematic overview of the complement cascade illustrating the three activation pathways (classical, lectin, and alternative) and the MAC.
Activated complement generates three major types of effectors: (1) anaphylatoxins (C3a and C5a), which are potent proinflammatory molecules that attract and activate leukocytes through interaction with their cognate G-protein–coupled receptors, C3a receptor (C3aR) and C5a receptor (C5aR); (2) opsonins (C3b, iC3b, and C3d), which decorate target surfaces through covalent bonding to facilitate transport and favor removal of target cells or immune complexes; and (3) the terminal membrane attack complex (MAC, C5b-9) that directly lyses targeted (opsonized) pathogens or damaged self cells (Table 1). These effectors allow the complement system to play an important role in host defense against bacteria, and in the removal of immune complexes and apoptotic cells, as indicated by the finding that individuals with inherited and acquired complement deficiencies are susceptible to bacterial infections and immune complex diseases.3 Over the past few years, other important functions of complement have been disclosed, including regulation of the adaptive immune response, promotion of tissue regeneration and angiogenesis, mobilization of stem cells, proper development of the central nervous system, and control of embryo implantation.4
Table 1.
The Main Physiologic Activities of the Complement System
| Activity | Complement Protein Responsible for the Activity |
|---|---|
| Host defense against infection | |
| Oponization | Covalently bound fragments of C3 and C4 (C3b, C4b) |
| Chemotaxis and activation of leukocytes | Anaphylatoxins (C5a, C3a); anaphylatoxin receptors on leukocytes (C5aR, C3aR) |
| Lysis of bacteria and cells | Membrane-attack complex (C5b-9) |
| Interface between innate and adaptive immunity | |
| Augmentation of antibody response | C3b and C4b and their fragments bound to immune complexes and to antigens; CR1-4 receptors on B cells and APC |
| Enhancement of T-cell response to APC | C3a and C5a, C3aR and C5aR on T cells and APC |
| Reduction of Treg function | C3a and C5a, C3aR and C5aR on T cells and APC |
| Disposal of waste | |
| Clearance of immune complexes from tissues | C1q; covalently bound fragments of C3 (C3b) and C4 (C4b), CR1 on erythrocytes, CR1-4 receptors on phagocytes |
| Clearance of apoptotic cells | C1q; covalently bound fragments of C3 (C3b) and C4 (C4b), CR1 on erythrocytes, CR1-4 receptors on phagocytes |
Abbreviations: APC, antigen presenting cells; Treg, regulatory T cells.
Unfortunately, the effector function of the system is not focused solely on the targets to be neutralized, but also may involve bystander cells. The end results depend on the extent and the persistence of the activation process. The undesired effects of complement activation are controlled by several complement regulators acting at different steps of the cascade and these are present in the fluid phase as well as on the surface of tissue and circulating cells.5 However, unrestricted complement activation easily can overcome the protection of the complement regulators and may result in extensive host tissue damage. This situation often is encountered in acute pathologic conditions, such as sepsis or ischemia-reperfusion, or in chronic diseases sustained and amplified by complement, activated by immune complexes, an ongoing inflammatory process, and/or by apoptotic/necrotic cells.
Among the human diseases that have been linked to complement dysregulation, several disorders of the kidney have been identified and studied extensively both clinically and experimentally. These studies not only have provided insights into the pathogenesis of many kidney diseases, but also have contributed significantly to our understanding of complement-mediated human tissue injury in general.
The Three Complement Activation Pathways
Three main pathways can activate the complement system: classical, lectin, and alternative (Fig. 1). The classical pathway (CP) uses C1 and is triggered by antigen–antibody immune complexes. Inactive C1 circulates as a serum molecular complex comprising six C1q molecules and two molecules each of the serine proteases C1r and C1s. After binding to a cognate antigen, the fragment crystallizable (Fc) portion of an IgG or IgM antibody interacts with the collagen-like tail of C1q. This interaction leads to a conformational change resulting in the sequential activation of C1r and C1s. The activated C1s then cleaves C4 and C2 into small inactive fragments (C4a and C2b) and larger active fragments (C4b and C2a). C4b binds to the sugar moieties of cell surface glycoproteins and binds noncovalently to C2a, to generate the classic pathway C3 convertase C4bC2a (Fig. 1). C4bC2a is an enzymatic complex that cleaves C3, the central component of the complement cascade, into the anaphylatoxin C3a and C3b. In addition to activation by IgG and IgM immune complexes, C1q also may be activated by apoptotic and necrotic cells and by acute phase proteins such as C-reactive protein.6
The lectin pathway (LP) resembles the CP in that its activation also leads to formation of the C4bC2a C3 convertase complex (Fig. 1). However, instead of relying on antibodies to recognize pathogenic components, the LP uses members of the collectin family of plasma proteins, namely mannose-binding lectins (MBLs) and ficolins, to identify patterns of carbohydrate ligands that are found on the surface of a wide variety of microorganisms. MBL consists of up to six trimeric subunits whose structure resembles a bouquet-like shape similar to that of C1q.1 The plasma concentration of MBL can vary up to 1000-fold in normal individuals. This variation largely is explained by single-nucleotide polymorphisms within exon 1 of the MBL-2 gene. Polymorphisms of the promoter region contribute further to the variation in MBL levels.7 Ficolins are homologous to MBL. Binding of MBL or ficolins to distinct sugar molecules on the pathogenic surface leads to activation of MBL-associated serine proteases (MASP-1, MASP-2 and MASP-3), which cleave C4 and C2 and generate C4bC2a in a reaction analogous to the classic pathway (Fig. 1).
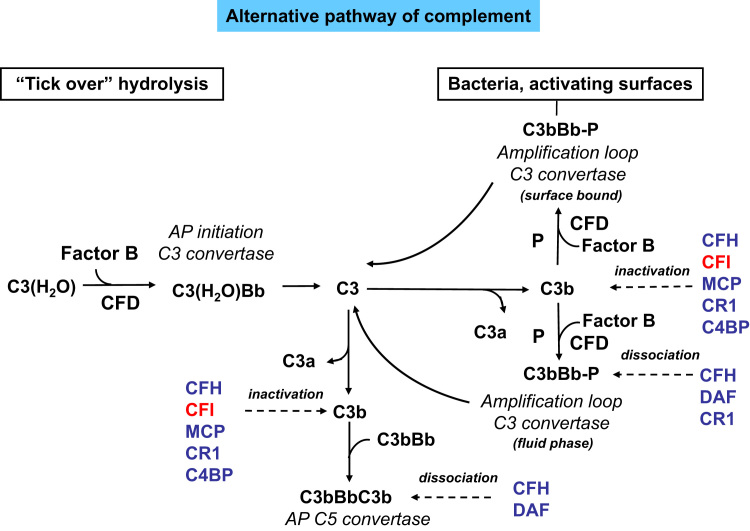
Although the classical and lectin pathways generally are activated upon recognition of exogenous materials, the alternative pathway (AP) is constitutively active at low levels in the normal host. This often is referred to as the tickover mechanism and allows the system to stay primed for rapid and robust activation (Fig. 1). Spontaneous hydrolysis of a thioester bond within C3 is thought to initiate the AP (Fig. 2). This leads to a conformational change in the structure of C3, resulting in a form of C3, referred to as C3(H2O), that functions similar to C3b with regard to its ability to bind factor B (CFB). The bound CFB then becomes a substrate for the serine protease factor D (CFD). Cleavage of CFB by CFD results in formation of the initiation AP C3 convertase C3(H2O)Bb, which, similar to the classic C3 convertase C4bC2a, can cleave C3 into C3a and C3b. The generation of C3b allows the AP to be fully activated either in fluid phase (fluid phase C3b) or on activating surfaces (surface bound C3b; Fig. 2) via formation of the amplification loop AP C3 convertase C3bBb (Figure 1, Figure 2).
Figure 2.
The alternative pathway of complement activation. The AP is activated continuously in plasma by low-grade hydrolysis of C3. The latter binds factor B to form a C3(H2O)B complex. CFD cleaves factor B to form the AP initiation C3 convertase that cleaves C3 to C3b. The activation then is amplified by the covalent binding of a small amount of C3b to hydroxyl groups on cell-surface carbohydrates and proteins of target cells such as bacterial cells. This C3b binds factor B, to form the amplification loop C3 convertase C3bBb. The binding of properdin (P) stabilizes this enzyme. C3 convertase enzymes cleave many molecules of C3, resulting in a positive feedback amplification loop. C3b also binds to the C3 convertase, forming the C5 convertase enzyme C3b2Bb. The AP is highly regulated to prevent nonspecific damage to host cells and limit the deposition of complement to the surface of pathogens. This fine regulation occurs through a number of membrane-anchored and fluid phase regulators. CFI (degrades C3b and C4b); CFH and CR1 both act as cofactors for factor I for C3b cleavage and favor the decay of the C3 convertase of the AP; C4BP: C4b binding protein (beside inhibiting the classic and the lectin pathways of complement, it also controls the alternative pathway by acting as a cofactor for factor I in the cleavage of C3b); MCP binds C3b and C4b and has cofactor activity for both the classic and the alternative pathways.
It should be noted that although the spontaneously generated C3(H2O)Bb is unique to AP, the C3b fragment generated from any of the pathways can bind to CFB and, with the participation of CFD, can form the AP C3 convertase C3bBb, which serves as an amplification loop for the entire complement system by rapidly augmenting the conversion of C3 to C3b necessary for full activation of the system and its downstream effects (Fig. 1).8 Newly formed C3bBb is stabilized by the plasma protein properdin that binds to the complex and slows its deactivation.8 Evidence also is available that properdin, in addition to serving as a stabilizer of AP C3 convertase, may initiate AP activation by interacting with surface AP C3 convertase or microbial antigens.9 Another notable finding of recent studies is the requirement of MASP1/3 for normal AP complement activity. Indeed, it has been shown that MASP1/3 cleave inactive CFD zymogen into the active form of CFD that normally is present in plasma. When MASP-1/3 are lacking, CFD circulates in plasma as the nonprocessed and inactive zymogen incapable of supporting AP complement activation.10
Apart from their roles in the classic, lectin, and alternative pathways of complement, C4b and C3b attach to immune complexes or to target bacterial cells through covalent binding; the opsonization of these targets by C4b, C3b, or their further cleavage fragments facilitates their transportation and clearance through the reticuloendothelial system. Indeed, complement activating immune complexes and opsonized target cells interact with complement receptor 1 (CR1) on human red blood cells via the bound C3b and C4b fragments and are delivered rapidly to the liver and spleen, where the target is handed over to the Kupffer cells or to the marginal zone macrophages and dendritic cells and are phagocytosed11 (Table 1). This transfer is mediated by complement receptors and cell-derived proteases and facilitated by Fc receptors present on the phagocytes. Mouse complement receptor-related y (crry) on erythrocytes plays a similar role as CR1 on red blood cells in human beings. Binding of another receptor, complement receptor of the immunoglobulin superfamily to C3b and its fragment inactive C3b (iC3b) also has been shown to be critical for the uptake of circulating immune complexes by both mouse and human Kupffer cells.12 Apoptotic cells bind C1q and fix C3- and C4-derived fragments. C1q plays an important role in the clearance of apoptotic cells in the body because C1q bound to syngeneic apoptotic cells not only activates the CP of complement (Table 1), but also binds directly to phagocytes.13 C1q deficiency indeed is associated with a severe form of systemic lupus erythematosus with overproduction of anti-DNA autoantibodies and accumulation of apoptotic cells.13, 14
iC3b and the final cleavage product of C3b, C3d, bind CR2 and play an important role in the cell-cycle control of B cells15 (Table 1). It later was found that the main function of the interaction of CR2 on B cells with antigen-bound C3d is to lower the threshold for B-cell activation by the B-cell antigen receptor.16 Immune complexes including antigens and C3d cross-linked on B cells through CR2 and B-cell receptors indeed are more effective in activating B cells than immune complexes alone.17 B cells also express CR1, but much less is known about the role of CR1-C3b signaling in B cells.16 Published studies would suggest that CR1 mediates inhibitory signals in human B cells by blocking B-cell–receptor–induced proliferation and inhibiting the differentiation of B cells to plasmablasts and their immunoglobulin production.16, 18 Inactive C3b also binds the leukocyte integrins CR3 and CR4 that belong to the superfamily of adhesion molecules (Table 1). CR3 is expressed on myeloid cells. It mediates the phagocytosis of particles opsonized with iC3b. Some pathogens, such as Staphylococcus epidermidis, directly bind CR3, without the need of complement. CR4 is expressed both by myeloid and lymphoid cells and it also is highly expressed on tissue macrophages, where it represents an important receptor for particles opsonized by iC3b (Table 1).
C5 Activation and the Terminal Pathway
C5 Cleavage
C5 is the initiator of the effector terminal phase of the complement system and shares the molecular structure consisting of two chains—α and β, linked by disulfide bonds, with C3 and C4, with the only difference being that C5 does not contain an internal thio-ester bond. The terminal phase is similar for the classical, lectin, and alternative pathways. The incorporation of C3b in the C3 convertases results in the formation of the C5 convertases: C3bBbC3b for the AP and C4bC2aC3b for the CP and LP (Fig. 1). These C5 convertases cleave C5 into C5a and C5b, ultimately resulting in the formation of the multimeric MAC (C5b-9) (Fig. 1).19 Other factors of the coagulation and fibrinolytic pathway including thrombin; human factors XIa, Xa, and IXa; and plasmin can cleave C5, independent of other complement factors, in the so-called extrinsic complement pathway.20
C5a, and the other anaphylatoxin C3a that generates from C3 cleavage, are potent bioactive molecules that can act on a wide variety of cell types expressing their high-affinity binding transmembrane receptors C5aR and C5L219, 21 for C5a and C3aR for C3a.22 C5aR are canonical G-protein–coupled signaling receptors; however, in contrast, C5L2 is unable to couple to G proteins.23 All these receptors are expressed on immune as well as nonimmune cells; however, C5L2 is expressed at much lower levels compared with C5aR.
C5a and C3a are potent inflammatory mediators targeting a broad spectrum of immune and nonimmune cells. By interacting with their G-protein–coupled receptors C5aR and C3aR, C5a and C3a regulate vasodilatation, increase the permeability of small blood vessels, and induce contraction of smooth muscles.24 In macrophages, neutrophils and eosinophils C5a and C3a can trigger an oxidative burst and in basophils can stimulate the release of histamine. In eosinophils, C5a and C3a regulate the production of eosinophil cationic protein, their adhesion to endothelial cells, and their migration. C5a is a powerful chemoattractant for macrophages, neutrophils, activated B and T cells, basophils, and mast cells,19, 25 the latter of which also migrate toward a C3a gradient (Table 1).
On the other hand, the other C5a receptor, C5L2, may function as a decoy receptor regulating the extracellular bioavailability of C5a, and so limiting the proinflammatory response to C5a.26 It also may act as a negative modulator of C5aR-mediated signal transduction through the β-arrestin pathway.27 However, recent studies have suggested that C5L2 has a proinflammatory role in experimental sepsis,28 allergic asthma,29 and also may control the development of T-helper 17 cells that are essential in asthma30 as well as autoimmune arthritis.31
It should be noted that once plasma C5a and C3a are released, plasma carboxypeptidases rapidly metabolize them, by cleaving the C-terminal arginine to less-potent forms: C5adesArg and C3adesArg.21 C5adesArg still retains signaling activity at C5aR; however, it has 10-fold to 100-fold reduced affinity for C5aR than intact C5a.32 Thus, this metabolism of C5a by plasma carboxypeptidases effectively limits the activity of C5a, without eliminating all of its functions. Interestingly, C5adesArg still retains high affinity for C5L2,33 which may enhance the proposed scavenger/decoy receptor activity of C5L2. In contrast, C3aR does not recognize the desarginate form (C3adesArg) of C3a.34
Formation and Roles of the Terminal Membrane Attack Complex (C5b-9)
In contrast to the early steps of complement activation, assembly of the cytolytic C5b-9 (Fig. 1) on the cell surface is a nonenzymatic process. It starts with the interaction of C5b, released from C5 convertase cleavage of C5, with C6. Binding of C7 to C5b6 leads to the formation of a more stable trimeric complex, C5b-7, which exposes transient lipid binding sites, allowing its association with the cell membrane. Interaction of C8 with C5b-7 results in the assembly of the tetrameric complex C5b-8, which promotes binding and polymerization of 10 to 16 molecules of C9. The final step of this process is the insertion of C5b-9 MAC complex into the cell plasma membrane, which causes cell death (Table 1). For a long time, the damaging effect of MAC has been associated almost exclusively to its cytolytic activity. Although this may be true for some cell targets under special conditions, as is the case of erythrocytes sensitized by antibodies of well-defined classes and subclasses and in sufficient numbers, the MAC does not appear, in general, to be very efficient, particularly on nucleated cells. Nucleated cells are able to withstand MAC insertion but they sustain sublethal injury, which leads to a cascade of cellular events as discussed in the article by Tamoko and Cybulsky.
Endothelial cells represent a potential target of MAC, which exerts a number of noncytolytic effects.19, 35 The complex promotes inflammation inducing the expression of adhesion molecules and the release of chemokines and platelet activating factor, which triggers the coagulation process, stimulating the expression of tissue factor. This favors the shedding of vesicles that support the formation of a prothrombinase complex, and contributes to modify the vascular tone, promoting the release of the vasodilator prostaglandin I2 and the vasoconstrictor thromboxane A2 from endothelial cells.
Complement Regulators
The powerful effector functions of complement have the potential to harm the host. The activation of CP and LP is largely dependent on foreign material, but under certain situations (eg, tissue ischemia and reperfusion), both pathways can be activated and cause autologous injury. More relevant to complement-related human diseases, deposition of C3b via AP activation and amplification is nondiscriminatory and, if not properly regulated, can damage host cells rapidly. Thus, it is essential that activity be down-regulated on host cells while efficient activation is permitted on foreign targets. Human beings and other mammals have developed a variety of both plasmatic and membrane-bound inhibitory proteins to regulate the location and activity of complement (Table 2).
Table 2.
Complement Regulatory Proteins in Human Beings and Their Functions
| Regulator | Function | Location |
|---|---|---|
| C1 inhibitor (C1-INH) | Inactivates C1r and C1s, MASP-1, and MASP-2 | Plasma |
| MCP | Cofactor for factor I–mediated cleavage of C3b and C4b | Membrane-bound |
| DAF | Destabilizes C3/C5 convertases of the CP and AP (decay accelerating activity) | Membrane-bound |
| CR1 | Decay accelerating activity as well as cofactor activity for factor I–mediated cleavage of C3b and C4b | Membrane-bound |
| C4 binding protein (C4BP) | Binds to C4b; decay accelerating and cofactor activity | Plasma |
| Factor H | Binds to C3b; has decay accelerating activity of the AP C3 and C5 convertases and cofactor activity | Plasma |
| Thrombomodulin | Increases CFH cofactor activity, activates TAFI-mediated C3a and C5a inactivation | Membrane-bound |
| Factor I | Degrades C3b and C4b aided by cofactors | Plasma |
| CD59 | Blocks the C9 association with C5b-8 to prevent C5b-9 formation on host cells | Membrane-bound |
| S-protein (vitronectin) | Binds to C5b-7 and inhibits C9 polymerization | Plasma |
| Clusterin (SP-40,40) | Binds to C5b-7 and inhibits generation of C5b-9 | Plasma |
Abbreviation: TAFI, thrombin activatable fibrinolysis inhibitor.
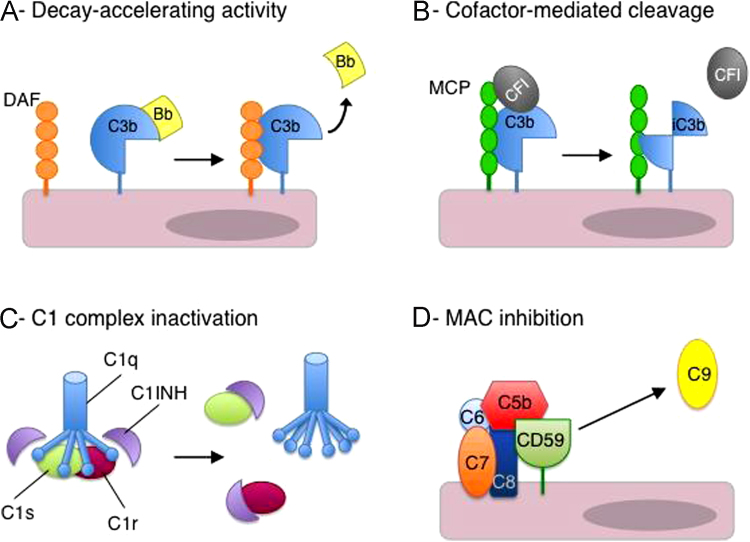
C1 inhibitor (C1INH, also termed SERPING1) irreversibly binds to and inactivates C1r and C1s of the CP and MASP-1 and MASP-2 of the LP, thus inhibiting the initiating steps of these activation pathways (Table 2, Fig. 3). Deficiency of C1INH results in episodic angioedema. C1INH, in addition to its role as a complement inhibitor, is the major inhibitor of factor XIIa and kallikrein of the contact system. The lack of inhibition of these enzymes by C1INH results in inappropriate bradykinin generation. This in turn mediates the increased vascular permeability characteristic of angioedema.36
Figure 3.
Mechanisms of complement regulation. (A) DAF (or CFH, CR1) destabilizes C3 convertases and accelerates the dissociation of C3bBb (depicted) and C4bC2a. (B) Cofactor activity: MCP (or CFH, CR1) binds to C3b and serves as a cofactor for CFI-mediated cleavage and inactivation of C3b (or C4b). (C) C1 complex inactivation. C1INH binds to C1r and C1s to inactivate the C1 enzyme complex. (D) MAC inhibition: CD59 inhibits C9 association with C5b-8 and prevents MAC (C5b-9) formation.
Complement factor H (CFH) and complement factor I (CFI) are the key plasma regulators of the AP of complement (Table 2). If one of them is missing, or totally dysfunctional, AP activation in plasma is vigorous and leads to secondary complement deficiency via overconsumption of C3 and other complement components. CFI is a plasma serine protease that is able to permanently inactivate C3b to iC3b by proteolysis, but needs a cofactor.37 iC3b then is cleaved into further fragments (C3dg and C3c) by CFI, again with the need for cofactor proteins.37 CFH is one such cofactors.38, 39 It is an abundant, single-chain glycoprotein circulating at a concentration of approximately 400 mg/L and is composed of 20 short consensus repeat domains, each consisting of approximately 60 amino acids. The major source of CFH is the liver, but extrahepatic synthesis has been shown in a variety of tissues including endothelial cells,40 glomerular mesangial cells,41 and, in rodents, podocytes.39 CFH regulates AP activation by competing with CFB in binding to C3b,42 by acting as a cofactor for CFI in the C3b cleavage,38, 39 and by decreasing the stability of the C3bBb convertase complex, accelerating the dissociation to C3b and Bb (decay accelerating activity) (Table 2). In addition to its regulatory activity in plasma, CFH is practically the only molecule that is involved in down-regulating AP activation on host structures that lack other surface-bound regulators such as basement membranes in kidney glomeruli.43 CFH also contributes to the protection of cellular surfaces such as endothelial cells thanks to its capability to bind heparan sulfates and cell surface glycosaminoglycans and the C3d part of C3b deposited on cell surfaces.44, 45 Therefore, it is clear that CFH has a physiological action both in the fluid phase and at the cell surface, particularly at the endothelial cell level. Deletion mutagenesis studies have shown that regulation of fluid-phase C3 activation requires the N-terminal five short consensus repeat domains46, 47 in which both the cofactor and decay-accelerating activities of CFH reside. In contrast, targeting of the protein to cell surfaces (surface recognition function) is mediated by C-terminal domains.48
CFI also cleaves the C4b component of the CP and LP C3 convertase C4bC2a in the presence of the plasma cofactor C4b-binding protein (Table 2). Of interest, C4b-binding protein also can favor C3b inactivation, although to a lesser degree (Fig. 2).
Membrane-bound complement regulators include membrane cofactor protein (MCP/CD46), decay-accelerating factor (DAF/CD55), and CR1 (CD35) (Table 2). MCP acts as a cofactor for C3b and C4b cleavage by CFI (Fig. 3), but it only protects those cells on which it is expressed.49 DAF accelerates the dissociation of the C3bBb AP C3 convertase similarly to CFH1, 50 (Fig. 3). DAF also decreases the stability of the C3 convertase of the classic and lectin pathways, C4bC2a, by accelerating its dissociation to C4b and C2a.51 CR1 on circulating cells mainly acts as an immune adherence receptor to facilitate the removal of C3b/C4b-opsonized immune complexes and pathogens from the circulation, but it also exerts complement inhibitory activities. CR1 has cofactor activity for CFI-mediated cleavage of C3b to iC3b, and thereafter to C3c and C3dg, and of C4b. In addition, it exerts decay-accelerating activities on the CP/LP and the AP C3 convertases (Table 2).51 More recently, the anticoagulant endothelial transmembrane protein thrombomodulin (THBD)52 has been shown to act also as a complement regulator via two distinct mechanisms (Table 2). THBD enhances CFI-mediated inactivation of C3b on the cell surface in the presence of the cofactor CFH.53 In addition, THBD is a critical cofactor for thrombin-mediated activation of the plasma procarboxypeptidase thrombin activatable fibrinolysis inhibitor.52, 54 Active thrombin activatable fibrinolysis inhibitor inhibits fibrinolysis by removing lysine residues from modified fibrinogen, but also des-arginates and inactivates the anaphylatoxins C3a and C5a.52, 55
In addition, the terminal MAC is regulated closely both in fluid phase and on cell membranes (Table 2, Fig. 3). Vitronectin, also known as protein S, is one of these regulators, which binds preferentially to C5b-7 and interacts with C9 to form SC5b-9, inhibiting its polymerization.56 Clusterin (Table 2) is another complement regulator that not only acts at the level of C7 as a component of C5b-7, preventing the insertion of this complex into the cell membrane, but also binds to C8 and to C9, inhibiting the polymerization of C9.57 These two inhibitors do not interfere with the full assembly of the complex, which, however, contains a substantially lower number of C9 molecules than MAC.
The CFH-related protein 1 (CFHR-1) has been reported as a novel regulator58 that inhibits the C5 convertase of the AP and binds to C5 and C5b-6, preventing the formation of MAC. A more recent study, however, indicated that CFHR1 along with CFHR2 and CFHR5 might act as complement activators through the formation of both homodimers and heterodimers. Dimerization confers avidity for tissue-bound complement fragments and enables these proteins to compete efficiently with CFH for ligand binding.59
CD59 is the key regulator of the terminal pathway (Table 2, Fig. 3). It inhibits C9 association with C5b-8 to prevent C5b-9 formation. Both CD59 and DAF are anchored to cell membranes by a glycosyl phosphatidylinositol molecule. Somatic mutations in the phosphatidylinositol glycan complementation class A gene in red blood cells3 result in a rare clonal disorder called paroxysmal nocturnal hemoglobinuria. As a result of defective phosphatidylinositol glycan complementation class A function, affected red cells lack all glycosyl phosphatidylinositol–linked membrane proteins to autologous complement-mediated lysis with consequent hemolytic anemia.
Complement and Adaptive Immunity
Immune cells, including T cells and antigen-presenting cells, produce complement products that have implications for organ transplantation and autoimmune diseases60, 61 (see the article by Cravedi and Heeger). Cognate T-cell––antigen-presenting cell interactions that result in T-cell activation are associated with the up-regulation and release of the AP complement components C3, CFB, and CFD by both cell types.61 The resultant activation products C3a and C5a bind to C3aR and C5aR expressed on T cells and augment the strength of the induced T-cell response.62 C3aR and C5aR signaling in T cells enhance T-cell proliferation and diminish T-cell apoptosis. The immune cell–derived and locally produced C3a and C5a also bind to C3aR/C5aR on antigen-presenting cells, inducing the release of cytokines (eg, interleukin-12 and interleukin-13) and up-regulating costimulatory molecules such as B7, further amplifying the immune response and directing T-cell phenotype toward interferon-γ–producing T-helper 1 immunity.61
More recent studies have shown that natural regulatory T cells express C3aR and C5aR and that signaling through these receptors inhibits natural regulatory T-cell function.63 Indeed, genetic and pharmacologic blockade of C3aR/C5aR signal transduction in mouse natural regulatory T cells augmented their suppressive function in vitro and in vivo, abrogated autoimmune colitis, and prolonged allogeneic skin allograft survival.63 Another study documented that when signals from C3aR and C5aR were blocked both in mouse and human CD4 T cells, this favored their transformation to induced Foxp3+ regulatory T cells in the presence of transforming growth factor β.64 The resulting induced Foxp3+ regulatory T cells exerted robust suppression on T-cell proliferation, had enhanced stability, and suppressed ongoing autoimmune disease when injected in mice with experimental autoimmune encephalomyelitis.64
Complement-dependent effects on alloreactive T-cell immunity regulate the phenotypic expression of immune-mediated injury in animal models. Further confirming a key role for C5a–C5aR interactions as pathogenic in transplant rejection are data indicating that C5aR blockade prolongs kidney transplant survival in rodents.65 This improved outcome is associated with an abrogation of intragraft mononuclear cell infiltration and a diminution in T-cell alloreactivity. In addition, mice deficient in either or both C5aR and C3aR develop less autoimmunity and are resistant to experimental allergic encephalomyelitis.62 Taken together, these results indicate that complement is a physiologically important regulator of alloreactive T-cell immunity and in autoimmunity.
Complement in the Kidney
Because of its highly specialized function, the kidney is subject to significant stress from exogenous factors (eg, pathogens, toxins, and cytokines filtered from the bloodstream). Consequently, renal function is dependent on a finely calibrated immune response including proper complement activation and regulation.
Although traditional thinking focuses on liver-derived serum complement as a principal pathogenic mediator of kidney injury, it is now abundantly clear that complement components are produced by parenchymal kidney cells as well.66
A comprehensive range of complement genes are expressed in the kidney.67 Some complement components that are produced primarily at extrahepatic sites (eg, C1q and CFD) are detected strongly in glomeruli. C1q, which is synthesized mainly in mononuclear phagocytes and epithelial cells, has been suggested to play an important role in the clearance of immune complexes and apoptotic bodies in the kidney.68, 69 Local expression of C1q could have a kinetic advantage over deposition of a circulating component. On the other hand, CFD, which is synthesized primarily by adipocytes, participates in the cleavage of CFB and thus contributes to AP function.
At variance with the glomerulus, tubules more strongly express the main components of complement synthesized in the liver (C2, C4, C3, and factor B).67 The glomerulus, because of its constant exposure to blood, is continuously in contact with plasma liver-derived complement factors, whereas the tubulointerstitium of the kidney is much more restricted in its access to circulating complement proteins. This area of the kidney has adapted to make its own complement proteins, possibly because of exposure to microorganisms ascending through the urinary tract. Thus, clustering of the components of the activation pathways in the tubule may act as a front-line defense against pathogens.
Four types of resident kidney cells are capable of synthesizing complement proteins when grown in tissue culture: glomerular epithelial cells, glomerular endothelial cells, mesangial cells, and proximal tubular cells.70, 71, 72, 73, 74 Secretion of C3 by such cells occurs spontaneously and may approach levels comparable with those produced by cultured hepatoma cells.74 In contrast, the expression of C4 often is detected weakly in cultured cells, even though the signal derived from tissue messenger RNA may be strong. This discrepancy between in vivo and in vitro expression may be owing to rapid loss of ability to synthesize C4 in vitro, as noticed in monocyte studies.75 Factor B also is detected weakly in vitro. However, many stimulators, including cytokines, growth factors, immune complexes, serum proteins, and human cytomegalovirus, can increase the expression of C3, C4, and factor B by cultured cells.66
Numerous factors have been shown to regulate the production of complement by renal cells.66 The effects of some of these signals are tissue-specific, and the response may vary even with different cell types in the same tissue. For example, interleukin-1 may induce a two-fold to three-fold increase in the renal expression of C2 in mice, but has no effect on hepatic synthesis of C2.76 Another example is tumor necrosis factor-α, which regulates the transcription of C3 in glomerular endothelial cells,74 but has no such effect in glomerular mesangial cells.71 The cytokines interleukin-2 and interferon-γ appear to play a major role in regulating the production of C3 and C4 by proximal tubular cells.70 Furthermore, interferon-γ induces changes in renal gene transcription whereas in hepatocytes it may act at the level of RNA translation to increase local complement synthesis.73 This local variation in the regulation of complement may allow fine-tuning of tissue levels expressed without alteration of circulating levels.
The transmembrane receptors for C3a and C5a (C3aR and C5aR), and the integral membrane CRs for C3b, iC3b, and C3dg, are expressed outside the kidney, particularly in cells of hematopoietic and immune lineage. These are important in renal injury through their infiltration of the kidney and/or by affecting kidney-directed immune responses. There is mounting evidence that intrinsic glomerular and tubular cell C3aR and C5aR expression and activation also can affect renal injury.77, 78 CR1 on podocytes and CR3 and CR4 in kidney dendritic cells have functions that remain poorly defined. These can be engaged by C3 and C5 activation products derived from systemic and local pools in renal injury. The deposition of the MAC complex, C5b-9 on glomeruli and tubules of diseased kidney, has led to investigation of the activity of the complex on renal cells. In mesangial and tubular cells, the complex has a dual effect. On one hand, C5b-9 stimulates both cell types to release cytokines and collagen, which cause tissue fibrosis,79 and on the other hand it is able to induce or enhance apoptosis.80
In addition to the complement activation pathway components, a number of complement regulatory proteins, particularly membrane-bound proteins, are synthesized within the kidney. These include DAF, MCP, CR1, and CD59. The distribution of these inhibitory proteins in normal and diseased kidneys has been studied comprehensively.81, 82, 83 These studies have shown considerable variation in the level of membrane regulators depending on cell type, suggesting that complement is regulated by distinct inhibitors within different sections of the kidney.84 Studies of human and mouse kidney have shown ubiquitous expression of CD59 on all major cell types within the kidney. DAF is found mostly on podocytes, endothelial cells, and in the juxtaglomerular apparatus. MCP is expressed throughout human renal tissues, but rodents do not normally express MCP beside spermatozoa. The rodent-specific complement regulator Crry is expressed ubiquitously in the kidney and is considered a functional homolog of human MCP. In human kidney, CR1 is restricted mostly to podocytes, although similar to MCP, rodents only have limited expression of CR1, which is generated by alternative splicing from the Cr1/2 gene.84
The renal tubule is relatively deficient in complement regulators, helping to explain the vulnerability of the renal tubule to complement attack.83 Cell-associated regulators at the C3 level, DAF CR1, and MCP are hardly detectable on the apical membrane of proximal tubular cells.66 Conversely, human proximal tubular cells produce CFH,85 the main soluble inhibitor of the AP. In addition, we found that CFH binds to heparan sulfate residues on human proximal tubular cell surface through its C-terminal domain.86 Importantly, surface-bound CFH limited C3 and C5b-9 deposits on the tubular cell surface, which indeed were enhanced in the presence of antibodies blocking either the N-terminal cofactor site or the C-terminal membrane binding site of CFH.86
The expression of regulatory molecules is up-regulated during the activation of complement,66 as occurs in a number of forms of renal disease.66, 82 Not surprisingly, overexpression of complement regulatory proteins in mice markedly ameliorated nephrotoxic serum nephritis.87 This indicates that complement regulatory proteins play a protective role against complement-mediated renal injury. The kidney therefore has a set of positive and negative internal controls with the ability to influence complement activation on the renal structures.
Complement activation affects a broad range of renal diseases moving from antibody-mediated glomerular diseases to thrombotic microangiopathies, progressive kidney diseases, and ischemia/reperfusion injury. A brief overview of the involvement of complement in kidney diseases that is discussed in detail in other articles in this issue is given below (Table 3).
Table 3.
Summary of the Main Complement-Associated Renal Diseases
| Disease | Pathogenesis | Complement Pathway |
|---|---|---|
| Lupus nephritis | Anti-DNA antibodies, accumulation of apoptotic cells | CP |
| Post-streptococcal glomerulonephritis | Antibody mediated, circulating or planted antigens | CP |
| Goodpasture syndrome | Anti-GBM antibodies | CP |
| IgA nephropathy | Deposition of polymeric IgA | CP/AP |
| ANCA-associated vasculitis | Antibodies directed against neutrophil components | CP/AP |
| Membranous nephritis | Antibody-antigen complexes subepithelially | CP AP/LP |
| MPGN I | Immune complexes, C3NeF, complement gene mutations | CP/AP |
| C3 glomerulopathies | C3NeF, complement gene mutations | AP |
| STEC-HUS | Shiga-toxin mediated endothelial injury/activation | AP |
| aHUS | Complement gene mutations, anti-CFH antibodies | AP |
| Tubulointerstitial injury in proteinuric progressive glomerulopathies | Proteins (including complement components) abnormally ultrafiltered by the glomeruli | AP |
| I/R injury | Ischemic tissue injury, oxygen radicals | AP/LP |
Complement in Kidney Diseases
Glomerulopathies
One of the fundamental protective actions of complement is the clearance of invasive pathogens and the removal of immune complexes and cell debris. This is evident in individuals with inherited C3 deficiency who develop recurrent bacterial infections and immune complex glomerulonephritis.3 In contrast to its physiologic function, complement activation leads to inflammation in several types of glomerulonephritis, such as systemic lupus erythematosus, IgA nephropathy, Goodpasture syndrome (anti–glomerular basement membrane [GBM] glomerulonephritis), antineutrophil cytoplasmic autoantibody (ANCA)-induced glomerulonephritis, and membranous nephropathy.77, 84
Anti-GBM–induced glomerulonephritis is characterized by immune complex deposition along the GBM. Many studies have shown that the complement system affects anti-GBM glomerulonephritis in human beings by amplifying antibody-mediated injury through CP and enhancing the inflammatory response through C5 activation.88 In the experimental model of this disease in mice, the nephrotoxic serum nephritis, deficiency of C3, or deficiency of C4 reduced renal disease. Subsequent studies showed that loss of DAF, Crry, CFH, and/or CD59 all exacerbated anti-GBM glomerulonephritis in mice, highlighting the relevance of complement control mechanisms in autoimmune kidney injury.84
Complement has been shown to play a key role in experimental (ANCA)-associated glomerulonephritis.89 Mice depleted of C3 with cobra venom factor and mice lacking C5 or treated with a C5 inhibitory antibody were protected from disease induced by injection of antimyeloperoxidase or anti–proteinase 3 antibodies. In the same model, no disease was seen in mice lacking CFB, whereas C4-deficient mice showed symptoms of ANCA-associated glomerulonephritis, which implicates AP activation and excludes a role for the CP and LP.89 The mechanistic link between ANCA-induced neutrophil activation and initiation of the AP complement system remains to be elucidated, and whether anticomplement therapy might be effective clinically has yet to be established.
Membranous nephropathy (MN) is another form of immune-mediated glomerular injury in which immune complexes of immunoglobulins and complement accumulate in a granular pattern along the outer side of the GBM, leading to glomerular visceral epithelial cell detachment90 (see the articles by Beck et al and Takano and Cybulsky). Seminal studies have identified several autologous antigens that are targets of antibody response in MN. In most patients with the idiopathic autoimmune form of MN, the autoantibodies are of the IgG4 and IgG1 type and are directed to the phospholipase A2-receptor.90, 91 These antibodies may affect glomerular visceral epithelial cells directly through interaction with the phospholipase A2-receptor.
The role of complement has been established clearly in experimental MN. Indeed, complement activation and local formation of C5b-9 is a prerequisite for the development of glomerular injury in rats with passive Heymann nephritis, an experimental model of MN, as documented by studies showing that complement inhibition with recombinant CR1 led to a reduction in proteinuria in this model.92
In human secondary MN, especially the forms associated with lupus, most studies found abundant C1q, whereas the role of the classic pathway in primary MN is uncertain because renal C1q deposits rarely are found. This fits with the IgG subclasses in immune deposits, predominantly IgG1 and IgG3 in secondary MN and predominantly poorly complement-fixing IgG4 in primary MN. On the other hand, C3, C4, CFB, MBL, and C5b-9 typically are present and co-deposited with IgG, suggesting that the lectin and the alternative pathways of complement activation could play a role (see articles in this issue by Ma et al and Takano and Cybulsky).
Inappropriate activation of the AP of complement system as a result of inefficient inhibition may lead to prolonged tissue damage and glomerular diseases. This mechanism recently was shown to underlie the Escherichia coli–associated and atypical forms of hemolytic uremic syndrome (aHUS).93 Sixty percent of aHUS cases are related to mutations in genes encoding complement regulatory proteins, CFH, MCP, CFI, and THBD, the alternative pathway C3 convertase components C3 and CFB, or inhibitory anti-CFH autoantibodies.94 Patients with combined mutations in 2 or 3 different complement genes also have been reported underlying the complexity of aHUS genetics.95
Similar to HUS, idiopathic forms of membranoproliferative glomerulonephritis (MPGN) are associated commonly with defective complement regulation. A recent immunofluorescence-based classification approach distinguishes those forms of MPGN with isolated C3 deposits, known as C3 glomerulopathies, and characterized by defective control of the AP of complement from MPGN type I with deposits of immunoglobulin and complement and characterized by activation of the CP by antigen-antibody immune complexes.96 However, such distinction is not accepted universally and evidence is accumulating that some cases of MPGN with C3 and immunoglobulin deposits also may involve abnormalities of AP regulation.97 C3NeF, an autoantibody stabilizing the AP C3-convertase, is found in 50% to 75% of patients with C3-glomerulopathies, but also is found in approximately half of patients with MPGN I. Auto-antibodies against CFH and CFB and complement gene mutations also have been reported.
Tubulointerstitial Injury in Progressive Kidney Diseases
Progressive glomerular diseases invariably are accompanied by tubulointerstitial damage, the extent of which is linked closely to an adverse renal outcome. The various proteins of the complement system are among the proteins appearing in the urine in nonselective glomerular proteinuria, leading to intratubular deposition of C3 and of C5b-9, and their activation also has been proposed to contribute to tubulointerstitial damage98 via cytotoxic, proinflammatory, and fibrogenic effects.
Abnormal C3 and C5b-9 staining in proximal tubular cells and along the brush border is a long known feature in chronic proteinuric diseases.98 Investigation using proteinuric models of glomerular injury showed less tubulointerstitial damage in complement-depleted rats and/or C6-deficient rats, or upon treatment with complement-inhibitory molecules.14 Evidence subsequently was provided that C3 and ultrafiltered plasma proteins co-localized to proximal tubular cells that were exposed to filtered protein overload in rats with remnant kidney before the accumulation of monocyte/macrophages in the interstitium.14, 98 Treatment with an angiotensin-converting enzyme inhibitor limited the excess load of both C3 and plasma proteins in proximal tubular cells and prevented the recruitment of inflammatory cells. In mice with protein overload–induced proteinuria, complement appeared to be an important effector of interstitial mononuclear cell infiltration and fibrogenesis, as shown by significant attenuation of injury in C3-deficient mice.99
Renal tubular cells synthesize C3 and other complement factors and exposure of cultured proximal tubular cells to total serum proteins up-regulated C3 messenger RNA expression and protein biosynthesis. Therefore, both excess ultrafiltration and protein-overload–induced proximal tubular cell synthesis of complement components could underlie complement-mediated injury in chronic proteinuric renal diseases.98
For determination of the injurious role of plasma-derived C3, as opposed to tubular cell–derived C3, C3-deficient kidneys were transplanted into wild-type mice99 before inducing protein-overload proteinuria. Protein overload led to the development of glomerular injury, accumulation of C3 in podocytes and proximal tubules, and tubulointerstitial changes. Conversely, when wild-type kidneys were transplanted into C3-deficient mice, protein overload led to a milder disease and abnormal C3 deposition was not observed.99 These data suggest that ultrafiltered C3 contributes more to tubulointerstitial damage induced by protein overload than locally synthesized C3.
Ischemia-Reperfusion Injury
In most organs, ischemia-reperfusion (I/R) injury is initiated by natural antibodies and activation of the CP of complement, but in the kidney immunoglobulins and CP activation do not appear to play a major role because, at variance with other organs, antibodies are not deposited in the kidney after I/R100 (see articles by Quigg et al and Thurman et al). The AP appears to be involved, as documented by findings that C3- or CFB-deficient mice are resistant to renal I/R injury, whereas C4 deficiency is not protective.101 Additional studies documented that murine C3-deficient kidneys are protected from postischemic damage after transplantation into syngeneic murine recipients with normal C3, whereas wild-type kidneys developed I/R injury upon transplantation into syngeneic C3-deficient recipients. These data indicate that kidney-derived C3, rather than circulating C3, drives the development of kidney I/R injury, at least in rodents. Small interfering RNA–induced down-regulation of the C5a anaphylatoxin receptor C5aR or administration of a C5aR antagonist prevented neutrophil infiltration and limited postischemic kidney injury in rodents, supporting a role for C5a–C5aR interactions in the pathogenesis of I/R injury in the kidney.61
All of the earlier-mentioned studies have been performed in rodents. Limited information is currently available from human beings and larger animals. In a recent study in swine, renal I/R injury resulted in the formation of C4d/C1q, C54/MBL, and MBL/MASP-2 deposits. Infusion of C1-inhibitor reduced complement deposits and infiltrating inflammatory leukocytes and limited tubular damage, which suggests that the CP/LP pathways of complement might contribute to kidney I/R injury.102
Conclusions
The involvement of the complement system in renal diseases is complex. There are multiple mechanisms of complement activation, and activation generates multiple biologically active fragments. Complement is involved in the resolution of injury as well; part of the complement activation seen in glomerulonephritis is the normal reaction to remove immune complexes and damaged cells from the glomerulus.
Eculizumab now has been used in dozens of patients with aHUS, MPGN, or at risk for humoral allograft rejection. Although these conditions are rare in the population, their studies have provided important insight to the pathogenesis of complement-mediated renal tissue injury as well as new understanding of mechanisms of action of complement regulatory proteins. These advances also have fueled many efforts to develop new targeted complement inhibitors and it is likely that in the near future complement-inhibitory drugs will be used in patients with more common types of renal diseases.
Footnotes
Financial support: Supported by a grant from Fondazione Telethon (GGP09075), the Fondazione ART per la Ricerca sui Trapianti ART ONLUS (Milan, Italy), and by a grant from the FP7 EU project (EURenOmics project, HEALTH-2012-305608).
Conflict of interest statement: none.
References
- 1.Walport M.J. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 2.Walport M.J. Complement. Second of two parts. N Engl J Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 3.Botto M., Kirschfink M., Macor P., Pickering M.C., Wurzner R., Tedesco F. Complement in human diseases: lessons from complement deficiencies. Mol Immunol. 2009;46:2774–2783. doi: 10.1016/j.molimm.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zipfel P.F., Skerka C. Complement regulators and inhibitory proteins. Nat Rev Immunol. 2009;9:729–740. doi: 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 6.Nauta A.J., Daha M.R., van Kooten C., Roos A. Recognition and clearance of apoptotic cells: a role for complement and pentraxins. Trends Immunol. 2003;24:148–154. doi: 10.1016/s1471-4906(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 7.Garred P., Honore C., Ma Y.J., Munthe-Fog L., Hummelshoj T. MBL2, FCN1, FCN2 and FCN3-The genes behind the initiation of the lectin pathway of complement. Mol Immunol. 2009;46:2737–2744. doi: 10.1016/j.molimm.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Lachmann P.J. The amplification loop of the complement pathways. Adv Immunol. 2009;104:115–149. doi: 10.1016/S0065-2776(08)04004-2. [DOI] [PubMed] [Google Scholar]
- 9.Kemper C., Atkinson J.P., Hourcade D.E. Properdin: emerging roles of a pattern-recognition molecule. Annu Rev Immunol. 2010;28:131–155. doi: 10.1146/annurev-immunol-030409-101250. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M., Ishida Y., Iwaki D., Kanno K., Suzuki T., Endo Y. Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D. J Exp Med. 2010;207:29–37. doi: 10.1084/jem.20090633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson A.L., Lindorfer M.A., Kennedy A.D., Foley P.L., Taylor R.P. Concerted clearance of immune complexes bound to the human erythrocyte complement receptor: development of a heterologous mouse model. J Immunol Methods. 2002;270:183–197. doi: 10.1016/s0022-1759(02)00296-x. [DOI] [PubMed] [Google Scholar]
- 12.Helmy K.Y., Katschke K.J., Jr, Gorgani N.N., Kljavin N.M., Elliott J.M., Diehl L. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 13.Taylor P.R., Carugati A., Fadok V.A., Cook H.T., Andrews M., Carroll M.C. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–366. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berger S.P., Roos A., Daha M.R. Complement and the kidney: what the nephrologist needs to know in 2006? Nephrol Dial Transplant. 2005;20:2613–2619. doi: 10.1093/ndt/gfi166. [DOI] [PubMed] [Google Scholar]
- 15.Melchers F., Erdei A., Schulz T., Dierich M.P. Growth control of activated, synchronized murine B cells by the C3d fragment of human complement. Nature. 1985;317:264–267. doi: 10.1038/317264a0. [DOI] [PubMed] [Google Scholar]
- 16.Erdei A., Isaak A., Torok K., Sandor N., Kremlitzka M., Prechl J. Expression and role of CR1 and CR2 on B and T lymphocytes under physiological and autoimmune conditions. Mol Immunol. 2009;46:2767–2773. doi: 10.1016/j.molimm.2009.05.181. [DOI] [PubMed] [Google Scholar]
- 17.Mongini P.K., Vilensky M.A., Highet P.F., Inman J.K. The affinity threshold for human B cell activation via the antigen receptor complex is reduced upon co-ligation of the antigen receptor with CD21 (CR2) J Immunol. 1997;159:3782–3791. [PubMed] [Google Scholar]
- 18.Kremlitzka M., Polgar A., Fulop L., Kiss E., Poor G., Erdei A. Complement receptor type 1 (CR1, CD35) is a potent inhibitor of B-cell functions in rheumatoid arthritis patients. Int Immunol. 2013;25:25–33. doi: 10.1093/intimm/dxs090. [DOI] [PubMed] [Google Scholar]
- 19.Woodruff T.M., Nandakumar K.S., Tedesco F. Inhibiting the C5-C5a receptor axis. Mol Immunol. 2011;48:1631–1642. doi: 10.1016/j.molimm.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Amara U., Flierl M.A., Rittirsch D., Klos A., Chen H., Acker B. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185:5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manthey H.D., Woodruff T.M., Taylor S.M., Monk P.N. Complement component 5a (C5a) Int J Biochem Cell Biol. 2009;41:2114–2117. doi: 10.1016/j.biocel.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Morgan B.P. Physiology and pathophysiology of complement: progress and trends. Crit Rev Clin Lab Sci. 1995;32:265–298. doi: 10.3109/10408369509084686. [DOI] [PubMed] [Google Scholar]
- 23.Okinaga S., Slattery D., Humbles A., Zsengeller Z., Morteau O., Kinrade M.B. C5L2, a nonsignaling C5A binding protein. Biochemistry. 2003;42:9406–9415. doi: 10.1021/bi034489v. [DOI] [PubMed] [Google Scholar]
- 24.Klos A., Tenner A.J., Johswich K.O., Ager R.R., Reis E.S., Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo R.F., Ward P.A. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 26.Gerard N.P., Lu B., Liu P., Craig S., Fujiwara Y., Okinaga S. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J Biol Chem. 2005;280:39677–39680. doi: 10.1074/jbc.C500287200. [DOI] [PubMed] [Google Scholar]
- 27.Bamberg C.E., Mackay C.R., Lee H., Zahra D., Jackson J., Lim Y.S. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J Biol Chem. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rittirsch D., Flierl M.A., Nadeau B.A., Day D.E., Huber-Lang M., Mackay C.R. Functional roles for C5a receptors in sepsis. Nat Med. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X., Schmudde I., Laumonnier Y., Pandey M.K., Clark J.R., Konig P. A critical role for C5L2 in the pathogenesis of experimental allergic asthma. J Immunol. 2010;185:6741–6752. doi: 10.4049/jimmunol.1000892. [DOI] [PubMed] [Google Scholar]
- 30.Lajoie S., Lewkowich I.P., Suzuki Y., Clark J.R., Sproles A.A., Dienger K. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11:928–935. doi: 10.1038/ni.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto M., Hirota K., Yoshitomi H., Maeda S., Teradaira S., Akizuki S. Complement drives Th17 cell differentiation and triggers autoimmune arthritis. J Exp Med. 2010;207:1135–1143. doi: 10.1084/jem.20092301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higginbottom A., Cain S.A., Woodruff T.M., Proctor L.M., Madala P.K., Tyndall J.D. Comparative agonist/antagonist responses in mutant human C5a receptors define the ligand binding site. J Biol Chem. 2005;280:17831–17840. doi: 10.1074/jbc.M410797200. [DOI] [PubMed] [Google Scholar]
- 33.Scola A.M., Higginbottom A., Partridge L.J., Reid R.C., Woodruff T., Taylor S.M. The role of the N-terminal domain of the complement fragment receptor C5L2 in ligand binding. J Biol Chem. 2007;282:3664–3671. doi: 10.1074/jbc.M609178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crass T., Raffetseder U., Martin U., Grove M., Klos A., Kohl J. Expression cloning of the human C3a anaphylatoxin receptor (C3aR) from differentiated U-937 cells. Eur J Immunol. 1996;26:1944–1950. doi: 10.1002/eji.1830260840. [DOI] [PubMed] [Google Scholar]
- 35.Dobrina A., Pausa M., Fischetti F., Bulla R., Vecile E., Ferrero E. Cytolytically inactive terminal complement complex causes transendothelial migration of polymorphonuclear leukocytes in vitro and in vivo. Blood. 2002;99:185–192. doi: 10.1182/blood.v99.1.185. [DOI] [PubMed] [Google Scholar]
- 36.Cugno M., Zanichelli A., Foieni F., Caccia S., Cicardi M. C1-inhibitor deficiency and angioedema: molecular mechanisms and clinical progress. Trends Mol Med. 2009;15:69–78. doi: 10.1016/j.molmed.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson S.C., Sim R.B., Lea S.M., Fremeaux-Bacchi V., Blom A.M. Complement factor I in health and disease. Mol Immunol. 2011;48:1611–1620. doi: 10.1016/j.molimm.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Zipfel P.F., Skerka C. Complement factor H and related proteins: an expanding family of complement-regulatory proteins? Immunol Today. 1994;15:121–126. doi: 10.1016/0167-5699(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 39.Pickering M.C., Cook H.T. Translational mini-review series on complement factor H: renal diseases associated with complement factor H: novel insights from humans and animals. Clin Exp Immunol. 2008;151:210–230. doi: 10.1111/j.1365-2249.2007.03574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwaeble W., Schwaiger H., Brooimans R.A., Barbieri A., Most J., Hirsch-Kauffmann M. Human complement factor H. Tissue specificity in the expression of three different mRNA species. Eur J Biochem. 1991;198:399–404. doi: 10.1111/j.1432-1033.1991.tb16028.x. [DOI] [PubMed] [Google Scholar]
- 41.van den Dobbelsteen M.E., Verhasselt V., Kaashoek J.G., Timmerman J.J., Schroeijers W.E., Verweij C.L. Regulation of C3 and factor H synthesis of human glomerular mesangial cells by IL-1 and interferon-gamma. Clin Exp Immunol. 1994;95:173–180. doi: 10.1111/j.1365-2249.1994.tb06033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conrad D.H., Carlo J.R., Ruddy S. Interaction of beta1H globulin with cell-bound C3b: quantitative analysis of binding and influence of alternative pathway components on binding. J Exp Med. 1978;147:1792–1805. doi: 10.1084/jem.147.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zipfel P.F., Heinen S., Jozsi M., Skerka C. Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol Immunol. 2006;43:97–106. doi: 10.1016/j.molimm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 44.Kazatchkine M.D., Fearon D.T., Austen K.F. Human alternative complement pathway: membrane-associated sialic acid regulates the competition between B and beta1 H for cell-bound C3b. J Immunol. 1979;122:75–81. [PubMed] [Google Scholar]
- 45.Manuelian T., Hellwage J., Meri S., Caprioli J., Noris M., Heinen S. Mutations in factor H reduce binding affinity to C3b and heparin and surface attachment to endothelial cells in hemolytic uremic syndrome. J Clin Invest. 2003;111:1181–1190. doi: 10.1172/JCI16651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhn S., Zipfel P.F. Mapping of the domains required for decay acceleration activity of the human factor H-like protein 1 and factor H. Eur J Immunol. 1996;26:2383–2387. doi: 10.1002/eji.1830261017. [DOI] [PubMed] [Google Scholar]
- 47.Sharma A.K., Pangburn M.K. Identification of three physically and functionally distinct binding sites for C3b in human complement factor H by deletion mutagenesis. Proc Natl Acad Sci U S A. 1996;93:10996–11001. doi: 10.1073/pnas.93.20.10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pangburn M.K. Host recognition and target differentiation by factor H, a regulator of the alternative pathway of complement. Immunopharmacology. 2000;49:149–157. doi: 10.1016/s0162-3109(00)80300-8. [DOI] [PubMed] [Google Scholar]
- 49.Liszewski M.K., Leung M., Cui W., Subramanian V.B., Parkinson J., Barlow P.N. Dissecting sites important for complement regulatory activity in membrane cofactor protein (MCP; CD46) J Biol Chem. 2000;275:37692–37701. doi: 10.1074/jbc.M004650200. [DOI] [PubMed] [Google Scholar]
- 50.Liszewski M.K., Farries T.C., Lublin D.M., Rooney I.A., Atkinson J.P. Control of the complement system. Adv Immunol. 1996;61:201–283. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- 51.Kim D.D., Song W.C. Membrane complement regulatory proteins. Clin Immunol. 2006;118:127–136. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 52.Conway E.M. Thrombomodulin and its role in inflammation. Semin Immunopathol. 2012;34:107–125. doi: 10.1007/s00281-011-0282-8. [DOI] [PubMed] [Google Scholar]
- 53.Delvaeye M., Noris M., DeVriese A., Esmon C., Esmon N., Ferrell G. Mutations in thrombomodulin in hemolytic-uremic syndrome. N Engl J Med. 2009;361:345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bajzar L., Manuel R., Nesheim M.E. Purification and characterization of TAFI, a thrombin-activable fibrinolysis inhibitor. J Biol Chem. 1995;270:14477–14484. doi: 10.1074/jbc.270.24.14477. [DOI] [PubMed] [Google Scholar]
- 55.Campbell W.D., Lazoura E., Okada N., Okada H. Inactivation of C3a and C5a octapeptides by carboxypeptidase R and carboxypeptidase N. Microbiol Immunol. 2002;46:131–134. doi: 10.1111/j.1348-0421.2002.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 56.Podack E.R., Kolb W.P., Muller-Eberhard H.J. The SC5b-7 complex: formation, isolation, properties, and subunit composition. J Immunol. 1977;119:2024–2029. [PubMed] [Google Scholar]
- 57.Tschopp J., Chonn A., Hertig S., French L.E. Clusterin, the human apolipoprotein and complement inhibitor, binds to complement C7, C8 beta, and the b domain of C9. J Immunol. 1993;151:2159–2165. [PubMed] [Google Scholar]
- 58.Heinen S., Hartmann A., Lauer N., Wiehl U., Dahse H.M., Schirmer S. Factor H-related protein 1 (CFHR-1) inhibits complement C5 convertase activity and terminal complex formation. Blood. 2009;114:2439–2447. doi: 10.1182/blood-2009-02-205641. [DOI] [PubMed] [Google Scholar]
- 59.Goicoechea de Jorge E., Caesar J.J., Malik T.H., Patel M., Colledge M., Johnson S. Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci U S A. 2013;110:4685–4690. doi: 10.1073/pnas.1219260110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lalli P.N., Strainic M.G., Yang M., Lin F., Medof M.E., Heeger P.S. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vieyra M.B., Heeger P.S. Novel aspects of complement in kidney injury. Kidney Int. 2010;77:495–499. doi: 10.1038/ki.2009.491. [DOI] [PubMed] [Google Scholar]
- 62.Strainic M.G., Liu J., Huang D., An F., Lalli P.N., Muqim N. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwan W.H., van der Touw W., Paz-Artal E., Li M.O., Heeger P.S. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210:257–268. doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Strainic M.G., Shevach E.M., An F., Lin F., Medof M.E. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:162–171. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gueler F., Rong S., Gwinner W., Mengel M., Brocker V., Schon S. Complement 5a receptor inhibition improves renal allograft survival. J Am Soc Nephrol. 2008;19:2302–2312. doi: 10.1681/ASN.2007111267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou W., Marsh J.E., Sacks S.H. Intrarenal synthesis of complement. Kidney Int. 2001;59:1227–1235. doi: 10.1046/j.1523-1755.2001.0590041227.x. [DOI] [PubMed] [Google Scholar]
- 67.Song D., Zhou W., Sheerin S.H., Sacks S.H. Compartmental localization of complement component transcripts in the normal human kidney. Nephron. 1998;78:15–22. doi: 10.1159/000044876. [DOI] [PubMed] [Google Scholar]
- 68.Walport M.J., Davies K.A., Botto M. C1q and systemic lupus erythematosus. Immunobiology. 1998;199:265–285. doi: 10.1016/S0171-2985(98)80032-6. [DOI] [PubMed] [Google Scholar]
- 69.Botto M., Dell'Agnola C., Bygrave A.E., Thompson E.M., Cook H.T., Petry F. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–59. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 70.Brooimans R.A., Stegmann A.P., van Dorp W.T., van der Ark A.A., van der Woude F.J., van Es L.A. Interleukin 2 mediates stimulation of complement C3 biosynthesis in human proximal tubular epithelial cells. J Clin Invest. 1991;88:379–384. doi: 10.1172/JCI115314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sacks S., Zhou W., Campbell R.D., Martin J. C3 and C4 gene expression and interferon-gamma-mediated regulation in human glomerular mesangial cells. Clin Exp Immunol. 1993;93:411–417. doi: 10.1111/j.1365-2249.1993.tb08193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou W., Campbell R.D., Martin J., Sacks S.H. Interferon-gamma regulation of C4 gene expression in cultured human glomerular epithelial cells. Eur J Immunol. 1993;23:2477–2481. doi: 10.1002/eji.1830231015. [DOI] [PubMed] [Google Scholar]
- 73.Sacks S.H., Zhou W., Pani A., Campbell R.D., Martin J. Complement C3 gene expression and regulation in human glomerular epithelial cells. Immunology. 1993;79:348–354. [PMC free article] [PubMed] [Google Scholar]
- 74.Sheerin N.S., Zhou W., Adler S., Sacks S.H. TNF-alpha regulation of C3 gene expression and protein biosynthesis in rat glomerular endothelial cells. Kidney Int. 1997;51:703–710. doi: 10.1038/ki.1997.101. [DOI] [PubMed] [Google Scholar]
- 75.Kulics J., Colten H.R., Perlmutter D.H. Counterregulatory effects of interferon-gamma and endotoxin on expression of the human C4 genes. J Clin Invest. 1990;85:943–949. doi: 10.1172/JCI114523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Falus A., Beuscher H.U., Auerbach H.S., Colten H.R. Constitutive and IL 1-regulated murine complement gene expression is strain and tissue specific. J Immunol. 1987;138:856–860. [PubMed] [Google Scholar]
- 77.Sacks S., Zhou W. New boundaries for complement in renal disease. J Am Soc Nephrol. 2008;19:1865–1869. doi: 10.1681/ASN.2007101121. [DOI] [PubMed] [Google Scholar]
- 78.Thurman J.M., Lenderink A.M., Royer P.A., Coleman K.E., Zhou J., Lambris J.D. C3a is required for the production of CXC chemokines by tubular epithelial cells after renal ishemia/reperfusion. J Immunol. 2007;178:1819–1828. doi: 10.4049/jimmunol.178.3.1819. [DOI] [PubMed] [Google Scholar]
- 79.Abe K., Li K., Sacks S.H., Sheerin N.S. The membrane attack complex, C5b-9, up regulates collagen gene expression in renal tubular epithelial cells. Clin Exp Immunol. 2004;136:60–66. doi: 10.1111/j.1365-2249.2004.02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nauta A.J., Daha M.R., Tijsma O., van de Water B., Tedesco F., Roos A. The membrane attack complex of complement induces caspase activation and apoptosis. Eur J Immunol. 2002;32:783–792. doi: 10.1002/1521-4141(200203)32:3<783::AID-IMMU783>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 81.Nangaku M. Complement regulatory proteins in glomerular diseases. Kidney Int. 1998;54:1419–1428. doi: 10.1046/j.1523-1755.1998.00130.x. [DOI] [PubMed] [Google Scholar]
- 82.Endoh M., Yamashina M., Ohi H., Funahashi K., Ikuno T., Yasugi T. Immunohistochemical demonstration of membrane cofactor protein (MCP) of complement in normal and diseased kidney tissues. Clin Exp Immunol. 1993;94:182–188. doi: 10.1111/j.1365-2249.1993.tb05998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ichida S., Yuzawa Y., Okada H., Yoshioka K., Matsuo S. Localization of the complement regulatory proteins in the normal human kidney. Kidney Int. 1994;46:89–96. doi: 10.1038/ki.1994.247. [DOI] [PubMed] [Google Scholar]
- 84.Lesher A.M., Song W.C. Review: complement and its regulatory proteins in kidney diseases. Nephrology (Carlton) 2010;15:663–675. doi: 10.1111/j.1440-1797.2010.01373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gerritsma J.S., Gerritsen A.F., De Ley M., van Es L.A., Daha M.R. Interferon-gamma induces biosynthesis of complement components C2, C4 and factor H by human proximal tubular epithelial cells. Cytokine. 1997;9:276–283. doi: 10.1006/cyto.1996.0164. [DOI] [PubMed] [Google Scholar]
- 86.Buelli S., Abbate M., Morigi M., Moioli D., Zanchi C., Noris M. Protein load impairs factor H binding promoting complement-dependent dysfunction of proximal tubular cells. Kidney Int. 2009;75:1050–1059. doi: 10.1038/ki.2009.8. [DOI] [PubMed] [Google Scholar]
- 87.Quigg R.J., He C., Lim A., Berthiaume D., Alexander J.J., Kraus D. Transgenic mice overexpressing the complement inhibitor crry as a soluble protein are protected from antibody-induced glomerular injury. J Exp Med. 1998;188:1321–1331. doi: 10.1084/jem.188.7.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Turnberg D., Cook H.T. Complement and glomerulonephritis: new insights. Curr Opin Nephrol Hypertens. 2005;14:223–228. doi: 10.1097/01.mnh.0000165887.75501.24. [DOI] [PubMed] [Google Scholar]
- 89.Xiao H., Schreiber A., Heeringa P., Falk R.J., Jennette J.C. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Glassock R.J. The pathogenesis of membranous nephropathy: evolution and revolution. Curr Opin Nephrol Hypertens. 2012;21:235–242. doi: 10.1097/MNH.0b013e3283522ea8. [DOI] [PubMed] [Google Scholar]
- 91.Beck L.H., Jr, Bonegio R.G., Lambeau G., Beck D.M., Powell D.W., Cummins T.D. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cybulsky A.V., Quigg R.J., Salant D.J. Experimental membranous nephropathy redux. Am J Physiol Renal Physiol. 2005;289:F660–F671. doi: 10.1152/ajprenal.00437.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Noris M., Mescia F., Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8:622–633. doi: 10.1038/nrneph.2012.195. [DOI] [PubMed] [Google Scholar]
- 94.Noris M., Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 95.Bresin E., Rurali E., Caprioli J., Sanchez-Corral P., Fremeaux-Bacchi V., Rodriguez de Cordoba S. Combined complement gene mutations in atypical hemolytic uremic syndrome influence clinical phenotype. J Am Soc Nephrol. 2013;24:475–486. doi: 10.1681/ASN.2012090884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.D’Agati V.D., Bomback A.S. C3 glomerulopathy: what’s in a name? Kidney Int. 2012;82:379–381. doi: 10.1038/ki.2012.80. [DOI] [PubMed] [Google Scholar]
- 97.Servais A., Noel L.H., Roumenina L.T., Le Quintrec M., Ngo S., Dragon-Durey M.A. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 98.Abbate M., Zoja C., Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 99.Abbate M., Zoja C., Corna D., Rottoli D., Zanchi C., Azzollini N. Complement-mediated dysfunction of glomerular filtration barrier accelerates progressive renal injury. J Am Soc Nephrol. 2008;19:1158–1167. doi: 10.1681/ASN.2007060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park P., Haas M., Cunningham P.N., Bao L., Alexander J.J., Quigg R.J. Injury in renal ischemia-reperfusion is independent from immunoglobulins and T lymphocytes. Am J Physiol Renal Physiol. 2002;282:F352–F357. doi: 10.1152/ajprenal.00160.2001. [DOI] [PubMed] [Google Scholar]
- 101.Quigg R.J. Complement and the kidney. J Immunol. 2003;171:3319–3324. doi: 10.4049/jimmunol.171.7.3319. [DOI] [PubMed] [Google Scholar]
- 102.Castellano G., Melchiorre R., Loverre A., Ditonno P., Montinaro V., Rossini M. Therapeutic targeting of classical and lectin pathways of complement protects from ischemia-reperfusion-induced renal damage. Am J Pathol. 2010;176:1648–1659. doi: 10.2353/ajpath.2010.090276. [DOI] [PMC free article] [PubMed] [Google Scholar]