Abstract
Megalin and cubilin are multifunctional endocytic receptors associated with many transporting epithelia. They play an essential role in transport of nutrients through the visceral yolk sac of rodents during embryogenesis. Here, we immunolocalise them to the endodermal layer of the human yolk sac, and to the syncytiotrophoblast and cytotrophoblast cells of placental villi. In villi, the protein level of both receptors increased with gestation. The mRNA for megalin remained constant, while that encoding cubilin increased with gestation. These results suggest megalin and cubilin may be important in human maternal–fetal transfer, and that they increase across gestation to facilitate this function.
Keywords: Transport, Endocytosis, Yolk sac, Megalin, Cubilin
1. Introduction
Our understanding of the nutritional pathways supporting development of the human conceptus during the first trimester has undergone major revision in the last few years. The absence of significant maternal and fetal blood flow to the placenta prior to 10 weeks has focused attention on alternative pathways, in particular histiotrophic nutrition from the endometrial glands and the role of the yolk sac [1]. Histiotrophic nutrition involves endocytic uptake of endometrial secretions by the endodermal layer of the visceral yolk sac or the trophoblast of the placenta [2]. In many species, the yolk sac and the chorio-allantoic placenta have collaborative roles in providing for fetal nutrition throughout gestation. The pathway has been characterised in detail in the visceral yolk sac placenta of the guinea pig [3,4], bat [5], and mouse and rat, where endocytosis and subsequent proteolysis of exogenous proteins account for the majority of amino acids supplied during organogenesis [6,7].
In the human, the trophoblast of the villous tree is the first transport epithelium to be confronted by the endometrial secretions. The apical border of the syncytiotrophoblast displays numerous coated pits [8], whereas caveolae cannot be detected [9]. In addition, immunohistochemistry has demonstrated uptake of maternal glycoproteins into larger endosomes during the first trimester, and subsequent co-localisation with lysosomal enzymes suggests some enter the digestive pathway [10]. Amino acids and other nutrients accumulate within the exocoelomic fluid [11], which bathes the secondary yolk sac. Interestingly, the outer mesothelial layer displays coated pits and endocytic vesicles [12], and also transporter proteins for folate, glucose and α-tocopherol [13–15]. These features suggest the yolk sac plays an absorptive role.
Megalin and cubilin are expressed in the endoderm of the inverted visceral yolk sac of rodents, and their functional significance during embryogenesis was recently reviewed [16,17]. Megalin has been localised to the syncytiotrophoblast layer of the placenta of the rat [18], and of the human at term [19]. Endocytic uptake of nutrients in epithelia is mediated by a dual-receptor complex formed through an interaction between megalin and cubilin [20]. Megalin (gp330) is a 600-kDa transmembrane protein, with a large extracellular domain and a short cytoplasmic tail believed to have signalling functions. Cubilin is a 460-kDa peripheral membrane protein. Cubilin lacks the transmembrane intracellular domain and therefore needs to interact with megalin to perform its endocytic function. The two co-localise in clathrin coated pits and endosomes, and have different ligand specificities. For example, transcobalamin-vitamin B12 and LDL are specific to megalin, and transferrin and HDL to cubilin [21].
Some epithelia such as the proximal convoluted tubule of the kidney are able to internalise and degrade a large variety of ligands, utilising receptors capable of binding several ligands. Most proteins that have not been retained by the glomerulus are thus reabsorbed by endocytosis [22]. Megalin and cubilin play an important role in the renal reabsorption of vitamins [20], iron (via endocytosis of transferrin) [23], and it is essential for maintaining Ca2+ metabolism [20]. The situation in the yolk sac epithelium is analogous, as the yolk sac is known to internalise and degrade proteins present in the maternal plasma [24]. Given their wide variety of ligands, megalin and cubilin may be essential in providing the embryo with vital substances such as vitamins, cholesterol, iron and calcium. The aim of this study was to study the protein and mRNA expression of these two proteins in the human yolk sac and placenta, and to quantify their expression across gestational age.
2. Methods
Human yolk sacs (n = 4) and first (7–8 wk, n = 5) and second (13–14 wk, n = 4 and 17 wk, n = 4) trimester placental samples were collected from elective surgical terminations of pregnancy, whereas samples of term placentas (39 wk, n = 5) were obtained from elective caesarean deliveries. All material was collected with informed written patient consent and approval of the Joint UCL/UCLH Committees on the Ethics of Human Research (05/Q0505/82). Tissues samples were snap frozen in liquid nitrogen for subsequent protein (Western blot) and mRNA analysis (quantitative real-time RT-PCR), or fixed in 4% paraformaldehyde and processed in paraffin wax for immunohistochemistry (IHC) [25]. All slides were immunostained under the same conditions for comparability. The cubilin antibody was from Santa Cruz (CA, USA) and megalin antibody from Abcam (Cambridge, UK). Negative controls were performed using non-immune serum.
2.1. Western blot
Frozen placental samples were homogenised in ice-cold RIPA buffer (1 ml of buffer per 100 mg tissue) containing 50 mM Tris–HCl, pH 7.4; 1% NP-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM Na3VO4; 1 mM NaF and complete mini protease inhibitor cocktail (Roche Diagnostics, East Sussex, UK). Tissue homogenates were centrifuged at 15,000× g, 4 °C for 10 min and the supernatants removed. Protein concentrations were determined using a BCA protein assay kit (Sigma Poole, UK). Lysates were mixed with 3× SDS PAGE sample buffer, heated to 70 °C for 3 min and allowed to return to room temperature. Equal amounts of protein (50 μg) were separated by 5% sodium dodecyl sulphate-polyacrylamide gel electrophoresis, transferred onto nitrocellulose membrane (Invitrogen, Paisley, UK) for 2.5 h, and subjected to immunoblot analysis. Membranes were blocked for 1 h at 25 °C in 5% milk diluted in Tris-buffered saline (TBS) and 0.1% Tween 20 and incubated with anti-cubilin (Santa Cruz, CA, USA) or anti-megalin (Abcam, Cambridge, UK) primary antibodies overnight at 4 °C. After washing and incubating with secondary antibodies, immunoreactive proteins were visualised by the ECL plus chemiluminescence system following the manufacturer's instructions (Amersham Biosciences, Bucks, UK). HiMark™ protein marker (Life Technologies) was run on each gel. The highest band was 460 kDa. We consistently detected a single band above this mark for megalin, corresponding to 600 kDa, and the cubilin protein band was detected just around the 460 kDa mark. Proteins were revealed and quantified using Image J software. Ponceau S expression served to normalise gel loading. The values are expressed as a percentage of the first trimester (7–8 wk) lysate for each experiment (100%).
2.2. RNA isolation and quantitative real-time RT-PCR analysis
Total RNA was isolated from human placentas using microRNeasy kit (Qiagen, Hilden, Germany). The RNA was quantified by spectrophotometry (Nanodrop Technologies, DE, USA). In brief, 20 μg of total RNA from each sample was reverse transcribed using a master mix containing SuperScript II Reverse Transcriptase (Invitrogen). The ABI PRISM 7700 Sequence Detection System (TaqMan) was used to perform real-time PCR according to the manufacturer's protocols. “Ct” values for each transcript (i.e. CSE, CBS) were compared with those for 18S rRNA. All primers and probes were obtained from Applied Biosystems (ABI, Warrington, UK) “Assays-on-Demand” and used a 5′ FAM reporter and 3′ nonfluorescent minor groove binder.
3. Results and discussion
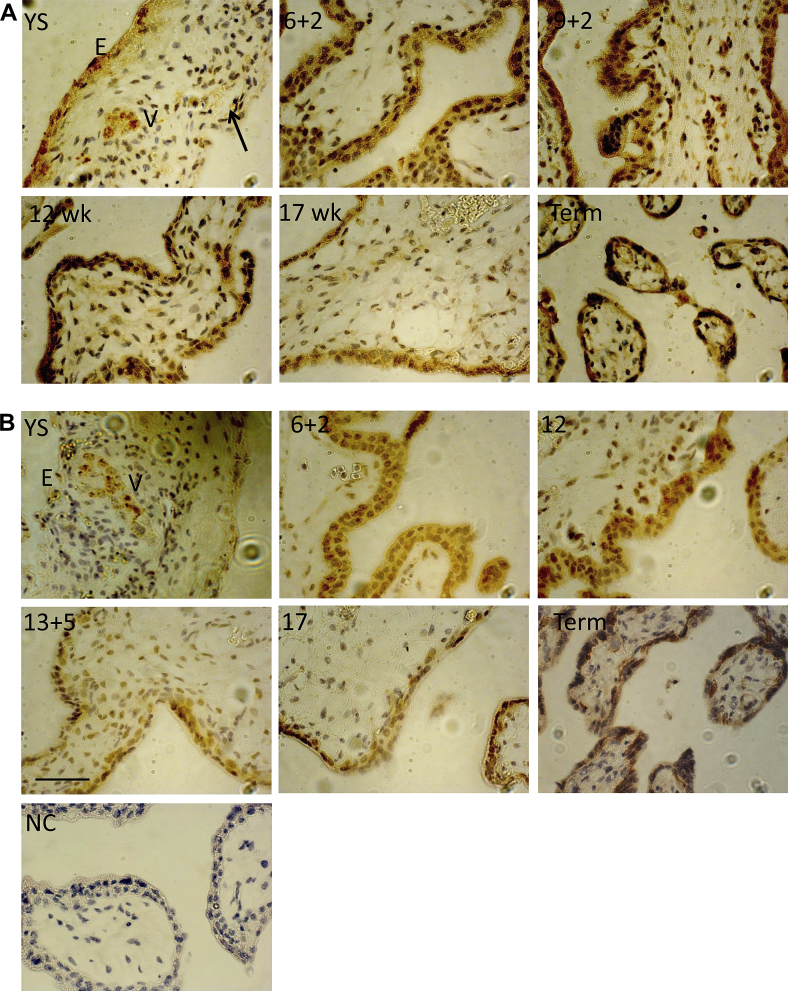
In the yolk sac, immunostaining for cubilin was strong in the cytoplasm of the cuboidal endodermal cells lining the cavity and forming the endodermal vesicles, whereas megalin showed only weak and patchy staining (Fig. 1). mRNA expression of the two membrane receptors was confirmed by RT-PCR in one yolk sac of 9.5 weeks gestational age (data not shown). Although based on a small number of specimens due to their rarity, this localisation matches that seen in the visceral yolk sac of rodents [17], suggesting that the two sacs perform similar roles in early pregnancy. Proteins in the exocoelomic fluid are capable of passing into the lumen of the yolk sac [11], and so may be endocytosed by the endoderm and digested within the lysosomal complexes present [26]. The localisation is, however, contrary to that of other transporter proteins, which are predominantly localised to the outer mesothelial layer [13–15]. This disparity may reflect differences in the transport functions of the two epithelia.
Fig. 1.
Immunolocalisation of cubilin and megalin in human placenta and yolk sac (YS) across gestation. Sections were immunostained with anti-cubilin (A) or anti-megalin (B) antibodies. In the yolk sac, staining for cubilin was strong in the endodermal epithelium (E) and the endodermal vesicles (V). Arrow denotes a capillary present in the vicinity of the external mesothelial layer. Immunoreactivity for megalin was more patchy, but present in the endodermal vesicles. In the villi, the syncytiotrophoblast and cytotrophoblast stained for both markers. Representative images are shown. The numbers superimposed on the images indicate the gestational age. YS – yolk sac; NC – negative control. Scale bar for all images = 50 μm.
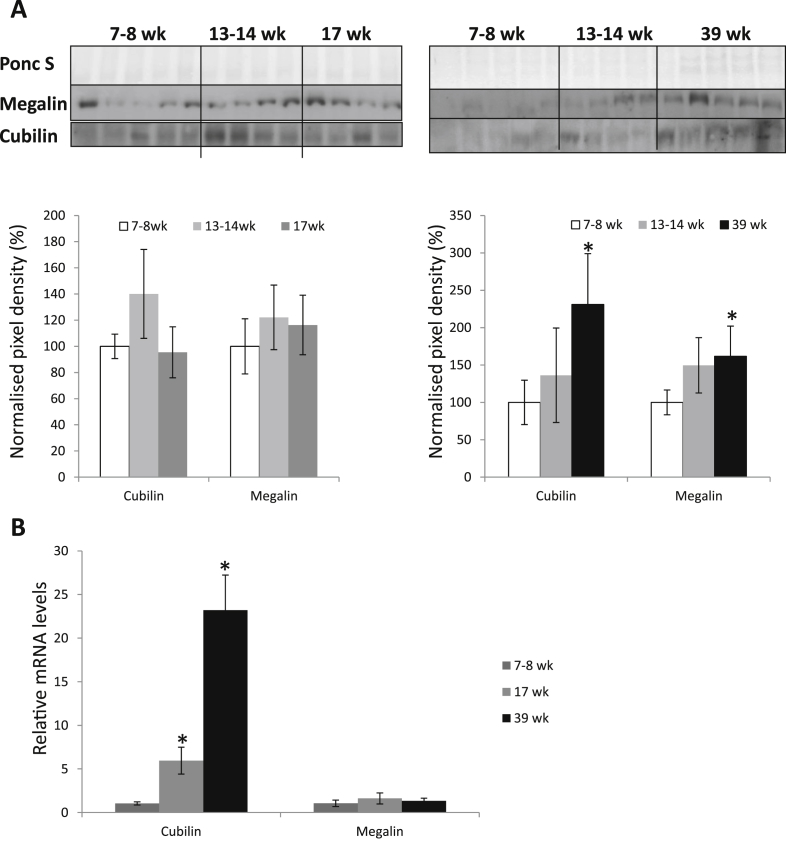
We detected megalin and cubilin by IHC in both the syncytiotrophoblast and cytotrophoblast cells of placental villi (Fig. 1). The protein level of both receptors increased with gestation. Both cubilin and megalin protein significantly increased in third trimester samples (p < 0.05), and there was a trend towards increased megalin and cubilin protein in the second trimester samples, compared to first trimester (Fig. 2A). Megalin mRNA did not vary significantly across gestation, whereas there was a significant increase in cubilin mRNA in the second and third trimester samples compared to first trimester samples (p < 0.05) (Fig. 2B). mRNA expression of megalin and cubilin was also confirmed in one yolk sac.
Fig. 2.
Protein and mRNA quantification of megalin and cubilin across gestation. A) Lysates from placental samples of 7–8 wk (n = 5), 13–14 wk (n = 4), 17 wk (n = 4) and 39 wk gestation (n = 5) were immunoblotted with antibodies against megalin and cubilin. Ponceau S expression served to normalise gel loading. Three groups of samples are displayed in each graph; gestational ages 7–8 wk, 13–14 wk and 17 wk are displayed in the left panel; and gestation ages 7–8 wk, 13–14 wk and 39 wk are displayed in the right panel. Normalised results (±SD) are plotted, expressing first trimester 7–8 wk samples as 100%. B) RNA was isolated and relative levels of megalin and cubilin mRNA were detected using quantitative real-time RT-PCR. Megalin and cubilin mRNA levels were normalised to the 18S RNA level. Significant differences (p < 0.05) are: * vs first trimester samples (One-Way ANOVA with Fisher's PLSD post-hoc test).
This is the first study to quantify mRNA and protein of megalin and cubilin in the human yolk sac and placenta. Megalin has previously been immunolocalised to the syncytiotrophoblast of term placentas [19], whereas in contrast Lundgren et al. [27] reported it to be present only in the underlying cytotrophoblast cells. These differences most likely reflect antibody specificity and the impact of antigen retrieval techniques. To our knowledge, cubilin has not been immunolocalised in the human placenta previously.
Together, these data indicate that megalin and cubilin may play a role in maternal–fetal endocytic transport during human pregnancy. In addition, they support the concept that the yolk sac functions in nutrient transfer during the crucial period of organogenesis. These receptors has been studied most extensively in the renal system, but potential ligands include vitamin B12 and D, folic acid, retinoic acid, albumin, transferrin, apolipoproteins, cholesterol, insulin and epidermal growth factor, calcium, immunoglobulins and aminoglycosides [20]. Megalin and cubilin endocytic receptors are thus of interest in nutrient transport in the embryo and in the fetus throughout gestation. Megalin has been proposed to serve as a ‘back-up’ in the transport of LDL and associated nutrients such as vitamin E. The megalin–cubilin dual-receptor complex could mediate the fetal delivery of HDL lipids, particularly cholesterol [28]. Cubilin has been demonstrated to mediate endocytosis of apolipoprotein A-I and HDL cholesterol during peri-implantation development in rodents [29], and genetic knockout is associated with developmental retardation and embryonic lethality at E7.5–13.5 [30]. There is evidence that the yolk sac allows transport of cholesterol from mother to the fetus [31], by degrading the protein component of internalised lipoproteins and transporting the reconditioned cholesterol to the embryo. The same process is likely to facilitate fetal transfer of micronutrients such as vitamins and minerals. Our study indicates that both megalin and cubilin increase across gestation to facilitate their transport functions to meet the demands of the growing fetus. Further studies are required to determine the functional importance of megalin and cubilin in normal and abnormal human development.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Burton G.J., Hempstock J., Jauniaux E. Nutrition of the human fetus during the first trimester – a review. Placenta. 2001;22(Suppl. A):S70–S77. doi: 10.1053/plac.2001.0639. [DOI] [PubMed] [Google Scholar]
- 2.Wooding F.P., Burton G.J. Springer; Berlin: 2008. Comparative placentation. Structures, functions and evolution; p. 301. [Google Scholar]
- 3.King B.F., Enders A.C. The fine structure of the guinea pig visceral yolk sac placenta. Am J Anat. 1970;127(4):394–414. doi: 10.1002/aja.1001270405. [DOI] [PubMed] [Google Scholar]
- 4.King B.F., Enders A.C. Protein absorption and transport by the guinea pig visceral yolk sac placenta. Am J Anat. 1970;129(3):261–287. doi: 10.1002/aja.1001290303. [DOI] [PubMed] [Google Scholar]
- 5.Enders A.C., Wimsatt W.A., King B.F. Cytological development of yolk sac endoderm and protein-absorptive mesothelium in the little brown bat, Myotis lucifugus. Am J Anat. 1976;146(1):1–30. doi: 10.1002/aja.1001460102. [DOI] [PubMed] [Google Scholar]
- 6.Ambroso J.L., Larsen S.V., Brabec R.K., Harris C. Fluorometric analysis of endocytosis and lysosomal proteolysis in the rat visceral yolk sac during whole embryo culture. Teratology. 1997;56(3):201–209. doi: 10.1002/(SICI)1096-9926(199709)56:3<201::AID-TERA3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Beckman D.A., Lloyd J.B., Brent R.L. Quantitative studies on the mechanisms of amino acid supply to rat embryos during organogenesis. Reprod Toxicol. 1998;12(2):197–200. doi: 10.1016/s0890-6238(97)00147-0. [DOI] [PubMed] [Google Scholar]
- 8.Ockleford C.D., Whyte A. Differentiated regions of human placental cell surface associated with exchange of materials between maternal and foetal blood: coated vesicles. J Cell Sci. 1977;25:293–312. doi: 10.1242/jcs.25.1.293. [DOI] [PubMed] [Google Scholar]
- 9.Lyden T.W., Anderson C.L., Robinson J.M. The endothelium but not the syncytiotrophoblast of human placenta expresses caveolae. Placenta. 2002;23(8–9):640–652. doi: 10.1053/plac.2002.0847. [DOI] [PubMed] [Google Scholar]
- 10.Burton G.J., Watson A.L., Hempstock J., Skepper J.N., Jauniaux E. Uterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancy. J Clin Endocrinol Metab. 2002;87:2954–2959. doi: 10.1210/jcem.87.6.8563. [DOI] [PubMed] [Google Scholar]
- 11.Jauniaux E., Gulbis B. Fluid compartments of the embryonic environment. Hum Reprod Update. 2000;6:268–278. doi: 10.1093/humupd/6.3.268. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Crussi F., Roth L.M. The human yolk sac and yolk sac carcinoma. An ultrastructural study. Hum Pathol. 1976;7(6):675–691. doi: 10.1016/s0046-8177(76)80079-2. [DOI] [PubMed] [Google Scholar]
- 13.Jauniaux E., Cindrova-Davies T., Johns J., Dunster C., Hempstock J., Kelly F.J. Distribution and transfer pathways of antioxidant molecules inside the first trimester human gestational sac. J Clin Endocrinol Metab. 2004;89:1452–1459. doi: 10.1210/jc.2003-031332. [DOI] [PubMed] [Google Scholar]
- 14.Jauniaux E., Johns J., Gulbis B., Spasic-Boskovic O., Burton G.J. Transfer of folic acid inside the first-trimester gestational sac and the effect of maternal smoking. Am J Obstet Gynecol. 2007;197(1)(58):e1–e6. doi: 10.1016/j.ajog.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Benirschke K., Burton G.J., Baergen R.N. 6th ed. Springer; Heidelberg: 2012. Pathology of the human placenta. [Google Scholar]
- 16.Fisher C.E., Howie S.E. The role of megalin (LRP-2/Gp330) during development. Dev Biol. 2006;296(2):279–297. doi: 10.1016/j.ydbio.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Zohn I.E., Sarkar A.A. The visceral yolk sac endoderm provides for absorption of nutrients to the embryo during neurulation. Birth Defects Res A Clin Mol Teratol. 2010;88(8):593–600. doi: 10.1002/bdra.20705. [DOI] [PubMed] [Google Scholar]
- 18.Zheng G., Bachinsky D.R., Stamenkovic I., Strickland D.K., Brown D., Andres G. Organ distribution in rats of two members of the low-density lipoprotein receptor gene family, gp330 and LRP/alpha 2MR, and the receptor-associated protein (RAP) J Histochem Cytochem. 1994;42(4):531–542. doi: 10.1177/42.4.7510321. [DOI] [PubMed] [Google Scholar]
- 19.Lambot N., Lybaert P., Boom A., Delogne-Desnoeck J., Vanbellinghen A.M., Graff G. Evidence for a clathrin-mediated recycling of albumin in human term placenta. Biol Reprod. 2006;75(1):90–97. doi: 10.1095/biolreprod.105.050021. [DOI] [PubMed] [Google Scholar]
- 20.Christensen E.I., Birn H. Megalin and cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3(4):256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- 21.Verroust P.J., Christensen E.I. Megalin and cubilin – the story of two multipurpose receptors unfolds. Nephrol Dial Transplant. 2002;17(11):1867–1871. doi: 10.1093/ndt/17.11.1867. [DOI] [PubMed] [Google Scholar]
- 22.Christensen E.I., Birn H., Verroust P., Moestrup S.K. Membrane receptors for endocytosis in the renal proximal tubule. Int Rev Cytol. 1998;180:237–284. doi: 10.1016/s0074-7696(08)61772-6. [DOI] [PubMed] [Google Scholar]
- 23.Kozyraki R., Fyfe J., Verroust P.J., Jacobsen C., Dautry-Varsat A., Gburek J. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc Natl Acad Sci U S A. 2001;98(22):12491–12496. doi: 10.1073/pnas.211291398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lloyd J.B. Cell physiology of the rat visceral yolk sac: a study of pinocytosis and lysosome function. Teratology. 1990;41(4):383–393. doi: 10.1002/tera.1420410404. [DOI] [PubMed] [Google Scholar]
- 25.Cindrova-Davies T., Yung H.W., Johns J., Spasic-Boskovic O., Korolchuk S., Jauniaux E. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am J Pathol. 2007;171(4):1168–1179. doi: 10.2353/ajpath.2007.070528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones C.J.P., Jauniaux E. Ultrastructure of the materno-embryonic interface in the first trimester of pregnancy. Micron. 1995;26:145–173. doi: 10.1016/0968-4328(95)00002-l. [DOI] [PubMed] [Google Scholar]
- 27.Lundgren S., Carling T., Hjalm G., Juhlin C., Rastad J., Pihlgren U. Tissue distribution of human gp330/megalin, a putative Ca(2+)-sensing protein. J Histochem Cytochem. 1997;45(3):383–392. doi: 10.1177/002215549704500306. [DOI] [PubMed] [Google Scholar]
- 28.Kozyraki R., Fyfe J., Kristiansen M., Gerdes C., Jacobsen C., Cui S. The intrinsic factor-vitamin B12 receptor, cubilin, is a high-affinity apolipoprotein A-I receptor facilitating endocytosis of high-density lipoprotein. Nat Med. 1999;5(6):656–661. doi: 10.1038/9504. [DOI] [PubMed] [Google Scholar]
- 29.Assemat E., Vinot S., Gofflot F., Linsel-Nitschke P., Illien F., Chatelet F. Expression and role of cubilin in the internalization of nutrients during the peri-implantation development of the rodent embryo. Biol Reprod. 2005;72(5):1079–1086. doi: 10.1095/biolreprod.104.036913. [DOI] [PubMed] [Google Scholar]
- 30.Smith B.T., Mussell J.C., Fleming P.A., Barth J.L., Spyropoulos D.D., Cooley M.A. Targeted disruption of cubilin reveals essential developmental roles in the structure and function of endoderm and in somite formation. BMC Dev Biol. 2006;6:30. doi: 10.1186/1471-213X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyne K.L., Woollett L.A. Transport of maternal LDL and HDL to the fetal membranes and placenta of the Golden Syrian hamster is mediated by receptor-dependent and receptor-independent processes. J Lipid Res. 1998;39(3):518–530. [PubMed] [Google Scholar]