Abstract
During general anesthesia positive pressure mechanical ventilation (MV) profoundly affects intrathoracic pressure and venous return, thus soliciting cardiopulmonary reflexes and modifying stroke volume. As a consequence heart period, approximated as the temporal distance between two consecutive R peaks on the ECG (RR), and systolic arterial pressure (SAP) variability series are usually highly correlated at the MV frequency (MVF) and this significant correlation is commonly taken as an indication of an active baroreflex. In this study the involvement of baroreflex was tested according to a time-domain linear Granger causality approach accounting explicitly for MV in two experimental protocols. In the first protocol volatile (VA) or intravenous (IA) anesthetic was administered in humans during pressure controlled MV (PCMV). In the second protocol IA was administered in pigs during PCMV or pressure support MV (PSMV). Causality analysis was contrasted with RR-SAP squared coherence. Significant coherence values at MVF were always found in both protocols. On the contrary, a significant causal link from SAP to RR was less frequently found in humans independently of the anesthesiological strategy and in animals during PCMV. PSMV was superior to PCMV in animals because it was able to better preserve a link from SAP to RR. During general anesthesia the involvement of baroreflex in governing RR-SAP variability interactions is largely overestimated by RR-SAP squared coherence and causality analysis can be exploited to rank anesthesiological strategies and MV modes according to the ability of preserving a working baroreflex.
Keywords: Baroreflex, Coherence analysis, Cardiovascular control, General anesthesia, Granger causality, Heart rate variability
1. Introduction
Baroreflex control of heart rate is an important short-term neural reflex aiming at guaranteeing the homeostasis of the organism through continuous adjustments of heart rate in response to arterial pressure changes (Smyth et al., 1969). Baroreflex sensitivity is usually depressed during general anesthesia. In particular, both volatile and intravenous anesthetics have been demonstrated to depress cardiac baroreflex sensitivity in humans (Tanaka and Nishikawa, 1999; Sato et al., 2005) and animals (Palmisano et al., 1991; Akine et al., 2001). Those studies evaluated the baroreflex sensitivity by administering a vasoactive agent capable to increase or decrease systolic arterial pressure (SAP) and by observing the magnitude of the evoked heart period changes. It was proposed that baroreflex sensitivity could be estimated without perturbing cardiovascular regulation from the analysis of the spontaneous beat-to-beat variability of heart period, approximated as the temporal distance between two consecutive R peaks on the ECG (RR), and SAP (Laude et al., 2004). The assessment of the “spontaneous” baroreflex sensitivity postulates a significant correlation between RR and SAP variabilities. Therefore, during general anesthesia it is common practice to check the significance of RR-SAP squared coherence at the rate of the mechanical ventilator (Beda et al, 2012) before estimating baroreflex sensitivity based on spectral or cross-spectral techniques (De Boer et al., 1985; Pagani et al., 1988) or to assess the significance of the RR-SAP correlation coefficient (Sato et al., 2005) when spontaneous baroreflex sequence method is exploited (Bertinieri et al., 1985).
Nevertheless, during general anesthesia positive pressure mechanical ventilation (MV) profoundly affects intrathoracic pressure and venous return and stimulates cardiopulmonary reflexes, thus influencing directly both SAP and RR variabilities (Beda et al., 2011). As a consequence RR and SAP series might be significantly correlated at the MV frequency (MVF) without any involvement of the baroreflex.
We hypothesize that causality analysis, specifically accounting for MV, provides different information about the involvement of the baroreflex during general anesthesia compared to squared coherence function and could be fruitfully exploited to assess the performance of anesthesiological treatments and MV modes in preserving a more physiological cardiovascular control.
The aim of this work is to compare a traditional tool for the assessment of the degree of linear association between RR and SAP (i.e. squared coherence) with a model-based linear approach for the estimation of Granger causality in the time domain. While coherence analysis does not take explicitly into account the temporal direction of the interactions, causality analysis assesses the statistical dependence only from SAP to RR and accounts for the confounding influences of MV on both RR and SAP variabilities. Comparison was carried out over two experimental protocols performed during general anesthesia. The fist protocol compares in humans under pressure controlled MV (PCMV) two different anesthesiological strategies involving the utilization of different anesthetics: volatile anesthetic (VA) versus intravenous anesthetic (IA). The second protocol compares two different MV modes: PCMV versus pressure support MV (PSMV) in animals under IA administration.
2. Materials and methods
2.1. General anesthesia in humans
Data belong to a database recorded during the NeuroMorfeo trial (Citerio et al., 2009, 2012) designed to compare VA and IA strategies under PCMV during neurosurgical procedures for elective craniotomy. The details of the experimental protocol are reported elsewhere (Citerio et al., 2009). Briefly, thirty-seven subjects (aged from 18 to 75 years) gave their written informed consent and were scheduled for craniotomy for supratentorial lesion. All subjects did not exhibit signs of intracranial hypertension, were in good physical state (ASA I-III, Glasgow Coma Scale equal to 15) and were randomly assigned to one of two anesthesiological strategies. Anesthesia was induced with propofol, a sedative-hypnotic agent, and remifentanil, as analgesic. After intubation of the trachea, patients underwent PCMV with an inspired mixture of air and oxygen (2:1) using a closed breathing system (fresh gas flow of 0.75 l min− 1 oxygen and 1.5 l min− 1 air during anesthesia) adjusted to achieve an end-tidal carbon dioxide of 30–35 mmHg. Ventilation was administered according to PCMV mode. The mean MVF was 0.24 and 0.25 Hz under administration of VA and IA respectively.
Anesthesia was maintained according to two anesthesiological strategies involving the administration of VA or IA. In eighteen subjects anesthesia was maintained through a VA strategy based on the administration of sevoflurane plus remifentanil, while in nineteen subjects it was maintained via IA strategy based on the administration of propofol plus remifentanil. The sevoflurane was administered in a 0.75 to 1.25 MAC range, while propofol was administered with continuous infusion at 10 mg Kg− 1 h− 1 for the first 10 minutes, then reduced to 8 mg Kg− 1 h− 1 for the next 10 minutes and reduced to 6 mg Kg− 1 h− 1 thereafter. The range of administration of remifentanil was from 0.05 to 0.1 μg Kg− 1 min− 1.
The dosage and the administration of drugs were strictly imposed during surgery for both groups. The protocol adhered to the principles of the Declaration of Helsinki All experimental procedures were approved by the Ethical Committee of the San Gerardo Hospital, Monza, Italy.
ECG (lead II) and invasive arterial pressure were continuously monitored and recorded one hour after the craniotomy event. Signals were sampled at 250 Hz and acquired directly through the patient monitor.
2.2. General anesthesia in animals
This protocol was originally designed to monitor respiratory sinus arrhythmia under different positive pressure MV modes (Beda et al, 2012). A detailed description of the experimental protocol can be found elsewhere (Beda et al., 2012). Briefly, eight healthy female juvenile pigs (32–42 kg) were anesthetized by administering propofol plus sufentanil, as analgesic. Animals were tracheally intubated with a cuffed tube (8 mm inner diameter) and instrumented with a catheter in the right femoral artery. Anesthesia was maintained through IA administration based on the continuous infusion of propofol (2–7 mg Kg−1 h−1) and sufentanil (0.3–1.5 μg Kg−1 h−1). MV was performed according to PCMV or PSMV modes. Only during PCMV muscle paralysis was achieved by infusion of atracurium (1 mg Kg−1). During PCMV the respiratory cycle was initiated by the ventilator at a fixed rate, while during PSMV it was triggered by the animal when his initial spontaneous inspiratory airflow exceeded a predefined threshold (5–7 l min−1). During PSMV the mean respiratory rate was 0.22 Hz. The MVF during PCMV was set in each animal according to the spontaneous respiratory rate observed during PSMV. In both modes, a positive driving pressure was delivered by the ventilator until a cycling-off condition occurred. More specifically, the positive driving pressure was terminated after a fixed time in the case of PCMV, while in the case of PSMV the cycling-off condition was reached when the airflow went below a given percentage of the peak inspiratory airflow (15–25%). These cycling off conditions were manually adjusted to achieve an inspiration/expiration ratio of 0.3. The inspiratory driving pressure was tuned cycle-by-cycle by an automatic control system (Beda et al., 2010) to achieve a target mean tidal volume of 12 ml kg−1 in all modes. The inspiration was followed by passive expiration. The animals underwent 30 minutes of each MV modes in a randomized order. When randomization procedure imposed the completion of the PCMV session before the PSMV one, a brief session of PSMV was carried out to find out the spontaneous respiratory rate of the animal. Throughout the experimental protocol, anesthetic agents were administered at constant infusion rates, the inspired fraction of O2 was 0.4 and the positive end-expiratory pressure was 5 cmH2O. The protocol adhered to the principles of the Guide for Care and Use of Vertebrate Animals in Research and Training. All experimental procedures were approved by the Animal Care Committee of the Faculty of Medicine Carl Gustav Carus, Dresden University of Technology, and by the Government of the State of Saxony.
One non-standard ECG lead and arterial blood pressure from the femoral artery were acquired synchronously during the last 20 minutes of each MV mode with sampling frequency of 2 kHz. Airflow was acquired continuously from the ventilator. The respiratory volume signal was obtained by integrating in time the airflow signal.
2.3. Data processing
After locating the R apex on the ECG using parabolic interpolation, RR was computed. SAP(i), where i is the cardiac beat counter, was assessed as the maximum arterial pressure inside RR(i). In the experimental protocol involving animals the respiratory volume signal was sampled in correspondence to the first R apex delimiting RR(i). This measure was indicated as RESP(i) and expressed in liters (l). In the experimental protocol involving humans RESP(i) was derived from respiratory–related ECG amplitude changes and it was expressed in arbitrary units (a.u.). RR(i), SAP(i) and RESP(i) measures were performed on a beat-to-beat basis, thus obtaining RR = {RR(i), i = 1,…,N}, SAP = {SAP(i), i = 1,…,N} and RESP = {RESP(i), i = 1,…,N} where N is the series length. N ranged from 200 to 250 samples. We selected sequences without evident artifacts and non stationarities. RR and SAP mean (μRR and μSAP) and variance (σ2RR and σ2SAP) were evaluated and expressed in ms, mmHg, ms2 and mmHg2 respectively. Data sequences were linearly detrended before any successive analysis.
2.4. Granger-causality test
Given the set of series Ώ = {SAP,RR,RESP}, SAP is said to Granger-cause RR in Ώ (i.e. SAP → RR) if SAP can provide an unique information about the future evolution of RR that cannot be derived from any of the series present in Ώ after excluding SAP (Granger, 1969). In other words, we say that SAP Granger-causes RR if RR can be better predicted in Ώ than in Ώ-{SAP} = {RR,RESP}. The interactions among the series in Ώ were described according to a linear time invariant parametric model (Lutkepohl, 2005). More specifically, the current sample of RR (i.e. RR(i)) depended on its own past values, i.e. the autoregressive (AR) part, plus the contributions of the present and past values of the remaining two signals (i.e. SAP and RESP). Both SAP and RESP were considered as exogenous (X) signals for RR, thus modeling a double X (XX) influence on RR. The number of past values of SAP, RR, and RESP was equal to q and q was optimized according to the Akaike figure of merit for multivariate processes (Akaike, 1974). We checked that the mean square prediction error of the ARXX model on RR in Ώ was significantly smaller than that of the ARX model on RR in Ώ-{SAP} with RESP as the unique X signal, thus indicating that the goodness of fit of the ARXX model was larger than that of the ARX one. The significance of the predictability improvement was tested according to the F statistic with p < 0.01 (Porta et al., 2012). The coefficients of the ARXX and ARX models were estimated using traditional least-squares approach and Cholesky decomposition method (Baselli et al., 1997). The series were first demeaned and, then, divided by the standard deviation, before performing causality test.
2.5. Squared coherence function
Squared RR-SAP coherence function (K2RR-SAP) was exploited to measure the degree of linear association between RR and SAP as a function of the frequency (K2RR-SAP(f)). The squared RR-SAP coherence function was assessed as the ratio of the square RR-SAP cross-spectrum modulus to the product of the power spectra. K2RR-SAP ranges from 0 to 1, indicating that SAP and RR are completely unrelated and perfectly linearly associated respectively. A parametric approach exploiting the bivariate AR model was chosen to estimate cross-spectrum and power spectra (Baselli et al., 1997). The model order was fixed to 10 and the coefficients of the model were identified via least squares approach (Baselli et al., 1997). K2RR-SAP(f) was sampled in correspondence of the MVF as detected in correspondence of the dominant peak of the power spectrum of the RESP series. The significance of K2RR-SAP(MVF) was assessed using the technique of surrogate data. Surrogate pairs were constructed simply by matching up the original RR series of a subject (or animal) with the original SAP series taken from another one. Only the matching of the RR series with the SAP one taken from the same subject (or animal) was prevented in the construction of the surrogate set; thus the number of surrogate pairs is equal to the number of subjects (or animals) minus 1. K2RR-SAP(MVF) was assessed from all surrogate pairs in each protocol. The 95th percentile of K2RR-SAP(MVF) distribution derived from surrogate was taken as the threshold for significance. If K2RR-SAP(MVF) computed over the RR and SAP relevant to the same subject (or animal) was larger than the threshold, the strength of the RR-SAP relation was deemed as significant.
2.6. Statistical analysis
The unpaired t-test, or Mann–Whitney rank sum test when appropriate, was applied to check whether time domain parameters depended on the anesthesiological strategy in humans. The paired t-test, or Wilcoxon signed rank test when appropriate, was applied to check whether time domain parameters depended on the MV strategy in animals. The χ2 test was performed to check the difference between the percentage of the subjects (or animals) exhibiting a significant K2RR-SAP(MVF) and that of the subjects (or animals) exhibiting a significant causal relation from SAP to RR. Values were reported as mean ± standard deviation. A p < 0.05 was always considered as significant.
3. Results
Table 1 reports time domain parameters obtained from humans in VA and IA groups. The RR mean, μRR, and the RR variance, σ2RR, were similar in VA and IA groups. The SAP mean, μSAP, and the SAP variance, σ2SAP, were significantly larger during IA.
Table 1.
Time domain parameters during VA and IA in humans.
| VA | IA | |
|---|---|---|
| μRR [ms] | 958.19 ± 161.13 | 925.00 ± 147.40 |
| σ2RR [ms2] | 582.59 ± 981.32 | 352.47 ± 744.93 |
| μSAP [mmHg] | 107.13 ± 9.24 | 117.22 ± 16.22* |
| σ2SAP [mmHg2] | 11.40 ± 15.47 | 15.58 ± 14.81* |
VA = volatile anesthetic; IA = intravenous anesthetic. Values are expressed as mean ± standard deviation. The symbol * indicates a significant difference between VA and IA with p < 0.05.
Table 2 reports time domain parameters obtained from pigs in PCMV and PSMV groups. While μRR, μSAP and σ2SAP were similar in PCMV and PSMV groups, σ2RR was significantly larger during PSMV.
Table 2.
Time domain parameters during PCMV and PSMV in pigs.
| PCMV | PSMV | |
|---|---|---|
| μRR [ms] | 652.26 ± 44.56 | 651.30 ± 76.20 |
| σ2RR [ms2] | 48.09 ± 47.90 | 662.53 ± 936.40* |
| μSAP [mmHg] | 121.92 ± 15.75 | 120.21 ± 15.66 |
| σ2SAP [mmHg2] | 3.69 ± 2.71 | 5.76 ± 2.79 |
PCMV = pressure controlled mechanical ventilation; PSMV = pressure support mechanical ventilation. Values are expressed as mean ± standard deviation. The symbol * indicates a significant difference between PCMV and PSMV with p < 0.05.
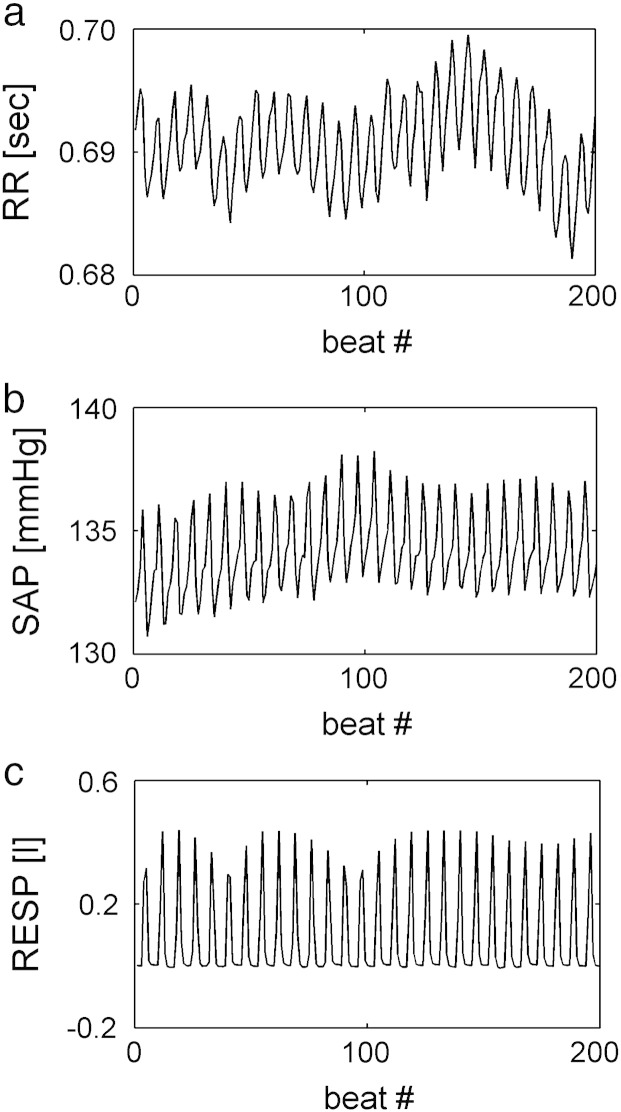
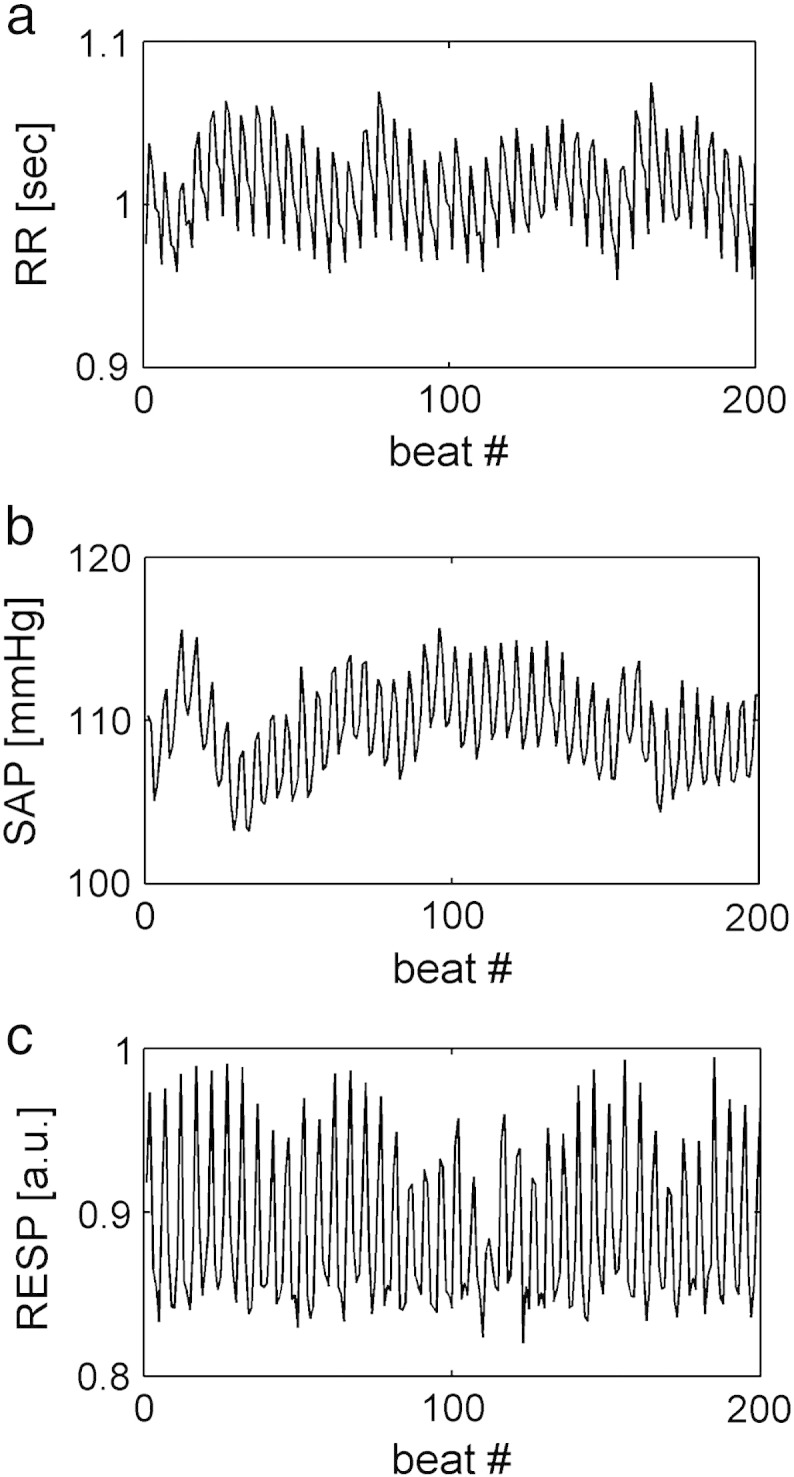
Two examples of RR, SAP and RESP series with no significant causal relation from SAP to RR are shown in Figs. 1a,b,c and 2a,b,c. The series depicted in Fig. 1 were recorded from a human subject, while those shown in Fig. 2 were acquired from an animal. In both figures the time series were obtained during PCMV under IA. In both figures RR and SAP series appear to be dominated by oscillations at the MVF. In these examples causality test suggests that the large fast fluctuations of the RR series do not occur in response to SAP changes, thus indicating a negligible involvement of baroreflex. Despite the virtually absent causal link from SAP to RR, K2RR-SAP(f) is close to 1 at the MVF and its harmonics (Fig. 3a,b) as a likely effect of the common action of the MV on both series.
Fig. 1.
RR (a), SAP (b) and RESP (c) series in a human subject with no significant causal relation from SAP to RR during PCMV under IA.
Fig. 2.
RR (a), SAP (b) and RESP (c) series in a pig with no significant causal relation from SAP to RR during PCMV under IA.
Fig. 3.
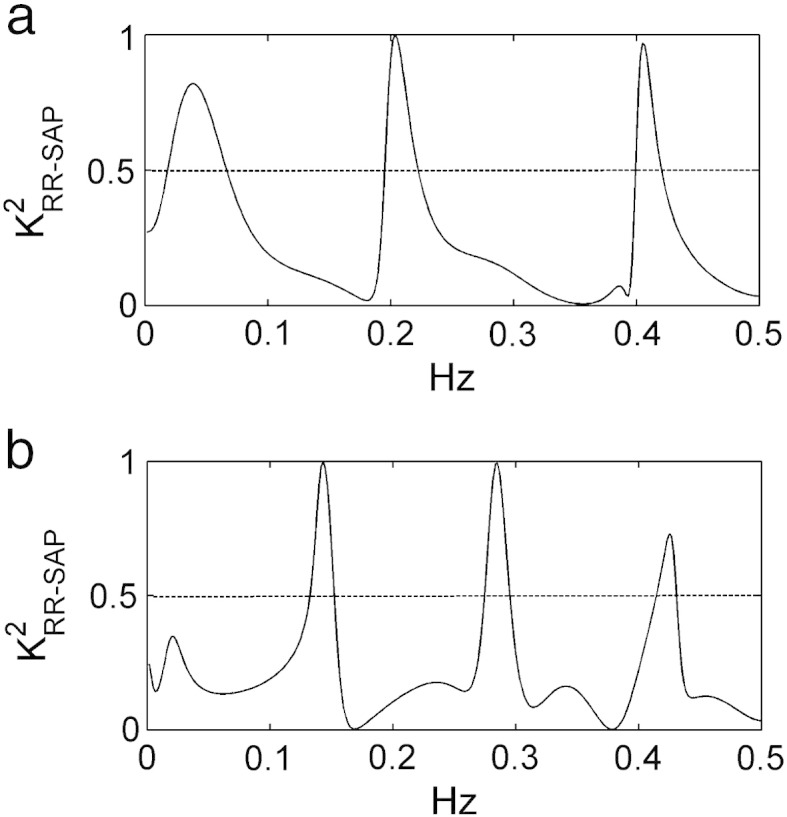
Squared coherence function, K2RR-SAP(f), computed in a human subject (a) and a pig (b) with no significant causal relation from SAP to RR. RR and SAP series are shown in Figs. 1a,b and 2a,b respectively. Despite the virtually absent causal link from SAP to RR, K2RR-SAP(f) exhibits values close to 1 in correspondence of the MVF and its harmonics, as a likely effect of the common perturbation on both series due to MV. The conventional threshold of significance for K2RR-SAP(f) (i.e. 0.5) is shown as a dotted line.
Tables 3 and 4 show indexes relevant to RR-SAP variability interactions in humans and animals respectively. Results are reported in terms of percentage of subjects showing a significant value of K2RR-SAP(f) at the MVF (i.e. K2RR-SAP(MVF)) and a significant causal relation from SAP to RR (i.e.. SAP → RR). Significant K2RR-SAP(MVF) was found in 100% of humans during PCMV under both VA and IA strategies (Tab.3) and in 100% of animals under IA during both PCMV and PSMV modes (Tab.4). A significant SAP → RR was detected in 39% and 63% of humans during PCMV under VA and IA respectively (Tab.3). The percentage of subjects with significant K2RR-SAP(MVF) was significantly higher than that with SAP → RR during PCMV under both VA and IA. A significant SAP → RR was detected in 62% and 100% of animals under IA during PCMV and PSMV respectively (Tab.4). Under IA during PCMV the percentage of animals with a significant SAP → RR was significantly smaller than that with a significant K2RR-SAP(MVF), while during PSMV coherence and causality analyses provided the same percentage (i.e. 100%).
Table 3.
Percentage of subjects showing significant RR-SAP interactions during VA and IA.
| VA | IA | |
|---|---|---|
| K2RR-SAP(MVF) | 100% | 100% |
| SAP → RR | 39%# | 63%# |
VA = volatile anesthetic; IA = intravenous anesthetic; MVF = mechanical ventilation frequency; K2RR-SAP(MVF) = squared coherence at MVF. The symbol # indicates a significant difference between coherence and causality analyses within the same experimental condition with p < 0.05.
Table 4.
Percentage of pigs showing significant RR-SAP interactions during PCMV and PSMV.
| PCMV | PSMV | |
|---|---|---|
| K2RR-SAP(MVF) | 100% | 100% |
| SAP → RR | 62%# | 100% |
PCMV = pressure controlled mechanical ventilation; PSMV = pressure support mechanical ventilation; MVF = mechanical ventilation frequency; K2RR-SAP(MVF) = squared coherence at MVF. The symbol # indicates a significant difference between coherence and causality analyses with the same experimental condition with p < 0.05.
4. Discussion
The main findings of this study can be summarized as follows: 1) RR-SAP squared coherence overestimates the role played by baroreflex in determining the association between RR and SAP during general anesthesia; 2) causality analysis accounting for MV is able to detect a causal link from SAP to RR during general anesthesia, thus suggesting a working baroreflex; 3) in humans accounting for MV decreases the probability of finding a significant causal relation from SAP to RR compared to a causality analysis based solely on RR and SAP variabilities, especially during PCMV under VA; 4) during PCMV mechanisms other than baroreflex are responsible for the high degree of association between RR and SAP at the MVF; 5) PSMV can preserve baroreflex control better than PCMV.
Under spontaneous closed loop conditions and in presence of small SAP changes it might happen that baroreflex contributes negligibly to the overall amount of RR variations especially in presence of a depressed baroreflex sensitivity. In this situation a sound procedure to assess baroreflex sensitivity based on spontaneous RR and SAP variabilities should advise that baroreflex sensitivity cannot be safely inferred. This warning is currently provided by the coherence function. If the coherence function is smaller than a threshold, RR and SAP series are irrelevantly linked and, thus, the estimate of the baroreflex sensitivity might be unreliable (De Boer et al., 1985). Usually the threshold is set at an arbitrarily, even though large, value (i.e. 0.5) (De Boer et al., 1985), or calculated based on the distribution of the coherence values derived analytically under the null hypothesis of uncoupling between two series with well-known statistical properties (Barres et al., 2004) or computed based on the construction of a distribution of coherence values derived empirically according to a set of uncoupled iso-distributed iso-spectral surrogates (Porta et al., 2002; Faes et al., 2004). However, a significant association between RR and SAP series might be solely due to Starling's law and diastolic runoff causing changes of SAP in response to modifications of RR (Baselli et al., 1994; Porta et al., 2002). Therefore, a coherence function value overcoming the threshold at a given frequency cannot be taken as a reliable proof of baroreflex involvement at that frequency. Conversely, only a causality test checking a specific temporal direction of the interactions (i.e. from SAP to RR) can provide a convincing evidence of the baroreflex involvement.
This study compares results of squared coherence analysis between RR and SAP variabilities with those provided by a causality test assessing the presence of a significant causal relation from SAP to RR in humans and animals during general anesthesia. We found that, regardless of the anesthetic administration mode and MV technique, in both humans and animals, RR and SAP variabilities were significantly correlated at the MVF. This finding is not surprising. Indeed, during general anesthesia under MV the amplitude of the RR and SAP oscillations at the MVF is not negligible (Beda et al., 2011). SAP changes at the MVF are the result of the modulation of the venous return, cardiac preload and afterload and stroke volume due to changes of intrathoracic pressure (Innes et al., 1993; Baselli et al., 1994) and of the link from RR to SAP due to diastolic runoff and Starling law (Baselli et al., 1994). RR changes at the MVF can be the result of the stimulation of cardiopulmonary receptors (Hakumaki, 1987), of the mechanical stimulation of sinus node due to changes of the intrathoracic pressure (Bernardi et al., 1989), of the coupling between respiratory centers and vagal outflow (Porta et al., 2000; Eckberg, 2003) and of baroreflex (De Boer et al., 1987). These mechanisms contribute to the raise of the values of RR-SAP squared coherence at the MVF above the threshold of significance observed in all cases in both protocols.
Conversely, causality analysis was able to recognize that RR variability was driven by SAP changes in a significantly smaller percentage of humans during PCMV under both IA and VA. The same observation held in animals under IA strategy during PCMV. Therefore, we conclude that during PCMV squared coherence overestimates the role of baroreflex in governing RR-SAP variability interactions and mechanisms other than baroreflex concur to determine the large RR-SAP correlation at the MVF. As a consequence of this finding we strongly advise that baroreflex sensitivity estimated from spontaneous RR and SAP variabilities during PCMV might be unreliable even in presence of a high value of squared coherence function at the MVF. In addition, this study supports the more physiological action of the PSMV mode compared to the PCMV one. Indeed, in animals under IA during PSMV the RR-SAP link was, at least partially, due to the solicitation of baroreflex. This finding is not in contrast with the possible action of mechanisms, different from baroreflex, capable of producing RR changes independent of SAP variations suggested in Beda et al. (2012). These mechanisms, working specifically at the MVF, might coexist with an active baroreflex working over different temporal scales (e.g. in the low frequency band), thus contributing all together to the overall amount of RR variability. A Granger causality analysis performed in the frequency domain (Baccalà and Sameshima, 2001; Kaminski et al., 2001; Porta et al., 2002) might be able to clarify this issue.
A previous study suggested that a significant causal relation from SAP to RR could be found in the majority of subjects during general anesthesia, thus suggesting that baroreflex was still working (Bassani et al., 2012) even though with a lower baroreflex sensitivity (Tanaka and Nishikawa, 1999; Sato et al., 2005). This finding was based on a Granger causality bivariate analysis performed solely over SAP and RR series. Since it was demonstrated that respiration is a latent confounder for RR-SAP causal interactions (i.e. disregarding respiration might introduce spurious causal links between RR and SAP series) (Porta et al., 2012), one of the major aim of this study was to verify if our previous conclusions about the relevance of the causal relation from SAP to RR (Bassani et al., 2012) were still valid even when accounting for MV. The present study confirms that causality from SAP to RR was still detectable even when the latent confounder of MV was accounted for. However, the percentage of subjects exhibiting causal relations from SAP to RR was decreased compared to our previous study (Bassani et al., 2012), especially during PCMV under VA, thus confirming the importance of accounting for MV when assessing RR-SAP causal relations.
5. Conclusions
This study exploits a time-domain approach to causality analysis, explicitly accounting for MV, as a tool to assess the performance of anesthesiological treatments and MV strategies. The analysis suggests a novel criterion for ranking anesthesiological treatments and MV strategies according to their ability to preserve the involvement of baroreflex in regulating RR-SAP variability interactions. According to this criterion PSMV ranked better than PCMV. In addition, the proposed analysis allows a more precise definition of the scope of applicability of the methods assessing spontaneous baroreflex sensitivity based on spectral and cross-spectral analyses during general anesthesia. Indeed, they should be applied only in those anesthesiological treatments and MV strategies capable to guarantee a significant causal relation from SAP to RR in a large percentage of cases, such as during PSMV, or, independently of the anesthesiological treatment and MV strategy, on an individual basis over those subjects preserving causality from SAP to RR. Future studies should compare different modalities utilized to acquire respiratory signal with the specific aim to understand if they lead to different conclusions in terms of causal relations among cardiovascular variables.
Acknowledgements
The NeuroMorfeo trial “Anaesthesiological strategies in elective craniotomy” (EudraCT 2007-005279-32) was fully financed by AIFA (FARM6FKJKK), the animal experiments were fully financed by the MedDrive Program of the Faculty of Medicine of the Technical University Dresden, and the Telethon Grant GGP09247 to A. Porta partially supported the study.
References
- Akaike H. A new look at the statistical novel identification. IEEE Trans. Autom. Control. 1974;19:716–723. [Google Scholar]
- Akine A., Suzuka H., Hayashida Y., Kato Y. Effects of ketamine and propofol on autonomic cardiovascular function in chronically instrumented rats. Auton. Neurosci-Basic Clin. 2001;87:201–208. doi: 10.1016/S1566-0702(00)00271-X. [DOI] [PubMed] [Google Scholar]
- Baccalà L., Sameshima K. Partial directed coherence: a new concept in neural structure determination. Biol. Cybern. 2001;84:463–474. doi: 10.1007/PL00007990. [DOI] [PubMed] [Google Scholar]
- Barres C., Cheng Y., Julien C. Steady-state and dynamic responses of renal sympathetic nerve activity to air-jet stress in sinoaortic denervated rats. Hypertension. 2004;43:629–635. doi: 10.1161/01.HYP.0000115384.01463.61. [DOI] [PubMed] [Google Scholar]
- Baselli G., Cerutti S., Badilini F., Biancardi L., Porta A., Pagani M., Lombardi F., Rimoldi O., Furlan R., Malliani A. Model for the assessment of heart period and arterial pressure variability interactions and respiratory influences. Med. Biol. Eng. Comput. 1994;32:143–152. doi: 10.1007/BF02518911. [DOI] [PubMed] [Google Scholar]
- Baselli G., Porta A., Rimordi O., Pagani M., Cerutti C. Spectral decomposition in multi-channel recordings based on multivariate parametric identification. IEEE Trans. Biomed. Eng. 1997;44:1092–1101. doi: 10.1109/10.641336. [DOI] [PubMed] [Google Scholar]
- Bassani T., Magagnin V., Guzzetti S., Baselli G., Citerio G., Porta A. Testing the involvement of baroreflex during general anesthesia through Granger causality approach. Comput. Biol. Med. 2012;42:306–312. doi: 10.1016/j.compbiomed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Beda A., Spieth P.M., Handzsuj T., Pelosi P., Carvalho N.C., Koch E., Koch T., de Abreu M.G. A novel adaptive control system for noisy pressure-controlled ventilation: a numerical simulation and bench test study. Intensive Care Med. 2010;36:164–168. doi: 10.1007/s00134-009-1665-3. [DOI] [PubMed] [Google Scholar]
- Beda A., Carvalho N.C., Güldner A., Koch T., Gama de Abreu M. Mechanical ventilation during anaesthesia: challenges and opportunities for investigating the respiration-related cardiovascular oscillations. Biomed. Tech. 2011;56:195–206. doi: 10.1515/BMT.2011.015. [DOI] [PubMed] [Google Scholar]
- Beda A., Guldner A., Simpson D.M., Carvalho N.C., Franke S., Uhlig C., Koch T., Pelosi P., de Abreu M.G. Effects of assisted and variable mechanical ventilation on cardiorespiratory interactions in anesthetized pigs. Physiol. Meas. 2012;33:503–519. doi: 10.1088/0967-3334/33/3/503. [DOI] [PubMed] [Google Scholar]
- Bernardi L., Keller F., Sanders M., Reddy P.S., Griffhth B., Meno F., Pinsky R. Respiratory sinus arrhythmia in the denervated human heart. J. Appl. Physiol. 1989;67:1447–1455. doi: 10.1152/jappl.1989.67.4.1447. [DOI] [PubMed] [Google Scholar]
- Bertinieri G., Di Rienzo M., Cavallazzi A., Ferrari A.U., Pedotti A., Mancia G. A new approach to analysis of the arterial baroreflex. J. Hypertens. 1985;3:S79–S81. [PubMed] [Google Scholar]
- Citerio G., Franzosi M.G., Latini R., Masson S., Barlera S., Guzzetti S., Pesenti A. Anaesthesiological strategies in elective craniotomy: randomized, equivalence, open trial — the NeuroMorpfeo trial. Trials. 2009;10:19. doi: 10.1186/1745-6215-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citerio G., Pesenti A., Latini R., Masson S., Barlera S., Gaspari F., Franzosi M.G. A multicentre, randomised, open-label, controlled trial evaluating equivalence of inhalational and intravenous anaesthesia during elective craniotomy. Eur. J. Anaesthesiol. 2012;29:371–379. doi: 10.1097/EJA.0b013e32835422db. [DOI] [PubMed] [Google Scholar]
- De Boer R.W., Karemaker J.M., Strackee J. Relationships between short-term blood pressure fluctuations and heart rate variability in resting subjects I: a spectral analysis approach. Med. Biol. Eng. Comput. 1985;23:352–358. doi: 10.1007/BF02441589. [DOI] [PubMed] [Google Scholar]
- De Boer R.W., Karemaker J.M., Strackee J. Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. Am. J. Physiol. 1987;253:H680–H689. doi: 10.1152/ajpheart.1987.253.3.H680. [DOI] [PubMed] [Google Scholar]
- Eckberg D.L. The human respiratory gate. J. Physiol. 2003;548:339–352. doi: 10.1113/jphysiol.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faes L., Pinna G.D., Porta A., Maestri R., Nollo G.D. Surrogate data analysis for assessing the significance of the coherence function. IEEE Trans. Biomed. Eng. 2004;51:1156–1166. doi: 10.1109/TBME.2004.827271. [DOI] [PubMed] [Google Scholar]
- Granger C.W.J. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37:424–438. [Google Scholar]
- Hakumaki M.O.K. Seventy years of the Bainbridge reflex. Acta Physiol. Scand. 1987;130:177–185. doi: 10.1111/j.1748-1716.1987.tb08126.x. [DOI] [PubMed] [Google Scholar]
- Innes J., De Cort S., Kox W., Guz A. Within-breath modulation of left ventricular function during normal breathing and positive-pressure ventilation in man. J. Physiol. 1993;460:487–502. doi: 10.1113/jphysiol.1993.sp019483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski M., Ding M., Truccolo W.A., Bressler S. Evaluating causal relations in neural systems: Granger causality, directed transfer function and statistical assessment of significance. Biol. Cybern. 2001;85:145–157. doi: 10.1007/s004220000235. [DOI] [PubMed] [Google Scholar]
- Laude D., Elghozi J.L., Girard A., Bellard E., Bouhaddi M., Castiglioni P., Cerutti C., Cividjian A., Di Rienzo M., Fortrat J.O., Janssen B., Karemaker J.M., Leftheriotis G., Parati G., Persson P.B., Porta A., Quintin L., Regnard J., Rudiger H., Stauss H.M. Comparison of various techniques used to estimate spontaneous baroreflex sensitivity (the EuroBaVar study) Am. J. Physiol. 2004;286:R226–R231. doi: 10.1152/ajpregu.00709.2002. [DOI] [PubMed] [Google Scholar]
- Lutkepohl H. Springer-Verlag; Berlin Heidelberg: 2005. New introduction to multiple time series analysis. [Google Scholar]
- Pagani M., Somers V.K., Furlan R., Dell'Orto S., Conway J., Baselli G., Cerutti S., Sleight P., Malliani A. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension. 1988;12:600–610. doi: 10.1161/01.hyp.12.6.600. [DOI] [PubMed] [Google Scholar]
- Palmisano B.W., Clifford P.S., Hoffmann R.G., Seagard J.L., Coon R.L., Kampine J.P. Depression of baroreflex control of heart rate by halothane in growing piglets. Anestesiology. 1991;75:512–519. doi: 10.1097/00000542-199109000-00019. [DOI] [PubMed] [Google Scholar]
- Porta A., Baselli G., Rimoldi O., Malliani A., Pagani M. Assessing baroreflex gain from spontaneous variability in conscious dogs: role of causality and respiration. Am. J. Physiol. 2000;279:H2558–H2567. doi: 10.1152/ajpheart.2000.279.5.H2558. [DOI] [PubMed] [Google Scholar]
- Porta A., Furlan R., Rimoldi O., Pagani M., Malliani A., van de Borne P. Quantifying the strength of linear causal coupling in closed loop interacting cardiovascular variability series. Biol. Cybern. 2002;86:241–251. doi: 10.1007/s00422-001-0292-z. [DOI] [PubMed] [Google Scholar]
- Porta A., Bassani T., Bari V., Pinna G.D., Maestri R., Guzzetti S. Accounting for respiration is necessary to reliably infer Granger causality from cardiovascular variability series. IEEE Trans. Biomed. Eng. 2012;59:832–841. doi: 10.1109/TBME.2011.2180379. [DOI] [PubMed] [Google Scholar]
- Sato M., Tanaka M., Umehara S., Nishikawa T. Baroreflex control of heart rate during and after propofol infusion in humans. Br. J. Anaesth. 2005;94:577–581. doi: 10.1093/bja/aei092. [DOI] [PubMed] [Google Scholar]
- Smyth H.S., Sleight P., Pickering G.W. Reflex regulation of the arterial pressure during sleep in man. A quantitative method of assessing baroreflex sensitivity. Circ. Res. 1969;24:109–121. doi: 10.1161/01.res.24.1.109. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Nishikawa T. Arterial baroreflex function in humans anaesthetized with sevoflurane. Br J. Anaesth. 1999;82:350–354. doi: 10.1093/bja/82.3.350. [DOI] [PubMed] [Google Scholar]