Abstract
Background
Oxytocin (OT) is a neuropeptide shown to attenuate inflammatory responses in both humans and animals, but the specific mechanism underlying these actions has not yet been identified. Preliminary research in humans suggests that monocytes (MOs) and macrophages (MPs) could be the target of OT’s anti-inflammatory actions. Here we present a series of ex vivo experiments in human MOs and MPs, testing whether OT attenuates these cells’ cytokine responses to a common bacterial product, lipopolysaccharide (LPS).
Methods
MO experiments were conducted using blood samples taken from healthy volunteers after obtaining informed consent. MPs were purchased frozen from a cell supplier. All samples were cultured under standard conditions: for 6 hours at 37°C in a 5% CO2 atmosphere. A number of variables were considered: Volunteer sex, method of MO isolation, LPS concentration, OT concentration, pre-incubation with OT, cytokines measured, and method of cytokine measurement.
Results
Regardless of the specific conditions, no attenuation of LPS-stimulated cytokine production by OT was observed in either MOs or MPs.
Conclusion
OT does not attenuate MO or MP inflammatory cytokine production following LPS stimulation. The previously observed anti-inflammatory properties of OT may be attributable to effects on other classes of immune cells or actions in other lymphoid compartments. Alternatively, OT’s effects on inflammation could be secondary to other neurohormonal changes it elicits.
Keywords: Oxytocin, monocytes, macrophages, lipopolysaccharide, inflammation, humans
Introduction
Oxytocin (OT) is a nonapeptide neurotransmitter and hormone produced and secreted centrally, by the hypothalamus and the posterior pituitary, and also peripherally, by reproductive organs, the thymus, and the adrenal glands [1]. At least three general physiological roles have emerged for OT: It is best characterized for its classical role in regulating parturition and lactation [2], and increasingly recognized as having a role in social interactions and social bonding [2–5]. Recent research has highlighted a third novel role of OT in regulating inflammatory responses. This has been observed across a wide range of inflammatory disease models in animals, including sepsis and infection [6,7], atherosclerosis [8,9], musculocutaneous flap survival [10], organ damage and inflammation [11–14], and burns [15,16]. In human models, OT has been shown to reduce systemic inflammation in a model of sepsis [17]. Given its systemic anti-inflammatory effects, OT is increasingly being considered as a potential therapeutic agent for inflammatory diseases but, as of yet, there is no agreement as to how and where OT is acting.
In humans, the monocyte (MO)/macrophage (MP) cell line has been singled out as a possible mechanistic link, which is especially of therapeutic interest given that MPs and their precursors, MOs, play a key role in initiating and sustaining inflammatory processes that contribute to chronic diseases, including depression, atherosclerosis, neurodegenerative disorders, and some cancers [18,19]. To date, two studies have explored OT’s anti-inflammatory potential in humans and assessed whether any effect could be mediated by OT’s action on MOs and/or MPs. The first study by Szeto, et al. [20] confirmed that OT receptor could be detected on healthy peripheral blood mononucleocytes and THP-1 MOs, isolated from a one-year-old Japanese boy with acute monocytic leukemia [21], and derived MPs, suggesting that OT is in fact capable of directly regulating these cells. THP-1-derived MPs were then activated with lipopolysaccharide (LPS), a molecule found on the surface of Gram-negative bacteria, and co-cultured with 0 to 100 pM OT for 6 hours under standard conditions. A clear attenuation of interleukin-6 (IL-6) secretion by the THP-1 MPs was observed, suggesting that MOs and MPs may be involved in OT’s systemic anti-inflammatory effects. This contrasts with the results of the second study by Clodi, et al. [17], which consisted of a randomized, placebo-controlled cross-over design where 10 healthy young males were given an intravenous bolus of LPS concurrent with administration of OT. Again, OT was shown to attenuate the systemic inflammatory cytokine response. However, when 4 samples of peripheral MOs were pre-incubated ex vivo with 0 to 100 nM for 30 minutes, then activated with LPS and cultured for 2, 4 or 24 hours, no attenuation of tumor necrosis factor-α (TNF-α), MO chemoattractant protein-1 production, or IL-6 was observed. As such, these results suggest that, although OT has systemic anti-inflammatory effects in male humans, MOs do not appear to mediate that effect.
These studies provide conflicting evidence about whether peripheral MOs and MPs are involved in OT’s systemic anti-inflammatory effects in humans. Furthermore, the work to date has been done exclusively in male cells, so it is not known if these results extend to cells isolated from females. Given that there are gender differences with respect to OT activity and function [2,22], it is important to determine whether any effects of OT are observable in cells from both male and female donors. Existing research has also focused on cell-line MPs, which may behave differently from primary MPs from healthy donors. Resolving these issues would have implications for our more general understanding of neuro-immune interactions, and for potential use of OT as an anti-inflammatory therapeutic agent. As such, the first goal of our experiments was to assess the viability of this particular mechanism; to determine whether or not OT clearly regulates inflammatory cytokine production in MOs and MPs taken from a sample of healthy human participants. The second goal of our experiments was to extend the work of Clodi, et al. [17] and Szeto, et al. [20] by assessing OT’s potential anti-inflammatory effect in MOs isolated from females, and in primary MPs from healthy humans. Finally, previous research measured quantified amount of cytokine secreted into the supernatant, but it is possible that intracellular cytokine production can be altered without a corresponding change in cytokine secretion. As such, we also incorporated intracellular staining and flow cytometry into our methods in order to differentiate between cytokine production vs. cytokine secretion. If OT does clearly affect MO and MP inflammatory cytokine secretion, we would expect to see a clear dose-response curve showing attenuation of cytokine production and/or secretion by increasing concentration of OT.
Methods
Cells
For experiments with MOs, whole blood was collected from healthy male and female volunteers, from 20 to 30 years of age, who provided informed consent and were compensated $10 for participating. Most OT-inflammation research focuses on male animals or human participants in order to avoid complications arising from hormone changes that occur during the menstrual cycle. However, if OT is to be used therapeutically, as an anti-inflammatory, we must know if its effects are gender dependent. As such, both sexes were included. Blood was taken via antecubetal blood draw by a trained phlebotomist into, unless otherwise stated, 5-mL sodium heparin Vacutainer tubes (BD Biosciences, Mississuaga, ON). All whole blood samples were diluted 10:1 with saline prior to culturing. For MP experiments, we purchased MO-derived, in vitro matured MPs from a cell supplier (AllCells, Emeryville, CA). All MPs used here were matured from a single donor’s peripheral MOs. Following the supplier’s instructions, cells were thawed, checked for viability, and stabilized for 24 to 72 hours prior to use in the cultures. All procedures and methods were approved by the University of British Columbia Research Ethics Board.
Culturing
Unless otherwise indicated, all culturing was done using flat-bottomed tissue culture plates with incubations lasting 6 hours at 37°C in 5% CO2, similar conditions for which positive results were reported by Szeto, et al. [20]. For experiments with MOs, 1600 µL of saline-diluted whole blood was added to each well, corresponding to roughly 6.2×105 MOs. For MP experiments, a cell counter was used to titrate suspensions to 5.0×105 cells in 800 µL of R10 medium per well. A “standard range” of OT concentrations, 10 to 1000 pM, was selected to encompass normal physiological levels [23,24]. However, some experiments included what would be considered supraphysiological levels (1000 to 10 000 pM OT). As such, OT concentrations in our experiments encompassed the whole range used in both the Szeto, et al. [20] and Clodi, et al. [17] analyses. Unless otherwise stated, cell cultures were stimulated with 50 ng/mL LPS.
Cytokine Measurement
Three methods were used to quantify cytokine synthesis and release: Intracellular cytokine staining (ICS) using fluorescence-activated cell sorting (FACS; FACSCaliber, BD Biosciences, Mississauga, ON), enzyme-linked immunosorbent assay (ELISA; R& D Systems, Minneapolis, MN), and electrochemiluminescence (ECL) on a Meso Scale Discovery multiplexing instrument (SECTOR Imager 2400, Gaithersburg, MD).
ICS was used to quantify intracellular cytokine production in response to LPS stimulation [see 25]. In these experiments, whole blood was co-cultured with LPS and OT at varying doses under varying conditions (see Table 1) for 6 hours. Each well also included a 0.1% Brefeldin A solution (BD Biosciences), a Golgi plug that prevents cytokine secretion from the cell. Following incubation, cultures were stored at 4°C over night and stained for ICS analysis the next day. All staining incubations were done at room temperature in the dark. First, red blood cells were lysed (Pharmlyse, BD Biosciences) and removed by washing, followed by re-suspension in staining buffer (1% fetal bovine serum in phosphate-buffered saline, BD BioSciences). Fc receptors were blocked by incubating for 15 minutes with 10% normal human serum. Cells were stained with CD-14 (10% APC-conjugated CD-14 Ab, BD BioSciences) during a 20 minute incubation, followed by a wash with staining buffer. Cells were fixed and permeabilized using a Cytofix/Cytoperm Plus Fixation/Permeabilization Kit (BD Biosciences) as per manufacturer’s instructions. Cells were then stained for intracellular IL-6 (4% PE-conjugated IL-6 antibody, BD BioSciences) during a 20 minute incubation. The samples were washed again and re-suspended in staining buffer prior to analysis on a FACSCaliber (BD Biosciences, Mississauga, ON). Data were analyzed with FlowJo (Tree Star Inc., Ashland, OR). Quadrants were set using data from concurrently run negative control samples (un-stimulated and non-stained samples), and positive and negative beads stained with appropriate antibodies.
Table 1.
Summary of experimental protocols tested. (PBMC = peripheral blood mononuclear cell)
| Experiment (Figure #) |
Participants | Cell type | LPS dose (ng/mL) |
OT doses (pM) |
Pre-incubation with OT (min) |
Cytokine Measurement |
|---|---|---|---|---|---|---|
| 1 (1) | Female | Monocytes (whole blood) | 50 | 0, 1, 10, 100, 1000 | 0 | FACS (CD14+ /IL-6 +) |
| Male | Monocytes (whole blood) | 50 | 0, 1, 10, 100, 1000 | 0 | FACS (CD14+ /IL-6+) | |
| 2 (1) | Female | Monocytes (whole blood) | 50 | 0, 1, 10, 100, 1000 | 0 | FACS (CD14+ /IL-6 +) |
| Monocytes (whole blood) | 50 | 0, 1, 10, 100, 1000 | 30 | FACS (CD14+ /IL-6 +) | ||
| Female | Monocytes (whole blood) | 50 | 0, 1, 10, 100, 1000 | 30 | FACS (CD14+ /IL-6 +) | |
| Monocytes (whole blood) | 50 | 0, 1, 10, 100, 1000 | 60 | FACS (CD14+ /IL-6 +) | ||
| 3 (1) | Male | Isolated PBMCs | 50 | 0, 1, 10, 100, 1000 | 30 | FACS (CD14+ IL-6 +) |
| Female | Isolated PBMCs | 50 | 0, 1, 10, 100, 1000 | 30 | FACS (CD14+ IL-6 +) | |
| 4 (2) | Female | Monocytes (whole blood) | 50 | 0, 1, 10, 100, 1000 | 0 | ELISA (IL-6) |
| Monocytes (whole blood) | 0.5 | 0, 1, 10, 100, 1000 | 0 | ELISA (IL-6) | ||
| 5 (3) | Male | Monocytes (whole blood) | 50 | 0, 1, 10, 100 | 30 | Meso Scale (TNFα, IL-6, IL-8, IL-1β) |
| 6 (2) | Male | Monocytes (whole blood) | 50 | 0, 0.1, 1, 10, 100, 1000, 10000 | 30 | ELISA (IL-6) |
| Female | Monocytes (whole blood) | 50 | 0, 0.1, 1, 10, 100, 1000, 10000 | 30 | ELISA (IL-6) | |
| Female | Monocytes (whole blood) | 50 | 0, 0.1, 1, 10, 100, 1000, 10000 | 30 | ELISA (IL-6) | |
| 7 (2, 4) | Male | Macrophages | 50 | 0, 0.1, 10, 100, 1000 | 0 | ELISA (TNFα); Mesoscale (TNFα, IL-6, IL-8, IL-1β) |
| Male | Macrophages | 50 | 0, 0.1, 10, 100, 1000 | 30 | ELISA (TNFα); Mesoscale (TNFα, IL-6, IL-8, IL-1β) | |
| Male | Macrophages | 50 | 0, 0.1, 10, 100, 1000 | 0 | Mesoscale (TNFα, IL-6, IL-8, IL-1β) | |
| Male | Macrophages | 50 | 0, 0.1, 10, 100, 1000 | 30 | Mesoscale (TNFα, IL-6, IL-8, IL-1β) | |
ELISA and ECL were used to assess cytokine release into supernatant. For both assays, whole blood was co-cultured with LPS and OT at varying doses under varying conditions (Table 1) for 6 hours. Following incubation, each well was centrifuged and the supernatant was harvested and stored at −30°C. MO cytokine production was analyzed using a human IL-6 DuoSet ELISA kit (R&D Systems, Minneapolis, MN). Plates were coated with antibody during the afternoon, left overnight at room temperature, and blocked at least one hour prior to assay. Plates were washed three times prior to adding sample and standards. Standards were prepared and diluted as per kit instructions (600-9.38 pg/mL). Supernatant samples were thawed immediately before assay, vortexed and centrifuged to remove any remaining solids, and underwent a 400× dilution. Both standards and samples were plated in duplicate. Plates were read at 450 nm on a Tecan Sunrise plate reader (Medford, MA). The average intra-assay CV for these analyses was 6.12%.
In some experiments we also quantified MPs production of TNF-α using the Quantikine ELISA kit (BD BiosSciences). As above, samples were thawed, vortexed and centrifuged immediately prior to assay. The manufacturer’s instructions were followed at each step and the plates were subsequently read at 490 nm on Tecan Sunrise plate reader (Medford, MA). Average CVs for these analyses were 7.36%.
MP supernatant samples and some MO samples were also assessed using ECL in order to obtain multiplex readouts for multiple cytokines. A Human Proinflammatory-4 II Tissue Culture kit containing prepared plates stained for IL-6, interleukin-8 (IL-8), interleukin-1β (IL-1β) and TNF-α was purchased from Meso Scale Discovery (Gaithersburg, MD). Samples were analyzed neat following the manufacturer’s instructions on a SECTOR instrument. Average intra-assay CVs for MO analyses were 11.2% for IL-8, 5.66% for TNF-α, 3.69% for IL-6, and 6.09% for IL- 1β. Average intra-assay CVs for MP analyses was 4.64% for IL-8, 1.54% for TNF-α, 5.12% for IL-6, and 14.2% for IL-1β.
Ligands
OT obtained from Cedarlane (Burlington, ON), the same supplier used in the Szeto, et al. [20] experiments, was diluted and prepared as per the manufacturer’s instructions. OT aliquots were stored at −80°C, and were thawed and diluted to working concentrations prior to cell culturing. LPS was obtained from Sigma-Aldrich (Oakville, ON) and stored at −30°C. Unless otherwise specified, LPS was diluted to a final concentration of 50 ng/mL prior to culturing.
Results
Experiment 1
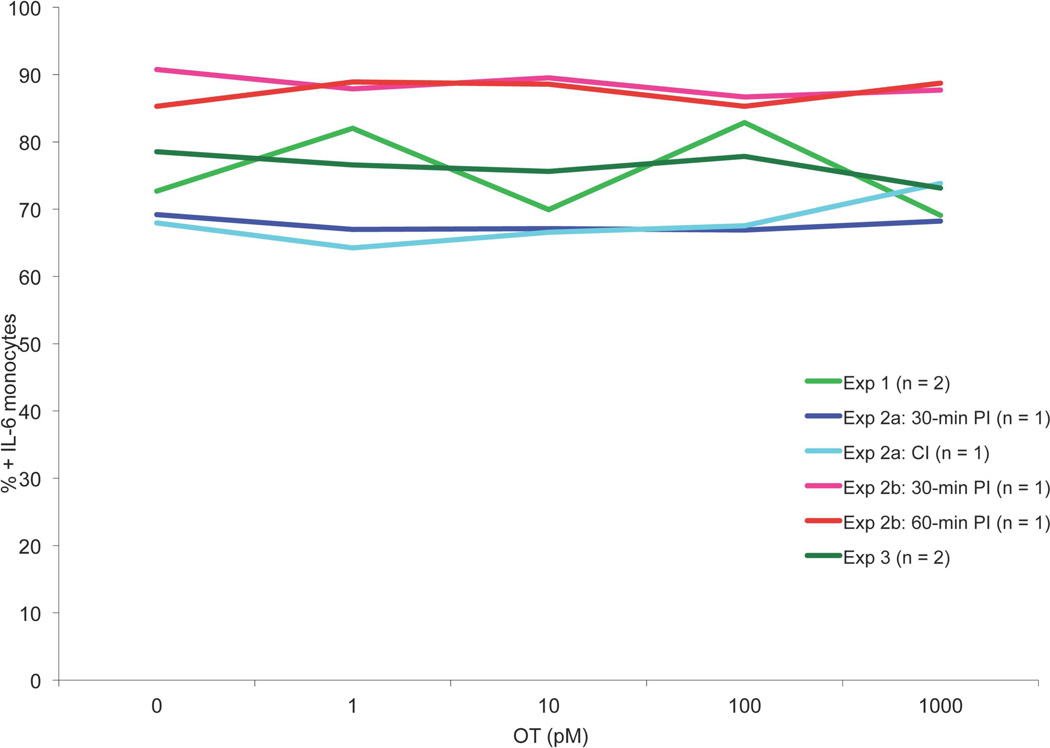
The goal of the first experiment was to determine if OT could be observed to modulate pro-inflammatory cytokine production by MOs stimulated with LPS. Whole blood samples were taken from a male and female volunteer, diluted 10:1 with saline solution, cultured with LPS and standard OT concentrations, and incubated as per above (please refer to Table 1 for a summary of all experimental protocols). After incubation, production of IL-6 was measured by ICS. Results were graphed and visually inspected for an attenuation curve. As can be seen in Figure 1, the curve was flat, suggesting that under these conditions OT did not attenuate LPS-stimulated IL-6 production in whole blood MOs, regardless of participant sex.
Figure 1.
MO ICS data showing the results from Experiments 1, 2 and 3. Experiment 1: Male and female whole-blood MOs incubated concurrently with 50 ng/mL LPS and 0–1000 pM OT, analyzed via FACS. Experiment 2: Male and female whole-blood MOs incubated with 50 ng/mL LPS and either concurrently or following a 30- or 60-minute pre-incubation with 0–1000 pM OT, analyzed via FACS. Experiment 3: Male and female isolated PBMCs incubated with 50 ng/mL LPS following a 30-minute pre-incubation with 0–1000 nM OT, analyzed via FACS. PI = pre-incubation, CI = concurrent incubation, n = number of participants per condition. Data are expressed as percentage of CD14+ cells that also stain IL-6+.
Experiment 2
It was possible that no attenuation was observable in Experiment 1 because MOs may need to be exposed to OT before stimulation occurs. This would be the case if OT regulates how cells prepare for challenge instead of the challenge response itself. To test this, we repeated Experiment 1 but introduced periods of pre-incubation with OT prior to stimulating MOs with LPS.
Two whole blood samples were taken from a female volunteer. Both samples were cultured as in Experiment 1 except the second sample was pre-incubated with OT for 30 minutes before adding LPS (Experiment 2a). IL-6 production was again analyzed via ICS and the results were graphed and inspected. Again, no attenuation was observed. Another two whole blood samples were taken from a different female volunteer and cultured as in Experiment 1 except one sample was pre-incubated with OT for 30 minutes and the other for 60 minutes (Experiment 2b). Inspection of FACS data again showed no clear attenuation of IL-6 production by OT in MOs (Figure 1).
Experiment 3
Prior research on OT regulation of uterine contractions suggests that calcium may play a role in OT signal transduction [26]. All our previous experiments used whole blood collected in a sodium heparin tube, which prevents clotting by chelating calcium ions. As such, it was possible that no action of OT had been observed because a key signal transducer was not available in culture. To assess this possibility, MOs were isolated from whole blood and cultured in a cell medium that replaced depleted calcium.
Blood samples were taken from a male and female volunteer using 8 mL CPT Vacutainer tubes (BD Biosciences, Mississauga, ON). Within 2 hours of the blood draw, the tubes were centrifuged for 20 minutes at 1800 RCF. Mononuclear cells present at the interface were harvested, washed in RPMI-1640, and then re-suspended in R10, a cell medium that contains calcium and other nutrients, at a final concentration of 106 cells per mL. Samples were pretreated with varying OT doses (Table 1) for 30 minutes prior to stimulation with LPS, and then incubated as per usual for 6 hours. Following incubation, the samples were prepared for ICS, analyzed by FACS, and results were graphed and visually inspected for attenuation of IL-6 production (Figure 1). Again, no attenuation of IL-6 production by OT concentration was observed, regardless of participant sex, suggesting that null results observed using whole blood samples was not because a key signal transduction molecule had been removed during sampling.
Experiment 4
ICS indexes cytokine production but not cytokine release. Since it is plausible that OT’s anti-inflammatory properties involve modulation of the latter process, we repeated Experiment 1 but assessed the magnitude of cytokine secretion into the culture supernatant by ELISA (Experiment 3a). We also tested whether the relatively high dose of LPS used in earlier experiments might have ‘washed out’ any effects of OT. As such, here we also repeated Experiment 1 but used a 100-fold lower dose of LPS (0.5 ng/mL; Experiment 3b).
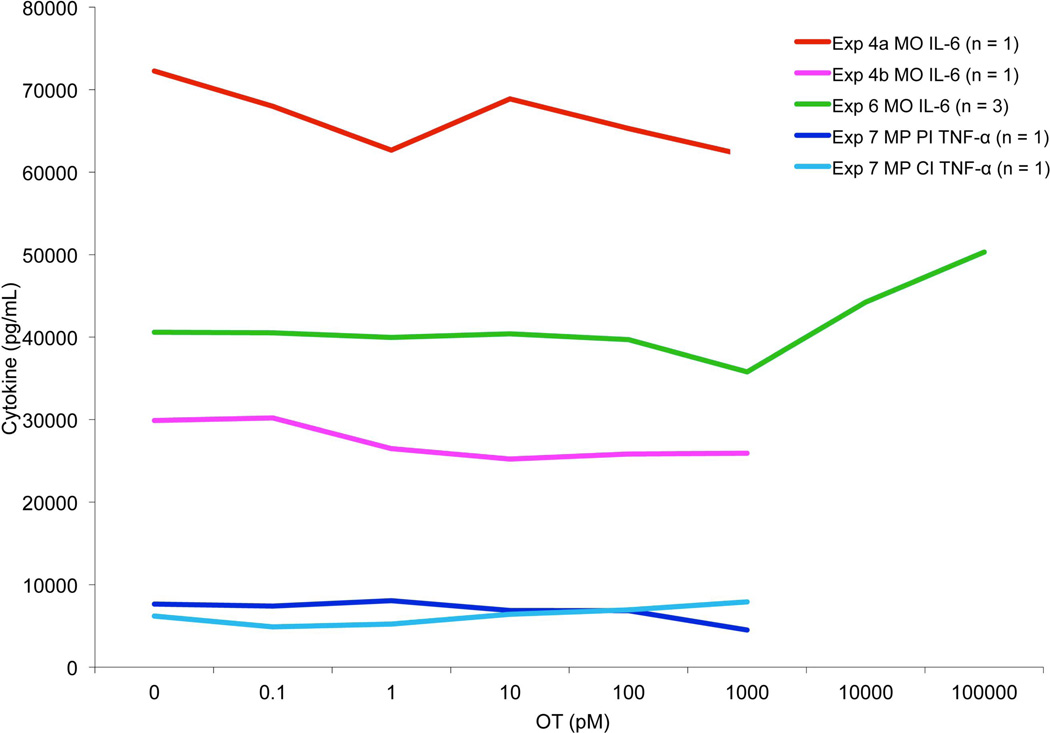
Two whole blood samples were taken from a female volunteer. The first sample was cultured as in Experiment 1 except no Golgi plug was added to the culture, allowing the MOs to freely secrete cytokines into the culture medium. The second sample was also cultured the same way except diluted LPS was used to challenge the MOs. Culture supernatant IL-6 was measured using ELISA, and the results were graphed and visually inspected for an attenuation curve. Again, no attenuation of IL-6 secretion by OT in MOs was observed. When the concentration of LPS was reduced, overall IL-6 secretion decreased but no attenuation curve emerged (Figure 2).
Figure 2.
MO and MP ELISA data, showing results from Experiments 4, 6 and 7. Experiment 4: Female whole-blood MOs incubated concurrently with 0–1000 pM OT and either 50 ng/mL or 0.5 ng/mL LPS, secreted IL-6 analyzed via ELISA. Experiment 6: Male and two female whole-blood MOs incubated with 50 ng/mL LPS following a 30-minute pre-incubation with 0–10 000 nM OT, secreted IL-6 analyzed via ELISA. Experiment 7: Male MPs incubated with 50 ng/mL LPS either concurrently or following a 30-minute pre-incubation with 0–1000 pM OT, secreted TNF-α analyzed via ELISA. PI = 30 minute pre-incubation, CI = concurrent incubation, n = number of participants per condition.
Experiment 5
After the results of Experiment 3 we concluded that LPS concentration was likely not masking any effect of OT and so we returned to the original LPS concentration. Again, it was possible that MOs needed to be pre-incubated with OT if this hormone regulates cell readiness for challenge. It is also possible that OT might not regulate MO IL-6 but other pro-inflammatory mediators instead.
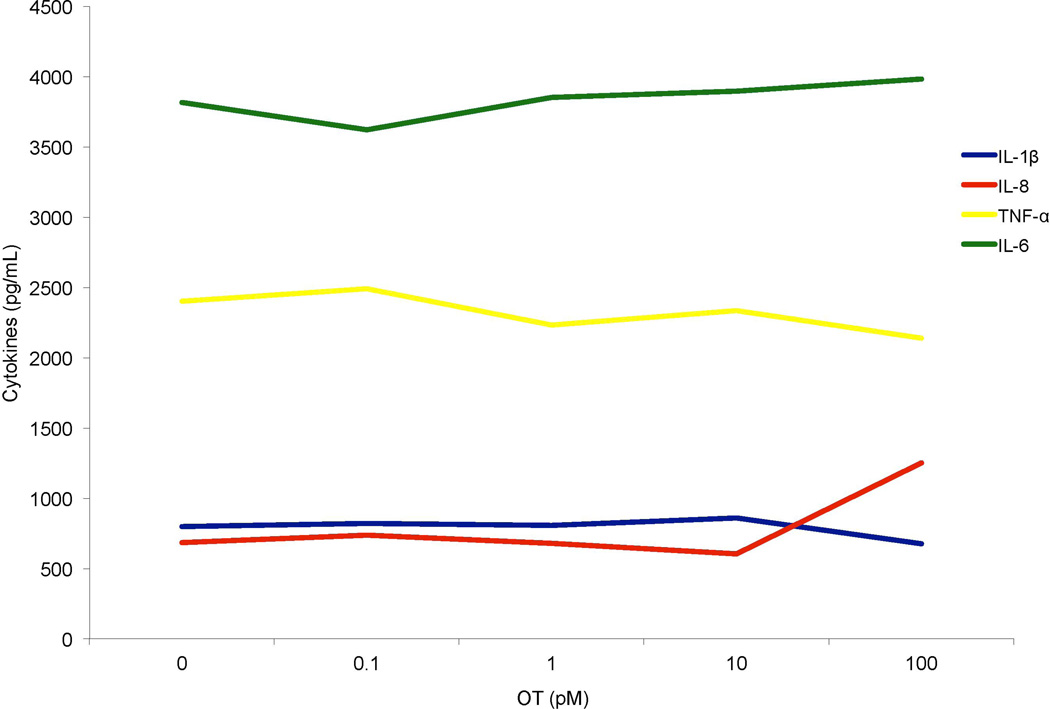
To test these possibilities, a whole blood sample was taken from a male volunteer and pre-incubated with OT for 30 minutes prior to LPS challenge. Culture supernatant was analyzed by mutiplex ECL and results were graphed. As can be seen in Figure 3, under these conditions OT did not attenuate MO secretion of IL-6, nor did it modulate LPS-stimulated production of other key pro-inflammatory mediators including IL-8, TNFα and IL-1β.
Figure 3.
MO ECL data, showing the results from Experiment 5: male whole-blood MOs incubated with 50 ng/mL LPS following a 30-minute pre-incubation with 0–100 pM OT, secreted cytokines (TNF-α, IL-6, IL-8 and IL-1β) analyzed via Meso Scale, n = 1.
Experiment 6
In the previous experiments we used OT doses within the physiological range for healthy adults. However, under certain circumstances, such as during birth, the circulating concentration of OT is much higher. It is possible that MOs only respond to OT under these supraphysiologic conditions. To test this notion, we stimulated MOs with a broader range of OT concentrations.
Whole blood samples were taken from a male and female volunteer. Samples were cultured with LPS under typical conditions but the OT concentrations used were 0, 0.1, 1, 10, 100, 1000 and 10 000 pM (Table 1). Culture supernatant IL-6 was measured using ELISA and graphed for visual inspection (Figure 2). Again, no clear pattern of IL-6 secretion attenuation by OT was observed, regardless of participant sex, even at the higher concentrations of OT.
Experiment 7
Based on the results of Experiments 1 through 6, which included cells from 8 different individuals, we concluded that OT does not act directly on primary human MOs to attenuate LPS-induced cytokine synthesis or release. However, as prior work shows that OTR concentration is much denser on the surface of MPs than MOs [20], we decided to re-conduct some of these studies with healthy human MPs on the assumption that these mature cells might be more OT responsive.
Thawed MPs were cultured with LPS, with OT co-incubation or pre-incubation at varying doses (see Table 1). Culture supernatant TNF-α was assessed via ELISA, and results were graphed and inspected for an attenuation curve. Again, no clear curve emerged (Figure 2).
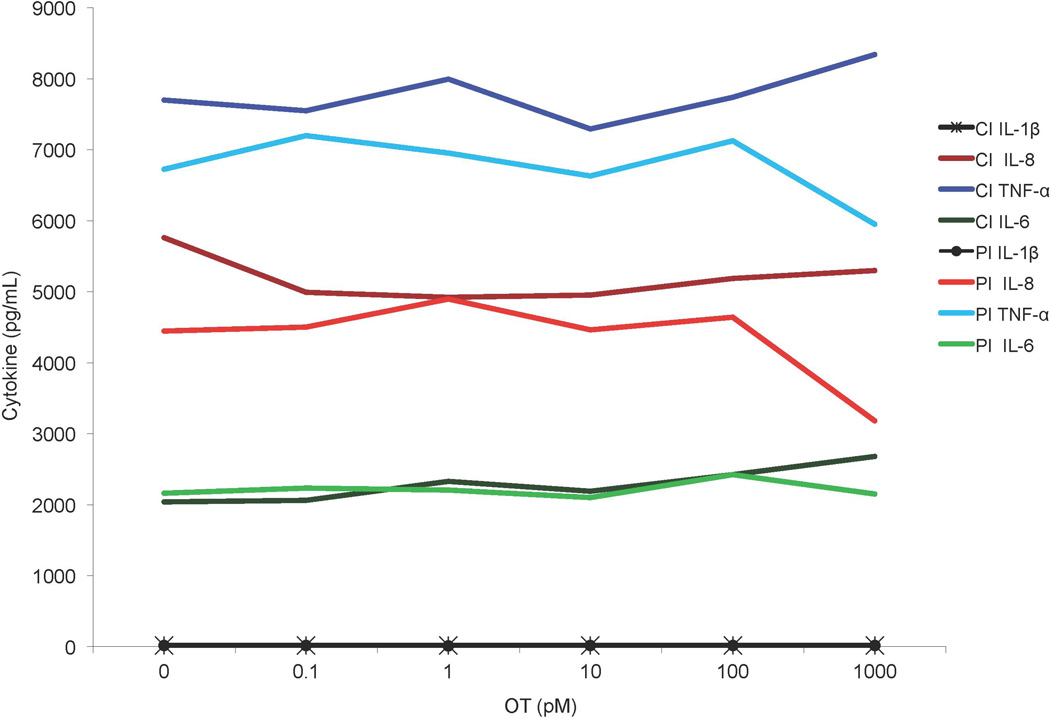
These MPs experiments were repeated in another set of studies but multiplex ECL methods were used, to determine if other cytokines might be affected. As Figure 4 shows, OT had no discernable influence on LPS-stimulated MPs production of IL-6, TNFα, IL-8 and IL-1β.
Figure 4.
MP ECL data, showing the results from Experiment 7: Two separate MP samples from the same male incubated with 50 ng/mL LPS either concurrently or following a 30-minute pre-incubation with 0–1000 pM OT, secreted cytokines (TNF-α, IL-6, IL-8 and IL-1β) analyzed via Meso Scale. PI = 30 minute pre-incubation, CI = concurrent incubation, n = 1.
Discussion
The purpose of this study was to assess whether OT can inhibit MO and/or MP cytokine production following activation with LPS, and to extend previous research by seeking to replicate any anti-inflammatory effect of OT in 1) female MOs, and 2) primary MPs from healthy human MP donors. OT has been shown to have systemic anti-inflammatory properties in humans and animals, but the specific mechanism of action is not understood. Work in human models has explored MO/MP cells as a potential underpinning mechanism, but the results are inconclusive: Clodi, et al. [17] found that in vivo OT attenuates LPS-evoked inflammatory cytokine production in a sample of male participants, but when this team challenged isolated peripheral MOs ex vivo with LPS, no attenuation of cytokine production was observed. Conversely, work by Szeto, et al. [20] suggested that OT does attenuate cytokine production in THP-1 derived MOs and MPs.
We did not observe any consistent anti-inflammatory effect of OT. Although we took care to base our experimental approach on Szeto, et al. [20], who reported IL-6 attenuation in THP-1 MPs co-treated with LPS and OT, we were unable to observe a similar pattern in the experiments reported here. The lack of attenuation by OT persisted even after we manipulated numerous factors that could have been masking the hormone’s inhibitory influences, including dosage and timing of administration, the method of cytokine measurement, the use of MOs vs. MPs, male vs. female participants, and calcium availability for signal transduction. Regardless of the conditions, we did not observe consistent dose-dependent inhibitory influences of OT on cytokine production. These results replicate and extend those obtained by Clodi, et al. [17], who did not observe OT effects on cytokine production by LPS-stimulated male MOs cultured ex vivo.
There are a number of reasons for why we and Clodi, et al. [17] may have been unable to observe an anti-inflammatory effect of OT. For example, it is always a possibility that cell lines behave differently from normal healthy cells, perhaps being more sensitive to OT than primary human MOs and MPs or due to differences in how OTR is expressed. It is also possible that MPs drive the OT anti-inflammation mechanism, and that somehow freezing and thawing compromised MP responses to OT in our experiments. This would have to be quite an isolated effect, however, as the MPs showed a high a degree of viability, as evidenced by their marked cytokine production following exposure to LPS. We did not evaluate this possibility here, but future research could explore it as an explanation for the conflicting results.
What does the overall pattern of findings [6,11,14–17,20] suggest about the mechanisms underlying OT’s systemic anti-inflammatory properties? Thus far, systemic administration of OT following a variety of immune challenges or injuries has led to reduced inflammation in both animals and humans [e.g.,6–16, 17]. Incubating LPS-activated primary MOs and MPs from healthy male and female donors with OT ex vivo, however, has yielded no clear, consistent attenuation of inflammatory cytokines. As such, we would argue that OT’s capacity to inhibit cytokine responses to systemically administered LPS [17] are unlikely to be mediated via direct influences on the function of MOs or MPs. Nonetheless, given that its receptor is expressed on these cells [20], OT could regulate some process relevant to MO/MP function that was not captured here that may have downstream consequences for systemic inflammation, for example, cell migration. It also remains possible that OT’s effects are dependent on specific micro-environmental conditions not replicated ex vivo. Further research is required to assess which aspects of MO and MP activity are regulated by the OTR and under what conditions. It would be particularly valuable to test whether OT has an effect on the activity of MPs harvested from lymphoid organs or tissues where these cells are resident.
With all that said, it also remains possible that OT’s actions are mainly exerted on other immune cells, like neutrophils, or are secondary to effects on other tissues. For example, vasculature tissue has been shown to express the OTR [20] and respond to systemic and centrally-administered OT [27]. OT could regulate the “leakiness” of the vasculature at sites of infection or injury, thereby affecting the number of immune cells that can migrate into the tissue or the kinds of activating signals expressed. Adipose tissue is also known to express the OTR (e.g., [28]. OT dosing in mice has been shown to reduce adipose tissue IL-6 secretion [8], which could also explain decreased systemic inflammation following OT exposure. Another scenario is that OT acts indirectly by regulating the secretion of other hormones with downstream antiinflammatory effects. For example, some researchers have suggested that OT may cross the blood brain barrier, act on the hypothalamic-pituitary-adrenal (HPA) axis to increase adenocorticotropic hormone (ACTH) release, which subsequently heightens corticosteroid secretion [29–31]. As such, through regulation of glucocorticoid secretion in the periphery, OT may have consequent anti-inflammatory effects on immune cells. Although this scenario is plausible in rats, in humans OT seems to have the opposite effect, reducing systemic output of cortisol [17,29,31]. As such, in humans at least, OT’s anti-inflammatory effects are probably not solely dependent on the HPA axis, though other centrally regulated neurohormonal mechanisms could be involved.
There are several limitations of our experiments. First, as is the case with all ex vivo studies, the conditions are imperfectly representative of the physiologic environment in which MOs and MPs operate. Second, we focused exclusively on LPS as a stimulus, because Clodi et al. (2008) showed that in vivo OT can abrogate its systemic inflammatory effects. However, in the body MOs and MPs are exposed to a much broader range of threats, not only from pathogens but also from injuries. Third, we used frozen MP matured ex vivo from MOs. Although the MPs were clearly viable, it is possible that the freezing and thawing process compromised their OT responsivity. It is also likely that MPs matured in tissue behave differently from MPs matured ex vivo. It will be important in future research to assess whether OT acts differently on MPs harvested from lymphoid organs or tissues where they are resident. Finally, our experiments involved cells from a fairly small number of individuals (n = 9), which precluded the use of formal statistical analyses. However, instead we calculated and inspected percent differences and found these to be small and/or inconsistent. This, coupled with the lack of a clear attenuation curve in our Figures, suggests that here OT did not inhibit cytokine production consistently, regardless of the donor sex, the dose, or the culture conditions. We could have done additional experiments with more subjects, but on the basis of consistently null findings this seemed unnecessary.
In summary, we conducted a series of experiments to assess whether LPS-activated MOs or MPs showed reduced cytokine production when incubated with OT in vitro, and extended previous research by including 1) female MOs, 2) primary human MPs, and (3) by examining cytokine secretion (ELISA) vs. cytokine production (ICS and FACS). Regardless of the conditions, no attenuation in cytokine production by OT was observed in either MOs or MPs. Although OT has been shown to have a systemic anti-inflammatory effect in both animals and humans, based on our work and work by Clodi, et al. [17] it appears that this effect is not mediated solely by MP or MO activity. Further research is necessary to understand how OT exerts its anti-inflammatory effect.
Acknowledgements
This research was supported by grant HD058052 from the National Institute of Child Health and Human Development.
References
- 1.Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 2.Carter C, Grippo A, Pournajafinazarloo H, Ruscio M, Porges S. Oxytocin, vasopressin and sociality. In: Neumann ID, Landgraf R, editors. Progress in brain research. vol 170. Elsevier; 2008. pp. 331–336. [DOI] [PubMed] [Google Scholar]
- 3.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends in cognitive sciences. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Frontiers in neuroendocrinology. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Kemp AH, Guastella AJ. The role of oxytocin in human affect: A novel hypothesis. Current Directions in Psychological Science. 2011;20:222–231. [Google Scholar]
- 6.Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegen BC. Oxytocin protects against sepsis-induced multiple organ damage: Role of neutrophils. The Journal of surgical research. 2005;126:73–81. doi: 10.1016/j.jss.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Petersson M, Wiberg U, Lundeberg T, Uvnas-Moberg K. Oxytocin decreases carrageenan induced inflammation in rats. Peptides. 2001;22:1479–1484. doi: 10.1016/s0196-9781(01)00469-7. [DOI] [PubMed] [Google Scholar]
- 8.Nation DA, Szeto A, Mendez AJ, Brooks LG, Zaias J, Herderick EE, Gonzales J, Noller CM, Schneiderman N, McCabe PM. Oxytocin attenuates atherosclerosis and adipose tissue inflammation in socially isolated apoe−/− mice. Psychosomatic medicine. 2010;72:376–382. doi: 10.1097/PSY.0b013e3181d74c48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed MA, Elosaily GM. Role of oxytocin in deceleration of early atherosclerotic inflammatory processes in adult male rats. International journal of clinical and experimental medicine. 2011;4:169–178. [PMC free article] [PubMed] [Google Scholar]
- 10.Petersson M, Lundeberg T, Sohlstrom A, Wiberg U, Uvnas-Moberg K. Oxytocin increases the survival of musculocutaneous flaps. Naunyn-Schmiedeberg's archives of pharmacology. 1998;357:701–704. doi: 10.1007/pl00005227. [DOI] [PubMed] [Google Scholar]
- 11.Dusunceli F, Iseri SO, Ercan F, Gedik N, Yegen C, Yegen BC. Oxytocin alleviates hepatic ischemia-reperfusion injury in rats. Peptides. 2008;29:1216–1222. doi: 10.1016/j.peptides.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Biyikli NK, Tugtepe H, Sener G, Velioglu-Ogunc A, Cetinel S, Midillioglu S, Gedik N, Yegen BC. Oxytocin alleviates oxidative renal injury in pyelonephritic rats via a neutrophil-dependent mechanism. Peptides. 2006;27:2249–2257. doi: 10.1016/j.peptides.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Tugtepe H, Sener G, Biyikli NK, Yuksel M, Cetinel S, Gedik N, Yegen BC. The protective effect of oxytocin on renal ischemia/reperfusion injury in rats. Regulatory peptides. 2007;140:101–108. doi: 10.1016/j.regpep.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegen BC. Oxytocin ameliorates oxidative colonic inflammation by a neutrophil-dependent mechanism. Peptides. 2005;26:483–491. doi: 10.1016/j.peptides.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Iseri SO, Gedik IE, Erzik C, Uslu B, Arbak S, Gedik N, Yegen BC. Oxytocin ameliorates skin damage and oxidant gastric injury in rats with thermal trauma. Burns : journal of the International Society for Burn Injuries. 2008;34:361–369. doi: 10.1016/j.burns.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Iseri SO, Dusunceli F, Erzik C, Uslu B, Arbak S, Yegen BC. Oxytocin or social housing alleviates local burn injury in rats. The Journal of surgical research. 2010;162:122–131. doi: 10.1016/j.jss.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 17.Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, Luger TA, Luger A. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. American journal of physiology Endocrinology and metabolism. 2008;295:E686–E691. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- 18.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Szeto A, Nation DA, Mendez AJ, Dominguez-Bendala J, Brooks LG, Schneiderman N, McCabe PM. Oxytocin attenuates nadph-dependent superoxide activity and il-6 secretion in macrophages and vascular cells. American journal of physiology Endocrinology and metabolism. 2008;295:E1495–E1501. doi: 10.1152/ajpendo.90718.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (thp-1) International journal of cancer Journal international du cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 22.Carter CS. Oxytocin and sexual behavior. Neuroscience and biobehavioral reviews. 1992;16:131–144. doi: 10.1016/s0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- 23.Amico JA, Seif SM, Robinson AG. Oxytocin in human plasma: Correlation with neurophysin and stimulation with estrogen. The Journal of clinical endocrinology and metabolism. 1981;52:988–993. doi: 10.1210/jcem-52-5-988. [DOI] [PubMed] [Google Scholar]
- 24.Kramer KM, Cushing BS, Carter CS, Wu J, Ottinger MA. Sex and species differences in plasma oxytocin using an enzyme immunoassay. Canadian Journal of Zoology. 2004;82:1194–1200. [Google Scholar]
- 25.Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- 26.Cassoni P, Sapino A, Marrocco T, Chini B, Bussolati G. Oxytocin and oxytocin receptors in cancer cells and proliferation. Journal of neuroendocrinology. 2004;16:362–364. doi: 10.1111/j.0953-8194.2004.01165.x. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki Y, Satoh S, Kimura M, Oyama H, Asano T, Shibuya M, Sugita K. Effects of vasopressin and oxytocin on canine cerebral circulation in vivo. Journal of neurosurgery. 1992;77:424–431. doi: 10.3171/jns.1992.77.3.0424. [DOI] [PubMed] [Google Scholar]
- 28.Zingg HH. Vasopressin and oxytocin receptors. Baillieres Clin Endocrinol Metab. 1996;10:75–96. doi: 10.1016/s0950-351x(96)80314-4. [DOI] [PubMed] [Google Scholar]
- 29.Gibbs DM. Vasopressin and oxytocin: Hypothalamic modulators of the stress response: A review. Psychoneuroendocrinology. 1986;11:131–139. doi: 10.1016/0306-4530(86)90048-x. [DOI] [PubMed] [Google Scholar]
- 30.Neumann ID. No stress response of the hypothalamo-pituitary-adrenal axis in parturient rats: Lack of involvement of brain oxytocin. Endocrinology. 2003;144:2473–2479. doi: 10.1210/en.2003-0037. [DOI] [PubMed] [Google Scholar]
- 31.Jezova D, Skultetyova I, Tokarev DI, Bakos P, Vigas M. Vasopressin and oxytocin in stress. Annals of the New York Academy of Sciences. 1995;771:192–203. doi: 10.1111/j.1749-6632.1995.tb44681.x. [DOI] [PubMed] [Google Scholar]