Abstract
Different mechanisms have been suggested to contribute to isoflurane-mediated neuroprotection. Previous studies have suggested that the protein Slit can abrogate neuronal death in mixed neuronal–glial cultures exposed to oxygen–glucose deprivation (OGD) and reperfusion (OGD/R). We hypothesized that isoflurane increases the expression of Slit and its receptor Robo when cortical neurons are exposed to OGD/R. To test this hypothesis, we exposed primary cortical neurons to OGD for 90 min and reperfusion for 24 h and investigated how isoflurane post-conditioning affected cell survival and expression of Slit2 and receptors Robo1 and Robo4. Cell survival increased after administration of isoflurane, as assessed by the lactate dehydrogenase assay, trypan blue analysis, and propidium iodide staining. Western blot analysis showed that cleaved caspase-3 was increased after OGD/R (P<0.01) but reduced by isoflurane post-conditioning. Real-time PCR and Western blot analysis showed that the expression levels of Slit2 and Robo1, but not Robo4, were increased after OGD/R (P<0.5)and increased even further by isoflurane post-conditioning (P<0.01). Our results suggest that isoflurane post-conditioning markedly attenuates apoptosis and necrosis of cortical neurons exposed to OGD/R possibly in part via elevation of Slit2/Robo1 expression. These findings provide a novel explanation for the pleiotropic effects of isoflurane that could benefit the central nervous system.
Keywords: Isoflurane, Neuroprotection, OGD;, Slit/Robo
1. Introduction
Patients at risk for perioperative stroke, or those who have suffered recent cerebral injury, may benefit from neuroprotective properties of anesthetic agents during surgery (Koerner and Brambrink, 2006). The effects of anesthetics or postoperative sedatives on functional deficit caused by an ischemic event could influence the choice of agent that an anesthesiologist makes. A large body of evidence suggests that isoflurane post-conditioning is neuroprotective in various experimental models and that it is capable of reducing early neural death (Cao et al., 2012; Fang Li et al., 2012; Lee et al., 2008; Li and Zuo, 2011). We hypothesized that the protein Slit and its receptor signaling pathway might contribute to the neuroprotective effect of isoflurane.
Three Slit isoforms (1, 2, and 3) and four Slit receptors (Robo 1–4) have been identified in mammals. The Slit–Robo signaling pathway was initially identified as an extracellular cue to guide axon path finding, promote axon branching, and control neuronal migration (Battye et al., 1999). It has been reported that Slit abolishes neuronal death in mixed neuronal–glial cultures exposed to oxygen–glucose deprivation (OGD) (Altay et al., 2007). This finding indicates that the protective effect of Slit occurs at least partly as a direct effect on neurons and/or neuron–glial interactions, and is not based solely on an indirect ability to reduce post-ischemic inflammation.
Studies have shown that induction of Slit2 expression contributes to peripheral nervous regeneration (Tanno et al., 2005) and angiogenesis (Legg et al., 2008). The work of Jones et al. (Jones et al., 2008) suggests that Slit2 can both positively and negatively regulate angiogenesis by binding to Robo1 and Robo4, respectively. Slit2, Robo1, and Robo4 may protect against edema formation and tissue injury under ischemic conditions (Acevedo et al., 2008).
To date, little attention has been paid to the effects of isoflurane post-conditioning on the expression of Slit and its receptor Robo. We hypothesize that isoflurane post-conditioning induces Slit expression to reduce neuronal death. To test this hypothesis, we exposed primary rat cortical neurons to OGD and resuscitation (OGD/R) and investigated the effect of isoflurane post-conditioning. We show that isoflurane post-conditioning induces Slit2/Robo1 expression, reverses the OGD/R-induced increase in caspase-3 protein, and protects neurons against OGD/R injury.
2. Results
Eight days after harvesting cortical tissue from neonatal rats, the percentage of neurons in the primary cultures was approximately 80%, as assessed by staining with NeuN (Supplementary data Fig. 1). This value is consistent with that of a previous study (Noh et al., 2005).
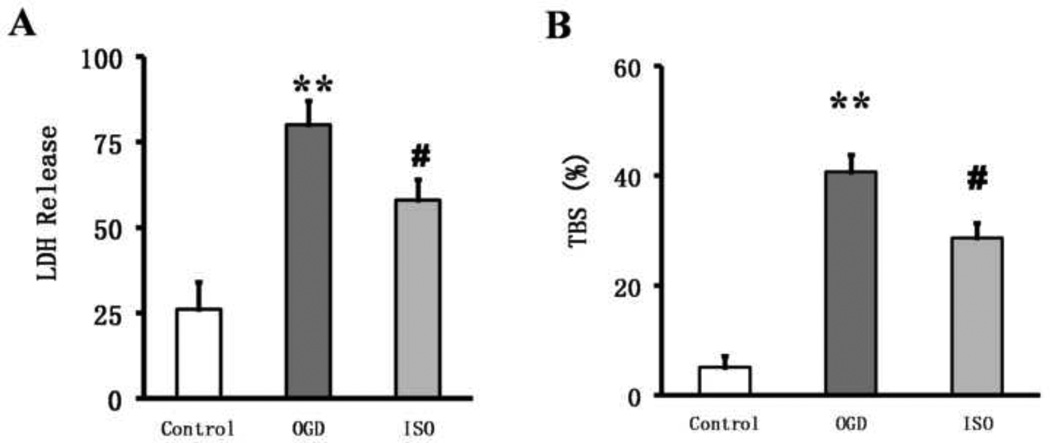
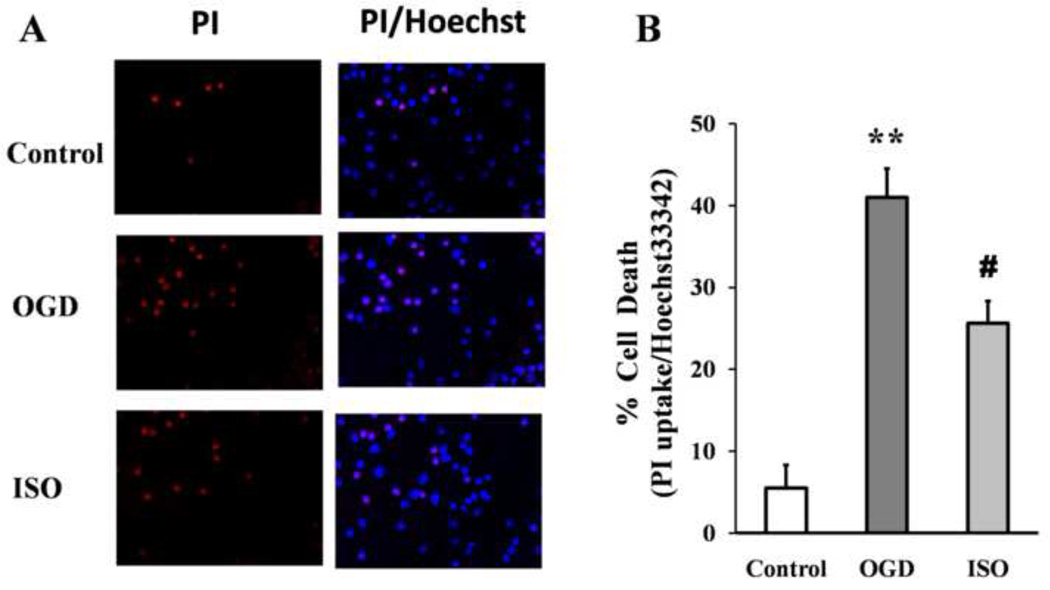
Cell death was significantly higher in cultures exposed to OGD/R than in control cultures. The LDH was 26.1±8.2% in control neuronal cultures and80.1±7.1% after OGD/R (P<0.01). However, after isoflurane post-conditioning, LDH decreased significantly to 58.1±6.1% (P<0.05 vs. OGD/R group; Fig. 1A). Trypan blue staining also revealed significant cell death after OGD/R. Based on trypan blue staining, the percentage of dead cells in control cultures was 5.1±2.1% but 40.7±3.1% in cultures exposed to 90 min of OGD (P<0.01). Isoflurane post-conditioning decreased trypan-blue–positive cells significantly to 28.7±2.7% (P<0.05 vs. OGD/R group; Fig. 1B). These results indicate that primary cortical neuron viability decreases sharply after OGD but that isoflurane post-conditioning can mitigate this loss of viability. Similar to the trypan blue study, propidium iodide (PI) staining showed neuronal death after OGD/R was 41.2±3.5% compared with that of the control group 5.5±2.8% (P<0.01). Isoflurane post-conditioning decreased the number of PI-positive cells to25.6±2.7% (P<0.05 vs. OGD/R group; Fig. 2).
Figure 1.
Isoflurane post-conditioning increases neuronal viability after oxygen–glucose deprivation (OGD) injury. (A) Lactate dehydrogenase (LDH) activity expressed as a percentage of total LDH (determined for each experiment by assaying the supernatant of sister cultures after 30 min of exposure to 1% Triton X-100) indicates that OGD increases cell death. Isoflurane (ISO) partially restored viability. The data were obtained from three independent experiments with at least eight wells per condition. (B)Trypan blue staining (TBS) showed that the viability of primary cultured cortical neurons was decreased with OGD exposure but partially restored by isoflurane post-conditioning. Data are presented as mean±SD (n=9/group). **P<0.01 vs. the control group. #P<0.05 vs. the OGD group.
Figure 2.
Isoflurane post-conditioning decreases neuronal death evaluated by propidium iodide (PI) staining after OGD, (A) PI (red) and Hoechst 33342 (Hoechst, blue) staining, Quantification of the PI-positive cells revealed that PI uptake increased after OGD indicating increased neuronal death. The number of PI-positive cells was decreased by isoflurane post-conditioning. Data are presented as mean ± SD (n=9/group). (B) **P<0.01 vs. the control group; #P<0.05 vs. the OGD group.
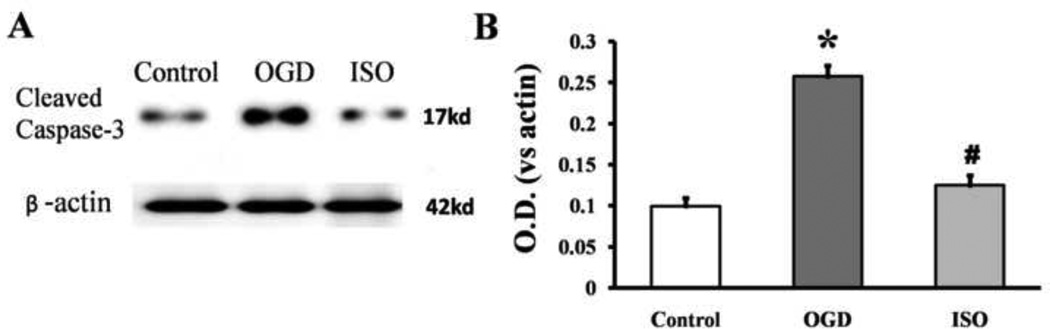
Western blot analysis showed that the expression of the active fragment caspase-3 was significantly elevated in the OGD/R group compared with that in the control group (P<0.05). With isoflurane post-conditioning, the level of the cleavedcaspase-3 decreased significantly (n=12/group; P<0.05 vs. control group, P<0.05 vs. OGD/R group; Fig. 3).
Figure 3.
Isoflurane post-conditioning inhibits oxygen–glucose deprivation (OGD)-evoked caspase-3 activation. The neurons were grown in standard control conditions or subjected to 90 min of OGD with or without exposure to 1 h of isoflurane (ISO) post-conditioning. All neurons were harvested at 24 h after OGD. (A) Representative Western blot of caspase-3 expression. (B) Optical density (OD) value of cleaved caspase-3 evaluated by Western blot analysis. Data are presented as mean ± SD (n=12/group). *P<0.05 vs. control. #P<0.05 vs. OGD group.
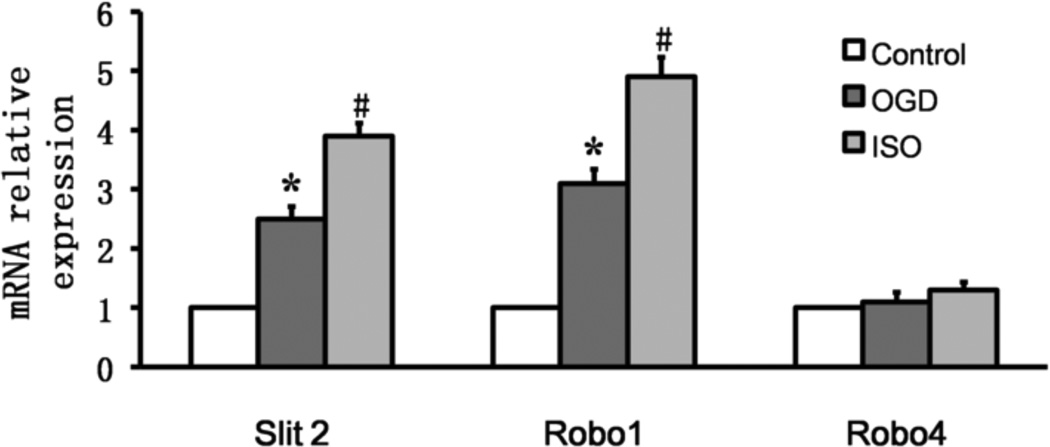
Real-time PCR showed that, compared with baseline in the control group, Slit2 and Robo1 mRNA expression levels increased after OGD/R and isoflurane post-conditioning (P<0.01 vs. control;P<0.01 vs. OGD/R). The level of Robo4 mRNA was unchanged under both conditions (P>0.05; n=9/group; Fig. 4).
Figure 4.
Isoflurane post-conditioning increases expression of Slit2 and Robo1 mRNA in cortical neurons exposed to oxygen-glucose deprivation (OGD). Data are presented as mean ± SD (n=9/group). *P<0.01 vs. control group after 24 h of reperfusion. #P<0.01 vs. OGD group after 24 h of reperfusion.
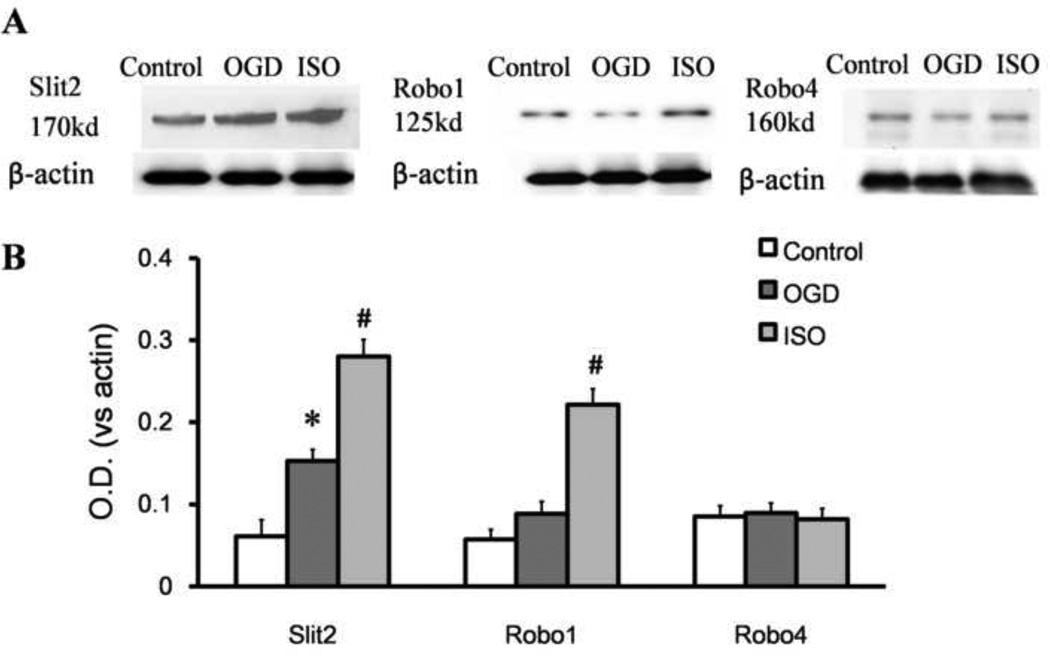
Western blot analysis also showed that expression of Slit2, Robo1, and Robo4 was minimal in the control group. The integrated density value ratio of Slit2 to β-actin was significantly greater in the OGD/R group that received isoflurane post-conditioning than in either the control group or untreated OGD/R group (both P<0.05). The integrated density of Robo1 after isoflurane post-conditioning increased significantly compared with that of the OGD/R group (P<0.05). However, the integrated density value ratio of Robo4 to β-actin did not differ among these three groups (all P>0.05; n=12/group, Fig. 5)
Figure 5.
Isoflurane post-conditioning promotes the expression of Slit2 and Robo1 after oxygen-glucose deprivation (OGD). The neurons were grown in standard control conditions or subjected to 90 min of OGD with or without 1 h of isoflurane post-conditioning. (A) Representative Western blot of Slit2, Robo1, and Robo4. (B) Optical density (OD) value of Slit2, Robo1, and Robo4 evaluated by Western blot analysis. Data are presented as mean ± SD (n=12/group). *P<0.05 vs. control. #P<0.05 vs. OGD group.
3. Discussion
In the present study, we found that OGD/R exposure caused significant damage to cultured cortical neurons by decreasing neuronal cell viability (as assessed by LDH release and trypan blue staining), by increasing neuronal death (as assessed by PI uptake) and by increasing apoptosis (as assessed by caspase-3 expression). These indicators of cell death and apoptosis were significantly attenuated by isoflurane post-conditioning. Our results demonstrate that isoflurane post-conditioning directly benefits cortical neurons subjected to OGD/R. The beneficial effect of isoflurane post-conditioning may be mediated by elevations in Slit2 and Robo1 expression.
Apoptotic cell death is orchestrated by the activation of a caspase cascade. Caspases are proteases that cleave target proteins, including other caspases (Nunez et al., 1998). Of the caspase family members, caspase-3 is commonly regarded as an “executioner” caspase because its activation by an upstream caspase usually signifies commitment to apoptosis (Hall et al., 1991). Emulsified isoflurane has shown cardioprotective effect against ischemic reperfusion injury by inhibiting apoptosis via the modulation of cleaved caspase expression in rats (Hu et al., 2013). In a rat model of hepatic ischemic reperfusion injury, isoflurane also showed protective effect by reducing the protein expression of caspase 3 (Ko et al., 2013). In a mouse model of subarachnoid hemorrhage, isoflurane (2%) post-conditioning reduced cleaved caspase-3 expression at 24 hours (Altay et al., 2012). The effect of isoflurane on the expression of the active fragment of caspase 3 in our OGD/R model suggests that isoflurane can attenuate apoptosis in primary cultured rat cortical neurons.
Cerebral ischemia/reperfusion is a complex process that results in cellular damage and death (Segura et al., 2008). Several studies have reported that isoflurane post-conditioning can induce neuroprotection against ischemic insult (Li and Zuo, 2011; Li et al., 2012). Furthermore, isoflurane post-conditioning is considered to be promising because its application does not require prediction of when a detrimental ischemic event will occur (Lin et al., 2011). Here, we have shown that isoflurane post-conditioning protects primary cultured cortical neurons against OGD/R injury. In our in vitro model, the neuroprotective effect of isoflurane post-conditioning may be mediated by increases in the expression of Slit2 and its receptor Robo1. Robo4 does not appear to be a major target for the protective effect of isoflurane. Slit2/Robo1 is a conserved ligand-receptor system that greatly affects the distribution, migration, axon guidance, and branching of neurons (Ypsilanti et al., 2010). By contrast, Robo4 is often detected in the vessel and has been reported to affect the formation of vessels (London and Li, 2011).
In Drosophila, Slit and its receptors play an important role in neuron–glia interactions by influencing the survival and migration of both cell types; moreover, interfering with these interactions alters their responsiveness to Slit–Robo signaling (Kinrade et al., 2001). Slit2 and its receptor Robo1 have been reported to be expressed in neurons in human brain and rat cortex which is consistent with our results (Fang et al.; Liu et al.;Mertsch et al., 2008). Slit combines with its signal-transducing receptor (Robo) which transmits the signal downstream further. Robo binds with itself by Ig domains I and II of the receptor’s cytoplasmic loop which probably serves as a neuronal receptor. Interestingly, Slit might also exert a direct neuroprotective effect through its association with Rho GTPase-mediated cytoskeletal rearrangements to stabilize the cellular architecture of the neuron itself (Altay et al., 2007). Moreover, inhibition of Rho GTPases or their effectors protects against in vivo ischemia/reperfusion injury in brain (Laufs et al., 2000; Trapp et al., 2001) and other organs (Bao et al., 2004). Modulation of Rho GTPase signaling cascades by Slit may help to maintain cellular integrity and function in ischemic brain secondary to its ability to prevent or reduce synaptic disruption and other changes in dendritic morphology crucial to neuronal viability (Hasbani et al., 2001).
In conclusion, this study confirmed that isoflurane at clinically relevant concentrations has a neuroprotective effect on rat cortical neurons in an in vitro model of ischemia and reperfusion. This beneficial effect may be mediated by slit2 and its receptor Robo1 for it is accompanied by the increases in the expression of Slit2 and Robo1, which might stabilize the cytoskeleton and thereby suppress neuronal death (apoptosis and necrosis). Additional research is needed to clarify the mechanism by which isoflurane enhances Slit2/Robo1 expression.
4. Material and Methods
All protocols were approved by the animal research committee of China Medical University.
4.1. Cell culture
Primary cultures of cortical neurons from newborn Sprague-Dawley rats were used as previously described (Meloni et al., 2002). Briefly, neonates were decapitated and cortical tissue was collected under sterile conditions. Meninges were removed and cortical tissue was dissociated in 5 mM L-cysteine (Sigma, St. Louis, MO, USA), 10 U/mL papain (ICN, Irvine, CA, USA), and 0.01% DNase I (Sigma) at 37°C. Dissociated neurons were washed in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL, Grand Island, NY, USA), centrifuged, and gently resuspended in DMEM medium with serum. After 24 h, the neurons were washed with phosphate-buffered saline (PBS) and gently resuspended in neuron-defined serum-free Neurobasal medium (Gibco-BRL) supplemented with B-27 (Gibco-BRL), 0.5 mM L-glutamine (Gibco-BRL), and 2 µg/mL gentamycin. The cells were plated at 8.5 × 105 cells/mL onto 100-mm culture dishes coated with 0.1 mg/mL poly-L-lysine (Sigma). The culture dishes were stored in a humidified atmosphere of 95% air–5% CO2 at 37°C. Cytosine arabinoside (10 µM) was changed twice weekly and experiments were performed on day 8 in vitro.
4.2. OGD and isoflurane conditioning
After 8 days in culture, cortical neurons were randomly divided into three groups: control, OGD/R, and OGD/R plus isoflurane. The OGD/R protocol has been described previously (Grabb and Choi, 1999). Cultures were washed three times with PBS and placed in glucose-free balanced salt solution (BSS; 116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 1.0 mM NaH2PO4, 26.2 mM NaHCO3, 1.8 mM CaCl2, 0.02 mM glycine, and 2 mg/L phenol red) that was bubbled with 100% N2 for 30 min. Cells were then placed in an anaerobic incubator (Thermo, Waltham, MA, USA)at 37°C for 90 min. OGD was terminated by removing the dishes from the anaerobic incubator, returning the stored preconditioned medium to the dishes, and returning the dishes to a standard cell incubator containing normal atmospheric oxygen and 5% CO2 at 37°C for 24 h. In the isoflurane post-conditioning experiments, cells were placed immediately after OGD into a Billups-Rothenburg chamber that was flushed with 2% isoflurane in 95% air and 5% CO2 at 6 L/min for 7–8 min. The chamber was then sealed for 1 h at 37°C. The isoflurane concentrations in the outlet gases from the chambers were confirmed to be 2% in the preconditioning experiment by the Datex™ infrared analyzer (Capnomac, Helsinki, Finland). After 1 h of isoflurane exposure, cells were replaced into standard incubators under normoxic conditions for 23 h. Control cell cultures not deprived of oxygen and glucose were exposed to BSS containing 6 mM glucose, were not bubbled with anaerobic gas, and were stored in the standard incubator.
4.3. Assessment of cell viability
Lactate dehydrogenase (LDH) activity was determined with an LDH cytotoxicity detection kit as described before (Kim et al., 2009). The cell culture medium was collected at the end of the experiment and centrifuged at 13,000 rpm for 10 min. One hundred microliters of the cell-free supernatant was transferred to 96-well plates and incubated with an equal volume of reaction mixture from the kit. The samples then were read in a spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA) with the absorbance wavelength at 492 nm and the reference wavelength at 655 nm. Background absorbance from cell-free buffer solution was subtracted from all absorbance measurements. Total intracellular LDH activity was determined by removing the cell culture medium from six-well plates, lysing the cells with 0.5% Triton X-100 for 30 min, and measuring LDH as described above. Percentage of cell death in the experimental samples was calculated by dividing the amount of LDH released in each sample by the total intracellular LDH. For trypan blue staining, 10 µL of 4% trypan blue solution was incubated for 2 min with 10 µL of cells collected from each plate; unstained live cells were counted on a hemocytometer (Lee et al., 2011).
4.4. Propidium Iodide Uptake Assay
Propidium iodide (PI) (Sigma, St. Louis, MO) (5 g/ml) was added to the neurons for 5 min and then post-fixed in 4% paraformaldehyde (PFA), washed with PBS, Nuclei were labeled with Hoechst 33342 (Invitrogen, Grand Island, NY). PI-positive cells were observed under microscope (Leica, Germany). Sections were analyzed by an investigator blinded to the experimental cohort using Image J software (version 1.42q; NIH, Bethesda, MD).
4.5. Real-time PCR assessment of Slit2, Robo1, and Robo4 mRNA expression
Total RNA was extracted from cultured cells with Trizolreagent (Invitrogen). RNA concentrations were determined and 500 ng of RNA was used. The primer sequences for Slit2, Robo1, and Robo4 were:
| Slit2: | 5'-GTGTGCTTCTGTCTGCTCTC -3' (F) |
| 5'-GCCAATGCCACTCATCTTCC -3' (R) | |
| Robo1: | 5'-CACCGAATGCTGCTGCCAAGT -3' (F) |
| 5'-CGCCACCATAACATCTGAAGG -3' (R) | |
| Robo4: | 5'-AGCTGAGAGCAGCCGTTGACT -3' (F) |
| 5'- CATTCAGCAGCCAGCGGATAG-3' (R) |
Real-time PCR was carried out on a 7500 Fast Real-Time PCR System (Applied Biosystems, Irvine, CA) with Power SYBR Green (Applied Biosystems). Glyceraldehyde 3-phosphate dehydrogenase was used as an endogenous control to normalize gene expression. In brief, the PCR mixtures were preheated at 50°C for 2 min and then at 95°C for 10 min to activate the AmpliTaq Gold DNA polymerase; then samples under went 40 cycles of amplification (95°C for 15 s; 60°C for 30 s; 68°C for 40 s). A final extension step was performed at 60°C for 10 min. Each reaction was carried out in triplicate. Graphs were plotted, and analysis was performed with the ΔΔCt method.
4.6. Western blot analysis
The neurons were preconditioned in 50 mM Tris-HCl (pH 7.4) containing 1 mM EDTA and protease inhibitor cocktail (Complete mini EDTA-free, Roche Molecular Biochemicals, Germany) and then homogenized. The crude homogenates were centrifuged at 1000g for 10 min at 4°C. The protein concentrations in the supernatants were determined with the BCA Protein Assay Reagent Kit (Pierce, USA). Equivalent amounts of protein sample (100 µg)were separated on 10% sodium dodecylsulphate-polyacrylamide gels and electro transferred onto nitrocellulose membrane (Bio-Rad, USA) as previously described (Kapinya et al., 2002). The membranes then were blocked with 5% nonfat milk in PBS containing 0.1% Tween-20 at room temperature for 1 h. Rabbit anti-rat polyclonal anti-Slit2, anti-Robo1, and anti-Robo4 antibodies (1:200 dilution, Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit anti-rat polyclonal cleaved caspase-3 antibody (1:500, Cell Signaling, Beverly, MA, USA) were used as primary antibodies, and anti-rabbit antibody (1:2,000 dilution, Santa Cruz) was used as a secondary antibody. Protein bands were detected with an ECL plus Western blot detection system (Amersham Biosciences, Piscataway, NJ, USA). Immunoreactivity was quantified with Scion Image Beta 4.02 Win software (Scion, USA).
4.7. Statistics
All quantitative data are expressed as mean ± SD. Multiple comparisons among groups were performed by one-way analysis of variance (ANOVA) followed by post hoc Bonferroni-Dunn tests. P<0.05 was considered to be statistically significant.
Supplementary Material
(A) Neuronal culture purity. NeuN positive cells (Red), Dapi positive cells (Blue); the purity of neurons is more than 80%. (B) Representative phase contrast pictures of neurons from control group, from oxygen-glucose deprivation (OGD)-treated group, and from Isoflurane (Iso)-treated group.
Highlights.
Clinical relevant concentration of Isoflurane has neuroprotective effect on neuron
Isoflurane post-conditioning attenuates apoptosis of neurons after OGD/R injury
This effect may be mediated by increases in the expression of Slit2 and Robo1
Acknowledgements
This work was supported by NSFC (81000824, 81101402, 30901404, 81171782), and NIH (K01AG031926, R01AT007317, R01NS078026). We thank Claire Levine for assistance with this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo LM, Weis SM, Cheresh DA. Robo4 counteracts VEGF signaling. Nature medicine. 2008;14:372–373. doi: 10.1038/nm0408-372. [DOI] [PubMed] [Google Scholar]
- Altay O, Hasegawa Y, Sherchan P, Suzuki H, Khatibi NH, Tang J, Zhang JH. Isoflurane delays the development of early brain injury after subarachnoid hemorrhage through sphingosine-related pathway activation in mice. Crit Care Med. 2012;40:1908–1913. doi: 10.1097/CCM.0b013e3182474bc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altay T, McLaughlin B, Wu JY, Park TS, Gidday JM. Slit modulates cerebrovascular inflammation and mediates neuroprotection against global cerebral ischemia. Exp Neurol. 2007;207:186–194. doi: 10.1016/j.expneurol.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W, Hu E, Tao L, Boyce R, Mirabile R, Thudium DT, Ma XL, Willette RN, Yue TL. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovasc Res. 2004;61:548–558. doi: 10.1016/j.cardiores.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Battye R, Stevens A, Jacobs JR. Axon repulsion from the midline of the Drosophila CNS requires slit function. Development. 1999;126:2475–2481. doi: 10.1242/dev.126.11.2475. [DOI] [PubMed] [Google Scholar]
- Cao L, Feng C, Li L, Zuo Z. Contribution of microRNA-203 to the isoflurane preconditioning-induced neuroprotection. Brain research bulletin. 2012;88:525–528. doi: 10.1016/j.brainresbull.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Li Q, Xu H, Sun Y, Hu R, Jiang H. Induction of inducible nitric oxide synthase by isoflurane post-conditioning via hypoxia inducible factor-1 alpha during tolerance against ischemic neuronal injury. Brain Res. 2012;1451:1–9. doi: 10.1016/j.brainres.2012.02.055. [DOI] [PubMed] [Google Scholar]
- Fang M, Liu GW, Pan YM, Shen L, Li CS, Xi ZQ, Xiao F, Wang L, Chen D, Wang XF. Abnormal expression and spatiotemporal change of Slit2 in neurons and astrocytes in temporal lobe epileptic foci: A study of epileptic patients and experimental animals. Brain Res. 1324:14–23. doi: 10.1016/j.brainres.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Grabb MC, Choi DW. Ischemic tolerance in murine cortical cell culture: critical role for NMDA receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:1657–1662. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11:292–298. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- Hasbani MJ, Viquez NM, Goldberg MP. NMDA receptors mediate hypoxic spine loss in cultured neurons. Neuroreport. 2001;12:2731–2735. doi: 10.1097/00001756-200108280-00028. [DOI] [PubMed] [Google Scholar]
- Hu ZY, Abbott GW, Fang YD, Huang YS, Liu J. Emulsified isoflurane postconditioning produces cardioprotection against myocardial ischemia-reperfusion injury in rats. J Physiol Sci. 2013;63:251–261. doi: 10.1007/s12576-013-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F, Mukouyama YS, Lindblom P, Seth P, Frias A, Nishiya N, Ginsberg MH, Gerhardt H, Zhang K, Li DY. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyper permeability. Nature medicine. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapinya KJ, Lowl D, Futterer C, Maurer M, Waschke KF, Isaev NK, Dirnagl U. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke. 2002;33:1889–1898. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- Kim JA, Li L, Zuo Z. Delayed treatment with isoflurane attenuates lipopolysaccharide and interferon gamma-induced activation and injury of mouse microglial cells. Anesthesiology. 2009;111:566–573. doi: 10.1097/ALN.0b013e3181af5b3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinrade EFV, Brates T, Tear G, Hidalgo A. Round about signalling, cell contact and trophic support confine longitudinal glia and axons in the Drosophila CNS. Development. 2001;128:207–216. doi: 10.1242/dev.128.2.207. [DOI] [PubMed] [Google Scholar]
- Ko JS, Gwak MS, Kim GS, Shin YH, Ryu S, Kim JS, Kim SJ, Kim ST. The protective effect of ischemic preconditioning against hepatic ischemic-reperfusion injury under isoflurane anesthesia in rats. Transplant Proc. 2013;45:1704–1707. doi: 10.1016/j.transproceed.2012.08.026. [DOI] [PubMed] [Google Scholar]
- Koerner IP, Brambrink AM. Brain protection by anesthetic agents. Curr Opin Anaesthesiol. 2006;19:481–486. doi: 10.1097/01.aco.0000245271.84539.4c. [DOI] [PubMed] [Google Scholar]
- Laufs U, Endres M, Stagliano N, Amin-Hanjani S, Chui DS, Yang SX, Simoncini T, Yamada M, Rabkin E, Allen PG, Huang PL, Bohm M, Schoen FJ, Moskowitz MA, Liao JK. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. Journal of Clinical Investigation. 2000;106:15–24. doi: 10.1172/JCI9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Li L, Jung HH, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Park HH, Koh SH, Choi NY, Lee KY. Amlodipine besylate and amlodipine camsylate prevent cortical neuronal cell death induced by oxidative stress. J Neurochem. 2011;119:1262–1270. doi: 10.1111/j.1471-4159.2011.07529.x. [DOI] [PubMed] [Google Scholar]
- Legg JA, Herbert JM, Clissold P, Bicknell R. Slits and Roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis. 2008;11:13–21. doi: 10.1007/s10456-008-9100-x. [DOI] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane postconditioning induces neuroprotection via Akt activation and attenuation of increased mitochondrial membrane permeability. Neuroscience. 2011;199:44–50. doi: 10.1016/j.neuroscience.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QF, Xu H, Sun Y, Hu R, Jiang H. Induction of inducible nitric oxide synthase by isoflurane post-conditioning via hypoxia inducible factor-1 alpha during tolerance against ischemic neuronal injury. Brain research. 2012;1451:1–9. doi: 10.1016/j.brainres.2012.02.055. [DOI] [PubMed] [Google Scholar]
- Lin D, Li G, Zuo Z. Volatile Anesthetic Post-Treatment Induces Protection Via Inhibition of Glycogen Synthase Kinase 3 Beta in Human Neuron-Like Cells. Neuroscience. 2011;179:73–79. doi: 10.1016/j.neuroscience.2011.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JB, Jiang YQ, Gong AH, Zhang ZJ, Jiang Q, Chu XP. Expression of Slit2 and Robo1 after traumatic lesions of the rat spinal cord. Brain Res. 113:43–48. doi: 10.1016/j.acthis.2009.08.003. [DOI] [PubMed] [Google Scholar]
- London NR, Li DY. Robo4-dependent Slit signaling stabilizes the vasculature during pathologic angiogenesis and cytokine storm. Current opinion in hematology. 2011;18:186–190. doi: 10.1097/MOH.0b013e328345a4b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni BP, Majda BT, Knuckey NW. Evaluation of preconditioning treatments to protect near-pure cortical neuronal cultures from in vitro ischemia induced acute and delayed neuronal death. Brain research. 2002;928:69–75. doi: 10.1016/s0006-8993(01)03361-3. [DOI] [PubMed] [Google Scholar]
- Mertsch S, Schmitz N, Jeibmann A, Geng JG, Paulus W, Senner V. Slit2 involvement in glioma cell migration is mediated by Robo1 receptor. J Neuro oncol. 2008;87:1–7. doi: 10.1007/s11060-007-9484-2. [DOI] [PubMed] [Google Scholar]
- Noh JS, Sharma RP, Veldic M, Salvacion AA, Jia X, Chen Y, Costa E, Guidotti A, Grayson DR. DNA methyltransferase 1 regulates reelin mRNA expression in mouse primary cortical cultures. Proc Natl Acad Sci U S A. 2005;102:1749–1754. doi: 10.1073/pnas.0409648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- Segura T, Calleja S, Jordan J. Recommendations and treatment strategies for the management of acute ischemic stroke. Expert opinion on pharmacotherapy. 2008;9:1071–1085. doi: 10.1517/14656566.9.7.1071. [DOI] [PubMed] [Google Scholar]
- Tanno T, Fujiwara A, Takenaka S, Kuwamura M, Tsuyama S. Expression of a chemorepellent factor, Slit2, in peripheral nerve regeneration. Bioscience, biotechnology, and biochemistry. 2005;69:2431–2434. doi: 10.1271/bbb.69.2431. [DOI] [PubMed] [Google Scholar]
- Trapp T, Olah L, Holker I, Besselmann M, Tiesler C, Maeda K, Hossmann KA. GTPase RhoB: an early predictor of neuronal death after transient focal ischemia in mice. Mol Cell Neurosci. 2001;17:883–894. doi: 10.1006/mcne.2001.0971. [DOI] [PubMed] [Google Scholar]
- Ypsilanti AR, Zagar Y, Chedotal A. Moving away from the midline: new developments for Slit and Robo. Development. 2010;137:1939–1952. doi: 10.1242/dev.044511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Neuronal culture purity. NeuN positive cells (Red), Dapi positive cells (Blue); the purity of neurons is more than 80%. (B) Representative phase contrast pictures of neurons from control group, from oxygen-glucose deprivation (OGD)-treated group, and from Isoflurane (Iso)-treated group.