Abstract
Purpose
Fenretinide induces apoptosis in malignant gliomas in vitro. This two-stage phase II trial was conducted to determine the efficacy of fenretinide in adults with recurrent malignant gliomas.
Patients and Methods
Twenty-two patients with anaplastic gliomas (AG) and 23 patients with glioblastoma (GBM) whose tumors had recurred after radiotherapy and no more than two chemotherapy regimens were enrolled. Fenretinide was given orally on days 1 to 7 and 22 to 28 in 6-week cycles in doses of 600 or 900 mg/m2 bid.
Results
Six of 21 (29%) patients in the AG arm and two of 23 (9%) patients in the GBM arm had stable disease at 6 months. One patient with AG treated at 900 mg/m2 bid dosage had a partial radiologic response. Median progression-free survival (PFS) was 6 weeks for the AG arm and 6 weeks for the GBM arm. PFS at 6 months was 10% for the AG arm and 0% for the GBM arm. Grade 1 or 2 fatigue, dryness of skin, anemia, and hypoalbuminemia were the most frequent toxicities reported. The trial was closed after the first stage because of the inadequate activity at the fenretinide doses used. The first-administration mean plasma Cmax for fenretinide was 832 ± 360 ng/mL at the 600 mg/m2 bid dosage and 1,213 ± 261 ng/mL at the 900 mg/m2 bid dosage.
Conclusion
Fenretinide was inactive against recurrent malignant gliomas at the dosage used in this trial. However, additional studies using higher doses of the agent are warranted based on the tolerability of the agent and the potential for activity of a higher fenretinide dosage, as suggested in this trial.
INTRODUCTION
The prognosis of patients diagnosed with malignant gliomas remains grave although treatments such as radical surgery, radiotherapy, and chemotherapy are of value in the management of these tumors.1–3 Despite advances in therapy, the overall impact of these treatments against malignant glioma remains limited and recurrences are the rule; hence, newer and more effective forms of therapy are clearly needed.
Retinoids are a class of natural and synthetic compounds with diverse biologic effects against malignancies, including growth arrest, differentiation, and angiogenesis inhibition.4 Preclinical studies have also shown that retinoids have growth-inhibitory effects against glioma.5 In addition, a phase II study of 13-cis-retinoic acid in patients with recurrent malignant brain tumors demonstrated evidence of antitumor activity.6 These data suggest that retinoids are active against malignant gliomas and warrant further study.
Fenretinide (4-hydroxyphenyl-retinamide), a synthetic retinoid, has activity against various types of malignant cells in preclinical studies and is under investigation as a chemopreventive agent.7–10 Unlike other retinoids, fenretinide induces apoptosis in vitro in tumor cells by generating free radicals and activating the ceramide pathway via retinoid receptor-dependent and -independent pathways.11,12 We previously reported that fenretinide is more potent than 13-cis-retinoic acid in growth inhibition of glioma cells at equimolar concentrations in vitro and induces apoptosis at concentrations of 3 to 5 µmol/L.13,14 In phase I studies in adults, plasma concentrations of up to 10 µmol/L (3.92 µg/mL) were achieved, suggesting that concentrations needed for antitumor activity were clinically achievable (J. Zwiebel, National Cancer Institute Cancer Therapy Evaluation Program [CTEP], personal communication).15 Animal studies suggest that fenretinide can cross the blood-brain barrier, probably due to its lipophilicity.16 These studies provide a strong rationale for evaluating the activity of fenretinide against gliomas.
In this study, we determined the efficacy of fenretinide in the treatment of patients with recurrent malignant glioma as assessed by progression-free survival (PFS) at 6 months. We also determined the objective measurable radiologic response rate, time to progression (TTP), overall survival, and toxicity of this agent.
PATIENTS AND METHODS
Patient Eligibility
Adults (≥ 18 years of age) with supratentorial malignant gliomas who had unequivocal tumor recurrence on a stable steroid dose as diagnosed by magnetic resonance imaging scan after radiotherapy and no more than two prior chemotherapy regimens were eligible for enrollment. Patients who had undergone resection of a recurrent tumor but had measurable residual disease were also eligible to participate if they had recovered from the effects of surgery. The glioblastoma multiforme (GBM) stratum included patients with GBM and gliosarcoma; the anaplastic glioma (AG) stratum included patients with anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), and mixed AG. Additional eligibility criteria included a Karnofsky performance score ≥ 70; a life expectancy of ≥ 8 weeks; and adequate bone marrow, liver, and renal function before starting therapy. The protocol was approved by the institutional review board of each participating institution and conducted in accordance with institutional and federal guidelines for human investigations. Patients were informed of the investigational nature of this study and signed informed consent forms that were approved by the institutional review boards. All patients in the reproductive age group were required to use adequate birth control (barrier methods) during and for 2 months after participation in this study. Patients who were pregnant or who had serious intercurrent medical illness and conditions that could potentially alter the metabolism of the drug were excluded from the study.
Evaluation During Study
Baseline and study laboratory evaluations included CBCs, serum chemistries (total protein, albumin, calcium, phosphorus, glucose, blood urea nitrogen, uric acid, creatinine, total bilirubin, alkaline phosphatase, lactate dehydrogenase, and ALT), lipid battery (triglycerides, cholesterol, low- and high-density lipoprotein), serum amylase and lipase, thyroid-stimulating hormone, thyroxine, and a serum pregnancy test for women of childbearing potential. A gadolinium-DTPA–enhanced magnetic resonance imaging was performed at baseline and before every cycle of treatment. In addition to undergoing a physical examination, patients completed a vision questionnaire and an M.D. Anderson Symptom Inventory questionnaire17 at the baseline evaluation and before each subsequent course of treatment.
Treatment Plan
Fenretinide, formulated as 100-mg capsules, was provided by the CTEP, and was administered at a dosage of 600 mg/m2 bid approximately 12 hours apart on days 1 to 7 and 22 to 28 of each 6-week period, constituting one treatment cycle. The dosage and schedule used in this trial were based on recommendations from the CTEP, which in turn were based on initial data from ongoing phase I studies. After nearly a year of accrual into the present trial, CTEP recommended a change in the phase II dosage based on final phase I data available; subsequently, four patients with AG were treated with fenretinide at the revised phase II dosage of 900 mg/m2 bid. To improve absorption of the drug, patients were advised to take it with a high-fat meal.18 Treatment was continued until the patient experienced tumor progression or toxicity.
Pharmacology and Correlative Studies
Fenretinide and N-(4-methoxyphenyl)-retinamide (4-MPR) levels were determined on day 1 of courses 1 and 4, at 0, 1, 3, 6, 9, and 12 hours after the first administration of the agent. Levels were also obtained on day 2 (12 hours after the second administration), and days 14 and 21. The protocol was amended to include a day 8 trough level for the 900 mg/m2bid dosage. Blood samples (7 mL) were collected in heparinized tubes wrapped in aluminum foil to prevent exposure to light and centrifuged immediately (1500 × g for 15 minutes at 4°C). The plasma was removed, placed in the light-resistant tubes provided to participating institutions, and stored at ≤ −20°C. Brain tissue, obtained during resection from one patient who developed progressive enhancement while undergoing fenretinide treatment, was protected from light, immediately frozen in liquid nitrogen, and stored at −70°C until further processing. The tissue homogenates were analyzed for fenretinide and 4-MPR. Plasma levels of fenretinide, 4-MPR, and retinol were analyzed by high-performance liquid chromatography as previously described,19 except that α-naphthoflavone was used instead of etretinate-free acid as the internal standard. Retinol-binding protein (RBP) was measured using a radial immunodiffusion assay validated for plasma RPB (Nanorid, The Binding Site, Birmingham, United Kingdom). Mean RBP reference concentrations were 53 mg/L (range, 39 to 67 mg/L) for healthy men and 46 mg/L (range, 33 to 60 mg/L) for healthy women.
The pharmacokinetic parameters for fenretinide were characterized by standard noncompartmental methods. The time intervals relative to the oral administration of fenretinide were used to determine the time to peak concentration (Cpmax) and the area under the plasma concentration-time curve (AUC). Cpmaxvalues were determined from each patient’s plasma concentration-time curve. Elimination rate constants were estimated by linear interpolation using the levels obtained on days 14 and 21. The AUC0–12 h was calculated using the linear trapezoidal rule. The percentage decrease in retinol and RBP levels after first administration of fenretinide was calculated by subtracting the 24-hour value from the baseline value divided by the baseline value × 100. Recovery of retinol or RBP to baseline values was determined by comparing the baseline value with the day 21 values.
End Points and Response Evaluation
The primary end point was the 6-month (26-week) PFS rate for patients treated with fenretinide. The secondary end points included the rate of measurable radiologic response, the TTP, the overall survival rate, and the toxicity of fenretinide in patients with recurrent malignant gliomas.
Statistical Design and Analysis
The study used a Simon Minimax two-stage phase II trial design with two strata (GBM and AG) using historical data for comparison. The historical values for comparison were obtained from a database of 375 patients with recurrent high-grade glioma (150 with AG and 225 with GBM) enrolled in eight previous phase II studies in which the 6-month PFS rate was 31% for patients with AG and 15% for patients with GBM.20 The hypothesis to be tested was H0, P < P0 v H1, P > P1, where P is the probability of PFS for 6 months, accepting a false-positive rate (α) ≤ 10% and a falsenegative rate (β) ≤ 10%. For the AG stratum, P0 was set to 20% and P1 was set to 40% (looking for a 20% improvement). These parameters lead to a two-stage design with a first stage of 21 patients stopping if no more than four patients responded or continuing accrual to a total of 45 patients, declaring success if more than 12 patients responded (with a 95% CI on the true response proportion from 26% to 56%). For the GBM stratum, the P0 was set to 10% and the P1 was set to 30% (looking for a 20% improvement). These parameters led to a two-stage design, with the first stage of 20 patients stopping if no more than two patients responded or continuing to a total of 40 patients, declaring success if more than six patients responded.
To accomplish the secondary objectives, the distributions of TTP and time to death were estimated using the Kaplan-Meier method. The proportions of assessable patients who had radiologic responses and those with PFS for 6 months in each of the major histologic subtypes were determined.
RESULTS
Patient Characteristics
Twenty-two patients were enrolled in the AG arm, including six patients with AO; three patients with mixed AG; 12 patients with AA; and one patient with initial diagnosis of AO, who was subsequently deemed inassessable for response after the central histologic review resulted in reclassification of his tumor as a grade 2 mixed glioma. All patients in this arm were assessable for toxicity and all but one for response. The last four patients in this arm who had recurrent AA were treated at a higher dose of 900 mg/m2 bid. Twenty-three patients were enrolled in the GBM arm, all of whom were assessable for toxicity and 22 of whom were assessable for response (21 with GBMs and one with gliosarcoma). The patients’ characteristics are listed in Table 1.
Table 1.
Patient Characteristics
| Characteristic | Anaplastic Glioma Arm | Glioblastoma Arm |
|---|---|---|
| No. of patients | 22 | 23 |
| Age, years | ||
| Median | 53 | 50 |
| Range | 29–76 | 26–75 |
| Sex | ||
| Male | 15 | 18 |
| Female | 6 | 5 |
| KPS | ||
| Median | 90 | 80 |
| Range | 70–100 | 70–100 |
Abbreviation: KPS, Karnofsky performance score.
End Point Evaluation
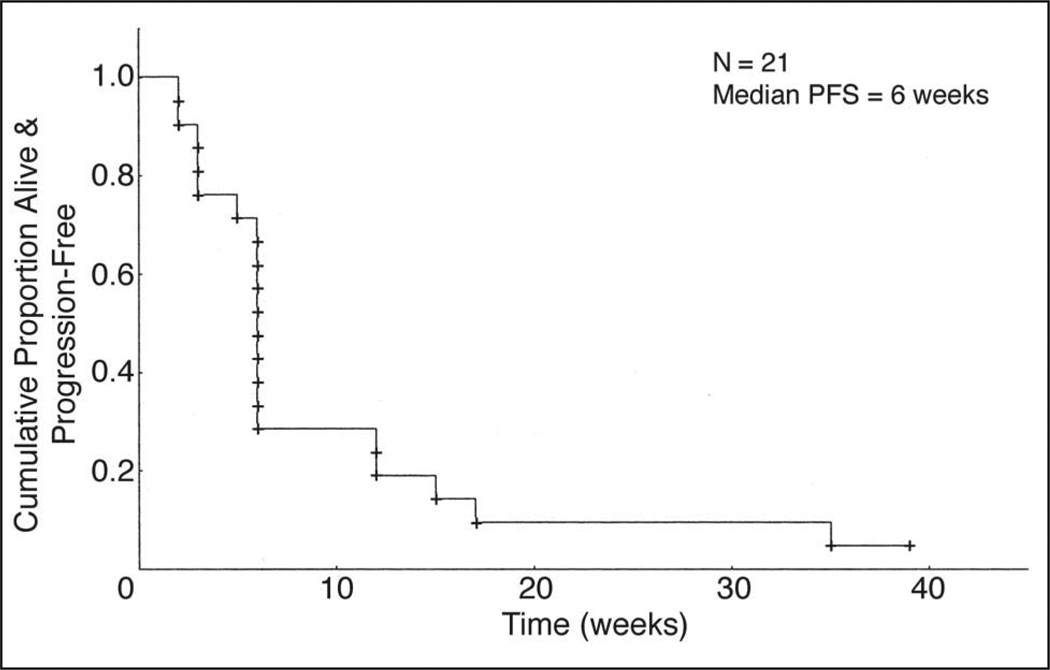
At the time of analysis, 15 of the 21 assessable patients with AG had experienced disease progression, and five patients had stable disease; the 6-month PFS rate was 10% (95% CI,3%to 36%) and the median PFS was 6 weeks (95% CI, 6 to 12 weeks; Fig 1). Median overall survival in this group was 30 weeks. One patient with an AA who was enrolled onto this study after her disease failed to respond to radiotherapy and chemotherapy (temozolomide plus marimastat), and who underwent fenretinide treatment at the 900 mg/m2 bid dosage, had a partial radiologic response. At the time of analysis, she had completed 15 cycles of fenretinide therapy and remained clinically stable (Fig 2).
Fig 1.
Kaplan-Meier curve for progression-free survival (PFS) for patients in the anaplastic glioma stratum.
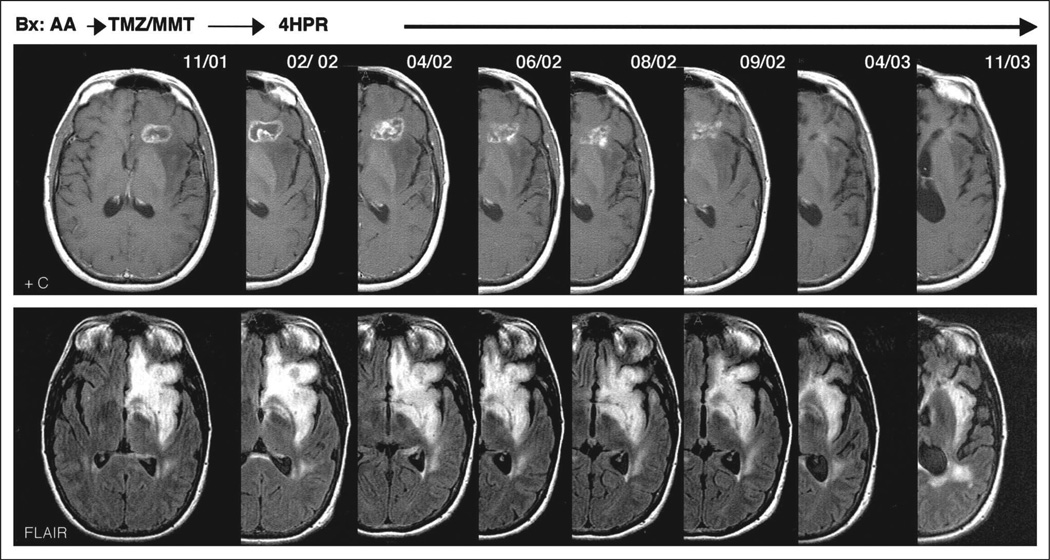
Fig 2.
Fenretinide-induced response in a patient with biopsy-proven recurrent anaplastic astrocytoma after radiation therapy failed a year before enrollment and temozolomide (TMZ) plus marimastat (MMT) subsequently. The patient has completed 13 cycles of fenretinide (900 mg/m2 bid) and remained progression free 18 months after initiation of therapy. AA, anaplastic astrocytoma; 4HPR, 4-hydroxyphenyl-retinamide.
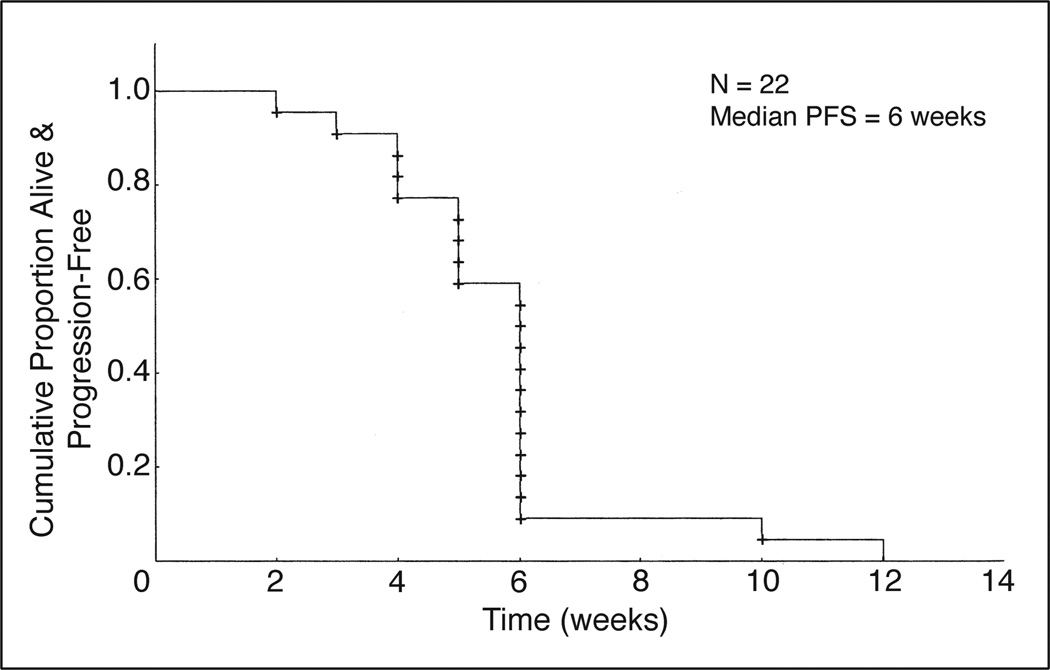
In the GBM arm, 23 patients enrolled onto the first stage were assessable for toxicity and all but one were assessable for response. Two (9%) patients had stable disease at 6 months; the remainder experienced disease progression. The median PFS was 6 weeks (95% CI, 5 to 6 weeks) and the PFS at 6 months was0%(Fig 3). Median overall survival was 16 weeks in this stratum. In accordance with the trial design, both the AG and GBM arms of the trial were closed to patient accrual after the first stage because the number of patients whose disease responded to treatment did not reach the preset limits that would permit starting the second stage.
Fig 3.
Kaplan-Meier curve for progression-free survival (PFS) for patients in the glioblastoma stratum.
Toxicity
In general, fenretinide was well tolerated, with only grade 1 or 2 toxicities reported in most patients in both arms of the trial (Table 2). Mild to moderate adverse events that were possibly linked to fenretinide were reported in 43 (95%) of the 45 patients enrolled onto the study. Results of the symptom inventory assessment (M.D. Anderson Symptom Inventory) are shown in Table 3. Of the several symptoms monitored and self-reported by the patients in this questionnaire, the only significant change after fenretinide therapy was worsening of fatigue from a mild to a moderate level. No improvements in symptoms were reported; however, it should be noted that the baseline symptoms reported in this test were graded as mild by all patients. The most common grade 1 toxicities included fatigue, headache, skin changes (dry skin, pruritus, and rash) and digestive tract symptoms (abdominal pain, cramping, diarrhea, stomatitis, and xerostomia). Grade 2 toxicities reported as possibly linked to fenretinide treatment included seizures and confusion. A patient with an AA who had been receiving treatment with fenretinide at the 600 mg/m2 bid dose for one cycle presented with headaches, nausea, and vomiting, and was found to have a small intracranial bleed in the region of the basal ganglia. He recovered without deficits and continued treatment without further events. Another patient, who was also undergoing treatment at the 600 mg/m2 bid dosage and was receiving oral anticoagulation with warfarin for deep venous thrombosis, died after developing an uncontrollable nasal bleed (international normalized ratio > 6.0). Of the four patients treated at the 900 mg/m2 bid dose, one had grade 3 vomiting, grade 2 speech impairment, and grade 1 memory impairment, which improved without residual symptoms. No other significant toxicity associated with the increased dosage was noted.
Table 2.
Toxicities Noted During Fenretinide Therapy Reported As Related to the Treatment
| Toxicity | Grade 1 | Grade 2 | Grade 5 |
|---|---|---|---|
| Abdominal pain | 2 | 0 | 0 |
| Abdominal cramping | 1 | 0 | 0 |
| Alopecia | 1 | 0 | 0 |
| Amylase increase | 1 | 0 | 0 |
| Anemia | 4 | 0 | 0 |
| Bleeding (without grade 3 or 4 thrombocytopenia) | 0 | 0 | 1 |
| CNS bleeding* | 0 | 0 | 0 |
| Cognitive disturbance | 2 | 0 | 0 |
| Confusion | 0 | 2 | 0 |
| Constipation | 1 | 0 | 0 |
| Diarrhea | 3 | 1 | 0 |
| Dizziness | 4 | 0 | 0 |
| Dry skin | 3 | 1 | 0 |
| Edema | 1 | 0 | 0 |
| Elevated transaminases | 2 | 0 | 0 |
| Elevated lactate dehydrogenase | 1 | 0 | 0 |
| Erythema multiforme | 0 | 1 | 0 |
| Fatigue | 8 | 3 | 0 |
| Headache | 3 | 0 | 0 |
| Hypercholesterolemia | 2 | 0 | 0 |
| Hypertriglyceridemia | 1 | 0 | 0 |
| Hypoalbuminemia | 3 | 0 | 0 |
| Hypokalemia | 1 | 0 | 0 |
| Hyponatremia | 1 | 0 | 0 |
| Hypophosphatemia | 0 | 1 | 0 |
| Infection without neutropenia | 0 | 1 | 0 |
| Insomnia | 2 | 0 | 0 |
| Leukopenia | 3 | 0 | 0 |
| Lymphocytopenia | 1 | 1 | 0 |
| Muscle weakness | 0 | 1 | 0 |
| Nausea or vomiting† | 2 | 1 | 0 |
| Night blindness | 1 | 0 | 0 |
| Photoposia | 1 | 0 | 0 |
| Pruritus | 1 | 0 | 0 |
| Seizures | 0 | 1 | 0 |
| Skin rash | 2 | 1 | 0 |
| Speech impairment | 0 | 1 | 0 |
| Stomatitis | 1 | 0 | 0 |
| Thrombocytopenia | 3 | 0 | 0 |
| Watery eyes | 2 | 0 | 0 |
| Xerostomia | 3 | 0 | 0 |
| Total | 67 | 16 | 1 |
Grade 3 and grade 4 CNS hemorrhage (intratumoral bleeding) was noted in one patient each.
One patient had grade 3 nausea.
Table 3.
MDASI Assessment Results
| Symptom | Mean Rating | |
|---|---|---|
| Baseline | Follow-Up | |
| Pain | 1.36 | 1.71 |
| Fatigue | 2.64 | 4.46* |
| Nausea | 0.57 | 0.64 |
| Sleep disturbance | 1.50 | 1.62 |
| Distress | 3.64 | 3.08 |
| Shortness of breath | 1.14 | 1.36 |
| Memory problems | 2.71 | 3.54 |
| Loss of appetite | 2.14 | 3.08 |
| Drowsy | 1.79 | 2.08 |
| Dry mouth | 1.86 | 2.38 |
| Sadness | 2.43 | 3.00 |
| Vomiting | 2.71 | 2.93 |
| Numbness or tingling | 2.71 | 2.77 |
| Interferes with activity | 2.50 | 2.31 |
| Interferes with mood | 3.50 | 2.77 |
| Interferes with work | 2.00 | 2.38 |
| Interferes with relationships | 2.79 | 3.00 |
| Interferes with walking | 2.14 | 2.00 |
NOTE. MDASI data from 14 individuals were available for analysis. Symptoms rated from 7 to 10 are considered severe, whereas 4 to 7 are moderate. The Wilcoxon signed rank test was used to compare the baseline and follow-up symptom ratings. There was a significant increase in fatigue ratings on treatment, but this did not appear to interfere significantly with the patients’ ability to function.
Abbreviation: MDASI, M.D. Anderson Symptom Inventory.
P < .05.
Pharmacology
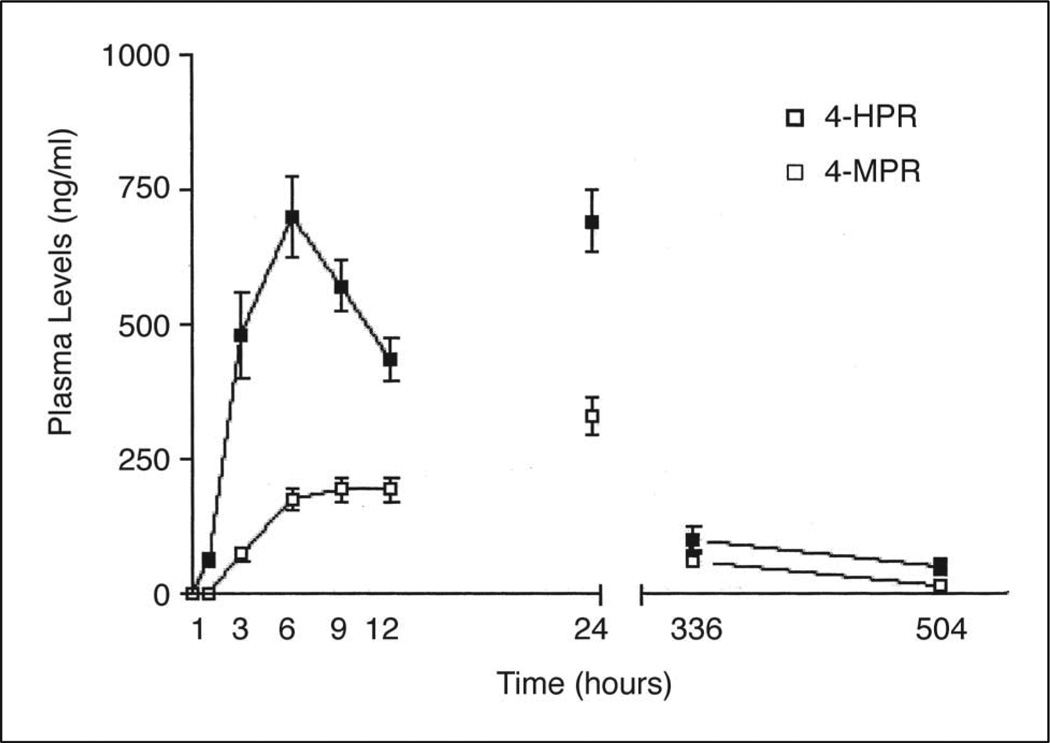
Thirty-two patients (15 with AG and 17 with GBM) had complete day 1 plasma concentration profiles for analysis: four of these patients underwent treatment with fenretinide at the 900 mg/m2 dosage (Table 4). Only one patient had a complete set of samples drawn on course 4 with an additional set obtained on course 13. At the 600 mg/m2 dose, the mean Cpmax was 832 ng/mL (2.1 µmol/L) for fenretinide and 232 ng/mL for its metabolite, 4-MPR. The AUC0–12 h values for fenretinide and 4-MPR were 5.8 and 1.6 µg × h/mL, respectively. At the 900 mg/m2 dosage, we observed proportionally higher mean Cpmax of fenretinide and 4-MPR (1,213 ng/mL [3.1µmol/L] and 337 ng/mL, respectively) and mean AUC0–12 h for fenretinide and 4-MPR (8.6 and 2.5 µg × h/mL, respectively). With the drug administration (with a high-fat meal) and sampling schedule used in this study, time to peak concentrations for fenretinide and 4-MPR occurred at a mean of 6.2 ± 2.6 and 9.8 ± 2.4 hours, respectively. After a single administration of fenretinide at a dose of 600 or 900 mg/m2, trough levels (12 hours) for fenretinide/4-MPR averaged 430/198 and 552/2.75 ng/mL, respectively. After the second administration, trough levels (24 hours) for fenretinide/4-MPR averaged 698/339 ng/mL at 600 mg/m2 and 879/414 ng/mL at 900 mg/m2. The data shown are from patients who received fenretinide at a dose of 600 mg/m2 (Fig 4); the data from the group who received the agent at a dose of 900 mg/m2 are not shown because of the small number of patients at this dose level. The harmonic mean terminal half-lives for fenretinide and 4-MPR were 91 ± 27 and 157 ± 73 hours, respectively, when evaluated in the interval between 7 and 14 days after drug interruption. The pharmacokinetic parameters for the patient who experienced a partial response at the 900 mg/m2 dosage were consistent with the population means. Blood samples were obtained during cycles 1, 4, and 13 from one patient with a low-grade oligodendroglioma who completed 21 cycles of fenretinide at 600 mg/m2 bid. There was little variation in the percentage decrease in retinol levels among the cycles (cycle 1, 57%; cycle 4, 51%; cycle 13, 48%). Cpmax and AUC0–12 h for fenretinide increased 1.4-and 1.3-fold, respectively between cycles 1 and 13.
Table 4.
Fenretinide (4-HPR) and 4-MPR Pharmacokinetic Parameters
| No. of Patients |
Dose (mg/m2) |
Cpmax (ng/mL) | AUC0–12 h (µg × h/mL) | Decrease in Retinol | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4-HPR | 4-MPR | 4-HPR | 4-MPR | |||||||||
| Value | SD | Value | SD | Value | SD | Value | SD | % | SD | Range | ||
| 26 | 600 | 832 | 360 | 232 | 125 | 5.84 | 2.47 | 1.64 | 0.93 | 45.83 | 10.05* | 25%–67% |
| 4 | 900 | 1213 | 261 | 337 | 113 | 8.60 | 2.17 | 2.45 | 0.83 | 50.33 | 5.03† | 45%–55% |
NOTE. Cpmax and AUC0–12 h reflective of 600 or 900 mg/m2 single administration. Retinol levels were assessed at baseline and 24 hours after fenretinide was first administered.
Abbreviations: 4-MPR, N-(4-methoxyphenyl)-retinamide; Cpmax, peak concentration; AUC, area under the concentration-time curve; SD, standard deviation.
n = 23
n = 3.
Fig 4.
Pharmacokinetic profiles of fenretinide (4-HPR) and N-(4-methoxyphenyl)-retinamide (4-MPR) in 26 patients. Plasma samples were obtained on day 1, course 1 at 0, 1, 3, 6, 9, and 12 hours, and trough levels at 24 hours, days 14 (336 hours) and 21 (504 hours) after the first administration of fenretinide at a dose of 600 mg/m2.
Brain tumor tissue was obtained at the time of surgery from a patient 16 days after the second cycle of fenretinide. Pathologic examination of the tissue specimens showed predominately necrosis with a few atypical cells. The central most necrotic portion of the resected tissue had low levels of fenretinide (2.9 ng/g wet weight) and 4-MPR (19.4 ng/g), but the peripheral solid component had higher levels of both fenretinide (211.2 ng/g) and 4-MPR (677.7 ng/g wet weight).
Retinol and Retinol-Binding Protein Levels
Retinol and RBP levels were available for 26 (600 mg/m2, n = 23; 900 mg/m2, n = 3) and 19 patients (600 mg/m2), respectively. Retinol levels were measured at baseline and 24 hours after first drug administration. The average percent decrease in retinol levels at the 600 and 900 mg/m2 dose level was 45.8% and 50.3%, respectively (Table 4). The baseline and 24-hour RBP levels were 71 ± 17.7 and 47 ± 46.5 ng/L, respectively. The average reduction in RBP was 36.5% ± 12.8%. The retinol levels of all but four patients at 600 mg/m2 and two patients at 900 mg/m2 returned to their baseline values before the second cycle. The changes in retinol and RBP levels were not associated with changes in visual function.
DISCUSSION
Unlike other retinoids, which are predominantly cytostatic, fenretinide induces apoptosis in several types of malignant cells and was well tolerated in phase I trials in both adults and children with solid tumors15,18,21 Our study is one of the earliest of several phase II trials to assess the efficacy of fenretinide against various solid tumors. All but four of the patients enrolled onto this trial were given fenretinide at a dose of 600 mg/m2 bid. Because more consistent plasma concentrations were achieved with higher doses without additional toxicity, a new phase II dosage of 900 mg/m2 bid was recommended by CTEP based on phase I data (data not published), and the last four patients enrolled in the AG arm were treated at this dose level.
Fenretinide did not demonstrate clinical efficacy against either AG or GBM at the doses and schedule used in this study. Several early treatment failures were seen in both the AG and GBM arms, suggesting that recurrent tumors continued to progress despite exposure to fenretinide as administered in this trial. However, of interest was the finding that one of the four patients with AG treated at the dosage of 900 mg/m2 bid showed a durable radiologic response and remained progression free, with no substantial toxicity after 13 cycles of therapy. This finding raises the possibility that fenretinide may have activity in a subset of patients with gliomas and that higher doses of the agent may be needed for antitumor efficacy.
A mean Cpmax of 2.1 µmol/L for fenretinide was achieved after the first administration of the agent at a dose of 600 mg/m2, whereas a higher mean Cpmax of 3.1 µmol/L was achieved with the 900 mg/m2 dose, suggesting that a dose-dependent increase in Cpmax and exposure (AUC0–12 h) was feasible in this patient population. Consistent with the half-life of fenretinide, 24-hour trough levels after the second administration were increased an average of 1.6-fold compared with the 12-hour trough levels. The increase in peak concentration and the AUC0–12 h of fenretinide observed in one patient between course 1 and course 13 was 1.4-fold and 1.3-fold, respectively. A two-fold increase in peak concentrations has been reported between days 1 and 28 on a daily single administration by other investigators.21
The β-phase half-life of fenretinide has been reported to be 25 to 27 hours with long-term daily administration.21,22 The half-lives of 91 hours for fenretinide and 157 hours for 4-MPR we report are probably an underestimate of the terminal elimination half-lives. The terminal half-live for fenretinide has been reported to range from 2.1 to 5.7 months and from 1.4 to 4.8 months for 4-MPR.22
Adverse effects attributable to fenretinide therapy were relatively mild and were limited mostly to grade 1 or 2 toxicities. Such adverse effects including fatigue, dry skin, anemia, and lipid abnormalities were characteristic of other retinoids but tended to be less frequent and milder. A patient who had a high international normalized ratio (> 6) while receiving oral anticoagulation for deep vein thrombosis died as a result of uncontrolled nasal bleeding while receiving fenretinide therapy. Another patient with an AG had a small intracranial bleed during the course of fenretinide therapy, resulting in a brief hospital admission, but recovered from the event and was able to resume therapy after discharge from the hospital until disease progression was noted. A previous report of a fatal intracerebral hemorrhage in a patient with chronic myelomonocytic leukemia after 3 weeks of treatment with fenretinide was attributed to an exacerbation of thrombocytopenia.23 Although the difference in cause for bleeding in these patients makes it less likely that these events were directly related to fenretinide therapy, a possible relationship of these events to the agent cannot be ruled out. Nyctalopia (night-blindness), another common adverse effect of retinoid therapy associated with decreased serum retinol levels, was not seen in this study despite careful assessment using a vision questionnaire aimed at identifying visual toxicity.24–27 Hepatotoxicity, a major limitation in the clinical use of other retinoids, was not a substantial adverse event in our patient population. Major adverse effects were not seen, perhaps due to the lower plasma levels of fenretinide found in this study compared with those in phase I trials, which were associated with more pronounced adverse events (unpublished data). Because fenretinide was formulated as 100-mg capsules, the number of capsules per administration ranged from nine to 18, which was perceived as inconvenient by several patients. Development of capsules incorporating a higher dose of the agent or of an intravenous formulation may alleviate this concern and permit administration of higher doses of fenretinide.
In breast cancer patients, fenretinide concentrations of approximately two-fold of that seen in plasma were detected in the contralateral normal breast tissue, suggesting that the drug accumulates in lipid-rich tissue.28 A recent study reported approximately five-fold higher breast tissue levels compared with plasma levels in patients with newly diagnosed breast cancer who underwent treatment with 100 to 300 mg of fenretinide 3 to 12 days before surgery.29 Given its lipophilicity, fenretinide was expected to cross the blood-brain barrier and reach sufficient concentration for activity in the tumor. We detected fenretinide and 4-MPR in resected brain tissue nearly 2 weeks after the last administration of the agent in a patient, indicating that the drug can not only cross the blood-brain barrier and accumulate in the brain tissue, but may also have a prolonged half-life in brain tissue.
The overall lack of major adverse events and the lack of activity suggest that higher doses of fenretinide could potentially be used in our patient population. However, there are several hurdles in using biologic agents such as fenretinide for therapeutic purposes that are becoming apparent in early studies.30 The optimal dose and schedule of fenretinide are uncertain and a definite maximum-tolerated dose has not been established. The complex biologic effects of fenretinide are difficult to evaluate in a clinical trial as a measure of the agent’s therapeutic activity. However, because the interest in fenretinide as a therapeutic agent against cancer is due to its ability to induce apoptosis, the measurement of apoptosis in the target tissue may be the optimal correlative measure to assess the activity of the agent and correlate it to clinical efficacy. Hence, future studies of this agent against gliomas may need to use higher doses of the agent, identify an optimal treatment schedule, and measure the degree of apoptosis and radiologic tumor response as correlative end points.
Acknowledgment
We thank Anne Sutton, Department of Scientific Publication, The University of Texas M.D. Anderson Cancer Center, for editorial assistance.
Supported in part by grant Nos. CA62399, CA62422, CA62412, CA16672, CA62455, CA62426, UO1CA62407-08, UO1CA62405, UO1CA62399, UO1CA62421, MO1-RR00079, MO1-RR00633, MO1-RR00056, MO1-RR0865, MO1-RR00042, and MO1-RR03186 from the National Institutes of Health, Bethesda, MD.
Footnotes
Authors’ Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Intl J Radiat Oncol Biol Phys. 1979;5:1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 2.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 3.Stewart LA. Chemotherapy in adult high-grade glioma: A systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 4.Love JM, Gudas LJ. Vitamin A, differentiation and cancer. Current Opin Cell Biol. 1994;6:825–831. doi: 10.1016/0955-0674(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 5.Bouterfa H, Picht T, Kess D, et al. Retinoids inhibit human glioma cell proliferation and migration in primary cell cultures but not in established cell lines. Neurosurgery. 2000;46:419–430. doi: 10.1097/00006123-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Yung WK, Kyritsis AP, Gleason MJ, et al. Treatment of malignant gliomas with high dose 13-cis retinoic acid. Clin Cancer Res. 1996;2:1931–1935. [PubMed] [Google Scholar]
- 7.Decensi A, Formelli F, Torrisi R, et al. Breast cancer chemoprevention: Studies with 4-HPR alone and in combination with tamoxifen using circulating growth factors as potential surrogate endpoints. J Cell Biochem Suppl. 1993;17G:226–233. doi: 10.1002/jcb.240531142. [DOI] [PubMed] [Google Scholar]
- 8.De Palo G, Camerini T, Marubini E, et al. Chemoprevention trial of contralateral breast cancer with fenretinide: Rationale, design, methodology, organization, data management, statistics and accrual. Tumori. 1997;83:884–894. doi: 10.1177/030089169708300603. [DOI] [PubMed] [Google Scholar]
- 9.Pienta KJ, Esper PS, Zwas F, et al. Phase II chemoprevention trial of oral fenretinide in patients at risk for adenocarcinoma of the prostate. Am J Clin Oncol. 1997;20:36–39. doi: 10.1097/00000421-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Decensi A, Bruno S, Costantini M, et al. Phase IIa study of fenretinide in superficial bladder cancer, using DNA flow cytometry as an intermediate end point. J Natl Cancer Inst. 1994;86:138–140. doi: 10.1093/jnci/86.2.138. [DOI] [PubMed] [Google Scholar]
- 11.Sun SY, Yue P, Lotan R. Induction of apoptosis by N-(4-hydroxyphenyl)retinamide and its association with reactive oxygen species, nuclear retinoic acid receptors, and apoptosis-related genes in human prostate carcinoma cells. Mol Pharmacol. 1999;55:403–410. [PubMed] [Google Scholar]
- 12.Maurer BJ, Metelitsa LS, Seeger RC, et al. Increase of ceramide and induction of mixed apoptosis/necrosis by N-(4-hydroxyphenyl)-retinamide in neuroblastoma cell lines. J Natl Cancer Inst. 1999;91:1138–1146. doi: 10.1093/jnci/91.13.1138. [DOI] [PubMed] [Google Scholar]
- 13.Puduvalli VK, Saito Y, Xu R, et al. Fenretinide activates caspases and induces apoptosis in gliomas. Clin Cancer Res. 1999;5:2230–2235. [PMC free article] [PubMed] [Google Scholar]
- 14.Saitoh Y, Goto T, Puduvalli VK, et al. Induction of apoptosis by N-(4-hydroxyphenyl)retinamide in glioma cells. Int J Oncol. 1999;15:499–504. doi: 10.3892/ijo.15.3.499. [DOI] [PubMed] [Google Scholar]
- 15.Parchment RE, Jasti BR, Kocarek TA, et al. Pharmacologic issues for fenretinide chemotherapy (4HPR, NSC-374551) Clin Cancer Res Suppl. 1999;5:3800s. (abstr 350) [Google Scholar]
- 16.Le Doze F, Debruyne D, Albessard F, et al. Pharmacokinetics of all-trans retinoic acid, 13-cis retinoic acid, and fenretinide in plasma and brain of rat. Drug Metab Dispos. 2000;28:205–208. [PubMed] [Google Scholar]
- 17.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Doose DR, Minn FL, Stellar S, et al. Effects of meals and meal composition on the bioavailability of fenretinide. J Clin Pharmacol. 1992;32:1089–1095. [PubMed] [Google Scholar]
- 19.Formelli F, Carsana R, Costa A, et al. Plasma retinol level reduction by the synthetic retinoid fenretinide: A one year follow-up study of breast cancer patients. Cancer Res. 1989;49:6149–6152. [PubMed] [Google Scholar]
- 20.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 21.Garaventa A, Luksch R, Piccolo MSL, et al. Phase I trial and pharmacokinetics of fenretinide in children with neuroblastoma. Clin Cancer Res. 2003;9:2032. [PubMed] [Google Scholar]
- 22.Formelli F, Clerici M, Campa T, et al. Five-year administration of fenretinide: Pharmacokinetics and effects on plasma retinol concentrations. J Clin Oncol. 1993;11:2036–2042. doi: 10.1200/JCO.1993.11.10.2036. [DOI] [PubMed] [Google Scholar]
- 23.Garewal HS, List A, Meyskens F, et al. Phase II trial of fenretinide [N-(4-hydroxyphenyl) retinamide] in myelodysplasia: Possible retinoid-induced disease acceleration. Leuk Res. 1989;13:339–343. doi: 10.1016/0145-2126(89)90071-4. [DOI] [PubMed] [Google Scholar]
- 24.Caruso RC, Zujewski J, Iwata F, et al. Effects of fenretinide (4-HPR) on dark adaptation. Arch Ophthalmol. 1998;116:759–763. doi: 10.1001/archopht.116.6.759. [DOI] [PubMed] [Google Scholar]
- 25.Modiano MR, Dalton WS, Lippman SM, et al. Ocular toxic effects of fenretinide. J Natl Cancer Inst. 1990;82:1063. doi: 10.1093/jnci/82.12.1063. [DOI] [PubMed] [Google Scholar]
- 26.Kingston TP, Lowe NJ, Winston J, et al. Visual and cutaneous toxicity which occurs during N-(4-hydroxyphenyl) retinamide therapy for psoriasis. Clin Exp Dermatol. 1986;11:624–627. doi: 10.1111/j.1365-2230.1986.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser-Kupfer MI, Peck GL, Caruso RC, et al. Abnormal retinal function associated with fenretinide, a synthetic retinoid. Arch Ophthalmol. 1986;104:69–70. doi: 10.1001/archopht.1986.01050130079024. [DOI] [PubMed] [Google Scholar]
- 28.Mehta RG, Moon RC, Hawthorne M, et al. Distribution of fenretinide in the mammary gland of breast cancer patients. Eur J Cancer. 1991;27:138–141. doi: 10.1016/0277-5379(91)90471-o. [DOI] [PubMed] [Google Scholar]
- 29.Sabichi AL, Modiano MR, Lee JJ, et al. Breast tissue accumulation of retinamides in a randomized short-term study of fenretinide. Clin Cancer Res. 2003;9:2400–2405. [PubMed] [Google Scholar]
- 30.Lang FF, Gilbert MR, Puduvalli VK, et al. Toward better early-phase brain tumor clinical trials: A reappraisal of current methods and proposals for future strategies. Neuro-oncol. 2002;4:268–277. doi: 10.1093/neuonc/4.4.268. [DOI] [PMC free article] [PubMed] [Google Scholar]