Abstract
Objectives
Despite significant medical advances, infective endocarditis (IE) remains an infection associated with high morbidity and mortality. The objective was to assess the safety and efficacy of high-dose daptomycin, defined as ≥8 mg/kg/day, in patients with confirmed or suspected staphylococcal and/or enterococcal IE.
Methods
This was a multicentre, retrospective observational study (2005–11). Adult patients, not undergoing haemodialysis, with blood cultures positive for staphylococci or enterococci and a definitive or possible diagnosis of IE, who received daptomycin ≥8 mg/kg/day (based on total body weight) for ≥72 h were included.
Results
Seventy patients met the inclusion criteria and comprised 33 (47.1%) with right-sided IE (RIE), 35 (50%) with left-sided IE (LIE) and 2 with both RIE and LIE. Several patients had concomitant sites of infection, with bone/joint infection being most prevalent (12.9%). Sixty-five patients received daptomycin as salvage therapy. Pathogens were isolated from 64 patients, with methicillin-resistant Staphylococcus aureus as the most common organism (84.4%), followed by vancomycin-resistant Enterococcus faecium (7.8%). The median (IQR) daptomycin dose was 9.8 mg/kg/day (8.2–10.0 mg/kg/day), and was similar in RIE and LIE patients (9.8 and 9.3 mg/kg/day, respectively). A total of 24 (34.3%) received combination therapy. For those patients with pathogens isolated (n = 64), the organism was eradicated in 57 (89.1%) patients. Among 64 clinically evaluable patients, 55 (85.9%) achieved clinical success. No patients required discontinuation of high-dose daptomycin due to creatine phosphokinase elevations.
Conclusions
Patients with both RIE and LIE had successful outcomes with high-dose daptomycin therapy. Additional clinical trials evaluating high daptomycin dosages in patients with IE are warranted.
Keywords: MRSA, infections, patient outcomes
Introduction
Infective endocarditis (IE) is a serious infection, which, despite important medical advances, remains associated with high morbidity and mortality.1,2 Complicating matters, drug resistance in pathogens that commonly cause IE, particularly methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE), have steadily increased during the past decade, severely hindering the choice of effective antimicrobial treatment.3,4 In fact, Fowler et al.5 revealed that S. aureus was the most common pathogen among a cohort of 1779 patients with IE, with MRSA isolated in >25% of these cases.
Vancomycin, a glycopeptide that has been available for >50 years, has been the primary treatment for invasive MRSA infections, including IE.6 However, the utilization of this antimicrobial has been questioned due to increasing reports of failure and decreased susceptibility.7–9 It is evident that there is a need for novel strategies in treating multidrug-resistant Gram-positive organisms in patients with serious infections such as IE. Daptomycin is an alternative to vancomycin for the treatment of serious infections, including IE,6,10,11 and is approved by the US FDA at 4 mg/kg/day for the treatment of complicated skin and skin structure infections and 6 mg/kg/day for the treatment of S. aureus bacteraemia, including right-sided endocarditis.12,13 However, based on its concentration-dependent activity, higher dosages may increase the rate of bacterial killing and reduce the emergence of resistance.14–16
The potential clinical response of higher doses is supported further both by in vitro pharmacokinetic/pharmacodynamic models utilizing high inocula of S. aureus and enterococci and in an animal model of IE against strains with reduced daptomycin susceptibility.17–19 Several case reports and post-marketing surveillance data have suggested that higher dosages may be safe and efficacious.20–23 A recent guideline recommends that, for patients with persistent MRSA bacteraemia and vancomycin failure, treatment with daptomycin at 10 mg/kg/day should be utilized;24 however, there are few clinical studies that have supported these recommendations.23,25,26 Although daptomycin is generally well tolerated, concerns about potential clinical or biochemical myositis as an adverse reaction warrant creatine phosphokinase (CPK) measurements weekly during therapy.27 Therefore, our objective was to assess the safety of high-dose daptomycin therapy, defined as ≥8 mg/kg/day, and the clinical response in patients with confirmed or suspected staphylococci and/or enterococcal IE in a multicentre evaluation.
Methods
Study design
From 2005 to 2011, a retrospective evaluation of high-dose daptomycin treatment was conducted at five medical centres in the USA.25 Participating institutions included: Detroit Medical Center, Detroit, MI, USA; Henry Ford Hospital, Detroit, MI, USA; Rush University Medical Center, Chicago, IL, USA; Sharp Memorial Hospital, San Diego, CA, USA; and Johns Hopkins Hospital, Baltimore, MD, USA. This study was approved by the Human Investigation Committee at each study site. Eligible subjects were all consecutive patients ≥18 years of age with positive blood cultures for staphylococcal or enterococcal species, who received daptomycin at ≥8 mg/kg/day (based on total body weight) for ≥72 h. Patients receiving any form of dialysis or renal replacement were excluded. The physicians caring for the patient at the time of initiation of therapy determined the treatment course. The IE subset included patients with a definite or possible diagnosis of IE as documented by the treating physician according to the modified Duke criteria.28
Clinical and demographic data collected included patient characteristics at the initiation of high-dose daptomycin therapy (e.g. age, gender, weight), presence of comorbid conditions (e.g. diabetes mellitus, renal disease, cerebral vascular accident), adverse events and the dose, frequency and duration of high-dose daptomycin therapy.
Clinical and microbiological outcomes
The primary outcomes were based on clinical and microbiological assessments of the primary diagnosis, IE, and were performed at the end of inpatient high-dose daptomycin therapy by the unblinded site investigator according to pre-defined clinical criteria. Clinical success included cure or improvement, defined as: (i) cure: signs and symptoms resolved and no additional antibiotic therapy was required and bacteraemia was cleared with negative cultures reported at the end of daptomycin therapy; and (ii) improvement: partial resolution of signs and symptoms and/or antibiotics were continued after inpatient high-dose daptomycin. Clinical failure was defined as an inadequate response to daptomycin therapy characterized by persistent, worsening or new/recurrent signs and symptoms or a positive culture reported at the end of daptomycin therapy. All patients meeting the inclusion criteria were evaluated for safety; clinical success was evaluated in the clinically evaluable population, which excluded patients for whom medical records did not contain all necessary information to determine response at the end of inpatient daptomycin therapy.
Pathogen information for Gram-positive isolates, site of infection and systemic Gram-positive therapy received before or during high-dose daptomycin were recorded for each patient. Organism identification and local susceptibility data were assessed for all positive blood cultures. Microbiological response was defined as organism eradication, organism persistence or no follow-up culture data available.
Additional clinical assessments included the presence and duration of fever (temperature ≥38.3°C), leucocytosis (≥10 × 103 cells/mm3), intensive care unit (ICU) admission, mechanical ventilation and positive blood culture (time from first positive blood culture to first day of 48 h of negative cultures). Patients' length of hospital stay and antibiotic therapy during hospitalization, discharge disposition and status 30 days post-discharge were also evaluated.
Safety assessments
Safety evaluations were recorded for all patients and included adverse events documented in the medical record by a treating physician as being suspected to be associated with daptomycin. Serum CPK levels, when available, were recorded as the baseline level, the level at the end of therapy and the highest level observed during therapy. CPK level elevations were defined as values >1000 IU/L [∼5× upper limit of normal (ULN)] in patients with unexplained signs and symptoms of myopathy and CPK levels >2000 IU/L (≥10× ULN) in patients without reported symptoms27 while on high-dose daptomycin therapy.
Statistical analysis
The primary analysis to evaluate drug utilization and outcomes was descriptive, with median (IQR), and proportions for categorical data. Additionally, characteristics potentially associated with clinical or microbiological outcome were compared using the χ2 test for categorical variables, and continuous variables were compared by Student's t-test or the Mann–Whitney U-test. A P value of <0.05 was considered significant. All calculations were computed using SPSS, version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Patients
A total of 70 patients with definitive or possible IE received high-dose daptomycin during the study period. Fifty-nine patients (84.3%) received vancomycin prior to high-dose daptomycin for a median (IQR) of 4 days (2–8 days). Eight patients (11.4%) received <8 mg/kg/day of daptomycin for a median of 4 days prior to being switched to high-dose daptomycin. Of the 70 patients, 33 (47.1%) had right-sided IE (RIE), 35 (50%) had left-sided IE (LIE), and 2 had both RIE and LIE; these two patients were analysed as part of the LIE group. Patient characteristics of the entire cohort and comparison of RIE versus LIE are displayed in Table 1. Compared with the LIE group, the RIE group were significantly younger, had a higher percentage of patients with liver disease and had a higher percentage of injection drug users. Several patients had concomitant sites of infection, with bone/joint infections being the most prevalent (12.9%), followed by skin/wound (11.4%) and hardware-related (8.6%) infections. Sixty-five of the 70 (92.9%) patients received daptomycin as salvage therapy; these patients failed to clear blood cultures while receiving another antimicrobial therapy directed at Gram-positive organisms prior to high-dose daptomycin.
Table 1.
Demographic and baseline clinical characteristics of patients
| Clinical characteristic | RIE (n = 33), median (IQR) or n (%) | LIE (n = 37), median (IQR) or n (%) | Total (n = 70), median (IQR) or n (%) |
|---|---|---|---|
| Age (years)a | 49 (40–58) | 56 (50–68) | 53 (44–63) |
| Gender | |||
| female | 17 (51.5) | 14 (37.8) | 31 (44.3) |
| male | 16 (48.5) | 23 (62.2) | 39 (55.7) |
| Race | |||
| white | 11 (33.3) | 14 (37.8) | 25 (35.7) |
| black | 21 (63.6) | 22 (59.5) | 43 (61.4) |
| other | 1 (3.0) | 1 (2.7) | 2 (2.8) |
| APACHE II score | 6 (4–9) | 8 (5–11) | 7 (5–10) |
| Weight (kg) | 66.7 (58.9–79.2) | 75 (62.9–86.1) | 71.5 (59.9–86.0) |
| Creatinine clearance (mL/min) | 71.7 (57.8–97.8) | 65.7 (40.8–91.6) | 69.7 (53.3–95.3) |
| Prior hospitalization ≤1 year | 18 (54.5) | 20 (54.1) | 38 (54.3) |
| Prior MRSA infection ≤1 year | 10 (30.3) | 5 (13.5) | 15 (21.4) |
| Prior vancomycin ≤30 days of indexed hospitalization | 5 (15.2) | 4 (10.8) | 9 (12.9) |
| Prosthetic device/hardware | 2 (6.1) | 6 (16.2) | 8 (11.4) |
| Renal disease (ARF or CKD) | 7 (21.2) | 10 (27) | 17 (24.3) |
| Injection drug usera | 25 (75.8) | 17 (45.9) | 42 (60) |
| Liver disease (hepatitis or cirrhosis)a | 16 (48.5) | 9 (24.3) | 25 (35.7) |
| Diabetes | 6 (18.2) | 9 (24.3) | 15 (21.4) |
APACHE II, Acute Physiology and Chronic Health Evaluation II; ARF, acute renal failure; CKD, chronic kidney disease.
aP < 0.05 between patients with RIE and LIE.
A pathogen was isolated in 64 patients (91.4%), with MRSA as the most common organism [54 patients (84.4%)], followed by vancomycin-resistant Enterococcus faecium [5 patients (7.8%)]. One patient had vancomycin-susceptible Enterococcus faecalis isolated, one patient had methicillin-resistant S. epidermidis isolated, one patient had methicillin-susceptible S. aureus (MSSA) isolated and two patients had both MRSA and Streptococcus viridans isolated. The distribution of organisms between the RIE and LIE groups was similar.
Overall, the median (IQR) daptomycin dose was 9.8 mg/kg/day (8.2–10.0 mg/kg/day), and was similar in RIE and LIE patients (9.8 and 9.3 mg/kg/day, respectively). Eleven patients (33.3%) with RIE received combination therapy compared with 13 (35.1%) in the LIE group. Antimicrobial agents co-administered with daptomycin included gentamicin (25.7%), gentamicin and rifampicin (20%), trimethoprim/sulfamethoxazole (7.1%), clindamycin (5.7%) and linezolid (5.7%). The primary pathogen isolated in these patients receiving combination therapy was MRSA. Patients with RIE received combination therapy for a median (IQR) of 3 days (1–7 days) versus 5 days (3–11 days) for those with LIE. There were no differences in outcomes in patients that received combination therapy compared with those that did not.
Clinical outcomes
The clinical courses of patients with RIE and LIE are displayed in Figure 1. Sixty-four (91.4%) patients were clinically evaluable, with 55/64 (85.9%) patients deemed clinical success. Nine (14.1%) patients were assessed as clinical failures and six (8.6%) patients were non-evaluable. Among the patients with RIE, 26 (86.7%) were assessed as clinical success, four (13.3%) failed therapy and three had indeterminate outcomes due to underlying conditions. Success and failure rates were similar in the LIE group, with 29 (85.3%) patients deemed clinical success, 5 (14.7%) assessed as clinical failure and 3 considered non-evaluable. MRSA was isolated from eight (88.9%) of the nine patients that were deemed clinical failures. No patients who failed therapy had any other concomitant sites of infection. Discharge dispositions were as follows: 29 (41.4%) were discharged home; 11 (15.7%) were discharged to skilled nursing facilities; 11 (15.7%) were discharged to rehabilitation facilities; 9 (12.9%) patients died during hospitalization; 3 (4.3%) patients were transferred to another hospital; and 7 (10%) patients had other dispositions. Thirty-day follow-up was available for 52/70 patients, with 44 (84.6%) alive and 8 (15.4%) died (either during hospitalization or within 30 days after discharge). Two of the nine patients that died had their death attributed to the infection. The first patient that died had MRSA LIE that required an aortic valve replacement, as well as MRSA meningitis with a haemorrhagic embolic cerebrovascular accident and subarachnoid haemorrhage. The second patient that died had E. faecalis aortic and mitral valve IE with concomitant multidrug-resistant Acinetobacter baumannii pneumonia.
Figure 1.
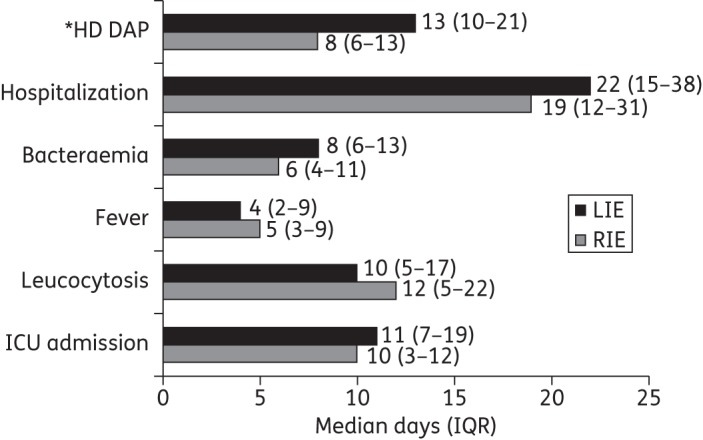
Clinical course of patients with RIE and LIE. *High-dose daptomycin treatment while in hospital.
Microbiological outcomes and organism characteristics
For those patients with pathogens isolated (n = 64), the organism was eradicated in 57 (89.1%) patients, persisted at end of daptomycin therapy in 6 (9.4%) patients [median (IQR) days of persistent bacteraemia: 21 days (7–30 days)] and 1 (1.6%) patient had no follow-up culture available. Each of the six patients who had persistent bacteraemia was infected with MRSA; data on subsequent antimicrobial therapy were available for three. Two of the six patients with persistent bacteraemia received combination therapy. One patient cleared his bacteraemia after receiving concomitant high-dose daptomycin (10 mg/kg), trimethoprim/sulfamethoxazole and rifampicin. Bacteraemia cleared in the second patient when he was switched from daptomycin to trimethoprim/sulfamethoxazole, and the last patient cleared on vancomycin (15 mg/kg) post-daptomycin therapy. Patients with RIE and LIE had similar microbiological outcomes.
The MIC50 and MIC90 values of daptomycin for baseline S. aureus isolates (n = 45) were 0.5 and 1 mg/L, respectively (range 0.38–2 mg/L). The MIC50 and MIC90 values for baseline S. aureus isolates for vancomycin were 1.5 (Etest) and 2 mg/L (broth microdilution) (range ≤0.5–2 mg/L). The daptomycin MIC50 and MIC90 for baseline enterococcal isolates were both 2 mg/L (range 1 to >4 mg/L). For vancomycin the MIC50 and MIC90 for baseline enterococcal isolates were both ≥64 mg/L (range 0.5 to ≥64 mg/L). Six (8.6%) patients developed non-susceptibility to daptomycin (Table 2); each had MRSA IE and had prior vancomycin exposure. Daptomycin non-susceptibility was identified after a median of 11 days of receiving high-dose daptomycin. Of note, five patients from the cohort of 70 patients received high-dose daptomycin as first-line therapy; none developed non-susceptibility to daptomycin.
Table 2.
Patients with MRSA IE developing non-susceptibility to daptomycin
| IE | DAP MIC (mg/L) | DAP MIC change | VAN MIC (mg/L) | VAN exposure (days) | Outcome |
|---|---|---|---|---|---|
| RIE | 0.38→4 | day 7 HD DAP | 1.5→2 | 17 | cleared on SXT |
| RIE | 1→4 | day 1 HD DAP | 2→2 | 5 | cleared on SXT |
| RIE | 0.5→4 | day 21 HD DAP | 1→2 | ≤30 days prior to admission | organism persisted |
| LIE | 1→4 | day 8 HD DAP | 2→2 | 2 | cleared on HD DAP |
| RIE/LIE | 0.5→4 | day 11 HD DAP | hVISA 2→4 | prior to admission VAN ×6 weeks | cleared on HD DAP |
| RIE/LIE | 1→2 | day 18 HD DAP | 1.5→2 | 20 | cleared on HD VAN |
DAP, daptomycin; VAN, vancomycin; HD, high-dose; hVISA, heterogeneous vancomycin-intermediate S. aureus; SXT, trimethoprim/sulfamethoxazole.
Safety
Two (2.9%) patients experienced mild or moderate adverse events attributed to high-dose daptomycin therapy. One patient experienced hyperkalaemia on day 7 of therapy and was switched to an alternative agent. The second patient developed thrombocytosis 5 days into therapy and remained on high-dose daptomycin, with the platelet count resolving. Median (IQR) values for baseline (n = 58), observed peak level (n = 51) and end-of-therapy (n = 37) available CPK levels were as follows: 45 (27–103), 74 (45–271) and 38 IU/L (26–97 IU/L), respectively. No patients developed an elevated CPK level that reached an abnormal value. There were 10 (14.3%) patients taking concomitant 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase inhibitors and none discontinued daptomycin therapy due to myopathy or myositis. Five (7.1%) patients with normal baseline CPK levels experienced an elevation in CPK (median 272 IU/L); however, all remained asymptomatic and none required discontinuation of high-dose daptomycin.
Discussion
We evaluated 70 patients with RIE or LIE who were treated with ≥8 mg/kg/day of daptomycin and found a success rate of 85.9% at the end of high-dose daptomycin therapy and 84.6% survival at 30 days. High-dose daptomycin was well tolerated, with only one patient requiring discontinuation of therapy due to an adverse event of hyperkalaemia, a previously unreported adverse event that may also have arisen from underlying conditions.
There are currently few data on the safety and clinical effectiveness of high-dose daptomycin in patients with IE, particularly with MRSA as the primary pathogen. Cunha et al.29,30 described two case reports of patients who had E. faecalis vancomycin-susceptible enterococcal bacteraemia and MRSA mitral valve acute bacterial endocarditis, both treated successfully with high-dose daptomycin, defined as 12 mg/kg/day. Durante-Mangoni et al.26 recently evaluated the efficacy and safety of high-dose daptomycin (defined as >6 mg/kg/day) in 25 patients with cardiac implantable electronic device-related IE due to staphylococci. The investigators noted an overall clinical success rate of 80% and microbiological eradication of 92%, with 20% of patients experiencing a significant CPK elevation; however, no patients required discontinuation of daptomycin due to adverse events or myopathy. Most studies describe the use of lower doses of daptomycin, the largest being that recorded in the Cubicin Outcomes Registry and Experience (CORE) database, where 49 patients with IE were evaluated.31 The median (range) dose of daptomycin was 6 mg/kg/day (4–7 mg/kg/day), with 55% patients receiving ≥6 mg/kg/day. Clinical outcomes were favourable, with 63% of patients assessed as clinical success. In a randomized registration trial13 6 mg/kg/day of daptomycin was compared with combination therapy with either a semi-synthetic penicillin or vancomycin plus initial gentamicin in patients with S. aureus bacteraemia and endocarditis. Among patients with RIE or bacteraemia, the clinical success rates of 70.0% for those who received daptomycin monotherapy and 68.7% among those who received the comparator combinations were similar to our results. However, in that study there were very few patients with LIE. Only a few of these patients received the full course of therapy and none of the patients with LIE due to MRSA were adjudicated as clinical successes. Additionally, the ascertainment of clinical success was based on a different definition compared with our study. The difference in outcomes may also be attributed to the high proportion of injection drug user (IDU)-related cases in our cohort. These patients tend to be younger with fewer comorbidities and often have better outcomes than non-IDUs.
Of note, the utilization of high-dose daptomycin has been shown to be beneficial in in vitro studies, in which there was enhanced killing and decreased emergence of resistance in simulated S. aureus and enterococci endocarditis vegetations in an in vitro pharmacokinetic/pharmacodynamic model treated with 8–12 mg/kg of daptomycin compared with dosages of 6 mg/kg.15,18,19,32,33 Additionally, since Enterococcus species typically have higher MICs of daptomycin than other Gram-positive organisms (0.5–4 versus 0.25–1 mg/L), patients with these serious infections may require higher dosages of daptomycin for optimal treatment.10,18,24,34
All the S. aureus and enterococci isolates were susceptible to daptomycin at onset of therapy. Six (8.6%) patients with MRSA IE developed non-susceptibility to daptomycin; all had prolonged prior vancomycin exposure, consistent with prior observations.35 From the patients with follow-up data available, all cleared their infection when switched to an alternative agent or on high-dose daptomycin.
We observed a low incidence of toxicity associated with high-dose daptomycin in this population, with only two patients experiencing either mild or moderate adverse reactions. The median end-of-therapy CPK level was 38 IU/L (26–97 IU/L). In a previous report, which included these cases, we described 250 patients with complicated Gram-positive infections receiving ≥8 mg/kg/day of daptomycin.25 High-dose daptomycin was well tolerated in the larger study cohort, with 10 (8.5%) patients with end-of-therapy CPK levels >200 IU/L (normal CPK levels are <200 IU/L) and no patients with values >604 IU/L at the end of therapy. Additionally, there was no significant correlation found between daptomycin dose and highest observed CPK level. Similarly, Figueroa et al.22 assessed the safety of ≥6 mg/kg of daptomycin in 61 patients. The mean dose that patients received was 8 mg/kg for a median of 25 days. The authors found that 3/61 (4.9%) patients experienced grade 3 CPK level elevations (levels >1000 IU/L), with all cases reversible by the discontinuation of daptomycin therapy.
There are several limitations of this study that should be noted. First, this was a subset analysis of a prior study25 that was not designed to collect specific information about the nature, diagnosis and management of IE, including surgical interventions, which are often indicated in patients at high risk of complications. However, the results of this study are still valuable since these patient cases were predominantly treatment-experienced and had serious comorbid conditions. Additionally, the retrospective observational nature of the study design did not allow for a matched cohort of patients who received standard dosages of daptomycin; however, since a majority of patients received daptomycin as salvage therapy, obtaining a comparable group would be difficult. Further, as a majority of our patients were placed on vancomycin prior to daptomycin, additional information regarding dosage and trough levels may have been beneficial. However, patients were placed on vancomycin for a short period of time (median 4 days) and our primary objective in this study was to evaluate the effectiveness of high-dose daptomycin. Lastly, retrospective, observational studies, such as our study, to assess toxicity are suboptimal for non-laboratory-based events.
In conclusion, high-dose daptomycin, defined as ≥8 mg/kg/day, may be an effective and safe antimicrobial for patients with either RIE or LIE. In addition, as noted above, it is important that our study revealed that patients with both RIE and LIE had successful outcomes with high-dose daptomycin therapy. Additional clinical trials evaluating high dosages of daptomycin in patients with IE are warranted.
Funding
This study was carried out as part of our routine work. M. J. R. is funded in part by NIH grant R21A1092055-01.
Transparency declarations
At the time of writing, R. K. had received speaking honoraria from Cubist Pharmaceuticals and Forest Laboratories, and served on the Advisory Board of Optimer Pharmaceuticals. R. K. is now employed by Cubist Pharmaceuticals and owns Cubist Pharmaceuticals stock. A. M. C. has received grant support from Cubist Pharmaceuticals, Forest Laboratories and the Michigan Department of Community Health. S. L. D. has served on the Advisory Board of Forest Laboratories. D. P. L. has received grant support from AstraZeneca, Cubist Pharmaceuticals and Cerexa, received speaking honoraria from AstraZeneca, Merck, Novartis, Forest and Cubist Pharmaceuticals, and served on the Advisory Board of Cerexa, Forest, RibX, Theravance and Cubist Pharmaceuticals. G. S. has received grant support from Forest Laboratories and speaking honoraria from Cubist Pharmaceuticals and Forest Laboratories. S. E. C. has received grant support from Cubist Pharmaceuticals and has consulted for Novartis. M. J. R. has received grant support from, consulted for or provided lectures for Cubist Pharmaceuticals, Forest Laboratories, Durata, Cepheid and Novartis. J. J. Z., C. W. C. and J. S.: none to declare.
References
- 1.Benito N, Miro JM, de Lazzari E, et al. Health care-associated native valve endocarditis: importance of non-nosocomial acquisition. Ann Intern Med. 2009;150:586–94. doi: 10.7326/0003-4819-150-9-200905050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez-Hidalgo N, Almirante B, Tornos P, et al. Contemporary epidemiology and prognosis of health care-associated infective endo-carditis. Clin Infect Dis. 2008;47:1287–97. doi: 10.1086/592576. [DOI] [PubMed] [Google Scholar]
- 3.Grundmann H, Aires-de-Sousa M, Boyce J, et al. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet. 2006;368:874–85. doi: 10.1016/S0140-6736(06)68853-3. [DOI] [PubMed] [Google Scholar]
- 4.Fridkin SK, Lawton R, Edwards JR, et al. Monitoring antimicrobial use and resistance: comparison with a national benchmark on reducing vancomycin use and vancomycin-resistant enterococci. Emerg Infect Dis. 2002;8:702–7. doi: 10.3201/eid0807.010465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler VG, Jr, Miro JM, Hoen B, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–21. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 7.Kullar R, Davis SL, Levine DP, et al. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: support for consensus guidelines suggested targets. Clin Infect Dis. 2011;52:975–81. doi: 10.1093/cid/cir124. [DOI] [PubMed] [Google Scholar]
- 8.Lodise TP, Graves J, Evans A, et al. Relationship between vancomycin MIC and failure among patients with methicillin-resistant Staphylococcus aureus bacteremia treated with vancomycin. Antimicrob Agents Chemother. 2008;52:3315–20. doi: 10.1128/AAC.00113-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khatib R, Johnson LB, Fakih MG, et al. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand J Infect Dis. 2006;38:7–14. doi: 10.1080/00365540500372846. [DOI] [PubMed] [Google Scholar]
- 10.Cha R, Grucz RG, Jr, Rybak MJ. Daptomycin dose-effect relationship against resistant gram-positive organisms. Antimicrob Agents Chemother. 2003;47:1598–603. doi: 10.1128/AAC.47.5.1598-1603.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mortin LI, Li T, Van Praagh AD, et al. Rapid bactericidal activity of daptomycin against methicillin-resistant and methicillin-susceptible Sta-phylococcus aureus peritonitis in mice as measured with bioluminescent bacteria. Antimicrob Agents Chemother. 2007;51:1787–94. doi: 10.1128/AAC.00738-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arbeit RD, Maki D, Tally FP, et al. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin Infect Dis. 2004;38:1673–81. doi: 10.1086/420818. [DOI] [PubMed] [Google Scholar]
- 13.Fowler VG, Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653–65. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 14.Akins RL, Rybak MJ. Bactericidal activities of two daptomycin regimens against clinical strains of glycopeptide intermediate-resistant Staphylo-coccus aureus, vancomycin-resistant Enterococcus faecium, and methicillin-resistant Staphylococcus aureus isolates in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother. 2001;45:454–9. doi: 10.1128/AAC.45.2.454-459.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose WE, Rybak MJ, Kaatz GW. Evaluation of daptomycin treatment of Staphylococcus aureus bacterial endocarditis: an in vitro and in vivo simulation using historical and current dosing strategies. J Antimicrob Chemother. 2007;60:334–40. doi: 10.1093/jac/dkm170. [DOI] [PubMed] [Google Scholar]
- 16.Rose WE, Leonard SN, Sakoulas G, et al. Daptomycin activity against Staphylococcus aureus following vancomycin exposure in an in vitro pharmacodynamic model with simulated endocardial vegetations. Anti-microb Agents Chemother. 2008;52:831–6. doi: 10.1128/AAC.00869-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chambers HF, Basuino L, Diep BA, et al. Relationship between susceptibility to daptomycin in vitro and activity in vivo in a rabbit model of aortic valve endocarditis. Antimicrob Agents Chemother. 2009;53:1463–7. doi: 10.1128/AAC.01307-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall AD, Steed ME, Arias CA, et al. Evaluation of standard- and high-dose daptomycin versus linezolid against vancomycin-resistant Enterococcus isolates in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother. 2012;56:3174–80. doi: 10.1128/AAC.06439-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose WE, Leonard SN, Rybak MJ. Evaluation of daptomycin pharmacodynamics and resistance at various dosage regimens against Staphylococcus aureus isolates with reduced susceptibilities to dapto-mycin in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother. 2008;52:3061–7. doi: 10.1128/AAC.00102-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz DE, Lindfield KC, Steenbergen JN, et al. A pilot study of high-dose short duration daptomycin for the treatment of patients with complicated skin and skin structure infections caused by Gram-positive bacteria. Int J Clin Pract. 2008;62:1455–64. doi: 10.1111/j.1742-1241.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 21.Mohr JF, Friedrich LV, Yankelev S, et al. Daptomycin for the treatment of enterococcal bacteraemia: results from the Cubicin Outcomes Registry and Experience (CORE) Int J Antimicrob Agents. 2009;33:543–8. doi: 10.1016/j.ijantimicag.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa DA, Mangini E, Amodio-Groton M, et al. Safety of high-dose intravenous daptomycin treatment: three-year cumulative experience in a clinical program. Clin Infect Dis. 2009;49:177–80. doi: 10.1086/600039. [DOI] [PubMed] [Google Scholar]
- 23.Moise PA, Hershberger E, Amodio-Groton MI, et al. Safety and clinical outcomes when utilizing high-dose (≥8 mg/kg) daptomycin therapy. Ann Pharmacother. 2009;43:1211–9. doi: 10.1345/aph.1M085. [DOI] [PubMed] [Google Scholar]
- 24.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–92. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 25.Kullar R, Davis SL, Levine DP, et al. High-dose daptomycin for treatment of complicated gram-positive infections: a large, multicenter, retrospective study. Pharmacotherapy. 2011;31:527–36. doi: 10.1592/phco.31.6.527. [DOI] [PubMed] [Google Scholar]
- 26.Durante-Mangoni E, Casillo R, Bernardo M, et al. High-dose daptomycin for cardiac implantable electronic device-related infective endocarditis. Clin Infect Dis. 2012;54:347–54. doi: 10.1093/cid/cir805. [DOI] [PubMed] [Google Scholar]
- 27.Product Information: Cubicin®, Daptomycin For Injection. Lexington, MA: Cubist Pharmaceuticals, Inc.; 2003. [Google Scholar]
- 28.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 29.Cunha BA, Mickail N, Eisenstein L. E. faecalis vancomycin-sensitive enterococcal bacteremia unresponsive to a vancomycin tolerant strain successfully treated with high-dose daptomycin. Heart Lung. 2007;36:456–61. doi: 10.1016/j.hrtlng.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Cunha BA, Krol V, Kodali V. Methicillin-resistant Staphylococcus aureus (MRSA) mitral valve acute bacterial endocarditis (ABE) in a patient with Job's syndrome (hyperimmunoglobulin E syndrome) successfully treated with linezolid and high-dose daptomycin. Heart Lung. 2008;37:72–5. doi: 10.1016/j.hrtlng.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Levine DP, Lamp KC. Daptomycin in the treatment of patients with infective endocarditis: experience from a registry. Am J Med. 2007;120(Suppl 1):S28–33. doi: 10.1016/j.amjmed.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 32.Tsuji BT, Rybak MJ. Short-course gentamicin in combination with daptomycin or vancomycin against Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Anti-microb Agents Chemother. 2005;49:2735–45. doi: 10.1128/AAC.49.7.2735-2745.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy S, Chambers HF. Daptomycin (LY146032) for prevention and treatment of experimental aortic valve endocarditis in rabbits. Antimicrob Agents Chemother. 1989;33:1522–5. doi: 10.1128/aac.33.9.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tedesco KL, Rybak MJ. Daptomycin. Pharmacother. 2004;24:41–57. doi: 10.1592/phco.24.1.41.34802. [DOI] [PubMed] [Google Scholar]
- 35.Sakoulas G, Alder J, Thauvin-Eliopoulos C, et al. Induction of daptomycin heterogeneous susceptibility in Staphylococcus aureus by exposure to vancomycin. Antimicrob Agents Chemother. 2006;50:1581–5. doi: 10.1128/AAC.50.4.1581-1585.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


