Abstract
HIV mother-to-child transmission (MTCT) is significantly reduced if antepartum viral load (apVL) is<50 copies/mL. Pharmacokinetic studies suggest increasing the dosage of lopinavir/ritonavir (LPV/r) in pregnancy. It is important to assess tolerance, safety, and rate of patients presenting a apVL<50 copies/mL when treating with increased dose of LPV/r during pregnancy. Confirmed HIV-infected pregnant women with a fetus at a gestational age of 14–33 weeks were randomly assigned to receive LPV/r 400/100 or 600/150 mg b.i.d. plus two nucleoside analogues (NRTIs). Treatment was discontinued in the case of alanine transaminase (ALT) of grade III elevation or higher, glucose, or triglycerides. Thirty-two women were randomized to the LPV/r 400/100 mg dose, and 31 women were randomized to the 600/150 mg dose. Overall, 9.4% of the women receiving the conventional dose, and 17.2% receiving the increased dose, discontinued treatment because of adverse events (p=0.29). The rates of gastrointestinal (GI) symptoms, laboratory abnormalities, preterm delivery, and low birth weight were similar in both groups. There were no cases of HIV MTCT. Among the women with a baseline VL>50 copies/mL assigned to the conventional dose group, 45% (95% confidence interval [CI] 62.5–27.5%) had a apVL>50 copies/mL compared with 10.5% (95% CI 21.6–0.6%) of those assigned to the increased dose group (p=0.01). There was no significant difference found for the patients with a baseline VL<50 copies/mL. In pregnant women with a baseline VL>50 copies/mL, it may be warranted to initiate LPV/r dosing at 600/150 mg, whereas the conventional dose is sufficient for pregnant women with a baseline VL<50 copies/mL.
Introduction
The magnitude of antepartum viral load (apVL) is directly related to the rate of mother-to-child transmission (MTCT) of HIV-1.1 An observational study found an 8% MTCT rate in pregnant women who received zidovudine monotherapy, a 3% rate for women who received dual antiretroviral therapy, and a 1.6% rate for women who received highly active antiretroviral therapy (HAART).2 Other studies that have examined HAART observed rates of MTCT<0.4% if the patient apVL was<50 copies/mL.3,4 To achieve an undetectable viral load, all current recommendations for the management of HIV-infected pregnant women propose the use of HAART.5–7 Ritonavir-boosted lopinavir (LPV/r) is the protease inhibitor (PI) of choice for the treatment/prophylaxis of HIV-infected pregnant women.
Physiological changes during pregnancy can significantly alter LPV serum levels, especially in the third trimester, which can compromise antepartum viral suppression and increase the risk of MTCT.8,9 Several pharmacokinetic studies have suggested that LPV/r dose should be increased during pregnancy, but there are no randomized clinical studies comparing conventional and increased doses of LPV/r during pregnancy.
The objective of the present study was to evaluate the safety, tolerability, and antepartum viral load with an increased dose of LPV/r during pregnancy, compared with a conventional dose, and to compare the rates of patients achieving an HIV RNA apVL of<50 copies/mL and the rates of adverse events affecting the pregnant women and their fetuses.
Methods
Study design
A computer algorithm was used to perform a block randomization with randomly selected block sizes of four and six, to allocate pregnant women to equally receive either 400 mg lopinavir in combination with 100 mg ritonavir (conventional dose) or 600 mg lopinavir in combination with 150 mg ritonavir (increased dose), b.i.d., in combination with two nucleoside analogues (NRTIs) selected at the discretion of the attending physician. Clinicians were not blinded to treatment arm. This protocol was approved by the Committee for Ethics in Research of the Universidade Federal de São Paulo (UNIFESP) Medical School, Brazil. The study was conducted between August 2008 and January 2012 at the reference center for HIV-infected pregnant women at the UNIFESP Medical School, Brazil.
Participants
We enrolled 63 pregnant women with a confirmed HIV infection that had not previously shown resistance to LPV/r and with a fetus whose gestational age was between 14 and 33 weeks. These women agreed to participate by signing an informed consent form. Women with blood glucose levels>126 mg/dL, triglycerides (TG) >500 mg/dL, and alanine transaminase (ALT) >2.5 times the upper limit of normality were excluded.
Intervention
After randomization, the pregnant women were evaluated monthly during pregnancy to monitor the occurrence of gastrointestinal (GI) symptoms (diarrhea, nausea, and vomiting) using a standardized questionnaire, changes in laboratory tests (TG, total and lipoprotein fraction cholesterol, fasting glucose, transaminases, creatinine, and bilirubin), CD4 count, and HIV-1 viral load. Gestational age and weight at delivery and antepartum viral load were assessed 15 days post-delivery. Following delivery, the LPV/r dose was reduced to the conventional dosing level for all of the pregnant women who had been receiving the increased dose level.
Study outcome and end-points
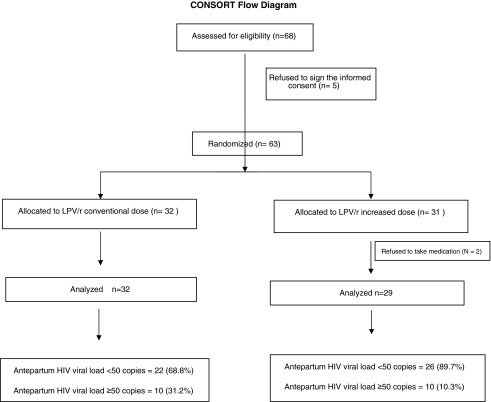
The primary outcome was an HIV RNA level<50 copies/mL in samples collected during the last 4 weeks of pregnancy, as illustrated in Fig. 1. The patients were followed until delivery or withdrawal of informed consent, or until they were removed from the study because of of grade III clinical symptoms (diarrhea, vomiting, or nausea) or laboratory abnormalities (glycemia, TG, ALT, or creatinine) according to the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events – DAIDS.10
FIG. 1.
CONSORT flow diagram.
Statistical analyses
The baseline characteristics of the increased and conventional LPV/r groups were compared using Student's t test for continuous variables and a χ2 test or Fisher's exact test for categorical variables. The rates of GI symptoms, laboratory abnormalities, preterm delivery, and low-birth weight were compared between the two groups using the χ2 test. Variables including the LPV/r group associated with an antepartum viral load<50 copies/mL by the univariate analysis were further analyzed using a logistic regression model. Differences with a p value of<0.05 were interpreted as a statistically significant. SPSS software version 20 (IBM, Armonk, NY) was used for all statistical analyses.
Results
All 61 patients were randomized. Thirty-two patients received the conventional dose, and 31 received the increased dose. Two women in the increased dose group were excluded for protocol violations. The mean time of LPV/r use was 85 days for the conventional dose group (interquartile range [IQR] 62–112) and 86 days for the increased dose group (IQR 62.25–114.50), and eight and ten patients, respectively, had been already receiving LPV/r prior to the randomization. The baseline demographic, clinical, anthropometric, and laboratory characteristics of the two groups are shown in Table 1.
Table 1.
Baseline Demographic, Clinical, Anthropometric, and Laboratory Characteristics of the Conventional Dose and Increased Dose Groups
| Conventional dose (Group 1) | Increased dose (Group 2) | p | |
|---|---|---|---|
| Number of patients | 32 | 29 | |
| Average age (years) | 28.5 | 29.4 | 0.67 |
| Average length of HIV infection (months) | 76 | 64.9 | 0.46 |
| Average CD4 (cells/mm3) | 475 | 531 | 0.41 |
| Average viral load (copies/mL) | 11.712 (IQR 25–4.929) | 49.933 (IQR 25–3.836) | 0.26 |
| Detectable viral load at randomization (n) | 20 | 19 | 1.0 |
| Average BMI | 24.85 | 25.16 | 0.72 |
| On HAART at baseline (n/%) | 21 (65%) | 18 (62%) | 0.79 |
| Prior PI (n/%) | 16 (50%) | 16 (55%) | 0.80 |
| Kaletra use at randomization (n/%) | 8 (25%) | 10 (34.4%) | 0.29 |
| Diarrhea (n) | 0.054 | ||
| No | 27 | 29 | |
| Yes | 5 (15.6%) | 0 (0%) | |
| Nausea (n) | |||
| No | 28 | 21 | 0.20 |
| Yes | 4 (12.5%) | 8 (27.5%) | |
| Vomiting (n) | |||
| No | 30 | 28 | 1.0 |
| Yes | 2 (6.25%) | 1 (3.4%) | |
| Glycemia (mg/dL) | 80 | 78 | 0.32 |
| Basal insulin (μUI/mL) | 6.8 | 6.5 | 0.87 |
| HOMA-IR | 1.4 | 1.24 | 0.57 |
| TG (mg/dL) | 193 | 186 | 0.75 |
| ALT (mg/dL) | 14 | 11 | 0.33 |
| TC (mg/dL) | 186 | 185 | 0.97 |
| HDL-c (mg/dL) | 57 | 56 | 0.74 |
| LDL-c (mg/dL) | 90 | 95 | 0.54 |
| Creatinine (mg/dL) | 0.53 | 0.52 | 0.68 |
BMI, body mass index; HAART, highly active antiretroviral therapy; HOMA-IR, Homeostasis Model of Assessment - Insulin Resistance; TG, triglycerides; ALT, alanine transaminase; TC, total cholesterol; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol.
Grade II or III diarrhea occurred only during the first month of LPV/r use. In pregnant women who were using LPV/r prior to the randomization, there were no (0/8) cases of diarrhea in those randomized to receive the conventional dose, whereas 10% (1/10) of patients randomized to receive the increased dose presented grade III diarrhea, and 20% (2/10) presented grade II diarrhea in the 1st month. For the pregnant women who did not use LPV/r prior to the randomization, 25% (6/24) in the conventional dose group presented with grade I or II diarrhea, and 36.9% (7/19) in the increased dose group presented diarrhea (p=0.51). In the pregnant women who were not using LPV/r prior to randomization, grade I nausea was reported in the 1st month by 20.8% (5/24) of the patients receiving the conventional dose, and by 42% (8/19) of the patients receiving the increased dose (p=0.18). Among the patients who had been already receiving LPV/r prior to the randomization, only 5.5% (1/19) of the patients receiving the increased dose presented grade I nausea. There was no need to use anti-emetic medications, decrease in fluid and nutrient intake, or loss of weight.
None of the pregnant woman had blood glucose levels higher than grade I, two patients in the conventional dose group were excluded because their TG levels had risen to grade III, and one patient from each group was excluded because their ALT levels had increased to level III.
The incidence of preterm birth was 19.3% in the conventional dose group and 17.8% in the increased dose group (p=0.60).
The percentage of women with an HIV-1 antepartum viral load of less than 50 copies/mL was significantly higher in the increased dose group (89.7%; 95% confidence interval [CI]: 0.78–1%) than in the conventional dose group (68.8%; 95% CI: 0.52–0.84%) (p=0.04). In both groups, detectable antepartum VL ranged from 84 to 872 copies/mL. As recommended by the Brazilian Ministry of Health, all pregnant women received zidovudine during labor, irrespective of antepartum VL.
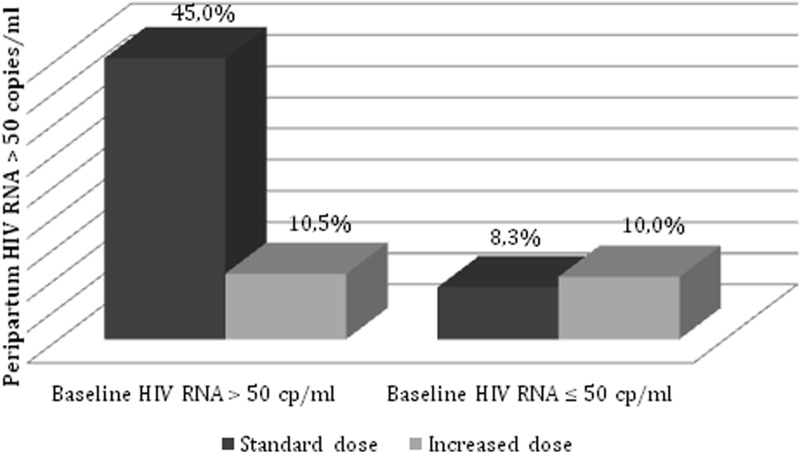
Among pregnant women with a baseline VL>50 copies/mL at the time of randomization, the percentage of women with an apVL<50 copies/mL at delivery was significantly higher in the patients assigned to the increased dose group (89.5%; 95% CI: 27.5–62.5%) than in the conventional dose group (55%; 95% CI: 0.6–21.6%) (p=0.01). However, in pregnant women with an undetectable baseline VL at randomization, there was no difference in the percentage patients with an undetectable apVL between the conventional dose group (8.3%; 95% CI: 0–0.17%) and the increased dose group (10%; 95% CI: 0–0.2%) (p=1.0), as illustrated in Fig. 2. There was no significant association among undetectable apVL and prior use of PI or body mass index (BMI). In addition to the grouping variable, VL at randomization and enfuvirtide use during pregnancy were also significantly associated with an undetectable apVL. However, in the logistic regression analysis that included these three variables in the model, only the receipt of the increased dose was independently associated with undetectable apVL levels (OR: 4.52, 95% CI: 1.01–20.4, p=0.04). There were no cases of HIV MTCT.
FIG. 2.
Rates of peripartum HIV RNA level >50 copies/mL among pregnant women treated with a conventional and increased dose of lopinavir/ritonavir according to baseline HIV RNA level.
Discussion
This study compared the efficacy, safety, and tolerability of an increased dose of LPV/r administered during the second and third trimester of pregnancy with the conventional dose. The percentage of patients with an apVL<50 copies/mL receiving the higher dose (89.7%) was significantly higher than among the patients receiving the conventional dose (68.8%) (p=0.04). There was no significant difference in the adverse event rates related to diarrhea and nausea or in laboratory abnormalities between the two groups. Likewise, the preterm delivery rates associated with the higher dose (17.8%) and the conventional dose (19.3%) were similar (p=0.6).
apVL
Pregnant women with a baseline HIV-1 VL>50 copies/mL assigned to the higher LPV/r dose (10.5%) achieved a greater rate of VL ≤50 copies/mL at delivery than those assigned to the conventional dose (45%) group (p=0.01). In contrast, pregnant women with undetectable baseline VLs had a similar rate of VL ≤50 copies/mL at delivery (p=1.0).
The risk of MTCT is directly correlated with the antepartum HIV VL.11 However, the threshold of apVL that can prevent MTCT remains uncertain. The current American recommendations for the use of antiretroviral drugs in pregnant HIV-1-infected women suggest a scheduled cesarean delivery at 38 weeks of gestation when the apVL is>1000 copies/mL or unknown, and also mandate intravenous zidovudine for women with an apVL>400 copies/mL.12–16 Therefore, should an apVL of 400 copies/mL be the optimal goal to minimize MTCT of HIV?
In the T-20 versus Optimized Regimen Only (TORO), Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir (RESIST), and Performance Of TMC114/ritonavir When evaluated in Treatment-Experienced patients with PI resistance (POWER) trials, an HIV RNA<50 copy end-point demonstrated the strongest durability over time, whereas HIV RNA levels<400 copies/mL were less sustainable over 48 weeks of treatment.17 Clinical trials of new antiretroviral drugs in highly experienced patients also presented high rates of HIV-RNA suppression<50 copies/mL. Subsequently, HIV RNA level suppression<50 copies/mL has become the standard efficacy end-point across trials of both naive and experienced patients. However, the ideal HIV RNA level threshold could be even lower. In 2012, a study analyzed 1247 non-pregnant patients on ART with a VL<50 copies/mL, and found that patients with viral loads of 40–49 copies/mL or those with viral loads<40 copies/mL but with a positive qualitative RNA test were at a greater risk of a sustained VL increase.18
These findings suggest that tests with lower limits of detection should be used to assess the efficacy of the response to ART, which is extremely relevant for the treatment and/or prophylaxis of HIV-infected pregnant women. In addition, a case of HIV MTCT in which the mother had a VL<400 copies/mL was recently described.19 This patient had a 239 copy/mL level during delivery, and the transmission occurred during the antepartum period. The newborn was HIV RNA-negative at 2 and 10 days, and tested positive at 1 month old.
A retrospective study conducted in England and Ireland between 2000 and 2006 with HIV-infected pregnant women found an MTCT rate of 0.1% among women with a apVL of<50 copies/mL, whereas the rate was 1.2% among women with apVLs between 51 and 999 copies/mL.4
A study conducted in France with>5000 HIV-infected pregnant women who used ART during pregnancy and did not breastfeed found a 0.7% MTCT rate for women with apVLs between 401 and 999 copies/mL, a 0.6% rate (95% CI 0.3–1.0%) for women with apVLs<than 400 copies/mL, and a rate of<0.4% (5/1338; 95% CI 0.1–0.9) for women with<50 copies/mL at delivery.3 In addition, 57% of the MTCT events from the women with a VL<400 copies/mL at delivery occurred during the antepartum period.
The 2012 British guidelines for the management of HIV infection in pregnant women recommend vaginal delivery for women using HAART with a VL<50 copies/mL at 36 weeks of gestation, and elective cesarean delivery for women with a VL>400 copies/mL independent of ART use. In women with loads between 50 and 399 copies/mL, elective cesarean delivery should be considered.6
The abovementioned considerations underscore the need for the lowest possible limit of HIV RNA detection at delivery to minimize MTCT.
Pregnant women with a VL<50 copies/mL at the time of randomization did not need to receive an increased dose of LPV/r to maintain an undetectable apVL at delivery. The concentration of LPV/r required to achieve an apVL<50 copies/mL is likely greater than that needed to maintain a VL<50 copies/mL. The evidence suggests that the risk of virological failure is 10-fold lower in patients with a VL<50 copies/mL for>72 months than in patients with a VL<50 copies/mL for<12 months.20 This study suggests that individuals with long-term viral suppression may be able to miss more doses (resulting in lower drug concentration) without experiencing viral rebound.
GI events
None of the pregnant women who were using LPV/r at the time of randomization and who were assigned to receive the conventional dose had diarrhea, most likely because they were already adapted to this medication. In contrast, those assigned to receive the increased dose had similar rates of diarrhea as the pregnant women who started ART with the conventional dose or increased dose during pregnancy: 30%, 25%, and 36.9%, respectively. Nausea, although it was more frequent in the increased dose group, was well tolerated, and there was no need to use anti-emetic medications, decrease in fluid and nutrient intake, or loss of weight. When using the conventional dose of LPV/r in men and non-pregnant women, diarrhea occurs in 39–57% of patients and nausea occurs in 11–15% of patients during the 1st year.21,22 Among patients who were exposed to HIV while receiving a prophylactic ART regimen with LPV/r at the conventional doses of tenofovir and emtricitabine, there was a 78% incidence of diarrhea and 59% incidence of nausea during the first 28 days of use.23 The lower rates of occurrence of diarrhea during pregnancy may be attributed to the constipation that occurs during pregnancy because of the progestational effect.24
Changes in laboratory tests
There was no notable increase in the blood glucose level of the pregnant patients evaluated despite evidence that suggests that there is an increased risk of gestational diabetes with the use of PIs.25,26
The use of the increased dose of LPV/r was not associated with a significant increase in total cholesterol (TC) or TG compared with the conventional dose. Hypertriglyceridemia and hypercholesterolemia have been reported in patients treated with the conventional dose of LPV/r.21,27 However, during pregnancy, there is an increase of TG in the first trimester.28 Lipids are used as an energy source for the fetus, and as precursors for bile acids and steroid hormones. Increased TG levels can be notably high independent of ART use.29,30
An increase in ALT levels was rarely observed in the present study. One patient using the increased dose presented with hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome during her pregnancy. One patient in the conventional dose group was excluded for having elevated asymptomatic grade III ALT levels. Pivotal studies on LPV/r have shown that increased ALT levels occur in ∼2% of patients.31,32
Prematurity
The occurrence of preterm delivery in the increased dose group (17.8%) was similar to that of the conventional dose group (19.3%). Recently, a study evaluating 1869 singleton births in the United States found that of 18.6% of preterm births, 10.2% were spontaneous.33 Additionally, the odds of preterm birth and spontaneous preterm birth were significantly greater among mothers who used PIs in the first trimester but not among mothers who used non-nucleoside reverse transcriptase inhibitors or triple nucleoside regimens during the first trimester. Individual PIs associated with a preterm birth were saquinavir, ritonavir, and LPV/r. The authors note that the mechanism of the first trimester effect is unclear, but could be related to changes in immune and inflammatory mediators. Further studies are needed to elucidate the specific drug effects and the interaction of the many factors that determine pregnancy outcome. Other published studies have determined an ∼20% rate of preterm birth among women using PIs.34–36
Conclusions
Compared with the conventional dose, the increased dose of LPV/r 600/150 mg was not associated with a greater frequency of GI symptoms, laboratory abnormalities, preterm delivery, or low birth weight. In pregnant women with a baseline VL>50 copies/mL, the dose of 600/150 mg of LPV/r seems warranted, whereas it does not seem necessary for those with a baseline VL<50 copies/mL.
Acknowledgments
Dr. Bonafe thanks the Brazilian Research Council (CNPq) for providing a research fellowship during the study.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Garcia PM. Kalish LA. Pitt J. Minkoff H. Quinn TC. Burchett SK, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341:394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 2.Cooper ER. Charurat M. Mofenson L. Hanson IC. Pitt J. Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acqir Immune Defic Syndr. 2002;29:484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 3.Warszawski J. Tubiana R. Le Chenadec J. Blanche S. Teglas JP. Dollfus C, et al. Mother-to-child HIV transmission despite antiretroviral therapy in the ANRS French Perinatal Cohort. AIDS. 2008;22:289–299. doi: 10.1097/QAD.0b013e3282f3d63c. [DOI] [PubMed] [Google Scholar]
- 4.Townsend CL. Cortina–Borja M. Peckham CS. de Ruiter A. Lyall H. Tookey PA. Low rates of mother-to-child transmission of HIV following effective pregnancy interventions in the United Kingdom and Ireland, 2000–2006. AIDS. 2008;22:973–981. doi: 10.1097/QAD.0b013e3282f9b67a. [DOI] [PubMed] [Google Scholar]
- 5.Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1- Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. [Jul 1;2013 ]. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf
- 6.British HIV Association. Guidelines for the management of HIV infection in pregnant women. HIV Med. 2012;13(Suppl. 2):87–157. doi: 10.1111/j.1468-1293.2012.01030_2.x. [DOI] [PubMed] [Google Scholar]
- 7.European AIDS Clinical Society Guidelines for treatment of HIV infected adults in Europe. Nov, 2012. http://www.europeanaidsclinicalsociety.org/images/stories/EACS–Pdf/EacsGuidelines-v6.1-2edition.pdf. [Jul 1;2013 ]. http://www.europeanaidsclinicalsociety.org/images/stories/EACS–Pdf/EacsGuidelines-v6.1-2edition.pdf Version 6.1.
- 8.Best BM. Stek AM. Mirochnick M. Hu C. Li H. Burchett SK, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acqir Immune Defic Syndr. 2010;54:381–388. doi: 10.1097/qai.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stek AM. Mirochnick M. Capparelli E. Best BM. Hu C. Burchett SK, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 10.Division of AIDS table for grading the severity of adult and pediatric adverse event version 1.0, December, 2004. Aug, 2009. http://rsc.techres.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf. [Jul 1;2013 ]. http://rsc.techres.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf Clarification.
- 11.Mofenson LM. Lambert JS. Stiehm ER. Bethel J. Meyer WA., 3rd Whitehouse J, et al. Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. Pediatric AIDS Clinical Trials Group Study 185 Team. N Engl J Med. 1999;341:385–393. doi: 10.1056/NEJM199908053410601. [DOI] [PubMed] [Google Scholar]
- 12.The International Perinatal HIV Group. The mode of delivery and the risk of vertical transmission of human immunodeficiency virus type 1–a meta-analysis of 15 prospective cohort studies. The International Perinatal HIV Group. N Engl J Med. 1999;340:977–987. doi: 10.1056/NEJM199904013401301. [DOI] [PubMed] [Google Scholar]
- 13.European Mode of Delivery Collaboration. Elective caesarean-section versus vaginal delivery in prevention of vertical HIV-1 transmission: A randomised clinical trial. Lancet. 1999;353:1035–1039. doi: 10.1016/s0140-6736(98)08084-2. [DOI] [PubMed] [Google Scholar]
- 14.Committee on Obstetric Practice. ACOG committee opinion scheduled cesarean delivery and the prevention of vertical transmission of HIV infection. Number 234, May 2000 (replaces number 219, August 1999) Int J Gynaecol Obstet. 2001;73:279–281. doi: 10.1016/s0020-7292(01)00412-x. [DOI] [PubMed] [Google Scholar]
- 15.Rodman JH. Flynn PM. Robbins B. Jimenez E. Bardeguez AD. Rodriguez JF, et al. Systemic pharmacokinetics and cellular pharmacology of zidovudine in human immunodeficiency virus type 1–infected women and newborn infants. J Infect Dis. 1999;180:1844–1850. doi: 10.1086/315152. [DOI] [PubMed] [Google Scholar]
- 16.Mirochnick M. Rodman JH. Robbins BL. Fridland A. Gandia J. Hitti J, et al. Pharmacokinetics of oral zidovudine administered during labour: A preliminary study. HIV Med. 2007;8:451–456. doi: 10.1111/j.1468-1293.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- 17.Hill A. Miralles D. Vangeneugden T. Lefebvre E. Should we now adopt the HIV-RNA<50 copy endpoint for clinical trials of antiretroviral-experienced as well as naive patients? AIDS. 2007;21:1651–1653. doi: 10.1097/QAD.0b013e3282703593. [DOI] [PubMed] [Google Scholar]
- 18.Doyle T. Smith C. Vitiello P. Cambiano V. Johnson M. Owen A, et al. Plasma HIV-1 RNA detection below 50 copies/ml and risk of virologic rebound in patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2012;54:724–732. doi: 10.1093/cid/cir936. [DOI] [PubMed] [Google Scholar]
- 19.Cressey TR. Stek A. Capparelli E. Bowonwatanuwong C. Prommas S. Sirivatanapa P, et al. Efavirenz pharmacokinetics during the third trimester of pregnancy and postpartum. J Acqir Immune Defic Syndr. 2012;59:245–252. doi: 10.1097/QAI.0b013e31823ff052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lima VD. Bangsberg DR. Harrigan PR. Deeks SG. Yip B. Hogg RS, et al. Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. J Acqir Immune Defic Syndr. 2010;55:460–465. doi: 10.1097/QAI.0b013e3181f2ac87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gathe J. da Silva BA. Cohen DE. Loutfy MR. Podzamczer D. Rubio R, et al. A once-daily lopinavir/ritonavir-based regimen is noninferior to twice-daily dosing and results in similar safety and tolerability in antiretroviral-naive subjects through 48 weeks. J Acqir Immune Defic Syndr. 2009;50:474–481. doi: 10.1097/QAI.0b013e31819c2937. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez–Garcia J. Cohen D. Johnson M. Sloan L. Fredrick L. Naylor C, et al. Short communication: Comparable safety and efficacy with once-daily versus twice-daily dosing of lopinavir/ritonavir tablets with emtricitabine+tenofovir DF in antiretroviral-naive, HIV type 1-infected subjects: 96 week final results of the randomized trial M05-730. AIDS Res Hum Retroviruses. 2010;26:841–845. doi: 10.1089/aid.2009.0307. [DOI] [PubMed] [Google Scholar]
- 23.Tosini W. Muller P. Prazuck T. Benabdelmoumen G. Peyrouse E. Christian B, et al. Tolerability of HIV postexposure prophylaxis with tenofovir/emtricitabine and lopinavir/ritonavir tablet formulation. AIDS. 2010;24:2375–2380. doi: 10.1097/QAD.0b013e32833dfad1. [DOI] [PubMed] [Google Scholar]
- 24.Carlin A. Alfirevic Z. Physiological changes of pregnancy and monitoring. Best Pract Res Clin Obstet Gynaecol. 2008;22:801–823. doi: 10.1016/j.bpobgyn.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Eastone JA. Decker CF. New-onset diabetes mellitus associated with use of protease inhibitor. Ann Intern Med. 1997;127:948. doi: 10.7326/0003-4819-127-10-199711150-00017. [DOI] [PubMed] [Google Scholar]
- 26.Visnegarwala F. Krause KL. Musher DM. Severe diabetes associated with protease inhibitor therapy. Ann Intern Med. 1997;127:947. doi: 10.7326/0003-4819-127-10-199711150-00016. [DOI] [PubMed] [Google Scholar]
- 27.Lafeuillade A. Hittinger G. Philip G. Lambry V. Jolly P. Poggi C. Metabolic evaluation of HIV-infected patients receiving a regimen containing lopinavir/ritonavir (Kaletra) HIV Clin Trials. 2004;5:392–398. doi: 10.1310/Q0TG-0V50-9JML-638U. [DOI] [PubMed] [Google Scholar]
- 28.Ghio A. Bertolotto A. Resi V. Volpe L. Di Cianni G. Triglyceride metabolism in pregnancy. Adv Clin Chem. 2011;55:133–153. doi: 10.1016/b978-0-12-387042-1.00007-1. [DOI] [PubMed] [Google Scholar]
- 29.Papadakis EP. Sarigianni M. Mikhailidis DP. Mamopoulos A. Karagiannis V. Acute pancreatitis in pregnancy: An overview. Eur J Obstet Gyn Reprod Biol. 2011;159:261–266. doi: 10.1016/j.ejogrb.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 30.Kayatas SE. Eser M. Cam C. Cogendez E. Guzin K. Acute pancreatitis associated with hypertriglyceridemia: A life-threatening complication. Arch Gynecol Obstet. 2010;281:427–429. doi: 10.1007/s00404-009-1183-0. [DOI] [PubMed] [Google Scholar]
- 31.Bánhegyi D. Katlama C. da Cunha CA. Schneider S. Rachlis A. Workman C. De Meyer S. Vandevoorde A. Van De Casteele T. Tomaka F. Week 96 efficacy, virology and safety of darunavir/r versus lopinavir/r in treatment–experienced patients in TITAN. Current HIV Res. 2012;10:171–181. doi: 10.2174/157016212799937218. [DOI] [PubMed] [Google Scholar]
- 32.Molina JM. Andrade-Villanueva J. Echevarria J, et al. Once daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet. 2008;372:646–655. doi: 10.1016/S0140-6736(08)61081-8. [DOI] [PubMed] [Google Scholar]
- 33.Watts DH. Williams PL. Kacanek D. Griner R. Rich K. Hazra R, et al. Combination antiretroviral use and preterm birth. J Infect Dis. 2013;207:612–621. doi: 10.1093/infdis/jis728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Senise J. Cruz R. Palacios R. Vaz M. Lacerda A, et al. Low birth weight and pre term delivery in relation to Lopinavir/ritonavir use in pregnancy. Am J Infect Dis. 2008;4:209–214. [Google Scholar]
- 35.Azria E. Moutafoff C. Schmitz T. Le Meaux JP. Krivine A. Pannier E, et al. Pregnancy outcomes in women with HIV type-1 receiving a lopinavir/ritonavir-containing regimen. Antivir Ther. 2009;14:423–432. [PubMed] [Google Scholar]
- 36.Powis KM. Kitch D. Ogwu A. Hughes MD. Lockman S. Leidner J, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis. 2011;204:506–514. doi: 10.1093/infdis/jir307. [DOI] [PMC free article] [PubMed] [Google Scholar]