Abstract
Aims
To study the feasibility and reliability of pocket-size hand-held echocardiography (PHHE) by medical residents with limited experience in ultrasound.
Methods and results
A total of 199 patients admitted to a non-university medical department were examined with PHHE. Six out of 14 medical residents were randomized to use a focused protocol and examine the heart, pericardium, pleural space, and abdominal large vessels. Diagnostic corrections were made and findings were confirmed by standard diagnostics. The median time consumption for the examination was 5.7 min. Each resident performed a median of 27 examinations. The left ventricle was assessed to satisfaction in 97% and the pericardium in all patients. The aortic and atrioventricular valves were assessed in at least 76% and the abdominal aorta in 50%, respectively. Global left-ventricular function, pleural, and pericardial effusion showed very strong correlation with reference method (Spearman's r ≥ 0.8). Quantification of aortic stenosis and regurgitation showed strong correlation with r = 0.7. Regurgitations in the atrioventricular valves showed moderate correlations, r = 0.5 and r = 0.6 for mitral and tricuspid regurgitation, respectively, similar to dilatation of the left atrium (r = 0.6) and detection of regional dysfunction (r = 0.6). Quantification of the abdominal aorta (aneurysmatic or not) showed strong correlation, r = 0.7, while the inferior vena cava diameter correlated moderately, r = 0.5.
Conclusion
By adding a PHHE examination to standard care, medical residents were able to obtain reliable information of important cardiovascular structures in patients admitted to a medical department. Thus, focused examinations with PHHE performed by residents after a training period have the potential to improve in-hospital diagnostic procedures.
Keywords: Echocardiography, Pocket-size, Hand-held, Point-of-care ultrasound, Bedside, Non-expert
Introduction
An early and correct diagnosis is a crucial step in the treatment of patients. A delayed or wrong diagnosis may delay the treatment, complicate inpatient workflow, and may in worst case scenario have a lethal outcome.1
During the recent two decades, the development of new digital technology and miniaturization of ultrasound scanners have moved these scanners from the echo-labs into the white coat pocket.2,3 This makes them an excellent clinical tool, available for any physician in different clinical settings as a point-of-care ultrasonography.4
These newly developed scanners have been studied in several clinical settings. In the hands of experienced users, pocket-size hand-held echocardiographic (PHHE) devices offer high-quality semi-quantitative assessment of cardiac structures, abdominal great vessels, and the pleural space at the physicians' point-of-care with a demonstrable clinical benefit.5–11
Medical history-taking and physical examination of most patients are performed by the residents in the emergency departments or bed wards. Few of these are skilled in ultrasonography and given the cost and the availability of the PHHE devices, non-expert users will frequently have such technology available for diagnostic use. Thus, we aimed to study the feasibility and reliability of PHHE in the hands of medical residents after a targeted training period in cardiovascular ultrasound.
Methods
Study population
This prospective observational study included 199 patients admitted to the medical department at Levanger Hospital, Norway. The patients were included in the period 4 April to 23 June 2011. The examination was performed by six medical residents taking part in the study. At study start, 12 medical residents were employed at the department, and half of them were randomized to participate in the study. During the study, another two residents joined the department, but they did not participate in the study. The residents have in-house call 24×7. Thus, the six participating residents covered ∼42% of the total period of inclusion. All emergency admissions during the time these six residents were on call were included in the study. There were no other criteria of inclusion. Only patients who did not consent to participate or did not stay long enough in the department to enable the necessary diagnostic procedures for the study were excluded. Due to logistic reasons, inclusion of patients was restricted to 199 of 446 available patients as standard diagnostic procedures and treatment had first priority.
The patients were admitted to the emergency room in a standard way. After having been triaged according to their symptoms, they were examined by the resident. Based on the medical history, physical examination, and supplemental tests, a preliminary diagnosis was made. Thus, usual care diagnostics were done prior to the examination with PHHE. All patients had standard follow-up according to their symptoms and findings. Patients, in whom pathology was suggested either by PHHE or by the standard clinical care, were referred for relevant gold-standard diagnostic follow-up. To improve the reliability of the sensitivity and specificity of the data, approximately 10 negatively described PHHE examinations per resident were randomly selected by the study committee and referred for reference imaging procedures as well. The study was approved by the Regional Committee for Medical Research Ethics, and conducted according to the second Helsinki Declaration. All the patients gave their informed consent to participate in the study.
Education of residents
The residents underwent a brief training program covering both the examination with PHHE and interpretation of the recordings. The program consisted of 4 h of lectures dealing with the theoretical basics and pitfalls of cardiovascular ultrasonography. Normal and pathological findings were demonstrated, and they were all provided with access to a virtual ultrasound-imaging library. All participating residents had a personal supervisor. Subsequently, the residents underwent 3 months of practical training, initially together with the supervisors in the echo-lab and in the radiology department, then using PHHE in the medical department with close connection to experienced ultrasonographers, having the opportunity to discuss their findings. They were encouraged to perform at least 100 examinations during the tutorial period. The actual numbers performed were median (interquartile range) 95 (80–225) examinations.
Pocket-size echocardiographic examination
The residents performed the PHHE examinations using a Vscan (version 1.2; GE Vingmed Ultrasound, Horten, Norway). This device offers B-mode and colour flow (CF) imaging. The total weight is 390 g including the phased array probe with bandwidth of 1.7–3.8 MHz. It provides two dimensional (2D) imaging and real time colour-Doppler within a sector that has fixed size, but is movable throughout the 2D sector. An algorithm enables automatic storage and loop recording of a cardiac cycle without ECG signal.12 Patient identification was performed by voice recording and the automatically assigned examination number. All images and recordings were saved on the device's micro-SD card and later transferred to a computer by commercial software (Gateway; GE Vingmed Ultrasound).
The pocket-size echocardiographic examinations were performed bedside, and when possible with the patients in the left-lateral decubitus position. The examinations included parasternal long- and short-axis views and apical four-chambers, two-chambers, and long-axis views. All views contained 2D and CF recordings. The patients were turned to supine position when examining the abdominal great vessels. The pleural space was recorded from supine or upright position. A standard examination protocol was used. Assessment of left- and right-ventricular function were done semi-quantitatively from the parasternal and apical positions, classified as normal/near normal, moderate, or severe dysfunction. The quantification was based on the systolic excursion of the atrioventricular plane for both ventricles. In addition, eye-balling of the left-ventricular ejection fraction as ≥45, 30–45, or <30% corresponded to normal/near normal, moderate, or severe dysfunction, respectively. With respect to the assessment of right-ventricular function, dilatation of the ventricle and/or diastolic shift to the left of the intraventricular septum was also included in the judgement. Severe regional dysfunction was classified as present or not. Valvular pathology and dysfunction was classified semi-quantitatively as mild, moderate, or severe. Quantification of stenosis was based on the amount of calcification and the movement of the cusps/leaflets. Quantification of the regurgitations was based on the CF jet and size and function of the adjacent chambers. The size of the left atrium (LA) was measured online from the parasternal position and quantified as normal (<40 mm), moderately dilated (40–50 mm), or severely dilated (>50 mm). Pericardial effusion was if present classified as significant or not based on visual judgement of the influence of the adjacent chambers. The inferior vena cava diameter was assessed from the subcostal position at the end expiration within 2 cm from the right atrial orifice. The size of the abdominal aorta was determined by the largest measured diameter. It was classified as aneurysmatic if the diameter exceeded 30 mm. Both pleural cavities were examined. If pleural effusion was present, this was graded as small or large amount. A large amount of pleural effusion was registered if the diameter between the thoracic wall and the lung exceeded 5 and 4.5 cm in the left or the right pleural cavity, respectively. The examinations of the different structures were judged by the residents as feasible if they were able to quantify the specific cardiac structures or function indices based on their recordings.
Validation of point-of-care pocket-size echocardiography
Standard echocardiography was performed in the hospital's echo-lab, under optimal conditions. The system used was a Vivid 7 scanner (GE Vingmed Ultrasound, Horten, Norway) using a 2.0 MHz phased-array transducer (M3S) with bandwidth 1.5–3.6 MHz. Second harmonic imaging was used. The recording of a cardiac cycle was ECG triggered. The standard examinations were performed independently by one of four experienced cardiologists blinded to the results of PHHE with a median (range) time delay of 21.1 (0.4–166) h. A complete echocardiographic examination was performed. Dimensions were measured from a parasternal view. Ejection fraction was measured by Simpson's rule from apical four- and two-chamber views.13 Valvular pathology was graded according to the recommendations from the European Association of Cardiovascular Imaging (EACVI) [former European Association of Echocardiography (EAE)].14–16 For the analyses of the patients who underwent both echocardiographic and radiographic examinations, the radiologists' classifications of pleural effusion [computer tomography (CT) or ultrasound] and the size of the abdominal aorta were preferred.
Statistical analysis
As the different echocardiographic and anthropometric measures partly were skewed compared with normal distribution, the basic characteristics are presented as mean ± standard deviation (SD) and (interquartile) range. Spearman's rho (r) was used for comparison of the ranking of pathology between the PHHE and the high-end echocardiographic examinations. Data are presented as r [95% confidence interval (CI)] with the 95% CI computed using bootstrapping. For comparison of continuous variables, Pearson's rho (r) and Bland–Altman statistics were used. Statistical analyses were performed using SPSS for Windows version 20.0 (SPSS, Inc., Chicago, IL, USA).
Results
Study population
Table 1 shows the baseline data of the 199 patients included in the study (107 men and 92 women). Mean ± SD (range) age was 65.6 ± 18.2 (17.1–98.5) years. The distribution of age was positively skewed compared with a normal distribution. The mean height was 170.9 ± 9.7 cm and the body mass index was 26.4 ± 5.6 kg/m2. At admission, atrial fibrillation was present in 33 (17%) patients, hypertension was present in 67 (34%) patients, 36 (18%) had known diabetes mellitus, and 20 (10%) had established heart failure. In total, cardiovascular disease defined as either angina pectoris, prior myocardial infarction, prior stroke, or established peripheral arterial disease was present in 71 (36%) of the patients. There were no significant differences in the basic characteristics of the 199 participants included in the study and the 247 participants not included in the study, but who were admitted to the hospital the days when the six residents performing PHHE examinations were on duty.
Table 1.
Basic characteristics of the 199 study participants
| Mean ± SD (range)a | |
|---|---|
| Age, years | 65.6 ± 18.2 (17.1–98.5) |
| Male, n (%) | 107 (53.8) |
| Height, cm | 170.9 ± 9.7 (150–196) |
| Body mass index, kg/m2 | 26.4 ± 5.6 (12–45) |
| Systolic blood pressure, mmHg | 143.9 ± 28.6 (74–245) |
| Diastolic blood pressure, mmHg | 75.0 ± 15.6 (24–120) |
| Heart rate, bpm | 82.8 ± 22.6 (40–160) |
| Atrial fibrillation, n (%) | 33 (16.6) |
| Known hypertension, n (%) | 67 (33.7) |
| Known diabetes, n (%) | 36 (18.1) |
| Known myocardial infarction, n (%) | 32 (16.1) |
| Known angina, n (%) | 17 (8.5) |
| Known heart failure, n (%) | 20 (10.1) |
| Known peripheral vessel disease, n (%) | 7 (3.5) |
| Known stroke, n (%) | 35 (17.6) |
| Known cardiovascular disease, n (%) | 71 (35.7) |
| Known cancer, n (%) | 16 (8.0) |
aData are presented as mean ± SD (range) unless otherwise specified.
Pocket-size hand-held echocardiography
The time consumption of the examination, including large vessels, was median (range) 5.7 (1.6–19.9) min. Each resident performed a median (interquartile range) of 27 (19–46) examinations. Table 2 shows the feasibility of PHHE. The left-ventricular (LV) function was assessed to satisfaction in nearly all of the patients (97%) and the pericardial space in all patients. The aortic and atrioventricular valves were assessed in at least 76% and the pulmonary valve in <50% of the patients. The vena cava inferior was assessed to satisfaction in 77% and the abdominal aorta in 50% of the population. This is also illustrated in Figure 1.
Table 2.
Feasibility of point-of-care pocket-size echocardiography
| Anatomic structure | Assessed to satisfaction (%) |
|---|---|
| Left ventricle | 194 (97) |
| Right ventricle | 172 (86) |
| Pericardium | 199 (100) |
| Left atrium | 173 (87) |
| Mitral valve | 177 (89) |
| Aortic valve | 171 (86) |
| Pulmonary valve | 97 (49) |
| Tricuspid valve | 152 (76) |
| Abdominal aorta | 99 (50) |
| Vena cava inferior | 154 (77) |
| Pleura | 190 (95) |
Figure 1.
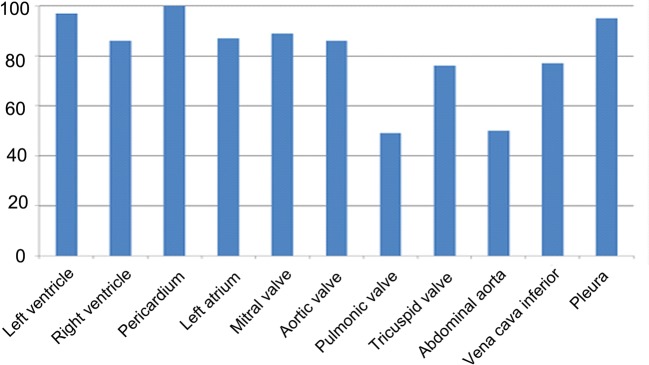
Feasibility of point-of-care pocket-size echocardiography. Feasibility (%) of the different cardiovascular structures when pocket-size echocardiography was performed by residents. The examinations of the different structures were judged by the residents as feasible if they were able to quantify the specific cardiac structures or function indices based on the recordings.
A total of 133 and 74 patients underwent high-end echocardiography and radiographic (CT or ultrasound) reference imaging, respectively. In total, 186 (93%) patients underwent reference imaging (Figure 2). For the different indices of cardiac structure or function, the available numbers of validated examinations are shown in Tables 3 and 4. Table 3 shows the correlations of semi-quantitative assessment of cardiovascular structures and function indices between PHHE and standard echocardiography. The classification of global left-ventricular function, pleural, and pericardial effusion showed very strong correlation with standard diagnostic procedures (Spearman's r ≥ 0.83, with variations between residents 0.70–0.93, 0.54–1.0, and 0.81–1.0, respectively). Regional left-ventricular function showed moderate correlation, r = 0.60 (variation between residents 0.53–0.61). The classification of aortic valve calcification/stenosis and regurgitation showed strong correlation with r = 0.67 (variation between residents 0.29–0.93) and r = 0.68 (variation between residents 0.33–1.0), respectively. Regurgitation of the atrioventricular valves showed moderate-to-strong correlations, r = 0.53 (variation between residents 0.34–0.80) for mitral and r = 0.61 (variation between residents 0.21–0.78) for tricuspid regurgitation, so did the degree of dilatation of the LA (r = 0.61) (variation between residents 0.23–0.76). No serious findings were missed. PHHE correlated strongly with standard diagnostics with respect to detect abdominal aortic aneurysms, r = 0.70. No aneurysms were missed, but there was one false positive diagnosis where the measurement of the aorta was 32 mm by PHHE and 28 mm by the abdominal CT. Figure 3 illustrates the reproducibility data of the abdominal aortic diameter. The maximal diameter of the inferior vena cava correlated only moderately with high-end echocardiography, Pearson's r = 0.45. Figure 4 illustrates the total number of misclassifications of global and regional ventricular and valvular pathology by PHHE compared with the reference. For the quantification of LV global function, LA size, and aortic stenosis, respectively, 7, 2, and 5% of the misclassifications were two degrees; all other misclassifications were only one degree. Figure 5 shows clinical examples of PHHE compared with reference method, and a clinical example is given in Supplementary material online, Videos S1 and S2.
Figure 2.
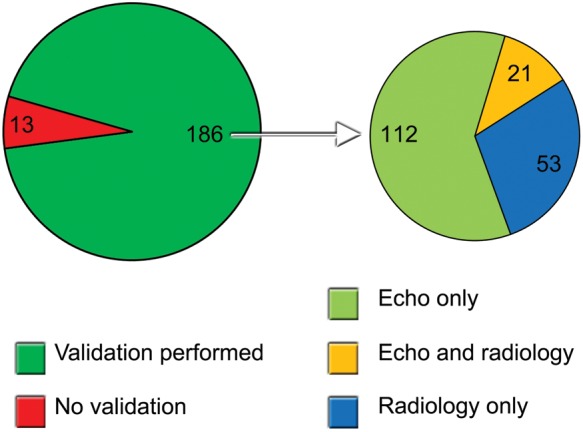
Validation of PHHE. Illustration of the number of patients that were validated with reference imaging (left) and by what kind of reference imaging (right). Echo, echocardiography.
Table 3.
Correlations of semi-quantitative classification of echocardiographic indices of pocket-size echocardiography and reference method
| n total | n pathology | R | 95% CI | |
|---|---|---|---|---|
| Global systolic function, left ventricle | 129 | 26 | 0.83 | 0.71–0.93 |
| Apparent regional dysfunction, left ventricle | 129 | 22 | 0.60 | 0.39–0.78 |
| Global systolic function, right ventricle | 115 | 10 | 0.44 | 0.10–0.72 |
| Size of left atrium | 117 | 68 | 0.61 | 0.48–0.72 |
| Aortic calcification and stenosis | 119 | 37 | 0.67 | 0.52–0.80 |
| Aortic regurgitation | 117 | 27 | 0.68 | 0.52–0.82 |
| Mitral regurgitation | 123 | 54 | 0.53 | 0.37–0.68 |
| Tricuspid regurgitation | 107 | 49 | 0.61 | 0.45–0.74 |
| Pericardial effusion | 131 | 4 | 0.86 | 0.57–1.00 |
| Pleural effusion | 151 | 20 | 0.83 | 0.67–0.94 |
| Abdominal aorta | 52 | 2 | 0.70 | 0.49–1.00 |
| Inferior vena cavaa | 94 | 0.45 | 0.24–0.62 |
Data presented as correlation coefficient (r) with 95% confidence interval achieved by bootstrapping.
n total, the total number who underwent both PHHE and reference imaging; n pathology, total number with the described pathology.
aContinuous variable, analysed by Pearson's correlation, all others analysed by Spearman's rank correlation.
Table 4.
Sensitivity, specificity, positive, and negative predictive value of point-of-care pocket-size echocardiography to detect at least moderate pathology compared with reference method
| n total | n pathology | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|
| LV dysfunction | 129 | 30 | 92 | 94 | 80 | 98 |
| RV dysfunction | 115 | 10 | 40 | 97 | 57 | 94 |
| LA enlargement | 117 | 68 | 62 | 94 | 93 | 64 |
| Aortic regurgitation | 117 | 27 | 82 | 89 | 69 | 94 |
| Aortic stenosis/calcification | 119 | 37 | 76 | 88 | 74 | 89 |
| Mitral regurgitation | 123 | 48 | 71 | 81 | 71 | 81 |
| Tricuspid regurgitation | 107 | 49 | 65 | 90 | 84 | 75 |
n total, the total number who underwent both PHHE and reference imaging; n pathology, total number with the described pathology; LV, left ventricle; RV, right ventricle; LA, left atrium; PPV, positive predictive value; NPV, negative predictive value.
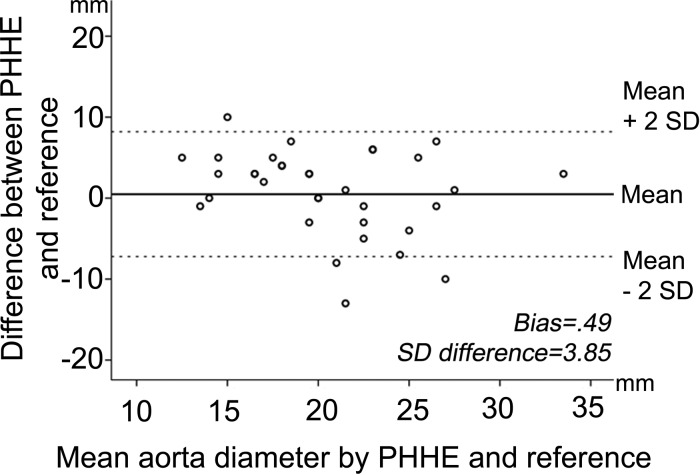
Figure 3.
Bland–Altman plot for the assessment of the abdominal aortic diameter using PHHE and reference imaging. Reproducibility for the assessment of the diameter of the abdominal aorta. Bland-Altman plot of difference between PHHE and reference imaging by the mean of the measurements.
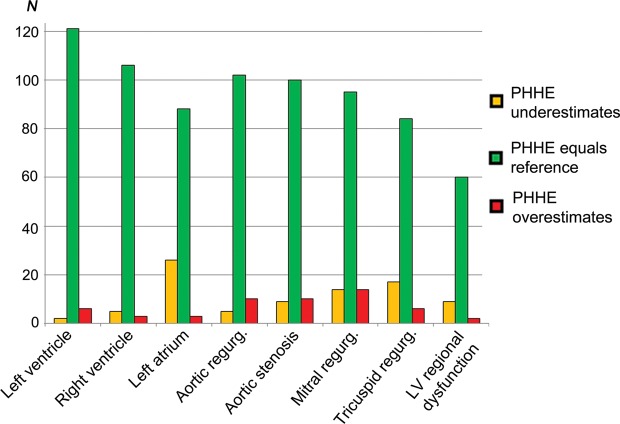
Figure 4.
Classification of ventricular and valvular pathology by PHHE compared with reference echocardiography. The agreement of PHHE and reference echocardiography in the quantification of ventricular and valvular pathology is illustrated. Over- and underestimation is the total numbers of misclassifications. In total, only 2% were misclassified by two degrees, the rest by one degree. LV, left ventricle; N, numbers; regurg, regurgitation.
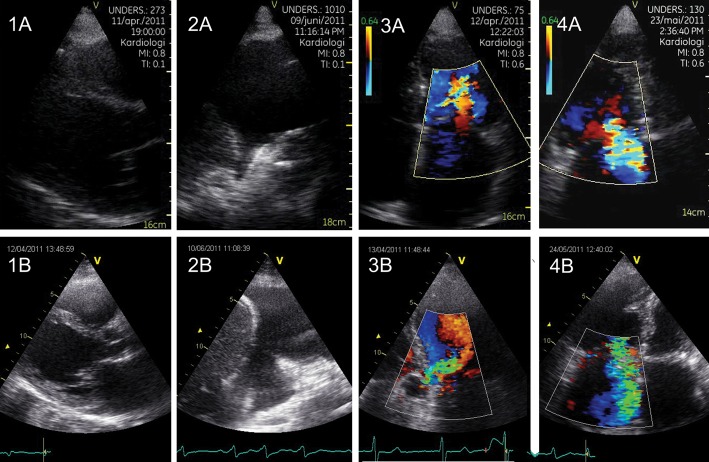
Figure 5.
Cases illustrating the comparison of PHHE with reference method. (A) shows images from the pocket-size device, while (B) shows images from the high-end Vivid 7 scanner (GE Vingmed Ultrasound). 1 (A and B): 54-year-old man with principal diagnosis of liver cirrhosis changed to dilated cardiomyopathy after PHHE. 2 (A and B): 70-year-old man with known heart failure concluded to be decompensated after finding the shown significant amount of pleural effusion, dilated vena cava inferior, and reduced LV function. 3 (A and B): 75-year-old man referred with stroke where PHHE revealed an unknown moderate aortic regurgitation (without importance for the acute treatment). 4 (A and B): 88-year-old woman admitted with heart failure. PHHE revealed dilated ventricles, the shown large tricuspid regurgitation, pleural effusion, and ascites due to hypervolaemia.
Table 4 shows the sensitivity, specificity, positive, and negative predictive values of PHHE to detect at least moderate pathology. There was high specificity and negative predictive values of detecting left- and right-ventricular dysfunction and aortic-valve pathology. On the contrary, the lower sensitivity and positive predictive values for the assessment of right-ventricular function and left-atrial size are mainly caused by some underestimation of pathology.
Discussion
Our study demonstrates that medical residents in <6 min can perform a bedside ultrasound examination of the heart, pleural space, and the abdominal great vessels after a 3 months training period and get reliable and clinically important diagnostic information beyond the standard physical examination.
The patients were included solely during the time when the participating residents were on call and represent otherwise an unselected population in our department. The population characteristics are also in line with patient characteristics from previous studies in similar settings.10,17,18
PHHE has in several studies showed a high feasibility and accuracy when performed by experts.6–9 Galderisi et al.8 showed slightly lower sensitivity and specificity when trainees performed PHHE compared with experts. Panoulas et al.19 showed improved diagnostic accuracy when medical students and junior doctors added a PHHE examination to history, physical examination, and ECG findings. Our results are in line with their findings when PHHE is performed by non-experts. The feasibility is overall very good, 75–100% for all structures except the pulmonic valve and the abdominal aorta which were assessed to satisfaction in approximately one-half of the patients. Inexperienced users may be less able to provide optimal image quality and need better image quality to be able to interpret the recordings compared with expert users, but we have no data to support this hypothesis. The abdominal aorta was assessed in a relatively small number of patients compared with expert studies.7,20 This may partly be explained by the fact that the residents did not register the aorta as assessed unless the entire length of the aorta was satisfactorily assessed. Secondly, patients were non-fasting, thereby reducing abdominal image quality, and BMI was ∼2 kg/m2 higher in whom the abdominal aorta was not assessed (P < 0.001). Nonetheless, there may have been too little focus on examining the great vessels during the training period.
The assessment of the global left-ventricular function and the pericardial and pleural space compared excellently with standard diagnostics. These are crucial issues in the cardiovascular ultrasound examination.21 The classification/assessment of valvular function showed moderate-to-strong correlation and we found high specificity and high negative predictive values for detecting at least moderate valvular pathology. Importantly, no serious findings were missed, neither according to aortic valve pathology or regurgitation of the atrioventricular valves. However, there was some under- and overestimation of both ventricular dysfunction and valvular pathology. This may be explained by less experienced users, a very sensitive colour mode, and the lack of spectral Doppler in the PHHE devices. We find the presented degree of misclassification of aortic stenosis, in line with the presented, but less pronounced overestimation of aortic stenosis related to the lack of spectral Doppler in recent studies.6,7 No moderate or severe aortic stenosis was missed. Atrioventricular valves regurgitations were missed more often compared with aortic regurgitations and this may be related to the higher number of atrioventricular regurgitations in the presented population. Due to moderate feasibility, the correlation of the aortic diameter was tested in only 52 patients and in these patients there was a strong agreement, and in the one misclassified, the difference was 4 mm. No aneurysms were missed by PHHE. The moderate agreement between PHHE and standard diagnostics in the assessment of the inferior vena cava may be explained by the period of time between PHHE and the standard echocardiography of median 21 h. Physiological variations and treatment effects may have influenced the results.22 In addition, measurements of the size of the LA and vena cava inferior may be influenced by the fact that the pocket-size device lacks ECG-cables and there are limited opportunities to ensure the correct timing in the cardiac or respiratory cycles.
Taking a thorough medical history and performing a physical examination will remain the cornerstones in the diagnostic procedure, but there is a need for improvement in diagnostic accuracy to decrease medical errors.1,23 PHHE is an excellent tool to provide further diagnostic information. As stated by the EACVI (former EAE) the users level of competence is very important in these devices.24 Experienced ultrasonographers can start using PHHE without limitations. In less-experienced users, targeted education and a training period are necessary and PHHE should be used only for targeted examinations depending on the skills of the user.
Even in the hands of relatively inexperienced residents, PHHE provides feasible and reliable information at the point-of-care and improves the diagnostic precision without significant time delay. However, it is important to state that PHHE cannot replace the standard echocardiographic examination performed by experts in the echo lab. It should remain a bedside imaging tool which allows for quick and important information without losing valuable time.
Limitations
In the study period, 1076 emergency admissions to the medical department were recorded and 84 of these patients declined consent. Out of the 446 patients randomized to receive PHHE examination, only 199 actually received it. This is mainly explained by busy working hours, hospital logistics, and the residents being informed to have a priority on standard diagnostics and treatment of patients.
The study was a single-centre study with a limited number of participating residents and patients. Consecutive patients were included and critical diagnosis such as aortic dissection and cardiac tamponade were not registered during the inclusion period. It is important to emphasize that in such cases, PHHE may offer a fast track to the correct diagnosis,10 but negative findings must not rule out further diagnostic tests if the clinician still suspects specific conditions.
Conclusion
By adding a point-of-care PHHE examination lasting <6 min, medical residents were able to obtain reliable information of important cardiac structures and great vessels in patients admitted to a medical department. Thus, a focused examination with PHHE performed by residents, after a targeted training period have the potential to improve in-hospital diagnostics and care.
Supplementary material
Supplementary material is available at European Heart Journal – Cardiovascular Imaging online.
Funding
This study is funded by the Nord-Trøndelag Health Trust, Norway and the Norwegian University of Science and Technology, Norway.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We thank all the participating doctors, nurses, and secretarial staff at Levanger hospital for their invaluable assistance with the inclusion and data collection for this study. OCM, GNA, HD, and BOH hold positions at MI Lab, a Centre of Research-based Innovation that is funded by the Research Council of Norway and industry. One of the industry partners is GE Vingmed Ultrasound. The Centre has a total budget of 124 million NOK for the 8 years period from 2007 to 2014, and the contribution from GE Vingmed Ultrasound to this budget is 7 million NOK (6%).
References
- 1.Burton JL, Underwood J. Clinical, educational, and epidemiological value of autopsy. Lancet. 2007;369:1471–80. doi: 10.1016/S0140-6736(07)60376-6. [DOI] [PubMed] [Google Scholar]
- 2.Roelandt JR. Ultrasound stethoscopy: a renaissance of the physical examination? Heart. 2003;89:971–3. doi: 10.1136/heart.89.9.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roelandt JR. Ultrasound stethoscopy. Eur J Intern Med. 2004;15:337–47. doi: 10.1016/j.ejim.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364:749–57. doi: 10.1056/NEJMra0909487. [DOI] [PubMed] [Google Scholar]
- 5.Dalen H, Haugen BO, Graven T. Feasibility and clinical implementation of hand-held echocardiography. Expert Rev Cardiovasc Ther. 2013;11:49–54. doi: 10.1586/erc.12.165. [DOI] [PubMed] [Google Scholar]
- 6.Prinz C, Voigt JU. Diagnostic accuracy of a hand-held ultrasound scanner in routine patients referred for echocardiography. J Am Soc Echocardiogr. 2011;24:111–6. doi: 10.1016/j.echo.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Andersen GN, Haugen BO, Graven T, Salvesen O, Mjolstad OC, Dalen H. Feasibility and reliability of point-of-care pocket-sized echocardiography. Eur J Echocardiogr. 2011;12:665–70. doi: 10.1093/ejechocard/jer108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galderisi M, Santoro A, Versiero M, Lomoriello VS, Esposito R, Raia R, et al. Improved cardiovascular diagnostic accuracy by pocket size imaging device in non-cardiologic outpatients: the NaUSiCa (Naples Ultrasound Stethoscope in Cardiology) study. Cardiovasc Ultrasound. 2010;8:51. doi: 10.1186/1476-7120-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prinz C, Dohrmann J, van Buuren F, Bitter T, Bogunovic N, Horstkotte D, et al. Diagnostic performance of handheld echocardiography for the assessment of basic cardiac morphology and function: a validation study in routine cardiac patients. Echocardiography. 2012;29:887–94. doi: 10.1111/j.1540-8175.2012.01728.x. [DOI] [PubMed] [Google Scholar]
- 10.Mjolstad OC, Dalen H, Graven T, Kleinau JO, Salvesen O, Haugen BO. Routinely adding ultrasound examinations by pocket-sized ultrasound devices improves inpatient diagnostics in a medical department. Eur J Intern Med. 2012;23:185–91. doi: 10.1016/j.ejim.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Skjetne K, Graven T, Haugen BO, Salvesen O, Kleinau JO, Dalen H. Diagnostic influence of cardiovascular screening by pocket-size ultrasound in a cardiac unit. Eur J Echocardiogr. 2011;12:737–43. doi: 10.1093/ejechocard/jer111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aase SA, Snare SR, Dalen H, Stoylen A, Orderud F, Torp H. Echocardiography without electrocardiogram. Eur J Echocardiogr. 2011;12:3–10. doi: 10.1093/ejechocard/jeq112. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. quiz 101–2. [DOI] [PubMed] [Google Scholar]
- 15.Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease) Eur J Echocardiogr. 2010;11:307–32. doi: 10.1093/ejechocard/jeq031. [DOI] [PubMed] [Google Scholar]
- 16.Lancellotti P, Tribouilloy C, Hagendorff A, Moura L, Popescu BA, Agricola E, et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 1: aortic and pulmonary regurgitation (native valve disease) Eur J Echocardiogr. 2010;11:223–44. doi: 10.1093/ejechocard/jeq030. [DOI] [PubMed] [Google Scholar]
- 17.de Groot-de Laat LE, ten Cate FJ, Vourvouri EC, van Domburg RT, Roelandt JR. Impact of hand-carried cardiac ultrasound on diagnosis and management during cardiac consultation rounds. Eur J Echocardiogr. 2005;6:196–201. doi: 10.1016/j.euje.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 18.Vourvouri EC, Koroleva LY, Ten Cate FJ, Poldermans D, Schinkel AF, van Domburg RT, et al. Clinical utility and cost effectiveness of a personal ultrasound imager for cardiac evaluation during consultation rounds in patients with suspected cardiac disease. Heart. 2003;89:727–30. doi: 10.1136/heart.89.7.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panoulas VF, Daigeler AL, Malaweera AS, Lota AS, Baskaran D, Rahman S, et al. Pocket-size hand-held cardiac ultrasound as an adjunct to clinical examination in the hands of medical students and junior doctors. Eur Heart J Cardiovasc Imaging. 2013;14:323–30. doi: 10.1093/ehjci/jes140. [DOI] [PubMed] [Google Scholar]
- 20.Dijos M, Pucheux Y, Lafitte M, Reant P, Prevot A, Mignot A, et al. Fast track echo of abdominal aortic aneurysm using a real pocket-ultrasound device at bedside. Echocardiography. 2012;29:285–90. doi: 10.1111/j.1540-8175.2011.01559.x. [DOI] [PubMed] [Google Scholar]
- 21.Martin LD, Mathews S, Ziegelstein RC, Martire C, Howell EE, Hellmann DB, et al. Prevalence of asymptomatic left ventricular systolic dysfunction in at-risk medical inpatients. Am J Med. 2013;126:68–73. doi: 10.1016/j.amjmed.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990;66:493–6. doi: 10.1016/0002-9149(90)90711-9. [DOI] [PubMed] [Google Scholar]
- 23.Combes A, Mokhtari M, Couvelard A, Trouillet JL, Baudot J, Henin D, et al. Clinical and autopsy diagnoses in the intensive care unit: a prospective study. Arch Intern Med. 2004;164:389–92. doi: 10.1001/archinte.164.4.389. [DOI] [PubMed] [Google Scholar]
- 24.Sicari R, Galderisi M, Voigt JU, Habib G, Zamorano JL, Lancellotti P, et al. The use of pocket-size imaging devices: a position statement of the European Association of Echocardiography. Eur J Echocardiogr. 2011;12:85–7. doi: 10.1093/ejechocard/jeq184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.