Abstract
The cerebellum has been implicated in both sensorimotor and cognitive function, but is known to undergo volumetric declines with advanced age. Individual differences in regional cerebellar volume may therefore provide insight into performance variability across the lifespan, as has been shown with other brain structures and behaviors. Here, we investigated whether there are regional age differences in cerebellar volume in young and older adults, and whether these volumes explain, in part, individual differences in sensorimotor and cognitive task performance. We found that older adults had smaller cerebellar volume than young adults; specifically, lobules in the anterior cerebellum were more impacted by age. Multiple regression analyses for both age groups revealed associations between sensorimotor task performance in several domains (balance, choice reaction time, and timing) and regional cerebellar volume. There were also relationships with working memory, but none with measures of general cognitive or executive function. Follow-up analyses revealed several differential relationships with age between regional volume and sensorimotor performance. These relationships were predominantly selective to cerebellar regions that have been implicated in cognitive functions. Therefore, it may be the cognitive aspects of sensorimotor task performance that are best explained by individual differences in regional cerebellar volumes. In sum, our results demonstrate the importance of regional cerebellar volume with respect to both sensorimotor and cognitive performance, and we provide additional insight into the role of the cerebellum in age-related performance declines.
Keywords: cerebellum, volume, sensorimotor performance, individual differences
Introduction
There is a large amount of inter-individual variation in both sensorimotor and cognitive performance, even among healthy individuals. Recently, the utility of investigating individual performance differences with respect to brain morphology has been highlighted across a variety of domains [1]. This individual differences approach allows one to better understand brain function and its relationship to behavior. In particular, investigating regional volumes of the cerebellum and their relationship to behavior may shed light on their specialized functions.
The cerebellum contributes to a variety of motor and cognitive tasks. Evidence for cerebellar involvement in these behaviors comes from both patient studies [2-5] and functional neuroimaging investigations [6-10]. Specifically, the cerebellum has been implicated in balance [2-4, 11-13], timing [14-15], sensorimotor adaptation [6], associative learning (eye-blink conditioning) [16-19], working memory [9,20, for a review see 21], and attention [22]. Additionally, the connectivity patterns between the cerebellum and the cerebral cortex in both nonhuman primates [23-24, for a review see 25] and humans [26-29] further support its role in both sensorimotor and cognitive behaviors.
Recent work provides evidence for a functional topography within the cerebellum [10, 30-31], such that the anterior cerebellum plus lobules VIIIa and VIIIb are associated with motor tasks, whereas the remainder of the posterior cerebellum is associated with cognitive and affective processing. Despite our increased knowledge of cerebellar topography, it is unknown how individual differences in regional cerebellar volumes may be related to task performance.
To date, investigations of individual differences in cerebellar volume and behavior have primarily focused on total cerebellar volume. Cerebellar volume has been related to eye-blink conditioning [32-33], processing speed [34], motor learning [35], learning a complex strategy-based video game [36], and balance in individuals with alcoholism [2,37]. Taken together, this literature demonstrates a link between cerebellar volume and both motor and cognitive performance, though the structure has been investigated as a whole, and regional volumes have not been considered.
Thus, while some inroads have been made towards understanding the relationships between cerebellar volume and performance, there is still relatively little known with respect to this structure. With two exceptions [2,38], previous studies have investigated overall cerebellar volume. In these exceptional cases, regions of the cerebellar vermis were associated with balance and cognitive function respectively [2,38]; however, subregions of the cerebellar hemispheres were not investigated. Because of the variety of behaviors that engage the cerebellum, and its known functional topography, investigating regional cerebellar volume is crucial.
Investigation of regional cerebellar volume may be particularly useful for understanding sensorimotor and cognitive aging. Older adults experience declines in both domains. While there have been several investigations into the impact of age-related brain changes on both cognitive function [cf. 39-41] and motor declines [reviewed in 42], this work has largely focused on the cerebral cortex. However, we know that the volume of the cerebellum in older adults is reduced relative to that of their younger counterparts [43-49]. Furthermore, five-year longitudinal data indicate volumetric decreases in the cerebellum [48]. Given this, investigations of regional cerebellar volume and behavior in older adults may provide important insight into why some individuals maintain performance into old age while others exhibit more declines.
Finally, investigating hemispheric differences associated with motor laterality is of interest. While laterality of cerebellar networks has been investigated using resting state functional connectivity in young adults [50], it is of interest to investigate this with respect to cerebellar volume in both motor and cognitive sub-regions. Such an investigation will provide greater insight into the functional topography of the cerebellum, as well as into the motor system.
Here, we had two main goals. First, we investigated regional cerebellar volumes in a sample of young and older adults. Specifically, we calculated the volumes of each lobule of the cerebellar hemispheres and vermis. To date, there have been no investigations of age differences in regional lobular cerebellar volumes. We hypothesized that, consistent with previous literature, there would be differences in overall volume as well as in lobular sub-volumes between the two age groups. Additionally, we investigated whether there are hemispheric differences in volume for motor and cognitive regions of the cerebellum. We hypothesized that the dominant hemisphere for motor function would be larger than the non-dominant, perhaps due to greater processing demands, while there would be no difference in volume across the two hemispheres in cognitive regions. Second, we took an individual differences approach to investigating relationships between regional cerebellar volume and a variety of behaviors. Participants completed a battery of motor and cognitive tasks. We predicted that there would be regionally specific correlations between behavioral performance and cerebellar volume, given the functional topography seen within the cerebellum [10, 30]. That is, we predicted that volumes of the anterior lobules and lobules VIIIa and VIIIb would be associated with sensorimotor task performance given their involvement in motor behaviors, while volumes of posterior lobules (with the exception of VIIIa and VIIIb) would be associated with cognitive task performance.
Method
Participants
We recruited 31 older (65.03 ± 6.42 years old, 8 females) and 23 young adults (22.04 ± 3.47 years, 14 females) from the University of Michigan and greater Ann Arbor community as part of a larger study. All participants were right-handed (based on self-report), healthy with no history of neurological or psychiatric disorders, and had no contraindications for MRI scanning. Participants signed a consent form approved by the University of Michigan Medical Institutional Review Board. All procedures were in accordance with the ethical standards laid out by the Declaration of Helsinki.
Structural Imaging
Structural MRI images were collected with a 3T GE Signa (General Electric Signa, Milwaukee, Wisconsin) scanner at the University of Michigan. We acquired a 110 slice (sagittal) inversion-prepped T1-weighted anatomical image using spoiled gradient-recalled acquisition in steady state (SPGR) image (flip angle = 15°, FOV = 260 mm, 1.4 mm slice thickness). These parameters provided coverage of the whole brain including the entire cerebellum.
Volume Calculations
We calculated the gray matter volume for each lobule in the right and left hemispheres and the vermis using the following procedure. The cerebellum was first extracted from the high-resolution (SPGR) anatomical images using the SUIT toolbox [51-52], implemented in SPM8 (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk). This resulted in a structural image of the isolated cerebellum, along with probability maps indicating the probability of each voxel in the volume belonging to the cerebellum and brainstem. We masked our structural images with the probability maps, yielding a structural image of the cerebellum and brainstem only, excluding any surrounding cortical tissue.
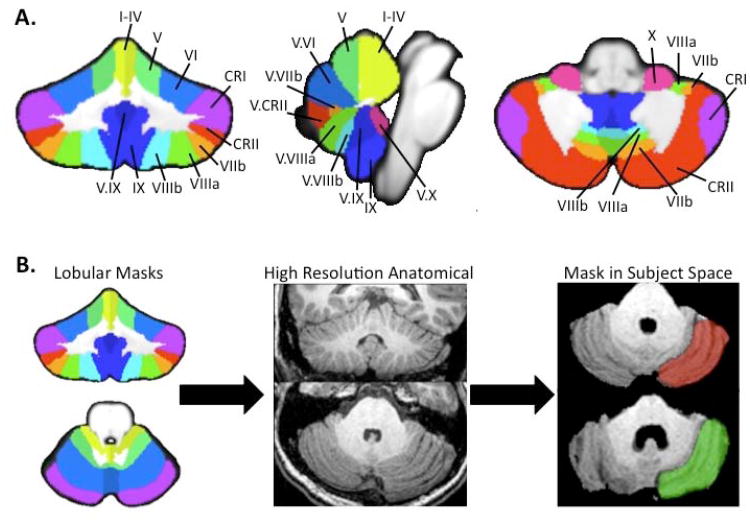
Next, we used the lobular regions described in the SUIT atlas [51-52] to determine individual lobular gray matter volumes of each subject for all lobules in the right and left hemispheres and the vermis. First, we created masks of each lobule of the right hemisphere, left hemisphere, and vermis using the probabilistic SUIT atlas. This resulted in 27 masks of the cerebellar gray matter. The lobules as defined by the SUIT atlas are depicted in Figure 1a. We were unable to create a mask for vermis Crus II. Second, the SUIT cerebellum template was normalized to each individual subject's cerebellar anatomical image (in native space) using Advanced Normalization Tools [53] (Penn Image Computing & Science Lab, http://www.picsl.upenn.edu/ANTS/). The transformation was first applied to the SUIT cerebellum, and then the resulting warp vectors were applied to the individual lobular masks. The result was a mask of each lobule normalized to individual subject space for each participant. Figure 1b provides a depiction of the processing stream described above.
Figure 1.
A) Lobules of the cerebellum as defined by the SUIT atlas presented on coronal (left), mid-saggital (center) and axial (right) slices. Labels are only included on the right hemisphere and vermis, though analysis was completed on both hemispheres. B) Anatomical imaging post processing stream. Masks created using the SUIT atlas (left) were then warped to individual subject's high-resolution anatomical images (center), resulting in subject-specific masks for each individual (right). Individual masked overlays for Right Crus I are presented for a representative young (red) and older adult (green).
These masks were loaded into MRICron (http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html) and converted to volumes of interest. They were overlaid onto each individual subject's structural scan and visually inspected to ensure accurate registration. We then used MRICron to calculate the descriptive statistics for each lobule, providing us with the gray matter volume of each lobule in cubic centimeters. This procedure was repeated for each individual participant.
Additionally, we calculated the total intracranial volume for all participants to normalize the cerebellar lobular volumes. We first segmented the gray matter, white matter, and cerebrospinal fluid using the segment function in SPM5. The VBM toolbox was used to get the total volume for each of these tissue types, in each individual subject, in native space. The volume of the gray matter, white matter, and cerebrospinal fluid were summed to get the total intracranial volume for each individual. Normalized lobular volumes were calculated by dividing the lobular volume by the total intracranial volume.
Behavioral Assessments
Participants were invited to return to our laboratory for a second day of behavioral testing. A subset of 10 young (21.7 ± 2.7 years old, 4 females) and 16 older (61.9 ±7 years old, 7 females) adults returned to the lab for this additional testing; this sample size is comparable to what has been used in previous studies investigating relationships between cerebellar volume and behavior [2, 32, 33]. A battery of motor and cognitive assessments was completed.
Cognitive and Executive Function
General cognitive function was assessed using the Montreal Cognitive Assessment (MOCA) [54]. Executive function was measured using the Trail-making task (A and B versions) [55]. We recorded the total score on the MOCA, and the time to complete the A and B portions of the Trail-making task, along with the difference between the two conditions (B-A, measured in seconds). The difference score was used in our subsequent analyses.
Working Memory
We administered a verbal working memory Sternberg task [56] similar to that used by Desmond and colleagues [57] using E-Prime 2.0 software (Psychology Software Tools, Inc). Four lowercase letters were presented in white, size 20 Courier New font on a black background around a centrally located fixation cross for 1500 msec. This was followed by a 3000 msec retention interval after which a capitalized letter was presented for 1500 msec. During the presentation of this letter, participants were asked to make a yes or no button press response to indicate whether or not the letter was a part of the previously viewed set. There was an additional 1500 msec after the presentation of the capitalized letter during which participants could make their response. This resulted in a total inter-trial interval of 7500 msec. Participants completed 144 trials (3 blocks of 48 trials each). We recorded accuracy (% correct) along with reaction time (msec) on correct trials for all participants.
Visuomotor Adaptation and Choice Reaction Time
A visuomotor adaptation task was administered using Presentation 14.5 software (Neurobehavioral Systems, Albany, CA) on a desktop computer [6, 58-59]. Targets (0.8 cm in diameter) were presented for 4 seconds in one of four locations: 4.8 cm to the right, left, above, or below a central starting position (0.8 cm in diameter). Participants controlled a cursor using their whole hand to move a standard gaming joystick (Logitech Extreme 3D joystick, Fremont, CA) placed on the desk in front of them. They were asked to move the cursor to the target circle as quickly and as accurately as possible and to maintain the cursor in the target circle until it disappeared. Upon disappearance of the target, participants were asked to release the joystick so the cursor would re-center itself. The next trial began 1 second later, resulting in a total inter-stimulus interval of 5 seconds. Participants performed 14 blocks of the task (24 trials per block), with the first two experimental blocks under veridical feedback conditions. This was followed by 10 adaptation blocks with visual feedback rotated 30° clockwise about the center of the screen, and finally two more blocks again under veridical feedback conditions.
The x and y coordinates from the joystick were recorded at a rate of 100 Hz. The data were analyzed offline using custom MATLAB (MathWorks, Inc, Natick, MA) programs. The data were first filtered with a dual low-pass Butterworth digital filter, using a cutoff frequency of 10 Hz, and then the resultant joystick path was calculated (square root of the sum of the squared x and y coordinates at each time point). The tangential velocity profile was then calculated through differentiation of the resultant position data. Movement onset and offset were computed through the application of Teasdale, Bard, Fleury, Young, and Proteau's [60] optimal algorithm to the velocity profile for each movement. Learning was assessed by measuring direction error (DE), which is the angle between a straight line from the start to the target position and a straight line from the start to the actual position attained at the time of peak velocity. We assessed performance during the adaptation and washout periods by fitting exponential functions to the trial-by-trial data [cf. 61]. These fits resulted in an intercept and decay constant that characterized the adaptation and washout performance. In our subsequent analyses we used the decay constant to describe adaptation and the intercept to model washout. The magnitude of the aftereffects during the washout period serves as an indicator of how much learning has occurred.
The first block of testing under veridical feedback served as a practice block. The second block of testing, still under veridical feedback, allowed us to measure choice reaction time. Here, we measured reaction time to move to the target (average over all trials).
Timing
Timing was measured using a synchronization-continuation tapping task [62] administered with E-Prime 2.0 software (Psychology Software Tools, Inc). Participants sat comfortably in front of the computer screen with their gaze centered on a fixation cross, and they were instructed to press the “z” key on the keyboard in synchrony with a periodic auditory tone sequence. Each trial began with the message “ready, go” presented on the computer screen, followed by the presentation of the tone sequence. Participants were instructed to synchronize their finger taps to the tone sequence when ready. After twelve synchronized finger taps, the tones were removed and participants continued tapping at the rate to which they synchronized for an additional 31 taps (30 time intervals). There were three time interval conditions: 500, 1000, and 1500 ms. Participants completed 5 trials of each of the three interval conditions with each hand (15 trials per hand), as well as practice trials prior to the start of each block. The order of hands and time interval condition were counterbalanced across participants. For our purposes in this study, only data from the dominant hand was analyzed.
We measured timing variability for each time interval condition during the un-paced (continuation) phase using the coefficient of variation (CV). CV was calculated by dividing the standard deviation of the inter-tap-interval by the mean duration of the inter-tap-interval. The mean CV for each hand and time interval was calculated. Analyses focused on the CV for the un-paced continuation phase (31 taps without the tone) completed using the dominant (right) hand. All three interval conditions were analyzed.
Balance
We assessed balance using the activities-specific balance confidence (ABC) scale [63] and a one-legged timed standing balance task. For the one-legged standing balance task participants stood on their dominant (right) leg with their arms crossed over their chest. They were timed (maximum of 90 seconds) from the point of taking their foot off the ground until it was placed back on the ground. A research assistant spotted the participants from behind throughout this process. Trials with eyes opened and closed were completed (three trials per condition). The averaged times standing on one leg from both the eyes opened and eyes closed conditions were used in our analyses.
Manual Dexterity
We assessed manual dexterity with the grooved pegboard (Lafayatte Instruments, Lafayette, IN). We quantified the time it took participants to fill all of the holes with “T” shaped pegs using the dominant (right) hand.
Statistical Analysis
All statistical analyses were performed using IBM SPSS 19 (IBM Corporation, Somers, NY, 2011). Across all of our analyses, in cases where the assumption of equal variance was violated, we used a Greenhouse-Geiser adjustment. The adjusted degrees of freedom and f- or t-values are reported.
Age Comparisons and Relationships with Volume
To compare intracranial volume we used a 3×2 tissue type (gray matter, white matter, cerebrospinal fluid) by age group mixed-model ANOVA. A 27×2 lobule by age group mixed-model ANOVA was computed to assess age differences in regional cerebellar volume. Follow-up pairwise t-tests were computed using a Bonferonni correction (p<.002). To account for the effect of gender we also included an additional 27×2×2 lobule by age group by gender mixed-model ANOVA.
To investigate laterality associated with motor dominance, we compared the left and right hemisphere volumes of the two motor representations within the cerebellum, along with a grouping of lobules associated with cognitive processing. The anterior motor representation included lobules I-IV and V, while the posterior motor representation included lobules VIIIa and VIIIb. The cognitive grouping consisted of Crus I, Crus II, and lobule VIIb, based on previous work implicating these regions in a variety of cognitive processes [10, 30-31]. We then computed a 3×2×2 region by hemisphere by age group mixed-model ANOVA.
Patterns of Lobular Volume Variance
A principal component analysis (PCA) was then performed on our 27 lobules of interest to reduce the number of factors in our multiple regression models investigating the relationships between regional cerebellar volume and behavior. In this case, regions would be grouped in a manner that accounts for significant portions of variance in the data, likely due to similarities in the volume of the lobules. Because of the potential for age differences in the solutions, we investigated the young and older adults separately. The corrected volumes (lobular volume/total intracranial volume) were entered into the analysis for each lobule across all subjects. The rotated solutions (using varimax rotation) were investigated for both age groups. In each case, scree plots were used to determine the number of significant components. We used Pearson's product-moment correlation to assess linear relationships between age and regional cerebellar volume (seven regions, as defined by our PCA; see Results). These analyses were evaluated using a Bonferonni correction (p<.007).
Relationships Between Regional Volume and Behavior
We first assessed age differences in behavioral performance using independent samples t-tests for all behaviors with the exception of timing. To investigate age differences in timing variability we used a 2 (age) by 3 (timing interval) mixed-model ANOVA.
Two separate sets of analyses were conducted to investigate the relationship between regional cerebellar volume (regions defined using PCA) and behavior. First, we investigated how individual differences in regional cerebellar volume across all participants were related to behavior. We used multiple regression with a backwards selection procedure. However, we ran separate models for both the right and left hemisphere to minimize colinearity, as there were strong correlations between homologous regions in both hemispheres. In both sets of models we included the vermis. Age was also included as we pooled our participants across age groups.
Second, differential relationships between regional volume and behavior in the two age groups were investigated in cases where there were no significant associations between behavioral performance and regional volume in our multiple regression analyses. In these cases, null findings on our combined multiple regression analyses may be due to differential involvement of regional cerebellar gray matter volume in behavior in the two age groups. Thus, we ran a correlation analysis between each cerebellar region and each behavioral measure in both the young and older adults. In cases where there were significant relationships in either age group, we then used Fisher's r-to-z transform and compared the strength of the two relationships between the age groups.
Results
Age Differences in Total Brain Volume
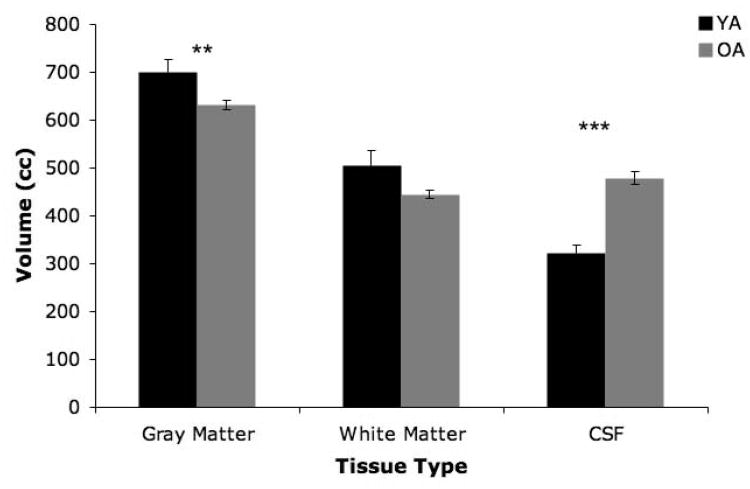
Our 2×3 age by tissue-type ANOVA investigating age differences in whole brain gray matter, white matter, and cerebrospinal fluid indicated that there was no significant main effect of age (F(1,58)=.472, p>.4; Figure 2). Importantly, this means that there was no significant difference in total intracranial volume between the two age groups. There was however a significant main effect of tissue type (F(1.62,94.02)=113.40, p<.001), and an age by tissue type interaction (F(1.62,94.02)=24.93, p<.001). Follow-up t-tests (using a Bonferonni correction, p<.01) indicated that this interaction was driven by significantly less gray matter in the older adults (t(58)=2.75, p<.01), and concomitantly, significantly greater volume of cerebrospinal fluid in the older adults (t(58)=−7.139, p<.001). There was no significant age difference in white matter volume when correcting for multiple comparisons, although there was a trend for older adults to have less (t(58)=2.26, p=.03).
Figure 2.
Age differences in cerebral tissue types (YA: black bars; OA: gray bars). There was no significant main effect of age, though there was a significant age by tissue type interaction. Follow-up t-tests indicated significant age differences in gray matter and CSF volume (**p<.01; ***p<.001). Error bars represent the standard error of the mean.
Age Differences in Cerebellar Lobular Volume and Hemispheric Effects
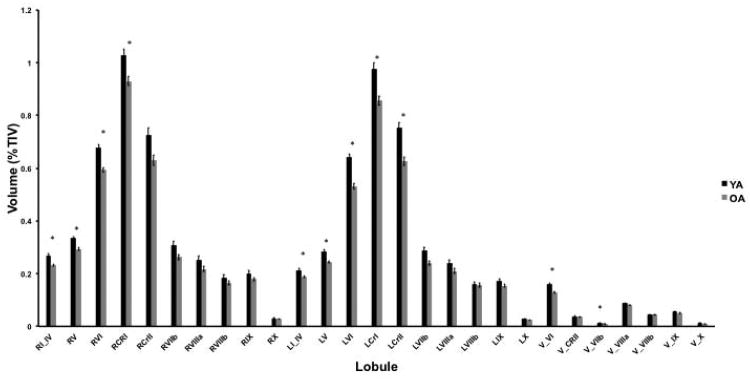
To test for age differences in lobular cerebellar volume we completed a 27×2 repeated measures ANOVA. This analysis revealed a significant main effect of age (F(1,51)=20.17, p<.001; Figure 3) indicating that young adults have larger cerebellar gray matter volume than older adults. There was also a significant main effect of lobule (F(4.26,217.14)=1985.66, p<.001) and a significant age by lobule interaction (F(4.26,217.14)=9.07, p<.001). Follow-up pairwise t-tests indicate significant age differences in the gray matter volumes of right lobules I-IV, V, VI, right Crus I, left lobules I-IV, V, VI, left Crus I, left Crus II, vermis lobule VI, and vermis lobule VIIb (in all cases t(52)>4.0, p<.001). Left lobule VIIb was nearly significant when correcting for multiple comparisons as well (t(52)=3.27, p=.002). This supports the hypothesis that there are regional age differences in cerebellar volume. Additionally, we investigated the impact of gender, as gender differences in cerebellar volume have been reported [64]. Using a 27×2×2 lobule by age group by gender mixed model ANOVA we found that there was a significant main effect of gender (F1,49)= 15.25, p<.001) such that females had larger cerebellar volume. As described above, there were also significant main effects of age and lobule. However, the age by gender interaction was not significant (F(1,49)= 1.18, p=.28) nor was the three way interaction (F(26,1274)= 1.20, p=.22), though there was a significant lobule by gender interaction (F(26, 1274)= 6.13, p<.001). Follow-up comparisons did not reveal any lobules that were significantly different based on gender, when correcting for multiple comparisons.
Figure 3.
Age differences in lobular volume (YA: black bars; OA: gray bars). There are significant main effects of age (F(1,51)=20.17, p<.001) indicating that older adults have smaller cerebellar gray matter volume. There is also a main effect of lobule (F(4.26,217.14)=1985.66, p<.001), along with a significant age by lobule interaction (F(4.26,217.14)=9.07, p<.001). *Indicates significant age differences in lobular volume as assessed using follow-up pairwise t-tests, corrected for multiple comparisons (p<.002). Error bars represent the standard error of the mean. Lobules are labeled using roman numerals. L: left hemisphere; R: right Hemisphere; V: vermis; CRI: Crus I; CRII: Crus II.
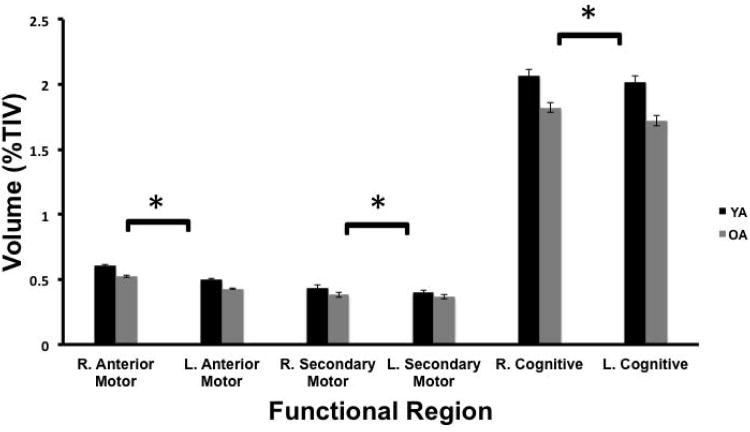
Next, we investigated hemisphere effects in motor and cognitive subregions of the cerebellum (as defined by previous functional imaging work). Our analysis revealed significant main effects of region (F(1.35, 70.07)=3042.83, p<.001; Figure 4) and hemisphere (F(1, 52)=63.99, p<.001), a significant region by age group interaction (F(1.35, 70.07)=16.15, p<.001), and a significant region by hemisphere interaction (F(1.51, 78.39)=10.95, p<.001). The hemisphere by age interaction was not significant (F(1,52)=.15, p>.70). The three-way region by hemisphere by age interaction was a strong trend, though not significant (F(1.51, 78.39)=3.01, p=.069). Given the significant region by hemisphere interaction, and the strong trend in the three-way interaction, we completed follow-up analyses investigating hemispheric differences by region. In all three regions, the effect of hemisphere was significant (in all cases F(1, 52)>8.8, p<.005). Across the anterior motor, posterior motor, and cognitive regions, the right hemisphere was larger. The effect sizes varied across these three regions (ηp2=.84, .14, and .23 for the anterior motor, posterior motor and cognitive regions, respectively) indicating that the hemispheric asymmetry effect is largest in the anterior motor regions of the cerebellum.
Figure 4.
Hemispheric differences in gray matter volume (%TIV) of motor and cognitive regions of the cerebellum (YA: black bars; OA: gray bars). The three-way repeated measures age by hemisphere by region interaction was nearly significant when using a Greenhouse-Geiser correction for sphericity (F(1.51, 78.39)=3.02, p=.068). We further conducted follow-up analyses to investigate the effect of hemisphere on the motor and cognitive regions. These analyses indicate significant effects of hemisphere for all three regions (in all cases, F(1,52)>8, p<.005), and are indicated with an asterisk. Error bars represent the standard error of the mean.
Patterns of Lobular Volume Variance
Our PCA in the young adults demonstrated a clearly organized solution. The rotated solution is presented in Table 1. Our scree plot indicated a 4-component solution for the young adults. Component one was made up of lobules of the posterior cerebellum, component two primarily of lobules of the anterior cerebellum, and component three was made up of lobules of the cerebellar vermis. The fourth component in the young adults was made up of left and right Crus I. Notably, these components generally followed the functional topography of the cerebellum such that motor and cognitive regions were separate.
Table 1.
Principal component analysis results in the young adults. The four significant components are highlighted in gray.
| Component | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| L. VIIIa | .949 | .119 | .084 | .126 | −.006 | −.021 |
| L. VIIIb | .937 | .045 | .129 | .118 | .133 | −.063 |
| L. VIIb | .922 | .144 | .065 | .077 | −.087 | .041 |
| R. VIIIb | .886 | .035 | .241 | −.030 | .237 | .163 |
| R. VIIIa | .833 | .082 | .218 | −.085 | .203 | .350 |
| R. VIIb | .787 | .159 | .228 | .000 | .146 | .463 |
| L IX | .771 | .170 | .285 | .281 | .266 | −.304 |
| R.IX | .735 | .275 | .427 | .206 | .256 | −.045 |
| L. Crus II | .702 | .210 | .098 | .425 | −.066 | .288 |
| R. VI | .112 | .812 | .275 | .083 | .210 | .063 |
| R. V | .169 | .808 | −.057 | −.029 | .052 | −.087 |
| L. V | .238 | .744 | .196 | .158 | .230 | .308 |
| R. I-IV | −.050 | .697 | −.144 | .540 | −.028 | −.213 |
| L. VI | .060 | .641 | .520 | .030 | .236 | .241 |
| L. I-IV | .021 | .632 | −.132 | .439 | .228 | .337 |
| V. VIIb | .326 | .609 | .516 | .071 | −.252 | .174 |
| V. VI | .136 | .589 | .155 | .493 | .021 | −.241 |
| V. Crus II | .174 | .501 | −.099 | .331 | −.447 | .344 |
| V. X | .274 | −.186 | .729 | .057 | .315 | .118 |
| V. IX | .497 | .209 | .704 | .214 | .216 | −.164 |
| V. VIIIb | .467 | .397 | .587 | .327 | −.017 | .107 |
| V. VIIIa | .357 | .381 | .562 | .322 | −.323 | .118 |
| R. Crus I | .065 | .037 | .280 | .757 | .183 | .124 |
| L. Crus I | .312 | .306 | .113 | .731 | .288 | .095 |
| L. X | .245 | .244 | .057 | .223 | .818 | .045 |
| R. X | .214 | .196 | .162 | .198 | .768 | .026 |
| R. Crus II | .487 | .157 | .298 | .350 | −.029 | .606 |
In the older adults our PCA yielded a three-component solution, based on the scree plot. The rotated solution is presented in Table 2. The first component was made up of lobules in the posterior region of the cerebellum, while the second component was made up of lobules in the anterior cerebellum. The third component included vermal lobules, along with right lobule V (anterior) and left lobule VIIIb (posterior). These latter two lobules also had strong loadings on components two and one, respectively. The remaining lobules were spread out across two additional non-significant components. In general, the older adult principal component analysis revealed an anterior, posterior, and vermal grouping in the cerebellum.
Table 2.
Principal component analysis results in the older adults. The three significant components have been highlighted in gray.
| Component | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| R. VIIIb | .944 | −.031 | .092 | .175 | .053 |
| R. VIIIa | .925 | −.001 | .151 | .061 | .054 |
| R. IX | .895 | .127 | .090 | .318 | .055 |
| R. VIIb | .874 | .252 | .261 | .022 | .080 |
| L. VIIIa | .842 | −.007 | .181 | .061 | .391 |
| L. IX | .828 | .040 | .225 | .362 | .122 |
| L. VIIb | .806 | .187 | .187 | .087 | .435 |
| L. Crus II | .621 | .341 | .492 | .236 | .300 |
| R. Crus II | .604 | .368 | .604 | .038 | −.024 |
| L. X | .599 | .433 | .021 | .564 | .200 |
| L. V | .038 | .944 | .068 | .148 | .097 |
| L. I-IV | .135 | .881 | .201 | .274 | .028 |
| L. VI | −.068 | .652 | .189 | .157 | .462 |
| R. I-IV | .282 | .638 | .375 | .262 | .282 |
| V. Crus II | .121 | .093 | .893 | .171 | .198 |
| R. V | .264 | .439 | .675 | .029 | .077 |
| V. VIIIa | .379 | .201 | .631 | .317 | .447 |
| V. VIIIb | .376 | .341 | .574 | .351 | .333 |
| L. VIIIb | .451 | −.138 | .476 | .294 | −.190 |
| V. X | .054 | .191 | .112 | .849 | −.095 |
| R. Crus I | .314 | .176 | .146 | .661 | .417 |
| V. IX | .476 | .271 | .495 | .622 | .170 |
| R. X | .544 | .219 | .200 | .588 | .166 |
| V. VIIb | .138 | .357 | .300 | .549 | .355 |
| V. VI | .162 | .131 | .030 | −.071 | .861 |
| L. Crus I | .379 | .092 | .131 | .307 | .721 |
| R. VI | −.004 | .227 | .454 | .182 | .600 |
Given the overall similarity between the patterns seen in the two groups, and the consistency of these patterns with anatomical divisions in the cerebellum, for our additional analyses we grouped the lobules together based on the young adult solution (Figure 5). The cerebellum was grouped into anterior, posterior, and vermal regions, separated by hemisphere. However, right and left Crus I were each evaluated individually.
Figure 5.
PCA components in the young adults were used to define sub-regions of the cerebellum for additional analysis. Overlays are presented on the right hemisphere only on both a coronal (left) and axial (right slice), to allow for comparison to the left hemisphere, though analysis was conducted on both hemispheres. Lobules not included in a component (left and right lobule X and right Crus II) were included in the posterior grouping given their anatomical location. Red: anterior cerebellum, corresponding to component two; Green: posterior cerebellar grouping corresponding to component one; Blue: Crus I, corresponding to component four; Yellow: cerebellar vermis corresponding to component three.
Finally, investigations of correlations between regional volume and age indicated that there were significant negative correlations with all cerebellar regions (in all cases r>.47, p<.001), with the exception of the right posterior cerebellum (Figure 6).
Figure 6.
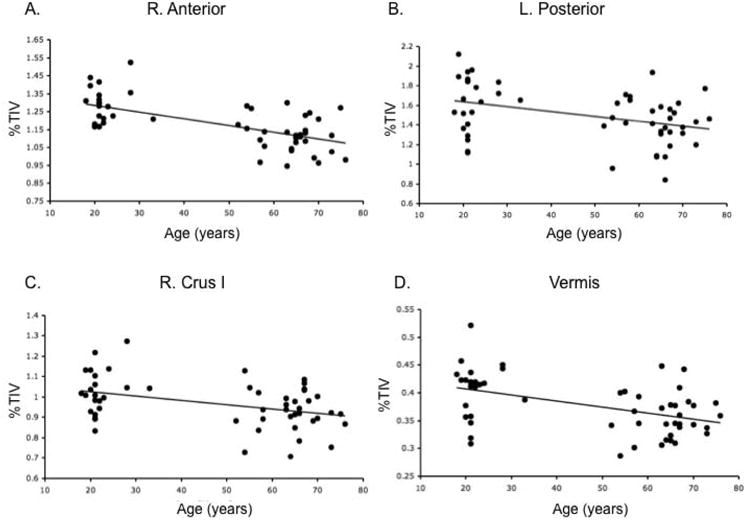
Correlations with age and regional cerebellar volume (% TIV). A) right anterior cerebellum (r=−.64), B) left posterior cerebellum (r=−.38), C) right Crus I (r=−.41), and D) the vermis (r=−.47). Additionally, there were significant negative correlations with the left anterior cerebellum (r=−.64) and left Crus (r=−.48), though just the right hemisphere is presented here for the sake of parsimony. These linear relationships indicate differences in cerebellar volume across adulthood.
Regional Cerebellar Volume and Performance
Table 3 lists the group mean scores on our behavioral tasks. There were significant age group differences in the time difference to complete the Trails A and B task (B – A, t(21.40)=-3.48, p<.01), reaction time on the Sternberg verbal working memory task (correct trials; (t(13.65)=-4.81, p<.001), standing balance with eyes opened (t(23)=2.93, p<.01) and closed (t(8.22)=2.44, p<.05), and time to complete the grooved pegboard test (t(22.96)=−3.83, p=.001), with older adults performing less well in each case. There were no significant age differences on the MOCA, working memory accuracy, balance confidence, choice reaction time, or learning rate and after effect magnitude on the joystick adaptation task. The lack of difference between the two age groups on the MOCA underscored the overall cognitive health of our two samples. Additionally, our 2 × 3 mixed-model ANOVA to investigate timing variability at the 500, 1000, and 1500 msec time intervals indicated that there was a significant main effect of age group (F(1,23)=6.40, p<.05), though there was no main effect of time interval (F(2, 1.63)=.062, p>.9) nor was there an age by time interval interaction (F(2, 1.63)=1.2, p>.3). In every case where there were significant age effects on performance, the young adults outperformed the older adults, with the exception of timing, where older adults performed better.
Table 3.
Mean task performance by age group.
| Task | Young Adult Mean (± SD) | Older Adult Mean (± SD) |
|---|---|---|
| Montreal Cognitive Assessment (Total Score) | 27.22 (1.79) | 26.56 (2.22) |
| Trails Score (Difference, Trails B - Trails A, msec)** | 12.26 (5.18) | 25.14 (13.09) |
| Working Memory Accuracy, % | 94.30 (6.29) | 89.19 (7.88) |
| Working Memory RT (Correct Trials, msec)*** | 712.28 (113.96) | 906.51 (72.91) |
| ABC 16 (% Confidence) | 95.79 (3.19) | 97.18 (3.02) |
| Standing Eyes Open Balance (Time, sec)** | 77.85 (23.41) | 46.39 (31.09) |
| Standing Eyes Closed Balance (Time, sec)* | 32.66 (32.18) | 6.32 (4.98) |
| Grooved Pegboard (Time to complete, sec)*** | 62.66 (5.87) | 75.02 (10.26) |
| Choice RT (msec) | 475.69 (136.68) | 621.46 (270.15) |
| Joystick Adaptation (decay constant) | .061 (.017) | .047 (.025) |
| Joystick Learning (Washout Intercept, Degrees) | 20.12 (5.91) | 15.85 (5.18) |
| 500 msec Timing (CV)† | .069 (.036) | .044 (.042) |
| 1000 msec Timing (CV)† | .080 (.082) | .034 (.018) |
| 1500 msec Timing (CV)† | .074 (.038) | .036 (.014) |
Significant age differences are indicated (
p<.05,
p<.01,
p<.001).
Timing was assessed using a 3×2 mixed-model ANOVA that indicated a significant main effect of age.
Multiple regression models were run separately for the right and left hemisphere to investigate relationships between individual differences in regional cerebellar volume and performance. Using a backwards selection method, several significant models emerged for both the right and left hemispheres with various behavioral measures, as well as with the vermis (see Tables 4 and 5 for the specific beta values). Working memory accuracy was related to left posterior cerebellar volume, such that larger volume resulted in better performance. While age was a significant predictor of working memory reaction time and the difference score on the Trails test (p<.001, in both instances), these models did not include any significant contributions by cerebellar volumes.
Table 4.
Significant models of task performance with right hemisphere cerebellar regions. While the model predicting CV500 emerged in both sets of analyses, we only report it here to eliminate redundancy.
| Predictor Variable | Beta | p |
|---|---|---|
| ABC 16: Adjusted R2=.146, F(1,24)=5.27, p<.05 | ||
| R. Posterior | −.424 | p<.05 |
| Eyes Closed Balance: Adjusted R2=.430, F(3,21)=7.03, p<.01 | ||
| Age | −.665 | p=.001 |
| R. Posterior | .478 | p<.05 |
| R. Crus I | −.421 | p<.05 |
| Choice RT: Adjusted R2=.134, F(1,24)=4.88, p<.05 | ||
| R. Crus I | −.411 | p<.05 |
| CV500: Adjusted R2=.221, F(2,22)=4.41, p<.05 | ||
| Age | −.470 | p<.05 |
| Vermis | −.506 | p<.05 |
Table 5.
Significant models of task performance with left hemisphere cerebellar regions.
| Predictor Variable | Beta | p |
|---|---|---|
| Working Memory Accuracy: Adjusted R2=.130, F(1,24)=4.73, p<.05 | ||
| L. Posterior | .406 | p<.05 |
| Choice RT: Adjusted R2=.237, F(1,24)=8.75, p<.01 | ||
| L. Crus I | −.517 | p<.01 |
| CV1000: Adjusted R2=.288, F(2,22)=5.86, p<.01 | ||
| Vermis | −.537 | p<.05 |
| L. Anterior | .704 | p<.01 |
A significant model predicting balance confidence on the ABC 16 scale emerged and included the right posterior cerebellum. Increased volume was associated with lower balance confidence. Also related to balance, there was a significant model predicting one-legged balance times while the eyes were closed, which included age, the right posterior cerebellum, and right Crus I. Both age and right Crus I volume were negatively associated with balance times, whereas the right posterior cerebellum was positively associated with balance times. Balance time with the eyes opened was associated only with age (p<.01).
Choice reaction time performance was significantly modeled by the volume of both right and left Crus I. In both hemispheres, larger volume of Crus I was associated with shorter choice reaction time. Our analyses also revealed a significant model predicting tapping variability at the 500 msec interval. This model included both age and volume of the vermis. Both were negatively associated with the coefficient of variation. That is, with older age and larger vermis volume, there was less variability. A similar model was revealed for tapping variability at the 1000 msec interval. This model included both volume of the vermis and that of the left anterior cerebellum. However, volume of the left anterior cerebellum was positively associated with tapping variability such that increases in volume were associated with increases in variability. Age was the only significant predictor of tapping variability at 1500 msec (p<.01).
There were no significant models predicting learning on the joystick task, while the intercept of our model of washout (indicative of the degree of learning), was only predicted by age (p<.05). Older adults exhibited numerically reduced aftereffects relative to the young adults (though there was no significant difference between the two groups), and this is likely driving this relationship. Finally, the model predicting performance on the grooved pegboard (time to complete) also only included age (p=.001).
Follow-up exploratory regression analysis was completed in the young and older adults separately, for lobules and behaviors that were not part of significant models in our multiple regression analyses. We discovered several interesting associations (Figure 7). First, the time to complete the grooved pegboard was negatively correlated with the volume of the right Crus I in young adults (r=−.72, p<.05). The pattern in the older adults was in the positive direction, though not significant (r=.32, p>.2; Figure 7a). Using a Fisher's r to z transform, we found that these two relationships were significantly different from one another (z=2.51, p<.01).
Figure 7.
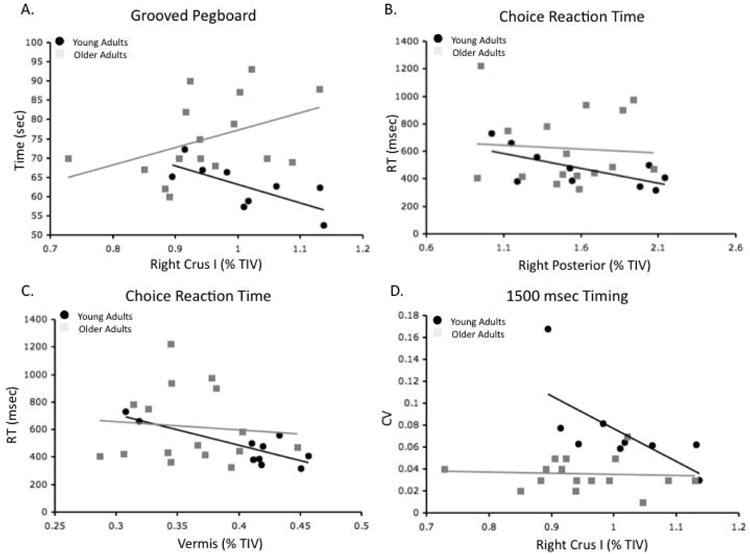
Differential relationships between regional cerebellar volume (%TIV) and performance in young and older adults. Differential relationships were seen between A) Grooved pegboard performance and right Crus I volume, B) Choice reaction time and the volume of the right posterior cerebellum, and C) Choice reaction time and the volume of the vermis, and D) 1500 msec timing and right Crus I volume, YA: black circles. OA: Gray Squares.
Second, while our multiple regression models pooling across all participants indicated that the volume of both the left and right Crus I significantly predicted choice reaction times, we found differing relationships in the two age groups for the right posterior cerebellum and the vermis. Right posterior cerebellar volume was significantly negatively correlated with choice reaction time in the young adults (r=−.68, p<.05; Figure 7b), while in the older adults there was no relationship at all (r=−.07, p>.7). There was a strong trend indicating that the two correlations were different (z=1.62, p=.052). It is also worth noting that there was a comparable trend indicating a negative correlation between the volume of the left posterior cerebellum and choice reaction time (r=−.61, p=.06). The pattern in the vermis was similar. Young adults showed a significant negative correlation between vermal volume and choice reaction time (r=−.83, p<.01; Figure 7c) and there was no relationship in the older adults (r=−.09, p>.7). Here, there was a significant difference between the two correlations (z=2.34, p<.01).
Finally, tapping variability at 1500 msec was differentially related to the volume of right Crus I (Figure 7d). In the young adults, there was a negative correlation (r=−.69, p<.05), indicating that larger Crus I volume was related to less tapping variability. In the older adults, there was no relationship (r=−.07, p>.7). The difference between these two correlations was a strong trend (z=1.58, p=.057).
Discussion
We observed significant age differences in cerebellar gray matter volume between young and older adults. Furthermore, our results demonstrated that individual differences in regional cerebellar volume are associated with sensorimotor and cognitive task performance. However, in some cases the relationships between regional volume and behavior differed in direction in the young and older adult samples. Taken together, our results further highlight the importance of the cerebellum to both sensorimotor and cognitive behaviors. They also indicate the importance of investigating regional cerebellar volume instead of the structure as a whole.
Aging and Lobular Volume
While past work has investigated age differences in cerebellar volume [43-46, 48-49], none has determined whether these age differences vary across cerebellar lobules. Here, we demonstrated that there are differential relationships between age and lobular gray matter volume in older adults, in addition to an overall age difference in cerebellar gray matter. This latter finding was consistent with the existing literature on this topic [43-46, 48-49]. Specifically, our analyses indicated that the gray matter volume of the anterior lobules and Crus I was particularly impacted by age. This was contrary to our hypothesis, which predicted greater age differences in posterior cerebellar volume. Indeed, over the course of development, the posterior cerebellum typically tracks the development of the prefrontal cortex [65]. In older adults, there are significant decreases in prefrontal cortical volume [66]. Given the known interactions between the posterior cerebellum and prefrontal cortex, both in terms of anatomical connections [23, 26] and in resting state functional networks [27-29, 67-6], volumetric differences were expected. However, it may be that once development is complete, the posterior cerebellum changes independently of the pre-frontal cortex. Further, it is important to note that Crus I is a posterior lobule. Thus, while the majority of the posterior cerebellum was not specifically impacted by aging, Crus I was affected.
We were also interested in investigating whether laterality has any impact on cerebellar regions associated with motor and cognitive function. As predicted, both the anterior and posterior motor regions of the cerebellum were larger in the right hemisphere of both the young and older adults, likely a result of all participants being right-handed. However, somewhat surprisingly, the lobules of the cerebellum associated with cognitive function were also larger in the right hemisphere. One likely explanation for the volumetric differences in these regions across the two hemispheres is the involvement of these lobules in language processing in addition to cognitive processing. While Crus I, Crus II, and lobule VIIb have been implicated in working memory and executive function [10, 30-31], they have also been implicated in language processing, particularly in the right hemisphere [10, 30-31]. Thus, the larger volume of the right hemisphere cognitive region as defined here may be due to interactions with the left cerebral hemisphere for language processing. Interestingly though, a comparison of the effect sizes across the three regions indicated that the hemispheric effect was much larger in the anterior motor region, likely linked to hand dominance. Previous investigations into cerebellar laterality using resting state networks demonstrated that the most strongly right-lateralized regions were in the posterior cerebellum [50]. Though the largest effects in our study were in the anterior cerebellum, in general our finding was consistent with previous work on cerebellar laterality.
Cerebellar Volume and Motor and Cognitive Function
Differences in regional volumes were related to both sensorimotor and cognitive behavior. However, the majority of the relationships implicated the posterior cerebellum and Crus I in the performance of sensorimotor tasks. In particular, both self-reported balance confidence and standing balance performance were associated with posterior cerebellum and Crus I volumes. Furthermore, in all cases, with the exception of the right posterior cerebellum in our model of standing balance (with eyes closed) these predictors were negatively associated with balance confidence and performance. Larger volumes were associated with poorer balance performance and confidence. This was somewhat surprising given previous work implicating the anterior vermis in balance [2, 69], though prefrontal cortex activity has also been seen during balance with the eyes closed [69] implicating higher cognitive functions as well. However, Crus I and the posterior cerebellum are typically implicated in cognitive tasks [9, 30]. A tendency for cognitive engagement during an automatic task such as balance may be detrimental to performance [70].
Though the posterior cerebellum included lobules VIIIa and VIIIb, which have known connections with the motor cortex in the non-human primate [24] and are part of resting-state motor networks [27, 68], this grouping also included Crus II and lobule VIIb. Crus II is connected to the prefrontal cortex in the primate brain [24] and is involved in resting state networks associated with the prefrontal cortex and cognitive function [27-29, 68, 71]. Lobule VIIb has also been implicated in these networks [71]. Given the regions making up the posterior cerebellum in this analysis, our results indicated that variability in cognitive performance associated with posterior cerebellar volume may be what was driving these relationships with balance. A similar argument could be made with respect to Crus I, as it is also associated with prefrontal cortical networks [27-29, 68, 71]. We speculated that larger volume may be associated with greater engagement of these cognitive networks. This in turn may have a negative impact on balance. Under dual-task motor and cognitive conditions, the automatic motor task suffers [70]. Additional cognitive processing associated with larger posterior cerebellum and Crus I volume may interfere with a more automatic task such as balance. Future work is however needed to test this hypothesis.
The volume of Crus I in both hemispheres was also associated with choice reaction time performance. However, in this case it was in the expected direction -- larger Crus I volume was related to shorter reaction times. Performance on a choice reaction time task is made up of multiple stages: stimulus processing, stimulus categorization, response preparation, and finally, response execution [72]. The preparation of responses has been proposed to be a race between multiple response options [73]. In the case of our task, this race may refer to the potential responses that can be made to the four targets. Thus, there is higher-level cognitive processing that occurs during the performance of such a task. It was Crus I, a region of the cerebellum associated with the prefrontal cortex and cognitive function, which was associated with performance on this task. These findings, coupled with those related to balance, indicated that cognitive processing may be the aspect of task performance that best captured individual differences in sensorimotor performance.
Interestingly, several of the differential relationships with age also included volumes of the posterior cerebellum and Crus I. In young adults, manual dexterity measured by performance on the grooved pegboard was negatively correlated with right Crus I volume. In the older adults the relationship (though not significant) was in the opposite direction. In this case, like in balance, the potential for additional cognitive processing may have been detrimental to the young adults. Furthermore, tapping variability at 1500 msec was negatively correlated with right Crus I volume in the young adults, though there was no relationship at all in the older participants. In this case, the young adults may be relying on cognitive strategies and/or recruiting different neural mechanisms to perform this task, consistent with the notion that tapping at intervals around 1000 msec or longer is more demanding of these resources [74-75]. Alternatively, the older adults, who performed quite well on this task, may have been highly motivated, and engaged in compensatory strategies.
Finally, the posterior cerebellum in young adults was negatively correlated with choice reaction time. As in our whole group result, in the young adults the larger volume of the posterior cerebellum may allow more efficient engagement of cognitive resources, whereas those may not be engaged as extensively in the older adults. One important caveat however, is that the posterior cerebellum included lobules VIIIa and VIIIb, which are associated with motor behavior [76]. Sensorimotor processes may be engaged more effectively in the young adults. The differential relationship between choice reaction time and the volume of the vermis followed the same pattern as that of the posterior cerebellum. However, in this case it may be that the vermis volume was beneficial for the execution of the motor response. It is connected with motor cortical regions [77], and is involved in resting state networks that include motor cortex [28].
Timing abilities have previously been tied to the cerebellum; for example, patients with cerebellar damage exhibit timing deficits, and neuroimaging studies have shown cerebellar activation in participants performing timing tasks [7, 14-15]. Here, we demonstrated that individual differences in the volume of the cerebellar vermis were associated with tapping variability at both 500 and 1000 msec intervals, and as previously described there were differential relationships in young and older adults at the 1500 msec interval. Larger volume was associated with better performance. Neuroimaging evidence indicates involvement of the vermis in timing [7, 78]. The role of the vermis has also been demonstrated using transcranial magnetic stimulation to the medial cerebellum [79-80]. Larger volume of the vermis may aid in the processing of time intervals and importantly, perhaps it aids in the execution of these precisely timed movements. However, it is also notable that in our model of tapping variability at 1000 msec, the left anterior cerebellum was also a significant component. It was positively associated with tapping variability. These responses were made with the right hand, and the cerebellum is cross-lateralized with respect to the cortex. Perhaps there was cross-talk between motor regions involved in the execution of tapping at this interval that was increased with greater volume in the left anterior cerebellum. This is however speculative, and in general, this result was somewhat surprising.
Overall, the majority of the relationships revealed in our analyses were with sensorimotor tasks, though it was the more cognitive regions of the cerebellum that were most related to sensorimotor performance. Interestingly, there were no relationships with our measures of executive function (set switching), though the cerebellum has been implicated in this domain [22]. Relationships with working memory performance were also surprisingly absent, with one exception. Working memory accuracy was significantly modeled by the volume of the left posterior cerebellum. While inferior aspects of the cerebellum (lobules VIIIa and VIIIb) have been implicated in maintenance during working memory [9, for a review see 21] in the verbal task used here, we would expect this relationship to be with the right posterior cerebellum. Perhaps bilateral recruitment of these networks associated with maintenance was beneficial to performance, though in general this finding was somewhat counterintuitive. Future studies may benefit from multiple measures of working memory and executive function to better assess the potential relationships between regional cerebellar volume and performance, particularly in terms of the lateralization of contributions. Furthermore, it is of note that we only investigated cerebellar gray matter volume. Inclusion of cerebellar white matter volume would also be informative in future studies, particularly given previous work demonstrating relationships between cerebellar white matter microstructure and motor learning [81].
Lastly, it is important to note that we did not assess alcohol consumption in our participants. Alcoholism has been linked to reduced cerebellar volume [2] and the structure is particularly susceptible to the effects of alcohol. In our sample, all participants were free of any neurological or psychological disorder, including substance abuse. However, this does not rule out the potential impact of alcohol consumption over the course of the lifespan in older individuals, nor does it rule out the impact of occasional binge drinking in either population. Future studies investigating cerebellar volume in aging populations would benefit from detailed analyses of the impact of alcohol consumption over the course of the lifespan.
Conclusions
The cerebellum is known to be involved in a wide variety of sensorimotor and cognitive behaviors. Furthermore, overall volume of the cerebellum is known to be smaller in older adults. Our individual differences approach to studying brain-behavior relationships provided evidence that regional cerebellar gray matter volume is indeed related to variability in sensorimotor performance. However, we found little evidence for relationships between regional cerebellar volume and cognitive performance. Interestingly, our results indicated that it is the more posterior regions of the cerebellum, often associated with cognitive processing, that are associated with sensorimotor performance variability. Finally, we also noted differential relationships between regional volume and sensorimotor performance in young and older adults. This may be due to differential engagement of these cognitive resources by age. Taken as a whole, these findings highlight both the importance of taking a regional approach when investigating cerebellar contributions to behavior, and the potential importance of regional cerebellar gray matter volume in performance in older adults.
Acknowledgments
J.A.B. was supported by NIH T32 AG000114 (S. Pletcher, PI). J.A.B (alumna) and R.D.S. (faculty) are members of the International Max Planck Research School on the Life Course (LIFE, www.imprs-life.mpg.de; participating institutions: MPI for Human Development, Humboldt-Universität zu Berlin, Freie Universität Berlin, University of Michigan, University of Virginia, University of Zurich). The authors wish to thank Lauren Wu and Maggie Smith for their help with data pre-processing.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest related to this manuscript.
References
- 1.Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci. 2011;12:231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan EV, Rose J, Pfefferbaum A. Physiological and focal cerebellar substrates of abnormal postural sway and tremor in alcoholic women. Biol Psychiat. 2010;67:44–51. doi: 10.1016/j.biopsych.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morton SM, Bastian AJ. Relative contributions of balance and voluntary leg-coordination deficits to cerebellar gait ataxia. J Neurophysiol. 2003;89:1844–1856. doi: 10.1152/jn.00787.2002. [DOI] [PubMed] [Google Scholar]
- 4.Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neuroscientist. 2004;10:247–259. doi: 10.1177/1073858404263517. [DOI] [PubMed] [Google Scholar]
- 5.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 6.Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Pütz B, et al. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- 7.Jueptner M, Rijntjes M, Wieller C, Faiss JH, Timmann D, Mueller SP, et al. Localization of a cerebellar timing process using PET. Neurology. 1995;45:1540–1545. doi: 10.1212/wnl.45.8.1540. [DOI] [PubMed] [Google Scholar]
- 8.Kim SG, Ugurbil K, Strick PL. Activation of a cerebellar output nucleus during cognitive processing. Science. 1994;265:949–951. doi: 10.1126/science.8052851. [DOI] [PubMed] [Google Scholar]
- 9.Chen SHA, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005;43:1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;4:489–501. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baloh RW, Jacobson KM, Beykirch K, Honrubia V. Static and dynamic posturography in patients with vestibular and cerebellar lesions. Arch Neurol. 1998;33:650–654. doi: 10.1001/archneur.55.5.649. [DOI] [PubMed] [Google Scholar]
- 12.Mauritz KH, Dichgans J, Hufschmidt A. Quantitative analysis of stance in late cortical cerebellar atrophy of the anterior lobe and other forms of cerebellar ataxia. Brain. 1979;102:461–482. doi: 10.1093/brain/102.3.461. [DOI] [PubMed] [Google Scholar]
- 13.Silfversköld BP. Cortical cerebellar degeneration associated with a specific disorder of standing and locomotion. Acta Neurol Scand. 1977;55:257–272. doi: 10.1111/j.1600-0404.1977.tb05646.x. [DOI] [PubMed] [Google Scholar]
- 14.Ivry RB, Keele SW, Diener HC. Dissociation of lateral and medial cerebellum in movement timing and movement execution. Exp Brain Res. 1988;73:167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- 15.Spencer RMC, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- 16.Gerwig M, Hajjar K, Dimitrova A, Maschke M, Kolb FP, Frings M, et al. Timing of conditioned eyeblink responses is impaired in cerebellar patients. J Neurosci. 2005;25:3919–3931. doi: 10.1523/JNEUROSCI.0266-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerwig M, Guberina H, Eßer AC, Siebler M, Choch B, Frings M, et al. Evaluation of multiple-session delay eyeblink conditioning comparing patients with focal cerebellar lesions and cerebellar degeneration. Behav Brain Res. 2010;212:143–151. doi: 10.1016/j.bbr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Dimitrova A, Gerwig M, Brol B, Gizewski ER, Forsting M, Beck A, et al. Correlation of cerebellar volume with eyeblink conditioning in healthy subjects and in patients with cerebellar cortical degeneration. Brain Res. 2008;1198:73–84. doi: 10.1016/j.brainres.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 19.Cheng DT, Disterhoft JF, Power JM, Ellis DA, Desmond JE. Neural substrates underlying human delay and trace eyeblink conditioning. P Natl Acad Sci USA. 2008;105:8108–8113. doi: 10.1073/pnas.0800374105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schumacher EH, Lauber E, Awh E, Jonides J, Smith EE, Koeppe RA. PET evidence for an amodal verbal working memory system. NeuroImage. 1996;3:79–88. doi: 10.1006/nimg.1996.0009. [DOI] [PubMed] [Google Scholar]
- 21.Marvel CL, Desmond JE. Functional topography of the cerebellum in verbal working memory. Neuropsychol Rev. 2010;20:271–279. doi: 10.1007/s11065-010-9137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen G, Buxton R, Wong E, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- 23.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 26.Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O, et al. Cognitive and motor loops of the human cerebro-cerebellar system. J Cognitive Neurosci. 2010;22(11):2663–2676. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- 27.Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, et al. Resting-state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat. 2012;6:31. doi: 10.3389/fnana.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernard JA, Peltier SJ, Benson BL, Wiggins JL, Jaeggi SM, Buschkuehl M, et al. Dissociable networks of the human dentate nucleus. Cereb Cortex. doi: 10.1093/cercor/bht065. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 31.Stoodley CJ, Valera EM, Schmahmann JD. An fMRI study of intra-individual functional topography in the human cerebellum. Behav Neurol. 2010;23:65–79. doi: 10.3233/BEN-2010-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodruff-Pak DS, Goldenberg G, Downey-Lamb MM, Boyko OB, Lemieux SK. Cerebellar volume in humans related to magnitude of classical conditioning. NeuroReport. 2000;3:609–615. doi: 10.1097/00001756-200002280-00035. [DOI] [PubMed] [Google Scholar]
- 33.Woodruff-Pak DS, Vogel IIIRW, Ewers M, Coffey J, Boyko OB, Lemieux SK. MRI-assessed volume of cerebellum correlates with associative learning. Neurobiol of Learn Mem. 2001;76:342–357. doi: 10.1006/nlme.2001.4026. [DOI] [PubMed] [Google Scholar]
- 34.Eckert MA, Keren NI, Roberts DR, Calhoun VD, Harris KC. Age-related changes in processing speed: unique contributions of cerebellar and prefrontal cortex. Front Hum Neurosci. 2010;4:1–14. doi: 10.3389/neuro.09.010.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microsc Res Tech. 2000;51:85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Basak C, Voss MW, Erickson KI, Root WR, Kramer AF. Regional differences in brain volume predict the acquisition of skill in a complex real-time strategy videogame. Brain Cognition. 2011;76:407–414. doi: 10.1016/j.bandc.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan EV, Desmond JE, Deshmuk A, Lim KO, Pfefferbaum A. Cerebellar volume decline in normal aging, alcoholism, and Korsakoff's syndrome: relation to ataxia. Neuropsychology. 2000;3:341–352. doi: 10.1037//0894-4105.14.3.341. [DOI] [PubMed] [Google Scholar]
- 38.MacLullich AMJ, Edmond CL, Ferguson KJ, Wardlaw JM, Starr JM, Seckl JR, et al. Size of the neocerebellar vermis is associated with cognition in healthy elderly men. Brain Cognition. 2004;56:344–348. doi: 10.1016/j.bandc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–96. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cognitive Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- 41.Raz N, Rodrigue KM, Kennedy KM, Acker JD. Vascular healthy and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology. 2007;21:149–157. doi: 10.1037/0894-4105.21.2.149. [DOI] [PubMed] [Google Scholar]
- 42.Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, et al. Motor control and aging: links to brain structural, functional and biochemical changes. Neurosci Biobehav Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luft AR, Skalej M, Schultz JB, Welte D, Kolb R, Bürk K, et al. Patterns of age-related shrinkage in cerebellum and brainstem observed in vivo using three-dimensional MRI volumetry. Cereb Cortex. 1999;9:712–721. doi: 10.1093/cercor/9.7.712. [DOI] [PubMed] [Google Scholar]
- 44.Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 45.Raz N, Dupuis JH, Briggs SD, McGavran C, Acker JD. Differential effects of age and sex on the cerebellar hemispheres and the vermis: a prospective MR study. Am J Neuroradiol. 1998;19:65–71. [PMC free article] [PubMed] [Google Scholar]
- 46.Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. Am J Neuroradiol. 2001;22:1161–1167. [PMC free article] [PubMed] [Google Scholar]
- 47.Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD. Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cereb Cortex. 2008;18:718–726. doi: 10.1093/cercor/bhm108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 49.Hoogendam YY, van der Geest JN, van der Lijn F, van der Lugt A, Niessen WJ, Krestin GP, et al. Determinants of cerebellar and cerebral volume in the general elderly population. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2012.02.012. In Press. [DOI] [PubMed] [Google Scholar]
- 50.Wang D, Buckner RL, Liu H. Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. J Neurophysiol. 2013;109:46–57. doi: 10.1152/jn.00598.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. NeuroImage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 52.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 53.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and nerodegenerative brain. Med Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 55.Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 56.Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 57.Desmond JE, Chen SHA, Shieh PB. Cerebellar transcranial magnetic stimulation impairs verbal working memory. Ann Neurol. 2005;8:53–560. doi: 10.1002/ana.20604. [DOI] [PubMed] [Google Scholar]
- 58.Inoue K, Kawashima R, Satoh K, Kinomura S, Sugiura M, Goto R, et al. A PET study of visuomotor learning under optical rotation. NeuroImage. 2000;11:505–516. doi: 10.1006/nimg.2000.0554. [DOI] [PubMed] [Google Scholar]
- 59.Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Contributions of spatial working memory to visuomotor learning. J Cognitive Neurosci. 2009;22:1917–1930. doi: 10.1162/jocn.2009.21351. [DOI] [PubMed] [Google Scholar]
- 60.Teasedale N, Bard C, Fleury M, Young DE, Proteau L. Determining movement onsets from temporal series. J Motor Behav. 1993;25:97–106. doi: 10.1080/00222895.1993.9941644. [DOI] [PubMed] [Google Scholar]
- 61.Benson BL, Anguera JA, Seidler RD. An explicit strategy improves performance but impairs sensorimotor adaptation. J Neurophysiol. 2011;105:2843–2851. doi: 10.1152/jn.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wing AM, Kristofferson AB. The timing of interresponse intervals. Percept Psychophys. 1973;13:455–460. [Google Scholar]
- 63.Powell LE, Myers AM. The activities-specific balance confidence (ABC) scale. J Gerontol A Biol Med Sci. 1995;50A:M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 64.Fan L, Tang Y, Sun B, Gong G, Chen ZJ, Lin X, et al. Sexual dimorphism and asymmetry in human cerebellum: an MRI-based morphometric study. Brain Res. 1353:60–73. doi: 10.1016/j.brainres.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 65.Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- 66.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RSR, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 67.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–2245. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ouchi Y, Okada H, Yoshikawa E, Nobezawa S, Futatsubashi M. Brain activation during maintenance of standing postures in humans. Brain. 122:329–338. doi: 10.1093/brain/122.2.329. [DOI] [PubMed] [Google Scholar]
- 70.Huxhold O, Li SC, Schmiedek F, Lindenberger U. Dual-tasking postural control: aging and the effects of cognitive demand in conjunction with focus of attention. Brain Res Bull. 2006;69:294–305. doi: 10.1016/j.brainresbull.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith EE. Choice reaction time: an analysis of the major theoretical positions. Psychol Bull. 1968;69:77–110. doi: 10.1037/h0020189. [DOI] [PubMed] [Google Scholar]
- 73.Osman A, Kornblum S, Meyer DE. The point of no return in choice reaction time: controlled and ballistic stages of response preparation. J Exp Psychol Human. 1986;12:243–258. doi: 10.1037//0096-1523.12.3.243. [DOI] [PubMed] [Google Scholar]
- 74.Lewis PA, Miall RC. Distinct systems for automatic and cognitively controlled time measurement: evidence from neuroimaging. Curr Opin Neurobiol. 2003;13:250–255. doi: 10.1016/s0959-4388(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 75.Ivry RB, Spencer RMC. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 76.Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp. 2001;13:55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coffman KA, Dum RP, Strick PL. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. P Natl Acad Sci USA. 2011;108:16068–16073. doi: 10.1073/pnas.1107904108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Penhune VB, Zatorre RJ, Evans AC. Cerebellar contributions to motor timing: a PET study of auditory and visual rhythm reproduction. J Cognitive Neurosci. 1998;10:752–765. doi: 10.1162/089892998563149. [DOI] [PubMed] [Google Scholar]
- 79.Théoret H, Haque J, Pascual-Leone A. Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neurosci Lett. 2001;306:29–32. doi: 10.1016/s0304-3940(01)01860-2. [DOI] [PubMed] [Google Scholar]
- 80.Grube M, Lee KH, Griffiths TD, Barker AT, Woodruff PW. Transcranial magnetic theta-burst stimulation of the human cerebellum distinguishes absolute, duration-based from relative, beat-based perception of subsecond time intervals. Front Psychol. 2010;1:171. doi: 10.3389/fpsyg.2010.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Della-Maggiore V, Scholz J, Johansen-Berg H, Paus T. The rate of visuomotor adaptation correlates with cerebellar white-matter microstructure. Hum Brain Mapp. 2009:4048–4053. doi: 10.1002/hbm.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]