Abstract
Cardiovascular disease is the leading cause of morbidity and mortality in postmenopausal women. Hypertension is a major risk factor for cardiovascular disease. The mechanisms responsible for postmenopausal hypertension have not been completely elucidated. However, various mechanisms have been implicated to play a role. For example, there is evidence that changes in estrogen/androgen ratios favoring increases in androgens, activation of the renin-angiotensin and endothelin systems, activation of the sympathetic nervous system, metabolic syndrome and obesity, inflammation, increased vasoconstrictor eicosanoids, and anxiety and depression may be important in the pathogenesis of postmenopausal hypertension. There is also evidence that hypertension is less well controlled in aging women than in aging men, but the reasons for this gender difference is not clear. Postmenopausal hypertension is likely multifactorial. Future studies will be necessary to determine the contribution of these systems listed above in mediating postmenopausal hypertension and to design treatment strategies that encompass these mechanisms to improve the quality of life of postmenopausal women as they age.
Keywords: androgens, estrogens, angiotensinogen, endothelin, leptin, 20-HETE, sympathetic activity
Cardiovascular disease is the leading cause of morbidity and mortality in men and women 1-10, but the incidence of cardiovascular disease-related deaths is higher in women than men 1,4-6. Hypertension is one of the leading risk factors for cardiovascular disease 1,4,6,11. Aging in both men and women is characterized by increases in blood pressure (BP) 1-10, but the age-related increases are more rapid in women than in men 6, 8-10, and the prevalence of hypertension in postmenopausal women is higher than in men 1,5-7,11. In the world in general, 25% of adult women are hypertensive, and in the United States, more than 75% of women over 60 years of age are hypertensive 8,9. In studies using the National Health and Nutrition Examination Survey (NHANES) 1999-2004 data set, the percentage of individuals with uncontrolled BP was 50.8 ± 2.1% in men and 55.9 ± 1.5% in women, although. women were more likely to have their BPs measured within the previous six months than men 11. Despite this, comparison of the NHANES III cohort with the NHANES IV cohort showed that women were more likely to have poorly controlled hypertension than men, although the drugs to treat hypertension were similar between men and women 11. Non-dipping of BP at night is associated with increased target organ damage 12-16. However, there is evidence that non-dipping in women in general is associated with greater target organ damage than in men 12,16, and postmenopausal women are more likely than pre-menopausal women to exhibit nocturnal non-dipping of BP 12. Thus while antihypertensive methods are similar between men and women and women are more likely to have their BPs measured, hypertension may be less well controlled in women. This suggests that perhaps the mechanisms responsible for hypertension in women may differ from the mechanisms in men.
The specific mechanisms responsible for the increased BP in women following menopause are not clear. Several physiological systems have been implicated in clinical studies. For example, postmenopausal women exhibit increases in plasma renin activity 17,18 and endothelin 19, compared to their premenopausal counterparts. Longitudinal studies have shown that serum androgen levels are increased in postmenopausal women, leading to alterations in estrogen/androgen ratios 20. In addition, Ward and colleagues reported that hypertension in postmenopausal women, not age-matched men, was associated with elevated excretion of 20-HETE, vasoconstrictor eicosanoids 21. Markers of oxidative stress are also increased in postmenopausal women 22-24, and oxidative stress has been shown to increase BP by reducing the bioavailability of nitric oxide 25. The incidence of obesity may be as high as 40% in postmenopausal women 26, and increases in body weight have been shown to be associated with increases in BP 27. Obesity is one component of the cluster of features known as the metabolic syndrome, that also includes insulin resistance, type II diabetes, hyperlipidemia, and hyperleptinemia, that could also impact BP 27. Rossi and colleagues reported that improvement in endothelial dysfunction and inflammation seen in response to antihypertensive medications was attenuated in postmenopausal women, aged 47-60 years, who exhibited symptoms of the metabolic syndrome 28. Thus the presence of metabolic syndrome not only may contribute to the hypertension but may affect response to treatment therapies in postmenopausal women.
Increased body weight, plasma leptin levels and aging have been shown to cause sympathetic activation 27,29,30. Whether sympathetic activity is increased in postmenopausal women is not clear, however. Czarnecka and colleagues reported that the levels of norepinephrine and leptin were higher in postmenopausal women than age-matched premenopausal women 31. However, Hogarth and colleagues found that both muscle sympathetic nerve activity (MSNA) and mulitunit bursts (b-MSNA were higher in age-matched normotensive and hypertensive men than normotensive and hypertensive women, respectively 32.
Chronic inflammation and increased levels of cytokines have been shown to contribute to hypertension in certain circumstances, such as in models of pre-eclampsia 33. Although inflammation and inflammatory cytokines are elevated with menopause 34, the role that chronic inflammation may be play in mediating postmenopausal hypertension is not clear. In addition to having a direct effect on BP, inflammation may also be a consequence of lack of psychological well-being in these aging women.
In this review, we will discuss the evidence that each of these factors may contribute to postmenopausal hypertension and will develop a unifying hypothesis that incorporates them all. There is also a brief mention of the potential role that depression and psychological well-being may play in contributing to postmenopausal hypertension.
The aged female SHR as a model of postmenopausal hypertension
In the past several years we have characterized an animal model of postmenopausal hypertension, the aging female spontaneously hypertensive rat. When young, these females have significantly lower BPs than do their male counterparts (see Figure 1) 35. With aging and cessation of estrus cycling (which occurs at 10-12 months of age), the BP increases in these females, such that by 16-18 months of age, their BPs are similar to or even higher than in age-matched males (see Figure 1). In addition, while serum estradiol levels are significantly reduced, serum androgens levels are elevated by 3-4 fold. Renal vascular resistance is also significantly elevated showing that the renal vasculature is constricted.
Figure 1. BPs in male and female spontaneously hypertensive rats with aging.
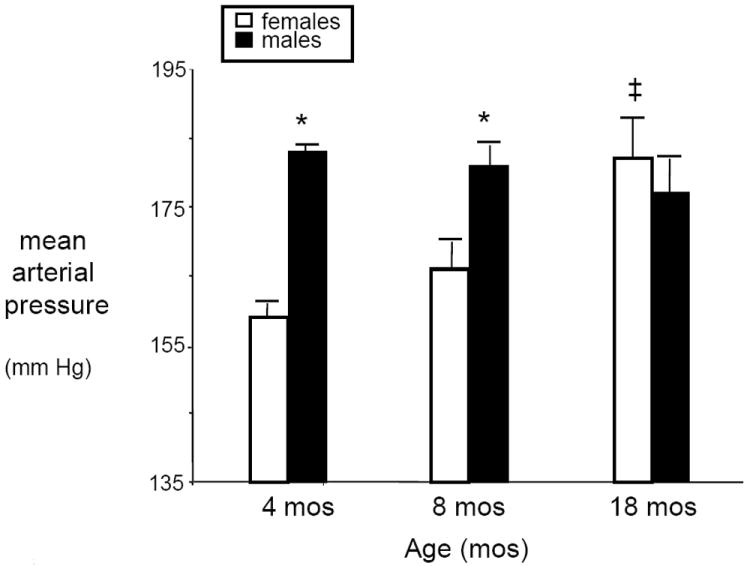
*, p<0.05, males compared with females, aged 4 and 8 months; ‡, p<0.05, females compared to females, aged 4 and 8 months.
Most clinical studies to discern the mechanisms that could be responsible for postmenopausal hypertension in women have yielded correlative data, rather than mechanistic data because of the limitation against invasive techniques, expense and difficulty of obtaining longitudinal data. In addition, most studies using animal models to study potential mechanisms responsible for postmenopausal hypertension have employed young, ovariectomized animals that do not exhibit the age-related changes that are present in women as they make the menopausal transition. Furthermore, more and more studies including the studies of the Women’s Health Initiative 36, HERS I and HERS II 37,38 have shown that hormone replacement therapy (HRT) in older individuals does not have the same beneficial cardiovascular effects as HRT in young women with premature ovarian failure 39. Thus it is imperative that animal studies take into consideration the effect of aging and cessation of estrus cycling as models of postmenopausal hypertension. Therefore, as we discuss the potential mechanisms responsible for postmenopausal hypertension that have been implicated in women, we will also discuss studies we and others have performed in various animal models to test these hypotheses.
Potential Mechanisms responsible for postmenopausal hypertension
Role of changes in estrogen/androgen ratios in mediating postmenopausal hypertension
Whether the presence of estradiol protects against increases in BP in premenopausal women, and conversely, whether the lack of estradiol contributes to hypertension in postmenopausal women is controversial and unknown. For example, Olszanecka and colleagues measured ambulatory BP in normotensive and hypertensive women, aged 40-60 years, and found that BP was similar in normotensive and hypertensive groups regardless of presence or absence of menopause 40. Unfortunately, there have been no studies to our knowledge in which ambulatory BP has been measured serially over the perimenopausal transition.
In experimental settings, many in vitro, estradiol has been shown to have a variety of effects that should be cardiovascular protective 41-43. However, despite the potential of estradiol to protect against cardiovascular disease, large clinical trials on the effect of hormone replacement therapy (HRT) in post-menopausal women do not support these previous findings. The results of the Women’s Health Initiative studies 36, and HERS I and HERS II 37,38 trials have not supported a role for HRT in prevention of either primary or secondary cardiovascular disease, respectively. Similarly, HRT has not been shown to consistently lower BP in post-menopausal women. As determined by 24 hr ambulatory BP monitoring, HRT typically results in changes of 3-5 mm Hg only 44-51. In some studies, only daytime BP was reduced 45-47; in others, only night time BP was reduced 48. In some studies only systolic BP 47, in others only diastolic BPs 49,50, were reduced with HRT. The mode of delivery of HRT, whether oral or transdermal, and the dose, whether high or low, may play a role in its efficacy, but the data are not consistent with which delivery mode is more effective. These studies all looked at short-term effects of HRT on BP; i.e. less than one year. Ichikawa and colleagues did find that transdermal hormone therapy for 12 and 24 months did reduce diastolic and mean BP in normotensive postmenopausal women 52. In contrast, Prelevic and colleagues studied healthy post-menopausal women who had been taking HRT for at least 5 yrs, and found that there was either no effect on BP or that BP was in fact higher in some women using HRT 53. Proponents of the beneficial role of estradiol in cardiovascular disease cite the use of progesterone in HRT as possibly negating the positive effects of estradiol 41,54. However, in women with surgical menopause and hysterectomy, estrogen replacement therapy (ERT), also did not result in significant sustained reductions in BP or reductions in cardiovascular disease risk 55. Thus reductions in estradiol that occur at menopause do not fully explain the progressive increases in BP in postmenopausal women, and estrogen replacement is not used as an antihypertensive in their treatment.
However, there is evidence that loss of estrogens at any age contributes to endothelial dysfunction, which is common in individuals with hypertension. Taddei and colleagues reported that in response to acetylcholine, an index of endothelial dysfunction, endothelium-dependent flow mediated vasodilation (FMD) was less attenuated with age in menstrual cycling hypertensive women than men, but after menopause, the FMD response was attenuated to the same extent in both women and men 56. Attenuated FMD is prognostic of coronary artery disease risk factors, including hypertension, in postmenopausal women 57. Women with premature ovarian failure before age 40 years also exhibit reduced brachial FMD compared to age-matched cycling women, but in these women, HRT with conjugated equine estrogen and medroxyprogesterone for 6 months reversed the endothelial dysfunction 55. In contrast, in the Women’s Angiographic Vitamin and Estradiol (WAVE) Trial, HRT had no beneficial effect on FMD in postmenopausal women 58. The fact that HRT protected against endothelial dysfunction in young women, but not in older women, both of whom had experienced menopause, supports the contention that aging may change the response to HRT and thus may independently contribute to increases in BP.
Endothelial dysfunction is typically characterized by reductions in nitric oxide (NO). Estradiol stimulates NO production since estradiol acutely increases intracellular calcium which activates endothelial NO synthase (eNOS) 59. In addition, estradiol upregulates synthesis of eNOS which would promote vasodilation and thus reductions in BP 41. Estradiol also upregulates superoxide dismutase 60, which removes superoxide and reduces oxidative stress. Superoxide binds to NO and renders NO unavailable for vasodilation 25. An intact NO system is necessary for antioxidants to reduce BP, however 61. So in situations of chronic hypertension when endothelial dysfunction is present and NO levels have been reduced for long periods, estradiol may not be able to reduce BP, just as other antioxidants are not. Furthermore, we have not been able to show that oxidative stress contributes to the control of BP in female hypertensive animals as it does in male animals 62-64, suggesting that oxidative stress may not be a factor in mediating hypertension in women either. This could explain why clinical trials with antioxidants have not be successful in reducing BP 65; e.g. the studies were not powered to separate women from men, and hypertension in women may be resistant to antioxidants.
Reductions in estradiol with menopause could affect the RAS. Animal studies have shown that estradiol downregulates the levels of angiotensin type I (AT1) receptors and angiotensin converting enzyme (ACE) levels 66,67, thus protecting against the activation of the renin-angiotensin system (RAS) and subsequent vasoconstriction. Therefore, reductions in estradiol would tend to activate the RAS. However, in normotensive postmenopausal women, HRT with transdermal 17β-estradiol and oral medroxyprogesterone reduced BP but had no effect on levels or expression of RAS components, including plasma renin activity, angiotensin I or II, aldosterone or angiotensin converting enzyme activity 52. Expression of AT1 receptors were not measured in this study. In contrast, treatment of postmenopausal women with AT1 receptor antagonists did improve endothelial dysfunction measured by FMD, whereas a calcium channel blocker did not 68, supporting a role for the RAS in contributing to postmenopausal hypertension. This possibility will be discussed in more detail later.
How androgens contribute to hypertension in postmenopausal women is also not clear. Studies in the Rancho Bernardo cohort in which serial androgen levels were measured showed that immediately following menopause, androgen levels are reduced, but that by 70 years of age, androgen levels are increased to levels found in premenopausal women 69. The ovary in postmenopausal women is a major source of androgen production 70,71, but increasing evidence supports local production of androgens, such as by the kidney 72. In men reduced levels of androgens are associated with cardiovascular disease 73-75. This is not the case in women in which elevated levels of androgens are associated with cardiovascular abnormalities. Young women with polycystic ovary syndrome (PCOS) who have elevated levels of plasma androgens and normal plasma estradiol levels have increased risk of cardiovascular disease not only when young but also following menopause 76,77. Thus the levels of androgens are indicative of differences in cardiovascular disease risk depending on gender, increased androgens predict cardiovascular disease in women; decreased androgen levels predict cardiovascular disease in men. Clinical studies have also shown that serum androgen levels increase with increasing body mass index in postmenopausal women, not premenopausal women 78. This is different than in men in which decreases in androgens are associated with increases in body mass index and obesity 74,75. In addition, elevated serum testosterone is associated with a higher risk of type II diabetes in postmenopausal women, but not age-matched men 79. Other mechanisms by which androgens could increase BP in postmenopausal women will be discussed in the context of the effect of androgens on other pro-hypertensive systems below.
Changes in estradiol receptors with aging and menopause
The lack of positive results in the WHI 35 and HERS I and II 36,37 trials with hormone replacement therapy and prevention of primary or secondary coronary artery disease suggest that aging may cause changes in estradiol and its mechanisms of biological activity. For example, a study in aging men and women in which estrogen receptor alpha (ERα) localization was determined by immunohistochemistry in biopsy samples from hypothalami, found that ERα was found mainly in the cellular cytosol in postmenopausal women, but in hypothalamic nuclei in age-matched men and premenopausal women 80. Longitudinal studies in female rats in which 17β-estradiol was replaced following ovariectomy at a young age, failed to maintain lower BP at 12 months of age compared to the levels that were attained at 4 months of age 81. Data is woefully missing with regard to how estradiol receptor expression changes in tissues important in BP control with aging in males or females. Furthermore, there are no studies to address to whether intracellular signaling of estradiol and its receptors, ERα, ERβ, and the plasma membrane-associated ER, GPR30, may change with aging.
Role of obesity and metabolic syndrome in postmenopausal hypertension
Obesity is rapidly becoming an epidemic in all of the developed countries in the world. In the United States, the incidence of obesity is increasing in individuals in all states with the highest incidence existing in the southeast 82,83. There are also ethnic differences in the incidence of obesity in the US. For example, 23.3% of non-Hispanic white women were found to be obese whereas 41.9% of non-Hispanic women were found to be obese 82. Obesity has been shown to increase after surgical menopause and to be increased in women who started HRT within 12 months of amenorrhea 84. These investigators also found that the incidence of obesity in women is associated with increases in free androgen index and reductions in sex hormone binding globulin. There is also evidence that even if women do not gain additional weight after menopause, there is a redistribution of body fat favoring an increase in abdominal fat gain rather than lower hip weight gain 85. Weight that accumulates in the abdominal area is associated with a higher incidence of cardiovascular disease than weight that is accumulated in the lower body 86.
The mechanisms by which weight gain or obesity cause hypertension are not clear. Increased body weight due to increased fat feeding in dogs increases BP that is prevented if the renal nerves have been severed 87, suggesting a sympathetic nervous system influence on the increase in BP with weight gain. Obesity is associated with increases in plasma leptin 27, and infusion of leptin increases BP in animals. Blockade of the sympathetic nervous system prevents this hypertensive effect 27. Leptin has been shown to activate the sympathetic nervous system via activation of melanocortin (MC) 4 receptors in the hypothalamus 27,30. Blockade of these receptors reduces BP in obese rats. Leptin has been shown to be increased in hypertensive postmenopausal women 40, and sympathetic activity has been shown to be higher in postmenopausal women than premenpausal women 31, but may be lower than in age-matched men 32.
Whether body weight alone or the combination of obesity and parameters of the metabolic syndrome, such as insulin resistance, hyperglycemia, hyperlipidemia, and hypertriglyceridemia, increase the risk of cardiovascular and renal disease and contribute to increased BP as well is controversial. Comparison of data from the Framingham Offspring, Atherosclerosis Risk in Communities, and Cardiovascular Health cohorts over more than 8 years, showed that abdominal obesity alone in these cohorts was not significantly associated with increased risk (odds ratio) of cardiovascular disease 88. However, inclusion of 1-2 parameters of metabolic syndrome and diabetes did significantly increase the odds ratio of contracting cardiovascular disease in both men and women, suggesting that the presence of metabolic abnormalities and diabetes are more indicative of cardiovascular disease risk than abdominal obesity alone.
Role of the renin-angiotensin system (RAS) in mediating postmenopausal hypertension
A major system for controlling BP and body fluid volume (i.e. pressure-natriuresis) is the renin-angiotensin system 89,90. Angiotensin II (Ang II) increases proximal sodium reabsorption by the kidney by stimulating epithelial transport 89,90. Thus the renin-angiotensin system monitors the levels of sodium and body fluid volume and adjusts its levels according. However, when Ang II levels are too high for the existing volume in the body, hypertension occurs 91. Similarly, if the body fluid volumes are perceived incorrectly, hypertension will occur. BP in women has been shown to be more salt-sensitive as they age than in men 92. Thus it is possible that there may be a blunting of the renin response to changes in salt in postmenopausal women. There may also be a genetic component of the RAS that contributes to postmenopausal hypertension since certain renin gene polymorphisms are associated with hypertension in women, aged 40-70 years, but not in men 93.
With regard to therapeutics, women may respond differently than men to blockade of the RAS. Miller and colleagues reported that while irbesartan, the AT1 receptor antagonist, reduced BP in both men and women, women developed Ang II insensitivity at a lower dose than did men, despite the fact that the levels of AT1 receptor expression in skin (by real time RT-PCR) was not different in men and women 94. In addition, Canzanello and colleagues found that non-Hispanic white ethnicity, female gender, higher plasma renin activity and lower body weight predicted a greater response to candesartan 95. Therefore, one reason for the lack of BP control in postmenopausal women, described previously 11, may be due to differential responses to drug therapy, including RAS blockers
Androgens can contribute to activation of the RAS by increasing intrarenal angiotensinogen 96,97,98. An increase in renin substrate could contribute to activation of the RAS if renin is not working at its enzymatic Vmax, which has not been studied in postmenopausal women, to our knowledge. In addition, animal studies support the notion that obesity and increases in leptin are associated with activation of the RAS 99. Thus postmenopausal increases in androgens and body weight could also activate the RAS leading to hypertension. As mentioned previously, the reduction in estrogens would also upregulate the AT1 receptor expression and ACE activity 41-43. Ovariectomy of animals is known to increase body weight, thus also creating the link between loss of estrogens, weight gain and activation of the RAS. In our postmenopausal hypertensive rats, while losartan, the AT1 receptor antagonist, decreased BP, it was not normalized 100, suggesting that RAS activation was not the only contributor to the postmenopausal increases in their BP.
Role of endothelin in postmenopausal hypertension
Chronic infusion of Ang II has been shown to stimulate synthesis of preproendothelin 101, another potent vasoconstrictor. When given chronically, endothelin causes increases in sodium reabsorption in the kidney and increases BP 102,103. Endothelin could also play a role in increasing BP by contributing to oxidative stress. In postmenopausal women, plasma endothelin levels have been shown to be increased 104, suggesting that endothelin may play a role in the increased BP following menopause. The biological activity of endothelin is mediated by two receptors, the ETA and ETB receptors. The majority of the vasoconstrictor action of endothelin is thought to be mediated via the ETA receptors 105. ETB receptors are thought to be coupled to nitric oxide, and when blocked, cause an increase in BP in Ang II-treated rats 106. These data support our contention that endothelin may contribute to the increased BP in postmenopausal rats and women. Whether there are changes in endothelin receptors in postmenopausal women has not been studied to our knowledge.
The mechanism by which endothelin itself increases in postmenopausal women is alo not clear. Endothelin synthesis can be upregulated by Ang II, as mentioned above 101, and ETA receptors have been shown to mediate Ang II hypertension 105,107. Furthermore, in women who have PCOS, which is characterized by hyperandrogenemia and increased plasma renin activity, plasma endothelin is also increased 108. The role played by estradiol in endothelin levels is controversial. While postmenopausal women have been shown to have elevated levels of endothelin, hormone replacement therapy with either micronized 17β-estradiol and didrogesterone or conjugated equine estrogen and medroxyprogeterone both increase endothelin levels in postmenopausal women 109. Elevated androgen levels that occur with menopause and aging could also increase endothelin levels. Studies in transsexual individuals who receive androgens for masculinization have been shown to have increased plasma endothelin levels 110. In contrast, activation of the RAS caused by androgen-mediated increases in angiotensinogen 97,98 could also lead to increases in endothelin since angiotensin II stimulates endothelial synthesis 101. Increased endothelin is also a factor in endothelial dysfunction that occurs with aging.
Studies in our postmenopausal rat model show that endothelin peptide levels are higher than in young controls and that blockade of the endothelin ETA receptor reduces their BP, but has no effect on BP in young female SHR or in age-matched old males 111. However, just as with RAS antagonists alone, ETA receptor antagonists alone failed to normalize the BP in these old females, again suggesting that a combination of factors contribute to the hypertension in postcycling old female SHR.
Role of inflammation in mediating postmenopausal hypertension
Menopause is associated with increases in C-reactive protein (CRP), an indicator of inflammation, and CRP levels have been shown to predict negative cardiovascular outcomes in postmenopausal women who use HRT and those who do not 112,113. Chronic inflammation, defined as increases in tumor necrosis factor- alpha (TNF-α) and inflammatory interleukins (IL), such as IL-6, is also a common finding in obese postmenopausal women 114. In fact, longitudinal studies in women as they transitioned from pre- to postmenopausal status showed that increases in inflammatory markers were mainly due to increases in visceral adiposity in the women 115.
Inflammation has been shown to increase BP in various models. For example, angiotensin II hypertension is attenuated in IL-6 knockout mice 116, suggesting that inflammation could contribute to hypertension in postmenopausal women who have activated RAS. Chronic inflammation is also a common finding in women with hyperandrogenism and PCOS, as measured by increases in C-reactive protein, TNF-α and IL-6. Tarkun and colleagues reported that the elevated levels of TNF-α and IL-6 correlated well with insulin resistance and hyperglycemia in women with PCOS 117, suggesting that androgens may increase inflammation. However, why TNF-α is increased with hyperandrogenemia in females is not clear, since in males, androgens are anti-inflammatory 118,119. Treatment of obese men with androgens causes a reduction in inflammatory cytokines 119. TNF-α has been shown to increase BP in some but not all circumstances. LaMarca and colleagues reported that TNF-α infusion in ovariectomized normotensive female rats replete with estradiol or progesterone did not increase their BP 120. Similar findings were made in normal pregnant female rats. However, in pregnant rats in which TNF-α is increased, such as the model of reduced uterine perfusion pressure (RUPP) that is hypertensive, etanercept, the TNF-α soluble receptor, significantly reduced BP 121.
Role of Eicosanoids in mediating postmenopausal hypertension
Arachidonic acid is converted to epoxyeicosotetraenoic acids (EETs) by epoxygenases or to 20-HETE by omega-hydroxylases. Ward and colleagues found that urinary excretion of 20-HETE, a vasoconstrictor, is higher in hypertensive women with endothelia dysfunction than in age-matched men 21. Androgens are known to increase the synthesis of 20-HETE via their effect on the synthesis of some of the subtypes of the omega-hydroxylases, that leads to hypertension 122,123. Wu and colleagues have also recently shown that androgen-mediated increases in 20-HETE activate NF-κB and increase BP which is prevented by blockade of both 20-HETE synthesis and IκB kinase inhibitors 124. We have preliminary data in our rat model of postmenopausal hypertension that suggest that 20-HETE may contribute to their hypertension. EETs are vasodilators that may be decreased following menopause, and thus contribute to postmenopausal hypertension. Future studies are necessary to follow up on these observations and hypotheses.
Role of anxiety and depression in mediating postmenopausal hypertension
Anxiety and depression may contribute to hypertension or women who are hypertensive may exhibit a higher rate of anxiety and depression. Depression and anxiety occurs at a significantly higher rate in women than in men 125. Depression and anxiety also are associated with increased risk of cardiovascular disease. For example, individuals with bipolar disorder have an increased risk of hypertension 126. Sympathetic activity can be increased with anxiety and chronic mental stress leading to increased BP, and this has been shown to be relevant in individuals with metabolic syndrome and hypertension 127. Sustained hypertension was also found to be associated with increased level of anxiety in a small Spanish cohort 128. Furthermore, ACE inhibitors used for the treatment of hypertension were found to reduce the occurrence of depression with anxiety 129. The mechanisms by which chronic anxiety and depression cause hypertension and may contribute to postmenopausal increases in BP are not clear and should be studied further.
Summary
In summary, the mechanisms responsible for postmenopausal hypertension are likely multifactorial. As shown in Figure 2, we hypothesize that changes in estrogen/androgen ratios that favor increases in androgens lead to activation of the RAS. Increases in androgens and Ang II can increase endothelin levels, and both Ang II and endothelin increase ω-hydroxylase activity (and 20-HETE synthesis) by increasing release of arachidonic acid from plasma membranes. Androgens promote synthesis of subtypes of ω-hydroxylases, such as cytochrome P450 4A2 and 4A8, in the vasculature. In combination with Ang II and endothelin, this leads to increases in vascular 20-HETE. In addition, we hypothesize that the increases in androgens with aging in postmenopausal women lead to increases in food intake and visceral adiposity leading to increases in leptin which in turn activate the sympathetic nervous system via the MC 4 receptors in the hypothalamus. Sympathetic activation would also increase intrarenal renin release and thereby also contribute to increases in Ang II. We further hypothesize that the combination of reductions in estrogens, increases in androgens, increases in visceral adiposity and increases in Ang II lead to increases in inflammatory cytokines, such as TNF-alpha, mainly via activation of NF-κB. The combination of increases in TNF-alpha, sympathetic activity, Ang II, endothelin and 20-HETE all lead to increases in renal vascular resistance and hypertension. We believe that hypertension may be controlled differently in postmenopausal women than age-matched men. Thus future studies are necessary to delineate the mechanisms responsible for postmenopausal hypertension and to develop treatment options specific for these women.
Figure 2. Schematic of potential mechanisms contributing to postmenopausal hypertension.

Abbreviations: Ang II, angiotensin II; Visc, visceral; TNF-α, tumor necrosis factor-alpha; 20-HETE, 20-hydroxyeicosatetraenoic acids.
Acknowledgments
The authors acknowledge the support of the National Institutes of Health RO1s HL66072 and HL69194 and PO1 HL51971 (to JFR), and the American Heart Association Scientific Development Grant #0830239N (to LLY).
The work is supported by NIH grants, as noted in acknowledgements (>$10,000) to JFR and AHA SGD (>$10,000) to LLY.
Footnotes
Conflict of interest disclosure: Neither Drs. Reckelhoff or Yanes have anything to disclose.
References
- 1.Ong KL, Tso AWK, Lam KS, Cheung BM. Gender differences in BP control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension. 2008;51:1142–1148. doi: 10.1161/HYPERTENSIONAHA.107.105205. [DOI] [PubMed] [Google Scholar]
- 2.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment and control of hypertension among United States adults 1999-2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension; analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high normal BP on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 5.Taddei S. BP through aging and menopause. Climacteric. 2009;12(Suppl 1):36–40. doi: 10.1080/13697130903004758. [DOI] [PubMed] [Google Scholar]
- 6.Perez-López FR, Chedraui P, Gilbert JJ, Pérez-Roncero G. Cardiovascular risk in menopausal women and prevalent related co-morbid conditions: facing the post-Women’s Health Initiative era. Fertil Steril. 2009;92:1171–1186. doi: 10.1016/j.fertnstert.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Sjoberg L, Kaaja R, Tuomilehto J. Epidemiology of postmenopausal hypertension. Int J Clin Pract Suppl. 2004;139:4–12. [PubMed] [Google Scholar]
- 8.Wiinberg N, Høegholm A, Christensen HR, Bang LE, Mikkelsen KL, Nielsen PE, Svendsen TL, Kampmann JP, Madsen NH, Bentzon MW. 24-h Ambulatory BP in 352 normal Danish subjects, related to age and gender. Am J Hypertension. 1995;8:978–986. doi: 10.1016/0895-7061(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 9.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of Hypertension in the US Adult Population. Results From the Third National Health and Nutrition Examination Survey, 1988-1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 10.Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11–18. doi: 10.1161/HYPERTENSIONAHA.108.120022. [DOI] [PubMed] [Google Scholar]
- 11.Kim JK, Alley D, Seeman T, Karlamangla A, Crimmins E. Recent changes in cardiovascular risk factors among women and men. J Women’s Health (Larchmont) 2006;15:734–46. doi: 10.1089/jwh.2006.15.734. [DOI] [PubMed] [Google Scholar]
- 12.Routledge FS, McFetridge-Durdle JA, Dean CR. Stress, menopausal status and nocturnal BP dipping patterns among hypertensive women. Can J Cardiol. 2009;25:e157–e163. doi: 10.1016/s0828-282x(09)70089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stolarz K, Staessen JA, O’Brien ET. Night-time BP: Dipping into the future? J Hypertension. 2002;21:2131–2133. doi: 10.1097/00004872-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A. Ambulatory BP. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory montoring. Unique aspects of BP during sleep. Hypertension. 2007;49:235–1241. doi: 10.1161/HYPERTENSIONAHA.107.087262. [DOI] [PubMed] [Google Scholar]
- 16.Verdecchia P, Schillaci G, Boldrini F, Guerrieri M, Porcellati C. Sex, cardiac hypertrophy and diurnal BP variatins in essential hypertension. J Hypertension. 1992;10:683–692. [PubMed] [Google Scholar]
- 17.Schunkert H, Danser AH, Hense HW, Derkx FH, Kurzinger S, Riegger GA. Effects of estrogen replacement therapy on the renin-angiotensin system in postmenopausal women. Circulation. 1997;95:39–45. doi: 10.1161/01.cir.95.1.39. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Vega F, Abellan J, Vegazo O, De Vinuesa SG, Rodriguez JC, Maceira B, de Castro SS, Nicolas RR, Luno J. Angiotensin ii type 1 receptor blockade to control BP in postmenopausal women: Influence of hormone replacement therapy. Kidney Int Suppl. 2002:S36–41. doi: 10.1046/j.1523-1755.62.s82.8.x. [DOI] [PubMed] [Google Scholar]
- 19.Komatsumoto S, Nara M. Changes in the level of endothelin-1 with aging. Nippon Ronen Igakkai Zasshi. 1995;32:664–669. doi: 10.3143/geriatrics.32.664. [DOI] [PubMed] [Google Scholar]
- 20.Jiroutek MR, Chen MH, Johnston CC, Longcope C. Changes in Reproductive Hormones and Sex Hormone-Binding Globulin in a Group of Postmenopausal Women Measured Over 10 Years. Menopause. 1998;5:90–4. [PubMed] [Google Scholar]
- 21.Ward NC, Rivera J, Hodgson J, Puddey IB, Beilin LJ, Falck JR, Croft KD. Urinary 20-hydroxyeicosatetraenoic acid is associated with endothelial dysfunction in humans. Circ. 2004;11:438–443. doi: 10.1161/01.CIR.0000136808.72912.D9. [DOI] [PubMed] [Google Scholar]
- 22.Helmersson J, Mattsson P, Basu S. Prostaglandin F(2alpha) metabolite and F2-isoprostane excretion in migraine. Clin Sci (Lond) 2002;102:39–43. [PubMed] [Google Scholar]
- 23.Signorelli SS, Neri S, Sciacchitano S, Di Pino L, Costa MP, Pennisi G, Ierna D, Caschetto S. Duration of menopause and behavior of malondialdehyde, lipids, lipoproteins and carotid wall artery intima-media thickness. Maturitas. 2001;39:39–42. doi: 10.1016/s0378-5122(01)00174-8. [DOI] [PubMed] [Google Scholar]
- 24.Estaban Castelao J, Gago-Dominguez M. Rick factors for cardiovascular disease in women: Relationship to lipid peroxidation and oxidative stress. Med Hypotheses. 2008;71:39–44. doi: 10.1016/j.mehy.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2003;284:R893–R912. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- 26.Lobo RA. Metabolic syndrome after menopause and the role of hormones. Maturitas. 2008;60:10–18. doi: 10.1016/j.maturitas.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: Role of sympathetic nervous system, leptin and melanocortins. J Biol Chem. 2010;85:17271–17216. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi R, Nuzzo A, Origliani G, Modena MG. Metabolic syndrome affects cardiovascular risk profile and response to treatment in hypertensive postmenopausal women. Hypertension. 2008;52:865–872. doi: 10.1161/HYPERTENSIONAHA.108.110478. [DOI] [PubMed] [Google Scholar]
- 29.Esler MD, Elkelis N, Lambert E, Straznicky N. Neural mechanisms and management of obesity-related hypertension. Curr Cardiol Rep. 2008;10:456–463. doi: 10.1007/s11886-008-0072-7. [DOI] [PubMed] [Google Scholar]
- 30.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 31.Czarnecka D, Pośnik-Urbańska A, Kawecka-Jaszcz K, Kolasińska-Kloch W, Wojciechowska W, Fedak D. Indices of autonomic nervous system activity in women with mild hypertension in the perimenopausal period. Kardiol Pol. 2009;67:243–251. [PubMed] [Google Scholar]
- 32.Hogarth AJ, Burns J, Mckintosh AF, Mary DA. Sympathetic nerve hyperactivity of essential hypertension is lower in postmenopausal women than men. J Hum Hypertension. 2008;22:544–549. doi: 10.1038/jhh.2008.31. [DOI] [PubMed] [Google Scholar]
- 33.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–5. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 34.Lee CG, Carr MC, Murdoch SJ, Mitchell E, Woods NF, Wener MH, Chandler WL, Boyko EJ, Brunzell JD. Adipokines, inflammation, and visceral adiposity across the menopausal transition: a prospective study. J Clin Endocrinol Metab. 2009;94:1104–10. doi: 10.1210/jc.2008-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fortepiani LA, Zhang H, Racusen LC, Roberts LJ, 2nd, Reckelhoff JF. Characterization of an animal model of postmenopausal hypertension in SHR. Hypertension. 2003;41:460–463. doi: 10.1161/01.HYP.0000046924.94886.EF. [DOI] [PubMed] [Google Scholar]
- 36.Burry KA. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. Curr Women’s Health Rep. 2002;2:331–332. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 37.Herrington DM. The HERS trial results: paradigms lost? Heart and Estrogen/progestin Replacement Study. Ann Int Med. 1999;131:463–466. doi: 10.7326/0003-4819-131-6-199909210-00012. [DOI] [PubMed] [Google Scholar]
- 38.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 39.Langrish JP, Mills NL, Bath LE, Warner P, Webb DJ, Kelnar CJ, Critchley HO, Newby DE, Wallace WH. Cardiovascular effects of physiological and standard sex steroid replacement regimens in premature ovarian failure. Hypertension. 2009;53:805–811. doi: 10.1161/HYPERTENSIONAHA.108.126516. [DOI] [PubMed] [Google Scholar]
- 40.Olszanecka A, Pośnik-Urbańska A, Kawecka-Jaszcz K, Czarnecka D, Fedak D. Adipocytokines and BP, lipids and glucose metabolism in hypertensive perimenopausal women. Kardiolo Pol. 2010;68:753–760. [PubMed] [Google Scholar]
- 41.Dubey RK, Oparil S, Imthurm B, Jackson EK. Sex hormones and hypertension. Cardiovasc Res. 2002;53:688–708. doi: 10.1016/s0008-6363(01)00527-2. [DOI] [PubMed] [Google Scholar]
- 42.Nickenig G, Bäumer AT, Grohè C, Kahlert S, Strehlow K, Rosenkranz S, Stäblein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Böhm M. Estrogen Modulates AT1 Receptor Gene Expression in Vitro and in Vivo. Circ. 1998;97:2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 43.Pelzer T, de Jager T, Muck J, Stimpel M, Neyses L. Oestrogen action on the myocardium in vivo: specific and permissive for angiotensin-converting enzyme inhibition. J Hypertens. 2002;20:1001–6. doi: 10.1097/00004872-200205000-00036. [DOI] [PubMed] [Google Scholar]
- 44.Affinito P, Palomba S, Bonifacio M, Fontana D, Izzo R, Trimarco B, Nappi C. Effects of hormone replacement therapy in postmenopausal hypertensive patients. Maturitas. 2001;40:75–83. doi: 10.1016/s0378-5122(01)00196-7. [DOI] [PubMed] [Google Scholar]
- 45.Cacciatore B, Paakkari I, Hasselblatt R, Nieminen MS, Toivonen J, Tikkanen MI, Ylikorkala O. Randomized comparison between orally and transdermally administered hormone replacement therapy regimens of long-term effects on 24-hr ambulatory BP in postmenopausal women. Am J Obstet Gynecol. 2001;184:904–9. doi: 10.1067/mob.2001.111246. [DOI] [PubMed] [Google Scholar]
- 46.Manhem K, Ahlm H, Milson I, Svensson A. Transdermal Oestrogen Reduces Daytime BP in Hypertensive Women. J Hum Hypertens. 1998;12:323–7. doi: 10.1038/sj.jhh.1000563. [DOI] [PubMed] [Google Scholar]
- 47.Kaya C, Cengiz SD, Cengiz B, Akgun G. Long-term effects of low dose 17beta-estradiol plus dydrogesterone on 24-hr ambulatory BP in healthy postmenopausal women: a 1 year, radomized, prospective study. Gynecol Endocrinol. 2007;23(Suppl1):62–67. doi: 10.1080/09513590701584956. [DOI] [PubMed] [Google Scholar]
- 48.Seely EW, Walsh BW, Gerhard MD, Williams GH. Estradiol With or Without Progesterone and Ambulatory BP in Postmenopausal Women. Hypertension. 1999;33:1190–4. doi: 10.1161/01.hyp.33.5.1190. [DOI] [PubMed] [Google Scholar]
- 49.Mortensen KH, Hansen KW, Erlandsen M, Christiansen JS, Gravholt CH. Ambulatory arterial stiffness index in Turner syndrome: the impact of sex hormone therapy. Horm Res. 2009;72:184–189. doi: 10.1159/000232495. [DOI] [PubMed] [Google Scholar]
- 50.Fisman EZ, Tenenbaum A, Shapira I, Motro M, Pines A. Lack of effects of transdermal estradiol on diastolic function: a randomized placebo-controlled double-blind short-term trial. Climacteric. 1999;2:174–80. doi: 10.3109/13697139909038059. [DOI] [PubMed] [Google Scholar]
- 51.de Carvalho MN, Nobre F, Mendes MC, Dos Reis RM, Ferriani RA, Silva de Sá MF. Low-dose transdermal hormone therapy does not interfere with the BP of hypertensive menopausal women: a pilot study. Blood Press Monit. 2008;13:277–283. doi: 10.1097/MBP.0b013e32830d4b60. [DOI] [PubMed] [Google Scholar]
- 52.Ichikawa A, Sumino H, Ogawa T, Ichikawa S, Nitta K. Effects of long term transdermal hormone replacement therapy on the renin-angiotesnsin-aldosterone system, plasma bradykinin levels and BP in normotensive postmenopausal women. Geriatr Gerontol Int. 2008;8:259–264. doi: 10.1111/j.1447-0594.2008.00474.x. [DOI] [PubMed] [Google Scholar]
- 53.Prelevic GM, Kwong P, Byrne DJ, Jagroop IA, Ginsburg J, Mikhailidis DP. A cross-sectional study of the effect of hormone replacement therapy on cardiovascular disease risk profile in healthy postmenopausal women. Fertil Steril. 2002;77:945–51. doi: 10.1016/s0015-0282(02)03078-9. [DOI] [PubMed] [Google Scholar]
- 54.Christ M, Seyffart K, Tillmann HC, Wehling M. Hormone replacement in postmenopausal women: impact of progestogens on autonomic tone and BP regulation. Menopause. 2002;9:127–36. doi: 10.1097/00042192-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Kalantaridou SN, Naka KK, Papanikolaou E, Kazakos N, Kravariti M, Calis KA, Paraskevaidis EA, Sideris DA, Tsatsoulis A, Chrousos GP, Michalis LK. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab. 2004;89:3907–13. doi: 10.1210/jc.2004-0015. [DOI] [PubMed] [Google Scholar]
- 56.Taddei S, Virdis A, Ghiadooni L, Mattei P, Sudano I, Bernini G, Salvetti A. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28:576–582. doi: 10.1161/01.hyp.28.4.576. [DOI] [PubMed] [Google Scholar]
- 57.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Card. 2008;51:997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 58.Kelemen M, Vaidya D, Waters DD, Howard BV, Cobb F, Younes N, Tripputti M, Ouyang P. Hormone therapy and antioxidant vitamins do not improve endothelial vasodilator function in postmenopausal women with established coronary artery disease: a substudy of the Women’s Angiographic Vitamin and Estrogen (WAVE) trial. Atherosclerosis. 2005;179:193–200. doi: 10.1016/j.atherosclerosis.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 59.Weiner CP, Lizasoain I, Baylis SA, Knowles RG, Charles IG, Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci USA. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Borrás C, Gambini J, Gómez-Cabrera MC, Sastre J, Pallardó FV, Mann GE, Viña J. 17beta-oestradiol up-regulates longevity related, antioxidant enzyme expression via ERK-1 and ERK2[MAPK]/NFkappaB cascade. Aging Cell. 2005;4:113–118. doi: 10.1111/j.1474-9726.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 61.Yanes L, Romero D, Iliescu R, Cucchiarelli VE, Fortepiani LA, Santacruz F, Bell W, Zhang H, Reckelhoff JF. Systemic arterial pressure response to two weeks of Tempol therapy in SHR: involvement of NO, the RAS, and oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R229–R233. doi: 10.1152/ajpregu.00530.2004. [DOI] [PubMed] [Google Scholar]
- 62.Sartori-Valinotti JC, Iliescu R, Fortepiani LA, Yanes LL, Reckelhoff JF. Sex differences in oxidative stress and the impact on BP control and cardiovascular disease. Clin Exp Pharm Physiol. 2007;34:938–945. doi: 10.1111/j.1440-1681.2007.04643.x. [DOI] [PubMed] [Google Scholar]
- 63.Lopez-Ruiz AF, Iliescu R, Reckelhoff JF. Refractory BP in female SHR to increased oxidative stress is not mediated by NO or by upregulation of renal antioxidant enzymes. Am J Physiol Regul Integr Comp Physiol. 2010;298:R266–71. doi: 10.1152/ajpregu.00471.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pechanova O, Simko F. Chronic antioxidant therapy fails to ameliorate hypertension: potential mechanisms behind. J Hypertension Suppl. 2009;27:S32–S36. doi: 10.1097/01.hjh.0000358835.25934.5e. [DOI] [PubMed] [Google Scholar]
- 65.McQueen MJ, Lonn E, Gerstein HC, Bosch J, Yusuf S. The HOPE (Heart Outcomes Prevention Evaluation) Study and its consequences. Scand J Clin Lab Invest Suppl. 2005;240:143–156. doi: 10.1080/00365510500236366. [DOI] [PubMed] [Google Scholar]
- 66.Nickenig G, Bäumer AT, Grohè C, Kahlert S, Strehlow K, Rosenkranz S, Stäblein A, Beckers F, Smits JF, Daemen MJ, Vetter H, Böhm M. Estrogen Modulates AT1 Receptor Gene Expression in Vitro and in Vivo. Circ. 1998;97:2197–2201. doi: 10.1161/01.cir.97.22.2197. [DOI] [PubMed] [Google Scholar]
- 67.Schunkert H, Danser AH, Hense HW, Derkx FH, Kürzinger S, Riegger GA. Effects of Estrogen Replacement Therapy on the Renin-Angiotensin System in Post-Menopausal Women. Circ. 1997;95:39–45. doi: 10.1161/01.cir.95.1.39. [DOI] [PubMed] [Google Scholar]
- 68.Wassmann K, Ghiassi A, Wassmann S, Böhm M, Nickenig G. AT1 Receptor antagonism improves endothelial dysfunction in postmenopausal women. Maturitas. 2006;53(2):176–83. doi: 10.1016/j.maturitas.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Mühlen D. Hysterectomy, oophorectomy and endogenous sex hormones levels in older women: the Rancho Bernardo study. J Endocrinol Metab. 2000;85:645–651. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- 70.Krug E, Berga SL. Postmenopausal hyperthecosis: function dysregulation of androgenesis in climacteric ovary. Obstet Gynecol. 1999;99:893–897. doi: 10.1016/s0029-7844(01)01588-5. [DOI] [PubMed] [Google Scholar]
- 71.Bui HN, Struys EA, Martens F, de Ronde W, Thienpont LM, Kenemans P, Verhoeven MO, Jakobs C, Dijstelbloem HM, Blankenstein MA. Serum testosterone levels measured by isotope dilution-liquid chromatography-tandem mass spectrometry in postmenopausal women versus those in women who underwent bilateral oophorectomy. Ann Clin Biochem. 2010;47:248–252. doi: 10.1258/acb.2010.009171. [DOI] [PubMed] [Google Scholar]
- 72.Quinkler M, Bumke-Vogt C, Meyer B, Bähr V, Oelkers W, Diederich S. The human kidney is a progesterone-metabolizing and androgen-producing organ. J Clin Endocrin Metab. 2003;88:2803–2809. doi: 10.1210/jc.2002-021970. [DOI] [PubMed] [Google Scholar]
- 73.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–8. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 74.Stanworth RD, Jones TH. Testosterone in obesity, metabolic syndrome and type 2 diabetes. Front Horm Res. 2009;37:74–90. doi: 10.1159/000176046. [DOI] [PubMed] [Google Scholar]
- 75.Allan CA, McLachlan RI. Androgens and obesity. Curr Opin Endocrinol Diabetes Obes. 2010;17:224–232. doi: 10.1097/MED.0b013e3283398ee2. [DOI] [PubMed] [Google Scholar]
- 76.Shaw LJ, Bairey Merz CN, Azziz R, Stanczyk FZ, Sopko G, Braunstein GD, Kelsey SF, Kip KE, Cooper-Dehoff RM, Johnson BD, Vaccarino V, Reis SE, Bittner V, Hodgson TK, Rogers W, Pepine CJ. Postmenopausal women with a history of irregular menses and elevated androgen measurements at high risk for worsening cardiovascular event-free survival: Results from NIH-NHLKBI Sponsored Women’s Ischemia Syndrome Evaluation. J Clin Endocrinol Metab. 2008;93:1276–1284. doi: 10.1210/jc.2007-0425. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship between androgen levels and BP in young women with polycystic ovary syndrome. Hypertension. 2007;49:1442–1447. doi: 10.1161/HYPERTENSIONAHA.106.083972. [DOI] [PubMed] [Google Scholar]
- 78.Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Dossus L, Micheli A, Arslan A, Lenner P, Shore RE, Krogh V, Koenig KL, Riboli E, Berrino F, Hallmans G, Stattin P, Toniolo P, Kaaks R. Body mass index, circulating levels of sex steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol. 2004;150:161–171. doi: 10.1530/eje.0.1500161. [DOI] [PubMed] [Google Scholar]
- 79.Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type Ii diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 80.Hestiantoro A, Swaab D. Changes in estrogen receptor-alpha and –beta in the infundibular nucleus of human hypothalamus are related to the occurrence of Alzheimer’s disease neuropathy. J Clin Endocrin Metab. 2004;89:1912–1925. doi: 10.1210/jc.2003-030862. [DOI] [PubMed] [Google Scholar]
- 81.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension. 2004;44:405–409. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 82.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 83.Center for Disease Control and Prevention. Vital signs: State-specific obesity prevalence among adults – United States 2009. MMWR Morb Mortal Wkly Rep. 2010;59:951–955. [PubMed] [Google Scholar]
- 84.Sutton-Tyrrell K, Zhao X, Santoro N, Lasley B, Sowers M, Johnston J, Mackey R, Matthews K. Reproductive hormones and obesity: 9 years of observation from the Study of Women’s Health Across the Nation. Am J Epidemiol. 2010;171:1212–1213. doi: 10.1093/aje/kwq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ozbey N, Sencer E, Molvalillar S, Orhan Y. Body fat distribution and cardiovascular disease risk factors in pre-and post-menopausal obese women with similar BMI. Endocrinol J. 2002;49:503–509. doi: 10.1507/endocrj.49.503. [DOI] [PubMed] [Google Scholar]
- 86.Ford ES, Li C, Zhao G, Tsai J. Trends in obesity and abdominal obesity among adults in the United States from 1999 to 2008. Int J Obes (Lond) 2010 doi: 10.1038/ijo.2010.186. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 87.Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates sodium retention and hypertension associated with obesity. Hypertension. 1995;25:893–897. doi: 10.1161/01.hyp.25.4.893. [DOI] [PubMed] [Google Scholar]
- 88.Wildman RP, McGinn AP, Lin J, Wang D, Muntner P, Cohen HW, Reynolds K, Fonseca V, Sowers MR. Cardiovascular disease risk of abdominal obesity vs metabolic abnormalities. Obesity (Silver Spring) 2010 doi: 10.1038/oby.2010.168. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hall JE, Guyton AC, Brands MJ. Control of sodium excretion and arterial pressure by intrarenal mechanisms and the renin-angiotensin system. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis, and Management. Second Edition. Raven Press; New York: 1995. pp. 1451–75. [Google Scholar]
- 90.Hall JE, Brands MJ, Henegar JR. Angiotensin II and Long-Term Arterial Pressure Regulation: The Overriding Dominance of the Kidney. J Am Soc Nephrol. 1999;10:S258–S265. [PubMed] [Google Scholar]
- 91.Romero JC, Reckelhoff JF. Role of Angiotensin and Oxidative Stress in Essential Hypertension. Hypertension. 1999;34:943–9. doi: 10.1161/01.hyp.34.4.943. [DOI] [PubMed] [Google Scholar]
- 92.Weinberger MH, Fineberg FS. Sodium and volume sensitivity of BP. Age and pressure change over time. Hypertension. 1991;18:67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]
- 93.Mansego ML, Redon J, Marin R, González-Albert V, Martin-Escudero JC, Fabia MJ, Martinez F, Chaves FJ. Renin polymorphisms and haplotypes are associated with BP levels and hypertension risk in postmenopausal women. J Hypertension. 2008;26:230–237. doi: 10.1097/HJH.0b013e3282f29865. [DOI] [PubMed] [Google Scholar]
- 94.Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Kennedy CR, Zimnplemann J, Gao W, Cattran DC, Scholey JW. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc, Nephrol. 2006;17:2554–2560. doi: 10.1681/ASN.2005101095. [DOI] [PubMed] [Google Scholar]
- 95.Canzello VJ, Baranco-Pryor E, Rahbari-Oskoui F, Schwartz GL, Boerwinkle E, Turner ST, Chapman AB. Predictors of BP response to the angiotensin receptor blocker candesartan in essential hypertension. Am J Hypertens. 2008;21:61–65. doi: 10.1038/ajh.2007.24. [DOI] [PubMed] [Google Scholar]
- 96.Reckelhoff JF. Gender differences in the regulation of BP. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 97.Chen Y-F, Naftilan AJ, Oparil S. Androgen-Dependent Angiotensinogen and Renin Messenger RNA Expression in Hypertensive Rats. Hypertension. 1992;19:456–463. doi: 10.1161/01.hyp.19.5.456. [DOI] [PubMed] [Google Scholar]
- 98.Ellison KE, Ingelfinger JR, Pivor M, Dzau VJ. Androgen Regulation of Rat Renal Angiotensinogen Messenger RNA Expression. J Clin Invest. 1989;83:1941–5. doi: 10.1172/JCI114102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hilzendeger AM, Morais RL, Todiras M, Plehm R, da Costa Goncalves A, Qadri F, Araujo RC, Gross V, Nakaie CR, Casarini DE, Carmona AK, Bader M, Pesquero JB. Leptin regulates ACE activity in mice. J Mol Med. 2010;88:899–907. doi: 10.1007/s00109-010-0649-7. [DOI] [PubMed] [Google Scholar]
- 100.Yanes LL, Romero DG, Iliescu R, Zhang H, Davis D, Reckelhoff JF. Postmenopausal hypertension: Role of the Renin Angiotensin System. Hypertension. 2010;56:359–363. doi: 10.1161/HYPERTENSIONAHA.110.152975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alexander BT, Cockrell KL, Rinewalt AN, Herrington JN, Granger JP. Enhanced renal expression of preproendothelin mRNA during chronic angiotensin II hypertension. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1388–1392. doi: 10.1152/ajpregu.2001.280.5.R1388. [DOI] [PubMed] [Google Scholar]
- 102.Wilkins FC, Jr, Alberola A, Mizelle HL, Opgenorth TJ, Granger JP. Systemic hemodynamics and renal function during long-term pathophysiological increases in circulating endothelin. Am J Physiol. 1995;268:R375–R381. doi: 10.1152/ajpregu.1995.268.2.R375. [DOI] [PubMed] [Google Scholar]
- 103.Mortensen LH, Pawloski CM, Kanagy NL, Fink GD. Chronic hypertension produced by infusion of endothelin in rats. Hypertension. 1990;15:729–33. doi: 10.1161/01.hyp.15.6.729. [DOI] [PubMed] [Google Scholar]
- 104.Komatsumoto S, Nara M. Changes in the level of endothelin-1 with aging. Nippon Ronen Igakkai Zasshi. 1995;32:664–669. doi: 10.3143/geriatrics.32.664. [DOI] [PubMed] [Google Scholar]
- 105.Ballew JR, Fink GD. Role of ET(A) receptors in experimental Ang II-induced hypertension in rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R150–R154. doi: 10.1152/ajpregu.2001.281.1.R150. [DOI] [PubMed] [Google Scholar]
- 106.Ballew JR, Fink GD. Role of endothelin ETB receptor activation in angiotensin II-induced hypertension: effects of salt intake. Am J Physiol Heart Circ Physiol. 2001;281:H2218–25. doi: 10.1152/ajpheart.2001.281.5.H2218. [DOI] [PubMed] [Google Scholar]
- 107.Sasser JM, Pollock JS, Pollock DM. Renal endothelin in chronic angiotensin II hypertension. Am J Physiol Regul Integr Comp Physiol. 2002;283:R243–R248. doi: 10.1152/ajpregu.00086.2002. [DOI] [PubMed] [Google Scholar]
- 108.Diamanti-Kandarakis E, Spina G, Kouli C, Migdalis I. Increase in endothelin-1 levels in women with polycystitic ovary syndrome and the beneficial effect of metformin treatment. J Clin Endocrin Metab. 2001;86:4666–73. doi: 10.1210/jcem.86.10.7904. [DOI] [PubMed] [Google Scholar]
- 109.de Kraker AT, Kenemans P, Smolders RG, Kroeks MV, van der Mooren MJ. Short-term effects of continuous combined oestrogen-progestogen therapy on several cardiovascular risk markers in healthy postmenopausal women; a randomized control trial. Eur J Obstet Gynecol Reprod Biol. 2009;142:139–144. doi: 10.1016/j.ejogrb.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 110.Polderman KH, Stehouwer CD, van Kamp GJ, Dekker GA, Verheugt FW, Gooren LJ. Influence of sex hormones on plasma endothelin levels. Ann Intern Med. 1993;118:429–432. doi: 10.7326/0003-4819-118-6-199303150-00006. [DOI] [PubMed] [Google Scholar]
- 111.Yanes LL, Romero DG, Cucchiarelli VE, Fortepiani LA, Gomez-Sanchez CE, Santacruz F, Reckelhoff JF. Role of endothelin in a model of postmenopausal hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R229–R233. doi: 10.1152/ajpregu.00697.2003. [DOI] [PubMed] [Google Scholar]
- 112.Kurtz EG, Ridker PM, Rose LM, Cook NR, Everett BM, Buring JE, Rexrode KM. Oral postmenopausal hormone therapy, C-reactive protein and cardiovascular outcomes. Menopause. 2010 doi: 10.1097/gme.0b013e3181e750dd. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rizzo M, Corrado E, Coppola G, Muratori I, Novo G, Novo S. Markers of inflammation are strong predictors of subclinical and clinical atherosclerosis in hypertensive women. Coron Artery Dis. 2009;20:15–20. doi: 10.1097/MCA.0b013e3283109065. [DOI] [PubMed] [Google Scholar]
- 114.Park HT, Cho SH, Cho GJ, Shin JH, Hong SC, Kim T, Hur JY, Kim YT, Kim SH. Relationship between serum adipocytokine levels and metabolic syndrome in menopausal women. Gynecol Endocrinol. 2009;25:27–31. doi: 10.1080/09513590802404021. [DOI] [PubMed] [Google Scholar]
- 115.Lee CG, Carr MC, Murdoch SJ, Mitchell E, Woods NF, Wener MH, Chandler WL, Boyko EJ, Brunzell JD. Adipokines, inflammaton and visceral adiposity across the menopausal transition: a prospective study. J Clin Endocrinol Metab. 2009;94:1104–1110. doi: 10.1210/jc.2008-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am J Physiol Heart Circ Physiol. 2006;290:H395–H340. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 117.Tarkun I, Arslan BC, Cantürk Z, Türemen E, Sahin T, Duman C. Endothelial dysfunction in young women with PCOS: relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab. 2004;89:5592–5596. doi: 10.1210/jc.2004-0751. [DOI] [PubMed] [Google Scholar]
- 118.Giltay EJ, Haider A, Saad F, Gooren LJ. C-reactive protein levels and ageing male symptoms in hypogonadal men treated with testosterone supplementation. Andrologia. 2008;40:398–400. doi: 10.1111/j.1439-0272.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- 119.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–8. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 120.LaMarca BD, Chandler DL, Grubbs L, Bain J, McLemore GR, Jr, Granger JP, Ryan MJ. Role of sex steroids in modulating tumor necrosis factor alpha-induced changes in vascular function and BP. Am J Hypertens. 2007;20:1216–1221. doi: 10.1016/j.amjhyper.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52:1161–1171. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nakagawa K, Marji JS, Schwartzman ML, Waterman MR, Capdevila JH. Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1055–1062. doi: 10.1152/ajpregu.00459.2002. [DOI] [PubMed] [Google Scholar]
- 123.Singh H, Schwartzman ML. Renal vascular cytochrome P450-derived eicosanoids in androgen-induced hypertension. Pharmacol Rep. 2008;60:29–37. [PubMed] [Google Scholar]
- 124.Wu C-C, Cheng J, Zhang FF, Gotlinger KH, Kelkar M, Zhang Y, Jat JL, Falck JR, Schwartzman ML. Androgen-dependcent hypertension is mediated by 20-HETE-induced vascular dysfunction: Role of IkappaB kinase. Hypertension. 2011 doi: 10.1161/HYPERTENSIONAHA.110.161570. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- 126.Goldstein BI, Fagiolini A, Houck P, Kupfer DJ. Cardiovascular disease and hypertension among adults with bipolar disorder in the United States. Biopolar Disord. 2009;11:657–662. doi: 10.1111/j.1399-5618.2009.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lambert E, Dawood T, Straznicky N, Sari C, Schlaich M, Esler M, Lambert G. Association between the sympathetic firing pattern and anxiety level in patients with metabolic syndrome and elevated BP. J Hypertension. 2010;28:543–550. doi: 10.1097/HJH.0b013e3283350ea4. [DOI] [PubMed] [Google Scholar]
- 128.García-Vera MP, Sanz J, Espinosa R, Fortún M, Magán I. Differences in emotional personality traits and stress between sustained hypertension and normotension. Hypertension Res. 2010;33:203–208. doi: 10.1038/hr.2009.210. [DOI] [PubMed] [Google Scholar]
- 129.Braszko JJ, Karwowska-Polecka W, Halicka D, Gard PR. Captopril and enalapril improve cognition and depressed mood in hypertensive patients. J Basic Clin Physiol Pharmacol. 2003;14:323–343. doi: 10.1515/jbcpp.2003.14.4.323. [DOI] [PubMed] [Google Scholar]
- 130.Roman RJ. P450 Metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2001;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 131.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-kappaB. J Appl Physiol. 2008;105:1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Takahashi M, Suzuki E, Takeda R, Oba S, Nishimatsu H, Kimura K, Nagano T, Nagai R, Hirata Y. Angiotensin II and tumor necrosis factor-alpha synergistically promote monocyte chemoattract protein-1 expression: Role of NF-kappa B, p38, and reactive oxygen species. Am J Physiol Heart Circ Physiol. 2008;294:H2879–H2888. doi: 10.1152/ajpheart.91406.2007. [DOI] [PubMed] [Google Scholar]


