Abstract
The formation of cotinine, the main proximate metabolite and a biomarker of nicotine exposure, is mediated primarily by CYP2A6. Our aim was to determine if higher cotinine levels in young children exposed to secondhand smoke (SHS) are a result of age-related differences in pharmacokinetics. Forty-nine participants, 2 to 84 months old, received oral deuterium-labeled cotinine, with daily urine samples for up to 10 days for cotinine half-life measurement. DNA from saliva was used for CYP2A6 genotyping. The estimate of half-life using a mixed effect model was 17.9 hrs (95%CI: 16.5, 19.3), similar to that reported in adults. There was no statistically significant effect of sex, race, age, or weight. Children with normal activity CYP2A6*1/*1 genotypes had a shorter half-life than those with 1–2 reduced activity variant alleles. Our data suggest that higher cotinine levels in SHS-exposed young children compared to adults are due to greater SHS exposure rather than different cotinine pharmacokinetics.
INTRODUCTION
Nicotine, the addictive substance in cigarettes that maintains smoking behaviors, is metabolized by the CYP2A6 enzyme to cotinine, which is pharmacologically inactive 1. In adults, cotinine has a mean half-life of 16–18 hours and is used as a quantitative biomarker for cigarette smoke consumption in smoking studies, and as a biomarker for second-hand smoke (SHS) exposure in non-smokers including infants and children 1–3.
Several pediatric studies have measured cotinine levels in plasma, saliva, urine, hair and occasionally meconium to document SHS exposure 2–7. Some of these studies have found substantially higher cotinine levels in infants and children relative to those found in adults exposed to SHS; some studies have found cotinine levels in children at levels comparable to active adult smokers 4, 8, 9.
There are several possible explanations for high levels of cotinine in children. One is inconsistent measurements of cotinine by different methods. Immunoassay methods, used in several pediatric studies, may cross react with substances other than cotinine and resulting in higher reported cotinine SHS levels compared to chromatography or chromatography–mass spectrometry methods, which generally have higher specificity. Alternatively there may be more systemic SHS exposure in the very young because their minute ventilation relative to body mass is higher and they may inhale more SHS relative to their body mass compared to adults. Third, there may be very high levels of SHS exposure if their parent or caretaker is a smoker and is holding or playing with the child while smoking. Finally, cotinine pharmacokinetics may be different, such that for the same nicotine exposure cotinine accumulates to higher levels in children compared to adults. Specifically, if the clearance of cotinine is slower or the volume of distribution is larger in children, then half-life will be longer compared to that seen in adults. Different pharmacokinetics in young children compared to adults has been observed for a number of other drugs 10, 11.
Two small studies in neonates and two larger studies in children have investigated the half-life of cotinine. Three studies used immunoassays (RIA) and found prolonged half lives relative to those reported for adults; one study used GC-MS and found the cotinine half-life in neonates to be similar to that in adults 9, 12–14. Etzel, et al, 12 collected urine twice daily for up to 6 days in hospitalized newborns of smokers, analyzed the samples by RIA for cotinine and found a mean half- life 68 hour with a range of 37 to 160 hours. Dempsey, et al, 13 collected daily blood samples for 3 days and urine samples for up to 7 days from newborns of smokers, and analyzed the samples for nicotine and cotinine by GC-MS. Although nicotine had a longer half-life than in adults, the cotinine half-life was similar to adults with a mean of 16.3 hrs, (95%CI 12.4–23.9) based on blood data, and 22.8 hrs (95%CI 19.5–25.8) based on urine data 13.
The two cotinine half-life studies in children kept SHS exposed children in a smoke-free hospital environment, and collected one or two daily urine samples. Leong et al 14 studied SHS exposed children who were admitted to the hospital as patients (non-research) and ranged in age from 0 months to 14.5 years, although 57% were under 2 years of age. Their urine was collected at admission and at 12, 24 and 48 hours. Among those under 2 years, the median half-life was 28.3 hours with a range of 6.3 to 285 hours 14. Collier et al 9 admitted 44 SHS exposed children less than 3 years of age for up to 6 days to a research ward, and collected urine several times daily. They found no difference in half-life between the sexes or between Whites and African Americans. For those under 1 year, the mean half-life was 108 hrs (SD +/− 152), for those 12–23 months old it was 86 hrs (+/−74), and for those 24–36 months old it was 40.5 hrs (+/− 10).
All four studies analyzed the urine for cotinine derived from the nicotine acquired from SHS. However these types of studies can be compromised by inadvertent ongoing exposure to nicotine by off-gassing from clothing and hair, or by surreptitious smoking by a visitor. The present study used oral doses of deuterium-labeled cotinine in SHS-exposed infants and children. Deuterium is a harmless, naturally occurring, non-radioactive isotope of hydrogen. Deuterium labeled cotinine has been widely studied and has the same pharmacokinetics as natural cotinine 15, 16. Labeling the cotinine allows the half-life to be studied independent of on-going SHS exposure, as it can be assayed using liquid chromatography-tandem mass spectrometry without interference from SHS derived cotinine.
Greater than 70% of nicotine is metabolized to cotinine by the liver enzyme CYP2A6, and cotinine is further metabolized exclusively by CYP2A6 to trans 3’-hydroxycotinine. CYP2A6 is genetically polymorphic and the clearance of cotinine is altered by genotype differences 1, 16. There is racial/ethnic variation in the frequency of CYP2A6 variant alleles among racial/ethnic groups 17. Therefore when comparing cotinine half-life among children of different racial/ethnic groups, it is important to consider the impact of genetic variation in CYP2A6.
The overall aim of our study was to determine the half-life of cotinine in the infants and young children to allow better interpretation of cotinine levels in babies and young children exposed to SHS, specifically those between 2 and 84 months of age, an understudied population. Measurement of disposition kinetics across this age range would also allow for the identification of developmental changes in CYP2A6 activity from infancy to early childhood. No data are available on the effect of CYP2A6 genotype on cotinine half-life in children. One pediatric study has found significant differences in cotinine levels associated with SHS exposure between young African American and Hispanic children 3, and this difference is well documented in adults 18. Therefore another aim of the study is to determine the effects of CYP2A6 genotype and of race on cotinine half-life in children. By using a mixed effects model (population) analysis of the pool data, we are able to handle imbalance in the data (e.g. unequal number of samples among individuals) and test for covariate effects.
RESULTS
Forty-nine subjects completed the study. Their age ranged from 2.5 to 82.4 months, with a median of 34.2 months and a mean of 34.6 months (SD +/− 21.9 months). Their weight ranged from 4.9 to 31.5 kg, with a median and a mean of 15 kg, (SD +/− 6.0 kg). Among the 44 subjects with successful genotyping, 23 (46.9%) had the CYP2A6*1/*1 genotype which corresponds to normal metabolic activity, 7 (14.3%) had the CYP2A6*1/*9 genotype and comprised the group of those with intermediate activity, while the remaining 28.6% had slow activity with 10 of these subjects having the CYP2A6*1/*17 genotype and 1 subject each for the remaining 4 genotypes (CYP2A6*1/*2, CYP2A6*1/*20, CYP2A6*9/*9, and CYP2A6*17/*17). By race/ethnicity, all white or Latino children (N=9) had CYP2A6*1/*1 genotype; among African American children (N=27), 13 had CYP2A6*1/*1, 7 had CYP2A6*1/*17, 4 had CYP2A6*1/*9 and 1 each had CYP2A6*1/*2, CYP2A6*1/*20 or CYP2A6*9/*9 genotype; among mixed race/ethnicity children (N=8), 3 each had CYP2A6*1/*9 or CYP2A6*1/*1, and 1 each had CYP2A6*1/*1 or CYP2A6*17/*17 genotype (Table 1). A higher percentage of younger compared to older children had *1/*1 genotypes (Table 2).
Table 1.
Prevalence of CYP2A6 genotype by race/ethnicity
| Genotypes | White | Black | Mix | Totals |
|---|---|---|---|---|
| *1/*1 | 9 | 13 | 1 | 23 |
| *1/*2 | 0 | 1 | 0 | 1 |
| *1/*9 | 0 | 4 | 3 | 7 |
| *9/*9 | 0 | 1 | 0 | 1 |
| *1/*17 | 0 | 7 | 3 | 10 |
| *17/*17 | 0 | 0 | 1 | 1 |
| *1/*20 | 0 | 1 | 0 | 1 |
| unknown | 3 | 2 | 0 | 5 |
| Total | 12 | 29 | 8 | 49 |
Table 2.
Distribution of CYP2A6 Genotype by Age
| Age Groups | N | %: *1/*1 (wild type) |
|---|---|---|
| < 1 year old (y.o.) | 11 | 64% |
| 1–1.9 y.o. | 7 | 71% |
| 2–2.9 y.o. | 8 | 37.5% |
| 3–3.9 y.o. | 8 | 25% |
| 4–4.9 y.o. | 7 | 43% |
| 5–6 y.o. | 8 | 37.5% |
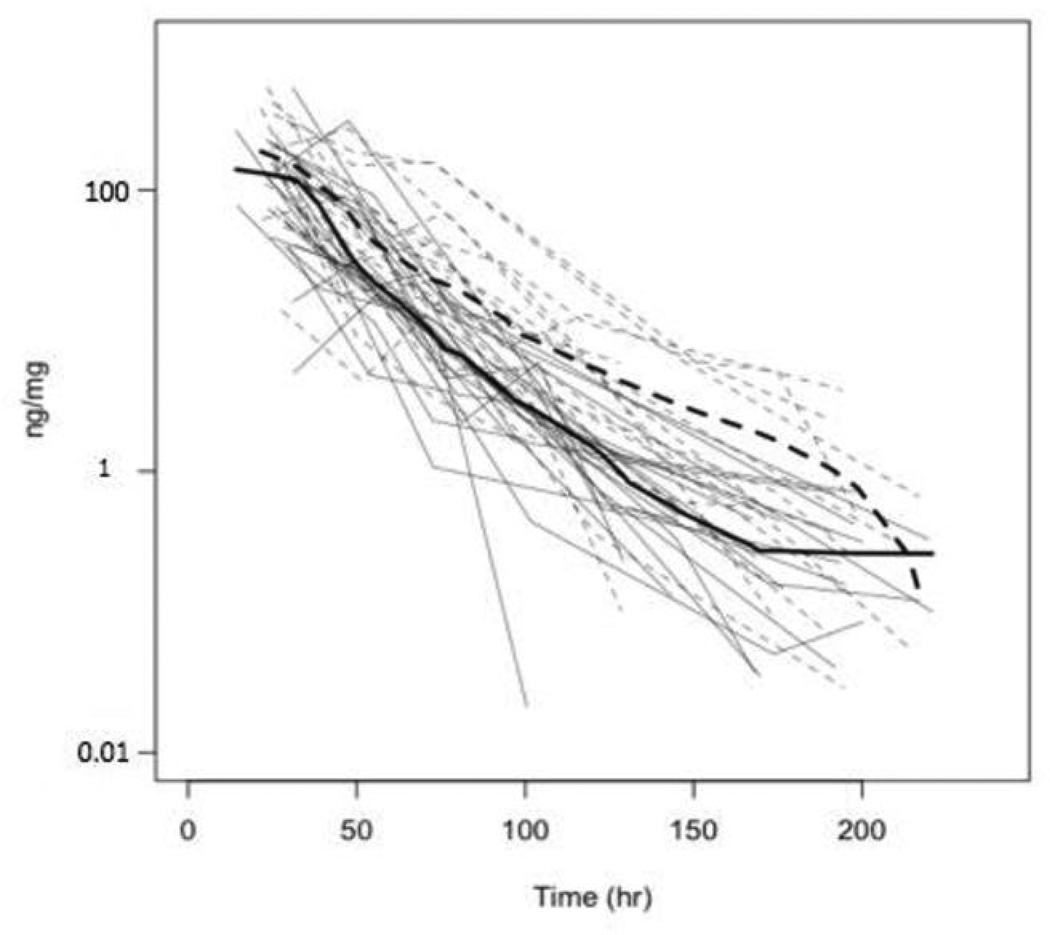
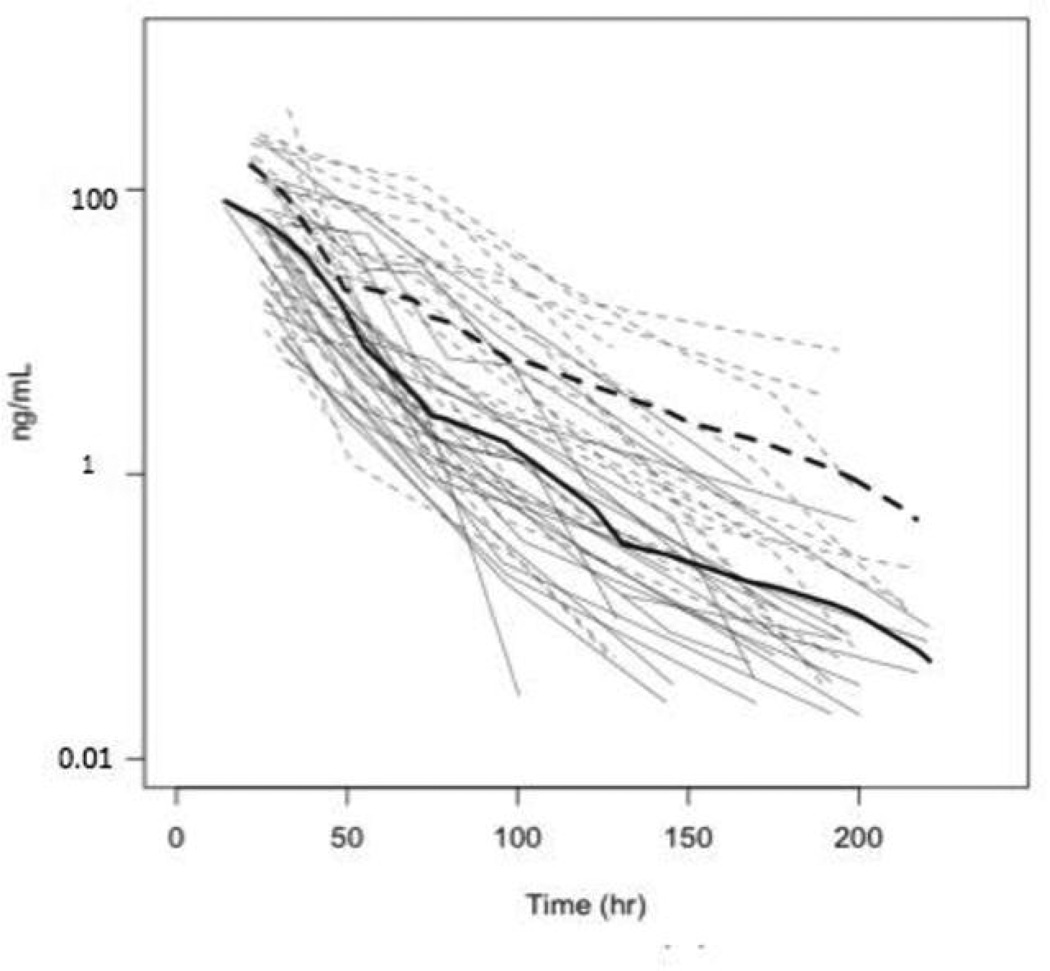
Urine cotinine levels, with and without correction for creatinine, over time for individual subjects are displayed in Figures 1 and 2. Table 3 displays the cotinine half-life estimates (95% confidence intervals, CI), shown by sex, race and age, based on analysis of urine levels with and without creatinine correction. Coincidentally, the overall half-life estimates and 95% CI (after rounding) are identical for creatinine-corrected and uncorrected data.
Figure 1.
Cotinine per mg creatinine (ng/mg) versus time. Data of individuals with normal genotype are shown with solid lines and those with intermediate or slow activity with dashed lines. Light grey lines are individual data and the dark lines are smoothed curves of the data from the corresponding genotype group.
Figure 2.
Uncorrected cotinine levels (ng/ml). Data of individuals with normal genotype are shown with solid lines and those with intermediate or slow activity with dashed lines. Light grey lines are individual data and the dark lines are smoothed of the data from the corresponding genotype group.
Table 3.
Half-Life Estimates by Sex, Race and Age
| N | Half-Life Point Estimate (95% CI) Creatinine-corrected |
Half-Life Point Estimate (95% CI) Creatinine-uncorrected |
||
|---|---|---|---|---|
| All subjects | 49* | 17.9 (16.5, 19.3) | 17.9 (16.5, 19.3) | |
| Sex Male | 25* | 18.4 (17.1, 19.7) | 18.5 (17.1, 19.9) | |
| Female | 24 | 17.4 (15.2, 19.6) | 17.4 (14.8, 19.6) | |
| Race | ||||
| White# | 12 | 17.5 (15.5, 19.5) | 17.7 (15.3, 20.1) | |
| Black | 29* | 18.1 (16.4, 19.8) | 17.7 (15.9, 19.5) | |
| Mixed | 8 | 18.2 (15.4, 21.0) | 18.8 (14.1, 23.5) | |
| Age Groups | ||||
| < 1 year old (y.o.) | 11 | 15.7 (13.6, 17.8) | 13.7 (12.0, 15.4) | |
| 1–1.9 y.o. | 7* | 18.5 (15.9, 21.1) | 18.4 (16.3, 20.5) | |
| 2–2.9 y.o. | 8 | 18.5 (15.6, 21.4) | 10.5 (7.0, 14.0) | |
| 3–3.9 y.o. | 8 | 17.2 (13.6, 20.8) | 16.8 (13.6, 20.6) | |
| 4–4.9 y.o. | 7 | 18.6 (15.7, 21.5) | 20.0 (16.0, 24.0) | |
| 5–6 y.o. | 8 | 19.3 (16.8, 21.8) | 19.6 (16.4, 22.8) | |
One 18-month old black male was excluded from the creatinine corrected urine half-life calculation because of missing creatinine levels, for a total of 48 subjects with the creatinine corrected results.
Included Hispanic-Whites
Figures 1 and 2 show smoothed population mean curves for cotinine in genetically normal and intermediate/slow metabolizers. Table 4 displays the estimates of half-life by genotype activity. Normal metabolizers had a significantly shorter creatinine-corrected urine cotinine half-life compared to intermediate and slow metabolizers.
Table 4.
Half-Life Estimates by Genotype Activity
| N | Point Estimate (95% CI) Creatinine-corrected |
Point Estimate (95% CI) Creatinine-uncorrected |
||
|---|---|---|---|---|
| CYP2A6 Genotype Activity | ||||
| Normal (*1/*1) | 23 | 16.4 (14.7, 18.1) | 16.2 (14.5, 17.9) | |
| Intermediate (*1/*9) | 7 | 19.6 (16.7, 22.5) | 20.0 (15.6, 24.4) | |
| Slow# (*1/*17, *1/*2, *1/*20, *9/*9, 17/*17) | 14 | 19.4 (16.6, 22.1) | 18.8 (15.9, 21.7) | |
| Intermediate/Slow# | 21 | 19.5 (17.4, 21.6) | 19.2 (16.8, 21.6) | |
| Unknown | 5 | 19.7 (16.7, 22.7) | 20.9 (16.4, 25.4) | |
|
Normal vs. Intermediate vs Slow vs. Unknown |
p | 0.022 | 0.096 | |
|
Normal vs. Intermediate/Slow vs. Unknown |
p | 0.006 | 0.036 | |
One 18 month old black male (CYP2A6*1/*17) was excluded from the creatinine corrected urine half-life calculations because of missing creatinine levels, for a total of 43 subjects for the creatinine corrected results. CI=confidence interval
For creatinine-corrected urine cotinine, there was no statistically significant effect (defined as p<0.01) of sex, race, or weight (all p > 0.2). Age was marginally significant (p = 0.056) when treated as a categorical variable (< 1 year old vs. > 1 year old) in the absence of genotype in the model, but not when genotype was included (p = 0.19).
For creatinine-uncorrected cotinine, genotype effect was marginally significant (p = 0.036) when the intermediate and slow variant groups were combined, but not when each group was treated separately or when either age or weight was in the model. There was a statistically significant effect of age and weight; however the model fit did not improve when both were present in the model, relative to the fit with either one alone, presumably because in growing infants and children age and weight are correlated. The effects of age and weight were marginally significant (p = 0.015 for age and p = 0.021 for weight) after correcting for genotype activity. Neither sex nor race had a significant effect (both p>0.3).
DISCUSSION
We present novel data on the half-life of cotinine using stable-isotope methodology in infants and young children. The use of labeled cotinine allowed us to study cotinine pharmacokinetics without potential confounding by ongoing environmental exposure to nicotine (and resulting generation of cotinine), and allows high sensitivity of measurement so as permit measurement of cotinine levels for several days after exposure. We provide novel data on the effects of age, sex and race/ethnicity and CYP2A6 genotype on cotinine elimination rate in the very young.
The estimate of the typical value of the half-life of cotinine, 17.9 hr, is similar to that observed in adult smokers and is similar to what we measured previously in neonates who had been exposed to tobacco smoke in utero 13. For creatinine-corrected half-life we found no effect of age on cotinine half-life. However we did see significant (when tested singularly) or marginally significant (when tested with genotype in the model) effects of age with creatinine-uncorrected measurements. There is a lower average cotinine half-life in children less than one year of age, but this is likely due to chance distribution from CYP2A6 genotype, such that a higher percentage of younger children had *1/*1 genotypes which are associated with shorter half-lives (Table 2). Overall our data suggest little or no developmental change in CYP2A6 activity after controlling for CYP2A6 genotype after 2 months of age. Our data are consistent with studies of CYP2A6 metabolic activity in livers from people of different age. For example, Al Koudsi et al found no relationship between age and either CYP2A6 protein levels or nicotine C-oxidation activity in a liver bank that included a number of livers from people below the age of 10 19. Others have reported similar findings, including no difference between fetal and adult livers 20–22.
We observed no significant effect of race but did find a strong effect of CYP2A6 genotype on cotinine half-life. Children with one or more reduced function variants (i.e. *2, *9, *17, *20) have a significantly longer estimate of typical half-life (19.5 hr) compared to those without such variants (16.4 hr). Similar genotype effects have been observed on cotinine clearance and half-life in adults 16. We expected to see an effect of race on half-life based on the higher prevalence of reduced function CYP2A6 alleles in African Americans compared whites. We did not find the expected half-life difference by race, even in univariate analysis, presumably because of relatively small sample size and inadequate power. Of note, as expected a higher frequency of African American and mixed ethnicity children had genotypes containing variants alleles relative to white children (48%, 89%, 0% respectively).
A limitation of using cotinine half-life as an indicator of developmental changes of CYP2A6 activity is that total cotinine clearance is determined not just by CYP2A6 activity, but also by enzymes involved in glucuronidation and N-oxidation, as well as by some renal clearance of unchanged cotinine 23. However, the similarity of half-life observed in the present study and that previously measured in adults, and the similar degree of impact of CYP2A6 genotype on cotinine half-life in adults and children, suggests a minimal effect of age on CYP2A6 activity. Because half-life is inversely proportional to clearance and directly proportional to volume of distribution of the central compartment, we cannot rule out a commensurate change with age in these two parameters resulting in no effect of age on half-life, but this this occurrence seems unlikely.
Our findings have implications for understanding tobacco smoke exposure in young children. First, our findings that the half-life of cotinine is similar in young children to that seen in adults, is inconsistent with some other studies in children 3, 9, 14. We suspect those studies suffer from methodologic problems of either nonspecific cotinine assays and/or persistent exposure to nicotine in the environment, with ongoing generation of cotinine. It is also possible that deep tissue stores of nicotine could result in prolonged production of cotinine and a longer half-life, although we did not observe that in our prior study in which we measured cotinine in neonates for several days following birth 13.
Second, we and others have found that cotinine levels in African American children are much higher than those observed in children of other race/ethnicities 3, 24. This could be due either to greater SHS exposure or to slower cotinine metabolism. The presence of reduced function CYP2A6 variants results in higher cotinine levels for any given level of nicotine intake 25. African Americans are known to have a higher frequency of reduced function CYP2A6 variants than Caucasians or Hispanics 17, 26. However the present study suggests that the effects of race or of reduced function CYP2A6 variants on half-life is relatively small. Assuming that half-life measured in urine reflects clearance of cotinine, the much higher levels of cotinine previously observed in African American children compared to white children cannot be accounted for solely by differences in cotinine metabolism. Greater SHS exposure is likely to account for more of the racial difference in cotinine levels.
In summary, we present novel data on the half-life of cotinine in infants and young children. The half-life of cotinine was similar to that observed in adults and was influenced by CYP2A6 genotype but not independently by age, sex or race/ethnicity. Our data will help in the interpretation of cotinine levels measured in SHS-exposed children.
METHODS
Overview
This was a study of the half-life of deuterium-labeled cotinine (COT-d2) which was dosed orally in infants and children, based on urine COT-d2 levels.
Subjects
Participants were infants and children, between 2 and 83 months of age, who by parental report were currently exposed to SHS in their homes. One hundred and seventy three parents and their infant or child were screened for eligibility, and 67.6% were not enrolled. Of those who were not enrolled, 47.7% declined to participate, and 52.3% were excluded for the following reasons: no SHS expose, 11%; parental time conflict, 10.3%; failed screening (non-medical), 9.4%; medical (e.g. asthma), 9.4%; race/ethnicity/sex enrollment cell full, 5.1%; age over 6 years, 2.6%; and reason not recorded, 5.1%. Fifty-six participants were enrolled, and 49 had sufficient urine data to be included in the present analysis. All participants were healthy by history and physical exam, had documented up to date immunizations and well baby/child visits, and had their medical records reviewed prior to enrollment. Exclusion factors were: currently on any medication or dietary supplements, asthma and other chronic illnesses, parental substance or alcohol abuse, and foster care placement. Fifty one percent were male, while the racial/ethnic distribution was 59% African American, 25% white or Latino, and 16% other or mixed race/ethnicity. Participants were aged 2.5 to 83.4 months old (m.o.), with the following age distribution: <12 m.o. (22.4%); 12–23 m.o. (14.3%); 24–35 m.o. (16.3%); 36–47 m.o. (16.3%); 48–59 m.o. (14.3%); and 60–83 m.o. (16.3%).
Recruitment and screening
Subjects were recruited via letters with flyers to pediatric offices/clinic and to churches, postings on Craig’s list, through participants in our adult smoking studies and by word of mouth from parents of study participants. Parents contacted us via our telephone recruitment line and a telephone screening was conducted to describe the study and determine that the participant met study criteria and had no exclusion factors. Those who met study criteria were scheduled for a screening visit, at which time the study was explained in detail and consent obtained. Other forms were filled out (demographic information, parent use of tobacco alcohol and drugs, SHS exposure history) and a home visit by the study coordinator was scheduled. At the home visit, the consent from the other parent was obtained by the study coordinator. Saliva and urine samples were obtained from the participant to verify that the participant would cooperate with sample collection. After the participant’s medical records were obtained and health and immunization status, and regularity of well baby/child visits verified, the participant was enrolled in the study. The study was approved by the Committee on Human Research at the University of California San Francisco, and parents were compensated for their time ($290 for one 10 hour stay on research ward and seven 1–2 hour home visits to collect urine and saliva samples).
Study Procedures
Parents and participants arrived at the Clinical Research Center (CRC) at San Francisco General Hospital by 9 A.M. There was no restriction upon eating or drinking relative to cotinine dosing, and age appropriate food was available for the participants and parents. Parents remained with the participants throughout their time at the CRC. After a baseline urine sample and a saliva sample for genotyping were collected, subjects were orally dosed with 0.05 mg/kg of deuterium-labeled cotinine (Cot-d4) solution. Deuterium labeling allows for measurement of cotinine that was administered for purposes of the study from natural cotinine derived from their SHS exposure. The deuterium-labeled cotinine was synthesized in our laboratory as described previously and has been used for many pharmacokinetic studies performed by our research group 27. An IND for the use of deuterium-labeled cotinine in infants and children was approved by the FDA. The dose selected was the lowest dose that would give us detectable Cot-d4 levels over 4 days assuming the pharmacokinetics were similar to weight-corrected adult values. Based on anticipated weight distribution, over 80% of subjects would receive a dose that was less than one tenth the oral adult dose (10 mg) that we have used in prior studies 28. A liquid solution of Cot-d4 was added to 1–2 ounces of participants’ preferred beverage. Participants and parents were discharged from the CRC 8 hours after dosing.
Research assistants made 5–6 visits spread over 10 days post dosing. The research assistant went to the participants’ home to collect a 20 ml spot urine sample on day 1, about 24 hours after dosing, and on days 2 and 3 post dosing. The first 13 subjects had 2 additional post dosing home visits while subjects 14–49 had 3 additional home visits for sample collection. The change was made to insure that 3 samples would be collected after the third day post dosing to be able to characterize a prolonged terminal half-life that might exist (e.g. days 4 or 5, days 7 or 8, and days 9 or 10). Toilet trained participants urinated into a plastic hood placed in the toilet to capture urine. For non-toilet trained children, the study coordinator placed a cotton ball into a clean diaper. After urination the urine was squeezed out of the cotton balls into a 20 ml plastic bottle with a tight lid. The minimum volume recovered by the cotton ball method was 5 ml, more than that required for the cotinine assay.
Genotyping
Saliva was collected using the Oragene-DNA saliva sponge kit (OG-250, CS1; dnagenotek.com). Three to five sponges on sticks were placed in the cheek pouches and allowed to absorb saliva (60 seconds). The sponges were then placed in the Oragene-DNA kit and covered with the liquid provided which preserves the DNA collected by the sponges; samples were frozen until shipped to the University of Toronto for genotyping.
DNA was extracted according the manufacturer’s instructions (Oragene). Briefly, 500 µl of Oragene solution containing saliva was mixed with 20 µl of PT-L2P (proprietary) solution, incubated on ice for 10 min and centrifuged for 5 min at room temperature at 13000 rpm. Ethanol was added to the supernatant to precipitate DNA. DNA of sufficient yield and quality was obtained for all but 5 subjects.
The DNA samples were genotyped for alleles which were relatively abundant in the Caucasians and African Americans and have established impact on CYP2A6 activity including CYP2A6*1B, *2, *4, *9, *12, *17, *20, *23, *24, *25, *26, *27, *28, *31, and *35, using a two-step gene-specific PCR amplification technique 17, 26, 29, 30.
Analytical Methods
Urine concentrations of deuterium-labeled cotinine were measured using liquid chromatography-tandem mass spectrometry using a published procedure, modified for determination of cotinine-d4, by using the corresponding ion transition m/z 181 > 84 31. The limit of quantitation for cotinine was 0.5 ng/ml.
Data Analysis
The measure of interest was the half-life of deuterium-labeled cotinine, estimated using urine cotinine concentrations. Analyses were done with and without correction for urine creatinine. A mixed-effects model analysis was carried out using the program NONMEM, Version 7.2.0 (Icon Development Solutions, Elliott City, MD) with first-order conditional estimation and interaction 32. Plots were done using the program R, Version 2.12.1. The structural model used was a mono-exponential decay with instantaneous input because the first sample was taken after most, if not all, of absorption had occurred..
The residual error was an exponential model (i.e., the error was additive to In-transformed values). The covariates tested in the pharmacokinetic data set were age, total body weight, race/ethnicity and CYP2A6 genotype. Goodness of fit (GOF) plots (individual and population predictions versus observation; population prediction and time versus conditional weighted residual) were used to check interim and final models.
Hypothesis testing for model development was based on the likelihood ratio test, which compares full versus reduced models with degrees of freedom (d.f.) equal to the difference in the number of parameters. Formal covariate testing was done in the order: i) singular addition, ii) stepwise addition of significant covariates (starting with covariate/parameter addition having greatest significance from step i, iii) singular deletion from a full model containing significant covariates based on step ii, and iv) stepwise deletion (starting with the covariate/parameter with least significance from step iii). A p-value of 0.01 (OFV −6.63 with 1 d.f.) was considered statistically significant; for steps (i-iii) of covariate tests, a less stringent critical p-value of 0.05 (OFV −3.84 with 1 d.f.) was applied. A nonlinear (power) model was used for models of continuous covariates, and a multiplicative model for categorical covariates. Standard errors reported are those obtained from the covariance step of the NONMEM program. Estimates of all parameters are given as their typical values and 95% confidence intervals (in parentheses).
Study Highlights.
What is the current knowledge on the topic?
Levels of cotinine, a metabolite and biomarker of nicotine exposure, are higher in young children exposed to secondhand smoke (SHS) compared to older children or adults. It is unknown whether this difference reflects different SHS exposure or agerelated changes in pharmacokinetics. Cotinine is metabolized primarily by CYP2A6.
What question did this study address?
The influence of age, sex, race and CYP2A6 genotype on cotinine half-life in infants and young children.
What this study adds to our knowledge?
The cotinine half-life in infants and young children is similar to that of adults and is influenced by CYP2A6 genotype but not independently age, sex or race.
How this might change clinical pharmacology and therapeutics?
Our data will help the interpretation of cotinine levels in SHS-exposure children. We provide novel information on developmental aspects of CYP2A6 activity in people.
ACKNOWLEDGMENTS
We thank Cecilia Yu, the research coordinator responsible for this study; Faith Allen, the data manager; Lisa Yu, Minjiang Duan, Trisha Mao and Lita Ramos, who performed the analytical chemistry; the nurses and dieticians on the CRC-CTSI research ward at San Francisco General Hospital and Marc Olmsted for editorial assistance.
This work was supported by the State of California Tobacco Related-Disease Research Program (grant no 15RT-0229), US Public Health Service grants DA020830, DA02277 and DA12393 from the National Institute on Drug Abuse, the University Endowed Chair in Addiction (RFT), CIHR (TMH-109787 RFT) CAMH, the CAMH foundation, the Canada Foundation for Innovation (#20289 and #16014) and the Ontario Ministry of Research and Innovation. Clinical studies were performed in part on the General Clinical Research Center at San Francisco General Hospital Medical Center with support of NIH/NCRR UCSF CTSI grant UL1 R024131.
Footnotes
CONFLICT OF INTEREST STATEMENT Dr. Tyndale has been involved in one-day workshops for Novartis and McNeil. As an Associate Editor for CPT, Dr. Tyndale was not involved in the review or decision process for this paper. Dr. Benowitz has been a consultant to several pharmaceutical companies 16 related to smoking cessation medications and has served as a paid expert witness in litigation against tobacco companies. The other authors have nothing to report.
References
- 1.Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 3.Dempsey DA, et al. Determination of Tobacco Smoke Exposure by Plasma Cotinine Levels in Infants and Children Attending Urban Public Hospital Clinics. Arch Pediatr Adolesc Med. 2012 doi: 10.1001/archpediatrics.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornelius MD, Goldschmidt L, Dempsey DA. Environmental tobacco smoke exposure in low-income 6-year-olds: parent report and urine cotinine measures. Nicotine Tob Res. 2003;5:333–339. doi: 10.1080/1462220031000094141. [DOI] [PubMed] [Google Scholar]
- 5.Tzatzarakis MN, et al. Hair nicotine/cotinine concentrations as a method of monitoring exposure to tobacco smoke among infants and adults. Hum Exp Toxicol. 2012;31:258–265. doi: 10.1177/0960327111422401. [DOI] [PubMed] [Google Scholar]
- 6.Derauf C, Katz AR, Easa D. Agreement between maternal self-reported ethanol intake and tobacco use during pregnancy and meconium assays for fatty acid ethyl esters and cotinine. Am J Epidemiol. 2003;158:705–709. doi: 10.1093/aje/kwg215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamer M, Ford T, Stamatakis E, Dockray S, Batty GD. Objectively measured secondhand smoke exposure and mental health in children: evidence from the Scottish Health Survey. Arch Pediatr Adolesc Med. 2011;165:326–331. doi: 10.1001/archpediatrics.2010.243. [DOI] [PubMed] [Google Scholar]
- 8.Irvine L, et al. What determines levels of passive smoking in children with asthma? Thorax. 1997;52:766–769. doi: 10.1136/thx.52.9.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collier AM, et al. Cotinine elimination and its use as a biomarker in young children involuntarily exposed to environmental tobacco smoke. Indoor Environ. 1994;3:353–359. [Google Scholar]
- 10.van den Anker JN, Schwab M, Kearns GL. Developmental pharmacokinetics. Handb Exp Pharmacol. 2011;205:51–75. doi: 10.1007/978-3-642-20195-0_2. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg H, van den Anker JN, Beijnen JH. Cytostatic drugs in infants: a review on pharmacokinetic data in infants. Cancer Treat Rev. 2012;38:3–26. doi: 10.1016/j.ctrv.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Etzel RA, Greenberg RA, Haley NJ, Loda FA. Urine cotinine excretion in neonates exposed to tobacco smoke products in utero. J Pediatr. 1985;107:146–148. doi: 10.1016/s0022-3476(85)80637-5. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey D, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and elimination kinetics in newborns. Clin Pharmacol Ther. 2000;67:458–465. doi: 10.1067/mcp.2000.106129. [DOI] [PubMed] [Google Scholar]
- 14.Leong JW, et al. The elimination half-life of urinary cotinine in children of tobacco-smoking mothers. Pulm Pharmacol Ther. 1998;11:287–290. doi: 10.1006/pupt.1998.0153. [DOI] [PubMed] [Google Scholar]
- 15.Jacob P, 3rd, Yu L, Wilson M, Benowitz NL. Selected ion monitoring method for determination of nicotine, cotinine and deuterium-labeled analogs: absence of an isotope effect in the clearance of (S)-nicotine-3',3'-d2 in humans. Biol Mass Spectrom. 1991;20:247–252. doi: 10.1002/bms.1200200503. [DOI] [PubMed] [Google Scholar]
- 16.Benowitz NL, Swan GE, Jacob P, 3rd, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin Pharmacol Ther. 2006;80:457–467. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Stable EJ, Herrera B, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280:152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- 19.Al Koudsi N, Hoffmann EB, Assadzadeh A, Tyndale RF. Hepatic CYP2A6 levels and nicotine metabolism: impact of genetic, physiological, environmental, and epigenetic factors. European journal of clinical pharmacology. 2010;66:239–251. doi: 10.1007/s00228-009-0762-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. Journal of Pharmacology and Experimental Therapeutics. 1994;270:414–423. [PubMed] [Google Scholar]
- 21.Shimada T, et al. Characterization of microsomal cytochrome P450 enzymes involved in the oxidation of xenobiotic chemicals in human fetal liver and adult lungs. Drug Metabolism and Disposition. 1996;24:515–522. [PubMed] [Google Scholar]
- 22.Parkinson A, Mudra DR, Johnson C, Dwyer A, Carroll KM. The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P 450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicology and applied pharmacology. 2004;199:193–209. doi: 10.1016/j.taap.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 23.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 24.Department of Health and Human Services, PHS. Vol. 2011. Washington DC: Government Printing Office; 2007. [Google Scholar]
- 25.Zhu AZ, et al. The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: the influence of genetics, race and sex. Cancer Epidemiol Biomarkers Prev. 2013 doi: 10.1158/1055-9965.EPI-12-1234-T. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mwenifumbo JC, et al. Novel and established CYP2A6 alleles impair in vivo nicotine metabolism in a population of Black African descent. Hum Mutat. 2008;29:679–688. doi: 10.1002/humu.20698. [DOI] [PubMed] [Google Scholar]
- 27.Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56:483–493. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 28.Dempsey D, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Ho MK, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85:635–643. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al Koudsi N, Ahluwalia JS, Lin SK, Sellers EM, Tyndale RF. A novel CYP2A6 allele (CYP2A6*35) resulting in an amino-acid substitution (Asn438Tyr) is associated with lower CYP2A6 activity in vivo. Pharmacogenomics J. 2009;9:274–282. doi: 10.1038/tpj.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacob P., 3rd Determination of the nicotine metabolites cotinine and trans-3'-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:267–276. doi: 10.1016/j.jchromb.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beal SL, Sheiner LB. NONMEM user's guide. University of California, San Francisco (CA): NONMEM Project Group; 1992. [Google Scholar]