Introduction
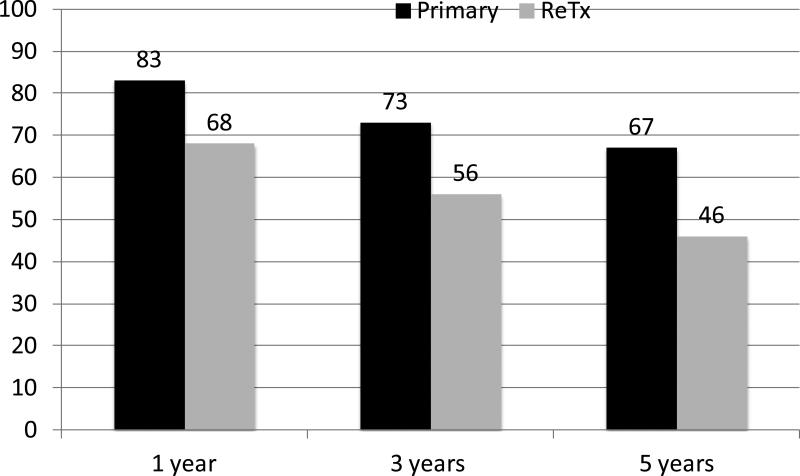
Donor livers are a scarce, life-saving resource. For patients whose lives depend upon liver transplantation, the policies defining priority for donor livers are of ultimate importance. Fair and just utilization of available livers requires that policy makers understand how to balance the needs and interests of each of the stakeholders. Although significant medical and surgical advances have been made in the last two decades, many patients who undergo liver transplantation will eventually have early complications or recurrent liver disease resulting in failure of their transplanted liver graft. When this occurs, repeat liver transplantation is often the only definitive treatment. Unfortunately, retransplantation has lower graft survival rates than primary transplant. [Figure 1] Numerous prognostic models have been developed to aid clinical decision-making as to whether or not pursuit of retransplantation can be justified based on the estimated survival after retransplantation. The fundamental principles of medical ethics i.e. autonomy, non-malfeasance, beneficence, justice and utility can further inform the rational application of prognostic models to retransplantation. Yet, to do so, will require a blunt and frank dialogue, within our transplant community and society at large, about the concepts of futility and rationing.
Figure 1.
Comparison of liver graft survival rates after primary and retransplantation at 1, 3, 5 years in the US for transplants performed 1997-2004, based on OPTN data as of September 2, 2011. [http://optn.transplant.hrsa.gov/latestData/rptStrat.asp, last accessed September 10, 2011.]
Medical and surgical advancements of the last 2 decades have established liver transplantation as a lifesaving intervention for patients with advanced liver disease that is now available in most regions of the world. In general, long term patient survival after liver transplantation is excellent, particularly when compared to the survival of patients with end stage liver disease without transplantation. For example, in the United States, patient survival after liver transplantation is 88%, 74% and 60% at 1, 5, and 10 years nationally, with single center reports of long term patient survival as high as 68% and 64% at 10 and 15 years, respectively.1, 2 The ubiquitous application of the procedure is limited by the scarcity of available liver grafts. Despite efforts to expand the donor pool and improve systems to triage liver grafts, death rates while waiting for liver transplantation remain high.1 Liver transplant candidates on the waiting list in the US with a MELD score of 15 and 40 have a mortality rate of 146 and 13,152 per 1000 patient-years, respectively, whereas the 1 year post-transplant mortality rate is 183.3 By comparison, patients undergoing repeat liver transplantation, have a 1 year mortality rate in the US of 264 deaths per 1,000 patient-years.4 Therefore, once decompensated liver disease occurs in the native liver or a liver graft, from an individual patient perspective, there is generally a net survival benefit with liver transplantation if a liver graft is available. Unfortunately, the gap between the supply of liver grafts and the demand for liver transplantation remains wide and without prospects to narrow in the foreseeable future.
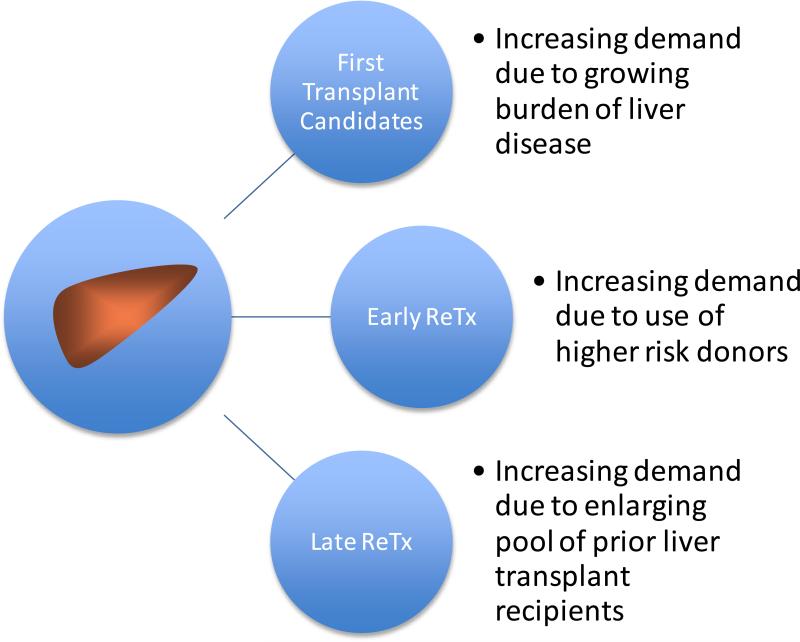
The global success of liver transplantation has extended the lives of many patients worldwide with an estimated 149,470 liver transplants performed in just the ten year period from 2000-2009.5 In the US, the Scientific Registry of Transplant Recipients estimated that as a result of the 88,160 liver transplants that were performed from 1988 to 2007, there were over 42,286 people living with a functioning liver graft in 2008. 6 Although estimates of the number of people living with a functional liver graft worldwide remains poorly quantified, assuming a 5 year survival rate of 40% to 70 % with an exponential survival distribution and an annual number of liver transplants of about 15,000 over the last 10 years, one can estimate that from the last decade alone, there would be 77,648 to 118,461 people worldwide living with a functioning liver graft in 2011. This large population of persons living with a functioning liver graft after a first transplant represents a growing pool of individuals who are at risk for graft failure, many of whom may eventually seek a second liver transplant. [Figure 2] Additionally, liver graft scarcity has motivated the transplant community to expand the donor pool by using liver grafts with a higher donor risk index (DRI) that are at greater risk of early graft failure from primary nonfunction (PNF), hepatic artery thrombosis (HAT) or ischemic type biliary injury. In the US during the period from 1999 to 2008, there was a 38% increase in the use of the lowest quality liver grafts (DRI>1.8) despite an increase in the risk of retransplantation with increasing DRI.1 In Europe, there was a steady rise in the median age (a key determinant of poor donor quality) of deceased liver donors from 40 to 50 years old during the same period. 7 Most patients with early graft complications will require retransplantation to achieve long term survival. In this context, the success of liver transplantation has the potential to exacerbate the scarcity of liver grafts by increasing demand from candidates seeking early and late retransplantation. Individual transplant centers can expect a greater demand for retransplantation which may justify a proactive review of center based resources and polices for these patients.
Figure 2.
Increasing demands for scarce liver grafts due to retransplantation (ReTx).
MELD, MELD allocation system and Retransplantation
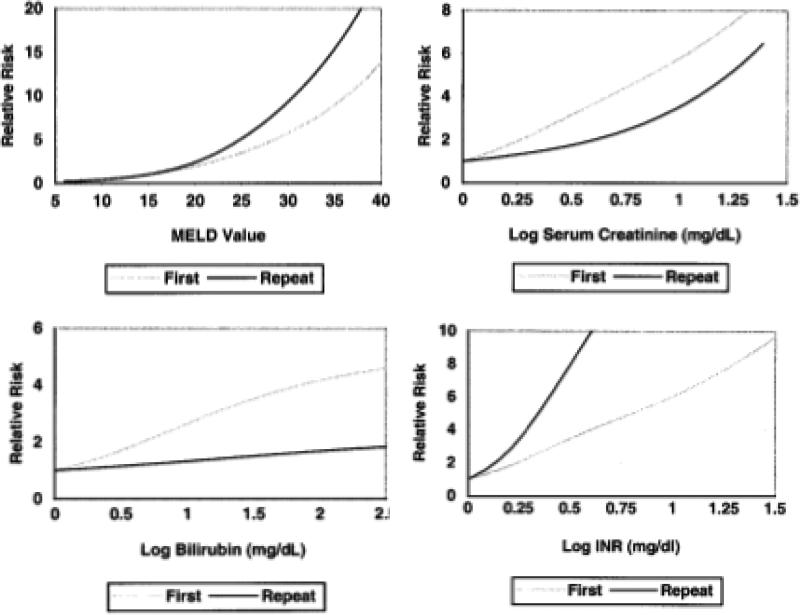
The majority of deceased donor liver graft allocation systems use an urgency based priority system with MELD as the primary measure of urgency. Allocation to retransplant candidates is generally under the same paradigm as to first transplant candidates. However, patients with very early liver graft failure from PNF or HAT are generally granted additional priority. For example, in the US patients meeting objective criteria for PNF or HAT within 7 days of transplant are granted the most urgent status, Status 1, and those with HAT occurring from 8 to 14 days are routinely granted an exceptional MELD score of 40. 8 Other retransplant candidates are prioritized by their laboratory MELD score. 8 In a study of the US population of candidates waiting for second (n=557) and first (n=8,431) liver transplants, Edwards et al found that the MELD score had reduced accuracy in predicting death while waiting for retransplant (c-statistic 0.79, 95% CI 0.75-0.85) as compared to first transplant (c-statistic 0.85, 95% CI 0.84-0.86). 9[Figure 3] When examining the impact of the individual laboratory components of the MELD, INR had a stronger correlation whereas creatinine and bilirubin each had a weaker correlation with waitlist mortality in retransplant candidates when compared to primary candidates.9 [Figure 3] Given the higher prevalence of intrinsic renal insufficiency and chronic cholestasis in prior liver transplant recpients10, this data suggest that worsening coagulopathy is an ominous sign in patients waiting for retransplantation and that the MELD score may underestimate the risk of waitlist mortality relative to primary transplant candidates.
Figure 3.
Comparison of waitlist mortality among candidates for first and repeat liver transplantation using MELD (a), Creatinine (b), Bilirubin (c), and INR (d). [Use with permission, Edwards et al Liver Transpl 2004;10:S10-6.
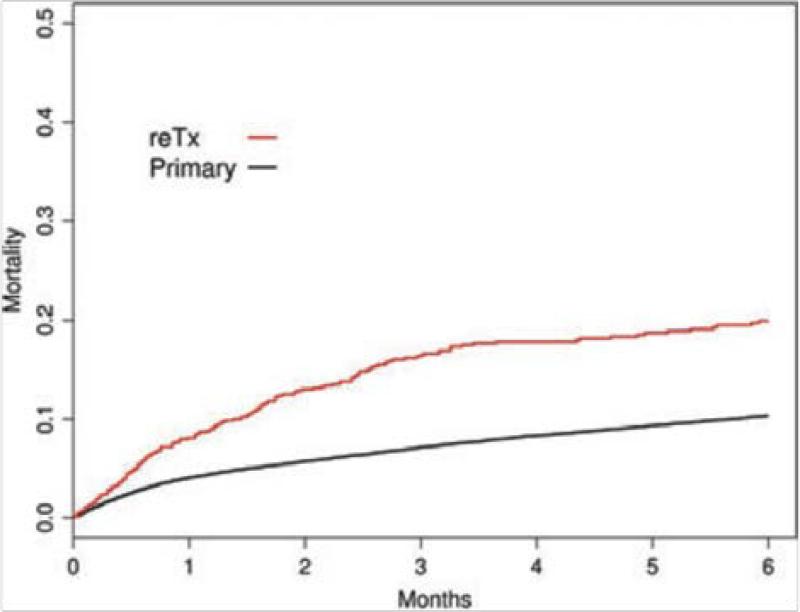
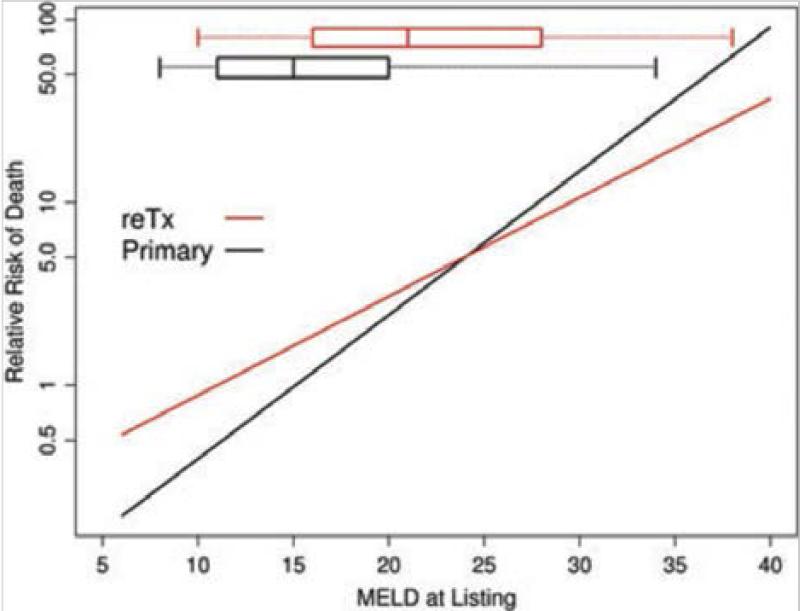
So are patients waiting for retransplantation disadvantaged under a MELD based allocation system? A recent study from Kim et al, concluded that implementation of MELD based allocation in the US did not adversely affect access to transplantation for retransplant candidates.10 This study of 2,081 retransplant and 45,943 primary transplant candidates, before and after implementation of MELD allocation in the US, found that despite higher waitlist mortality rates in retransplant candidates when compared to primary transplant candidates 1) the relative probability of a retransplant candidate surviving to receive a liver graft increased more under MELD allocation than for those waiting for a first liver transplant and 2) rates of waitlist death for both retransplant and primary transplant candidates were largely unaffected by MELD allocation. [Figures 4 and 5] Importantly, in a plot of waitlist mortality verses MELD score at the time of listing, retransplant candidates with a low MELD score (<25) had a greater mortality than primary candidates yet at higher MELD scores (25-40) the waitlist mortality was lower for retransplant candidates. [Figure 6] This latter analysis highlights the influence of bias introduced by candidate selection process. Further evidence of this selection bias is demonstrated in both the Edwards et al and Kim et al studies by comparing the demographics and MELD scores at listing of retransplant and primary transplant candidates. Retransplant candidates were younger and had higher MELD scores at listing than primary transplant candidates.9,10 From these studies, one can conclude that even though the MELD score underestimates waitlist of mortality in retransplant candidates, that with implementation of the MELD allocation system retransplant candidates had a greater relative increase in access to liver grafts compared to primary transplant candidates yet remained at a greater absolute risk of waitlist mortality, particularly when the MELD score is low. Furthermore, the candidate selection process has a powerful and important influence on access to retransplantation that is difficult to quantify in such retrospective studies of the allocation system alone.
Figure 4.
Mortality in six months following waitlist registration in primary and retransplantation (ReTx) [Used with permission, Kim et al Am J Transplant 2010;10:2652-7]
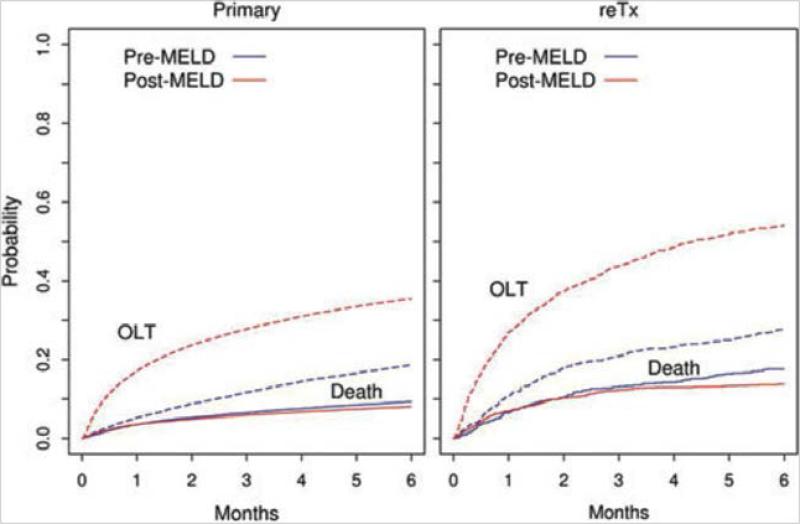
Figure 5.
Probability of liver transplantation (OLT) or death for primary and retransplantation (ReTx) candidates in the pre- and post-eras. Solid curves represent death and dotted ones LTx. [Used with permission, Kim et al Am J Transplant 2010;10:2652-7]
Figure 6.
Relation between listing and MELD score and waiting list mortality in primary transplant and retransplant candidates (ReTX). Bars and whiskers represent median, 25 to 75 percentiles and 2 to 98 percentile of MELD score at listing. Primary=black, ReTX=red. [Used with permission, Kim et al Am J Transplant 2010;10:2652-7]
Several factors outside of the liver allocation system can influence which liver transplant recipients with liver graft failure will ultimately undergo retransplantation. These include several factors related to the individual patient, physician judgment, transplant center policies and experience as well as geographic donor organ availability. Once graft failure is recognized, the patient's desire for and ability to endure retransplantation is assessed. If the patient seeks retransplantation and the clinical care team endorses it, the next step typically is a formal medical, surgical and psychosocial evaluation. If the patient is deemed an appropriate candidate and is registered on the waiting list for retransplantation, appeals for MELD exceptions can be considered based on special circumstances. Whether such an appeal is submitted and accepted can be influenced by the patient's degree of advocacy, the physician's medical judgment, the merits of the appeal and the reviewer's interpretation of that appeal. Retransplant candidates are generally sicker and seek a more technically demanding procedure than primary candidates. These factors can have a varied influence on the surgical team's likelihood of accepting an available liver graft that is based on their experience and their perceived probability of another potentially more appropriate liver graft offer. 11, 12 Geographic variations in organ availability and practice patterns can play a large role in graft acceptance. 13 Lastly, while patients wait for an appropriate liver graft, repeated assessments of the patient's medical status and continued desire for the procedure inform bedside clinical decisions. This complex process is highly reliant on sound and ethical medical judgment and realistic patient expectations within the context of severe liver graft scarcity.
Application of Medical Ethics to Retransplantation
Rational application of the fundamental principles of medical ethics can assist the transplant community in liver graft allocation policy development and bedside decision-making. [Table 1] As stewards of a scarce public resource, the transplant community must balance the care for the individual patient with our obligations to society at large. Fulfillment of this duty requires reconciling these potentially conflicting roles. Although liver graft allocation policies should strive to be objective and fair for all those in need, the complex process outlined above entails several factors that are difficult or impossible to address objectively. To preserve the public's and our patient's trust, polices should be as transparent and open as practically possible. The availability of donor organs for transplantation is highly dependant on the public's willingness to donate organs. Loss of public trust, risks lower organ donation rates and reduced access to transplant for our patients. Society at large is therefore an important stakeholder, yet it is our patients whose lives hang in the balance. As most patients with liver graft failure would have some potential benefit from retransplantation, how can the clinical transplant team decline a patient's request for the procedure? When and how can we say no?
Table 1.
Working Definitions of Ethical Principles
| Ethical Principle | Working Definition |
|---|---|
| Autonomy | The right of the patient to accept or refuse any treatment, relates to the patients being able to make informed decisions on their own behalf, rather than being subjected to paternalistic decisions being made for them by health care providers |
| Non-malfeasance | Doing no harm or, even more appropriately, no further harm |
| Beneficence | Implies that the healthcare providers must provide benefits in the best interest of the individual patient while balancing benefit and risks |
| Justice | Implies the concern and duty to distribute limited health resources equally with in a society and the decision of who gets what treatment (fairness and equality) |
| Utility | Maximizing the net benefit to society from a, generally scarce, resource |
| Dignity and Honesty | The right of patients to be treated with dignity and information should be honest without suppressing important facts |
Such decision-making unfortunately is never simple or easy. Precedence exists in other historical examples of scarce resources such as the advent of insulin in 1922, penicillin in 1942, hemodialysis in early 1960's and antiretroviral HIV medications in the 1980's.14, 15 Lessons from these experiences have informed medical ethicists and clinicians of the pitfalls and challenges when distributing scarce life saving medical treatments. Such decisions can be inherently value laden and benefit from efforts to avoid prejudicial bias introduced by subjective assessments of social worth. Medical criteria are not completely value free but often can inform decision-making once a paradigm is chosen, i.e. whether to give priority to the most urgent cases, to those with the best chance of survival or to those with the best chances of quality of life afterwards. Although most liver transplant allocation systems are currently based the degree of urgency, predicted outcome after the procedure often plays both explicit and implicit roles. For example, in the US, patients with hepatocellular carcinoma are granted additional priority while their tumor stage is within the Milan criteria and the predicted risk of tumor recurrence after transplant remains reasonably low. 8 However, if the tumor burden progresses beyond the Milan criteria, the additional priority for transplant is removed. Even though the individual patient with excess tumor burden has the potential of a longer life with a transplant as opposed to that without a transplant, objective policy is in place to redirect liver grafts to other patients. Placed in the context of medical ethics principles, distributive justice (duty to distribute limited health resources equally with in a society), utility (duty to maximize the net benefit to society) and nonmalfeasance (doing no harm) guide this policy with patient autonomy (right of the patient to accept or refuse any treatment) and beneficence (best interest of the individual patient) taking a secondary role. [Table 1] This paradigm may serve as guideline for considering decision-making in candidate selection and allocation policy for retransplantation.
When considering candidate selection in retransplantation, there are two seminal decision nodes 1) the decision to place the patient on the waiting list and 2) the decision to proceed with retransplantation when a liver graft becomes available. In retransplant candidate selection decisions, rational approaches can be elucidated though the systematic application of ethical principles. The autonomy of individual patients to accept or refuse retransplantation should preclude proceeding with the procedure if the patient does not desire it. However, ethicists addressing similar issues in the context autonomy and end-of-life care, have highlighted the limits of individual autonomy stating that refusing to let people do things to you is one thing but demanding that people do things for you is another, particularly if doing so may be at the expense of others.16 In this light, a patient with graft failure rightfully can refuse retransplantation yet the principle of autonomy would not support the patient's demand to undergo the procedure. The principle of non-malfeasance provides little guidance in candidate selection decision-making unless there is no feasible chance of survival with retransplantation. It does however, serve as an important reminder that retransplantation has greater morbidity and mortality than primary transplant. A physician's duty to beneficence obliges one to balance the risks and the benefits of retransplantation in the individual patient with liver graft failure. This assessment is dynamic and dependent on the clinical status of the patient over time and highly reliant on the professional judgment of physician. Dignity and honesty are imperative to avoid degradation of patient-physician trust.
This process begins with a commitment to avoid placing patients on the waiting list that the clinical team knows that they would not to proceed with retransplantation. Additionally, it requires a frank discussion with listed patients that over time they may become too sick for the procedure. When making decisions to list a candidate or to proceed when a graft is available, the risks of retransplantation may exceed the benefits in the clinical team's judgment. In this case, there are reasonable grounds to not proceed with the procedure. The more common and more controversial scenario is that there is a potential net benefit to the individual patient but that expected benefit is significantly less than that of another patient. Here, decision makers can rely on the principles of distributive justice and utility.
There at least two aspects of distributive justice and utility that may apply to decision making in retransplantation.17, 18 Firstly, one may consider that retransplant candidates may be more or less deserving than primary transplant candidates. Apart from acute liver graft failure, the arguments that retransplant candidates are more deserving than primary candidates are unconvincing when closely scrutinized. The arguments for higher priority for the retransplant candidate suggest that there is an obligation of the system to make up for suffering endured by the patient due to a failed first transplant or that the transplant teams have a duty not to abandon their patients.17, 19 In the setting of acute liver graft failure, currently polices described above, generally allow for additional priority within the pool of all liver transplant candidates. However, Ubel et al have argued that on the basis of having received a prior transplant, retransplant candidates are neither more nor less deserving than primary transplant candidates. They contend that retransplant candidates with iatrogenic causes of liver graft failure (i.e. PNF, HAT) are not more deserving than first liver transplant candidates with native liver failure from natural causes. Additionally, they argue that retransplant candidates are not less deserving of a second liver graft, while others are waiting for a first transplant, because the retransplant candidate may have previously been denied equal access to other public resources such as primary health care, education or income. Whether or not this broad interpretation of balancing non-health care injustices with additional heath care resources holds is arguable.
The second and more convincing argument is based on utility. Ubel et al argue that a utilitarian interpretation of distributive justice (the greatest utility to society) justifies lower priority to the transplant candidate who has a lower predicted graft survival.17 They cite the generally lower graft survival rates in retransplant recipients as grounds for reduced priority for retransplant candidates. This recommendation would support the incorporation of survival models into liver graft allocation for patients seeking retransplantation in a manner similar to that for patients with hepatocellular carcinoma. That is, while the predicted post-transplant survival after retransplantation is estimated to be similar to primary transplant candidates, retransplant candidates would have at least equal urgency based priority to a liver graft. Yet at some point, when the predicted survival reaches a minimum threshold, the goal of the allocation system would no longer be to promote equivalent priority among retransplant and primary transplant candidates but would rather be to grant a relative advantage to primary transplant candidates. The mechanics of such a change in allocation may be complex yet evidence based approaches with serial re-evaluations, similar to what has been done with HCC priority modifications in the US, would serve as a path forward.20
Absent formal policy measures guiding decision making in allocation of liver grafts to retransplant candidates, the decision of whether or not to proceed with use of a liver graft for retransplantation relies heavily on the professional judgment of the bedside clinicians. No change in allocation can or should replace this clinical judgment yet the emotional involvement of the clinical team can make difficult decisions about the appropriate use of liver grafts even harder. The transplant clinician's role as steward of a scarce public resource is best not enacted at the bedside but unfortunately it often must. When this occurs, the risk-benefit discussions with the patient and family can take two paths. The hard road entails a frank and decisively more difficult explanation that despite some potential benefit to the patient, the liver graft will go to another patient with a greater expected benefit. The easier road is a conversation about the patient's individual risks and benefits, which cites to some degree, the futility of proceeding with retransplantation. However, in the vast majority of candidates, retransplantation is not futile and care decisions made on this basis can obscure an honest and frank discussion with the patient.
Futility and Retransplantation
The concept of medical futility, though often cited as the rationale for withholding an intervention, may obscure or limit the application of more appropriate ethical principles in decision-making regarding retransplantation. Notably, a practical working definition for futility remains quite controversial.16, 21, 22 The components of futility that are generally agreed upon include the inability to achieve a specific interventional related goal and the probability of achieving that goal.23 In liver transplantation, the intervention related goal most often is long term liver graft and patient survival. Additional goals, such as avoiding undue harm to the recipient and returning to an improved quality of life are no less important, though are value laden, and therefore more vexing to apply in this context. The dominant challenge in the application of futility in liver transplantation is one of perspective. From the patient's perspective, any potential benefit might exclude futility. From the ethicist perspective, lack of success of 100 similar cases has been suggested as a working threshold to define futility. 21, 22 From a societal perspective, given the current liver graft scarcity, it is unlikely that survival rates as low as 1% would be considered satisfactory. The transplant community has struggled to define an appropriate minimum threshold for graft and patient survival after retransplantation. Although several minimum thresholds have been considered, none have suggested graft survival rates less than 50% at 1 year. 24-26 Given the discrepant perspectives of what defines retransplant success, futility is an impractical and potentially divisive principle to apply in bedside decision-making and care discussions with patients.
Futility verse Rationing
Although there is some overlap in the concepts of futility and rationing, the distinctions between them are illustrative in how they may be applied to decision-making in retransplantation. [Table 2] The juxtaposition of these overlapping concepts has been articulated by Jecker and Schneiderman.27 When rationing criteria refer to medical benefit, the meaning of futility and rationing share common features. For example, when access to an intervention is based on medical benefit and that estimated benefit is very low, both futility and rationing might be implicated to withhold that intervention. Futility is generally applied to decisions regarding individual patients yet rationing can be applied to the general population, subgroups or individuals. Rationing is defined by the principle of maximizing distributive justice within a population based on the degree of scarcity whereas, futility is defined by the available empirical evidence that predicts the likelihood of not achieving a desired outcome. Lastly, rationing implies an influence of resource scarcity whereas futility does not. That is, under conditions of scarcity, even beneficial interventions may be denied based on rationing.
Table 2.
Comparison of the Characteristics of Futility and Rationing
| Characteristic | Futility | Rationing |
|---|---|---|
| Generally applied to | Individual | Individual or a Population |
| Based on | Threshold for unacceptable likelihood of success | Distributive justice and fair allocation of resources |
| Defined by | Empirical evidence | Degree of scarcity |
| Requires Scarce Resource | No | Yes |
| Could reasonably be based on cost | No | Yes |
| Often refers to Therapeutic benefit | Yes | Yes |
| Requires assessment of Therapeutic Benefit | Yes | No |
Rationing and Retransplantation
When considering candidate selection and allocation decisions in retransplantation, rationing provides a more practical and forthright construct than futility. Unlike other medical rationing, the indisputable gap between the supply and demand for scarce donor organs, makes rationing in transplant decision-making an inevitable reality. Although cost and capacity for higher volume within transplant centers could also create a need to ration access to liver transplantation, the lack of sufficient organs to satisfy the demand establishes the need for rationing as a fact rather than a hypothetical case. Using organ scarcity as the foundation of decision-making lays the framework for stakeholder expectations. Defining minimum thresholds for success remains integral to decision-making but the discrepant perspectives of how success is defined is much less troublesome within the construct of rationing than that of futility. When rationing is the expectation of all the stakeholders, including the patient, the goal of retransplantation is not merely to achieve any possible benefit for an individual patient regardless of how small. Rather, the goal is to exceed a pre-determined, minimum expected graft survival. In doing this, bedside decision-making and conversations with patients and their families are no less difficult but are likely to be more forthright and honest.
Allocation polices based on incorporating outcome measures for retransplant patients would be consistent with the principle of utility and the utilitarian application of distributive justice. If created in a fair and transparent manner, such polices have the potential to minimize discrepant expectations of patients and the transplant team during bedside decision-making. Patients with a poor expected outcome would not be placed on the waiting list for retransplantation. Patients already on the waitlist who had clinical deterioration and resultant poor expected outcome with retransplantation would either be reduced in priority, be made temporally inactive or be removed from the waiting list.
There are at least two major challenges when applying rationing and outcome measures in retransplant medical decision making: 1) determining what is the minimum threshold for acceptable graft survival and 2) who determines that threshold. The transplant community must work with the public to identify what is an appropriate minimum threshold for graft survival after retransplantation. Surveys of the general public indicate support of a pluralistic approach, balancing utilitarianism with other principles including equal opportunity and personal responsibility yet none quantified a minimum graft survival. In a survey study, Ratcliff et al quantified the tradeoffs of utilitarianism and other principles using a conjoint analysis. In their survey conducted in the United Kingdom (UK), 303 university employees were asked to allocate organs among two groups that varied based on several potential patient characteristics. Among the characteristics tested, expected survival after transplant was the strongest relative attribute (32%), then alcoholic etiology of liver disease (30%), age (24%), time on waiting list (6%), and prior transplantation (6%). 28 In another of survey of 138 members representing the general public in Pittsburgh Pennsylvania, Ubel et al found that poor survival after liver transplant was associated with a preference for a lower priority for transplant, an effect that was stronger if the candidate was seeking retransplantation. 18 Another survey study in the UK that included 1000 members of the general public found that a candidate's age and outcome were the two most important factors when allocating liver grafts. 29
The liver transplant community has been working to define a minimum acceptable threshold for graft survival at national consensus conferences. A national colloquium in the United Kingdom in 1998 concluded that in that country a patients should be offered liver transplantation only if there is an expected 5 year survival of greater than 50%.24 These criteria were recommended for decisions regarding placing patients on the waiting list as well as for removing them from the waiting list. Additionally, they recommended that allocation should aim to maximize outcome in preference to allowing every potential recipient an equal chance to receive an organ. With regard to retransplantation, this group suggested that their 50% 5 year survival guideline should be relaxed for early retransplant candidates i.e. those with PNF, but should hold firm for late retransplantation.24 In 2008, the UK Liver Advisory Group concurred with the previous UK recommendation of a 5 year 50% survival threshold yet cautioned against formal de-listing criteria until evidence based guidelines could be developed.25 A US based consensus conference in December 2003 also addressed this topic. 26 Although a minimum graft survival threshold of approximately 50% was suggested, the time point after transplantation was left undefined but 1 and 5 year time points were considered.26 For allocation, the MELD based urgency based system was lauded yet in the case of retransplantation, a modified system that incorporated both pre-and post-transplant survival was considered. The allocation schema for patients with large hepatocellular carcinomas was identified as a potential road map for developing de-listing criteria. As discussed above, that schema provides priority to hepatocellular cancer within Milan criteria but removed the additional priority if the tumor exceeds Milan criteria. The group acknowledged that since transplant candidates with poor estimated post-transplant survival represent those with the highest mortality without transplant, any criteria to define a minimum acceptable survival threshold should be strongly evidence based and bias in favor of proceeding with transplantation.26 Importantly, all of these conferences raised concerns about the application of models with limited accuracy in predicting post-transplant survival.24-26 Although it is clear that a consensus on a minimum survival threshold is difficult to achieve, it is imperative to work toward that goal. A starting point for this discussion would be to develop guidelines to avoid listing retransplant candidates with 5 year graft survival below 50% and to de-list or inactivate retransplant candidates when the expected 1 year survival falls below 50%.
Prognostic Models in Retransplantation
Several mathematical models have been developed to predict survival after retransplantation using multivariate regression methods and were previously and recently summarized.30, 31 Among the different predictive factors examined, preoperative serum total bilirubin and serum creatinine have consistently provided the most significant prognostic power. Other important factors identified include HCV status, recipient age, donor age, warm and cold ischemia times, UNOS status (ICU, hospital ward, ambulatory), mechanical ventilator support, and interval to retransplantation. Post-retransplantation survival rates are higher with younger recipients, longer intervals between transplants (> 2 months), and retransplantation before severe decompensation. Whereas patients with emergent/urgent early (< 1 week up to 2 months) retransplantation, graft primary non-function, physical debility, MELD score > 25, creatinine > 2mg/dL, total bilirubin > 13 mg/dL, Child-Turcotte-Pugh score>/=10, donor age > 60 years, donation after cardiac death, cold ischemic time > 12 hours, warm ischemic time> 75 minutes, fared worse. Such prognostic models can be used to risk stratify candidates for repeat liver transplantation with, at best, moderate accuracy, c-statistic about 0.65 for 1 year survival.32 The most significant challenges to further improvement in the accuracy of these models include the inability to model random operative and perioperative events that are by definition unpredictable. Additionally, as the vast majority of these models were developed on patients that already had undergone retransplantation, not in patients being considered for the procedure, a selection bias may limit the generalizability to clinical decision-making when selecting candidates to place on the waiting list. Improved evidence-based models developed in liver recipients not yet listed for retransplant are needed. Prospective registries of prior transplant candidates being considered for relisting may aid this goal. This is particularly true with elective retransplantation for chronic graft failure or in geographic areas of extreme organ shortage, where the clinical status of the candidate can change during a prolonged waiting period. However, once a candidate is on the waiting list for retransplantation, the prognostic models can be used to inform decision-making of whether or not to proceed with retransplantation when a liver graft becomes available. Models that account for both patient and donor characteristics, such donor age and donation after cardiac death, are more complex but may provide a more accurate estimate the probability of graft and patient survival after retransplantation. Such models already exist, yet their complexity has limited their general acceptance.33
Conclusions
The concept of medical futility, though often cited as the rationale for withholding an intervention, may obscure or limit the application of more appropriate ethical principles in decision-making in retransplantation. When considering candidate selection and allocation decisions in retransplantation, rationing provides a more practical and forthright construct than futility. Using organ scarcity as the origin of decision-making lays framework for stakeholder expectations. Defining the minimum thresholds for success remains integral to decision-making but discrepant perspectives of how success is defined are much less troublesome within the construct of rationing than within that of futility. When rationing is the expectation of all the stakeholders, including the patient, the goal of retransplantation is not merely to achieve any possible benefit to an individual patient but rather the goal is to exceed a pre-determined minimum expected graft survival. Although it is clear that a consensus minimum threshold for graft survival is difficult to achieve, it is imperative to work toward that goal. A starting point for this discussion would be to develop guidelines to avoid listing retransplant candidates with an expected 5 year graft survival below 50% and to de-list or inactivate retransplant candidates when the expected 1 year survival falls below 50%. An evidence based, utilitarian approach with serial re-evaluations, similar to what was done with HCC priority modifications in the US, may serve as a path forward.
Key points.
In the context of scarcity of available liver grafts, rationing provides a more useful construct than futility when approaching decision-making in candidate selection and organ allocation for retransplantation.
Assessments of public preferences, ethicist's analysis and consensus conferences in the transplant professional community suggest support for outcome based candidate and organ allocation decisions in liver retransplantation.
Although it is clear that a consensus on a minimum threshold for graft survival after retransplantation is difficult to achieve, a starting point for this discussion would be to develop guidelines to avoid listing retransplant candidates with a 5 year graft survival below 50% and to de-list or inactivate retransplant candidates when the expected 1 year survival falls below 50%.
Acknowledgments
Funding: This work was funded in part by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (DK076565) and by the Agency for Heathcare Reseach and Quality (DK076565).
Abbreviations
- HAT
Hepatic artery thrombosis
- HCC
Hepatocellular carcinoma
- MELD
Model for End Stage Liver Disease
- PNF
Primary non-function
- ReTx
retransplant
- UK
United Kingdom
- US
United States
References
- 1.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999-2008. Am J Transplant. 2010 Apr;10(4 Pt 2):1003–19. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 2.Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, et al. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg. 2005 Jun;241(6):905–16. doi: 10.1097/01.sla.0000164077.77912.98. discussion 16-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005 Feb;5(2):307–13. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 4.OPTN [September 2,2011];OPTN/STRR Annual Report Table 9.7a. http://wwwustransplantorg/annual_reports/current/907a_prevorgtx_lihtm.
- 5. [September 2, 2011];Global observatory on donation and transplantation World Transplant Data Reports. wwwtransplant-observatoryorg.
- 6.OPTN [September 2, 2011];OPTN/SRTR Annual Report Data Tables. http://wwwustransplantorg/annual_reports/current/data_tables_section9htm. 9/2/2011.
- 7. [September 2, 2011];Eurotransplant. Eurotransplant Annual Report 2010. http://wwweurotransplantorg/cms/indexphp?page=annual_reports.
- 8.OPTN [September 2, 2011];Organ Distribution: Allocation of Livers. http://optntransplanthrsagov/PoliciesandBylaws2/policies/pdfs/policy_8pdf.
- 9.Edwards E, Harper A. Does MELD work for relisted candidates? Liver Transpl. 2004 Oct;10(10 Suppl 2):S10–6. doi: 10.1002/lt.20271. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Larson JJ, Lim YS, Kim WR, Pedersen RA, Therneau TM, et al. Impact of MELD on waitlist outcome of retransplant candidates. Am J Transplant. 2010 Dec;10(12):2652–7. doi: 10.1111/j.1600-6143.2010.03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reese PP, Yeh H, Thomasson AM, Shults J, Markmann JF. Transplant center volume and outcomes after liver retransplantation. Am J Transplant. 2009 Feb;9(2):309–17. doi: 10.1111/j.1600-6143.2008.02488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volk ML, Reichert HA, Lok AS, Hayward RA. Variation in organ quality between liver transplant centers. Am J Transplant. 2011 May;11(5):958–64. doi: 10.1111/j.1600-6143.2011.03487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation. 2011 Feb 27;91(4):479–86. doi: 10.1097/TP.0b013e3182066275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blagg CR. The early history of dialysis for chronic renal failure in the United States: a view from Seattle. Am J Kidney Dis. 2007 Mar;49(3):482–96. doi: 10.1053/j.ajkd.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 15.McGough LJ, Reynolds SJ, Quinn TC, Zenilman JM. Which patients first? Setting priorities for antiretroviral therapy where resources are limited. Am J Public Health. 2005 Jul;95(7):1173–80. doi: 10.2105/AJPH.2004.052738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baily MA. Futility, autonomy, and cost in end-of-life care. J Law Med Ethics. 2011;39(2):172–82. doi: 10.1111/j.1748-720X.2011.00586.x. Summer. [DOI] [PubMed] [Google Scholar]
- 17.Ubel PA, Arnold RM, Caplan AL. Rationing failure. The ethical lessons of the retransplantation of scarce vital organs. JAMA. 1993 Nov 24;270(20):2469–74. doi: 10.1001/jama.270.20.2469. [DOI] [PubMed] [Google Scholar]
- 18.Ubel PA, Loewenstein G. The efficacy and equity of retransplantation: an experimental survey of public attitudes. Health Policy. 1995 Nov;34(2):145–51. doi: 10.1016/0168-8510(95)00714-4. [DOI] [PubMed] [Google Scholar]
- 19.Eguchi S, Soyama A, Mergental H, van den Berg AP, Scheenstra R, Porte RJ, et al. Honoring the contract with our patients: outcome after repeated re-transplantation of the liver. Clin Transplant. 2011 Mar-Apr;25(2):E211–8. doi: 10.1111/j.1399-0012.2010.01389.x. [DOI] [PubMed] [Google Scholar]
- 20.Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant. 2010 Jul;10(7):1643–8. doi: 10.1111/j.1600-6143.2010.03127.x. [DOI] [PubMed] [Google Scholar]
- 21.Schneiderman LJ, Jecker NS, Jonsen AR. Medical futility: its meaning and ethical implications. Ann Intern Med. 1990 Jun 15;112(12):949–54. doi: 10.7326/0003-4819-112-12-949. [DOI] [PubMed] [Google Scholar]
- 22.Schneiderman LJ, Jecker NS, Jonsen AR. Medical futility: response to critiques. Ann Intern Med. 1996 Oct 15;125(8):669–74. doi: 10.7326/0003-4819-125-8-199610150-00007. [DOI] [PubMed] [Google Scholar]
- 23.Berger JT, Rosner F, Potash J, Kark P, Farnsworth P, Bennett AJ. Medical futility: towards consensus on disagreement. HEC Forum. 1998 Mar;10(1):102–18. doi: 10.1023/a:1008827206192. [DOI] [PubMed] [Google Scholar]
- 24.Neuberger J, James O. Guidelines for selection of patients for liver transplantation in the era of donor-organ shortage. Lancet. 1999 Nov 6;354(9190):1636–9. doi: 10.1016/S0140-6736(99)90002-8. [DOI] [PubMed] [Google Scholar]
- 25.Neuberger J, Gimson A, Davies M, Akyol M, O'Grady J, Burroughs A, et al. Selection of patients for liver transplantation and allocation of donated livers in the UK. Gut. 2008 Feb;57(2):252–7. doi: 10.1136/gut.2007.131730. [DOI] [PubMed] [Google Scholar]
- 26.Olthoff KM, Brown RS, Jr., Delmonico FL, Freeman RB, McDiarmid SV, Merion RM, et al. Liver Transpl; Summary report of a national conference: Evolving concepts in liver allocation in the MELD and PELD era.; Washington, DC, USA. December 8, 2003; Oct, 2004. pp. A6–22. [DOI] [PubMed] [Google Scholar]
- 27.Jecker NS, Schneiderman LJ. Futility and rationing. Am J Med. 1992 Feb;92(2):189–96. doi: 10.1016/0002-9343(92)90111-n. [DOI] [PubMed] [Google Scholar]
- 28.Ratcliffe J. Public preferences for the allocation of donor liver grafts for transplantation. Health Econ. 2000 Mar;9(2):137–48. doi: 10.1002/(sici)1099-1050(200003)9:2<137::aid-hec489>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Neuberger J, Adams D, MacMaster P, Maidment A, Speed M. Assessing priorities for allocation of donor liver grafts: survey of public and clinicians. BMJ. 1998 Jul 18;317(7152):172–5. doi: 10.1136/bmj.317.7152.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biggins SW, Beldecos A, Rabkin JM, Rosen HR. Retransplantation for hepatic allograft failure: prognostic modeling and ethical considerations. Liver Transpl. 2002 Apr;8(4):313–22. doi: 10.1053/jlts.2002.31746. [DOI] [PubMed] [Google Scholar]
- 31.Carrion JA, Navasa M, Forns X. Retransplantation in patients with hepatitis C recurrence after liver transplantation. J Hepatol. 2010 Nov;53(5):962–70. doi: 10.1016/j.jhep.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Rosen HR, Prieto M, Casanovas-Taltavull T, Cuervas-Mons V, Guckelberger O, Muiesan P, et al. Validation and refinement of survival models for liver retransplantation. Hepatology. 2003 Aug;38(2):460–9. doi: 10.1053/jhep.2003.50328. [DOI] [PubMed] [Google Scholar]
- 33.Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009 Apr;9(4 Pt 2):970–81. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]