Abstract
We conducted a cluster-randomized trial to assess the impact of a school-based water treatment, hygiene, and sanitation program on reducing infection with soil-transmitted helminths (STHs) after school-based deworming. We assessed infection with STHs at baseline and then at two follow-up rounds 8 and 10 months after deworming. Forty government primary schools in Nyanza Province, Kenya were randomly selected and assigned to intervention or control arms. The intervention reduced reinfection prevalence (odds ratio [OR] 0.56, 95% confidence interval [CI] 0.31–1.00) and egg count (rate ratio [RR] 0.34, CI 0.15–0.75) of Ascaris lumbricoides. We found no evidence of significant intervention effects on the overall prevalence and intensity of Trichuris trichiura, hookworm, or Schistosoma mansoni reinfection. Provision of school-based sanitation, water quality, and hygiene improvements may reduce reinfection of STHs after school-based deworming, but the magnitude of the effects may be sex- and helminth species-specific.
Introduction
Over two billion individuals worldwide are infected with soil-transmitted helminths (STHs) with school-aged children exhibiting the greatest STH morbidity.1,2 The STH infection is directly related to fecal exposure, either through ingestion or skin exposure. Chronic intense infection can adversely affect growth and cognitive development in school-aged children.3 Fortunately, much of the morbidity associated with STH infection can be reversed cheaply and safely by periodic chemotherapy, typically using anthelmintics.4–8 Treatment of school-aged children—usually through school-based deworming—can also reduce infection rates among untreated children and community members.9,10
In the absence of control measures aimed at reducing exposure, treatment of STH infections is followed by re-infection, necessitating repeated treatments.11,12 The benefits of such treatment can be sustained by efforts to reduce environmental exposure to infection through improved sanitation and hygiene behaviors.13,14 Although household coverage of water and sanitation is measured by the United Nations Children's Fund (UNICEF)/World Health Organization (WHO) Joint Monitoring Program for Water and Sanitation, little is known about global access of water, sanitation, and hygiene (WASH) in schools.15 In 2008, UNICEF estimated that only 46% of schools in their priority countries had water supply and 37% had toilets.16
Few studies have assessed the impact of a school WASH program in mitigating STH infection. A recent meta-analysis of the effect of sanitation on STH infection reported mainly cross-sectional surveys of low quality17; only two studies reported results on intensity of STH infection and few intervention studies were identified. Availability and use of sanitation facilities was associated with significant protection against STH infection: the overall odds ratios (ORs) were 0.54 (95% confidence intervals [CIs]: 0.43–0.69) for Ascaris lumbricoides, 0.58 (CI: 0.45–0.75) for Trichuris trichiura, and 0.60 (CI: 0.48–0.75) for hookworm.
Here, we report the results of a cluster-randomized trial that investigated the impact of a school-based WASH program in reducing reinfection with STH species after anthelmintic treatment compared with pupils that received deworming alone. The study was nested within a larger trial assessing the health impact of improved access to school-based WASH on absenteeism and diarrheal diseases.18
Methods
Background and study design.
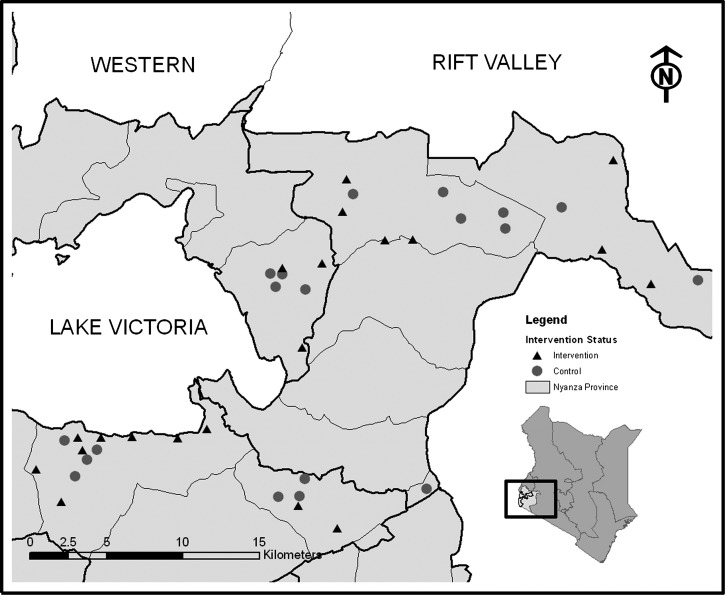
This investigation was a cluster-randomized trial assessing the impact of improved school WASH access on STH infection of primary school pupils. We compared infection patterns of STH among pupils in schools that received deworming plus a comprehensive school-based water treatment, sanitation, and hygiene intervention (arm 1: intervention) to those who received deworming only (arm 2: control). The investigation was conducted in 40 government primary schools in Nyanza Province, western Kenya, between 2007 and 2009 (Figure 1). The primary study outcomes were the prevalence and intensity of four common STH species: hookworms (Ancylostoma duodenale and Necator americanus), roundworm (A. lumbricoides), and whipworm (T. trichiura). Secondary outcomes included the prevalence and egg count of the trematode, Schistosoma mansoni.
Figure 1.
Schools randomly selected for inclusion in the study of helminth reinfection in Nyanza Province, Kenya.
Study area and school selection.
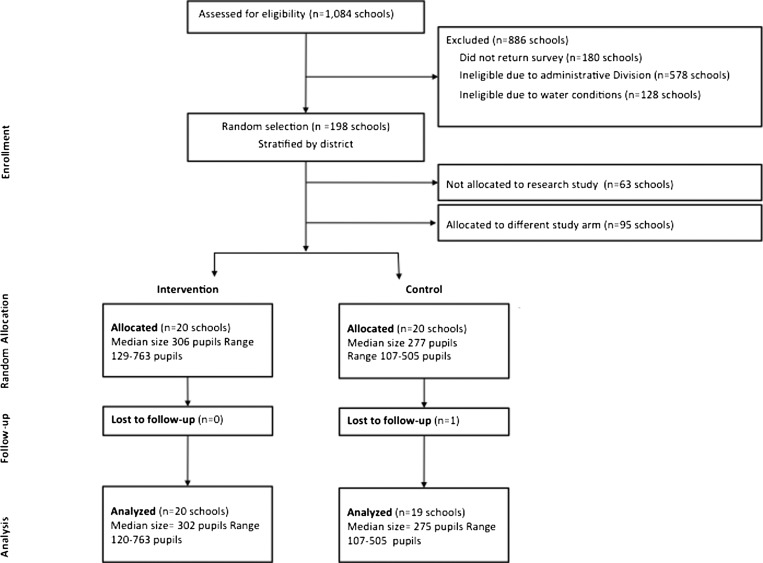
This study took place in eastern and southern portions of Nyanza Province, Kenya (Figure 1). Forty schools—20 intervention and 20 control—were randomly selected for inclusion in this study (Figure 2). Schools were eligible for participation only if they did not meet the pupil-latrine ratio of 30:1 for boys and 25:1 for girls per the Government of Kenya standards,19 and reported a water source within 1 km of the school during the dry season. The original study frame for the trial was selected using a rapid assessment of water and sanitation access conducted in conjunction with the Ministry of Education.18 All random selection and allocation was conducted by the research manager using a random number generator in Microsoft Excel (Redmond, WA). One control school was excluded because of deworming activities after study enrollment, but before baseline data collection, and was not included in the analysis.
Figure 2.
Flow diagram of school random selection and allocated as part of cluster-randomized design.
Intervention.
Intervention schools received hygiene promotion, water treatment technology, and sanitation infrastructure, which included commercially manufactured hand washing and drinking water storage containers and a 1-year supply of point-of-use water treatment product distributed by Population Services International with the brand name WaterGuard. The number of latrines provided at each school was based on enrollment; schools received between four and seven latrines. One parent and one teacher at each school were trained on hygiene behavior change, health education, and proper maintenance of sanitation and water storage facilities. Intervention schools received the hygiene promotion and water treatment hardware between May and June 2007; latrines were constructed between May 2007 and November 2008. Control schools received sanitation improvements and hygiene education after the final round of data collection.
After each of the three data collection rounds (baseline, follow-up 1, and follow-up 2), all children in study schools (intervention and control) received mass treatment of STH infections using a single oral dose of albendazole (400 mg). Albendazole is highly efficacious in treating infection with A. lumbricoides (93.9% cure rate) and hookworm (78.8%), but is less effective against T. trichiura (43.6%).8 Children found infected with S. mansoni were given praziquantel at 40 mg/kg,20 unlike the deworming of all pupils in study schools conducted for STH infections.
Sample size calculation.
On the basis of previous surveys in the region, we assumed baseline levels of infection of 42% for A. lumbricoides, 55% for T. trichiura, and 77% for hookworm.9 We based our power calculation on A. lumbricoides reinfection, given the high cure rate of albendazole, which is a parasite most correlated with poor sanitation and hygiene, rapid reinfection, and because of its direct fecal–oral transmission.2,8,11 We assumed reinfection to previous levels at follow-up and a conservative intra-cluster correlation of 0.18; we estimated the need for 20 schools per arm and 25 pupils per school for power to detect a 20% difference in infection rate between intervention and control using α = 0.05 and β = 0.2. In the final data collection round, the number of pupils per school was increased to 30 per school to account for unusable samples, though we were not always able to get a sufficient number of pupils. The detectable effect size is smaller than reductions found as part of a public sewerage and drainage intervention in Salvador, Brazil.21
Pupil selection and stool sampling.
Children enrolled in the study were randomly selected from school registers in grades 3–5 and were eligible if they were between 7 and 13 years of age and were dewormed by the project in the previous year (during follow-up rounds). We sampled 3,120 pupils attending public primary school at three time points (975 at baseline in May 2007; 975 at follow-up 1 in April 2008; and 1,170 at follow-up 2 in February 2009) and asked to supply a stool sample the day of the site visit. Stool samples were examined microscopically within 1 hour of preparation using the Kato-Katz method22,23; each stool sample was processed on two separate slides and read by different laboratory technicians. The mean of the two readings was calculated and designated as the value for that pupil. As a quality check, a random selection of 10% of slides were examined again by a different microscopist and if the number of worm eggs was different by 10%, slides were then reread.24
Surveys.
We recorded pupils' age, grade, sex, self-reported geophagy (soil-eating behavior),25 and observation of shoe wearing.26 School-level assessments, by interview with head teacher and direct observation, included indicators of school WASH access. Data from a random selection of 25 households within the school catchment areas were used to develop aggregate school and community-level baseline variables on socio-economic and sanitation characteristics. Household heads (preferably females because of familiarity with WASH conditions) were interviewed about their WASH attitudes and practices, and observations were collected on latrine conditions and household assets. Household assets were used to estimate socio-economic status using principal component analysis.27 An asset score was calculated from questions derived from the 2003 Kenya Demographic and Health Surveys (DHS) with water and sanitation indicators removed.28 Continuous asset scores were grouped into quintiles. Data from the household survey were used to compute aggregated community variables. School surveys, pupil data, and laboratory data were collected on paper surveys. Data were manually double-entered using Microsoft Access 2003.
Analysis methods.
Data were cleaned and analyzed using SAS version 9.2 (Cary, NC) and Stata version 10 (College Station, TX). The impact of the intervention was analyzed in terms of the prevalence of infection (proportion of individuals infected) and the intensity of infection (the number of worms harbored by an individual).29 Intensity of infection is indirectly estimated by the concentration of eggs per gram of feces (EPG), and is a critical measure of morbidity and is directly related to the rate of transmission (and hence the rate of exposure).30 The risk of morbidity is strongly related to the size of the number of worms harbored by an individual, hence studies in the intensity of infection have the greatest public health relevance.31
To test the impact of the intervention, we developed multivariable population-level regression models using generalized estimating equations. For prevalence of infection, we applied multivariable logistic regression. The effect of the intervention on quantitative egg counts was assessed using a log-linear model, assuming a negative binomial distribution with a log link.30 We assessed confounding by a priori determined covariates: data collection round, sex, age, observed shoe wearing, and reported geophagy.25,32 Because of sex-stratified findings from other trial outcomes, as a sub-analysis, we report sex-stratified estimates of effect.18 All models controlled for cluster-level baseline worm infection levels.29 Point estimates accounted for pupil selection weights. Standard errors were adjusted for the study design, including clustering at the school level and geographic strata33; we present both round-specific models and pooled estimates with sex-stratified results.
Ethics statement.
Ethics approval was obtained from the Institutional Review Board of Emory University in Atlanta, Georgia; the Ethical Committee of London School of Hygiene and Tropical Medicine, United Kingdom; and the Ethical Review Committee of Great Lakes University of Kisumu, Kenya. School head teachers and members of school management committees provided written consent in loco parentis. A meeting with parents and school administrators at participating schools was also conducted at baseline to explain the procedures, benefits of the program, and benefits and risks to participation. Pupils provided oral assent in the trial before providing a stool sample collection.
Results
Baseline pupil-, school-, and community-level characteristics.
Pupil characteristics, and cluster-aggregated data for school and community-level variables are found in Table 1. A total of 915 (93.8%) pupils at baseline provided stool samples that could be analyzed. Pupil-level covariates, such as age and sex were similar at baseline between the intervention and control schools. The mean age in each group was 11.2 years. Measured socio-economic indicators were similar between intervention and control schools. At baseline, schools randomly allocated to the intervention arm had higher mean enrollment (354 pupils) compared with controls (292 pupils). These schools also had a higher ratio of boys per latrine (98.1/latrine versus 63.4/latrine) and girls per latrine (83.3/latrine versus 59.1/latrine). Fewer than 40% of schools had access to an improved water source during the dry season and only 76% met the original inclusion criterion of a dry season source within 1 KM. Measured community covariates, such as percent of households headed by females alone, percent of mothers who completed primary school, community latrine coverage, and the proportion of the households in the lowest socio-economic quintile were comparable between the intervention and control communities at baseline (Table 1).
Table 1.
Aggregate school and household characteristics at baseline among randomly selected schools and communities in Nyanza Province Kenya, February 2007
| Variables | Intervention | Control |
|---|---|---|
| Pupil variables | N = 470 | N = 445 |
| Mean percent girls sampled (SD) | 49.4 (10.0) | 51.6 (10.4) |
| Mean age of pupils sampled (SD) | 11.2 (0.6) | 11.2 (0.6) |
| Pupil wearing shoes (%) | 260 (55.4) | 231 (52.0) |
| Pupil reports soil eating at school (%) | 50 (10.7) | 47 (10.6) |
| Pupil reports soil eating at home (%) | 229 (48.8) | 208 (46.9) |
| School conditions | N = 20 | N = 19 |
| No. schools with electricity at school (%) | 0 (0) | 0 (0) |
| No. schools with iron sheet roofing throughout school (%) | 19 (95) | 20 (100) |
| No. schools with cement floor throughout school (%) | 4 (20) | 2 (10) |
| Mean school enrollment in number of pupils (SD) | 353.6 (162.6) | 291.6 (101.7) |
| Mean proportion girls (SD) | 49.8 (2.9) | 49.4 (3.8) |
| Mean proportion of pupils who are orphans (SD) | 12.3 (8.0) | 13.1 (6.1) |
| Mean pupil/teacher ratio (SD) | 33.3 (12.1) | 29.2 (8.1) |
| Mean months water is available throughout year (SD) | 2.9 (2.6) | 2.7 (3.4) |
| No. schools where dry season source is < 1,000 m | 14 (74) | 13 (72) |
| No. schools where dry season water source is improved* (%) | 7 (35) | 7 (37) |
| Mean distance to dry season water source in meters (SD) | 900.0 (1047.6) | 832.1 (1502.8) |
| Pupil/latrine ratio greater than 3 times government standard | ||
| Number of schools with boys/latrine ratio > 90:1 (%) | 4 (20) | 2 (10) |
| Number of schools with girls/latrine ratio > 75:1 (%) | 1 (5) | 2 (10) |
| Household demographics† | N = 20 | N = 19 |
| Female headed households (mean %) | 35.4 (20.0) | 27.2 (14.2) |
| Female head of household completed primary school (mean %) | 56.1 (16.9) | 52.7 (14.7) |
| Household currently using improved drinking water source† (mean %) | 62.0 (33.0) | 63.8 (30.1) |
| Latrine coverage in community (mean %) | 49.1 (22.5) | 50.9 (22.0) |
| Parent of households in lowest wealth quintile (mean %) | 21.2 (13.4) | 15.3 (10.7) |
Point estimate is the mean and (standard deviation) unless otherwise indicated.
Improved sources are defined by the UNICEF/WHO joint monitoring program (wssinfo.org).
Household statistics are aggregated from household surveys and are presented as mean percentages (and standard deviations of the mean).
At baseline, 467 (51%) of pupils reported soil eating either at school or home (Table 1). This behavior was more common among girls (56%) than boys (46%, P < 0.001), but not between pupils in intervention (52%) and control (50%, P = 0.50) schools. Shoes were observed worn by 491 (54%) of pupils at baseline and was similar between intervention (55%) and control (52%, P = 0.30). More girls (60%) wore shoes than boys (47%, P < 0.0001).
Baseline helminth infection.
Overall, 37.2% of children were infected with at least one STH species Table 2, whereas 7.0% of children harbored two or more STH species (data not shown). A number of imbalances in infection levels were observed between study arms. The prevalence of any STH species and infection with at least two species of STH was higher in the intervention arm (40.4% and 8.2%, respectively) compared with the control arm (33.8% and 5.8%). The prevalence and mean egg count of A. lumbricoides was higher (14.2%, 1,094 EPG) among children in the intervention arm, compared with control (9.2%, 506 EPG). Balance between intervention and control schools was more similar for other worms. Calculated intra-cluster correlations were lower than expected for A. lumbricoides (0.04), T. trichiura (0.08), hookworm (0.13), and S. mansoni (0.11).
Table 2.
Prevalence and eggs per gram of feces for soil transmitted helminths (STH) and schistosomiasis at baseline and two follow-up rounds by worm type in Nyanza Province, Kenya 2007–2009
| Variable* | Baseline (May 2007) | Follow-up 1 (April 2008) | Follow-up 2 (February 2009) | |||||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | P† | Intervention | Control | P† | |
| (N = 20) | (N = 19) | (N = 20) | (N = 19) | (N = 20) | (N = 19) | |||
| Multiple STH infection | ||||||||
| % with at least 1 STH infection | 40.4 (25.9) | 33.8 (23.8) | 17.8 (13.0) | 22.8 (13.2) | 0.19 | 19.0 (12.4) | 17.9 (14.4) | 0.71 |
| % with at least 2 STH infections | 8.2 (8.8) | 5.8 (7.4) | 3.6 (4.4) | 4.0 (5.2) | 0.34 | 3.3 (3.7) | 2.7 (5.6) | 0.62 |
| % with at least 3 STH infections | 0.8 (1.6) | 0.7 (2.1) | 0.2 (0.9) | 1.3 (3.0) | 0.06 | 0.0 (0.0) | 0.0 (0.0) | 0.75 |
| Ascaris lumbricoides | ||||||||
| Prevalence | 14.2 (13.9) | 9.2 (14.2) | 6.2 (5.8) | 7.4 (7.4) | 0.10 | 6.2 (6.9) | 8.6 (14.6) | 0.03 |
| Arithmetic mean egg count | 1094 (1488) | 506 (1049) | 462 (829) | 589 (902) | 0.03 | 395 (623) | 796 (1337) | 0.004 |
| Trichuris trichiura | ||||||||
| Prevalence | 5.9 (7.2) | 4.4 (4.9) | 5.3 (7.7) | 6.4 (7.5) | 0.41 | 7.3 (6.1) | 6.5 (6.5) | 0.88 |
| Arithmetic mean egg count | 13.7 (31.1) | 7.1 (11.6) | 10.3 (17.3) | 8.4 (13.1) | 0.62 | 23.0 (70.5) | 33.1 (62.0) | 0.46 |
| Hookworm | ||||||||
| Prevalence | 29.4 (19.0) | 27.7 (21.2) | 10.0 (9.5) | 14.3 (13.1) | 0.50 | 8.8 (7.2) | 5.4 (5.8) | 0.54 |
| Arithmetic mean egg count | 145.4 (153.1) | 104.1 (164.1) | 36.2 (71.0) | 42.9 (71.5) | 0.35 | 34.4 (48.7) | 31.8 (54.1) | 0.50 |
| Schistosoma mansoni | ||||||||
| Prevalence | 6.1 (11.3) | 2.7 (4.1) | 7.8 (10.8) | 4.9 (6.6) | 0.18 | 9.7 (17.4) | 4.8 (11.5) | 0.69 |
| Arithmetic mean egg count | 13.6 (27.5) | 12.6 (32.9) | 13.6 (21.0) | 8.5 (15.0) | 0.98 | 31.9 (102.0) | 15.2 (35.7) | 0.62 |
Sample size: 915 pupils at baseline, 946 at follow-up 1, and 1113 at follow-up 2.
Point estimates are cluster-level means (standard deviations).
P values based on a cluster-level difference in difference.
Unadjusted effect of worm infection at follow-up.
Stool samples for 946 (97.0%) pupils at the first follow-up and 1,113 (95.1%) pupils at the second follow-up were analyzable (Table 2). At follow-up, a similar proportion of pupils were infected with at least one worm within intervention (17.8% in follow-up 1, 19.0% in follow-up 2) compared with control (22.8% and 17.9%, respectively) schools. The difference in differences (DID) between intervention and control schools was −11.6% (P = 0.19) at follow-up 1 and −5.5% (P = 0.71) for follow-up 2. For A. lumbricoides prevalence, the crude DID between intervention and control was −6.2% (P = 0.10) and −7.4% (P = 0.03), respectively, for each follow-up round. Mean egg counts were lower for intervention schools at both follow-up rounds (−462 and −395 EPG) than in control schools (−589 EPG [P = 0.03], −796 EPG [P = 0.004]). For hookworm prevalence, DID was −6.0% (P = 0.50) and +1.7% (P = 0.54) for each follow-up round, respectively. Difference in EPG were 48.0 (P = 0.35) for follow-up 1 and 37.8 (P = 0.50) for follow-up 2. No differences were found for DID of T. trichiura or S. mansoni for either worm prevalence or egg count.
Soil eating, shoe wearing, and school conditions at follow-up.
Although the proportion of children practicing geophagy did not vary by study arm at follow-up among all pupils (intervention: 16.8%, control: 21.0%, P = 0.16), there was a significant difference between the proportion of girls compared with the proportion of boys practicing geophagy (29.4% versus 18.5%, P < 0.001). Similarly, at follow-up girls were more likely to wear shoes (73.3%) than boys (59.8%, P < 0.001).
Table 3 reports the availability of WASH facilities at baseline and follow-up by study group. Although water supply improvements were not provided by the program (only storage containers), intervention schools increased access to drinking water (from 32% to 85%) and hand washing water (from 5% to 85%). This change was significant compared with controls (P < 0.01 for both). No schools had soap at baseline; at follow-up, 25% of intervention schools had purchased and were providing soap, whereas no control schools were providing soap. Construction of latrines at intervention schools resulted in improved pupil to latrine ratios from 89:1 at baseline to 32:1 at follow-up. There was no change in access to an improved water source in the dry season.
Table 3.
Uptake of water treatment, sanitation, and hygiene improvements during the course of the trial, by intervention and control schools in Nyanza Province, Kenya, February 2007 and February 2009
| Variable | Baseline | Final | P | ||
|---|---|---|---|---|---|
| Intervention (N = 20) | Control (N = 19) | Intervention (N = 20) | Control (N = 19) | ||
| School dry season water source is improved* | 7 (37%) | 8 (44%) | 8 (40%) | 11 (58%) | 0.63 |
| Drinking water provided | 6 (32%) | 8 (44%) | 17 (85%) | 6 (32%) | < 0.01 |
| Chlorine residual in drinking water | 0 (0%) | 0 (0%) | 9 (45%) | 0 (0%) | < 0.01† |
| Hand washing water available | 1 (5%) | 4 (22%) | 17 (85%) | 6 (32%) | < 0.01 |
| Soap available | 0 (0%) | 0 (0%) | 5 (25%) | 0 (0%) | < 0.01† |
| Mean (SD) pupils per latrine | 89 (87) | 60 (41) | 32 (13) | 52 (25) | 0.07 |
| Mean (SD) boys per latrine | 84 (98) | 62 (49) | 33 (12) | 46 (30) | 0.17 |
| Mean (SD) girls per latrine | 90 (84) | 64 (34) | 34 (18) | 61 (42) | 0.03 |
Values are n (%) or means (standard deviation).
Improved sources based on definitions established by the UNICEF and WHO Joint Monitoring Program.
P values compare intervention and control for final round using Fischer's exact test.
Multivariable adjusted models of species infection.
No confounders were found to appreciably change the estimates of effect or standard errors, therefore all models account for baseline infection only Tables 4 and 5. Pupils in the intervention had an OR of 0.48 (95% CI 0.22–1.06) and lower egg count (RR 0.32, 95% CI 0.12–0.82) of A. lumbricoides compared with controls (Table 4). Results for follow-up 1 were similar. Children attending intervention schools were 44% less likely to be infected with A. lumbricoides compared with those children in control schools (OR 0.56, 95% CI 0.31–1.00). Ascaris lumbricoides egg count was significantly lower for pupils in intervention schools (RR 0.34, 95% CI 0.15–0.75) (Table 5). The effect of the intervention on egg count differed by sex (P = 0.03): among girls, those in the intervention schools had lower egg count (RR 0.29, 95% CI 0.11–0.74, P < 0.01) compared with those in the control. There was no effect of the intervention for boys (RR 1.01, 95% CI 0.46 species-specific −2.28, P = 0.98). Geophagy and shoe wearing were not significant effect modifiers in any of the models.
Table 4.
Estimate effect comparing helminth infection among pupils in schools that received hygiene promotion, sanitation, safe water treatment provision compared with control schools for each follow-up round in Nyanza Province, Kenya 2007–2009
| Variable | A. lumbricoides | T. trichiura | Hookworm | S. mansoni | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Estimate | 95% CI | P | Estimate | 95% CI | P | Estimate | 95% CI | P | |
| Prevalence | OR | OR | OR | OR | ||||||||
| Follow-up 1 | 0.68 | 0.41–1.14 | 0.14 | 0.74 | 0.32–1.68 | 0.47 | 0.52 | 0.27–1.00 | 0.05 | 1.05 | 0.44–2.52 | 0.90 |
| Follow-up 2 | 0.48 | 0.22–1.06 | 0.07 | 0.86 | 0.46–1.63 | 0.65 | 1.27 | 0.71–2.23 | 0.41 | 1.52 | 0.39–5.95 | 0.54 |
| Egg count | RR | RR | RR | RR | ||||||||
| Follow-up 1 | 0.38 | 0.14–1.02 | 0.06 | 1.22 | 0.44–3.34 | 0.69 | 0.77 | 0.31–1.91 | 0.57 | 1.04 | 0.37–2.96 | 0.93 |
| Follow-up 2 | 0.32 | 0.12–0.82 | 0.02 | 0.35 | 0.12–1.04 | 0.06 | 0.48 | 0.15–1.48 | 0.19 | 0.47 | 0.13–1.64 | 0.23 |
OR = odds ratio; RR = rate ratio. Models controlled for school-aggregated baseline egg counts.
Table 5.
Estimate effect comparing helminth infection among pupils by sex in schools that received hygiene promotion, sanitation, safe water treatment provision compared with control schools for each follow-up round in Nyanza Province, Kenya, 2007–2009
| Variable | A. lumbricoides (N = 2,047) | T. trichiura (N = 2,059) | Hookworm (N = 2,047) | S. mansoni (N = 2,059) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | P | Estimate | 95% CI | P | Estimate | 95% CI | P | Estimate | 95% CI | P | |
| Prevalence | OR | OR | OR | OR | ||||||||
| Follow-up 1 and 2 | 0.56 | 0.31–1.00 | 0.05 | 0.82 | 0.45–1.45 | 0.48 | 0.80 | 0.47–1.36 | 0.40 | 1.29 | 0.45–3.66 | 0.49 |
| Girls | 0.48 | 0.19–1.26 | 0.13 | 1.13 | 0.56–2.27 | 0.71 | 1.22 | 0.59–2.54 | 0.58 | 1.44 | 0.35–5.88 | 0.60 |
| Boys | 1.02 | 0.46–2.28 | 0.96 | 0.60 | 0.32–1.14 | 0.12 | 0.66 | 0.34–1.27 | 0.20 | 0.59 | 0.26–1.34 | 0.20 |
| Egg count | RR | RR | RR | RR | ||||||||
| Follow-up 1 and 2 | 0.34 | 0.15–0.75 | < 0.01 | 0.55 | 0.21–1.48 | 0.24 | 0.58 | 0.27–1.26 | 0.16 | 0.63 | 0.23–1.70 | 0.35 |
| Girls | 0.29 | 0.11–0.74 | 0.01 | 0.54 | 0.13–2.27 | 0.54 | 3.45 | 1.21–9.88 | 0.02 | 1.11 | 0.26–4.66 | 0.88 |
| Boys | 1.01 | 0.31–3.33 | 0.98 | 0.62 | 0.21–1.80 | 0.37 | 0.17 | 0.06–0.57 | < 0.01 | 0.30 | 0.11–0.88 | 0.03 |
OR = odds ratio; RR = rate ratio. Models controlled for school-aggregated baseline egg counts. Calculated for two follow-up rounds, 8–10 months after school-wide deworming.
For infection with T. trichiura, our data revealed an OR of 0.86 (CI 0.46–1.63) and an RR of 0.35 (95% CI 0.12–1.04) during follow-up 2 (Table 4). Results for follow-up 1 were similar for prevalence, but not for egg count (RR 1.22, 95% CI 0.44–3.34). For the pooled follow-up, we did not find evidence for the effect of the intervention on infection prevalence (OR 0.82, 95% CI 0.45–1.45) or egg count (RR 0.55, 95% CI 0.21–1.48) (Table 5). Soil eating significantly modified the overall effect on egg count (P = 0.006). Pupils that practiced geophagy showed a significant reduction in risk of infection with T. trichiura (RR 0.33, 95% CI 012–0.92) compared with controls; pupils that did not report soil eating were not significantly different from the null (RR 2.97, 95% CI 0.86–10.3).
No significant difference was found for hookworm egg count for follow-up 1 (RR 0.77, 95% CI 0.31–1.91) or follow-up 2 (RR 0.48, 95% CI 0.15–1.48) (Table 4). The interaction between the intervention and data collection round was significant for prevalence of hookworm: we found a difference in hookworm prevalence during follow-up 2 (OR 0.52, 95% CI 0.27–1.00), but not during follow-up 2 (OR 1.27, 95% CI 0.71–2.23). For the pooled follow-up period, we found no overall effect of the intervention on hookworm prevalence (OR 0.80, 95% CI 0.47–1.36) or egg count (RR 0.58, 95% CI 0.27–1.26) (Table 5). The results for egg count differed significantly by sex (P < 0.001); the intervention was protective for boys, but increased the odds of infection for girls. Egg count was significantly modified by shoe wearing (P = 0.02) and soil eating (P = 0.05). Pupils without shoes were significantly impacted by the intervention (RR 0.30, CI 0.11–0.82, P = 0.02); those with shoes exhibited no statistical difference (RR 0.52, CI −0.34–1.40, P = 0.23). Stratified analysis by soil eating behavior did not reveal effect estimates significantly different than the null.
There was no significant impact of the intervention on the prevalence (OR 1.29, 95% CI 0.45–3.66) or intensity (RR 0.63, 95% CI 0.23–1.70) of S. mansoni infection (Table 5). The differences were significantly different by sex for both prevalence (P = 0.04) and egg count (P = 0.002); the effect of the intervention on egg count was significant for boys (RR 0.30, 95% CI 0.11–0.88). Other effect modifiers were not significant.
Discussion
To our knowledge, this is the first cluster-randomized trial to assess the impact of school-based sanitation and hygiene improvements on reinfection with different STH species following mass anthelmintic treatment. Our findings reveal mixed results for the impact of improved school-based WASH infrastructure on STH infection, with evidence for sex-specific effects of the intervention for different STH species. The role of behaviors—such as shoe wearing and geophagy—may moderate the effect of the intervention. Our analysis is technically an assessment of the difference between infection among pupils in each time point for the intervention and control arms. Throughout the manuscript we refer to STH “reinfection,” rather than “infection” to clarify that all pupils in the study were dewormed before each follow-up round. However, we are not longitudinally following individual children and thus do not know their baseline status, we are relying on a test that has poor detection at low levels of infection, and we did not test for cure rate. Understanding of what we mean by the WASH intervention preventing “reinfection” should be interpreted as such.
We found considerable differences in the prevalence and intensity of reinfection with A. lumbricoides in the intervention schools compared with the control schools. These findings were consistent for each of the follow-up rounds. A sex-stratified analysis revealed that these differences between the intervention and control schools were only significant among girls. This sex-specific effect of the intervention on A. lumbricoides infection on girls was consistent with findings from this same trial that the intervention has a considerable impact on absence among girls, but not boys.18 There is no evidence that girls are biologically more susceptible to STH infection34; rather, these results suggest that improving access to WASH reduces the exposure to feces for girls more than it does for boys. Girls may be less likely to urinate or defecate in the open than boys, thus may disproportionately benefit when latrines are new or clean, or when hand washing water and soap are available.
We found significant, but not universal improvement in WASH conditions in the intervention arm schools. The proportion of schools that had detectable chlorine in drinking water increased and pupil/latrine ratios all improved. Even though no soap or water provided (only hygiene education and water containers), intervention schools provided more drinking water, hand washing water, and soap for hand washing. However, these levels of fidelity to the intervention are sub-optimal, indicating that a program able to achieve higher coverage would increase the impact on STH infection.
Previous cross-sectional and longitudinal studies have shown that improved WASH characteristics are associated with lower levels of A. lumbricoides infection.17,24,35–37 The current study found WASH impacts on the prevalence of A. lumbricoides to other intervention trials of household- and school-based hygiene promotion programs that have shown a reduction in A. lumbricoides reinfection on primary school-age children.38,39 In Brazil, Moraes and colleagues40 found great reductions in the prevalence of infection because of community sanitation improvement.
Because of similar exposure pathways for A. lumbricoides and T. trichiura—typically from ingestion of feces on hands or from eating unwashed or undercooked food—we would expect similar findings for both species. In this study, we found no evidence of the effect of the intervention on T. trichiura, either overall or by sex. Differences in egg count during follow-up 2 were suggestive of an effect, but not a statistically significant one. A possible reason for this finding is the known low efficacy of a single dose of albendazole in treating T. trichiura.8,41 However, pupils in the intervention schools who practiced geophagy had lower levels of T. trichiura reinfection compared with those in the control.25 Those who practiced geophagy may have reduced risk of environmental exposure to helminth eggs. Alternatively, geophagy practice could serve as a proxy for other parameters representing marginalized populations who were impacted by the intervention.
The effect of the intervention on hookworm reinfection among boys but not girls was unexpected. These results may be correlated to the greater effect of the intervention on children without shoes. Because hookworm is transmitted usually through contact with infected feces through the sole of the foot, legs, and buttocks, our results reflect the finding that children without shoes are more susceptible to hookworm infection, and boys are more likely than girls to go without shoes. Pupils without shoes showed a significantly and substantially greater difference of hookworm reinfection at follow-up compared with those in controls. These children may have greater contact with contaminated soil, and provision of new latrines could substantially reduce exposure. Shoe wearing is likely associated with economic status, a factor not able to be assessed in this analysis, but points to the effect of the intervention on more marginalized pupils. The effectiveness of the intervention at the first follow-up, but not the second may be explained by the diminishing effect of the latrine construction in mitigating hookworm reinfection.
Limitations.
Although the impact of the intervention on A. lumbricoides reinfection was substantial and significant, we cannot rule out that our findings are a consequence of baseline imbalances and differential rate patterns of reinfection between study arms. We expect that infection would revert to baseline levels in as few as 6 months42–44; our follow-up was 10 months after deworming. However, we saw a secular trend of decreased prevalence of helminth infection among children in control schools. The rate of reinfection crucially depends on the intensity of parasite transmission (as measured by the basic reproductive number, R0), the efficacy of treatment and the percentage of the overall community, which was treated as the result of treating school children,2,30 three factors that are unknown. Multivariable analysis adjusted for aggregate baseline levels of outcome measures for each model.
A number of additional limitations are recognized. First, because of the high turnover of pupils and laboratory and budget constraints, we were unable to follow the same individuals throughout the course of the project. Thus, our baseline measures are only for aggregate measures at the school. Because individual propensity for reinfection is a considerable risk factor, using school-level aggregate data induces imprecision in the findings.45,46 Second, our study was powered with infection rates from a previous study that were considerably higher than what we found at baseline, limiting our study power.9 A recent review of worm infection in East Africa reported median prevalence estimates in Nyanza Province of A. lumbricoides (18.5%), T. trichiura (11.9%), and hookworm (17.6%).47 Third, there was considerable heterogeneity in delivery and uptake of the intervention. Only 25% of schools in the intervention reached the Government of Kenya standards for pupil/latrine ratios, only 25% had soap available for hand washing. We observed hand washing water and soap at only 25% of schools at follow-up. On the other hand, this study should be regarded as an effectiveness trial of a real-world intervention. As such, some schools were more successful at improving access to clean latrines and hygiene behaviors of their pupils. Fourth, a quarter of the schools did not meet the original eligibility criterion of having a water source within 1 KM, though this likely biased our results towards the null by limiting the impact of the hygiene intervention and ability to maintain latrines. Finally, a single stool sample using Kato-Katz has been shown to be inaccurate relative to other diagnostic methods,48 although may have sensitivities of > 90% for both A. lumbricoides and T. trichiura.49 This limitation is mitigated by the use of this method for both study arms and that increased detection of low-burden individuals may have biased our results towards the null.
Conclusions
Deworming alone cannot eliminate STH infection if schools and communities lack adequate WASH facilities, and the gains from deworming will only be sustained through improved WASH access. An increasing number of national governments and international organizations are implementing school-based deworming as part of an integrated school health program.50 Implementing WASH programs, including school-based programs, is complex, especially as it is difficult to ensure uniform implementation across schools. Furthermore, poor WASH facilities in schools is only one source of exposure to helminth infection, and any effect on improved WASH facilities is likely to be mediated by differences in other, individual- or household- mediated, routes of fecal exposure.51 Our findings provide initial support for the benefit of improved WASH in schools when implemented alongside school-based deworming, but show that the effect is not consistent among boys and girls, among sub-groups with different exposure-related patterns of behavior, or for different worms. Additional studies assessing the impact of school WASH are warranted to better understand the impact of school-level interventions and how this impact is augmented by other individual- or community-based efforts to reduce exposure.
ACKNOWLEDGMENTS
We are indebted to the staffs of CARE - Kenya, Great Lakes University of Kisumu, Water.org, and the Kenya Water and Health Organization for their diligent efforts in Kenya. Lily Lukorito played a considerable role in stool sampling. We acknowledge the work of Dr. Alfred Luoba who passed away before the completion of the project. SJB is supported by a Wellcome Trust Senior Fellowship in Basic Biomedical Science (098045).
Footnotes
Financial support: The project was funded by the Bill & Melinda Gates Foundation and led by CARE – USA.
Authors' addresses: Matthew C. Freeman, Emory University, Environmental Health, Atlanta, GA, E-mail: mcfreem@emory.edu. Thomas Clasen, London School of Hygiene and Tropical Medicine, Infectious and Tropical Diseases, London, UK, E-mail: thomas.clasen@lshtm.ac.uk. Simon J. Brooker, London School of Hygiene and Tropical Medicine, Infectious and Tropical Diseases, London, UK, and KEMRI Wellcome Trust, Nairobi, Kenya, E-mail: simon.brooker@lshtm.ac.uk. Daniel O. Akoko, Great Lakes University of Kisumu, Tropical Institute of Community Health and Development, Kisumu, Kenya, E-mail: obotedaniel@yahoo.com. Richard Rheingans, University of Florida, Environmental and Global Health, Gainesville, FL, and Emory University, Global Health, Atlanta, GA, E-mail: rrheing@ufl.edu.
References
- 1.Crompton DW. How much human helminthiasis is there in the world? J Parasitol. 1999;85:397–403. [PubMed] [Google Scholar]
- 2.Hotez PJ, Bundy DAP, Beegle K, Brooker S, Drake L, de Silva N, Montresor A, Engels D, Jukes M, Chitsulo L, Chow J, Laxminarayan R, Michaud CM, Bethony J, Correa–Oliveira R, Xiao SH, Fenwick A, Savioli L. Helminth Infections: Soil-Transmitted Helminth Infections and Schistosomiasis. Disease Control Priorities in Developing Countries. New York: Oxford University Press; 2006. pp. 467–482. [Google Scholar]
- 3.Dickson R, Awasthi S, Williamson P, Demellweek C, Garner P. Effects of treatment for intestinal helminth infection on growth and cognitive performance in children: systematic review of randomized trials. BMJ. 2000;320:1697–1701. doi: 10.1136/bmj.320.7251.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sur D, Saha DR, Manna B, Rajendran K, Bhattacharya SK. Periodic deworming with albendazole and its impact on growth status and diarrhoeal incidence among children in an urban slum of India. Trans R Soc Trop Med Hyg. 2005;99:261–267. doi: 10.1016/j.trstmh.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Taylor-Robinson DC, Jones AP, Garner P. Deworming drugs for treating soil-transmitted intestinal worms in children: effects on growth and school performance. Cochrane Database Syst Rev. 2007;((4)):CD000371. doi: 10.1002/14651858.CD000371.pub3. [DOI] [PubMed] [Google Scholar]
- 6.Gulani A, Nagpal J, Osmond C, Sachdev HP. Effect of administration of intestinal anthelmintic drugs on hemoglobin: systematic review of randomized controlled trials. BMJ. 2007;334:1095. doi: 10.1136/bmj.39150.510475.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JL, Brooker S. Impact of hookworm infection and deworming on anemia in non-pregnant populations: a systematic review. Trop Med Int Health. 2010;15:776–795. doi: 10.1111/j.1365-3156.2010.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 9.Miguel E, Kremer M. Worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica. 2004;72:159–217. [Google Scholar]
- 10.Bundy DAP, Wong MS, Lewis LL, Horton J. Control of geohelminths by delivery of targeted chemotherapy through schools. Trans R Soc Trop Med Hyg. 1990;84:115–120. doi: 10.1016/0035-9203(90)90399-y. [DOI] [PubMed] [Google Scholar]
- 11.Jia T-W, Melville S, Utzinger J, King CH, Zhou X-N. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1621. doi: 10.1371/journal.pntd.0001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yap P, Du Z-W, Wu F-W, Jiang J-Y, Chen R, Zhou X-N, Hattendorf J, Utzinger J, Steinmann P. Rapid re-infection with soil-transmitted helminths after triple-dose albendazole treatment of school-aged children in Yunnan, People's Republic of China. Am J Trop Med Hyg. 2013;89:23–31. doi: 10.4269/ajtmh.13-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asaolu SO, Ofoezie IE. The role of health education and sanitation in the control of helminth infections. Acta Trop. 2003;86:283–294. doi: 10.1016/s0001-706x(03)00060-3. [DOI] [PubMed] [Google Scholar]
- 14.Henry FJ. Reinfection with Ascaris lumbricoides after chemotherapy: a comparative study in three villages with varying sanitation. Trans R Soc Trop Med Hyg. 1988;82:460–464. doi: 10.1016/0035-9203(88)90162-9. [DOI] [PubMed] [Google Scholar]
- 15.WHO, UNICEF . Progress on Sanitation and Drinking-Water: 2010 Update. WHO/UNICEF Joint Monitoring Program for Water Supply and Sanitation. Geneva: WHO; 2010. [Google Scholar]
- 16.UNICEF . Water, Sanitation and Hygiene Annual Report. New York: UNICEF; 2009. [Google Scholar]
- 17.Ziegelbauer K, Speich B, Mäusezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: systematic review and meta-analysis. PLoS Med. 2012;9:e1001162. doi: 10.1371/journal.pmed.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman MC, Greene LE, Dreibelbis R, Saboori S, Muga R, Brumback B, Rheingans R. Assessing the impact of a school-based water treatment, hygiene, and sanitation program on pupil absence in Nyanza Province, Kenya: a cluster-randomized trial. Trop Med Int Health. 2012;17:380–391. doi: 10.1111/j.1365-3156.2011.02927.x. [DOI] [PubMed] [Google Scholar]
- 19.Republic of Kenya Ministry of Education . National School Water, Sanitation and Hygiene Promotion Strategy 2008–2015. Nairobi: 2008. [Google Scholar]
- 20.Tukahebwa EM, Vennervald BJ, Nuwaha F, Kabatereine NB, Magnussen P. Comparative efficacy of one versus two doses of praziquantel on cure rate of Schistosoma mansoni infection and re-infection in Mayuge District, Uganda. Trans R Soc Trop Med Hyg. 2013;107:397–404. doi: 10.1093/trstmh/trt024. [DOI] [PubMed] [Google Scholar]
- 21.Moraes LR, Cancio JA, Cairncross S. Impact of drainage and sewerage on intestinal nematode infections in poor urban areas in Salvador, Brazil. Trans R Soc Trop Med Hyg. 2004;98:197–204. doi: 10.1016/s0035-9203(03)00043-9. [DOI] [PubMed] [Google Scholar]
- 22.Katz N, Chavez A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 23.WHO . Basic Laboratory Methods in Medical Parasitology. Geneva: World Health Organization; 1991. [Google Scholar]
- 24.Stothard JR, Imison E, French MD, Sousa-Figueiredo JC, Khamis IS, Rollinson D. Soil-transmitted helminthiasis among mothers and their pre-school children on Unguja Island, Zanzibar with emphasis upon ascariasis. Parasitology. 2008;135:1447–1455. doi: 10.1017/S0031182008004836. [DOI] [PubMed] [Google Scholar]
- 25.Luoba AI, Wenzel Geissler P, Estambale B, Ouma JH, Alusala D, Ayah R, Mwaniki D, Magnussen P, Friis H. Earth-eating and reinfection with intestinal helminths among pregnant and lactating women in western Kenya. Trop Med Int Health. 2005;10:220–227. doi: 10.1111/j.1365-3156.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 26.Alemu A, Atnafu A, Addis Z, Shiferaw Y, Teklu T, Mathewos B, Birhan W, Gebretsadik S, Gelaw B. Soil transmitted helminths and Schistosoma mansoni infections among school children in Zarima town, northwest Ethiopia. BMC Infect Dis. 2011;11:189. doi: 10.1186/1471-2334-11-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 28.Gwatkin DR, Rustein S, Johnson K, Suliman E, Wagstaff A, Amouzou A. Socio-economic differences in health, nutrition, and population: Kenya. Country Reports on HPN and Poverty. Washington, DC: The World Bank; 2007. [PubMed] [Google Scholar]
- 29.Anderson RM, Schad GA. Hookworm burdens and fecal egg counts: an analysis of the biological basis of variation. Trans R Soc Trop Med Hyg. 1985;79:812–825. doi: 10.1016/0035-9203(85)90128-2. [DOI] [PubMed] [Google Scholar]
- 30.Anderson RM, May RM. Infectious Diseases of Humans. Oxford: Oxford University Press; 1991. [Google Scholar]
- 31.Brooker S, Bundy DA. Soil-transmitted helminths (geohelminths) In: Cook GC, Zumla AI, editors. Manson's Tropical Diseases. London: Elsevier; 2008. pp. 1515–1548. [Google Scholar]
- 32.Humphries D, Mosites E, Otchere J, Twum WA, Woo L, Jones-Sanpei H, Harrison LM, Bungiro RD, Benham-Pyle B, Bimi L, Edoh D, Bosompem K, Wilson M, Cappello M. Epidemiology of hookworm infection in Kintampo North Municipality, Ghana: patterns of malaria coinfection, anemia, and albendazole treatment failure. Am J Trop Med Hyg. 2011;84:792–800. doi: 10.4269/ajtmh.2011.11-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayes R, Moulton LH, Morgan BTJ. Cluster randomized controlled trials. New York: Chapman and Hall/CRC Press; 2009. [Google Scholar]
- 34.Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197–288. doi: 10.1016/S0065-308X(04)58004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunawardena GS, Karunaweera ND, Ismail MM. Effects of climatic, socio-economic and behavioral factors on the transmission of hookworm (Necator americanus) on two low-country plantations in Sri Lanka. Ann Trop Med Parasitol. 2005;99:601–609. doi: 10.1179/136485905X51436. [DOI] [PubMed] [Google Scholar]
- 36.Traub RJ, Robertson ID, Irwin P, Mencke N, Andrew Thompson RC. The prevalence, intensities and risk factors associated with geohelminth infection in tea-growing communities of Assam, India. Trop Med Int Health. 2004;9:688–701. doi: 10.1111/j.1365-3156.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- 37.Knopp S, Mohammed KA, Stothard JR, Khamis IS, Rollinson D, Marti H, Utzinger J. Patterns and risk factors of helminthiasis and anemia in a rural and a peri-urban community in Zanzibar, in the context of helminth control programs. PLoS Negl Trop Dis. 2010;4:e681. doi: 10.1371/journal.pntd.0000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gungoren B, Latipov R, Regallet G, Musabaev E. Effect of hygiene promotion on the risk of reinfection rate of intestinal parasites in children in rural Uzbekistan. Trans R Soc Trop Med Hyg. 2007;101:564–569. doi: 10.1016/j.trstmh.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Kanoa B, George E, Abed Y, Al-Hindi A. Evaluation of the relationship between intestinal parasitic infection and health education among school children in Gaza City, Beit-Lahia Village and Jabalia Refugee Camp, Gaza Strip, Palestine. The Islamic University Journal. 2006;14:39–49. (Series of Natural Studies and Engineering) [Google Scholar]
- 40.Moraes LR, Cancio JA, Cairncross S, Huttly S. Impact of drainage and sewerage on diarrhoea in poor urban areas in Salvador, Brazil. Trans R Soc Trop Med Hyg. 2003;97:153–158. doi: 10.1016/s0035-9203(03)90104-0. [DOI] [PubMed] [Google Scholar]
- 41.Olsen A, Namwanje H, Nejsum P, Roepstorff A, Thamsborg SM. Albendazole and mebendazole have low efficacy against Trichuris trichiura in school-age children in Kabale District, Uganda. Trans R Soc Trop Med Hyg. 2009;103:443–446. doi: 10.1016/j.trstmh.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Hlaing T, Saw T, Lwin M. Reinfection of people with Ascaris lumbricoides following single, 6-month and 12-month interval mass chemotherapy in Okpo village, rural Burma. Trans R Soc Trop Med Hyg. 1987;81:140–146. doi: 10.1016/0035-9203(87)90306-3. [DOI] [PubMed] [Google Scholar]
- 43.Hall A, Anwar KS, Tomkins AM. Intensity of reinfection with Ascaris lumbricoides and its implications for parasite control. Lancet. 1992;339:1253–1257. doi: 10.1016/0140-6736(92)91593-w. [DOI] [PubMed] [Google Scholar]
- 44.Elkins DB, Haswell-Elkins M, Anderson RM. The importance of host age and sex to patterns of reinfection with Ascaris lumbricoides following mass anthelmintic treatment in a South Indian fishing community. Parasitology. 1988;96:171–184. doi: 10.1017/s0031182000081749. [DOI] [PubMed] [Google Scholar]
- 45.Quinnell RJ. Genetics of susceptibility to human helminth infection. Int J Parasitol. 2003;33:1219–1231. doi: 10.1016/s0020-7519(03)00175-9. [DOI] [PubMed] [Google Scholar]
- 46.Pullan RL, Kabatereine NB, Quinnell RJ, Brooker S. Spatial and genetic epidemiology of hookworm in a rural community in Uganda. PLoS Negl Trop Dis. 2010;4:e713. doi: 10.1371/journal.pntd.0000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brooker S, Kabatereine NB, Smith JL, Mupfasoni D, Mwanje MT, Ndayishimiye O, Lwambo NJ, Mbotha D, Karanja P, Mwandawiro C, Muchiri E, Clements AC, Bundy DA, Snow RW. An updated atlas of human helminth infections: the example of East Africa. Int J Health Geogr. 2009;8:42. doi: 10.1186/1476-072X-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Habtamu K, Degarege A, Ye-Ebiyo Y, Erko B. Comparison of the Kato-Katz and FLOTAC techniques for the diagnosis of soil-transmitted helminth infections. Parasitol Int. 2011;60:398–402. doi: 10.1016/j.parint.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 49.Tarafder M, Carabin H, Joseph L, Balolong E, Jr, Olveda R, McGarvey S. Estimating the sensitivity and specificity of Kato-Katz stool examination technique for detection of hookworms, Ascaris lumbricoides and Trichuris trichiura infections in humans in the absence of a ‘gold standard’. Int J Parasitol. 2010;40:399–404. doi: 10.1016/j.ijpara.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WHO . Accelerating Work to Overcome the Global Impact of Neglected Tropical Disease: A Roadmap for Implementation. Geneva: World Health Organization; 2012. [Google Scholar]
- 51.Cairncross S, Blumenthal U, Kolsky P, Moraes L, Tayeh A. The public and domestic domains in the transmission of disease. Trop Med Int Health. 1996;1:27–34. doi: 10.1046/j.1365-3156.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]