Abstract
Calcified Taenia solium granulomas are the focus of repeated episodes of perilesional edema and seizures in 50% of persons with calcifications, history of seizures, and a positive serology for cysticercosis. The pathophysiology is unclear but recent studies suggest the edema is caused by inflammation. We report two new cases and four other published cases where cessation of corticosteroids appeared to result in recurrence or new appearance of perilesional edema around calcifications. This suggests that perilesional edema is an immune-mediated phenomenon.
Introduction
Neurocysticercosis is an infection of the larval cyst stage of the cestodes, Taenia solium and is responsible for 29% of epilepsy in endemic areas worldwide.1 Humans harbor the adult tapeworm in the small intestine, which consists of a head, neck, and a long array of increasingly mature segments or proglottids each containing 30,000–50,000 infectious ova released in the feces as liberated ova or proglottids. After ingestion of ova in feces by pigs or after the accidental ingestion of ova from contaminated hands or in food or water by humans, the developing oncospheres migrate into the blood stream, lodge in tissues throughout the body but mostly develop into viable cysts in the brain, muscles, and subcutaneous tissues. Tapeworms develop after ingestion of undercooked pork by humans, fulfilling the life cycle.2
Viable parenchymal cysts incite little inflammation until recognized by the host, which commonly results in cyst degeneration and seizures. The end-stage granuloma, consisting of varying amounts of host inflammatory cells and degenerated cyst components, frequently calcifies resulting in typical calcifications on computed tomography (CT) imaging. Recent studies indicate calcifications are important foci of seizure activation.3,4 The presence of enhancement around a subset of calcifications5–9 the episodic occurrence of perilesional edema and seizure activity localized to some calcifications,3,4,10 and the presence of significant inflammation in two excised lesions11 (Nash and others, in preparation), strongly suggest the continued presence of inflammation associated with a subset of calcifications. This hypothesis implies that immune responses are periodically induced, perhaps by intermittent antigen release10 or after episodic loss of suppressive immune inhibition, or some combination of both.
We describe two patients who developed perilesional edema around calcifications in the setting of corticosteroid withdrawal after treatment of parenchymal neurocysticercosis. We reviewed four similar cases (Table 1) whose histories suggest the presence of perilesional edema was initiated in the setting of corticosteroid withdrawal after treatment.
Table 1.
Summary of cases with perilesional edema induced by corticosteroid withdrawal or summary of cases
| Case number | Age (year), sex (reference) | Known calcifications | Context | Relation to steroid taper |
|---|---|---|---|---|
| 1 | 37 y/o F12 | Yes | Treated 7 years earlier, proven calcifications without edema | Stopped 7 days earlier |
| 2 | 8 y/o F13 | Yes, documented asymptomatic calcifications | Developed symptomatic pons enhancing lesion | Within 1 week |
| 3 | 48 y/o F5,14 | Yes, 55 calcifications | Multiple episodes of perilesional edema | 2 days after taper |
| 4 | 43 y/o F15 | Yes | Viable cyst and calcification, undergoing anthelminthic and corticosteroids treatment | Stopped 10 days earlier, multiple calcifications with edema |
| 5 | 34 y/o F (Mejia and Nash 2013) | Yes | Viable single cyst that calcified after treatment. Multiple seizure episodes during taper or after stopping | 1 and 6 days after completing taper |
| 6 | 28 y/o M (Mejia and Nash 2013) | Yes | Treatment of viable cysts with 2 known previous calcifications without enhancement or edema | 4 days after stopping steroids MRI showed 2 calcifications with perilesional edema |
MRI = magnetic resonance imaging.
Case Report
Patient 1.
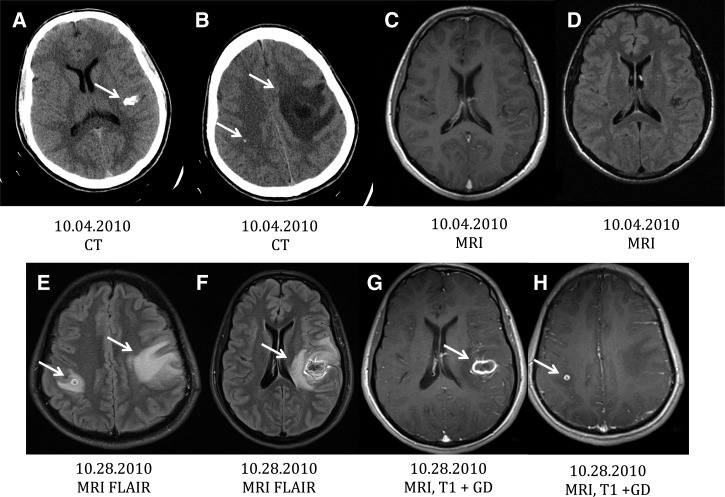
A 28-year-old man from Guatemala presented to a local hospital with a 2-day history of progressive right arm and leg weakness and a constant left sided headache. An initial CT and magnetic resonance imaging (MRI) revealed a large cyst occupying the left motor strip (Figure 1B). Because of the size of the cyst, the large degree of edema and worsening neurologic condition, this lesion was excised. Furthermore, visualized on imaging was a single small round calcified lesion in the right parietal lobe (Figure 1B), a viable cyst in the left parietal convexity (not shown), and a large calcified lesion in the Sylvian fissure (Figure 1A), abutting upon the left frontotemporal lobe. He was treated with a 10-day course of albendazole 800 mg every 12 hours and 10 days of dexamethasone 16 mg daily with a short taper and levetiracetam 500 mg twice a day. Four days after stopping steroids, the patient began to have fevers of 101°F, increasing the left sided headaches and decreasing strength of the right extremities. On his initial visit to National Institutes of Health (NIH), 24 days after initial hospitalization and 9 days after he developed fever, follow-up MRI and CT examinations (Figure 1E–H) unexpectedly revealed that the two previously quiescent calcified lesions developed significant perilesional edema and enhancement (Figure 1E–H). The prior viable cysts were degenerating and the edema surrounding the excised cyst had decreased (not shown). He was restarted on corticosteroids. His symptoms resolved on a slow taper of corticosteroids and serial MRI's showed gradual improvement. The patient subsequently developed another spontaneous perilesional edema episode involving the Sylvian fissure calcification about 1.5 years later (images not shown).
Figure 1.
Patient 1: Magnetic resonance imaging (MRI) and computed tomography (CT) images at presentation (A–D) and initial evaluation at National Institutes of Health (NIH) (E–H). (A and B) show calcifications in the left Sylvian fissure (A) and right parietal (B) region without perilesional edema on MRI fluid attenuated inversion recovery (FLAIR) sequences, (C and D), respectively. (B) A mass is present in the left motor strip with massive edema that differs in location from the Sylvian fissure calcification. (E) and (F) are MRI FLAIR sequences showing the development of edema and enhancement (G and H, MRI T1 with gadolinium) around both calcifications.
Patient 2.
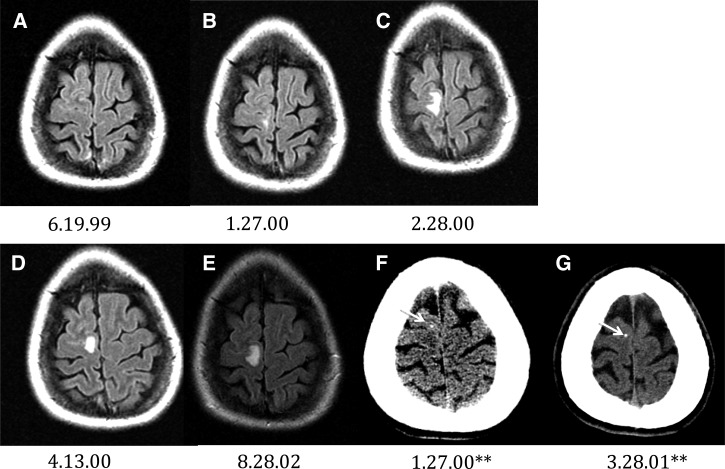
A 34-year-old female was well until September 22, 1998 when she suddenly experienced left leg shaking followed by a generalized seizure. Another focal seizure involving only the left leg occurred 5 days later. A series of CT and MRI scans revealed a single circular enhancing edematous non-calcified lesion in the medial frontal aspect of the right frontal lobe (not shown). A brain abscess was suspected and the patient was treated with phenytoin and 8 weeks of intravenous antibiotics. A follow-up MRI 1 month later, showed the perilesional edema had disappeared, but enhancement persisted (not shown). An informal opinion by NIH at the request of her caring physicians suggested that neurocysticercosis was a possibility prompting a serological examination at a commercial laboratory, which was subsequently positive. A second positive serology confirmed the results of the first test. She was treated with a 14-day course of albendazole and corticosteroids in November of 1998. The NIH was officially consulted on June 19, 1999. An MRI at this date did not show perilesional edema (Figure 2A) and a Western blot serology for cysticercosis, performed at the Centers for Disease Control and Prevention (CDC) was positive. She did well for about the next 6 months, but on 1/9/2000 focal seizures of the left foot reoccurred and imaging revealed the reappearance of perilesional edema around the lesion (not shown) prompting a course of high dose corticosteroids. Subsequently, on 1/27/2000 the patient, who by this time was asymptomatic, was reevaluated at NIH. A CT scan revealed a small calcification had developed within the lesion (Figure 2F) and the associated edema had decreased considerably (Figure 2B). The lesion became densely calcified over the next 14 months (Figure 2G). Shortly after the corticosteroids were stopped, on 2/12–14/2000 numbness of the face, left foot focal seizures, and perilesional edema around the calcification reoccurred. High dose corticosteroids were restarted by her private physician and the patient was subsequently reevaluated at NIH on 2/28/2000. The patient was asymptomatic and MRI imaging showed persisting perilesional edema (Figure 2C). By 3/14/2000, perilesional edema was no longer present and the patient was weaned off corticosteroids on 4/12/2000. However, the next day on 4/13/2000 (Figure 2D) the patient again experienced focal seizures involving the left foot and the reappearance of perilesional edema around the lesion. The perilesional edema resolved by 5/05/2000 (not shown). Over the next 2 years, despite appropriate anti-seizure medication, the patient experienced multiple episodes consisting of various combinations of focal seizures of the left foot, dysesthesias of the face and/or left side of the body accompanied by the reappearance of perilesional edema. Episodes, which were treated with high-dose corticosteroids followed by a taper, resulted in regression of perilesional edema and disappearance of focal seizures followed by repeated episodes when steroids were decreased or stopped. Perilesional edema was again documented on 8/28/2002 (Figure 2E) following evaluation for focal left foot seizures. Eventually, following a taper lasting months, recurrent seizures did not reoccur when corticosteroids were stopped. The patient was lost to follow-up. Although the patient had traveled widely in the United States and Europe, there was no travel to Latin America or known endemic regions. Furthermore, there was no history of more than casual contact with persons from endemic regions including domestic employees nor was there a history of ingestion of exotic or likely contaminated food.
Figure 2.
Patient 2: Waxing and waning presence of perilesional edema around a calcifying granuloma. All are magnetic resonance imaging fluid attenuated inversion recovery (MRI FLAIR) sequences with the exception of the computed tomography (CT) images (**).
Results
Both of the newly reported cases and the four previously published reports (Table 1) show a clear association between stopping and/or tapering corticosteroids and new presence of perilesional edema associated with symptoms. Case 1 (Table 1) was a 37 y/o female who developed headache, nausea, vomiting, and decreased mentation 1 week after completing a 5-day course of corticosteroids for erythema nodosum.12 She had presented about 9 years earlier with seizures and multiple viable parenchymal cysts caused by T. solium. Subsequently, she required a ventriculoperitoneal shunt for hydrocephalus and was treated successfully with albendazole for multiple parenchymal cysts that had all calcified 4 years before her present illness. The MRI and CT showed edema and increased enhancement and edema around three of the calcifications. Prior imaging, the latest 3 months earlier, revealed enhancement but no edema around the same calcifications.
Case 2 presented with esotropia caused by an enhancing calcification in the left pons.13 Multiple calcifications caused by neurocysticercosis were incidentally documented following brain imaging for minor head trauma about 3 years earlier. She experienced multiple episodes of perilesional edema around the left pons calcification, some of which clearly occurred shortly after stopping corticosteroids. In addition, parenchymal calcifications changed from non-enhancing to enhancing lesions in at least one episode. Case 3, who was previously reported, was followed prospectively because of recurring seizures, most commonly associated with intermittent perilesional edema, around a subset of 55 calcifications.5,14 Perilesional edema episodes were initially treated with high-dose dexamethasone followed by tapers of varying duration. Over a 4-year period she experienced 12 clinical episodes of seizures or focal neurological deficits, 6 of which occurred during the taper just after stopping steroids. Most recurrent episodes of perilesional edema occurred when the patient was tapered from 16 mg/dexamethasone. Episodes were documented at dosages during the steroid taper of 5 mg every other day, 0.5 mg every other day, 3 mg/day, and 2 days after completing a taper.
Poeschl and others15 reported an individual (case 4) who developed perilesional edema around 8 calcifications 8 days after completion of a course of albendazole and corticosteroids for treatment of viable cysts. Earlier imaging documented neither edema nor enhancement associated with these calcifications.
The timing of perilesional associated with taper or cessation of corticosteroid varied from during the taper to 4 weeks after stopping corticosteroids. Some of the variability may be the result of difficulty in recognizing the beginning of symptoms and delay in obtaining confirmatory MRIs. Although it is possible those spontaneously occurring perilesional edema episodes could randomly coincide with decreased or cessation of corticosteroid dosing, the repetitive association of symptoms and perilesional edema is compelling. Five of the six patients with more than one calcification showed perilesional edema involving multiple calcifications that suggests a mechanism(s) not inherently limited to a specific lesion (Table 1). On the other hand, spontaneously occurring perilesional edema episodes may occur repeatedly around one calcification or a small subset of calcifications.
Discussion
Perilesional edema around calcifications occurs spontaneously in 50% of persons in endemic regions with a history of seizures, with only calcifications and a positive serology for T. solium infection.3 The association between cessation or decrease in corticosteroids during a taper of drug dosing, which is commonly administered to lessen symptoms caused by brain edema of any cause including neurocysticercosis, was unexpected. We observed a close association in our patients and subsequently realized that a number of reports in the literature were in fact unrecognized examples of perilesional edema events worsened or initiated after cessation or dose lowering during the taper of corticosteroids. This association was previously unappreciated. Although in some instances albendazole was administered along with corticosteroids, several lines of evidence suggest the decrease in corticosteroids and not administration of albendazole triggered perilesional edema episodes. Albendazole administrative has no effect on calcified cysts. When antihelminthics were first used, treatment with praziquantel was routinely used because of the possibility of live non-visualized cysts in patients with only calcifications. No induced inflammation occurred (Nash T, personal communication), which is consistent with the general knowledge that calcifications are dead cysts (Nash and others, in preparation)10,11,16–18; additionally, albendazole was not always given and therefore could play no role in the process. In three of the six cases, albendazole was only administered after the initial presentation, and in one patient was not administered before one of several episodes. The histopathology of two calcified lesions associated with repeated episodes does not show live or viable parasite (Nash and others, in preparation).11 Therefore, corticosteroids are implicated because its administration was invariably associated with one or more episodes, whereas albendazole was variably given before perilesional edema episodes occurred and does not affect dead cysts.
There are two favored hypotheses of the causative mechanism(s), an immune inflammatory cause and edema following seizure activity19–21; currently, most of the evidence favors inflammatory mechanisms. Although a few calcified lesions have been described as grossly chalky white lesions22 containing little host response and sometimes recognized parasite remnants16,17,22,23; nevertheless, a subset of calcifications appear to be associated with inflammation. Enhancement is frequently seen around some calcifications suggesting the presence of enough inflammation to cause blood brain barrier disruption5–9; the histopathology of calcified lesions is sparse. A few illustrations in texts indicate little on-going inflammation,16,22,23 which is contrasted with the histological descriptions of the entire calcified lesion surgically removed from 2 patients with episodes of perilesional edema,11 (Nash and others, in preparation). The calcified lesions showed significant mononuclear inflammatory reaction. Activation of microglial astrocytes and monocytes has also been measured using a ligand to translocator protein 18 kDa in patients with recent episodes of perilesional edema. There was a significant increase in uptake in the affected regions indicating the presence of inflammation.18 Furthermore, the presence of calcification in a lesion does not exclude the presence of inflammation. In patient 2, the T. solium granuloma was imaged frequently and showed gradual calcification. Even though there was a small amount of calcification, the granuloma behaved very much like the previously non-calcified granuloma. It is likely that some calcified lesions, for reasons that are unclear, still maintain a certain degree of inflammation causing blood brain barrier dysfunction and enhancement and a propensity to cause perilesional edema. Inflammation as a result of non-calcified degenerating lesions is caused by host immune response to parasite antigen, which may wax and wane in intensity sometimes resulting in seizures or focal symptoms. The episodic appearance of inflammation and edema around calcified cysts is reminiscent of degenerating non-calcified cysts and suggests a similar pathophyisological mechanism, which could be either intermittent release or recognition of parasite antigen by the host or periodic loss of immune suppression.10
The second favored hypothesis is edema, which occurs secondary to seizure activity. Several lines of evidence do not favor this idea. The edema following seizures is mostly described in persons with status epilepticus.19,20,21 Most patients with perilesional edema in calcific disease do not develop status epilepticus and even 10% are asymptomatic.3 Electroencephalographs are usually not abnormal in the few perilesional edema patients studied close to the presenting seizure (Nash T, personal communication). The edema associated with seizure activity is diffuse and not angiocentric, which is the pattern in perilesional edema24; finally, perilesional edema is a relatively common event in calcified neurocysticercosis.3 The edema caused by seizures in epilepsy appears to be less common. Another possibility is inflammation will be partially decreased with steroids, but will revert to its initial degree as the steroids are tapered as may have occurred in some episodes in case 2. However, in cases 1, 4, 6, and some episodes of cases 2 and 3, perilesional edema was not present initially, but became evident after stopping corticosteroids. Although it is conceivable that corticosteroids could result in remodeling of the calcification and release of antigen, a hypothesized unproven mechanism, it may be more likely that corticosteroids causes transient loss of immune suppression that results in overt inflammation and edema. If true, seizures caused by perilesional edema may be controlled by manipulation of the immune system.14
Footnotes
Financial support: This study was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases; U.S. National Institutes of Health.
Authors' addresses: Rojelio Mejia, Baylor College of Medicine, National School of Tropical Medicine, Houston, TX, and National Institute of Allergy and Infectious Diseases, Laboratory of Parasitic Diseases, Bethesda, MD, E-mail: rojelio.mejia@bcm.edu. Theodore E. Nash, National Institute of Allergy and Infectious Diseases, Laboratory of Parasitic Diseases, Bethesda, MD, E-mail: tnash@niaid.nih.gov.
References
- 1.Ndimubanzi PC, Carabin H, Budke CM, Nguyen H, Qian YJ, Rainwater E, Dickey M, Reynolds S, Stoner JA. A systematic review of the frequency of neurocyticercosis with a focus on people with epilepsy. PLoS Negl Trop Dis. 2010;4:e870. doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nash TE, Garcia HH. Diagnosis and treatment of neurocysticercosis. Nat Rev Nephrol. 2011;7:584–594. doi: 10.1038/nrneurol.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nash TE, Pretell EJ, Lescano AG, Bustos JA, Gilman RH, Gonzalez AE, Garcia HH. Cysticercosis Working Group in Peru Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: a prospective cohort and nested case-control study. Lancet Neurol. 2008;7:1099–1105. doi: 10.1016/S1474-4422(08)70243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nash TE, Del Brutto OH, Butman JA, Corona T, Delgado-Escueta A, Duron RM, Evans CA, Gilman RH, Gonzalez AE, Loeb JA, Medina MT, Pietsch-Escueta S, Pretell EJ, Takayanagui OM, Theodore W, Tsang VC, Garcia HH. Calcific neurocysticercosis and epileptogenesis. Neurology. 2004;62:1934–1938. doi: 10.1212/01.wnl.0000129481.12067.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nash TE, Pretell J, Garcia HH. Calcified cysticerci provoke perilesional edema and seizures. Clin Infect Dis. 2001;33:1649–1653. doi: 10.1086/323670. [DOI] [PubMed] [Google Scholar]
- 6.Gupta RK, Awasthi R, Rathore RKS, Verma A, Sahoo P, Paliwal VK, Prasad KN, Pandey CM, Narayana PA. Understanding epileptogenesis in calcified neurocysticercosis with perfusion MRI. Neurology. 2012;78:618–625. doi: 10.1212/WNL.0b013e318248deae. [DOI] [PubMed] [Google Scholar]
- 7.Sheth TN, Pillon L, Keystone J, Kucharczyk W. Persistent MR contrast enhancement of calcified neurocysticercosis lesions. AJNR Am J Neuroradiol. 1998;19:79–82. [PMC free article] [PubMed] [Google Scholar]
- 8.White AC., Jr Neurocysticercosis: a major cause of neurological disease worldwide. Clin Infect Dis. 1997;24:101–113. doi: 10.1093/clinids/24.2.101. [DOI] [PubMed] [Google Scholar]
- 9.Del Brutto OH. Prognostic factors for seizure recurrence after withdrawal of antiepileptic drugs in patients with neurocysticercosis. Neurology. 1994;44:1706–1709. doi: 10.1212/wnl.44.9.1706. [DOI] [PubMed] [Google Scholar]
- 10.Nash T. Edema surrounding calcified intracranial cysticerci: clinical manifestations, natural history, and treatment. Pathogens and Global Health. 2012;106:275–279. doi: 10.1179/2047773212Y.0000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ooi WW, Wijemanne S, Thomas CB, Quezado M, Brown CR, Nash TE. Short report: a calcified Taenia solium granuloma associated with recurrent perilesional edema causing refractory seizures: histopathological features. Am J Trop Med Hyg. 2011;85:460–463. doi: 10.4269/ajtmh.2011.11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheth TN, Lee C, Kucharczyk W, Keystone J. Reactivation of neurocysticercosis: case report. Am J Trop Med Hyg. 1999;60:664–667. doi: 10.4269/ajtmh.1999.60.664. [DOI] [PubMed] [Google Scholar]
- 13.Park SY, Barkovich AJ, Weintraub PS. Clinical implications of calcified lesions of neurocysticercosis. Pediatr Infect Dis J. 2000;19:581–583. doi: 10.1097/00006454-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Mitre E, Talaat KR, Sperling MR, Nash TE. Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis. 2007;44:549–553. doi: 10.1086/511040. [DOI] [PubMed] [Google Scholar]
- 15.Poeschl P, Janzen A, Schuierer G, Winkler J, Bogdahn U, Steinbrecher A. Calcified neurocysticercosis lesions trigger symptomatic inflammation during antiparasitic therapy. AJNR Am J Neuroradiol. 2006;27:653–655. [PMC free article] [PubMed] [Google Scholar]
- 16.Escobar A. Pathology of the nervous system. In: Palacios E, Rodriguez-Carbajal J, Taveras JM, editors. Cysticercosis of the Central Nervous System. Springfield, IL: Charles Thomas; 1983. pp. 27–54. [Google Scholar]
- 17.Escobar A, Weidenheim K. The pathology of neurocysticercosis. In: Singh G, Prabhakar S, editors. Taenia solium Cysticercosis: From Basics to Clinical Science. Wallingford, Oxon, UK: CABI Publishing; 2002. pp. 289–305. [Google Scholar]
- 18.Masahiro F, Mahanty S, Zoghbi S, Araneta M, Hong J, Pike V, Innis RB, Nash TE. PET reveals inflammation around calcified Taenia solium granulomas with perilesional edema. PLoS ONE. 2013;8:e74052. doi: 10.1371/journal.pone.0074052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milligan TA, Zamani A, Bromfield E. Frequency and patterns of MRI abnormalities due to status epilepticus. Seizure. 2009;18:104–108. doi: 10.1016/j.seizure.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Raghavendra S, Ashalatha R, Krishnamoorthy T, Kesavadas C, Thomas SV, Radhakrishnan K. Reversible periictal MRI abnormalities: clinical correlates and long-term outcome in 12 patients. Epilepsy Res. 2007;73:129–136. doi: 10.1016/j.eplepsyres.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Cole AJ. Status epilepticus and periictal imaging. Epilepsia. 2004;45((Suppl 4)):72–77. doi: 10.1111/j.0013-9580.2004.04014.x. [DOI] [PubMed] [Google Scholar]
- 22.Henneberg R. Die tierischen Parasiten des Zentralnervensystems. In: Lewandowsky M, editor. Handbuch der Neurologie. 1912. pp. 642–683. editor. [Google Scholar]
- 23.Marquez-Monter H. Cysticercosis. In: Marcial-Rojas R, editor. Pathology of Protozoal and Helminthic Diseases. Baltimore, MD: Williams & Wilkins Company; 1971. pp. 592–617. [Google Scholar]
- 24.Nash TE, Patronas NJ. Edema associated with calcified lesions in neurocysticercosis. Neurology. 1999;53:777–781. doi: 10.1212/wnl.53.4.777. [DOI] [PubMed] [Google Scholar]