Abstract
Ticks in the nostrils of humans visiting equatorial African forests have been reported sporadically for decades, but their taxonomy and natural history have remained obscure. We report human infestation with a nostril tick in Kibale National Park, Uganda, coincident with infestation of chimpanzees in the same location with nostril ticks, as shown by high-resolution digital photography. The human-derived nostril tick was identified morphologically and genetically as a nymph of the genus Amblyomma, but the mitochondrial 12S ribosomal RNA or the nuclear intergenic transcribed spacer 2 DNA sequences of the specimen were not represented in GenBank. These ticks may represent a previously uncharacterized species that is adapted to infesting chimpanzee nostrils as a defense against grooming. Ticks that feed upon apes and humans may facilitate cross-species transmission of pathogens, and the risk of exposure is likely elevated for persons who frequent ape habitats.
Introduction
Africa contains a great diversity of ticks, many of which transmit diseases of considerable importance to global human and animal health.1,2 Over several decades, sporadic cases of nostril ticks have been reported in persons who have visited equatorial African forests. In 1960, Walton documented three cases of nostril ticks in visitors to the forests of western Uganda, including Kibale Forest,3 which is now Kibale National Park, the location of our study. Walton speculated that such ticks “might normally infest the nasal passages of anthropoid apes,” on the basis that chimpanzees (Pan troglodytes schweinfurthii) were common in each forest where nostril ticks had been reported. Morphologic examination proved inconclusive at the time, but it was hypothesized that these nostril ticks might be nymphal stages of A. paulopunctatum, which infests wild suids.4 In 2008, Aronsen and Robbins reported feeding to repletion of a nostril tick in a researcher visiting Kibale National Park to study primates and elephants.5 Morphologic examination showed the tick to be a nymph of the genus Amblyomma, consistent with earlier descriptions of Walton, but identification to species was again not possible.
In this study, we report molecular characterization of a nostril tick from a researcher visiting Kibale National Park, Uganda. In addition, using high-resolution digital photography, we document coincident infestation of many young chimpanzees in Kibale with nostril ticks. Our analyses suggest that nostril ticks may commonly infest wild apes and may sporadically infest human visitors to ape habitats.
Materials and Methods
Kibale National Park, western Uganda (0°13′–0°41′N, 30°19′–30°32′E) is a semi-deciduous forested park with an area of 795 km2 that contains a high diversity and biomass of wild non-human primates.6 Chimpanzees have been studied continuously in Kibale since 1987, and research projects of varying durations have focused on other non-human primates in Kibale, other animals, plants, persons, and the physical environment.7 These activities create a community of researchers and associated personnel who spend considerable time in the forest. Such activities increase rates of microbial transmission between humans and wild apes.8,9
A multi-decade, concerted effort has led to a remarkable degree of habituation of the Kanyawara community of chimpanzees in Kibale,10,11 which at the time of our study contained approximately 55 chimpanzees. As part of the regular monitoring of this chimpanzee community, digital photographs are obtained. In 2011 and 2012, several young chimpanzees were observed with nostril ticks, and efforts were made to document these cases by using high-resolution photography. Approximately 45 chimpanzees were regularly photographed during the study period. Photographs were taken opportunistically at close range with both digital SLR and point-and-shoot cameras. The cameras were set to a high ISO speed (at least 2,000), high frames per second (at least 6), and a wide aperture (f/2.8 at 200 mm). Effort was made to shoot at an angle that captured the interior of the nostrils. A chimpanzee was considered infested if a smooth, rounded, tick-like object, able to be differentiated from debris, was seen partially or fully occluding the nostril in one or more photograph.
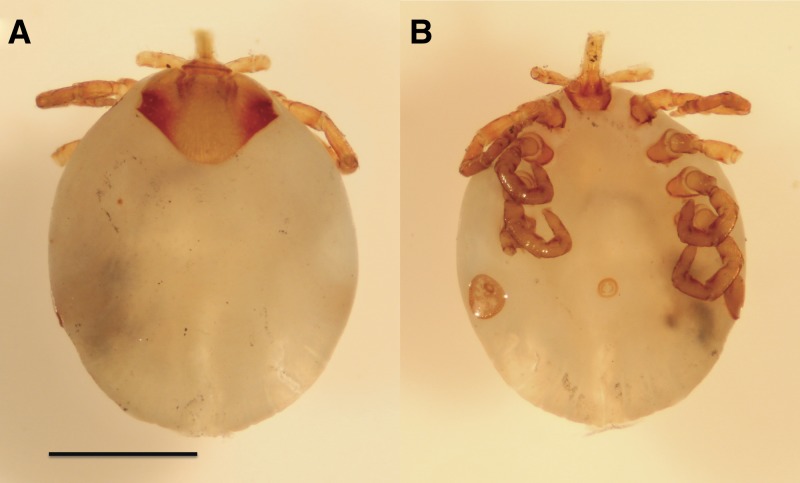
In June 2012, immediately upon returning to the United States after approximately three weeks in Kibale, one of us (TLG) discovered a tick in his right nostril. The tick was attached to the upper lateral nasal cartilage at the level of the rhinion (osseocartilaginous junction with the nasal bone). As reported in previous studies,3,5 the infestation caused mild irritation but no epistaxis. The tick was removed intact by using forceps and placed in RNAlater solution (Invitrogen, Grand Island, NY). Dorsal and ventral photographs of the tick were obtained by using a SMZ-745T Zoom Stereo Photo Microscope with 2× auxiliary objective (Nikon Instruments, Melville, NY), and a leg plus an additional tibia and tarsus from a second leg were removed for DNA extraction.
Total DNA was extracted by using the DNeasy Blood and Tissue kit (QIAGEN, Valencia, CA) and used as template in separate touchdown polymerase chain reactions to amplify a 360-basepair fragment of the tick mitochondrial 12S ribosomal RNA (rRNA)12 and the 1,041-basepair tick nuclear 5.8S–28S rRNA intergenic transcribed spacer 2 (ITS2).13 DNA extracts from two ticks of known identity (A. maculatum and A. americanum, collected in Texas) were used as positive controls. After purification of the amplicons (ExoSAP-IT; Affymetrix, Santa Clara, CA), bidirectional Sanger sequencing was performed on an ABI 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA) at Eton Bioscience (San Diego, CA).
Based on preliminary assessments of tick morphology and DNA sequences, 12S rRNA and ITS2 sequences of all African Amblyomma ticks in GenBank were downloaded and used in phylogenetic analyses. Sequences were aligned by using Clustal Omega14 with manual adjustment. Phylogenetic trees were constructed from aligned sequences by using the neighbor-joining method15 implemented in the computer program MEGA5,16 with a maximum composite likelihood distance correction and 1,000 bootstrap replicates of the data. Sequences of 12S rRNA and ITS2 from the nostril tick, A. maculatum, and A. americanum were deposited in Genbank (accession numbers KC538939–KC538944).
Results
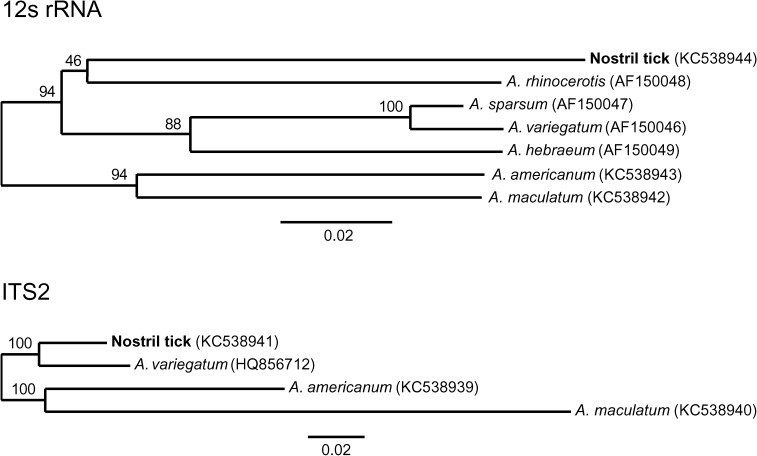
During January 2011–July 2012, high-resolution photography identified nine chimpanzees with ticks in their nostrils of 45 chimpanzees photographed, resulting in a period prevalence of 20% (95% confidence interval = 10.9–33.8%; Figure 1). Some chimpanzees were photographed with nostril ticks on more than one occasion, including in alternating nostrils, indicating independent infestations (Figure 1). The tick removed from the nostril of the researcher was identified morphologically as a partially engorged nymph belonging to the genus Amblyomma (Figure 2), but species-level morphologic identification was not possible; it is generally not possible in practice to identify wild-caught African nymphal ticks of this genus to the species level based on morphologic characteristics.17 DNA sequences of 12S rRNA and ITS2 did not match any sequences in GenBank but were closest to A. rhinocerotis in the case of 12S rRNA (85.2% nucleotide similarity) and A. variegatum in the case of ITS-2 (94.4% nucleotide similarity). Phylogenetic trees of 12S rRNA and ITS2 showed the nostril tick to cluster with known African Amblyomma species but to be divergent from any published sequence13,18 (Figure 3).
Figure 1.
Nostril ticks in young chimpanzees in Kibale National Park, Uganda, 2011–2012. A, Thatcher, born in November 2011, with right nostril tick on February 27, 2012. B, Thatcher with a left nostril tick on July 24, 2012. C, Thatcher with both nostrils occluded by ticks on December 2, 2011. D, Betty, born in January 2011, with a left nostril tick on January 23, 2011. Photo credits: Andrew B. Bernard, Ronan M. Donovan, and Jessica A. Hartel.
Figure 2.
Tick removed from a human nostril in July 2012 after field research in Kibale National Park, Uganda. A, Dorsal. B, Ventral. Note that leg 4 on the right side and the tibia and tarsus of leg 2 on left side were removed for DNA extraction. Scale bar = 1 mm. Photo credit: Gabriel L. Hamer.
Figure 3.
Phylogenetic trees of nostril tick and other ticks of the genus Amblyomma from 12S ribosomal RNA (rRNA) (top) and nuclear intergenic transcribed spacer 2 (ITS2) (bottom) sequence alignments constructed by using the neighbor-joining method. All African Amblyomma species in Genbank were included, as were A. americanum and A. maculatum from Texas that were processed along with the nostril tick as positive controls. Alignment lengths were 319 and 1,015 nucleotide positions, respectively. GenBank accession numbers are shown in parentheses. Numbers above branches indicate bootstrap values based on 1,000 resamplings of the data. Scale bars indicate nucleotide substitutions per site.
Discussion
We are aware of only two previous reports of infestations of human nostrils with ticks, and these infestations both occurred in visitors to the forests of western Uganda.3,5 Anecdotally, however, such infections are not uncommon. For example, the researcher infested in the current study recalls two prior tick infestations in his nostrils during two previous visits to Kibale over approximately nine years. In addition, two of us (JAH and RWW) recall hosting nostril ticks during visits to Kibale, and reports of such infestations from other researchers and field personnel in Kibale occur intermittently.
The nostril tick that we identified was a member of the genus Amblyomma, as has been reported,3,5 but DNA sequences of this particular species did not previously exist in GenBank. Thus, this tick is either a recognized species that has not yet been sequenced or an unrecognized species. Adult specimens would likely be necessary to make this determination, which might necessitate either trapping an adult or allowing a nymph to feed to repletion and molt. To date, however, our efforts to recover ticks from the forest floor in locations frequented by chimpanzees have been unsuccessful, and we have not yet had the opportunity to allow a nymph to feed to repletion. In Africa, a total of 22 tick species in the genus Amblyomma have been described,19 of which 10 are known to occur in Uganda and 5 others possibly occur there.17 Limited genetic data on these species are available; for example, as of July 7, 2013, mitochondrial 12S rRNA or ITS2 sequences of only four such species were represented in GenBank, and even fewer African Amblyomma species were represented by sequences of other loci.
We note that we likely underestimated infestation rates of chimpanzees because ticks may have been present at times when animals were not photographed, and ticks may have been present but minimally engorged or attached deep within the nostril and therefore not visible. We also note that it is not definitive that the tick removed from the human nostril was of the same species as the ticks observed in chimpanzee nostrils. Despite the remarkable degree of habituation of the Kanyawara chimpanzees, it remains impractical to collect ticks directly from the nostrils of these wild apes. Nevertheless, the coincidence of the human infestation with a high frequency of chimpanzee infestation in the same location is suggestive.
Despite close daily observation and high-resolution photography, we did not observe engorged ticks on the bodies of the chimpanzees studied, even on hairless regions. Grooming is a frequent activity among chimpanzees, and its importance for social bonding cannot be overstated.20 A tick that specializes on chimpanzees would clearly benefit from defenses against grooming, such as seeking an attachment site anatomically inaccessible to manual removal; infestation of the nostril would appear to be ideal in this regard. In this light, it is noteworthy that the chimpanzee louse (Pediculus schaeffi) will suddenly become stiff and motionless when exposed to light, even falling from the hair like a piece of debris, presumably to evade detection when the hair of a chimpanzee is parted during grooming.21 Such countermeasures to grooming may be common adaptations of primate ectoparasites.
Many Ambloymma ticks are three-hosts ticks, requiring blood meals from three separate vertebrate hosts, with off-host time spent on the forest floor or on vegetation, including underbrush and the forest canopy.22 In sub-Saharan Africa, Amblyomma spp. ticks transmit Ehrlichia ruminantium, Rickettsia africae, and Coxiella burnetii, which are the agents of heartwater disease, African tick bite fever, and Q fever, respectively.4,23,24 Our findings raise the possibility that nostril ticks could transmit these or similar diseases between humans and chimpanzees, as well as to and from other terrestrial or arboreal species on which the ticks feed.4 The risks of zoonotic transmission may be increased for researchers, research personnel, and others who spend time in forested habitats frequented by chimpanzees.8,9
Finally, we note that we did not discover our nostril tick until after the researcher had returned to his home country, similar to a previous report.5 The subtle host-seeking and attachment behavior that must accompany tick infestation of the highly innervated mammalian nostril supports the hypothesis that this tick is adapted to infesting nasal passages. More generally, although infestation with nostril ticks is a rare event, a great many persons visit wild ape habitats each year for such purposes as tourism and research.7 Rapid international travel of persons unknowingly infested with nostril ticks and other similar parasites could, under the right circumstances, lead to the establishment of exotic tick populations or the transmission of tick-borne diseases far from their countries of origin.25,26
ACKNOWLEDGMENTS
We thank the Uganda Wildlife Authority and the Uganda National Council for Science and Technology for granting us permission to conduct research in Kibale National Park; the Makerere University Biological Field Station, the Kibale Chimpanzee Project, and the Kibale EcoHealth Project for providing support in the field; and Lorenza Beati-Ziegler and Richard G. Robbins for helpful comments and discussion.
Footnotes
Financial support: This study was supported in part by National Institutes of Health grant TW009237 as part of the joint National Institutes of Health–National Science Foundation Ecology of Infectious Disease program and the United Kingdom Economic and Social Research Council.
Authors' addresses: Sarah A. Hamer, Department of Veterinary Integrative Biosciences, Texas A&M University, College Station, TX, E-mail: shamer@cvm.tamu.edu. Andrew B. Bernard, Merchantville, NJ, E-mail: andrew.b.bernard@gmail.com. Ronan M. Donovan, Helena, MT, E-mail: ronmdon@gmail.com. Jessica A. Hartel, Department of Biological Sciences, University of Southern California, Los Angeles, CA, E-mail: hartelj@gmail.com. Richard W. Wrangham, Department of Human Evolutionary Biology, Harvard University, Cambridge, MA, E-mail: wrangham@fas.harvard.edu. Emily Otali, Makerere University Biological Field Station, Fort Portal, Uganda, E-mail: eotali@yahoo.co.uk. Tony L. Goldberg, Department of Pathobiological Sciences, University of Wisconsin-Madison, Madison, WI, E-mail: tgoldberg@vetmed.wisc.edu.
References
- 1.Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cumming GS. Using habitat models to map diversity: pan-African species richness of ticks (Acari: Ixodida) J Biogeogr. 2000;27:425–440. [Google Scholar]
- 3.Walton GA. A tick infecting the nostrils of man. Nature. 1960;188:1131–1132. doi: 10.1038/1881131a0. [DOI] [PubMed] [Google Scholar]
- 4.Van der Borght-Elbl A. Ixodid Ticks (Acarina, Ixodiae) of Central Africa. Volume 5. Tervuren, Belgium: Musee Royal de l'Afrique Centrale; 1977. [Google Scholar]
- 5.Aronsen GP, Robbins RG. An instance of tick feeding to repletion inside a human nostril. Bulletin of the Peabody Museum of Natural History. 2008;49:245–248. [Google Scholar]
- 6.Struhsaker T. Ecology of an African Rain Forest: Logging in Kibale and the Conflict between Conservation and Exploitation. Gainesville, FL: University Press of Florida; 1997. [Google Scholar]
- 7.Wrangham R, Ross E. Science and Conservation in African Forests: The Benefits of Long-Term Research. Cambridge, UK: Cambridge University Press; 2008. [Google Scholar]
- 8.Goldberg TL, Gillespie TR, Rwego IB, Wheeler ER, Estoff EE, Chapman CA. Patterns of gastrointestinal bacterial exchange between chimpanzees and humans involved in research and tourism in western Uganda. Biol Conserv. 2007;135:511–517. [Google Scholar]
- 9.Rwego IB, Isabirye-Basuta G, Gillespie TR, Goldberg TL. Gastrointestinal bacterial transmission among humans, mountain gorillas, and livestock in Bwindi impenetrable National Park, Uganda. Conserv Biol. 2008;22:1600–1607. doi: 10.1111/j.1523-1739.2008.01018.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith TM, Machanda Z, Bernard AB, Donovan RM, Papakyrikos AM, Muller MN, Wrangham R. First molar eruption, weaning, and life history in living wild chimpanzees. Proc Natl Acad Sci USA. 2013;110:2787–2791. doi: 10.1073/pnas.1218746110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson ML, Kahlenberg SM, Wells M, Wrangham RW. Ecological and social factors affect the occurrence and outcomes of intergroup encounters in chimpanzees. Anim Behav. 2012;83:277–291. [Google Scholar]
- 12.Hickson RE, Simon C, Cooper A, Spicer GS, Sullivan J, Penny D. Conserved sequence motifs, alignment, and secondary structure for the third domain of animal 12S rRNA. Mol Biol Evol. 1996;13:150–169. doi: 10.1093/oxfordjournals.molbev.a025552. [DOI] [PubMed] [Google Scholar]
- 13.Beati L, Patel J, Lucas-Williams H, Adakal H, Kanduma EG, Tembo-Mwase E, Krecek R, Mertins JW, Alfred JT, Kelly S, Kelly P. Phylogeography and demographic history of Amblyomma variegatum (Fabricius) (Acari: Ixodidae), the tropical bont tick. Vector Borne Zoonotic Dis. 2012;12:514–525. doi: 10.1089/vbz.2011.0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthysse JG, Colbo MH. The Ixodid Ticks of Uganda: Together with Species Pertinent to Uganda because of Their Present Known Distribution. College Park, MD: Entomological Society of America; 1987. [Google Scholar]
- 18.Beati L, Keirans JE. Analysis of the systematic relationships among ticks of the genera Rhipicephalus and Boophilus (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. J Parasitol. 2001;87:32–48. doi: 10.1645/0022-3395(2001)087[0032:AOTSRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Voltzit OV, Keirans JE. A review of African Amblyomma species (Acari, Ixodida, Ixodidae) Acarina. 2003;11:135–214. [Google Scholar]
- 20.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge, MA: Harvard University Press; 1986. [Google Scholar]
- 21.Kuhn HJ. Parasites and the phylogeny of the cararrhine primates. In: Chiarelli B, editor. Taxonomy and Phylogeny of Old World Primates with References to the Origin of Man. Torino, Italy: Rosenberg and Seller; 1967. pp. 187–195. [Google Scholar]
- 22.Sonenshine DE. Biology of Ticks. New York: Oxford University Press; 1991. [Google Scholar]
- 23.Cazorla C, Socolovschi C, Jensenius M, Parola P. Tick-borne diseases: tick-borne spotted fever rickettsioses in Africa. Infect Dis Clin North Am. 2008;22:531–544. doi: 10.1016/j.idc.2008.03.009. ix–x. [DOI] [PubMed] [Google Scholar]
- 24.Walker JB. The tick vectors of Cowdria ruminantium (Ixodidea, Ixodidae, Genus Amblyomma) and their distribution. Onderstepoort J Vet. 1987;54:353–379. [PubMed] [Google Scholar]
- 25.Randolph SE, Rogers DJ. The arrival, establishment and spread of exotic diseases: patterns and predictions. Nat Rev Microbiol. 2010;8:361–371. doi: 10.1038/nrmicro2336. [DOI] [PubMed] [Google Scholar]
- 26.Hamer SA, Goldberg TL, Kitron UD, Brawn JD, Anderson TK, Loss SR, Walker ED, Hamer GL. Wild birds and urban ecology of ticks and tick-borne pathogens, Chicago, Illinois, USA, 2005–2010. Emerg Infect Dis. 2012;18:1589–1595. doi: 10.3201/eid1810.120511. [DOI] [PMC free article] [PubMed] [Google Scholar]