Abstract
Alternatives to culture are needed in high burden countries to assess whether response to treatment of multidrug-resistant-tuberculosis (MDR-TB) is satisfactory. The objective was to assess the association of weight gain and treatment outcome. The methods included analysis of clinical, bacteriologic, and weight from 439 MDR-TB patients in the Philippines. Odds ratios (ORs) were calculated to determine whether 5% weight gain during the first 6 months of treatment was associated with outcome. Three hundred and ten (71%) patients were cured and 129 (29%) had poor outcomes (death, defaulted, or failed treatment). Fifty-three percent were underweight (body mass index [BMI] < 18.5 kg/m2) before treatment. Five percent weight gain after completing 3 months of treatment was associated with good outcome among patients who were underweight before treatment (OR 2.1; 95% confidence interval [CI], 1.05 to 4.4). Baseline weight and degree of weight change during the first 6 months of treatment can help identify persons who are more likely to have poor outcomes and require other interventions.
Introduction
Identification of surrogate markers of treatment response and risk for poor outcome would be of benefit to tuberculosis (TB) programs as tools for individual patient management. Patients with markers of poor response could be targeted for longer treatment, post-treatment surveillance, or other interventions. The most important outcomes for patients during treatment of a disease are survival, disability, and capacity for functioning in daily life1; the outcomes measured in TB treatment programs mainly focus on microbiological endpoints such as sustained sputum culture conversion for cure and persistent smear or culture positivity for failure. In several earlier studies involving patients, the majority of whom had drug-susceptible or likely drug-susceptible TB, body weight gain was associated with cure after TB treatment.2–6 Self-reported resolution and reappearance of symptoms also has been shown to be associated with microbiologically defined success and relapse.7
Because multidrug-resistant-TB (MDR-TB) treatment averages 18 to 24 months in duration, there is a great need to identify early indicators of a good response to treatment. Now that treatment of MDR-TB is being rolled out to resource-poor countries where access to sputum culture is limited, simple measures of treatment response need to be considered. Most patients with MDR-TB are chronically ill and malnourished.8–12 Weight gain could be a potentially useful biomarker of response to MDR-TB treatment. Weight gain is simple to measure, inexpensive to obtain, and easily accessible. Nearly all TB clinics have a scale and most weigh their patients monthly during treatment.
The Philippines is one of the 27 countries with the highest burden of MDR-TB worldwide, with an estimated 8,800 new patients with MDR-TB in 2010.13 The programmatic management of drug-resistant TB (PMDT) initiative is expanding services to cover the entire country; however, laboratory capacity will not be rolled out as rapidly as clinical capacity. Monitoring weight gain during treatment could be a practical tool to identify patients in whom treatment is failing and who may benefit from earlier re-evaluation, longer treatment, or more frequent follow-up. To further assess the value of monitoring weight gain during treatment as a predictor of treatment outcome, we analyzed prospectively collected data combined with retrospectively collected data on weight in a large treatment MDR-TB treatment cohort with good follow-up and serial bacteriological monitoring.
Methods
Study population.
The Preserving Effective TB Treatment Study (PETTS) was a multinational, prospective observational study whose primary objective was to determine risk factors for amplification of drug resistance during the treatment of MDR-TB.14 Eligibility criteria for PETTS included culture-confirmed MDR-TB, 18 years of age or older, and initiation of second-line drug treatment within 30 days of the baseline positive sputum culture. From January 2005 to June 2008, 787 patients with MDR-TB were started on treatment in the Philippines national PMDT. Of these, 482 patients (60%) were enrolled into the PETTS study. Because this cohort had regular follow-up and monthly sputum cultures, we used this cohort for analysis. The study collected demographic and clinical data including baseline height and weight. Monthly weights during treatment were abstracted from patient charts and entered into the study database.
Treatment regimens and monitoring.
Treatment of MDR-TB in the Philippines was initiated in 1999 by the Tropical Disease Foundation, a non-government organization in Manila, as the first Green Light Committee-approved program. Treatment was individualized based on drug susceptibility testing results. Human immunodeficiency virus (HIV) testing of patients was not done. Patients were followed up in the clinic weekly during the first 2 months and then monthly. At each visit, adherence with treatment, TB symptoms, and drug side effects were reviewed. All clinics were equipped with simple beam balance scales. Patients were weighed weekly during each scheduled visit during the first 6 months of treatment and monthly thereafter. Sputum smears and cultures were performed monthly on Lowenstein-Jensen media. Drug susceptibility testing was performed using published methods at a single quality controlled laboratory at baseline and then after 4 months of treatment if cultures were still positive.15
Prescribed treatment regimens included at least four drugs to which the patient's isolate was susceptible, including a second-line injectable drug during the first 6 months or until the patient was culture negative for 4 months, a fluoroquinolone that the patient had not previously received, and at least two group four drugs (cycloserine, prothionamide, para-aminosalicyclic acid). Dosages were based on the Partners in Health MDR-TB treatment handbook.16 All drugs were manufactured under good manufacturing practice. Doses were administered by directly observed therapy (DOT) 6 days per week at three MDR-TB treatment centers in Manila. A health care worker or trained lay supervisor watched the patient swallow each dose of drugs and looked in the patient's mouth after dosing to assure that the drugs were swallowed and not cheeked. Food baskets and reimbursement for public transport were given to low income patients as defined by the Philippines national economic scheme. Roughly 70% of our patients received such assistance. Patients who missed doses for more than 6 days were traced by home visits. This was a routine strategy used by the treatment centers to prevent long-term default.
MDR-TB treatment outcomes.
Baseline weight was the weight recorded before or during the first week of treatment. Monthly weight was the weight taken during the last week of the treatment month or the first week of the subsequent month if the former was not available. Underweight was defined as a body mass index (BMI) of < 18.5 kg/m2.17 Five percent weight gain was defined as an increase by 5% in the baseline weight calculated as the difference in weights at the two times divided by the weight at the earlier time × 100. Final MDR-TB treatment outcomes were based on international consensus and World Health Organization (WHO) guidelines distinguishing the following categories: cure, completion, default, failure, and death.18,19 Cure was defined as completion of treatment with five or more negative cultures over at least 12 months after the last positive culture or having completed 468 doses after sustained culture conversion. According to the WHO guidelines, an exception was made for a single positive culture with low colony counts during the last 12 months followed by at least three negative monthly cultures. Culture conversion was defined as the month when the culture first converted to negative followed by 2 months of negative cultures. Completion of treatment was defined as completion of therapy with bacteriological conversion persisting through the end of treatment but fewer than five negative cultures or < 12 months of follow-up after the last positive culture. Default was defined as missing all doses of anti-TB treatment for at least two consecutive months without ever returning to treatment. Treatment was considered to have failed if the patient had more than one positive sputum culture during the final 12 months of treatment or terminated treatment early. For this analysis, patients were classified as having favorable treatment outcome if they were cured or completed treatment. Poor outcomes included those who defaulted, died, or experienced treatment failure.
Data management and analysis.
Treatment records were initially captured on a standardized paper form and double-entered into an Epi Info 3.3.2 database (CDC, Atlanta, GA).20 Analyses were performed with Stata (version 9.2, Stata, College Station, TX). Receiver operating characteristic (ROC) curves was used to determine suitable cutoffs for weight gain after 1–6 months of treatment that would predict favorable outcome21; the association of weight gain during therapy and treatment outcome varied according to initial weight. We used body weight as a continuous variable in our initial analysis but did not detect an effect of weight gain on treatment outcome. As done in several previously published studies of weight gain and treatment outcome in drug-susceptible and drug-resistant TB,3,4,9–12,22 we next stratified baseline BMI using a WHO Nutrition Unit-recommended cut-point for underweight (< 18.5 mg/kg2) to identify patients at risk because of thinness or underweight for public health, disease control program, and interventional studies.23 When we stratified the population as underweight or not using this cut-point (BMI < 18.5 kg/m2), we found an effect on treatment outcome.
We used ROC curve analyses to determine the cutoffs (based on an acceptable trade-off between sensitivity and specificity to detect a good outcome) to obtain the proportion of subjects who gained weight at the end of each treatment month relative to baseline. The ROC curves were created considering treatment outcome versus weight gain of 1% after 1 month of treatment, 2% after 2 months of treatment, 3% after 3 months of treatment, and 4% after 4 months of treatment of the two groups. We used 5% gain over the first part of treatment because a weight gain of this magnitude (corresponding to a weight gain of 2.5 kg in a 50 kg patient) was likely reproducible, clinically important, and represented a true increase in lean body mass. In comparing patient characteristics of those with favorable outcomes versus poor outcomes, we used the χ2 test for categorical variables and independent samples t test for continuous variables. P values < 0.05 were considered significant. To quantify the magnitude of the effect of 5% weight gain on outcome, odds ratios (ORs) with 95% confidence intervals (CI), adjusted for age, sex, and other factors that were significant in the univariate analyses, were calculated.
Ethical considerations.
The study protocol was approved by the Tropical Disease Foundation and the U.S. Centers for Disease Control and Prevention institutional review boards. All patients gave informed consent for study participation.
Results
Four hundred and eighty-two patients with culture-confirmed MDR-TB were enrolled in the PETTS study at MDR-TB treatment centers in Manila from January 2005 to June 2008. Forty-three patients were excluded from this analysis because no baseline weight was recorded in the medical record. The remaining 439 patients constitute the population for this study. Three hundred and ten (71%) patients had favorable treatment outcomes and 129 (29%) had poor outcomes. Of those with poor outcomes, 81 (63%) defaulted, 33 (26%) died, and 15 (12%) patients failed. After 3 months of treatment of MDR-TB, 345 patients who had both baseline and Month 3 weights. Of these, 83 (24%) had poor outcomes.
The median waiting time from screening to treatment was 205 days (interquartile range [IQR] 157–285). The median number of months of treatment was 20.3 months (IQR 19.1–22.5). Culture conversion occurred after 1 month of MDR-TB treatment of 58% (256 of 439) of the patients, by the end of 2 months for 82% (360 of 439), and by the end of 3 months in 92% (403 of 439) of patients. The median time to culture conversion was 1 month (IQR 1–2 months; range 1–16 months). The mean weight and BMI for all patients was 47.4 kg (SD 11.2 kg) and 18.5 kg/m2 (SD 4.2 kg/m2), respectively. Fifty-three percent (233 of 439) of all patients were underweight before treatment.
In a univariate analysis (Table 1), the baseline characteristics of patients who were cured versus those who had poor outcome were similar except for culture conversion and treatment interruptions. Poor treatment outcome was associated with late (after 3 or more months of treatment) sputum culture conversion and any treatment interruption. Poor outcome was not associated with being underweight before treatment, age, sex, admission to hospital, or cavitary lesions on a chest radiograph. Those with poor outcomes had a lower BMI though not more likely to meet the “underweight” category.
Table 1.
Baseline sociodemographic and clinical characteristics of 439 patients with MDR-TB included in this analysis*
| Characteristic | Outcome N (%) | P value | |
|---|---|---|---|
| Favorable outcome N = 310 | Poor outcome N = 129 | ||
| Male | 190 (61) | 81 (63) | 0.768 |
| Age (years; mean ± SD) | 38.1 + 11.8 | 38.5 + 12.5 | 0.723 |
| Culture conversion to negative (N = 434) | |||
| Late (after 3 months or more) | 41 (13) | 33 (27) | 0.001 |
| Early (1 to 2 months) | 269 (87) | 91 (73) | |
| Number of previous TB episodes | |||
| 3 or more | 177 (57) | 67 (52) | 0.322 |
| 1 or 2 episodes | 133 (43) | 62 (48) | |
| Previous TB treatment | |||
| First-line drugs only | 266 (86) | 114 (88) | 0.473 |
| First- and second-line drugs | 44 (14) | 15 (12) | |
| Previous treatment with a fluoroquinolone | 42 (14) | 14 (11) | 0.441 |
| Previous treatment with second-line injectable drug | 5 (2) | 3 (2) | 0.611 |
| Weight (kg; mean ± SD) | 48.0 (10.9) | 45.8 (11.8) | 0.08 |
| Body mass index (BMI; kg/m2 ± SD) | 18.8 (4.4) | 17.8 (3.8) | 0.02 |
| Underweight (BMI < 18.5 kg/m2) | 161 (52) | 72 (56) | 0.458 |
| Diabetes mellitus | 83 (27) | 34 (26) | 0.928 |
| Highest educational attainment | |||
| Primary | 46 (14.8) | 30 (23.3) | 0.098 |
| Secondary | 134 (43.2) | 48 (37.2) | |
| Post-secondary | 130 (41.9) | 51 (39.5) | |
| Marital status | |||
| Single/widowed/separated | 118 (38.1) | 43 (33.3) | 0.349 |
| Married/cohabiting | 192 (61.9) | 86 (66.7) | |
| Used | 218 (70) | 70 (61) | 0.064 |
| Waiting time to treatment ≥ 6 months | 202 (66) | 74 (58) | 0.15 |
| Contact with any TB patient | 180 (61) | 79 (64) | 0.564 |
| Admission to TB facility | 32 (10) | 16 (12) | 0.525 |
| Cavitary disease on baseline chest radiograph | 158 (52) | 58 (46) | 0.34 |
| Failed earlier TB treatment | 176 (57) | 78 (61) | 0.476 |
| Any treatment interruption | 59 (19) | 82 (64) | < 0.001 |
Values represent the number of patients and percentages unless otherwise noted.
TB = tuberculosis; BMI = body mass index.
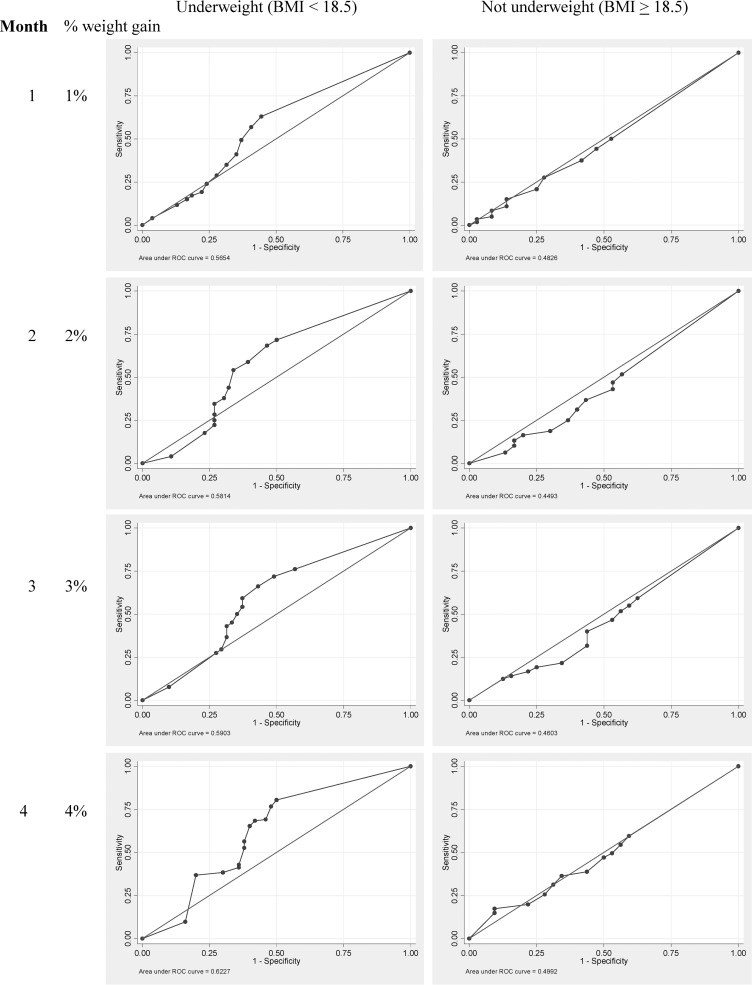
The overall cumulative percentage weight gain from baseline through the completion of 6 months of treatment of MDR-TB was 5.6% (IQR 1.4 to 11.7) (Table 2). This table reflects the numbers of patients who had weights at each month. The ROC curves showed weight gain during the first 4 months of treatment using the threshold definitions and was associated with a good outcome for patients who were underweight (≤ 18.5 kg/m2) before treatment (Figure 1 ).
Table 2.
Cumulative percent weight gain from baseline per month of anti-TB treatment (N = 356)*
| Month | Median (IQR) | ||
|---|---|---|---|
| Overall | Patients with baseline BMI < 18.5 | Patients with baseline BMI ≥ 18.5 | |
| 1 (N = 356) | 0.9 (−3.0–4.6) | 1.3 (−2.6, 5.9) | 0.7 (−3.2, 3.2) |
| 2 (N = 362) | 1.9 (−3.0–6.4) | 2.8 (−2.0, 8.2) | 0.7 (−3.7, 5.1) |
| 3 (N = 345) | 3.1 (−1.9–8.6) | 3.9 (−0.2, 10.8) | 2.5 (−4.3, 5.9) |
| 4 (N = 335) | 4.0 (−2.2–9.7) | 5.8 (−0.6, 12.4) | 2.4 (−3.8, 7.2) |
| 5 (N = 324) | 4.8 (−2.2–11.5) | 6.1 (−0.5, 14.2) | 3.6 (−3.0, 8.3) |
| 6 (N = 314) | 5.6 (−1.4–11.7) | 7.6 (0, 14.7) | 3.2 (−2.7, 9.1) |
Only patients with baseline and the respective monthly weight were included in the analysis. Four patients defaulted at Month 3, one defaulted at Month 4, two defaulted and two died at Month 5, and five defaulted and three died at Month 6.
TB = tuberculosis; IQR = interquartile range; BMI = body mass index.
Figure 1.
Receiver operating characteristics (ROC) curve to determine sensitivity and specificity of different percentage weight change during the first 4 months of multidrug-resistant-tuberculosis (MDR-TB) treatment (N = 439).
In a multivariate analysis (Table 3), controlling for age, sex, culture conversion, and any treatment interruption, weight gain of 5% after 3 months of treatment was associated with good outcome among underweight patients (OR 2.1; 95% CI, 1.05 to 4.4). This association was also significant with 5% weight gain after 4, 5, and 6 months of treatment with the strongest association observed after 4 months of treatment (OR 2.8; 95% CI, 1.3 to 6.2).
Table 3.
Multivariate analysis of body weight gain during treatment and treatment outcome (N = 439)
| Weight gain from baseline | Underweight (BMI < 18.5) | Not underweight (BMI ≥ 18.5) | ||||
|---|---|---|---|---|---|---|
| Cured | Poor outcome | Adjusted OR (95% CI)* | Cured | Poor outcome | aOR* (95% CI) | |
| Month 1 | ||||||
| > 5% | 42 (73.7) | 15 (26.3) | 0.9 (0.4, 1.9) | 18 (78.3) | 5 (21.7) | 1.1 (0.4, 3.2) |
| ≤ 5% | 104 (72.7) | 39 (27.3) | ref | 102 (76.7) | 31 (23.3) | ref |
| Month 2 | ||||||
| > 5% | 56 (76.7) | 17 (23.3) | 1.2 (0.6, 2.5) | 32 (74.4) | 11 (25.6) | 0.6 (0.2, 1.3) |
| ≤ 5% | 92 (70.2) | 39 (29.8) | ref | 96 (83.5) | 19 (16.5) | ref |
| Month 3 | ||||||
| > 5% | 71 (79.8) | 18 (20.2) | 2.1 (1.1, 4.4) | 38 (73.1) | 14 (26.9) | 0.6 (0.3, 1.3) |
| ≤ 5% | 71 (68.3) | 33 (31.7) | ref | 82 (82.0) | 18 (18.0) | ref |
| Month 4 | ||||||
| > 5% | 75 (79.8) | 19 (20.2) | 2.8 (1.3, 6.2) | 44 (80.0) | 11 (20.0) | 1.1 (0.5, 2.5) |
| ≤ 5% | 57 (64.8) | 31 (35.2) | ref | 77 (78.6) | 21 (21.4) | ref |
| Month 5 | ||||||
| > 5% | 78 (80.4) | 19 (19.6) | 2.8 (1.3, 5.9) | 48 (75.0) | 16 (25.0) | 0.6 (0.3, 1.4) |
| ≤ 5% | 51 (63.0) | 30 (37.0) | ref | 68 (82.9) | 14 (17.1) | ref |
| Month 6 | ||||||
| > 5% | 80 (78.4) | 22 (21.6) | 2.3 (1.1, 5.3) | 51 (81.0) | 12 (19.1) | 0.7 (0.3, 1.7) |
| ≤ 5% | 47 (67.1) | 23 (32.9) | ref | 68 (86.1) | 11 (13.9) | ref |
Adjusted for age, sex, culture conversion, and any treatment interruption.
BMI = body mass index; OR = odds ratio; CI = confidence interval; aOR = adjusted odds ratio.
The sensitivity of 5% weight gain during the first 3 months of treatment to predict good outcome was 50% (95% CI, 41.5 to 58.5). The specificity of 5% weight gain to predict poor outcome was 64.7% (95% CI, 50.1 to 77.6). The positive predictive value was 80% (95% CI, 70 to 85.6) and the negative predictive value was 32% (95% CI, 23 to 41.6).
Discussion
Weight gain during the first 3 months of treatment was an important predictor of long-term treatment success in underweight patients starting treatment of MDR-TB. More than 5% weight gain during the first 3 months of treatment was associated with good outcome. This association remained consistent after 4, 5, and 6 months of treatment after controlling for age, sex, culture conversion, and treatment interruption. Weight gain was not associated with outcome among patients with BMI equal or > 18.5 kg/m2 at the time they started treatment. The majority of poor outcomes in this cohort occurred in patients who defaulted (63%). The association of default and failure to gain weight may be caused by factors such as poor drug tolerability and side effects or limited income and ability to afford food stuffs—information not fully captured in our study.
Our results add to knowledge about weight gain and response to TB treatment. In an earlier study by Khan and colleagues3 among 857 United States and Canadian patients with drug-susceptible TB, patients who had a weight deficit of 10% or more of their ideal body weight at diagnosis and who gained at least 5% of their baseline weight during the first 2 months of treatment had a lower risk for later relapse. A study in 27 Indian patients with MDR-TB treated with individualized therapy reported that weight gain after 6 months of therapy was a predictor of successful outcome.24 Among patients with MDR-TB in Peru, treatment failure and death were associated with low BMI (< 20 mg/kg2) before treatment.12 A recent study in Vietnam with 2,609 patients treated in DOTS clinics showed that those patients who had a baseline weight of < 40 kg and had a weight gain of more than 5% after 2 months of treatment had a lower risk of poor outcome.22 Our data generally confirm these results and suggest that weight gain during the first 3 months of treatment is an important indicator of outcome in patients who are underweight.
Although 5% weight gain during early treatment of MDR-TB in our study was associated with good outcomes only in patients who were underweight (BMI < 18.5 kg/m2) before treatment, most patients with MDR-TB are underweight. In recent published treatment cohorts and clinical trials of adults with MDR-TB, 32 to over 75% of patients were underweight with a pre-treatment BMI of < 18.5 kg/m2.8–11,24,25 Underweight has also been shown to be an independent significant risk factor for poor treatment outcome and TB-related and all-cause mortality in patients treated for MDR-TB.11 Although the sensitivity of weight gain in predicting good outcome was only 50%, these high-risk patients can be readily identified and weight gain may be a useful indicator for programs where cultures are costly or not readily available.
Our study has several limitations. First, body weight was abstracted from patients' treatment cards and baseline weights and weights at some follow-up visits were not available for all patients. Missing weights were more frequent for patients with poor outcomes (data not shown). Second, the patients in this cohort were adults with MDR-TB of varying chronicity who were treated by a single DOTS-Plus program and may not be generalizable to all patients with MDR-TB. Third, most of the patients were poor and baseline malnutrition may have been caused by limited access to food, anorexia, and increased catabolism of energy and nutrients because of TB2,5; similarly, weight gain during treatment might have been due, in part, to improved access to food supplements provided during treatment and response to anti-TB drugs. However, provision of food supplements and transport assistance was based on income and economic status and not on body weight. Fourth, lack of weight gain might be related to poor tolerance of some second line drugs such as ethionamide that frequently causes vomiting. Fifth, patients were not tested for HIV co-infection. The prevalence of HIV infection in the Philippines26 and the prevalence of HIV co-infection in patients with TB13 is low (< 1%) and our results are unlikely to be confounded by HIV co-infection. Sixth, the simple beam balances used to measure patient weights were not calibrated regularly; however, these scales are among those most widely used in TB clinics in high burden countries. A 5% weight gain (corresponding, for example, to 2.5 kg in a 50 kg patient) can be measured reliably and is likely to be clinically relevant3; finally, this analysis derived a 5% weight gain criterion that needs validation in further studies in different populations.
Among the strengths of our study are that it is based on a large cohort of 439 patients with proven MDR-TB who received directly observed treatment and who were followed with serial clinical and bacteriological assessments at regular intervals. Smears and cultures were performed at an experienced, quality controlled laboratory. More than half of our patients were underweight before treatment therefore we had reasonable statistical power to detect an association between weight gain during treatment and treatment outcome in both groups. Body weight was recorded weekly during the first 6 months of treatment by well-trained staff.
In conclusion, a weight gain of 5% or more above baseline weight during the first 3 months of treatment of MDR-TB was associated with good outcome for patients who were underweight before treatment. In resource-limited countries, cultures are not available in most areas. Recording baseline body weight and height and following change in weight monthly during the first 6 months of treatment may be a practical tool to identify patients at risk for poor treatment outcome who require greater medical attention. We encourage others treating patients for MDR-TB and conducting clinical trials of new drugs and regimens for drug-resistant TB to collect data on body weight during treatment to better define the impact of weight change and treatment outcome. We also recommend that the association of early 5% body weight gain with good treatment outcome be validated in future MDR-TB treatment cohorts.
Disclaimer: The conclusions and data interpretations presented in this report are solely those of the authors and do not necessarily represent the official position of the U.S. Government or the authors' institutions.
Footnotes
Authors' addresses: Ma Tarcela Gler, Ruffy Guilatco, and Janice C. Caoili, Tropical Disease Foundation, PMDT, Makati City, Metro Manila, Philippines, E-mails: tarcelasg@yahoo.com, rsguilatco@gmail.com, and janice.caoili@gmail.com. Julia Ershova and Peter Cegielski, Centers for Disease Control and Prevention, Division of TB Elimination, Atlanta, GA, E-mails: jhe3@cdc.gov and gzc2@cdc.gov. John L. Johnson, Case Western Reserve University School of Medicine, Tuberculosis Research Unit, Department of Medicine, Cleveland, OH, E-mail: jlj@case.edu.
References
- 1.Temple R. A regulatory authority's opinion about surrogate endpoints. In: Nimmo WS, Tucker GT, editors. Clinical Measurement in Drug Evaluation. New York: John Wiley; 1995. pp. 3–22. [Google Scholar]
- 2.Harries AD, Nkhoma WA, Thompson PJ, Nyangulu DS, Wirima JJ. Nutritional status in Malawian patients with pulmonary tuberculosis and response to chemotherapy. Eur J Clin Nutr. 1988;42:445–450. [PubMed] [Google Scholar]
- 3.Khan A, Sterling TR, Reves R, Vernon A, Horsburgh CR. Lack of weight gain and relapse risk in a large tuberculosis treatment trial. Am J Respir Crit Care Med. 2006;174:344–348. doi: 10.1164/rccm.200511-1834OC. [DOI] [PubMed] [Google Scholar]
- 4.Krapp F, Veliz JC, Cornejo E, Gotuzzo E, Seas C. Bodyweight gain to predict treatment outcome in patients with pulmonary tuberculosis in Peru. Int J Tuberc Lung Dis. 2008;12:1153–1159. [PubMed] [Google Scholar]
- 5.Onwubalili JK. Malnutrition among tuberculosis patients in Harrow, England. Eur J Clin Nutr. 1988;42:363–366. [PubMed] [Google Scholar]
- 6.Vasantha M, Gopi PG, Subramani R. Weight gain in patients with tuberculosis treated under directly observed treatment short-course (DOTS) Indian J Tuberc. 2009;56:5–9. [PubMed] [Google Scholar]
- 7.Bark CM, Dietze R, Okwera A, Quelapio MI, Thiel BA, Johnson JL. Clinical symptoms and microbiological outcomes in tuberculosis treatment trials. Tuberculosis (Edinb) 2011;91:601–604. doi: 10.1016/j.tube.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Deun A, Maug A, Salim MA, Das PK, Sarker MR, Daru P, Reider HL. Short, highly effective, standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 9.Franke MF, Appleton SC, Bayona J, Arteaga F, Palacios E, Llaro K, Shin SS, Becerra MC, Murray MB, Mitnick CD. Risk factors and mortality associated with default from multidrug-resistant tuberculosis treatment. Clin Infect Dis. 2008;46:1844–1851. doi: 10.1086/588292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin SS, Pasechnikov AD, Gelmanova IY, Peremitin GG, Strelis AK, Mishustin S, Barnashov A, Karpeichik Y, Andreev YG, Golubchikova VT, Tonkel TP, Yanova GV, Yedilbayev A, Rich ML, Mukherjee JS, Furin JJ, Atwood S, Farmer PE, Keshavjee S. Adverse reactions among patients being treated for MDR-TB in Tomsk, Russia. Int J Tuberc Lung Dis. 2007;11:1314–1320. [PubMed] [Google Scholar]
- 11.Kim DH, Kim HJ, Park SK, Kong SJ, Kim YS, Kim TH, Kim EK, Lee KM, Lee SS, Park JS, Koh WJ, Lee CH, Kim JY, Shim TS. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med. 2008;178:1075–1082. doi: 10.1164/rccm.200801-132OC. [DOI] [PubMed] [Google Scholar]
- 12.Mitnick C, Bayona J, Palacios E, Shin S, Furin J, Alcantara F, Sanchez E, Sarria M, Becerra M, Fawzi MC, Kapiga S, Neuberg D, Maguire JH, Kim JY, Farmer P. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization . Global Tuberculosis Control. Geneva: WHO/HTM/TB/2011.16; 2011. WHO Report 2011. [Google Scholar]
- 14.Dalton T, Cegielski P, Aaksilp S, Asencios L, Caoili JC, Cho SN, Erokhin VV, Ershova J, Gler MT, Kazennyy BY, Kim HJ, Kliiman K, Kurbatova E, Kvasnovsky C, Leimane V, van der Walt M, Via LE, Volchenkov GV, Yagui MA, Kang H. Global PETTS Investigators Akksilp R, Sitti W, Wattanaamornkiet W, Andreevskaya SN, Chernousova LN, Demikhova OV, Larionova EE, Smirnova TG, Vasilieva IA, Vorobyeva AV, Barry CE, 3rd, Cai Y, Shamputa IC, Bayona J, Contreras C, Bonilla C, Jave O, Brand J, Lancaster J, Odendaal R, Chen MP, Diem L, Metchock B, Tan K, Taylor A, Wolfgang M, Cho E, Eum SY, Kwak HK, Lee J, Lee J, Min S, Degtyareva I, Nemtsova ES, Khorosheva T, Kyryanova EV, Egos G, Perez MT, Tupasi T, Hwang SH, Kim CK, Kim SY, Lee HJ, Kuksa L, Norvaisha I, Skenders G, Sture I, Kummik T, Kuznetsova T, Somova T, Levina K, Pariona G, Yale G, Suarez C, Valencia E, Viiklepp P. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet. 2012;380:1406–1417. doi: 10.1016/S0140-6736(12)60734-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gler MT, Macalintal LE, Raymond L, Guilatco R, Quelapio MI, Tupasi TE. Multi-drug resistant TB among previously treated patients in the Philippines. Int J Tuberc Lung Dis. 2011;15:652–656. doi: 10.5588/ijtld.10.0400. [DOI] [PubMed] [Google Scholar]
- 16.Rich ML. The PIH Guide to the Medical Management of Multidrug-Resistant Tuberculosis. International edition. Boston, MA: Partners in Health; 2003. pp. 1–146. [Google Scholar]
- 17.ExpertPanel on the Identification, Evaluation, and Treatment of Over- weight in Adults Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Am J Clin Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis: Emergency Update 2008. Geneva: WHO/HTM/TB/2008; 2008. [Google Scholar]
- 19.Laserson KF, Thorpe LE, Leimane V, Weyer K, Mitnick CD, Riekstina V, Zarovska E, Rich ML, Fraser HS, Alarcón E, Cegielski JP, Grzemska M, Gupta R, Espinal M. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9:640–645. [PubMed] [Google Scholar]
- 20.U.S. Centers for Disease Control and Prevention Epi Info. 2005. http://wwwn.cdc.gov/epiinfo/html/downloads.htm Available at. Accessed December 4, 2011.
- 21.Buyse M. Towards validation of statistically reliable biomarkers. Eur J Cancer. 2007;(Suppl 5):89–95. [Google Scholar]
- 22.Hoa NB, Lauritsen JM, Reider HL. Changes in bodyweight and tuberculosis outcome in Vietnam. Int J Tuberc Lung Dis. 2012;17:61–66. doi: 10.5588/ijtld.12.0369. [DOI] [PubMed] [Google Scholar]
- 23.Bailey KV, Ferro-Luzzi A. Use of body mass index of adults in assessing individual and community nutritional status. Bull World Health Organ. 1995;73:673–680. [PMC free article] [PubMed] [Google Scholar]
- 24.Dhingra VK, Rajpal S, Mittal A, Hanif M. Outcome of multi-drug resistant tuberculosis cases treated by individualized regimens at a tertiary level clinic. Indian J Tuberc. 2008;55:15–21. [PubMed] [Google Scholar]
- 25.Diacon A, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L, Pistorius C, Krause R, Bogoshi M, Churchyard G, Venter A, Allen J, Palomino JC, De Marez T, van Heeswijk RP, Lounis N, Meyvisch P, Verbeeck J, Parys W, de Beule K, Andries K, McNeeley DF. The diarylquinoline TMC 207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360:2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 26.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Report: UNAIDS Report on the Global AIDS Epidemic. Geneva: UNAIDS/10.11E/JC1058E; 2010. [Google Scholar]