Abstract
The highly populated floodplains of the Bengal Delta have a long history of endemic and epidemic cholera outbreaks, both coastal and inland. Previous studies have not addressed the spatio-temporal dynamics of population vulnerability related to the influence of underlying large-scale processes. We analyzed spatial and temporal variability of cholera incidence across six surveillance sites in the Bengal Delta and their association with regional hydroclimatic and environmental drivers. More specifically, we use salinity and flood inundation modeling across the vulnerable districts of Bangladesh to test earlier proposed hypotheses on the role of these environmental variables. Our results show strong influence of seasonal and interannual variability in estuarine salinity on spring outbreaks and inland flooding on fall outbreaks. A large segment of the population in the Bengal Delta floodplains remain vulnerable to these biannual cholera transmission mechanisms that provide ecologic and environmental conditions for outbreaks over large geographic regions.
Introduction
Cholera, an acute diarrheal illness caused by the bacterium Vibrio cholerae, remains a major public health threat in the developing world. Seasonal recurrence of this infectious disease in endemic areas, the role of climate in its proliferation, and recent epidemic outbreaks across different continents have greatly challenged scientists, epidemiologists, and public health professionals. The life cycle of V. cholerae is intricately linked to two types of processes with vastly different spatial and temporal scales.1 Despite major advances in the understanding of the microbiologic and genetic level processes surrounding the bacterium, the role of the large-scale hydrologic, ecologic, and climatic (referred to as macro-scale) processes in propagating the disease in space and time is not well understood. For example, the existence of an aquatic environmental reservoir of V. cholerae and its association with marine plankton species has been firmly established.2 However, the role of river discharge or ocean temperature in aiding the bacterium growth or propagation of the disease is not well understood.3
A large number of investigations of cholera outbreaks and the role of environmental factors have been published, especially since 2005. Colwell1 and Lobitz and others4 were among the first to suggest large-scale role of climate processes on cholera outbreaks in the Bengal delta region. Pascual and others,5,6 Koelle and others,7 and Cash and others8 have suggested a strong relationship of Bengal cholera with sea-surface temperature in the Pacific Ocean and the El Nino-Southern Oscillation phenomenon. Constantin de Magny and others9 and Emch and others10 have also suggested the role of regional precipitation and influence of the ocean climate on regional cholera outbreaks. It should be noted that most of these studies have relied on surveillance data from Matlab, a rural coastal town in Bangladesh. The geographic and hydrologic dissimilarities between Matlab and other affected areas were reported as early as 1978.11 Bengal cholera outbreaks are subject to strong seasonal influences in regional hydroclimatology and display a wide variation in location, time of occurrence and magnitude.12 It is generally accepted that cholera outbreaks across the region are under reported and that the disease burden has been severely underestimated. Studies using Matlab as the main data source thus provide a limited picture of the spatial and temporal variability of cholera outbreaks and underlying processes for the entire Ganges-Brahmaputra-Meghna (GBM) basin.
Akanda and others12,13 recently showed how regional droughts or floods in the GBM basin region affect the magnitude of cholera outbreaks in spring and in fall, respectively. The physical basis of these findings is that the highly asymmetric hydrology of the GBM region causes drastic reduction of freshwater flows in the dry season, accelerating the saline front movement from Bay of Bengal towards inland freshwater; the inward movement of seawater and increased salinity of the estuarine rivers provide an optimal growth environment for the bacterium. An asymmetric influence of the regional rivers on cholera suggests seasonal cholera in multiple locations is associated with underlying macro-scale climatic drivers throughout the region, i.e., in coastal areas in the spring and inland regions in the fall. Salinity of estuarine rivers and water inundation in the inland floodplains has been suggested to influence cholera outbreaks in the Bengal Delta.12,14 However, these hypotheses were not examined by detailed modeling studies or undertaking an investigation directly linking estuarine salinity to spring cholera outbreaks. Similarly, detailed flood impact studies to quantitatively link local and regional floods with water and sanitation breakdown and subsequent fall cholera outbreaks in different locations across the Bengal Delta region have not been conducted.
In this study, we investigate how such macro-scale environmental drivers physically modulate cholera dynamics in disease-prone areas and affect populations over large geographic areas in the Bengal Delta. With long-term cholera surveillance data from the inland mega-city of Dhaka, and recent surveillance data from additional locations in the floodplains and regional surveillance centers, we can identify the seasonal nature of cholera outbreaks in multiple locations with respect to the underlying macro-scale climatic drivers throughout the region. Ability to provide a rapid assessment of underlying drivers, as a precursor to epidemic outbreaks, would greatly aid in identifying population centers at increased risk of epidemics. More specifically, we address the following three questions in this study. The first question is Do seasonal GBM stream flow patterns show a macro-scale asymmetric relationship with spring and fall disease incidence in different surveillance locations? The second question is Does estuarine salinity intrusion over large areas increase risk of cholera in the dry season, and do inland flood patterns and water stagnation in lowland areas lead to elevated levels of cholera incidence in the fall? The third question is Can we estimate the size of the population at risk in response to macro-scale environmental mechanisms of dual seasonal outbreaks, based on detailed simulation of salinity and flooding in the Bengal Delta floodplains?
Materials and Methods
Study area and surveillance locations.
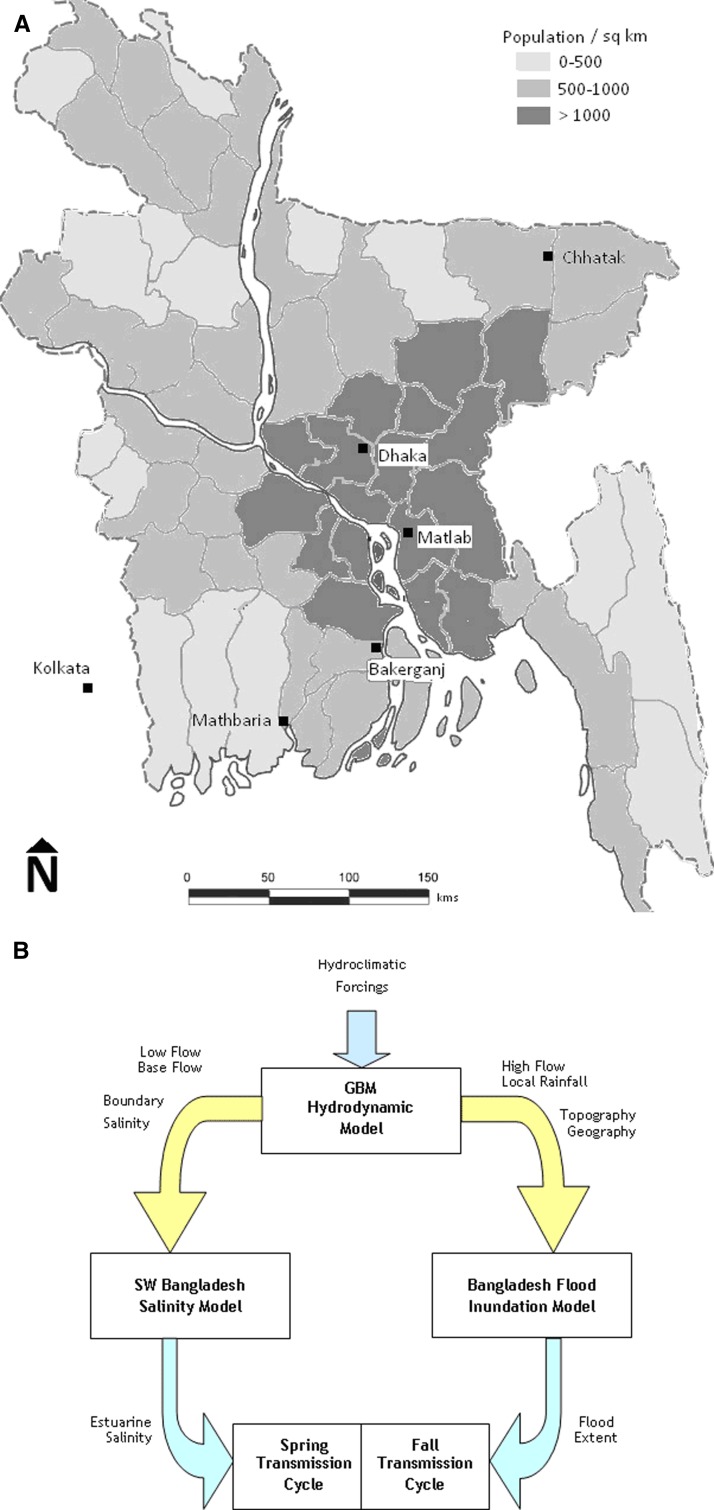
Much of the literature on epidemiology, transmission, propagation, and control of cholera involves long-term datasets generated by the International Center for Diarrheal Disease Research (icddr,b) in Matlab, Bangladesh, where cholera is endemic and extensive surveillance and associated hydroclimatic data are available. The icddr,b has maintained a sophisticated disease surveillance program in Dhaka since 1980, where cholera incidence is assessed by testing for V. cholerae among a sequential sample of patients visiting the hospital each week. These data and cholera incidence records from Matlab (1998–2007), Bakerganj (1998–2007), Chhatak (1999–2005), and Mathbaria (2003–2007) in Bangladesh, where the icddr,b maintains satellite surveillance operations (Figure 1 and Table 1) were used in the study. Kolkata cholera incidence is recorded by the National Institute of Cholera and Enteric Diseases in India.
Figure 1.
A, District-wise population density map of Bangladesh, showing surveillance areas used in this study and location of major rivers in the region. B, Schematic of conceptual model setup for simulation of estuarine salinity and flood inundation. GBM = Ganges-Brahmaputra-Meghna.
Table 1.
Ecosystem type, average cholera incidence, and data length for six locations
| Location | Ecosystem type | Data period |
|---|---|---|
| Dhaka, Bangladesh | Urban, floodplain | 1980–2007 |
| Matlab, Bangladesh | Rural, floodplain | 1998–2007 |
| Mathbaria, Bangladesh | Rural, coastal | 2003–2007 |
| Chhatak, Bangladesh | Rural, upstream | 1999–2005 |
| Bakerganj, Bangladesh | Semi-urban, coastal | 1998–2007 |
| Kolkata, India | Urban, coastal | 1998–2007 |
Bangladesh is situated on alluvial floodplains in the downstream parts of the Ganges-Brahmaputra-Meghna River basin encompassing the Bengal Delta (Figure 1). The hydroclimatology of this region is highly asymmetric in time and space, with more than 80% of the annual precipitation occurring during the four monsoon months of June through September. Average annual rainfall ranges from 1,500 mm in the Ganges basin catchments in the west to more than 4,000 mm in the Meghna basin areas in eastern Bangladesh.15 As a result, the entire GBM basin region experiences an asymmetric pattern of water availability with severe scarcity of water predominating during the winter and spring months in downstream, followed by abundance of water in summer in floodplains along the river corridors and lower elevation delta areas.16
Dhaka, a mega-city with 12 million persons and a population of more than 23 million persons in the Greater Dhaka region, is a freshwater ecosystem in central Bangladesh, surrounded by several tributaries of the GBM rivers. Matlab (population = 250,000) is a cholera-endemic area in southeastern Bangladesh, located near the confluence of the GBM rivers. However, it is less than 100 kilometers from the coast of the Bay of Bengal and is subject to tidal fluctuations. Matlab is sustained predominantly by an agriculture sector and better access to healthcare compared with other rural locations in Bangladesh.
Bakerganj is a district town in southern Bangladesh adjacent to the main GBM outlet, known as the Meghna Channel, and prone to major flood events, typically during the later months of the monsoon season.15 This region has a large fishing industry, with numerous estuarine channels leading to the Bay of Bengal. Population exposure to brackish water is high. Mathbaria, a coastal locality close to the Bay of Bengal, is known for freshwater scarcity because the region is served by one major river from the north, the Gorai. Chhatak, located inland and farthest from the coast, experiences heavy rainfall during monsoon months and is prone to flash floods.
Mathbaria experiences cholera outbreaks predominantly in the spring, whereas Chhatak cholera outbreaks occur in the fall. Dhaka, Matlab, and Bakerganj, located along the central floodplains of the GBM river system, have the characteristic dual cholera outbreak pattern. For comparative purposes, additional cholera incidence data were obtained from Kolkata, a major city in eastern India with a population of more than 20 million persons, which is also located in the Bengal Delta and supplied by a distributary of the Ganges River. It experiences biannual cholera peaks in the spring and fall like the other locations, according to the recent surveillance records for 1998–2007.
Seasonal and interannual variability analysis.
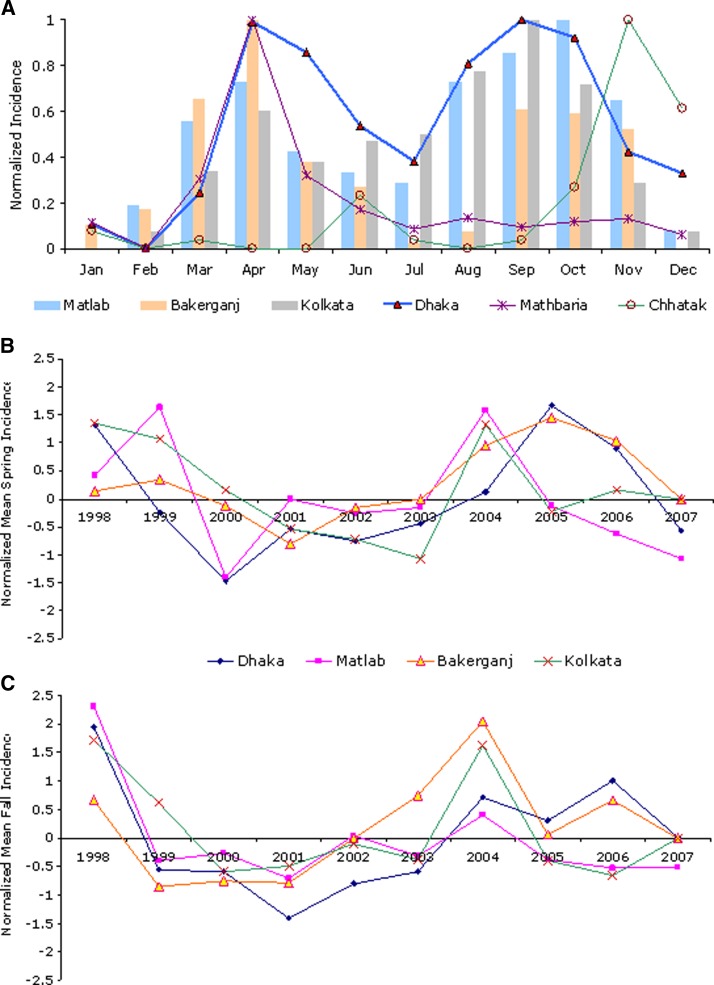
Monthly and seasonal occurrences of cholera were compared over multiple surveillance locations throughout the region. Monthly climatologic incidence (average monthly incidence for the duration of the study period) was computed for the surveillance centers and the data were analyzed to obtain monthly and seasonal incidence patterns. A list of the surveillance centers and the length of the data period is shown in Table 1. Monthly average cholera incidence with confidence intervals is shown in Figure 2A. For seasonal analysis, cholera time series was disaggregated into two seasonal components, spring (March–May) and fall (October–December), for each of the six sites. Seasonal cholera incidence anomalies were calculated as deviations from the mean for 1998–2007 and normalized with respective standard deviations. Mathbaria and Chhatak were excluded from the anomaly analysis because of a shorter temporal length of available data. However, the single peak nature of these locations (Mathbaria in the spring and Chaatak in the fall) enabled effective comparison of incidence of these locations with the dual peak signature of other the four locations with respective interannual variation. A comparison of mean seasonal cholera incidence anomalies is shown in Figure 2B and C.
Figure 2.
Comparison of normalized A, mean monthly cholera incidence; B, mean spring incidence anomaly; and C, mean fall incidence anomaly for selected locations, Bangladesh.
Macro-scale role of regional rivers.
If the hypothesis of large-scale hydroclimatic controls of cholera dynamics is valid, a dual role of river discharge for all the surveillance locations along these major rivers would be seen, namely, region-wide inverse relationship between low flow and spring cholera and a positive relationship between fall cholera with high flow. To test this hypothesis, seasonal low and high flow volumes for the Ganges and the Brahmaputra Rivers were compared with mean spring and fall cholera incidence anomalies for all four locations, respectively. Mean stream flow values for the lowest flow months (February and March) and the two highest flow months (July and August) were used to develop a seasonal low and high flow time series. Kolkata is situated on the Bhagirathi-Hooghly River system in eastern India, which is directly supplied by upstream diversions from the Ganges River.17 Thus, only stream flow data from the Ganges River were used for the Kolkata analysis.
Salinity and flood extent simulation.
Lack of salinity data for the estuarine ecosystems and detailed flood information post-monsoon has prevented previous studies from analyzing the role of these variables on cholera transmission. A physically based mathematical model was used to circumvent the need for intensive data collection and estimate flow and salinity data at various ungauged sites in the Bengal Delta. MIKE-11, a one-dimensional river modeling system, developed by the Danish Hydraulic Institute (Copenhagen, Denmark),18 was used to simulate dominant hydrologic processes in inland floodplains and coastal ecosystems of this large river basin.
After successful calibration of the model for the GBM system and coastal region, we tested seasonal and interannual scenarios or options with the model to determine the impact of hydroclimatic changes on hydrologic and ecological indicators relevant to cholera. Results for different seasonal forcing were post-processed using the MIKE-11 Advection-Dispersion module and geographic information system tools to develop estuarine salinity and floodplain inundation maps (Figure 1B). Matlab (Mathworks, Inc., Natlick, MA) and Excel (Microsoft Corporation, Redmond, WA) were used for statistical analyses and ArcGIS (ESRI, Redlands, CA) was used for flood area analysis and visualization.
The Nedbør-Afrstrømnings Model18 generates overland flow from upstream flow, rainfall, evaporation and groundwater and uses it to express lateral inflow for the Hydro-Dynamic module, which uses an implicit finite difference solution of the Saint Venant equations18 and known boundary conditions at upstream inflow points and downstream tidal water level boundaries to estimate water levels at river reach control points. For validation of the environmental component of the model, runoff values are compared with water level and flow observations from existing gauging stations across the GBM river system inside Bangladesh.19 The model has been extensively calibrated against measured flow and observed river stage values using parameters such as Manning's Number, channel geometry, and riverbed soil type.
The Hydro-Dynamic module outputs are subsequently used as input to the Advection–Dispersion module to simulate salinity of river water), and to geographic information system to compute flooded land area. The Advection–Dispersion module applies advective transport with the mean flow volume and dispersive transport for concentration gradient to solve for one-dimensional conservation of mass. Observed salinity concentrations are used at all model boundary locations as initial conditions. The salinity model was calibrated separately for the dry (January–April) and wet (July–October) seasons to reflect seasonal variation of salinity in the rivers.20 The geographic information system module uses detailed digital elevation models of the GBM spatial domain and outputs of river water levels at locations across the basin to compute depth and extent of flooding. Monthly salinity and flood area statistics were calculated for affected districts in Bangladesh and analyzed with cholera incidence data at surveillance locations.
Role of estuarine salinity and flood inundation.
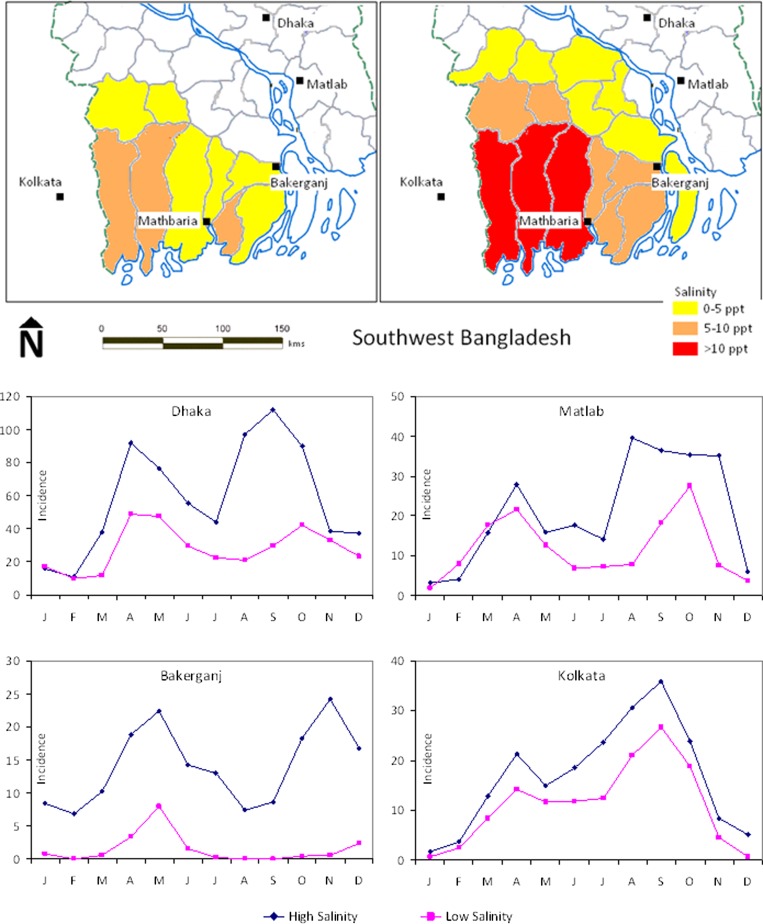
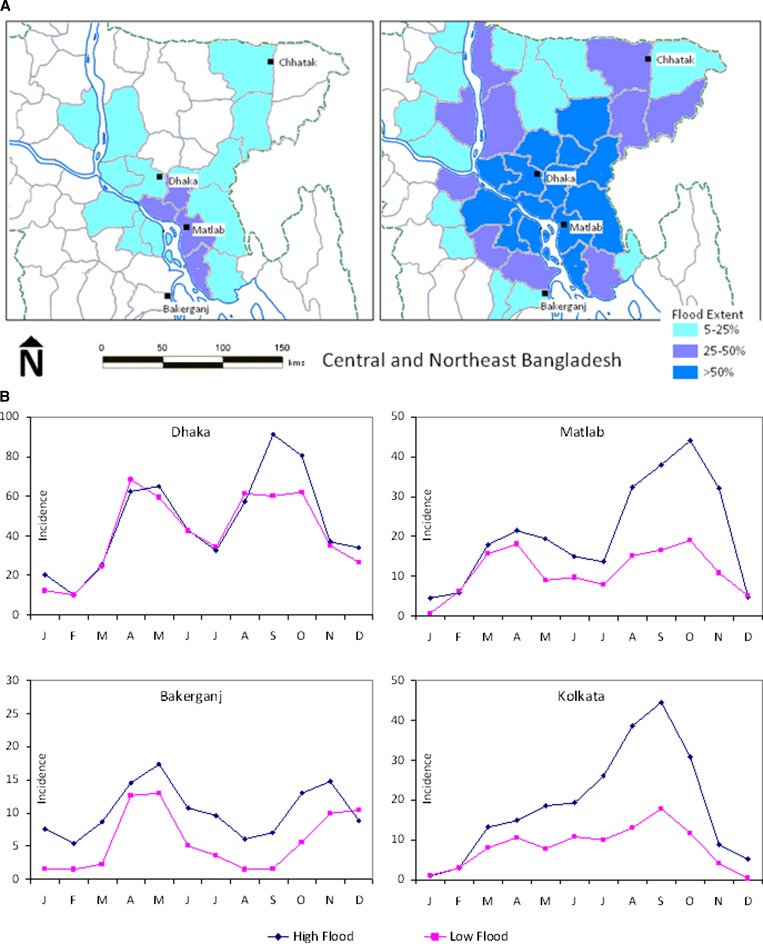
Seasonal associations were analyzed by using Pearson correlation coefficients calculated for March–May salinity and spring cholera, and July–October flooding and corresponding fall cholera (Table 2). The analysis was extended by examining the pattern of monthly cholera in all four (Dhaka, Matlab, Kolkata and Bakerganj) surveillance locations after high and low salinity in the estuaries. The 10 year analysis for 1998–2007 was divided into high and low bins, based on salinity during outbreak seasons, and average monthly cholera in selected sets of five high and five low years across all four surveillance locations (Figure 4 ). To establish evidence for flood inundation perpetuating the progression of cholera in the fall, the 10 year (1998–2007) period of analysis was divided into high and low bins, based on the extent of flooding in the wet season (July–October), and average of the monthly cholera cases in Dhaka, Matlab, Kolkata, and Bakerganj, for the selected five high and five low years (Figure 5).
Table 2.
Correlation between seasonal mean cholera incidence and large-scale hydroclimatic controls in the Ganges-Brahmaputra-Meghna Basin region*
| Location | Cholera season | Low flow | Estuarine salinity | High flow | Flood extent |
|---|---|---|---|---|---|
| Dhaka, Bangladesh (n = 20) | Spring | −0.48† | 0.68‡ | − | − |
| Fall | −0.48† | 0.86‡ | 0.69‡ | 0.77‡ | |
| Matlab, Bangladesh (n = 10) | Spring | −0.64† | 0.41 | − | − |
| Fall | −0.52 | 0.36 | 0.86‡ | 0.81† | |
| Bakerganj, Bangladesh (n = 10) | Spring | −0.44 | 0.67† | − | − |
| Fall | −0.23 | 0.63† | 0.40 | 0.37 | |
| Kolkata, India (n = 10) | Spring | −0.79† | 0.63† | − | − |
| Fall | −0.67† | 0.25 | 0.81† | 0.79† |
n = no. of years of data used in the analysis (n = 20: 1988–2007; n = 10: 1998–2007).
0.05 > P > 0.01.
P < 0.01.
Figure 4.
Monthly cholera incidence in Dhaka, Matlab, and Bakerganj, Bangladseh, and Kolkata, India, and high and low estuarine salinity in southwestern Bangladesh, 1988–2007.
Figure 5.
Monthly cholera incidence in Dhaka, Matlab, Bakerganj, Bangladesh, and Kolkata, India, and high and low flood inundation in central and northeastern Bangladesh, 1988–2007.
Estimating population at risk.
The district-wide population database (Bangladesh Bureau of Statistics, 2011)21 of Bangladesh was used to estimate the population at risk of cholera in each district. We argue that the population of a district is not constantly at risk because of spatial and temporal changes in hydroclimatic and ecologic forcings and resulting environmental conditions. Thus, the percentage of population in each district as a function of the seasonal drivers of cholera was used to estimate monthly population vulnerability. However, because location-specific cholera data are not available, the risk of transmission is assumed to be uniform across all locations within each district. This limitation has been further addressed in the discussion section of this report.
During the dry season, coastal districts in the south and central districts contiguous with Dhaka and the river corridors are vulnerable to cholera. Salinity at different locations along the rivers and coastal points was used to estimate average salinity in districts, and a variance weighted average value in the central districts away from coastal areas was used to estimate the fraction of population at risk. In the absence of representative cholera data at the district level, mean, median, and variance estimates of the regional surveillance center were used to estimate the range of cholera incidence in each district. For subsequent wet seasons, district-wise percentage inundation values of flood extent were used to estimate the fraction of the district population at risk of cholera in the fall due in a post-monsoon environment.
Results
Dual peaks of cholera can be observed for all four floodplain locations (Dhaka, Matlab, Bakerganj, and Kolkata), with the first outbreak in the spring and a second major outbreak later in the year, during the fall (Figure 2A). The winter and peak monsoon months exhibited the lowest number of cases in all locations. On average, more cholera cases occurred in the spring and fall in all four areas and fewer cases in the winter (December–February) and summer (June–August). However, a significant peak in cholera cases occurs in Mathbaria in the spring, coinciding with the spring peak in the four floodplain locations (Dhaka, Matlab, Bakerganj, and Kolkata). Conversely, Chhatak experiences large outbreaks only in the fall, following the fall peak in the same four floodplain locations. Similar seasonal anomalies at all locations suggest that cholera in Dhaka, Matlab, Kolkata, and Bakerganj is influenced by similar macro-scale environmental drivers during the respective seasons are shown in Figure 2B and C.
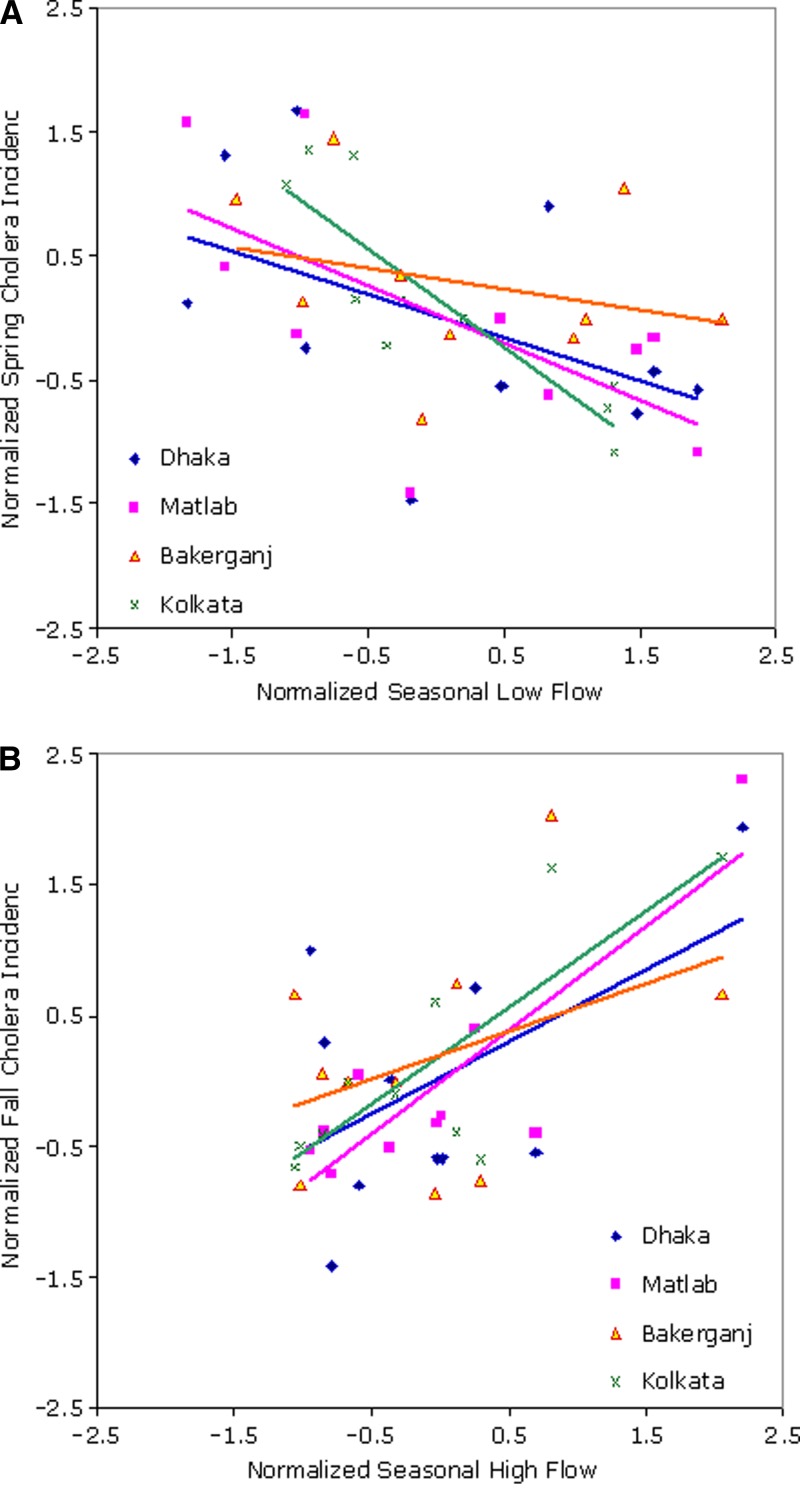
Seasonal low-flow and high-flow anomalies for the major rivers compared with average spring (March–May) and fall (September–November) cholera anomalies for each location (Ganges River for Kolkata) are shown in Figure 3 . A key observation shown in Figure 3A and B is reversal of linear relationships between stream flow and cholera incidence between spring and fall. Seasonal low-flow volumes are negatively correlated with spring cholera cases in all locations, and high-flow volumes show a strong positive relationship with cholera in the fall. Thus, scarcity of water in the major river channels increases the risk for cholera throughout the southern region in spring, and abundance of water in the monsoon season leads to large epidemics in the fall.
Figure 3.
Mean seasonal cholera incidence and stream flow anomaly in Bengal Delta for A, dry and B, wet seasons, Bangladesh, 1998–2007.
Cross-correlation values between salinity and spring cholera in March–May and extent of floods and fall cholera in July–October, respectively, for Dhaka, Bakerganj, Kolkata, and Matlab are shown in Table 2. The consistent yet contrasting dual influence of flow volumes (negative correlation in spring and positive in fall) across all surveillance locations strengthens earlier proposed hypotheses by Akanda and others.12,13
Higher cholera incidence is seen during spring for high salinity years and greater spatial coverage of salinity intrusion in coastal Bengal Delta, with a smaller number of cholera cases after lower salinity (Figure 4). The consistent picture of variability and the clear separation between high- and low-salinity years suggest a significant relationship between estuarine salinity and enhanced growth of V. cholerae in spring, contributing to cholera in subsequent months of the year. Also, cholera incidence is distinctly different for each location between high and low flood years (Figure 5 ). The estimated population at risk for cholera in the affected districts caused by salinity and flood processes in spring and fall is summarized in Table 3.
Table 3.
Average estimated seasonal population vulnerability in spring and fall seasons caused by macro-scale cholera outbreaks at selected districts in Bangladesh*
| Region | Population at risk | |
|---|---|---|
| Spring | Fall | |
| Dhaka | 13,878 | 15,329 |
| Sylhet | 0 | 3,138 |
| Barisal | 619 | 585 |
| Khulna | 826 | 56 |
| Comilla | 1,240 | 3,708 |
| Faridpur | 172 | 862 |
| Jessore | 301 | 82 |
| Noakhali | 1,047 | 2,028 |
Population vulnerable to potential cholera outbreaks, in thousands. Average estimates calculated over 1998–2007 simulation period. Population Data Source: Bangladesh Bureau of Statistics, 2011.21
Discussion
A number of studies have suggested that estuarine salinity and inland flooding can significantly affect cholera transmission in the Bengal Delta region. The lack of reliable and continuous data on the incidence of cholera, notably, poor coverage over time and space, hinders research on the coast-climate-cholera connection. Also, most of the published literature on Bengal cholera and climate have data only for Matlab, a coastal town in Bangladesh and, consequently, provides an incomplete understanding of cholera in the Bengal Delta region. With surveillance data for six locations in the region, we were able to assess the role of macro-scale drivers in of seasonal cholera cycles and strengthen the plausibility of a coastal bacterial reservoir enhanced by estuarine salinity regimes and post monsoon contamination due to flooding.
The results of our analyses show that large areas of the highly populated floodplains in this region are at high risk of epidemic cholera in the spring and fall because of large spatial coverage of salinity intrusion and flooding, respectively (Figures 4 and 5). Population centers along the floodplain corridors of the GBM river system remain vulnerable to seasonal and interannual variability of cholera transmission mechanisms with respect to underlying macro-scale hydroclimatic drivers during the spring and fall. A striking similarity across different geographic locations over a 10-year period suggests the same large-scale hydroclimatic processes have an effect on the incidence of cholera. Seasonal processes (Figures 2–5) over a large extent of the Bengal Delta region modulate cholera incidence, thus making it possible to predict the timing and magnitude of cholera outbreaks.
A coastal reservoir of V. cholerae indicates cholera would occur after increased salinity, and lower cholera incidence after decreased salinity in the coastal areas. Similarly, if monsoon floods have an effect on cholera incidence in the fall, more cholera cases would occur in regions affected by monsoon floods and post-monsoon inundation. Cholera outbreaks can be local and independent, occurring simultaneously in various locations under favorable environmental conditions.22,23 Thus, natural disasters such as a drought or a flood and resulting salinity and inundation conditions increase the likelihood of outbreaks in population centers along the floodplains, making them concurrently vulnerable. Improved understanding of macro-scale drivers and the ability to estimate these underlying physical drivers also aids identification of population centers at risk of epidemics. For example, if the extent of flooding can be estimated as early as July or August for specific geographic locations, a valuable two or three month lead-time could be provided to target prevention efforts before the major fall outbreak in October.
The World Health Organization has long supported the development of an early warning system for epidemic cholera outbreaks that use climatic and environmental information. Several attempts have been made to predict cholera outbreaks, but an early warning system based on physical understanding of underlying processes has yet to be developed and implemented at an operational level. The lack of understanding of the underlying transmission mechanisms has hindered the accurate estimation of population at risk to cholera outbreaks in affected areas. The findings of the present study do not directly represent a causal connection; however, it identifies two plausible and important physical variables in the transmission process, estuarine salinity and inland flooding.
The World Health Organization estimates that only a fraction of the actual number of cholera cases worldwide is reported to the global database annually.24 Population burden from endemic and epidemic cholera thus remains largely unknown for developing countries. In the Bengal Delta region of southern Asia, environmental and hydroclimatic factors are strong drivers of seasonal cholera,12 but are not typically accounted for in the estimation of population vulnerability. In contrast to the findings in the study by of Ali and others,24 in which they argue for a constant risk throughout the year for the whole population, our observation and results show that the size of the population at risk is highly dependent on the magnitude and physical extent of underlying hydroclimatic and environmental drivers. As suggested by these results, a large segment of the population living on floodplains along major river corridors remain vulnerable to the biannual cholera over large geographic regions in either spring, fall, or both in response to the seasonal hydrology.
For this study, risk of cholera transmission was assumed to be distributed evenly across a district (a provincial unit in Bangladesh has 64 districts (Figure 1), which is a limitation of this study. Because of the lack of cholera surveillance data from individual towns, villages, or small geographic units, it is not possible to know the spatial distribution of cholera transmission risk except with Matlab.10 However, it would be reasonable to assume cholera risk across a smaller geographic region such as a district, which is homogeneous in terms of ecosystem functions, access to water resources, and the influence of large-scale hydroclimatic environmental processes. For future research, better health surveillance data and higher resolution climate and hydrologic data would be invaluable to understand these processes in more detail.25–27
A cholera warning system based on the geographic specificity of salinity and flood signatures holds the promise of predicting the future intensity and location of epidemics. Such a system can potentially provide public health authorities with spatially specific warnings regarding the extent and magnitude of impending outbreaks, especially in slum areas lacking in necessary water infrastructure, to alert medical personnel and start implementing preventive measures. The framework presented based on the variability of macro-scale seasonal hydroclimatic forcings has the potential to provide public health authorities in Bangladesh a more accurate estimate of population numbers at risk, and a spatially focused warning capability essential to support planning, preparation, and early intervention among vulnerable populations.
ACKNOWLEDGMENTS
We thank the Institute of Water Modeling (Dhaka, Bangladesh), particularly Zahirul Haque Khan, Shume Akhtar and Khan Wahid Palash for help with MIKE11 simulations.
Footnotes
Financial support: This study was supported, in part, by a research challenge grant (1RC1TW008587-01) from the National Institutes of Health under the American Recovery and Reinvestment Act (2009). Rural cholera incidence data collection was supported by National Institutes of Health grant RO1-A13912901 as well as by grants from the US National Science Foundation (NSF 0809783 and NSF 0741600).
Authors' addresses: Ali Shafqat Akanda, Department of Civil and Environmental Engineering, University of Rhode Island, Kingston, RI, E-mail: akanda@egr.uri.edu. David M. Gute, and Shafiqul Islam, Department of Civil and Environmental Engineering, Tufts University, Medford, MA, E-mail: david.gute@tufts.edu, shafiqul.islam@tufts.edu. Antarpreet S. Jutla, Department of Civil and Environmental Engineering, West Virginia University, Morgantown, WV, E-mail: asjutla@mail.wvu.edu. Anwar Huq, and Rita R. Colwell, Biomolecular Sciences Building, University of Maryland, College Park, MD, E-mail: huqanwar@gmail.com, rcolwell@umd.edu. R. Bradley Sack, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, E-mail: rsack@jhsph.edu. Munirul Alam, Centre for Food and Waterborne Diseases, International Centre for Diarrhoeal Disease Research, Mohakhali, Dhaka 1212, Bangladesh, E-mail: munirul@icddrb.org.
References
- 1.Colwell RR. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 2.Worden AZ, Seidel M, Smriga S, Wick A, Malfetti S, Bartlett D, Azam F. Trophic regulation of Vibrio cholerae in coastal marine waters. Environ Microbiol. 2006;8:21–29. doi: 10.1111/j.1462-2920.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- 3.Jutla AS, Akanda AS, Griffiths J, Colwell RR, Islam S. Warming oceans, phytoplankton, and river discharge: implications for cholera outbreaks. Am J Trop Med Hyg. 2011;85:303–308. doi: 10.4269/ajtmh.2011.11-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobitz B, Beck L, Huq A, Wood B, Faruque A, Colwell R. Climate and infectious disease: Use of remote sensing for detection of Vibrio cholerae. Proc Natl Acad Sci USA. 2000;97 doi: 10.1073/pnas.97.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pascual M, Rodo X, Ellner SP, Colwell RR, Bouma MJ. Cholera dynamics and El Niño-Southern Oscillation. Science. 2000;289:1766–1769. doi: 10.1126/science.289.5485.1766. [DOI] [PubMed] [Google Scholar]
- 6.Pascual M, Chaves LF, Cash B, Rodo X, Yunus M. Predicting endemic cholera: the role of climate variability and disease dynamics. Clim Res. 2008;36:131–140. [Google Scholar]
- 7.Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. Refractory periods and climate forcing in cholera dynamics. Nature. 2005;436:696–700. doi: 10.1038/nature03820. [DOI] [PubMed] [Google Scholar]
- 8.Cash BA, Rodo X, Kinter JL., III Links between tropical Pacific SST and cholera incidence in Bangladesh: role of the eastern and central tropical Pacific. J Clim. 2008;21:4647–4663. [Google Scholar]
- 9.Constantin de Magny G, Murtugudde R, Sapiano MR, Nizam A, Brown CW, Busalacchi AJ, Yunus M, Nair GB, Gil AI, Lanata CF, Calkins J, Manna B, Rajendran K, Bhattacharya MK, Huq A, Sack RB, Colwell RR. Environmental signatures associated with cholera epidemics. Proc Natl Acad Sci USA. 2008;105:17676–17681. doi: 10.1073/pnas.0809654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emch M, Feldacker C, Yunus M, Streatfield PK, Dinh Thiem V, Canh do G, Ali CM. Local environmental predictors of cholera in Bangladesh and Vietnam. Am J Trop Med Hyg. 2008;78:823–832. [PubMed] [Google Scholar]
- 11.Briscoe J. The role of water supply in improving health in poor countries. Am J Clin Nutr. 1978;81:2100–2113. doi: 10.1093/ajcn/31.11.2100. [DOI] [PubMed] [Google Scholar]
- 12.Akanda AS, Jutla AS, Alam M, Constantin de Magny G, Alam M, Sack RB, Huq A, Colwell RR, Islam S. Hydroclimatic influences on seasonal and spatial cholera transmission cycles: implications for public health intervention in Bengal Delta. Water Resour Res. 2011;47:W00H07. [Google Scholar]
- 13.Akanda AS, Jutla AS, Islam S. Dual peak cholera transmission in Bengal Delta: a hydroclimatological explanation. Geophys Res Lett. 2009;36:L19401. [Google Scholar]
- 14.Miller CJ, Drasar BS, Feachem RG. Cholera and estuarine salinity in Calcutta and London. Lancet. 1982;1:1216–1218. doi: 10.1016/s0140-6736(82)92340-6. [DOI] [PubMed] [Google Scholar]
- 15.Mirza MM. Three recent extreme floods in Bangladesh: a Hydro-meteorological analysis. Nat Hazards. 2003;28:35–64. [Google Scholar]
- 16.Akanda AS. South Asia's water conundrum: hydroclimatic and geopolitical asymmetry, and brewing conflicts in the eastern Himalayas. Intl J River Basin Management. 2012;10:307–315. [Google Scholar]
- 17.Rahman MM, Hassan MQ, Islam MS, Shamsad SZ. Environmental impact assessment on water quality deterioration caused by decreased Ganges flow. Environ Geol. 2000;40:31–40. [Google Scholar]
- 18.Danish Hydraulic Institute . Scientific Documentation on MIKE-11 NAM-HD-AD and MIKE-11 GIS. Copenhagen: DHI Press; 2008. Release 9.2. [Google Scholar]
- 19.Nishat B, Rahman SM. Water resources modeling of the Ganges-Brahmaputra-Meghna River basins using satellite remote sensing data. J Am Water Resour Assoc. 2009;45:1313–1327. [Google Scholar]
- 20.Wahid S, Babel M, Bhuiyan A. Hydrologic monitoring and analysis in Sundarbans mangrove ecosystem, Bangladesh. J Hydrol (Amst) 2007;332:381–395. [Google Scholar]
- 21.Bangladesh Bureau of Statistics (BBS), Bangladesh 2011. http://www.bbs.gov.bd Available at.
- 22.Stine O, Alam M, Tang L, Nair GB, Siddique AK, Faruque SM, Huq A, Colwell RR, Sack RB, Morris JG., Jr Seasonal cholera from multiple small outbreaks in rural Bangladesh. Emerg Infect Dis. 2008;14:831–833. doi: 10.3201/eid1405.071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alam M, Islam A, Bhuyan NA, Rahim N, Hossain A, Khan GY, Ahmed D, Watanabe H, Izumiya H, Faruque AS, Akanda AS, Islam S, Sack RB, Huq A, Colwell RR, Cravioto A. Clonal transmission, dual peak, and off-season cholera in Bangladesh. Infection Ecology and Epi. 2011;1:7273. doi: 10.3402/iee.v1i0.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali M, Lopez AL, You YA, Kim YE, Sah B, Maskery B, Clemens J. The global burden of cholera. Bull World Health Organ. 2012;90:209–218A. doi: 10.2471/BLT.11.093427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jutla AS, Akanda AS, Huq A, Faruque A, Colwell R, Islam S. A water marker monitored by satellites to predict endemic cholera. Remote Sensing Letters. 2013 doi: 10.1080/2150704X.2013.802097. doi:10.1080/2150704X.2013.802097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jutla AS, Whitcombe E, Hasan H, Haley B, Akanda AS, Huq A, Alam M, Sack B, Colwell R. Environmental factors influencing epidemic cholera. Am J Trop Med Hyg. 2013;89:597–607. doi: 10.4269/ajtmh.12-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jutla AS, Akanda AS, Islam S. A framework for predicting endemic cholera using satellite derived environmental determinants. Environmental Modelling and Software. 2013 doi:http://dx.doi.org/10.1016/j.envsoft.2013.05.008. [Google Scholar]