Abstract
In Houston, we have been monitoring the immune response to West Nile virus (WNV) infection in a large cohort of study participants since 2002. Using enzyme-linked immunosorbent assay techniques, serum from 163 participants was tested for the presence of anti-WNV immunoglobulin M (IgM) and IgG antibodies. We found that 42%, 34%, and 23% of study participants had either positive or equivocal results when tested for anti-WNV IgM antibodies approximately 1, 6, and 8 years post-infection, respectively. Conversely, almost one-half of study participants (46%) had undetectable anti-WNV IgG antibodies by 8 years post-infection. This study is the first study to calculate the slope of the rate of decay of antibodies over time as well as show persistence of detectable anti-WNV IgM antibodies up to 8 years post-infection. These findings warrant additional investigation, particularly the determination of whether persistence of IgM is related to persistent infection with WNV.
Introduction
West Nile virus (WNV) infection is typically diagnosed through a combination of findings, including clinically compatible illness and positive results from specific laboratory tests.1,2 Patients suspected of WNV infection typically have serum or cerebrospinal fluid (CSF) tested for the presence of anti-WNV immunoglobulin M (IgM) antibodies using an IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA). IgM is considered a marker of acute infection, with decline to undetectable levels expected around 2–3 months post-infection with most viral diseases.
During the WNV encephalitis epidemic in Bucharest, Romania in 1996, patterns of IgM and IgG reactivity in ELISAs were evaluated.3 Anti-WNV IgM antibodies were detectable in serum as early as the second day after onset of encephalitis. IgG antibody response seemed to occur 4–5 days after onset of illness. IgM was still present in more than 50% of convalescent sera collected 2 months after the onset of central nervous system (CNS) illness.
Other studies have also documented persistence of IgM antibodies over an extended period of time. During an outbreak in Greece in 2010, researchers noted that 41% of patients continued to experience IgM persistence up to 180 days post-infection.4 After the WNV outbreak in New York City in 1999, Roehrig and others5 studied the antibody response in 29 patients diagnosed with encephalitis. Serial bleeds identified anti-WNV IgM persistence approximately 1.4 years post-onset in 7 of 12 patients who still had evidence of IgM antibodies on previous serial bleeds. At 9 months post-onset, those patients who were older (> 65 years of age) seemed to be more likely to have detectable IgM antibodies, and at 1 year post-onset, those patients who presented with encephalitis seemed to be more likely to have IgM persistence compared with meningitis. Unfortunately, the sample size was small, and therefore, no statistically significant difference was seen among any groups at any time.
IgM antibody persistence can hinder diagnosis in successive years in areas affected by large epidemics.3 With WNV now endemic in the United States, the kinetics of IgM and IgG antibody response are important to understand. This paper presents the findings from our study, where we evaluated a large cohort of WNV-positive patients in Houston over an 8-year period to determine the duration of detectable IgM antibodies, the rate of decay of IgM and IgG antibodies, and whether the antibody response is different in patients based on demographics, comorbidities, and social behaviors.
Methods
Study population.
Study participants were identified through routine disease surveillance conducted by Harris County Public Health and Environmental Services, City of Houston Department of Health and Human Services, and Gulf Coast Regional Blood Center. Methods for confirming WNV status were previously described.6 Participants who agreed to take part were enrolled into the study. A total of 163 study participants took part in this study. After giving their consent, participants were interviewed to collect demographic, medical history, and social history data at the time of acute WNV infection. Blood specimens were collected every 6 months for analysis. Participation in blood collections was based on availability of the study subject; therefore, not all participants took part in each follow-up collection. This study was reviewed and approved by the University of Texas Health Science Center Committee for the Protection of Human Subjects (HSC-SPH-03-039) and complied with the Health Insurance Portability and Accountability Act.
WNV ELISA.
Using ELISA techniques, serum was tested for the presence of anti-WNV IgM and IgG antibodies. Centers for Disease Control and Prevention (CDC) provided in-kind protocols, reagents, positive and negative control serum, normal antigen (sucrose–acetone-extracted suckling mouse brain antigen), and technical support for performing these analyses. The protocol provided by the CDC for the MAC-ELISA was followed.7,8 Briefly, anti-IgM (capture antibody) was coated on 96-well microtiter plates. This step was followed sequentially by the patient's serum (1:400 dilution) and the viral (Focus Diagnostics, Cypress, CA) and normal antigens. The presence of antigen was detected by using enzyme-conjugated antiviral antibody, and a colorimetric result was generated by the interaction of the enzyme and chromogenic substrate. Samples were run in duplicate, and each plate included a positive and negative control. An average absorbance of each plate was determined at 450 nm and calculated between the two wells of each patient sample. The optical density of the positive control was divided by the optical density of the negative control. If this value was greater than three, then the test was validated. The optical density of the patient serum was divided by the optical density of the negative control to determine the P/N ratio. A negative result was defined as a P/N < 2. An equivocal result was defined as a P/N ≥ 2 and < 3. A positive result was defined as a P/N ≥ 3. Normal antigen was also run in duplicate for each patient to help eliminate false positives. The mean optical density on the positive patient specimen against the viral antigen had to be at least two times greater than the mean optical density of the patient specimen against the normal antigen. Positive ELISA results with high background against normal antigen were repeated. If high background was detected again, then the sample was excluded from analysis.
To determine the presence of anti-WNV IgG antibodies, the CDC protocol was also followed.7 Briefly, viral group-reactive monoclonal antibody was coated on a 96-well plate followed sequentially by known viral or normal antigen, patient serum, enzyme-conjugated human IgG, and lastly, substrate for the conjugate used. Samples were run in duplicate with positive and negative controls, and an average absorbance of each sample was determined at 405 nm. The P/N ratio was calculated and interpreted in the same manner as IgM.
Data analysis.
Descriptive statistics were performed to illustrate host immunologic response. Linear regression was used to determine the slope of antibody decay over time. All P/N observations were included. Univariate logistic regression analysis was performed to assess potential associations between having detectable anti-WNV IgM antibodies (P/N ≥ 2) and demographics, comorbidities, and social behaviors at 1 and 5 years post-infection. For the purposes of this study, we defined history of chronic alcohol abuse as consuming either 3.5 or more alcoholic drinks in one sitting at least one time per week and/or 15 or more alcoholic drinks in a week as reported by the study participant. Tobacco use was based on self-report of regular usage. Multivariate logistic regression analysis was also performed to establish independent risk factors and test for potential confounding. All risk factors on univariate analysis with P ≤ 0.25 were included in multivariate analysis. A backward step elimination of the highest non-significant value method was used. Only those factors with P ≤ 0.10 were considered significant in the multivariate analysis. All calculations were run using STATA v12.0 software (College Station, TX) and NCSS 2007 (Kayesville, UT).
Results
A total of 163 participants took part in this longitudinal study. Our study population consisted of 62% males and 83% whites. The average age at acute disease onset was 52 years (range = 9–88 years). The study population was comprised of 56% neuroinvasive disease cases (38% encephalitis and 18% meningitis) and 44% non-neuroinvasive disease cases (28% febrile and 16% asymptomatic).
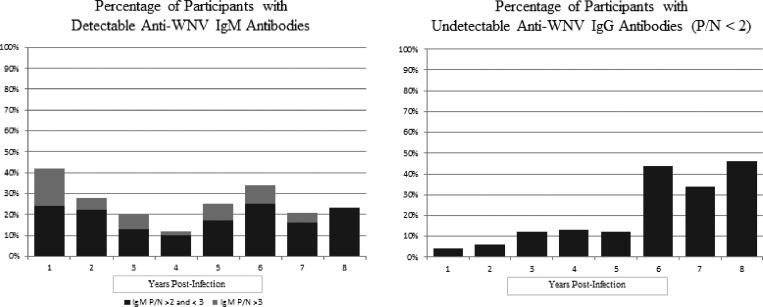
The median anti-WNV IgM and IgG P/N values by year post-acute WNV infection are listed in Table 1. At 1 year post-infection, 42% of participants had positive or equivocal IgM antibodies (P/N ≥ 2); 34% of participants had positive or equivocal IgM antibodies at 6 years post-infection, and 23% of participants had positive or equivocal IgM antibodies at 8 years post-infection (Figure 1). Interestingly, the percentage of study participants with detectable IgM antibodies seems to decline over years 1–4 but then increases again from years 5 to 8. Anti-WNV IgG antibodies steadily declined over the study period. At 1, 6, and 8 years post-infection, 4%, 44%, and 46% of participants tested negative for anti-WNV IgG antibodies, respectively. Interestingly, 7 of 11 (64%) and 4 of 5 (80%) of those participants with detectable IgM were found to be negative for IgG antibodies at 6 and 8 years, respectively.
Table 1.
Descriptive summary of antibody response among cohort participants by years post-WNV infection
| 1 Year (N = 91) | 2 Years (N = 79) | 4 Years (N = 68) | 6 Years (N = 32) | 8 Years (N = 22) | |
|---|---|---|---|---|---|
| Median IgM P/N (range) | 2.37 (0.80–18.70) | 1.74 (0.60–7.60) | 1.72 (0.70–6.54) | 1.72 (0.13–5.84) | 1.61 (0.14–4.08) |
| Median IgG P/N (range) | 8.12 (0.90–18.10) | 6.22 (0.70–20.00) | 5.89 (0.70–20.00) | 6.45 (0.84–20.00) | 2.50 (0.86–6.80) |
| IgM positive (P/N ≥ 3)* | 16 (18) | 5 (6) | 1 (2) | 3 (9) | 0 (0) |
| IgM equivocal (P/N = 2.0–2.99)* | 22 (24) | 17 (22) | 7 (10) | 8 (25) | 5 (23) |
| IgM negative (P/N < 2)* | 53 (58) | 57 (72) | 60 (88) | 21 (66) | 17 (77) |
| IgG negative (P/N < 2)* | 4 (4) | 5 (6) | 9 (13) | 14 (44) | 10 (46) |
Reported as crude number (%).
Figure 1.
Percentages of participants (N = 163) with detectable IgM or undetectable IgG by year post-infection.
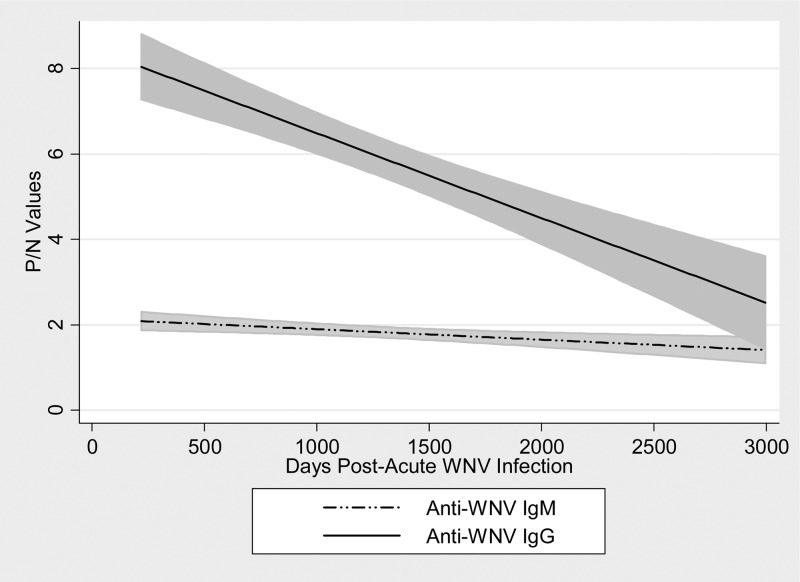
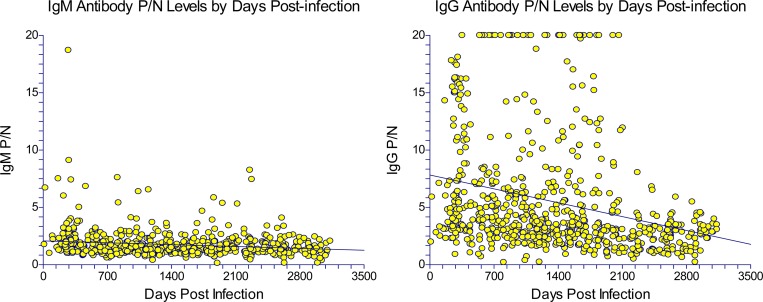
The linear regression of anti-WNV IgM and IgG P/N value change over time is noted in Figures 2 and 3. Using 664 observations of anti-WNV IgM P/N results, we estimated the equation of the straight line relating the P/N value and the number of days post-acute WNV infection as anti-WNV IgM P/N value = (2.076) + (−0.0002) (days post-infection). The slope of the line was significantly different than zero (P = 0.0001, t value = −3.99). Using 685 observations of anti-WNV IgG P/N results, we estimated the equation of the straight line relating the P/N value and the number of days post-acute WNV infection as anti-WNV IgG P/N value = (7.823) + (−0.0017) (days post-infection). The slope of the line was significantly different than zero (P < 0.0001, t value = −7.72).
Figure 2.
Regression lines with 95% confidence interval areas for anti-WNV IgM and IgG P/N values over days after acute WNV infection.
Figure 3.
Scatter plots of anti-WNV IgM (N = 664 data points) and IgG (N = 685 data points) P/N levels over time among 163 study participants.
As seen in Table 2, we found the following variables on univariate analysis appropriate for inclusion in the multivariate model: sex (P = 0.16), race (P = 0.13), history of chronic alcohol abuse (P = 0.04), and history of chronic tobacco use (P = 0.06). On multivariate analysis, we identified the following variables as significantly associated with having detectable IgM antibodies at 1 year post-infection: sex (P = 0.04), history of chronic alcohol abuse (P = 0.04), and history of tobacco use (P = 0.06). The odds ratio (OR) for male sex was found to be protective (OR = 0.4), indicating that females are more likely to have persistent IgM antibodies than men. Those participants with a history of chronic alcohol abuse had 4.6 greater odds of elevated IgM titers at 1 year post-infection, and those participants with a history of tobacco use had 2.4 greater odds of elevated IgM titers at 1 year post-infection. The same analysis was repeated for 5 years post-infection (data not shown). Based on univariate analysis, age 65 years and older (P = 0.18) and history of hypertension (P = 0.09) were included in the multivariate model. Only hypertension neared significance (OR = 3.5; 95% confidence interval = 0.7 to 1.2; P = 0.09).
Table 2.
Univariate and multivariate logistic regression analysis: association between detectable IgM antibodies (IgM P/N ≥ 2) at 1 post-infection and reported demographics, comorbidities, and social behaviors at the time of initial infection
| 1 Year post-infection | ||||||
|---|---|---|---|---|---|---|
| All cases (%; N = 92) | Number IgM P/N ≥ 2 (%; N = 38) | OR (95% CI) | Univariate P value | Multivariate OR (95% CI)* | Multivariate P value* | |
| Demographics | ||||||
| Sex (male) | 58 (63) | 21 (55) | 0.5 (0.2–1.3) | 0.16 | 0.4 (0.1–0.95) | 0.04 |
| White, non-Hispanic race | 72 (78) | 33 (87) | 2.4 (0.8–7.3) | 0.13 | NS | NS |
| Age ≥ 65 years | 27 (29) | 11 (29) | 0.9 (0.4–2.3) | 0.90 | ||
| Neuroinvasive WNV disease | 66 (72) | 29 (76) | 1.4 (0.5–3.6) | 0.49 | ||
| Encephalitis from WNV | 42 (46) | 17 (45) | 0.9 (0.4–2.1) | 0.82 | ||
| Comorbidities | ||||||
| Hypertension | 38 (41) | 15 (39) | 0.9 (0.4–2.0) | 0.71 | ||
| Diabetes | 16 (17) | 6 (16) | 0.8 (0.3–2.4) | 0.67 | ||
| Chronic alcohol abuse | 11 (12) | 8 (21) | 4.4 (1.1–18.1) | 0.04 | 4.6 (1.0–20.3) | 0.04 |
| Tobacco use | 49 (53) | 25 (66) | 2.3 (0.98–5.5) | 0.06 | 2.4 (0.95–6.2) | 0.06 |
CI = confidence interval; NS = not significant.
Only those variables with P ≤ 0.25 were entered into the multivariate model.
Discussion
We found a higher than expected proportion of participants with detectable anti-WNV IgM antibodies several years post-infection. In this study, we identified positive or equivocal detection of IgM antibodies in 42% of cases having measurable antibodies approximately 1 year post-infection and 23% of cases having measurable antibodies at 8 years post-infection. This study is the first study to show longitudinal persistence of IgM in a large cohort of cases for this length of time. Because IgM and IgG are used to identify acute cases of WNV infection, developing an understanding of the kinetics of antibody response is critical in being able to diagnose acute infection. Persistence of IgM from previous transmission seasons can result in false interpretation of test results in patients suspected of acute arboviral disease. Although overall decline of IgM antibodies is expected, extended high titers could possibly represent viral persistence.9
When we began this study in 2003, we used the only nationally accepted protocol for determination of IgM and IgG antibodies at that time, which was based on the CDC recommendations and previously published studies. With the introduction of Food and Drug Administration (FDA)-approved commercially available kits through Focus Diagnostics and PanBio, Inc. a couple of years into the study,10 we made the decision to continue to use the CDC protocol to remain consistent with methods and interpretations. In 2010, we began to use the commercially available kits by PanBio. Although those data are not presented in this report, we continued to find IgM persistence with the use of the commercially available kits. Not including equivocal results, we found 23% and 6% positive for IgM antibodies at 6 and 8 years post-infection, respectively. This result is a higher prevalence of positives (P/N ≥ 3) than what we found after the CDC MAC-ELISA protocol (9% and 0% at 6 and 8 years, respectively) (Table 1). These findings support our conclusion of persistence of detectable IgM antibodies up to 8 years after infection, and our use of the CDC MAC-ELISA protocol might possibly have given us results that underrepresent the true prevalence of persistent IgM.
In previously published studies, the persistence of anti-WNV antibodies was previously documented for more than 1 year after infection.4,5,11 Roehrig and others5 found a higher proportion of IgM persistence in patients over the age of 65 years and cases with a clinical presentation of encephalitis. In this study, we were able to examine a larger cohort of WNV-positive study participants, and we did not find any statistical associations between IgM persistence and age (either as a continuous or categorical variable) or disease severity. Unlike the work by Roehrig and others5, we found several inconsistencies in odds ratios for neuroinvasive disease and IgM persistence over time. Odds ratios fluctuated between increased risk, protective, and null without any evidence of trend. Even after refining disease categories (encephalitis, meningitis, fever, and asymptomatic), no significant associations were found. Based on our longitudinal data, we did not find any associations between IgM persistence and acute WNV disease severity.
We did find that social behaviors, specifically tobacco use and chronic alcohol abuse, were statistically associated with detectable IgM antibodies at 1 year post-infection. Substance abuse has known maleficent effects on the immune system.12,13 Substance abuse is likely causing an underlying immunosuppressant condition, resulting in chronic viral infection. This study was the first to include social histories in evaluation of antibody persistence, and these findings should be considered when exploring the mechanisms behind IgM persistence.
We discovered a substantial decline in detectable IgG antibodies over the study period, which is seen in Figure 1, with almost one-half (46%) of participants negative for IgG antibodies by 8 years post-infection. It is postulated that this finding could be influenced by the nature of our population being older. Advanced age is associated with both severe WNV disease and decline in humoral immunity.14,15 The average age of our study population at the time of acute infection was 52 years, and 56% of our population had neuroinvasive WNV disease; therefore, it is possible that these factors could influence the decline in IgG antibodies overtime. Statistically, being negative for IgG antibodies at 6 years post-infection was not associated with any factors, including age or severity of illness from WNV. Finally, we did not expect to find 64% and 80% of those participants with detectable IgM antibodies at 6 and 8 years, respectively, to be IgG-negative. These findings warrant additional investigation.
Interestingly, two participants in this study were confirmed positive in CSF for WNV during acute infection by both ELISA and Plaque Reduction Neutralization Test (PRNT). However, both have been negative (P/N < 2) for both IgM and IgG on all serial blood draws since that time. We did not expect to find consistently negative results in these patients. Sera from these cases were tested for evidence of neutralizing antibodies by both hemagglutination inhibition and PRNT, and both had positive titers identified. Similar findings have been reported with experimentally infected primates.16 The reasons behind negative detection of IgM and IgG antibodies are unclear.
We examined several demographic, comorbidity, and social history variables and their associations with persistence of IgM antibody response; however, the true reason for continued detection of IgM could be related to other factors, including viral persistence. Persistent infection with WNV has been well-documented in multiple species, including humans.17–22 Hepatitis C virus (HCV) is also a member of the Flaviviridae family, and presence of IgM anti-HCV antibodies serves as a marker of active viral replication and associated liver disease during the chronic stage of infection.23 Biologically, this association is understandable, and our finding of persistent IgM in WNV-infected patients leads to an urgent need to further document and understand the pathology of persistent WNV infection in humans.
Several studies have documented a high percentage of WNV patients with elevated IgM levels up to 1 year post-infection.3–5,11 One of the strengths of this current study is that we had the opportunity to capture repeated measures of antibody levels in a large cohort population over a substantial period of time. By doing so, we were able to show continued IgM persistence in one-fifth of participants up to 8 years post-infection. Persistent IgM response could lead to misdiagnosis of acute WNV disease. Physicians should be particularly mindful to diagnose acute WNV disease based on both the presence of clinically compatible illness and laboratory evidence of infection, preferably by the presence of anti-WNV IgM antibodies in CSF. Additional research is needed to understand the mechanisms and possible pathology related to extended levels of IgM antibodies over such a long period of time.
ACKNOWLEDGMENTS
We thank Robert Tesh, Melissa Resnick, Liliana Rodriguez, Emily Herrington, Vicki Miller, Monica Sierra, Josh Hellums, Charuta Kale, and Anne Hause for assistance with blood collections and laboratory testing procedures. We also thank the study participants for their willingness to contribute to this study.
Footnotes
Financial support: This study was supported in part by National Institutes of Health, National Institute of Allergy and Infectious Diseases Grants K23 AI057341, AI U19 089992, N01 50027 HHSN266200500027C, and 5R01AI091816-01; Department of Defense/Army Grant W81XWH-04-2-0031; and the Gillson Longenbaugh Foundation.
Authors' addresses: Kristy O. Murray, Department of Pediatrics, Baylor College of Medicine, Houston, TX, E-mail: KMurray@bcm.edu. Melissa N. Garcia and Rodion Gorchakov, Department of Pediatrics, Section of Tropical Medicine, Baylor College of Medicine, Houston, TX, E-mails: mnolan@bcm.edu and rodion@bcm.edu. Chris Yan, University of Texas Health Science Center at San Antonio, School of Medicine, San Antonio, TX, E-mail: yanc@livemail.uthscsa.edu.
References
- 1.Craven RB, Roehrig JT. West Nile virus. JAMA. 2001;344:1858–1859. doi: 10.1001/jama.286.6.651. [DOI] [PubMed] [Google Scholar]
- 2.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2:519–529. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 3.Tardei G, Ruta S, Chitu V, Rossi C, Tsai TF, Cernescu C. Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile virus infection. J Clin Microbiol. 2000;38:2232–2239. doi: 10.1128/jcm.38.6.2232-2239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papa A, Danis K, Athanasiasdou A, Delianidou M, Panagiotopoulos T. Persistence of West Nile virus immunoglobulin M antibodies, Greece. J Med Virol. 2011;83:1857–1860. doi: 10.1002/jmv.22190. [DOI] [PubMed] [Google Scholar]
- 5.Roehrig JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, Campbell GL. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis. 2003;9:376–396. doi: 10.3201/eid0903.020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray KO, Baraniuk S, Resnick M, Arafat R, Kilborn C, Cain K, Shallenberger R, York T, Martinez D, Malkoff M, McNeely W, Elgawley N, Khuwaja SA. Clinical investigation of hospitalized West Nile virus cases in Houston, 2002–2004. Vector Borne Zoonotic Dis. 2008;8:167–174. doi: 10.1089/vbz.2007.0109. [DOI] [PubMed] [Google Scholar]
- 7.Johnson AJ, Martin DA, Karabatsos N, Roehrig JT. Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J Clin Microbiol. 2000;38:1831. doi: 10.1128/jcm.38.5.1827-1831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for rountine diagnosis of arboviral infections. J Clin Microbiol. 2000;38:1823–1826. doi: 10.1128/jcm.38.5.1823-1826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray KO, Walker C, Herrington E, Lewis JA, McCormick J, Beasley D, Tesh RB, Fisher-Hoch S. Persistent infection with West Nile virus years after initial infection. J Infect Dis. 2010;201:2–4. doi: 10.1086/648731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawlins ML, Swenson EM, Hill HR, Litwin CM. Evaluation of an enzyme immunoassay for detection of immunoglobulin M antibodies to West Nile virus and the importance of background subtraction in detecting nonspecific reactivity. Clin Vaccine Immunol. 2007;14:665–668. doi: 10.1128/CVI.00480-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prince HE, Tobler LH, Lape-Nixon M, Foster GA, Stramer SL, Busch MP. Development and persistence of West Nile virus-specific immunoglobulin M (IgM), IgA, and IgG in viremic blood donors. J Clin Microbiol. 2005;43:4316–4320. doi: 10.1128/JCM.43.9.4316-4320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAllister-Sistilli CG, Caggiula AR, Knopf S, Rose CA, Miller AL, Donny EC. The effects of nicotine on the immune system. Psychoneuroendocrinology. 1998;23:175–187. doi: 10.1016/s0306-4530(97)00080-2. [DOI] [PubMed] [Google Scholar]
- 13.Cook RT. Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol Clin Exp Res. 1998;22:1927–1942. [PubMed] [Google Scholar]
- 14.Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70:179–189. doi: 10.1111/j.1399-0039.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 15.Murray K, Baraniuk S, Resnick M, Arafat R, Kilborn C, Cain K, Shallenberger R, York TL, Martinez D, Hellums JS, Hellums D, Malkoff M, Elgawley N, McNeely W, Khuwaja SA, Tesh RB. Risk factors for encephalitis and death from West Nile virus infection. Epidemiol Infect. 2006;134:1325–1332. doi: 10.1017/S0950268806006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wertheimer AM, Uhrlaub JL, Hirsch A, Medigeshi G, Sprague J, Legasse A, Wilk J, Wiley CA, Didier P, Tesh RB, Murray KO, Axthelm MK, Wong SW, Nikolich-Žugich J. Immune response to the West Nile virus in aged non-human primates. PLoS One. 2010;5:e15514. doi: 10.1371/journal.pone.0015514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tesh RB, Siirin M, Guzman H, Travassos da Rosa APA, Wu X, Duan T, Lei H, Nunes MR, Xiao S-Y. Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis. 2005;192:287–295. doi: 10.1086/431153. [DOI] [PubMed] [Google Scholar]
- 18.Tonry JH, Xiao S-Y, Siirin M, Chen H, Travassos APA, Tesh RB. Persistent shedding of West Nile virus in the urine of experimentally infected hamsters. Am J Trop Med Hyg. 2005;73:320–324. [PubMed] [Google Scholar]
- 19.Pogodina VV, Frolova MP, Malenko GV, Fokina GI, Koreshkova GV, Kiseleva LL, Bochkova NG, Ralph NM. Study on West Nile virus persistence in monkeys. Arch Virol. 1983;75:71–86. doi: 10.1007/BF01314128. [DOI] [PubMed] [Google Scholar]
- 20.Siddharthan V, Wang H, Motter NE, Hall JO, Skinner RD, Skirpstunas RT, Morrey JD. Persistent West Nile virus associated with a neurological sequela in hamsters identified by motor unit number estimation. J Virol. 2009;83:4251–4261. doi: 10.1128/JVI.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Appler KK, Brown AN, Stewart BS, Behr MJ, Demarest VL, Wong SJ, Bernard KA. Persistence of West Nile virus in the central nervous system and periphery of mice. PLoS One. 2010;5:e10649. doi: 10.1371/journal.pone.0010649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart BS, Demarest VL, Wong SJ, Green S, Bernard KA. Persistence of virus-specific immune responses in the central nervous system of mice after West Nile virus infection. BMC Immunol. 2011;12:6. doi: 10.1186/1471-2172-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quiroga JA, Herrero M, Castillo I, Navas S, Pardo M, Carreño V. Long-term follow-up study of serum IgM antibody to hepatitis C virus (HCV), HCV replication, and liver disease outcome in chronic HCV. J Infect Dis. 1994;170:669–673. doi: 10.1093/infdis/170.3.669. [DOI] [PubMed] [Google Scholar]