Abstract
Objective
To determine the effects of coculturing endometrial epithelial cells (eEC) with paired endometrial stromal fibroblasts (eSF) on cell-specific gene expression and cytokine secretion patterns.
Design
In vitro study.
Setting
University research laboratory.
Patient(s)
Endometrial biopsies were obtained from premenopausal women.
Intervention(s)
Polarized eEC and subject-paired eSF were cultured for 12.5 hours alone (monoculture) or combined in a two-chamber coculture system without cell-cell contact. Cells and conditioned media were analyzed for global gene expression and cytokine secretion, respectively. Purified, endometrial tissue-derived eEC and eSF isolated by fluorescent activated cell sorting (FACS) were used as noncultured controls.
Main Outcome Measure(s)
Cell-specific global gene expression profiling and analysis of secreted cytokines in eEC/eSF cocultures and respective monocultures.
Result(s)
Transepithelial resistance, diffusible tracer exclusion, expression of tight junction proteins, and apical/basolateral vectorial secretion confirmed eEC structural and functional polarization. Distinct transcriptomes of eEC and eSF were consistent with their respective lineages and their endometrial origin. Coculture of eEC with eSF resulted in altered cell-specific gene expression and cytokine secretion.
Conclusion(s)
This coculture model provides evidence that interactions between endometrial functionally polarized epithelium and stromal fibroblasts affect cell-specific gene expression and cytokine secretion underscoring their relevance when modeling endometrium in vitro.
Keywords: Endometrium, coculture, polarized epithelium, stroma, microarray, cytokines
Human endometriumis composed of a single layer of polarized, columnar epithelial cells (eEC) that interface with the uterine lumen and resident and transient endometrial cells in the underlying stroma (1, 2). It undergoes dynamic, cyclic temporalspatial changes in responsetocirculating ovarian steroid hormones, during which growth, cellular differentiation, shedding, and subsequent renewal occur (1–3). These processes are associated with conceptus signaling during nidation and the invasive phase of implantation (3, 4), with the endometrium playing a vital role in pregnancy establishment and maintenance. Several pathological conditions, including hormonal disorders and endometrial cancers, are associated with this tissue (5–8), which can also serve as a portal of entry for pathogenic microbes (9) as well as a propagation zone for virulent agents, including human immunodeficiency virus (HIV) (9, 10). Developing an in vitro model of human endometrial cell communication with fidelity to in vivo cellular functions holds promise for advancing understanding of the multiple roles the endometrium plays in health and disease.
Several endometrial in vitro models exist and are used as conventional tools to study endometrial function. The eEC or endometrial stromal fibroblasts (eSF) are commonly cultured in monoculture to assess effects of steroid hormones, hormone receptor modulators, and agents that could enter the uterine lumen (11–13). However, a limitation of in vitro monoculture systems is the absence of paracrine interactions that normally influence cell function and behavior in vivo. Indeed, rodent epithelial/stromal transplantation studies show that the stimulation of eEC mitogenesis by estrogen is mediated through the stromal cells (14). In addition, a fundamental property of the endometrial epithelium is cellular polarity, with cells connected via junctional proteins forming a tight epithelium and displaying functionally specialized plasma membrane compartments: apical (luminal) and basolateral (stromal) (15). Studies using eEC often do not provide models that account for this polarized environment. Polarized eEC secrete cytokines apically to exert their action on cells in the uterine lumen and also basolaterally to act on cells in the underlying stroma (16).
The use of endometrial coculture models is less prevalent than monocultures owing in part to the inherent complexity of cocultures and also because of the known difficulty and fastidiousness of growing normal human endometrial epithelial cells in long-term culture, which is further compounded if a functionally polarized epithelium is a requisite for physiologically meaningful studies. Notwithstanding these limitations, the use of endometrial coculture models has been well documented. Early studies show that the mesenchyme facilitates development and differentiation of the epithelium (17). Cunha and colleagues demonstrated that steroid-receptor positive stroma is necessary to drive hormonally regulated epithelial morphogenesis in the murine prostate and the female reproductive tract (18–20), underscoring the importance of epithelial-stromal paracrine signaling. Coculture of eEC and eSF has been reported to restore the steroid-induced suppression of an epithelial-specific metalloproteinase observed in whole tissue explants (21), as well as induce development of ultrastructural epithelial polarity and microvilli (22). Also, inhibition of eEC thymidine incorporation and stimulation of glycodelin secretion, suggesting eEC differentiation, were observed only in cocultures with eSF, which allowed cell-cell interactions (23).
The objective of the current study was to build upon existing endometrial epithelial/stromal coculture models by establishing a coculture system that demonstrates preservation of cell lineage markers (compared with in vivo cells) and functional epithelial polarity to determine the effects of paracrine interactions on the transcriptomes of cultured eEC and eSF. The utility of this model derives from its potential to investigate how exogenous challenges to the epithelium (implanting embryos, hormones, pharmaceuticals, infectious agents, seminal plasma) may affect endometrial cellular responses and function, thereby providing a valuable complement to in vivo studies often limited because of ethical or regulatory constraints.
Materials and Methods
Tissues Procurement and Processing
Human endometrial tissue samples were obtained in accordance with the guidelines of the Declaration of Helsinki Written informed consent was obtained from all subjects. The study was approved by the Committee on Human Research of the UniversityofCalifornia, San Francisco (UCSF). Endometrial tissue samples were processed on the day of collection, and primary cell cultures were initiated immediately after tissue processing. Subjects were premenopausal women (ages 28–53) and confirmed not to be pregnant. Details of their clinical history and cycle phase at the time of tissue sampling are in the Supplemental Data available at online at www.fertstert.org, Supplemental Tables 1–9. Briefly, samples used for culture experiments were obtained during the early (n = 2), mid (n = 1), and late (n = 1) secretory phases. Additional samples for validation studies (n = 3) were obtained in the proliferative phase. Tissue samples were obtained through the National Institutes of Health Specialized Cooperative Centers Program in Reproduction and Infertility Research Human Endometrial Tissue and DNA Bank at UCSF under established standard operating procedures (24). Endometrial tissue samples included six biopsies (obtained using the Pipelle Endometrial Suction Curette, Cooper Surgical) from subjects undergoing oocyte retrieval, hysteroscopy, or laparoscopic surgery for benign conditions and one hysterectomy specimen.
Endometrial tissue was digested with 6.4 mg/mL collagenase type I; 125 U/mL hyaluronidase in Hanks buffered salt solution with Ca++ Mg++. Contaminant red cells were lysed with 0.155 M NH4Cl, 0.01 M KHCO3, 0.1 mM EDTA, pH 7.3, and the dissociated cellular elements were DNase treated (4 mg/mL) and then size fractionated with a 40-μm cell strainer (BD Biosciences) to separate single cells from fragments of endometrial epithelial sheets and glands. Selective attachment to plastic dishes was used as the final step to separate endometrial epithelial and stromal cells (25). The single-cell eSF fraction was established in primary culture and serially passaged as described elsewhere (26) in stromal cell medium (SCM): 75% phenol red-free Dulbecco's modified eagle medium (DMEM)/25% MCDB-105 supplemented with10% charcoalstripped fetal bovine serum (FBS) and 5 μg/mL insulin. The eEC were plated on Matrigel-coated dishes (BD Biosciences) with defined keratinocyte serum-free medium (KSFM; Gibco) and achieved 75% confluence within 10–14 days.
Epithelial and Stromal Cell Coculture Experimental Design
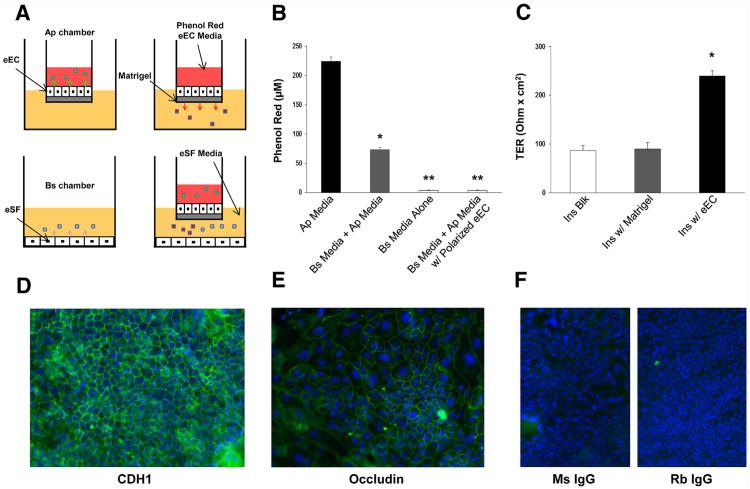
We used a two-chamber coculture system without direct cell-cell contact between chambers (Fig. 1A), which allows separate analysis of secreted products in the apical and basolateral chambers when cells in the upper chamber/insert form a functionally competent tight epithelial barrier. The two cell types in our coculture system have different media requirements for optimal long-term culture (KSFM for eEC and SCM for eSF), and exposure of eSF cultures to KSFM resulted in reduced growth and viability. Likewise, exposure of eEC cultures to SCM or other high serum media reduced growth and altered morphology. Therefore, we conducted preliminary time course experiments using as coculture medium a low serum formulation modified from that reported for endometrial cocultures by Arnold et al. (23) and compatible with eSF cultures (75% phenol red-free DMEM/25% MCDB-105 supplemented with 1% FBS and 1 mg/mL bovine serum albumin). The functional integrity of polarized eEC cultures exposed to this coculture medium was monitored by leakage of phenol red from the apical to the basolateral chamber (see next section). Results showed a functionally competent tight epithelium through 12.5 hours but compromised functional integrity of the tight epithelial barrier at later time points, resulting in phenol red leakage from the apical into the basolateral chamber. This pilot study defined the time frame for our coculture experiments. Primary eEC cultures were harvested at 50%–75% confluency using Accutase (EMD Millipore), and 105 eEC were seeded into hanging inserts (24-well size, polyethylene terephthalate membrane, 1 μm pore Millicell hanging cell culture inserts, EMD Millipore) coated with Matrigel (BD Biosciences) and placed in 24-well plates in KSFM (0.2 mL in insert/apical chamber; 1 mL in well/basolateral chamber). Culture medium was renewed every 2-3 days, and eEC achieved confluency and were functionally polarized within 2-4 weeks, as shown by increased transepithelial resistance (TER), lack of diffusible tracer exchange between chambers (see next section), and immunolocalization of tight junction proteins between adjoining cells (see immunofluorescence below). Patient-paired eSF were harvested at passage 2, and 105 cells were plated on uncoated plastic 24-well plates that accommodate the hanging inserts; confluency was achieved within 2–4 days. For coculture, polarized eEC insert cultures were transferred to the 24-well plates containing confluent subject-paired eSF cultures, resulting in a basolateral chamber with resident eSF. The culture medium in both the apical and basolateral chambers in cocultures was replaced with the coculture medium as described, and phenol red (32 mg/L) was added to the medium in the apical chamber of cocultures to confirm that no media exchange occurred between the apical and basolateral chambers during the course of the experiment. Replicate eEC and eSF monocultures were processed in parallel under identical conditions to cocultures as individual cell type controls. Mono- and cocultures were incubated for 12.5 hours, conditioned media were collected from the apical and basolateral chambers, and cells were processed for RNA extraction.
Figure 1.
The endometrial coculture system. (A) Polarized confluent eEC in monoculture secrete factors into the apical chamber (Ap) of the insert. Factors are also secreted into the basolateral (Bs) chamber. Products secreted in each chamber do not mix. Confluent eSF grown in monoculture secrete products into the Bs chamber. When grown in coculture, there are apically secreted products, and in the Bs chamber, a heterogeneous mix of basolateral eEC and eSF secreted products. The eEC medium in the Ap chamber contains phenol red used as diffusible tracer to assess epithelial integrity. The eSF medium in the Bs chamber has low phenol red. (B) Diffusible tracer exclusion. Phenol red concentration in eEC medium of the Ap chamber (Ap Media). Phenol red concentration in the Bs chamber before (Bs Media Alone) and after equilibration for 12.5 hours with eEC medium in Matrigel-coated inserts without (Bs Media+Ap Media) or with confluent eEC (Bs Media+Ap Media w/Polarized eEC). Values represent the mean ± SEM of experiments using cell preparations from four different subjects. *P<.05 compared with Bs Media; **P<.05 compared with Bs Media+Ap Media. (C) TER was measured for uncoated (Ins Blk) or Matrigel-coated (Ins Blk w/Matrigel) cell-free inserts or inserts with confluent eEC (Ins w/eEC). Values represent the mean ± SEM of experiments using cell preparations from four different subjects. *P<.05 compared with Ins Blk. (D) Indirect immunofluorescence for the epithelial adherens junction protein e-cadherin (CDH1). Green fluorescence shows pericellular immunolocalization in confluent polarizied eEC. (E) Indirect immunofluorescence for the tight junction protein occludin. Green fluorescence shows pericellular immunolocalization in confluent polarized eEC. (F) Nonimmune mouse (Ms IgG) and rabbit (Rb IgG) controls show no background fluorescence. Blue nuclear fluorescence from DAPI. Original magnification ×50. Shown are representative samples of cultures derived from four subjects.
TER and Phenol Red Exclusion
TER was measured in eEC insert cultures using the Millicell ERS System (EMD Millipore) compared with baseline TER of cell-free inserts with or without Matrigel coating. Functional integrity/competence of the tight epithelial barrier was further assessed by determining whether phenol red added to the apical chamber would leak into the basolateral chamber. Phenol red levels were measured in the apical and basolateral chambers at the beginning and end of each experiment by absorbance at 559 nm using a Beckman Coulter DU 530 spectrophotometer (Beckman Coulter). Only experiments with no detectable levels of phenol red leakage into the basolateral chamber were included for analysis of the conditioned media. Phenol red concentrations were calculated from absorbance values using the extinction coefficient of phenol red.
Immunofluorescence
Indirect immunofluorescence was conducted following previously reported methods (27). Briefly, cells cultured in Matrigel-coated (eEC) or noncoated (eSF) chamber slides were fixed in 2% paraformaldehyde, permeabalized with 0.1% Triton X-100, blocked with 10% normal goat serum, and incubated overnight at 4°C with the following primary antibodies: mouse anti-human keratin 18 (1:200; C-7785, Sigma Aldrich), vimentin (1:200; V-6389, Sigma Aldrich), e-cadherin (ab1416 Abcam), or rabbit anti-occludin (ab31721, Abcam). Cells were then washed 3 times with phosphate-buffered saline/0.1% Tween 20 buffer and incubated for 1 hour at room temperature with the corresponding Alexafluor 488 conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (1:250; A-11001 and A-11008, respectively; Invitrogen) and then washed 3 times with buffer. For specificity controls, the primary antibodies were substituted with the corresponding mouse or rabbit nonimmune IgG. Chamber slides were mounted with ProLong Gold Antifade Reagent with 4′,6-diamidino-2-phenylindole (DAPI; P-36931, Invitrogen) and viewed on a Leica DM 5000 microscope equipped with epifluorescence optics (Leica Microsystems, Inc.). Counts of keratin 18 (KRT18)- and vimentin-positive cells were done in four random × 40 fields per sample.
Fluorescence Activated Cell Sorting (FACS)
We isolated eEC and eSF by FACS from whole endometrial tissue to test the fidelity of in vitro cultured cell gene expression compared with in vivo derived cells. Details can be found in the Supplemental Materials portion of this manuscript, found online at www.fertstert.org.
RNA Isolation
Total RNA was isolated from cultured eEC and eSF using the Nucleospin RNA purification kit (Machery Nagel) following the manufacturer's protocol including DNase treatment. For FACS-sorted cell populations, total RNA was isolated using the Arcturus PicoPure RNA Isolation Kit (Applied Biosystems) and DNase treated using RNase-Free DNase Set (Qiagen). The purity and integrity of all RNA samples were confirmed through Nanodrop (Nanodrop) and Bioanalyzer (Agilent), respectively.
Microarray Analysis
RNA from cultured and FACS-sorted cells (n = 3 each) were further processed for analysis on Affymetrix Human Gene 1.0 ST Arrays (Affymetrix) with updated annotations, probing 36,079 transcripts and 21,014 genes, as reported elsewhere (28). Briefly, RNA was reverse transcribe/amplified into cDNA, and sense-strand cDNA targets were fragmented/labeled and hybridized to Affymetrix Human Gene 1.0 ST Arrays. The quality of the amplified cDNA and fragmented cDNA was assessed using the Bioanalyzer, and only samples meeting yields and quality standards were used for hybridization. Intensity values of different probe sets (genes) were imported into GeneSpring GX 11.02 software (Agilent Technologies) and processed using the robust multiarray analysis algorithm for background adjustment, normalization, and log2 transformation of perfect match values. RMA16 was used as the background correction algorithm for ST array technology. Differential expression analysis was performed for the following comparisons between eEC and eSF in monoculture and coculture: [1] eEC monoculture (eECmono) versus eSF monoculture (eSFmono); [2] eEC coculture (eECco) versus eSF coculture (eSFco); [3] eECmono versus eECco; [4] eSFmono versus eSFco. Differential expression analysis was also conducted on highly pure, noncultured, eEC versus eSF populations isolated from endometrial tissue by FACS (eECFACS vs. eSFFACS). Analysis output includes only genes with ≥ 1.5-fold change and P<.05 by two-way analysis of variance (ANOVA) with Benjamini-Hochberg multiple-testing correction for false discovery rate. The use of a 1.5-fold cutoff for biologically relevant analysis is consistent with previous reports (29, 30).
Fluidigm-Based Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Validation of 108 selected differentially expressed genes was conducted on a total of 24 cDNA samples derived from cultured eEC and eSF (eEC and eSF derived from n = 3 subjects, in mono and coculture) and from eEC and eSF FACS-isolated cells from n = 3 subjects by qRT-PCR using the Fluidigm 48.48 and the 96.96 Dynamic Array Integrated Fluidic Circuits and the Biomark System (Fluidigm), as described elsewhere (28), with the following modifications. The optimal dilution of preamplified cDNA used for downstream analysis was determined, since it is different for RNA isolated from sorted cells, cultured cells, and tissues. Thus, the 1:5 (RNA from FACS cells) and 1:50 (RNA from cultured cells) dilutions used were determined by dilution curve analysis, which in turn evaluates the efficiency of the primers used (all primers used for these experiments exhibited a slope that equated to 90%–110% amplification efficiency). The dilution that generated the earliest exponential amplification of the diluted preamplified cDNA was used for subsequent analysis. The comparative Ct method was used to obtain relative expression for each grouping comparison, where the amount of target normalized to heat shock protein HSP90AB1 for cultured cells, and beta actin for FACS-sorted cells, was represented by delta Ct (DCt). These housekeeping genes were chosen from a pool and were selected for the stability of expression between cell types and treatment variables. Expression was then normalized to an internal calibrator for cultured and sorted cells and represented as delta delta Ct (DDCt), and total fold change was calculated by 2DDCt (ABI User Bulletin 2).
Luminex Multiplex Cytokine Assays
Conditioned media were centrifuged at 13,000 × g for 5 minutes to remove cellular debris, and supernatants were analyzed for secreted cytokines using a custom multiplex Luminex kit (EMD Millipore), which included interleukin (IL)1A, -B, -2, -4, -5, -6, -8, -10, tumor necrosis factor alpha (TNFA), interferon gamma (IFNG), granulocyte macrophage colony stimulating factor (CSF2), macrophage inflammatory protein 1 α (CCL3), β (CCL4), monocyte chemoattractant protein 1 (CCL2), 3 (CCL7), fractalkine (CX3CL1), and secreted chemokine (c-c motif) ligand 5 (CCL5). All protocols were based on manufacturer's specifications. Briefly, conditioned media were incubated in prewet Luminex plates overnight with antibody-coated, fluorescent-dyed capture microspheres specific for each analyte, followed after washing by detection antibodies and streptavidin-phycoerythrin. The washed microspheres with bound analytes were resuspended in sheath fluid and analyzed on a Bioplex (Biorad) bead sorter. Standard curves and high/low range positive controls were used to determine the concentration of each cytokine. Additional controls for background noise and interference included unconditioned media with/without phenol red. To ensure the appropriate level of sensitivity, samples with <50 beads for each cytokine target were excluded from the analysis. Each sample was run in duplicate, and results for each sample were repeated independently on at least two different plates. Data were adjusted for media volume and normalized to cell number.
Statistical Analysis
Differential expression analysis of microarray data was conducted using Genespring. Fluidigm qRT-PCR data were analyzed by t tests to determine significant differences in the expression of cell-specific markers in eEC versus eSF or between mono- versus coculture in each cell type using R-Commander (2011) and Microsoft Excel (2010). Statistical analysis of epithelial TER and phenol red exclusion data were performed on R-Commander using ANOVA with Tukey's post hoc analysis. Secreted cytokine data were analyzed using preconceived orthogonal contrasts with pairwise comparisons of specific experimental groups with the Statistical Analysis System software (SAS, 2011).
Results
eEC Structural and Functional Polarization
A main requisite for a physiologically relevant coculture model is the formation of functionally competent polarized epithelium. Structurally, this requires the formation of tight junctions that separate apical and basolateral compartments and enable the vectorial secretion of molecules into these discrete compartments. We modeled the endometrial epithelium, as previously done by other investigators (23), by culturing eEC on Matrigel-coated inserts with discrete apical and basolateral compartments that are accessible for analysis (Fig. 1A). Then it was important to determine the functional competence of the tight epithelial barrier, which requires not only the establishment of TER but also verifying that there was no exchange of diffusible molecules between the apical and basolateral compartments. Therefore, TER was measured in confluent epithelial cultures, and phenol red added to the apical chamber was used as diffusible tracer to assess exchange between apical and basolateral compartments. The baseline concentration of phenol red in the apical chamber was 224.8 ±7.1 μM (Fig. 1B). In cell-free inserts, phenol red diffused through the 1μm pores of the membrane and across the Matrigel layer, equilibrating with the medium in the lower chamber, thus raising the phenol red concentration in the latter from 3.3 ± 1.1 to 73.6 ± 3.5 μM (P<.05). With a confluent eEC monolayer in the insert, the phenol red concentration in the basolateral chamber of cocultures remains unchanged, confirming formation of an impermeable, tight epithelial layer in the cocultures through 12.5 hours. Insert cultures with confluent eEC also had increased TER (225 ±15 Ohm × cm2) compared with cell-free uncoated or Matrigel-coated inserts (P<.05; Fig. 1C). Confluent eEC cultures were examined by immuno-fluorescence for the presence and cellular localization of e-cadherin (CDH1) and occludin, major protein constituents of adherens and tight junctional complexes. As shown in Figure 1D and E, confluent epithelial cultures displayed pericellular immunereactivity for CDH1 and occludin, consistent with tight junction localization.
Culture Morphology and Immunofluorescence
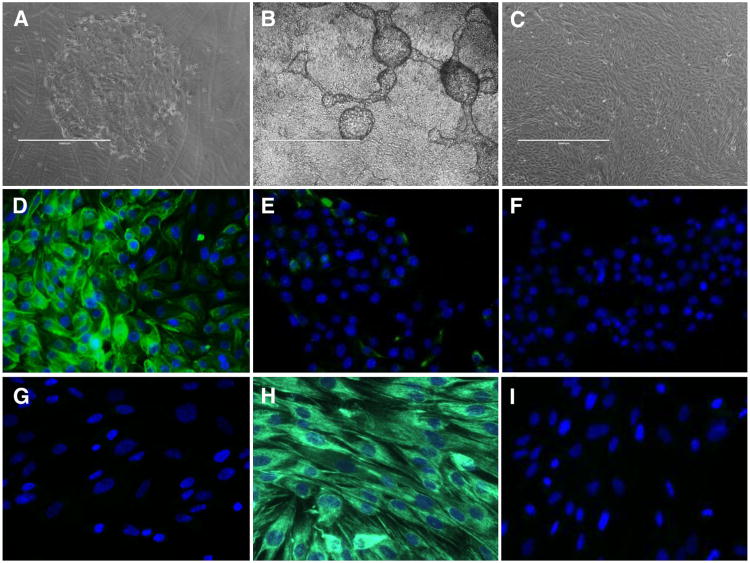
Primary eEC cultures showed expanding colonies of actively growing cells (Fig. 2A), which when subcultured onto Matrigel-coated inserts formed dense confluent monolayers with characteristic domes and ridges (Fig. 2B), distinct from the typical monolayer morphology of eSF (Fig. 2C). Immunofluorescence showed that eEC cultures comprised predominantly (94% ± 3.1%) KRT18-positive cells (Fig. 2D) with minimal presence (4.3% ± 1.5%) of vimentin-immunoreactive cells (Fig. 2E), consistent with previous reports (20). The eSF cultures were 100% vimentin-positive and had no KRT18-positive cells (Fig. 2G and H). Nonimmune IgG controls are shown in Figure 2F and I.
Figure 2.
Culture morphology and immunocytochemistry of eEC and eSF. Phase contrast microscopy (A–C), original magnification ×40, scale bar represents 1,000 μm. Primary eEC in culture on Matrigel-coated dish (A). Confluent passage 1 eEC culture on Matrigel-coated hanging insert (B). Confluent passage 2 eSF (C). Indirect immunofluorescence (D–I), green fluorescence shows antibody reactivity, blue fluorescence shows nuclear DAPI reactivity, original magnification ×400. Passage 1 eEC grown on Matrigel show cytoskeletal reactivity for KRT18 (D), rare isolated vimentin-positive cells (E), and no reactivity with nonimmume IgG (F). Passage 2 eSF show no KRT18 reactivity (G), cytoskeletal immunolocalization of vimentin (H), and no reactivity with nonimmume IgG (I). Results are representative of experiments using cells isolated from four subjects.
Transcriptome Analysis of Cultured and Noncultured eEC and eSF
Endometrial cell cultures were characterized by microarray analysis, and differential gene expression was assessed based on cell type (eEC vs. eSF) and culture condition (mono- vs. coculture) for the following comparisons: [1] eEC monoculture (eECmono) versus eSF monoculture (eSFmono); [2] eEC coculture (eECco) versus eSF coculture (eSFco); [3] eECmono versus eECco; [4] eSFmono versus eSFco. In addition, we tested the fidelity of in vitro cultured cell-specific gene expression compared with that in noncultured in vivo derived pure eEC and eSF populations isolated from endometrial tissue by FACS (eECFACS vs. eSFFACS). The complete lists of differentially expressed genes for these comparisons are shown in Supplemental Tables 5–9 (available online at www.fertster.org). Of a total of 3,010 differentially expressed genes in cocultured eEC versus eSF (>1.5 fold; P<.05), 70 were validated by qRT-PCR. Validated genes up-regulated in eECco versus eSFco are shown in Supplemental Table 2, and those up-regulated in eSFco versus eECco are shown in Supplemental Table 3. The observed pattern of up-regulated genes for each cell type revealed a unique signature consistent with the respective epithelial (e.g., keratins, junctional proteins, mucins) or mesenchymal (e.g., vimentin, interstitial collagens) lineage and with the differential gene expression of in vivo derived eEC and eSF (Supplemental Table 4). In addition, eEC and eSF differentially expressed genes characteristic of their corresponding endometrial cell type (e.g., WNT7A, AREG, MMP7 in eEC; HOXA11, WNT5A, MMP2, IGFBPs in eSF). Moreover, the expression of lineage-specific genes by eEC and eSF was not altered by the culture condition (e.g., monoculture vs. coculture; coculture data are shown in Supplemental Tables 2 and 3; monoculture data are shown in Supplemental Tables 5 and 6).
The Effect of Coculture on eEC and eSF Gene Expression
The transcriptomes of eEC and eSF were distinctly affected when cocultured with the corresponding epithelial/stromal counterpart (eECmono vs. eECco; eSFmono vs. eSFco; Table 1), clearly indicating the functional relevance of reciprocal eEC-eSF interactions in regulating cell-specific gene expression. The complete lists are shown in Supplemental Tables 8 and 9 (available online at www.fertster.org). In eEC, genes associated with endometrial immunity, including defensins and the cytokine TGFA as well as the epithelial sodium channel gene SCNN1G, which is required for epithelial barrier function (31), were down-regulated in monoculture compared with coculture (Table 1). In eSF, multiple genes associated with endometrial immunity were also down-regulated in mono- compared with coculture (Table 1). These included the cytokines IL32, CCL7, CXCL2, CCL2, and IL8. Expression of the antiviral serine protease SERPINB2(32) was also reduced in monocultured compared with cocultured eSF.
Table 1. Select genes differentially expressed in mono- versus coculture.
| eECmono versus eECco | eSFmono versus eSFco | ||||
|---|---|---|---|---|---|
| Fold change | Fold change | ||||
| Gene | Array | PCR | Gene | Array | PCR |
| GAS5 | 1.9 | NPA | PRELP | 2.1 | 2.0 |
| ATXN7L1 | 1.6 | NPA | STMN2 | 1.9 | 1.8 |
| LRRFIP1 | 1.5 | NC | TGFB2 | 1.8 | 1.8 |
| CDC20 | 1.5 | NC | LEF1 | 1.7 | NC |
| SDF2 | −1.6 | −1.5 | MMP11 | 1.6 | 1.6 |
| CTSL2 | −1.6 | NC | GRIA4 | 1.6 | 3.1 |
| ALDOC | −1.7 | −1.8 | OLFML2B | 1.5 | 2.1 |
| TMPRSS2 | −1.7 | NC | JAM2 | −1.5 | −1.8 |
| DEFB103B | −1.7 | −2.3 | IL7R | −1.6 | −2.1 |
| TGFA | −1.7 | −1.7 | CX3CL1 | −1.6 | −13.6 |
| ANGPTL4 | −1.8 | −2.4 | APOL1 | −1.6 | −1.8 |
| TMEM86A | −1.9 | −2.3 | SERPINB2 | −1.7 | −2.2 |
| ANKRD37 | −2.0 | −2.6 | DRAM | −1.7 | −2.0 |
| SCNN1G | −2.3 | −1.7 | NFKB2 | −1.7 | −3.0 |
| IL32 | −2.2 | −2.7 | |||
| TNFAIP3 | −2.7 | −8.0 | |||
| CCL7 | −3.0 | −11.9 | |||
| NFKBIA | −3.0 | −4.3 | |||
| CLDN1 | −3.1 | −5.9 | |||
| CXCL2 | −3.3 | −25.6 | |||
| TNFSF18 | −3.7 | −3.1 | |||
| ICAM1 | −4.5 | −38.1 | |||
| SOD2 | −6.1 | −6.5 | |||
| CCL2 | −7.6 | −5.3 | |||
| CXCL1 | −10.0 | −13.7 | |||
| IL8 | −12.7 | −52.6 | |||
Note: Minimum 1.5-fold change; n = 3; P< .05. NPA = no primers available; (−) = down-regulation; NC = no change.
Differential Patterns of Cytokine Secretion in the Coculture Model
The coculture model allows analysis of cell- (eEC vs. eSF) and compartment- (apical vs. basolateral) specific secreted products. We chose to measure endometrial cytokines that would be relevant to endometrial physiology, given that they are produced by eEC and eSF and play a role in endo-metrial remodeling/establishment of pregnancy (33–35) and/or are the focus of endometrial disease models (10). Therefore, selected cytokines were measured in conditioned media of apical and basolateral compartments of monocultures and cocultures. Cell-specific cytokines were delineated by comparison of eEC cytokines (both apical and basolateral) versus eSF cyto-kines (Table 2). The apically secreted eEC cytokines IL1A, -4, -6, -8, CSF2, TNFA, and CX3CL1 did not differ in cocul-ture compared with monoculture. However, apical CCL3 and CCL4 levels were increased, and CCL2 decreased (P<.05) in coculture compared with monoculture (Table 2). Comparison of apical versus basolateral cytokine secretion in eEC monocultures showed clear indication of selective differential vectorial apical versus basolateral secretion for particular cytokines including CCL2, -3, and -4, CX3CL1, IL1A, -4, -6, and TNFA, as evidenced by significant differences (P<.05 or detectable vs. nondetectable) in their respective apical versus basolateral concentrations (Table 2). In cocultures, the basolateral chamber (containing the combined eEC basolateral and eSF secretion) had detectable levels of CCL2, CCL7, CSF2, CX3CL1, IL6, and IL8. Comparison of the latter with the sum of the corresponding eEC basolateral plus eSF cytokines secreted in monoculture showed that basolateral CCL2 was increased (P<.05) in cocultures, whereas CSF2 and CX3CL1 were decreased (P<.05). In addition, TNFA was present in monoculture eEC basolateral secretion but unde-tectable in the basolateral chamber of cocultures (Table 2).
Table 2. Analysis of select cytokines secreted by eEC into the apical and basolateral compartments and by eSF.
| eECmono | eSFmono | eECco/eSFco | |||
|---|---|---|---|---|---|
| Cytokine | AS (pg/105 cells) | BS (pg/105 cells) | SS (pg/105 cells) | AS (pg/105 cells) | BS + SS (pg/105 cells) |
| CCL2 | 97.42 ± 5.4 | 72.76 ± 1.4a | 492.9 ± 17.9b | 70.4 ± 2.3c | 1,695.1 ± 31.8d |
| CCL3 | 5.76 ± 0.3 | ND | ND | 16.23 ± 0.7c | ND |
| CCL4 | 1.0 ± 0.2 | ND | ND | 2.32 ± 0.1c | ND |
| CCL5 | ND | ND | ND | ND | ND |
| CCL7 | ND | ND | 13.8 ± 1.0 | ND | 20.11 ± 0.14 |
| CSF2 | 13.7 ± 5.1 | 13.7 ± 1.1 | 1.0 ± 0.6b | 23.2 ± 12.0 | 5.3 ± 1.7d |
| CX3CL1 | 56.27 ± 5.3 | ND | 46.3 ± 7.0 | 43.18 ± 1.7 | 17.7 ± 1.76d |
| IFNG | ND | ND | ND | ND | ND |
| IL1A | 47.4 ±11.1 | ND | ND | 42.3 ± 35.1 | ND |
| IL1B | ND | ND | ND | ND | ND |
| IL2 | ND | ND | ND | ND | ND |
| IL4 | 12.4 ± 2.3 | ND | ND | 11.6 ± 2.4 | ND |
| IL5 | ND | ND | ND | ND | ND |
| IL6 | 113.5 ± 38.5 | 15.6 ± 5.4a | 22.6 ± 18.4b | 57.9 ± 16.1 | 43.5 ± 19.0 |
| IL8 | 807.0 ± 140.1 | 573.75 ± 213.2 | 128.3 ± 24.88b | 627.38 ± 70.3 | 1,085.7 ± 501 |
| IL10 | ND | ND | ND | ND | ND |
| TNFA | 23.55 ± 4.22 | 9.55 ± 4.92a | ND | 17.75 ± 7.1 | ND |
Note: AS = apical; BS = basolateral; eFS = SS; ND =nondetectable.
P< .05 between eECmono AS and eECmono BS.
P< .05 between eECmono AS + BS and eSFmono SS.
P< .05 between eECmono AS and eECco/eSFco AS.
P< .05 between eECco/eSFco SS + BS and eECmono BSmono + eSFmono.
Discussion
The data presented herein suggest that an endometrial coculture system with confluent, functionally polarized epithelium can provide important and unique insights into the transcriptome as well as the cytokine-secretory activity of endometrial eEC and eSF. Building on previously established principles, the strengths of this model include the following: [1] it has discrete compartments wherein well-defined and characterized subject-paired eEC and eSF can engage in functionally relevant cell interactions, while at the same time both cells and their products can be analyzed separately; [2] eEC and eSF show characteristic transcriptome signatures consistent with those of the corresponding pure epithelial and stromal fibroblast cell populations FACS-isolated from human endometrium; [3] it has a structurally competent tight epithelium displaying functional polarity with vectorial apical/basolateral secretion; and [4] there is clear indication of paracrine interactions between the cell compartments, as evidenced by differential gene expression and secretory activity in cocultured cells compared to their monocultured counterparts.
Establishment of the Endometrial Epithelial and Stromal Phenotypes
To model the endometrium properly, several key requirements have to be met by the coculture model. First and foremost, eEC and eSF have to retain demonstrably in culture their in vivo phenotype. To determine in vitro phenotypic stability, we examined the expression of multiple genes representative of the epithelial and mesenchymal lineages as well as genes characteristic of the endometrial epithelial and stromal fibroblast cell populations. Purity of the cell cultures was inferred from the mutually exclusive differential expression of lineage-/tissue-specific genes in the eEC and eSF populations. In addition, we used the well-established conventional markers of eEC and eSF culture purity, KRT18 and vimentin (21, 36, 37), respectively, in both gene expression and protein studies. Independent confirmation of cell-specific expression of KRT18 and vimentin in eEC and eSF, respectively, is provided by our differential gene expression data of highly pure endometrial eEC versus eSF cell populations isolated by FACS using independent cell surface selection markers (Supplemental Table 2).
Epithelium
Our data showed that the cultured eEC express genes specific to the epithelial lineage, including EPCAM (TACSTD1 [28]); keratins 23, 7, and 18 (38, 39); mucins 16, 20, and 1 (28, 40–42); integrins B6 and B8 (27, 43, 44); and laminins C2 and B3 (45–48), while also expressing genes associated with the endometrial epithelium, including amphiregulin (49, 50), epiregulin (51, 52), and the endometrial epithelial defensins (53, 54). Moreover, comparison of the differential expression of these lineage-/endometrial-specific genes in cultured eEC and eSF and in the corresponding noncultured highly pure cell populations that were FACS-sorted from endometrial tissue confirmed the consistency of the in vitro and in vivo lineage phenotypes, implying phenotypic stability of eEC and eSF in culture. Indeed, our results (Supplemental Table 2) indicate that the aforementioned genes in eEC and eSF were similarly expressed in both cultured and noncultured cells. Microarray data were validated with qPCR, and certain genes, including KRT18, CDH1, occludin, and some of the cytokine genes, were further validated at the protein level through immuno-fluorescence/immunoassay.
Interestingly, WNT7A, HBEGF, and KRT13 transcripts, all known to be restricted to the endometrial luminal epithelium, were prominently expressed in eEC cultured on inserts. WNT7A is localized to the luminal epithelium in human endometrium (55), and HBEGF, an endometrial growth factor in the luminal epithelium, plays a vital role in promoting blastocyst attachment and invasion in mice and presumably in humans (50, 56, 57). KRT13 was recently shown to be a marker of luminal, but not glandular, epithelium in human endometrium and used to define a luminal phenotype for the human endometrial carcinoma cell line ECC-1 (58). Therefore, eEC cultured on Matrigel-coated inserts prominently express genes whose products are restricted in vivo to the luminal endometrial epithelium, suggesting a luminal endometrial epithelial phenotype of eEC in this model. However, it is not clear from the current data whether this reflects a homogeneous cell population with luminal phenotype or whether the eEC cultures are a heterogeneous population containing cells with both luminal and glandular phenotypes. Additional in situ hybridization/immunolocalization studies would be required to determine the luminal/glandular phenotypic heterogeneity of these eEC cultures and their potential as a viable model of the luminal endometrial epithelium.
Stroma
Genes up-regulated in cultured eSF compared with in eEC and indicative of endometrial stromal phenotypes were largely consistent with the literature, including members of the WNT family and the HOXA axis (59–61), PDGFRB (28), and the stromal collagens (62, 63). Gene expression of eSF markers, for example, vimentin and secreted cytokines, was validated at the protein level through immunofluorescence and secreted protein measurements. Although vimentin has been shown to be expressed in a number of cell types, within normal endometrium its stromal localization is well established, and its use as a marker for the identification of eSF in cell cultures is reported in multiple models (21, 36, 37). The expression of PDGFRB is well documented in endometrial stromal fibroblasts, as well as in mesenchymal stem cells, and is indeed used as a selection marker for FACS isolation of these endometrial cell types (28, 64). Our current transcriptome data of highly pure endometrial cell populations isolated by FACS demonstrate the differential expression of PDGFRB in eSF versus eEC (Supplemental Table 5), which parallels the differential expression observed in cultured eSF versus eEC (Tables 1 and 2).
Together our data support the in vitro phenotypic stability of eEC and eSF in the coculture model.
Cell-Specific Expression of Cytokine Genes and Cytokine Production
The cytokines assayed herein were chosen based on their presence in the endometrium and the roles they play regarding innate uterine immunity and endometrial function (33–35). There are many other secreted factors in the endometrium, including growth factors, noncytokine immune modulators, and matrix proteinases. These molecules all play important roles in endometrial function. Given the limitations of this model, which provided low yields of conditioned media, we chose to focus on endometrial cytokines because of their importance in endometrial physiology and pathophysiology. Cell-specific patterns of cytokine gene expression were largely supported by the immunosecretory patterns. However, there were some cytokines that were detectable at the protein level but not differentially expressed using microarray or qRT-PCR. For example, IL4 and IL6 showed no differences between eEC and eSF at the transcript level, but the proteins were secreted exclusively (IL4) or at significantly higher levels (IL6) by eEC. Conversely, IL1B was highly expressed at the transcript level in eEC compared to eSF, but secreted protein was undetectable. These inconsistencies suggest that further investigation is warranted into storage and post-trancriptional and post-translational activities of some genes and proteins, which may also identify alternative pathways regulating cytokine production and secretion in human endometrium.
Effect of Coculture on Cell-Specific Transcriptomes
In addition to the set of genes differentially expressed between cell types, several genes associated with endome-trial immunity and repair were differentially expressed in individual cells types in coculture compared to monoculture. In cocultured eEC, genes associated with innate immunity (the defensin DEFB103B) (65, 66), cytokines (TGFA), wound healing-associated factors (ANGPTL4)(67), and the sodium channel maintenance factor associated with epithelial barrier function (SCNN1G)(31) were up-regulated compared to monocultured eEC. In eSF, the majority of genes associated with immune function were also up-regulated in coculture compared to monoculture. These primarily included cytokines (CCL2, CXCL1, IL8, CXCL2, CCL7) and an immune-related serine protease inhibitor (SERPINB2). Together these data suggest that when modeling immune responses in endometrium, coculture studies provide additional information, compared with monocultures using epithelial or stromal cells, since multiple genes associated with the host defense mechanism, wound healing, and cytokine-regulated immune responses are blunted in monocultured eEC and eSF, implying dependence on paracrine stimulation. Moreover, since cytokines are implicated in cellular proliferation and tissue remodeling during hormonal exposure, in vitro studies using hormones in monoculture models for disease or functional pathway analysis must take this into consideration.
Effect of Coculture on Cell-Specific Cytokine Production
Several observations on cytokine secretion by endometrial cells in coculture highlight the additional valuable insights afforded by this model. For example, monocultured eEC produced TNFA secreted basolaterally through the basal lamina (represented by the Matrigel layer coating the inserts in our model). However, TNFA was undetectable in the basolateral chamber when eEC and eSF were cocultured. We speculate that this cytokine may bind to receptors on the eSF and participate in eEC/eSF paracrine signaling–a possibility supported by the documented presence of TNFA receptors in eSF (68). Alternatively, TNFA may be proteolytically degraded and/or metabolized and thereby cleared from the culture medium. Also, CCL2, a chemotactic cytokine that recruits monocytes, lymphocytes, and dendritic cells to sites of tissue injury and inflammation, was secreted in monoculture by eSF and to a lesser degree by eEC basolaterally. However, CCL2 levels in thebasolateral chamber of cocultures were significantly higher than the sum of the monocultured eSF + basolateral eEC secretions, suggesting potentiation of CCL2 production in the cocultures through eEC/eSF paracrine signaling.
It is of note that IL4, CCL3, and CCL4 were secreted primarily apically by the eEC and that apical secretion of CCL3 and CCL4 increased in coculture. Given that the epithelial monolayer in the coculture system has similarities to the luminal epithelium in vivo, it is tempting to speculate that these three cytokines may play a role in the uterine lumen microenvironment. CCL3 and CCL4 act as potent chemokines recruiting monocytes and natural killer cells and inducing proliferation of peripheral blood lymphocytes in response to infection (69, 70), as well as promoting wound repair (71). Endometrial CCL3 and CCL4 are also known to inhibit HIV infection by binding to their cognate receptor CCR5 (72) and to an HIV coreceptor on target immune cells thereby preventing HIV binding and entry (73). The fact that CCL3 and CCL4 are apically secreted into the lumen suggests that these chemokines could play an important role in adaptive immunity against HIV infections in the upper female reproductive tract. One of the functions of IL4 is to promote the differentiation of macrophages to the "alternatively activated" anti-inflammatory/repair (M2) phenotype (74– 76). Thus, IL4 may participate in endometrial luminal repair, potentially after menstruation and/or tissue insult.
Limitations of the Model
The establishment of functional polarity, cellular purity/phenotypic stability, and the demonstration of alterations to global gene expression clearly represent important advances in the development of endometrial coculture models, however, there are limitations. For example, while paracrine signaling is present in the in vitro model, in vivo paracrine signaling involves cell adjacencies with distances and volumes of extracellular space not necessarily recapitulated in the in vitro model, leading to different gradients or absolute concentrations of secreted products participating in paracrine cell-to-cell communication. Other methodologies, such as cellular attachment onto opposite sides of a membrane (allowing limited contact) or eEC growth on or embedded within an eSF-containing matrix (23), may offer some advantages, but they also carry with them additional limitations compared with the current model, as, for example, limited surface for cell growth or limited ability for analyzing separately individual cell types and their products, as we have successfully done with our model.
It is also acknowledged that in the current study we have not addressed the critical role of the ovarian steroids estradiol and progesterone in the mono- and cocultures, and this represents an important and highly relevant element missing at this time. The multiplier effect of analyzing two cell types and their interactions and the limited yields and growth potential of the eEC in culture thus far have curtailed the possibilities to include the additional experimental groups required to study hormonal effects. Ongoing efforts in our lab focus on overcoming these technical challenges to enhance the efficiency of this model and ultimately address experimentally the role of ovarian steroids in endometrial epithelial-stromal interactions.
Finally, it should be noted that additional studies are needed to demonstrate that the documented changes have occurred in direct response to specific paracrine signals from the other side of the barrier. Achieving this will be an important validation of the model.
Summary
In summary, the endometrial coculture system described herein builds on previously developed models and further identifies cell-specific global gene expression and cytokine production changes resulting from eEC-eSF interactions in coculture. Our coculture model provides a valuable complement to monoculture studies, and the use of polarized eEC offers additional possibilities for in vitro modeling of human endometrial cellular physiological and pathological processes. Future directions include study of paracrine interactions in the context of ovarian-derived steroid hormone regulation of normal endometrial function and in endometrial disorders such as endometriosis and endometrial cancers.
Supplementary Material
Acknowledgments
This project was supported by the National Institutes of Health (NIH) grant no. NIH AI083050-04 (to L.C.G.), the Eunice Kennedy Shriver National Institute of Child Health and Human Development Specialized Cooperative Centers Program in Reproduction and Infertility Research grant no. U54HD 055764 (to L.C.G.), University of California, San Francisco/NIH T32 Fellowship on Integrated Training in Reproductive Sciences 2011–2012 grant no. 5T32HD007263-28 (to J.C.C.), and the Ruth L. Kirschstein National Research Service Awards grant no. 1F32HD074423-01 (to J.C.C.).
The authors thank Zara Zelenko, Brittni Johnson, Christine Van Dyke, Nicole Persson, and Drs. Warner Greene, Barbara Shacklett, Ruth Greenblatt, and Karen Smith-McCune for helpful discussions. The authors also appreciate the advice of Amy Hamilton and Florence Ng (Fluidigm) for their technical guidance regarding Fluidigm-based gene expression assays and Greg Hamilton at the UCSF Helen Diller Family Comprehensive Cancer Center Core Services. Finally, the authors acknowledge Linda Ta, Yanxia Hao, and the University of California, San Francisco Genomics Core at the Gladstone Institute, the National Institutes of Health Specialized Cooperative Centers Program in Reproduction and Infertility Research Human Endometrial Tissue and DNA Bank, and the University of Oulu, Oulu University Hospital, Oulu, Finland.
Footnotes
J.C.C. has nothing to disclose. D.W.E. has nothing to disclose. T.T.P. has nothing to disclose. M.R.M. has nothing to disclose. F.B. has nothing to disclose. R.H.M. has nothing to disclose. J.S.T. has nothing to disclose. K.C.V. has nothing to disclose. L.C.G. has nothing to disclose. J.C.I. has nothing to disclose.
References
- 1.Aplin J, Fazleabas A, Glasser S, Giudice L. The endometrium: molecular, cellular, and clinical perspectives. 2d. London: Informa Healthcare; 2008. [Google Scholar]
- 2.Giudice LC. Elucidating endometrial function in the post-genomic era. Hum Reprod Update. 2003;9:223–35. doi: 10.1093/humupd/dmg019. [DOI] [PubMed] [Google Scholar]
- 3.Irwin J, Giudice L. The decidua. San Diego: Academic Press; 1998. [Google Scholar]
- 4.Aghajanova L, Hamilton AE, Giudice LC. Uterine receptivity to human embryonic implantation: histology, biomarkers, and transcriptomics. Semin Cell Dev Biol. 2008;19:204–11. doi: 10.1016/j.semcdb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giudice LC. Genomics' role in understanding the pathogenesis of endometriosis. Semin Reprod Med. 2003;21:119–24. doi: 10.1055/s-2003-41318. [DOI] [PubMed] [Google Scholar]
- 6.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364:1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 7.Saso S, Chatterjee J, Georgiou E, Ditri AM, Smith JR, Ghaem-Maghami S. Endometrial cancer. Br Med J. 2011;343:d3954. doi: 10.1136/bmj.d3954. [DOI] [PubMed] [Google Scholar]
- 8.Savaris RF, Groll JM, Young SL, DeMayo FJ, Jeong JW, Hamilton AE, et al. Progesterone resistance in PCOS endometrium: a microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J Clin Endocrinol Metab. 2011;96:1737–46. doi: 10.1210/jc.2010-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGowan I. Microbicides: a new frontier in HIV prevention. Biologicals. 2006;34:241–55. doi: 10.1016/j.biologicals.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Yeaman GR, White HD, Howell A, Prabhala R, Wira CR. The mucosal immune system in the human female reproductive tract: potential insights into the heterosexual transmission of HIV. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S57–62. [PubMed] [Google Scholar]
- 11.Aghajanova L, Giudice LC. Effect of bisphenol A on human endometrial stromal fibroblasts in vitro. Reprod Biomed Online. 2011;22:249–56. doi: 10.1016/j.rbmo.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalitkumar PG, Lalitkumar S, Meng CX, Stavreus-Evers A, Hambiliki F, Bentin-Ley U, et al. Mifepristone, but not levonorgestrel, inhibits human blastocyst attachment to an in vitro endometrial three-dimensional cell culture model. Hum Reprod. 2007;22:3031–7. doi: 10.1093/humrep/dem297. [DOI] [PubMed] [Google Scholar]
- 13.Wyrick PB, Knight ST, Gerbig DG, Jr, Raulston JE, Davis CH, Paul TR, et al. The microbicidal agent C31G inhibits Chlamydia trachomatis infectivity in vitro. Antimicrob Agents Chemother. 1997;41:1335–44. doi: 10.1128/aac.41.6.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–40. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fish EM, Molitoris BA. Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med. 1994;330:1580–8. doi: 10.1056/NEJM199406023302207. [DOI] [PubMed] [Google Scholar]
- 16.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–46. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 17.McLoughlin CB. Mesenchymal influences on epithelial differentiation. Symp Soc Exp Biol. 1963;17:359–88. [PubMed] [Google Scholar]
- 18.Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool. 1976;196:361–70. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- 19.Cunha GR, Chung LW, Shannon JM, Taguchi O, Fujii H. Hormone-induced morphogenesis and growth: role of mesenchymal-epithelial interactions. Recent Prog Horm Res. 1983;39:559–98. doi: 10.1016/b978-0-12-571139-5.50018-5. [DOI] [PubMed] [Google Scholar]
- 20.Neubauer BL, Chung LW, McCormick KA, Taguchi O, Thompson TC, Cunha GR. Epithelial-mesenchymal interactions in prostatic development. II. Biochemical observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J Cell Biol. 1983;96:1671–6. doi: 10.1083/jcb.96.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osteen KG, Rodgers WH, Gaire M, Hargrove JT, Gorstein F, Matrisian LM. Stromal-epithelial interaction mediates steroidal regulation of metallo-proteinase expression in human endometrium. Proc Natl Acad Sci U S A. 1994;91:10129–33. doi: 10.1073/pnas.91.21.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schatz F, Gordon RE, Laufer N, Gurpide E. Culture of human endometrial cells under polarizing conditions. Differentiation. 1990;42:184–90. doi: 10.1111/j.1432-0436.1990.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 23.Arnold JT, Kaufman DG, Seppala M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod. 2001;16:836–45. doi: 10.1093/humrep/16.5.836. [DOI] [PubMed] [Google Scholar]
- 24.Sheldon E, Vo KC, McIntire RA, Aghajanova L, Zelenko Z, Irwin JC, et al. Biobanking human endometrial tissue and blood specimens: standard operating procedure and importance to reproductive biology research and diagnostic development. Fertil Steril. 95:2120–2. 1e–12. doi: 10.1016/j.fertnstert.2011.01.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirk D, King RJ, Heyes J, Peachey L, Hirsch PJ, Taylor RW. Normal human endometrium in cell culture. I. Separation and characterization of epithelial and stromal components in vitro. In Vitro. 1978;14:651–62. doi: 10.1007/BF02616162. [DOI] [PubMed] [Google Scholar]
- 26.Irwin JC, Kirk D, King RJ, Quigley MM, Gwatkin RB. Hormonal regulation of human endometrial stromal cells in culture: an in vitro model for decidualization. Fertil Steril. 1989;52:761–8. doi: 10.1016/s0015-0282(16)61028-2. [DOI] [PubMed] [Google Scholar]
- 27.Erikson DW, Burghardt RC, Bayless KJ, Johnson GA. Secreted phospho-protein 1 (SPP1, osteopontin) binds to integrin alpha v beta 6 on porcine trophectoderm cells and integrin alpha v beta 3 on uterine luminal epithelial cells, and promotes trophectoderm cell adhesion and migration. Biol Reprod. 2009;81:814–25. doi: 10.1095/biolreprod.109.078600. [DOI] [PubMed] [Google Scholar]
- 28.Spitzer TL, Rojas A, Zelenko Z, Aghajanova L, Erikson DW, Barragan F, et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod. 2012;86:58. doi: 10.1095/biolreprod.111.095885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalman MR, Deeter A, Nimishakavi G, Duan ZH. Fold change and p-value cutoffs significantly alter microarray interpretations. BMC Bioinformatics. 2012;13(Suppl 2):S11. doi: 10.1186/1471-2105-13-S2-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan L, Hillman JD, Progulske-Fox A. Microarray analysis of quorum-sensing-regulated genes in Porphyromonas gingivalis. Infect Immun. 2005;73:4146–54. doi: 10.1128/IAI.73.7.4146-4154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charles RP, Guitard M, Leyvraz C, Breiden B, Haftek M, Haftek-Terreau Z, et al. Postnatal requirement of the epithelial sodium channel for maintenance of epidermal barrier function. J Biol Chem. 2008;283:2622–30. doi: 10.1074/jbc.M708829200. [DOI] [PubMed] [Google Scholar]
- 32.Schroder WA, Major L, Suhrbier A. The role of SerpinB2 in immunity. Crit Rev Immunol. 2011;31:15–30. doi: 10.1615/critrevimmunol.v31.i1.20. [DOI] [PubMed] [Google Scholar]
- 33.Jones RL, Hannan NJ, Kaitu'u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004;89:6155–67. doi: 10.1210/jc.2004-0507. [DOI] [PubMed] [Google Scholar]
- 34.Kelly RW, King AE, Critchley HO. Cytokine control in human endometrium. Reproduction. 2001;121:3–19. doi: 10.1530/rep.0.1210003. [DOI] [PubMed] [Google Scholar]
- 35.Salamonsen LA, Hannan NJ, Dimitriadis E. Cytokines and chemokines during human embryo implantation: roles in implantation and early placentation. Semin Reprod Med. 2007;25:437–44. doi: 10.1055/s-2007-991041. [DOI] [PubMed] [Google Scholar]
- 36.Overton CE, Fernandez-Shaw S, Hicks B, Barlow DH, Starkey P. In vitro culture of endometrial stromal and gland cells as a model for endometriosis: the effect of peritoneal fluid on proliferation. Fertil Steril. 1997;67:51–6. doi: 10.1016/s0015-0282(97)81855-9. [DOI] [PubMed] [Google Scholar]
- 37.Richards RG, Brar AK, Frank GR, Hartman SM, Jikihara H. Fibroblast cells from term human decidua closely resemble endometrial stromal cells: induction of prolactin and insulin-like growth factor binding protein-1 expression. Biol Reprod. 1995;52:609–15. doi: 10.1095/biolreprod52.3.609. [DOI] [PubMed] [Google Scholar]
- 38.Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunoloc-alization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab. 1997;82:1621–8. doi: 10.1210/jcem.82.5.3919. [DOI] [PubMed] [Google Scholar]
- 39.Sun TT, Eichner R, Nelson WG, Tseng SC, Weiss RA, Jarvinen M, et al. Keratin classes: molecular markers for different types of epithelial differentiation. J Invest Dermatol. 1983;81:109s–15s. doi: 10.1111/1523-1747.ep12540831. [DOI] [PubMed] [Google Scholar]
- 40.Blalock TD, Spurr-Michaud SJ, Tisdale AS, Heimer SR, Gilmore MS, Ramesh V, et al. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:4509–18. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- 41.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–34. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 42.Lloyd KO, Burchell J, Kudryashov V, Yin BW, Taylor-Papadimitriou J. Comparison of O-linked carbohydrate chains in MUC–1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J Biol Chem. 1996;271:33325–34. doi: 10.1074/jbc.271.52.33325. [DOI] [PubMed] [Google Scholar]
- 43.Bowen JA, Hunt JS. The role of integrins in reproduction. Proc Soc Exp Biol Med. 2000;223:331–43. doi: 10.1046/j.1525-1373.2000.22348.x. [DOI] [PubMed] [Google Scholar]
- 44.Pilewski JM, Latoche JD, Arcasoy SM, Albelda SM. Expression of integrin cell adhesion receptors during human airway epithelial repair in vivo. Am J Physiol. 1997;273:L256–63. doi: 10.1152/ajplung.1997.273.1.L256. [DOI] [PubMed] [Google Scholar]
- 45.Lu W, Ebihara N, Miyazaki K, Murakami A. Reduced expression of laminin-5 in corneal epithelial cells under high glucose condition. Cornea. 2006;25:61–7. doi: 10.1097/01.ico.0000179932.21104.3c. [DOI] [PubMed] [Google Scholar]
- 46.Olsen J, Kirkeby LT, Brorsson MM, Dabelsteen S, Troelsen JT, Bordoy R, et al. Converging signals synergistically activate the LAMC2 promoter and lead to accumulation ofthe laminin gamma 2 chain in human colon carcinoma cells. Biochem J. 2003;371:211–21. doi: 10.1042/BJ20021454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian J, Niu J, Li M, Chiao PJ, Tsao MS. In vitro modeling of human pancreatic duct epithelial cell transformation defines gene expression changes induced by K-ras oncogenic activation in pancreatic carcinogenesis. Cancer Res. 2005;65:5045–53. doi: 10.1158/0008-5472.CAN-04-3208. [DOI] [PubMed] [Google Scholar]
- 48.Salo S, Haakana H, Kontusaari S, Hujanen E, Kallunki T, Tryggvason K. Laminin-5 promotes adhesion and migration of epithelial cells: identification of a migration-related element in the gamma2 chain gene (LAMC2) with activity in transgenic mice. Matrix Biol. 1999;18:197–210. doi: 10.1016/s0945-053x(99)00012-8. [DOI] [PubMed] [Google Scholar]
- 49.Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol. 1995;9:691–705. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- 50.Giudice LC. Potential biochemical markers of uterine receptivity Hum Reprod. 1999;14(Suppl 2):3–16. doi: 10.1093/humrep/14.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- 51.Das SK, Das N, Wang J, Lim H, Schryver B, Plowman GD, et al. Expression of betacellulin and epiregulin genes in the mouse uterus temporally by the blastocyst solely at the site of its apposition is coincident with the “window” of implantation. Dev Biol. 1997;190:178–90. doi: 10.1006/dbio.1997.8694. [DOI] [PubMed] [Google Scholar]
- 52.Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14:1147–61. doi: 10.1210/mend.14.8.0498. [DOI] [PubMed] [Google Scholar]
- 53.King AE, Fleming DC, Critchley HO, Kelly RW. Differential expression of the natural antimicrobials, beta-defensins 3 and 4, in human endometrium. J Reprod Immunol. 2003;59:1–16. doi: 10.1016/s0165-0378(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 54.MasCasullo V, Fam E, Keller MJ, Herold BC. Role of mucosal immunity in preventing genital herpes infection. Viral Immunol. 2005;18:595–606. doi: 10.1089/vim.2005.18.595. [DOI] [PubMed] [Google Scholar]
- 55.Gaetje R, Holtrich U, Karn T, Cikrit E, Engels K, Rody A, et al. Characterization of WNT7A expression in human endometrium and endometriotic lesions. Fertil Steril. 2007;88:1534–40. doi: 10.1016/j.fertnstert.2007.01.128. [DOI] [PubMed] [Google Scholar]
- Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M et al. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–83. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 57.Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst. implantation Dev Genet. 1997;21:102–8. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 58.Mo BV, Vendrov AE, Palomino WA, DuPont BR, Apparao KB. Lessey BA ECC-1 cells: a well-differentiated steroid-responsive endometrial cell line with characteristics of luminal epithelium. Biol Reprod. 2006;75:387–94. doi: 10.1095/biolreprod.106.051870. [DOI] [PubMed] [Google Scholar]
- 59.Hayashi K, Burghardt RC, Bazer FW, Spencer TE. WNTs in the ovine uterus potential regulation of periimplantation ovine conceptus development. Endocrinology. 2007;148:3496–506. doi: 10.1210/en.2007-0283. [DOI] [PubMed] [Google Scholar]
- 60.Hu J, Gray CA, Spencer TE. Gene expression profiling of neonatal mouse uterine development. Biol Reprod. 2004;70:1870–6. doi: 10.1095/biolreprod.103.026336. [DOI] [PubMed] [Google Scholar]
- 61.Taylor HS, Vanden Heuvel GB, Igarashi P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod. 1997;57:1338–45. doi: 10.1095/biolreprod57.6.1338. [DOI] [PubMed] [Google Scholar]
- 62.Hewitt KJ, Shamis Y, Hayman RB, Margvelashvili M, Dong S, Carlson MW, et al. Epigenetic and phenotypic profile of fibroblasts derived from induced pluripotent stem cells. PLoS One. 2011;6:e17128. doi: 10.1371/journal.pone.0017128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeong JA, Ko KM, Bae S, Jeon CJ, Koh GY, Kim H. Genome-wide differential gene expression profiling of human bone marrow stromal cells. Stem Cells. 2007;25:994–1002. doi: 10.1634/stemcells.2006-0604. [DOI] [PubMed] [Google Scholar]
- 64.Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22:2903–11. doi: 10.1093/humrep/dem265. [DOI] [PubMed] [Google Scholar]
- 65.Geng LN, Yao Z, Snider L, Fong AP, Cech JN, Young JM, et al. DUX4 activates germline genes, retroelements, and immune mediators: implications for facioscapulohumeral dystrophy. Dev Cell. 2012;22:38–51. doi: 10.1016/j.devcel.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zapata W, Rodriguez B, Weber J, Estrada H, Quinones-Mateu ME, Zimermman PA, et al. Increased levels of human beta-defensins mRNAin sexually HIV-1 exposed but uninfected individuals. Curr HIV Res. 2008;6:531–8. doi: 10.2174/157016208786501463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L, et al. Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J Biol Chem. 2010;285:32999–3009. doi: 10.1074/jbc.M110.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishida M, Nasu K, Fukuda J, Kawano Y, Narahara H, Miyakawa I. Down-regulation of interleukin-1 receptor type 1 expression causes the dysregulated expression of CXC chemokines in endometriotic stromal cells: a possible mechanism for the altered immunological functions in endometri-osis. J Clin Endocrinol Metab. 2004;89:5094–100. doi: 10.1210/jc.2004-0354. [DOI] [PubMed] [Google Scholar]
- 69.Maurer M, von StebutE. Macrophage inflammatory protein-1. Int JBiochem Cell Biol. 2004;36:1882–6. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 70.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–83. [PubMed] [Google Scholar]
- 71.DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM. MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J Clin Invest. 1998;101:1693–8. doi: 10.1172/JCI1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, et al. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol. 2006;7:1209–16. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5:835–43. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 74.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 75.Kodelja V, Muller C, Tenorio S, Schebesch C, Orfanos CE, Goerdt S. Differences in angiogenic potential of classically vs alternatively activated macrophages. Immunobiology. 1997;197:478–93. doi: 10.1016/S0171-2985(97)80080-0. [DOI] [PubMed] [Google Scholar]
- 76.Martinez FO, Helming L, Gordon S. Alternative activation of macro-phages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.