Abstract
Background
The Extension for Community Healthcare Outcomes (ECHO) model was developed to improve access to care for complex health problems such as hepatitis C virus (HCV) infection for underserved populations. Using videoconferencing technology, ECHO trains primary care providers to treat complex diseases.
Methods
A prospective cohort study compared treatment of HCV at the University of New Mexico (UNM) HCV clinic to treatment by primary care clinicians at 21 ECHO sites in rural areas and prisons in New Mexico. A total of 407 treatment naive patients with chronic HCV were enrolled. The primary end point was a sustained viral response (SVR).
Results
The rate of SVR was 57.5% (84/146) for patients treated at UNM and 58.2% (152 /261) at ECHO sites (P=0.89); difference between SVR rates 0.7% (95% CI -9.2%, 10.7%). In genotype 1 infection the SVR rate was 45.8% (38 /83) at UNM and 49.7% (73 /147) at ECHO sites (P=0.57). Serious adverse events occurred in 13.7% of the UNM HCV clinic cohort and 6.9% of the ECHO cohort.
Conclusions
This study demonstrates that the ECHO model is an effective way to treat HCV in underserved communities. Implementation of this model would allow other states and nations to treat more patients with HCV.
Background
The Extension for Community Healthcare Outcomes (ECHO) model was developed by the University of New Mexico Health Sciences Center (UNMHSC), as a platform for both service delivery and outcomes research. 1,2 The objectives of ECHO are to: 1) improve access to best practice care for hepatitis C virus (HCV) infection for minorities and underserved populations, and 2) demonstrate the safety and efficacy of the ECHO model-based treatment for HCV in rural communities, and 3) to compare effectiveness to a university based clinic treatment. This innovative paradigm makes specialized medical resources of academic medical centers (AMC) more accessible outside of urban areas.
An estimated 170 million patients worldwide are chronically infected with HCV with 3.2 million in the United States.3,4 Many patients were infected in the 1970s and 1980s which is leading to a rising tide of cirrhosis and hepatocellular carcinoma (HCC).5 Chronic HCV infection accounts for 10,000 deaths each year in the United States and is the leading cause for liver transplantation.6,7
Fortunately, treatment for HCV is available, cost effective and cures 45% of patients with genotype 1 and 75% of patients with genotypes 2 and 3.8,9,10,11 Sustained virologic response (SVR) permanently halts the progression of liver disease, reverses fibrosis in many patients and reduces the risk of HCC. However, treatment is complex. Pegylated interferon and ribavirin are associated with serious side effects that require aggressive management by multi-disciplinary experts.9,10,11
Despite advances in treatment and remarkable improvements in cure rates, very few persons with chronic HCV are receiving treatment. The total number of prescriptions for HCV antiviral medications declined from 2002 to 2007. If this trend continues, it's estimated that treatment will prevent only 14.5% of potential liver-related deaths caused by HCV between 2002 and 2030.12 Members of racial and ethnic minorities and older patients are less likely to receive needed care.13,14,15,16
The reasons for poor quality and insufficient access to HCV treatment are complex and not completely understood. Historically, few primary care clinicians have offered HCV treatment in rural areas and prisons due to lack of training.17 In 2004, patients from rural areas faced an appointment delay of 6 months to be seen at the University of New Mexico (UNM) HCV clinic and had to travel up to 250 miles. A typical genotype 1 patient would have to make an average of 18 trips during the course of their treatment. Major barriers to care also exist for prison inmates, and according to the Department of Corrections data 40% of 6000 inmates in the New Mexico Department of Corrections (NMDOC) are infected with HCV. As of 2003, not a single patient in the correctional system had received HCV treatment.
Lack of access to specialty care services at community-based health centers (CHC) is a major problem, particularly for uninsured patients.18,19 CHCs are often the most culturally appropriate and accessible choices, particularly in rural areas, with the benefit of ongoing trust and relationships with patients. Therefore, these can be ideal places to deliver complex HCV care if they can access the needed expertise.
Methods
ECHO Model
Using state-of-the-art telehealth technology, ECHO trains and supports primary care providers from underserved areas to develop knowledge and self-efficacy so they can deliver best practice care for complex health conditions like chronic HCV. At each of these ECHO partner sites, participants include a lead clinician (a physician, nurse practitioner, or physician's assistant) as well as a nurse or medical assistant who will help manage patient care. None of the community practice sites had treated HCV patients before joining the ECHO network.
Community providers take part in weekly HCV clinics, called “Knowledge Networks” by joining a videoconference or calling into a teleconference line. (See online supplement at www.nejm.org ) The providers present their cases by sharing patient medical histories, lab results, treatment plans, and questions about best practices and individual challenges. UNMHSC specialists from the fields of hepatology, infectious diseases, psychiatry, and pharmacology provide advice and clinical mentoring during these clinics. Working together, the community providers and specialists manage patients following evidence-based protocols. These case-based discussions are supplemented with short didactic presentations by inter-disciplinary experts to improve content knowledge.
This case-based approach creates a “Learning Loop“ which builds deep knowledge, skills and self-efficacy in several ways. Longitudinal co-management of patients with specialists allows community providers to practice their expanded knowledge and skills in a manner that builds self-efficacy in handling real-world situations with their actual patients, while ensuring that they follow best practices as they learn. Learning from other community-based providers with similar challenges and patient profiles is facilitated through shared case management decision making.
There are currently 16 community sites and 5 prisons that deliver HCV treatment using the ECHO model. Since ECHO's inception in 2003 there has been over 5,000 case presentations and 800 patients treated. We conducted a prospective cohort study to assess the safety and efficacy of ECHO model-based treatment in comparison to university clinic-based HCV treatment. Our hypothesis was that when HCV treatment is delivered using the ECHO model it is as effective as that provided on-site at the AMC.
Study population
Patients were included in the ECHO and active control (UNM) cohorts if they: 1) were treatment-naïve, 2) had evidence of chronic HCV with detectable HCV RNA, 3) were between the ages of 18 and 65, and 4) initiated treatment between September 7, 2004 and February 29, 2008 if they had genotypes 1 or 4) or between September 7, 2004 and August 15, 2008 (if they had genotypes 2 or 3). Since genotype 1 and 4 require longer duration of treatment, this distinct timing allowed us to identify a definitive outcome for all subjects within the cohort prior to December 31, 2009.
Exclusion criteria included an absolute neutrophil count (ANC) < 1500 per cubic millimeter, platelet count < 75,000 per cubic millimeter, creatinine > 2.0 mg/dL, co-infection with HIV or hepatitis B, history of a solid organ transplant and decompensated liver disease.
Study design
A prospective cohort study design was used. All patients received standard HCV treatment (per the ECHO clinical protocol) with pegylated interferon at standard doses and weight- based ribavirin (for all genotypes). Early in the study period, duration of treatment was based on genotype alone (48 weeks for genotype 1 and 24 weeks for non-1 genotypes). Starting September 2006, treatment was extended for slow responders. Growth factors were used as clinically indicated. Clinical adverse events were monitored throughout the study. The AST to platelet ratio index (APRI) was used to estimate fibrosis and cirrhosis. The higher the APRI score the more likely a patient is to have significant fibrosis.
The study was approved by the UNMHSC Institutional Review Board. A waiver of informed consent was obtained as all patients received standard of care and data collected was considered part of routine care.
End Point
The primary end point was a sustained virologic response (SVR), defined as an undetectable HCV RNA level 24 weeks after the end of treatment. All patients who received at least one dose of interferon were included in the analysis. Subjects without follow-up data were considered to be treatment failures.
Assessment of Safety
Safety was assessed by laboratory tests and visits on weeks 1,2,4, and monthly thereafter. Serious adverse events were reported and investigated. An independent data and safety monitoring committee evaluated all serious adverse events.
Statistical Analysis
Continuous variables are expressed as mean ± SD. Group differences in continuous variables were analyzed by student's t-test and 95% Confidence Interval or the Mann Whitney U-test. P-values < 0.05 were considered statistically significant. Since this study was not randomized, multivariate analysis was used to verify that a difference between the two treatments did not appear after adjusting for patient factors. Stepwise logistic regression was used to identify patient predictors of SVR that might be confounders including age, sex, minority status, marital status, employment status, housing status, route of transmission, height, weight, body mass index (BMI), HCV viral load, genotype, APRI score, blood urea nitrogen (BUN), creatinine , aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, total bilirubin, total protein, albumin, white blood cell count (WBC), absolute neutrophil count (ANC), hemoglobin, red blood cell distribution width (RDW), mean corpuscular volume (MCV), platelet count.
Results
Patients
During the study period, 519 patients were started on HCV treatment. One hundred and twelve subjects were excluded, leaving 407 subjects for analysis (Figure 1). There were baseline differences between the two cohorts (Table 1). The ECHO cohort included a significantly higher proportion of men (96% of patients treated in the prison system were male) were more likely to be Hispanic, and had a higher mean weight and BMI. The UNM HCV clinic subjects were older. Fifty-six percent of both groups had genotype 1.
Figure 1.
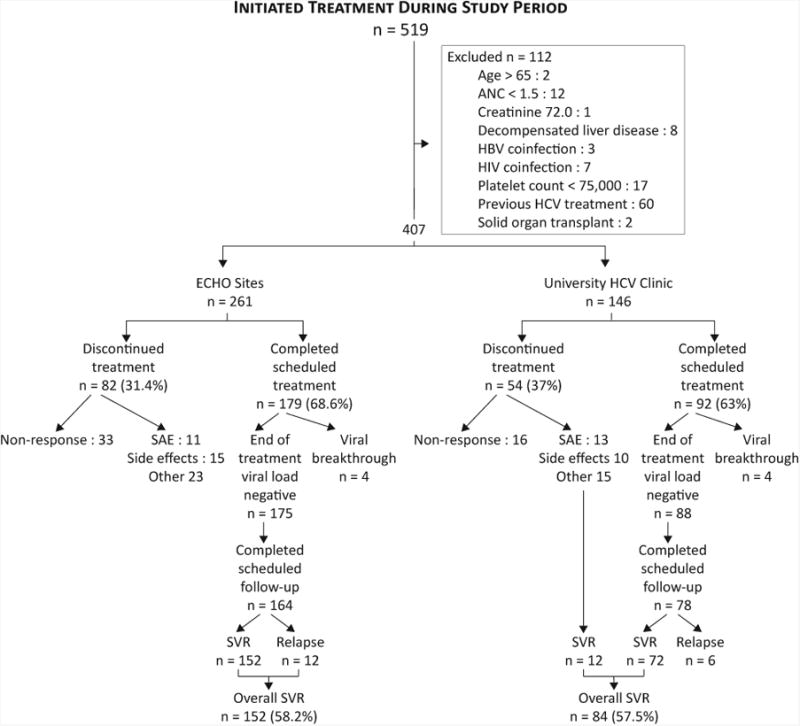
Table 1. Comparison of ECHO and University subjects.
| Characteristic | ECHO sites n = 261 | University Clinicn = 146 | P-value |
|---|---|---|---|
| Age - yr | 41.9 ± 9.8 | 45.4 ± 9.8 | 0.001 |
| Male sex – no. (%) | 190 (72.8%) | 66 (45.2%) | <0.001 |
| Race α – no. (%) * | |||
| Caucasian | 244 (95.3%) | 134 (91.8%) | 0.15 |
| American Indian | 8 (3.1%) | 3 (2.1%) | 0.53 |
| African American | 4 (1.6%) | 3 (2.1%) | 0.72 |
| Asian or Pacific Islander | - | 6 (4.1%) | 0.001 |
| Ethnicityα – no. (%)** | |||
| Hispanic | 156 (64.5%) | 60 (41.4%) | <0.001 |
| Any Minority | 166 (67.8%) | 72 (49.3%) | <0.001 |
| Weight (pounds) | 188 ± 35 | 177 ± 39 | 0.007 |
| BMI (Kg/M2) mean *** | 29.4 ± 5.3 | 28.1 ± 5.7 | 0.03 |
| ≤ 24.9 Normal | 47 (19.1%) | 45 (31.2%) | 0.006 |
| 25.0 – 29.9 Overweight | 97 (39.4%) | 54 (37.5%) | 0.71 |
| ≥ 30 Obese | 102 (41.5%) | 45 (31.2%) | 0.05 |
| ALT (Unit/Liter) | 103 ± 78 | 97 ± 73 | 0.44 |
| APRI**** score ŧ | 0.935 ± 0.910 | 0.938 ± 0.847 | 0.97 |
| Baseline Log10 viral load | 5.92 ± 0.94 | 5.84 ± 1.01 | 0.43 |
| Genotype 1 | 147 (56.3%) | 83 (56.8%) | 0.50 |
Race and ethnicity reported by provider
5 missing race
20 missing ethnicity
17 missing BMI
APRI=[(AST/upper limit of normal)/platelet count (109/L)] × 100
Virologic Response
The rate of SVR for ECHO sites was 152/261 (58.2%) and was not different from that for the UNM HCV clinic, 84/146 (57.5%).The difference was 0.7% with a 95% CI of (-9.2%, 10.7%). The overall SVR rate in genotype 1 patients was 48.3%. (See Table 2 for SVR rates by genotype and site.) Stepwise multivariable logistic-regression analyses identified several patient factors as independent predictors of SVR: genotype 1, ALT, APRI score (Table 3). When adjusted for patient characteristics, SVR did not differ by site of treatment (adjusted OR=1.04; 95% CI 0.671 to 1.60).
Table 2. SVR by Genotype and Site (number/percent) *.
| Genotype | ECHO Sites | University Clinic | Difference** and95 % CI | P-value |
|---|---|---|---|---|
| All genotypes | 152/261 (58.2%) | 84/146 (57.5%) | +0.7 (-9.2, 10.7) | 0.89 |
| Genotype 1 | 73/147 (49.7%) | 38/83 (45.8%) | +3.9 (-9.5, 17.0) | 0.57 |
| Genotype 2 or 3 | 78/112 (69.7%) | 42/59 (71.2%) | -1.5 (-15.2, 13.3) | 0.83 |
SVR rates not reported separately for 6 subjects with genotypes 4 or 6.
%SVR (ECHO - University Clinic) and 95% CI is 95% confidence interval
TABLE 3. Multivariate logistic model for SVR.
| Variable | Univariate Model | Best Multivariate Model | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI for OR | P-value | Adjusted OR | 95% CI for OR | P-value | |
| ECHO | 1.029 | 0.683 to 1.551 | 0.89 | 1.097 | 0.708 to 1.700 | 0.68 |
| ALT | 1.052* | 1.01 to 1.09 | 0.01 | |||
| WBC** | 0.861 | 0.762 to 0.972 | 0.02 | |||
| APRI*** | 0.43 | 0.299 to 0.620 | < 0.001 | |||
| Genotype 1 | 0.404 | 0.261 to 0.624 | < 0.001 | |||
per 10 unit change
per 1000 change
APRI=[(AST/upper limit of normal)/platelet count (109/L)] × 100
Note 1. Hosmer-Lemeshow P = 0.443; showing goodness-of-fit
Note 2. Best Multivariate Model obtained by stepwise logistic regression of SVR
Note 3: Other candidate variables included: age, sex, minority status, marital status, employment status, housing status, route of transmission, height, weight, BMI, HCV viral load, BUN, creatinine, AST, alkaline phosphatase, total bilirubin, total protein, albumin, hemoglobin, RDW, MCV, ANC, platelet count
Note 4. The comparison of the Univariate to Multivariate model shows that the similarity of ECHO versus UNM with respect to SVR is not significantly modified by the “best” covariates even though these covariates are important predictors of SVR.
Safety
Overall, serious adverse events (SAE) were more frequent in the UNM HCV clinic cohort (13.7%) than in the ECHO cohort (6.9%; P = 0.02). SAE's requiring termination of treatment were also more common in the UNM HCV clinic cohort (8.9%) compared to the ECHO cohort (4.2%; P = 0.05). (Table 4)
Table 4. Serious Adverse Events (SAE) and Discontinuation of Treatment According to Treatment Group.
| Adverse Event | Project ECHON=261 | UNMHSC HCV ClinicN=146 | P-value |
|---|---|---|---|
| Serious Adverse Event – no. (%) | |||
| Any | 18 (6.9) | 20 (13.7) | P=0.02 |
| Treatment Related | 13 (5.0) | 15(10.3) | |
| According to Clinical Category | |||
| Hematological Disorders | 2 (1.4) | ||
| Cardiovascular Disorders | 3 (2.1) | ||
| Gastrointestinal & Hepatobiliary Disorders | 7 (2.7) | 4 (2.7) | |
| Infections | 3 (1.1) | 5 (3.4) | |
| Psychiatric Disorders | 2 (1.4) | ||
| Other Disorders | 5 (1.9) | 4 (2.7) | |
| Discontinuation of Treatment – no. (%) | |||
| For Serious Adverse Event | 11 (4.2) | 13 (8.9) | P=0.05 |
Discussion
In this community-based study, we were able to demonstrate high rates of cure for HCV treatment delivered through the ECHO model. The SVR rates in our ECHO cohorts of 58% overall and 48% in genotype 1 patients were similar to those observed in our study's comparison group treated at the academic medical center and the rates reported in licensing trials for HCV treatment.9,10,11 Previous community-based treatment studies have failed to replicate the results of licensing trials. For example, the SVR rate was 34% for genotype 1 patients in the Weight-Based Dosing of Peginterferon alfa 2-b and Ribavirin (WIN-R) trial.20 The Veteran's Affairs experience at 121 facilities showed an SVR rate of 20% for genotype 1 patients.21
Our study cohort, particularly at the ECHO sites, was predominately Hispanic. We met our goal of increasing treatment for underserved and minority patients. A recent study by the Latino Study Group showed significantly lower rates of SVR in Hispanic genotype 1 patients compared to non-Hispanics (34% vs. 49%).22 We did not see a similar ethnic difference in SVR. Recent research suggests that disparities in treatment for minorities may be due to geography and location of the patient. 23,24,25 Treatment through ECHO overcomes this barrier by bringing expertise and clinical resources to the rural clinician that may not otherwise be widely available, positively affecting outcomes.
The study design has three principal limitations. First, there was no comparison group of patients being treated in rural settings without the ECHO model. The barriers to treatment are so formidable and concerns for safety so great that almost no cases are currently treated in rural and frontier areas of New Mexico. The second limitation was an inability to randomize providers to ECHO and active control groups because we could not ethically encourage control providers to treat HCV without training; and patients could not be randomized due to the nature of the study. Third, multivariate models can adjust for differences in patient variables that are measured but do not address those that are not or cannot be measured.
Although the inclusion of practice site (Project ECHO versus University) was not significant in the multivariate model for SVR, the confidence interval for its odds ratio was quite broad. These results are consistent with a substantial difference in outcomes in ECHO compared with University care. The study was not large enough to establish equivalence.
The results of this study demonstrate that the ECHO model is an effective way to treat HCV in rural and underserved communities. By implementing this model other states and nations can potentially treat a much higher portion of patients infected with HCV, thereby preventing an enormous burden of illness and death. There are a number of potential explanations for this success. Community providers, particularly CHCs, provide coordinated, patient-centered care in facilities proximate to their patients. Patients are likely to have greater trust with local providers who can be culturally competent for their specific communities. This may enhance patient adherence, allow more frequent in-person visits and otherwise allow greater direct contact with the clinician. As a result, providers may be better able to comply with best practice protocols, ensure close lab test assessment, offer tailored patient education, and provide greater and timelier management of side effects. In addition, the fact that hepatitis and primary care are delivered by the same clinician ensures better integration and fewer communication challenges.
As a result of the success of the model for HCV, ECHO has now expanded to 255 sites. These clinics address common and complex health issues including substance use disorders, cardiac risk reduction, chronic pain, asthma, rheumatology and multiple other diseases. The project demonstrates that technology and inter-disciplinary collaboration can be used to leverage scarce specialty care resources.
In conclusion we have shown that treating a complex disease such as HCV using the ECHO model has similar effectiveness as treatment at an AMC. ECHO represents a needed change in conventional paradigms of AMCs and specialist care being available only in urban areas. ECHO has potential for replication in the United States and abroad as community providers and academic specialists partner to respond to an increasingly diverse range of chronic health issues.
Supplementary Material
Contributor Information
Sanjeev Arora, Email: SArora@salud.unm.edu.
Karla Thornton, Email: KThornton@salud.unm.edu.
Glen Murata, Email: GMurata@salud.unm.edu.
Paulina Deming, Email: PDeming@salud.unm.edu.
Summers Kalishman, Email: SKalish@salud.unm.edu.
Denise Dion, Email: DMDion@salud.unm.edu.
Brooke Parish, Email: BParish@salud.unm.edu.
Thomas Burke, Email: tburke@salud.unm.edu.
Wesley Pak, Email: WPak@salud.unm.edu.
Jeffrey Dunkelberg, Email: jeffrey-dunkelberg@uiowa.edu.
Martin Kistin, Email: MKistin@salud.unm.edu.
John Brown, Email: JoBrown@salud.unm.edu.
Steven Jenkusky, Email: sjenkusky@phs.org.
Miriam Komaromy, Email: miriamkomaromy@gmail.com.
Clifford Qualls, Email: cqualls@salud.unm.edu.
References
- 1.Arora S, Geppert CM, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82:154–60. doi: 10.1097/ACM.0b013e31802d8f68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora S, Thornton K, Jenkusky SM, Parish B, Scaletti JV. Project ECHO: linking university specialists with rural and prison-based clinicians to improve care for people with chronic hepatitis C in New Mexico. Public Health Rep. 2007;122(2):74–7. doi: 10.1177/00333549071220S214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hepatitis C: WHO/CDS/CSR/LYO/2003? Hepatitis C: World Health Organization; 2002. [Accessed June 29, 2010]. at http://www.who.int/csr/disease/hepatitis/Hepc.pdf. [Google Scholar]
- 4. [Access June 28, 2010];Hepatitis C FAQs for health professionals: Centers for Disease and Prevention. 2010 at http://www.cdc.gov/hepatitis/HCVfaq.htm.
- 5.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality and survival trends in the United States from 1975 to 2005. J Clin Onc. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wise M, Bialek S, Finelli L, Bell BP, Sorvillo F. Changing trends in hepatitis C-related mortality in the United States, 1995-2004. Hepatology. 2008;47:1128–35. doi: 10.1002/hep.22165. [DOI] [PubMed] [Google Scholar]
- 7.Freeman RB, Jr, Steffick DE, Guidinger MK, Farmer DG, Berg CL, Merion RM. Liver and intestine transplantation in the United States, 1997-2006. Am J Transplant. 2008;8:Part 2:958–76. doi: 10.1111/j.1600-6143.2008.02174.x. [DOI] [PubMed] [Google Scholar]
- 8.Tran JA, Joseph TA, Saab S. Treating hepatitis C in the prison population is cost-saving. Hepatology. 2008;48:1387–95. doi: 10.1002/hep.22509. [DOI] [PubMed] [Google Scholar]
- 9.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. T Lancet. 2001;358:958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 10.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 11.Hadziyannis SJ, Sette H, Jr, Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–55. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 12.Volk ML, Tocco R, Saini S, Lok ASF. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50:1750–5. doi: 10.1002/hep.23220. [DOI] [PubMed] [Google Scholar]
- 13.Kanwal F, Hoang T, Spiegel BM, et al. Predictors of treatment in patients with chronic hepatitis C infection – role of patient verses nonpatient factors. Hepatology. 2007;46:1741–9. doi: 10.1002/hep.21927. [DOI] [PubMed] [Google Scholar]
- 14.Shim M, Khaykis I, Park J, Bini EJ. Susceptibility to hepatitis A in patients with chronic liver disease due to hepatitis C virus infection: missed opportunities for vaccination. Hepatology. 2005;42:688–95. doi: 10.1002/hep.20830. [DOI] [PubMed] [Google Scholar]
- 15.Rousseau CM, Ioannou GN, Todd-Stenberg JA, et al. Racial differences in the evaluation and treatment of hepatitis C among veterans: a retrospective cohort study. Am J Public Health. 2008;98:846–52. doi: 10.2105/AJPH.2007.113225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butt AA, Justice AC, Skanderson M, Rigsby MO, Good CB, Kwoh CK. Rate and predictors of treatment prescription for hepatitis C. Gut. 2007;56:385–9. doi: 10.1136/gut.2006.099150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spaulding AC, Weinbaum CM, Lau DT, et al. A framework for management of hepatitis C in prisons. Ann Intern Med. 2006;144:762–9. doi: 10.7326/0003-4819-144-10-200605160-00010. [DOI] [PubMed] [Google Scholar]
- 18.Cook NL, Hicks LS, O'Malley AJ, Keegan T, Guadagnoli E, Landon BE. Access to specialty care and medical services in community health centers: lack of access to specialty services is a more important problem for CHCs than previously thought. H Affairs. 2007;26:1459–68. doi: 10.1377/hlthaff.26.5.1459. [DOI] [PubMed] [Google Scholar]
- 19.Adashi EY, Geiger HJ, Fine MD. Health care reform and primary care: the growing importance of the community health center. N Engl J Med. 2010;362:2047–50. doi: 10.1056/NEJMp1003729. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson IM, Brown RS, Jr, Freilich B, et al. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis c patients: a randomized trial. Hepatology. 2007;46:971–81. doi: 10.1002/hep.21932. [DOI] [PubMed] [Google Scholar]
- 21.Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of U.S. veterans to treatment for the hepatitis C virus. Heptol. 2007;46:37–47. doi: 10.1002/hep.21662. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Torres M, Lennox JJ, Sheikh MY, et al. Peginterferon alfa-2a and ribavirin in latino and non-latino whites with hepatitis C. N Engl J Med. 2009;360:257–67. doi: 10.1056/NEJMoa0805062. [DOI] [PubMed] [Google Scholar]
- 23.Epstein AM, Ayanian JZ, Keogh JH, et al. Racial disparities in access to renal transplantation. N Engl J Med. 2000;343:1537–45. doi: 10.1056/NEJM200011233432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med. 2004;351:575–84. doi: 10.1056/NEJMsa040609. [DOI] [PubMed] [Google Scholar]
- 25.Epstein AM. Health care in America – still too separate, not yet equal. N Engl J Med. 2004;351:603–5. doi: 10.1056/NEJMe048181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


