Abstract
Fumonisins (FB) are mycotoxins found in maize. The purpose of this study was to 1) determine the relationship between FB1, FB2 and FB3 intake and urinary excretion in humans, 2) validate a method to isolate urinary FB on C18-SPE cartridges for international shipment, and 3) test the method using samples from Guatemala. Volunteers (n=10) consumed 206 grams/day of tortillas and biscuits prepared from masa flour and a product containing maize flour. Volunteers estimated their daily urine output and samples were analyzed for FB1, FB2 and FB3 and hydrolyzed FB1. Only FB1 was detected in urine suggesting lower absorption of FB2 and FB3. Excretion was highly variable peaking soon after consumption began and decreasing rapidly after consumption stopped. Within five days after consumption ended FB1 was not detected in urine. In a study with eight volunteers, the average total urinary FB1 was 0.5% of the intake. FB1 was detected in 61% (107/177) of the samples collected in Guatemala. The results support the use of urinary FB1 to assess ongoing exposure in population based studies. However, relating the FB1 concentration in urine to dietary intake of FB by individual subjects will be complicated due to inter-individual variability and the rapidity of clearance.
Keywords: Fumonisin, Fusarium verticillioides, Urinary fumonisin B1
INTRODUCTION
Fusarium verticillioides is a fungal pathogen of maize that produces FB, potent inhibitors of ceramide synthases [1]. FB cause animal diseases [reviewed in 2], and are implicated in human carcinogenesis [3], neural tube defects [4] and stunting in children [5]. While there are many forms of FB, those most common in maize are FB1, FB2 and FB3 [6, 7]. Where maize is a dietary staple, the probable daily intake of FB indicate that many maize consumers will exceed the provisional maximum tolerable daily intake (PMTDI) of 2 µg/kg b.w. (FB1, FB2 and FB3 alone or in combination) recommended by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) [8].
In the USA, Mexico and Central America maize-based foods are eaten in large amounts and are often produced through a process called nixtamalization [9]. The process of nixtamalization involves alkaline treatment of maize prior to cooking and reduces the total FB and increases the hydrolyzed FB (HFB); FB lacking both of the tricarballylic acid side chains [10]. HFB1 is less toxic in animals compared to the parent compound [11, 12]. Nonetheless, in areas of Central America where conditions are conducive to growth of F. verticillioides there is considerable potential for human exposure to high levels of FB because maize consumption is high [13] and even after nixtamalization there is still significant amounts of FB in the masa flour.
In humans, FB1 is excreted in both feces and urine [14, 15]. Studies in areas of the world where maize is consumed in large amounts have used urinary FB1 to evaluate human exposure. These studies show that even though the concentration of FB1 in the urine is low, the marker is useful for demonstrating the correlation between the amount of maize-based food consumed and levels of urinary FB1 [15]. It is also useful for identifying high and low exposure populations [16], and for validating intervention strategies including sorting and washing of the maize [17] and ingestion of calcium montmorillonite (NovaSil) [18].
Little is known about the kinetics of absorption and excretion of FB in humans. Nonetheless, urinary FB1 has been used to estimate intake in humans using assumptions about absorption and the kinetics of excretion based on studies in laboratory and farm animals. In animals, FB1 is rapidly but poorly absorbed from the gastrointestinal tract [19, 20, 21]. Once absorbed there is no evidence that FB are metabolized. The majority of the FB1 ingested is excreted in the feces unchanged or as the fully hydrolyzed or partially hydrolyzed form; lacking one of the two tricarballylic acid side chains. Relative to feces, a much smaller amount of FB1 is excreted in urine (< 2%) and the little that is excreted in urine is the parent compound.
Based on the few studies conducted in humans it is likely that much of what is known about excretion in animal studies is also true in humans. For example, in humans very little FB1 is detected in urine [15, 16, 17, 18] relative to what has been reported in feces [14]. In the one study where the transfer to urine could be indirectly estimated the percent FB1 was calculated to be 0.075% (0.054–0.104%) [17]. All of the studies of urinary excretion in humans have analyzed only FB1 [15, 16, 17, 18]. Thus, there is no published human information documenting urinary excretion of FB2 and FB3.
The specific objectives of this study were 1) determine the quantitative relationship between FB1, FB2 and FB3 dietary intake and urinary excretion in humans consuming maize-based foods in amounts approximating consumption where maize is a dietary staple, 2) develop and validate a method to isolate urinary FB1, FB2 and FB3 on C18-SPE cartridges for international shipment for analysis by LCMS, and 3) test the method using urine samples collected from humans in Guatemala.
MATERIALS AND METHODS
Chemicals and standards
Acetonitrile (ACN) and water were HPLC grade and formic acid was reagent grade. FB1, FB2 and FB3 (>95% pure) and also qualitative standards of HFB2 and HFB3 were provided as a gift from Ronald Plattner, National Center for Agricultural Utilization Research, USDA-ARS, Peoria, IL, USA. HFB1 was prepared from pure FB1 by hydrolysis in 2 N KOH (70°C overnight) followed by cleanup over a 35 cc C18 SepPak® column (Waters, Milford, MA USA) [10]. N-(1-deoxyfructos-1-yl) FB1 was a gift from Lauren S. Jackson USFDA, Summit-Argo, IL USA). A qualitative standard of fumonisin C1 was a gift from Jennifer Tonos (USDA, ARS, Crop Genetics and Production Research Unit, Stoneville, MS). U-[13C34]-FB1-solution (25 µg/ml) OEKANAL® was purchased from Sigma-Aldrich Laborchemikalien, Seelze, Germany.
Liquid chromatography/ mass spectrometry (LCMS) quantitation of FB
The LCMS method for quantitation of FB1, FB2 and FB3 was similar to that described previously [22, 23]. Additional details about the validation of the analysis of the uncooked and cooked maize-based foods and modifications of the LCMS method are described in the Supporting Information Tables S1, S2 and S3 and Figure S1.
Purchase of masa flour and maize-based foods in the USA and extraction and isolation of FB
Maize masa flour (produced in the USA, n=11, 2 kg each) and a maize-based hot cereal/atol (traditional maize flour-based Central American product produced in Guatemala, n=9, 0.45 kg each) were purchased from grocery stores in the USA in 2007 and 2009 and analyzed for FB. Samples were removed directly from the manufactures packages without additional mixing and extracted and processed using C18-SPE cartridges (Sep-Pak® Classic C18 cartridges, Waters Corporation, Milford, MA, USA) following the procedure described for shelled maize [24]. The recoveries from the uncooked and cooked foods were determined experimentally (Supporting Information Table S1). The recovery of U-[13C34]-FB1 internal standard closely approximated the recoveries of FB1, FB2 and FB3 (Supporting Information Table S1). Thus, the quantitation of FB2 and FB3 based on the recovery of U-[13C34]-FB1 provides a reasonably accurate estimate of the FB2 and FB3 in the tortillas and biscuits. Analysis of maize masa flour and maize-based hot cereal/atol purchased for this study revealed that all contained detectable levels of FB1, FB2 and FB3 at a ratio, on average, of 1.00:0.30:0.15 and 1.00:0.25:0.09 in the masa flour and the hot cereal/atol, respectively (Supporting Information Table S4)
Preparation of tortillas and maize-biscuits and FB analysis
Tortillas were prepared according to the recipe provided by the manufacturer of the masa flour. The only ingredients added were salt and water (0.67 g table salt and 133.6 ml tap water/100g masa flour). The tortillas were formed using a tortilla press and cooked on a griddle at medium high for approximately 2 minutes/side. The maize flour biscuits were prepared using the maize-based hot cereal/atol following a recipe from the North American Millers Association. The only ingredients added were baking powder, sugar, almond extract and water (4 g baking powder, 24 g cane sugar, 1.25 ml almond extract and 105 ml water/100g maize-based hot cereal/atol). The biscuits were baked at 163°C for 20 to 25 min. Tortillas and biscuits were cooled to ambient temperature and then stored in sealed plastic bags at 4°C.
Samples of the uncooked masa flour and maize-based hot cereal/atol and the cooked tortillas and biscuits were lyophilized and weighed. The cooked materials were ground into a fine powder using a high speed electric blender to the same consistency (based on visual appearance and tactile properties) as the uncooked materials. The average dry weight of tortillas and biscuits was 19.2 g±0.7 g (±SD, n=19) and 18.2 g±0.4 g (±SD, n=18), respectively. After mixing, a total of 150 ng of U-[13C34]-FB1 was added to samples (2.5 g to 10 g) of each material which was again mixed and then allowed to dry under vacuum overnight before extracting. The dried samples were extracted and processed using C18-SPE cartridges, and analyzed by LCMS as described in the online Supporting Information Tables S5 and S6. Samples were analyzed for U-[13C34]-FB1, FB1, FB2 and FB3 and HFB1, HFB2 and HFB3. Unless stated otherwise, the quantitation of all FB was based on the recovery of the U-[13C34]-FB1 internal standard.
Studies in humans in the USA and Guatemala
Table 1 summarizes the studies conducted in humans and briefly outlines the specific objectives of each study. A total of four studies were conducted and are described in detail below. Briefly, three studies (Study 1, Study 2 and Study 3) were conducted in the USA under controlled conditions and Study 4 was conducted in Guatemala a country where maize is a dietary staple consumed in large amounts and frequently containing FBs [13]. Study 1, 2 and 3 were intended to model for a brief period (3 or 6 days) the levels of maize consumption and FB intake that are common in Guatemala [13]. In Guatemala maize is a dietary staple and people consume maize-based foods every day at every meal over their entire life. The levels of FB in the commercial products purchased in the USA (Supporting Information Table S4) and the cooked tortillas and biscuits (Supporting Information Tables S5 and S6) prepared from those commercial products are similar to what is seen in Guatemala [13].
Table 1.
Summary of the studies conducted and the primary objectives of each study.
| Study name | Duration (days abstain/consume/abstain) |
Number of volunteers (number of urine samples expected to be collected) |
Objectives |
|---|---|---|---|
| Study 1 | 11 days (3/3/5) |
n=1 (n=54, 1 PM sample during 3 abstaining days before consuming and then every urination during 3 days consuming and the following 5 days abstaining) |
|
| Study 2 | 14 days (3/6/5) |
n=1 (n=25, 1 PM sample during 3 abstaining days before consuming and then 1 AM and 1 PM during 6 days consuming and the following 5 days abstaining) |
|
| Study 3 | 11 days (3/3/5) |
n=8 (n=88, 1 sample/day/volunteer) |
|
| Study 4 | Daily (all consuming) |
n=177 (n=177, 1 sample/volunteer) |
|
See Supplemental Table 7.
Urinary FB excretion in humans consuming known amounts of FB in controlled studies (Study 1, 2 and 3)
Ten healthy (self described) volunteers (2 females and 8 males) were recruited in Athens, Georgia USA to participate in studies to determine the kinetics of urinary excretion and to develop and validate a method for isolating urinary FB1, FB2 and FB3 using C18-SPE cartridges. The human-subjects research protocol and informed consent form were approved by the University of Georgia Human Subjects Office Institutional Review Board (Project Number 2009-10769-2). All consenting volunteers were asked to do the following: 1) answer questions about their health, age, weight and activity level, 2) receive instructions about maize-based foods to avoid during the periods requiring abstaining from maize-based foods, and 3) participate in training on the procedure for collecting daily urine samples and estimating daily urine output. The average age of volunteers was 45.8 years old (range 24 to 64 yr) and the average height and weight was 178 cm (157 to 191 cm) and 91 kg (73 to 132 kg), respectively.
Study 1 was conducted to obtain urine and preliminary kinetic data for methods development. A healthy consenting volunteer (n=1) was asked to provide urine samples after abstaining from eating maize-based foods for three days. The volunteer was asked to consume six tortillas and five biscuits per day for 3 days followed by 5 days abstaining from any maize-based foods. The volunteer was asked to collect urine samples for FB analysis at each urination during the 3 days of consuming and the following 5 days of abstaining. Urine samples for FB analysis were collected and the urine volume measured at each urination during the entire 3 days of consuming and the following 5 days of abstaining. FB intake was based on the concentration of FB in the cooked tortillas and biscuits (Supporting Information Table S5).
In Study 2, a single (n=1) consenting volunteer was asked to do the same as described in Study 1 but with six days consuming tortillas and biscuits (3 days abstaining, 6 days consuming, 5 days abstaining). This volunteer was asked to measure the volume of every urination and to collect a urine sample in the morning (AM sample) and again in the evening (PM sample) at least 2 hours after consuming the evening meal for FB analysis. Urinary creatinine was measured using the Jaffe’s Method [25] (http://www.searo.who.int/en/Section10/Section17/Section53/Section481_1755.htm). FB intake was based on the concentration of FB in the cooked tortillas and biscuits (Supporting Information Table S5).
In Study 3, eight volunteers (n=8) were asked to abstain from eating any maize-based foods for 3 days followed by 3 days consuming six tortillas and five biscuits per day followed by 5 days abstaining from maize-based foods and to collect daily urine samples and estimate and record the total volume of urine produced each day for the entire 11 days of the study. In all studies (Study 1, 2 and 3) the volunteers were instructed to space the consumption of foods so they would be consumed in the morning, afternoon and evening each day. Samples of uncooked materials were analyzed for FB before the study began and cooked materials were analyzed after the study was completed (Supporting Information Table S6).
Urinary FB in humans consuming unknown amounts of FB in Guatemala (Study 4)
In order to test the method for collecting, processing and shipping the C18-SPE extracts of the urine from Guatemala to the USA a total of 101 males and 76 female consenting volunteers between the ages of 18 and 70 years old were recruited in the Departments of Chimaltenango (male, n=54; female, n=40) and Escuintla (male, n=47; female, n=36). The human subjects research protocol and informed consent form was approved by the Comité Institucional de Etica of the Instituto de Nutrición de Centro América y Panamá (Project Number CIE REV003/2010). Consenting volunteers were asked to complete a questionnaire concerning their consumption of maize-based foods. Individuals consuming ≥ 410 grams of maize-based food/day and whose self-reported health was good or “average” were invited to participate in the study and to provide a urine sample. The height, weight, age and information about maize consumption were recorded as part of the questionnaire. All volunteers reported they ate maize-based foods every day and tortillas comprised over 96% of the food consumed and over 72% of the volunteers consumed maize-based food only a few hours before providing the urine sample. Demographic information is summarized in Supporting Information Table S7. The urine sample was stored on ice until it could be frozen at −20°C. The urine was processed and FB were isolated on C18-SPE cartridges as described above and in the online Supporting Information materials except that after the final wash of the loaded cartridge the excess solvent was removed and the cartridges were wrapped with Parafilm™ and express shipped to the USDA laboratory in Athens. Maize being sold for human consumption was collected from vendors in both Departments at the same time as the urine sampling and analyzed for FB1, FB2 and FB3. For additional details on FB stability, processing and shipping of the C18-SPE cartridges see Riley et al. [24].
Statistical Analysis
Statistical analysis was performed using SigmaStat software (Jandel Scientific, San Rafael, CA, USA). When many groups were compared, one-way analysis of variance was used, followed by post-hoc multiple comparisons unless the data failed normality or was of unequal variance in which case the Mann-Whitney rank sum test was used. When only two groups were compared, a Student t test or Mann-Whitney rank sum test was used. The Chi Square test was used to test for differences in incidence or frequency. All data are expressed as mean ±SD, and differences among or between means was considered to be significant if the probability (p) was <0.05.
RESULTS
All volunteers in Study 1, 2 and 3 conducted in the USA abstained from eating maize-based foods for 3 days before consuming the tortillas and biscuits. A spot urine sample was collected on each of the three abstaining days and FB1 was never detected in any of the samples that were analyzed.
FB1 Detection in Urine (Study 1)
In Study 1, the single volunteer consumed 206 grams (daily total) of maize-based food (6 tortillas and 5 biscuits) in three meals (morning, afternoon, evening) for 3 days (72 h). The concentration of FB1 in the tortillas and biscuits consumed was 1.37 µg/g and 1.63 µg/g, respectively (Supporting Information Table S5). Compared to the uncooked starting material, the tortillas and biscuits contained significantly less FB1 and total FB (FB1+FB2+FB3) (Supporting Information Table S5). Based on the cooked foods the single volunteer consumed 306 µg of FB1/day and the FB1 intake was 4 µg/kg b.w./day.
Of eight urine samples analyzed over the course of 26 h, all were positive for FB1 (8/8) but there was no FB2 or FB3 detected (0/8). The absence of FB2 and FB3 in the spot urine samples in the preliminary study was unexpected. It was hypothesized that the SPE cartridges were not binding or releasing FB2 and FB3. To test this hypothesis, the eight urine samples were spiked with FB3 and it was confirmed that the C18-SPE were effectively binding FB3 (Fig. 1). During the development of the method (Study 1 and 2) it was found that recoveries of FB2 and FB3 were slightly, but not significantly, greater (8% and 23%, respectively) than FB1 (Supporting Information Table S8) a result consistent with the estimated LODs for FB1, FB2 and FB3 (Supporting Information Table S3) which showed that the LOD were similar for all three FBs.
Figure 1.

(A) Chromatogram showing the separation, retention times, and area (number in parentheses) under the peaks for a standard solution containing 1 pg/µl of FB1, FB2 and FB3. In all the other panels (B, C, D, E, and F) the chromatograms are from urine samples spiked with FB3 before C18-SPE clean up. In the FB1 positive samples (C, D and E) the areas and the ng FB1/ml urine are shown in parentheses. (B) A urine sample after 3 days of not consuming any maize products (PreD3). (C) The urine sample at 2.75 h after consuming two tortillas and two biscuits containing FB1, FB2 and FB3 (D1-2.75 h). (D) The urine sample at 26 h after consuming four additional tortillas and three additional biscuits (D1-26 h) and (E) the urine after 72 h and (F) 96 h abstaining from consuming any additional maize-based foods (PostD3 and PostD4). None of the peaks eluting near the retention time of FB2 had the base peak or mass spectra of FB2 (data not shown). The urinary FB1 levels for each 24 h interval calculated based on the recovery of FB3 are shown in Supporting Information Table S9.
Time-Course of FB1 Urinary Excretion (Study 1 and Study 2)
In Study 1, the first meal of two tortillas and two biscuits was at 07:30 and the first urination was at 10:15 at which time FB1 was detected in the urine sample. The FB3 added to the urine samples before extraction (20 ng total/sample) was used as an internal standard for quantification of FB1. Based on the FB3 recovery, the concentration of FB1 at 10:15 on day 1 of dosing (Fig. 1C, D1-2.75 h) was 0.217 ng FB1/ml and at 26 h after the first meal the level of FB1 in the urine was 0.78 ng FB1/ml (Fig. 1D, D1-26 h). Three days after the last meal (Fig. 1E, PostD3) only a trace (0.1 ng/ml) of FB1 was detectable, and 4 days post dosing (Fig.1F, PostD4) no FB1 was detected. Based on the total FB1 intake (918 µg over 3 days) it was estimated that the cumulative percent FB1 excreted in the urine was 0.78% (Supporting Information Table S9).
Study 2 also used a single volunteer (n=1) and was conducted similar to Study 1 described above except that the tortillas and biscuits were consumed for 6 days. Urine samples were collected in the morning and in the evening and the urine volume for every urination was measured throughout the period of consumption and for 5 days post-consumption. As in Study 1, no FB2 or FB3 was detected in any of the FB1 positive urine samples (0/18). The total FB1 excreted in the urine per day (Fig. 2A) and the cumulative FB1 intake and cumulative FB1 excreted in the urine (Fig. 2B) was calculated based on the average of the AM and PM FB1 concentration in the urine (Fig. 3A) and the total daily urine volume. In total, 0.42 % of FB1 consumed was excreted in the urine (Fig. 2B, inset). The first evening urine sample collected after consuming the first three meals (D1-11.5 h) contained 0.82 ng FB1/ml (Fig. 3A), a value similar to that seen in the 26 h sample in the preliminary study (Fig. 1D). The highest urinary FB1 concentration was 1.40 ng/ml in the urine sample collected in the evening on consuming day 5 (Fig. 3A). After four days of not consuming tortillas or biscuits, FB1 was not detected in the urine (Fig. 3A).
Figure 2.
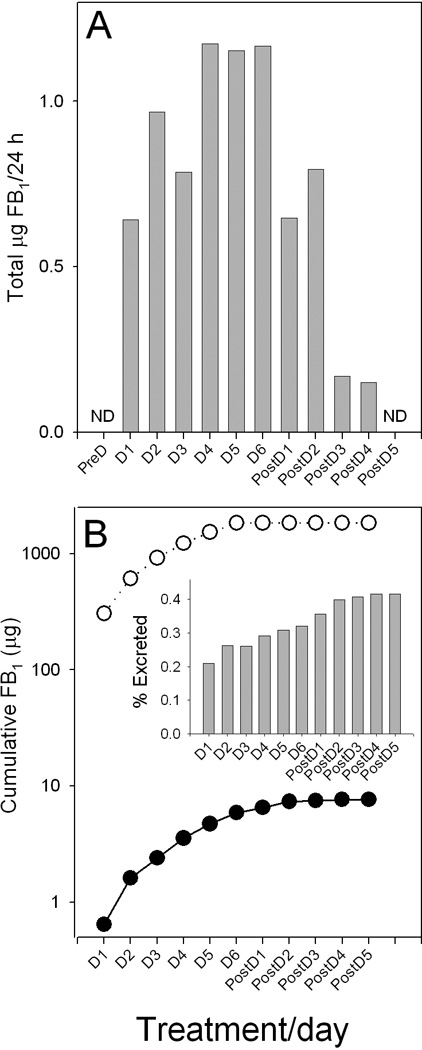
(A) Total daily urinary FB1 excreted (µg/24 h) by a volunteer abstaining from consuming maize-based foods for 3 days (PreD), 6 days consuming six tortillas and five biscuits/days (D1–D6) followed by 5 days abstaining (PostD1-PostD5). (B) Cumulative FB1 intake (open circles) and cumulative excretion in the urine (solid circles) over the entire period of consuming (D1-D6) and post-consuming (PostD1-5). Inset in (B) is the percentage of the cumulative FB1 intake excreted in the urine at each time period. ND= not detected.
Figure 3.
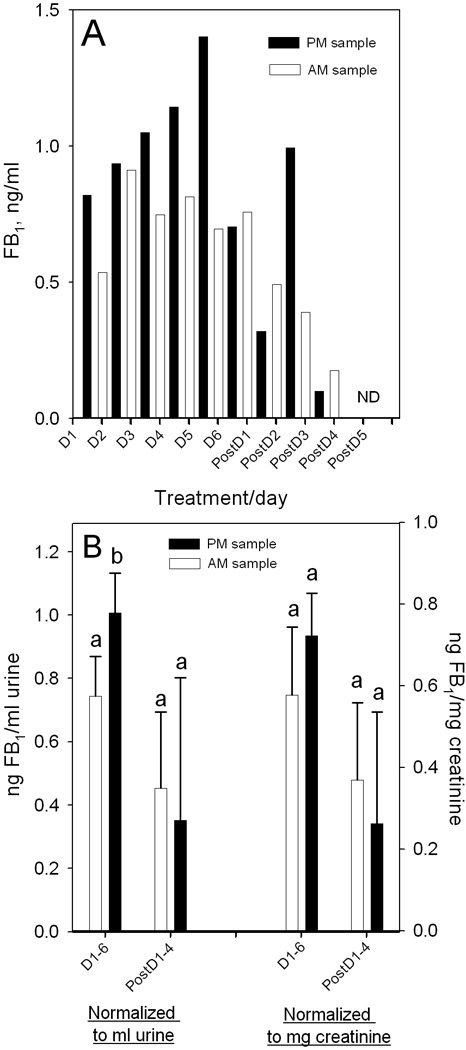
(A) FB1 in evening (PM, shaded bars) and morning (AM, light bars) urine samples from a single volunteer during 6 days of consuming 206 g of tortillas and biscuits (D1–D6) followed by 5 days abstaining from maize-foods (PostD1-5). (B) Comparison of the FB1 concentration expressed as ng/ml urine or ng/mg creatinine in AM and PM urine samples while consuming (D1-6, n=6) or abstaining (PostD1-5, n=4) from maize-foods. Pairs of bars with differing superscripts are significantly different (p<0.05).
Levels of FB1 in Morning and Evening Spot Urine Samples (Study 2)
The urinary FB1 in the AM and PM samples were similar although during the period of tortilla and biscuit consumption the PM levels were generally higher and post consumption the PM levels were generally lower (Fig. 3B). This was apparent regardless of whether FB concentration was normalized to urine volume or creatinine. The difference between PM versus AM samples was statistically significant (p<0.05, n=6) in samples collected on days 1 to 6 when normalized to urine volume (Fig. 3B).
Inter-individual Variability in FB1 Excretion (Study 3)
A third study, Study 3, using eight volunteers (n=8) was conducted that was similar to Study 1 and Study 2 described above except that urine samples were collected once per day in the evening. The tortillas and biscuits were consumed for 3 days. The mean concentration of FB1 in tortillas and biscuits was 1.52 µg/g and 0.89 µg/g, respectively (Supporting Information Table S6) and the calculated intake was 256 µg of FB1/day/volunteer resulting in an average intake of 2.94±0.55 µg/kg b.w./day (±SD, n=8).
The total FB1 excreted in the urine (Fig. 4A) and the percent of the total FB1 intake excreted in the urine (Fig. 4A, inset) was calculated based on the single FB1 concentration in the urine in the PM sample and the total estimated daily urine volume. In total, 0.50% ± 0.24% (±SD, n=8) of the FB1 consumed was excreted in the urine. All subjects had detectable levels of urinary FB1 on consuming days 1, 2 and 3 and because of the high variability there was no significant difference in the mean total urinary FB1 on any of the consuming days or post dosing days 1 and 2. By post dosing day 3 there was no FB1 detected in the urine. Normalization of total urinary FB to the weight of each volunteer did not reduce the variability (Fig. 4B). The average maximum concentration of FB1 in the urine samples (1.36 ng/ml ± 0.76 ng/ml; ±SD, n=8) was similar to the highest concentration (1.40 ng/ml) found in Study 2, the 6-day study described above (Fig. 3A). FB2 and FB3 were not detected in any of the FB1 positive spot urine samples analyzed (0/26).
Figure 4.
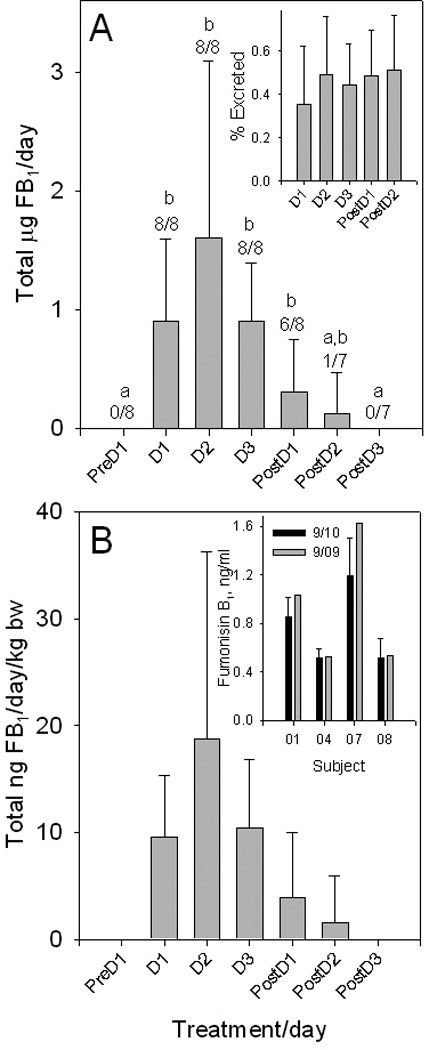
(A) Mean urinary FB1 excreted per day (µg/24 h) by eight volunteers abstaining from consuming maize-foods for 3 days (PreD1), 3 days consuming 6 tortillas and 5 biscuits/days (D1–D3) followed by 3 days abstaining (PostD1-PostD3) (The PostD4 and PostD5 urine samples were not analyzed and samples for PostD2 and 3 were not available for one volunteer). The number of volunteers that were FB1 positive over the total urine samples analyzed is shown above each error bar. Mean values with differing superscripts are significantly different. Inset in (A) is the mean cumulative FB1 excreted in the urine as a percentage of the cumulative FB1 intake. (B) FB1 concentration (ng/ml) normalized to body weight (The results of the statistical analysis was the same as in (A)). Inset in (B) is the results of the re-analysis ((September 2010=9/10, n=3 replicates) of urine samples from four volunteers stored frozen and analyzed 1 year after the initial analysis (September 2009=9/09, n=1).
Stability of FB1 in Stored Urine Samples
Collecting of spot urine samples was used in sampling urine in Guatemala and those samples were stored frozen for up to a month after collection. Samples of the urine collected and analyzed from four volunteers in September of 2009 were reanalyzed in September of 2010 (Fig. 4B, inset). The results show that FB1 concentration in the urine stored frozen at −20°C for 1 year was very similar to that in the original urine sample. Thus, FB1 was stable in the frozen urine for up to 1 year.
FB1 Levels in Urine of Maize Consumers in Guatemala (Study 4)
A method for assessing FB contamination of maize in Guatemala by express shipping maize extracts processed in Guatemala for analysis at the USDA laboratory in Athens has been used successfully for several years [24, 13]. In order to test if the modified process would work equally well with human urine, volunteers were recruited from two Departments in Guatemala (Study 4). Healthy volunteers (male and female) from these two communities were recruited in March of 2011 and asked to complete a questionnaire and if their consumption of maize-based foods, based on recall, was greater than or equal to 410 g/day then they were asked to provide a urine sample after giving informed consent. Samples of maize (1 to 2 kg) sold for human consumption in the local market was collected at the same time in March 2011. Analysis of the maize showed low levels of FB in the maize (Fig. 5A). The average level of FB1 in the maize purchased in Chimaltenango and Escuintla were 0.39±0.34 mg/kg (n=5) and 0.33±0.42 mg/kg (n=10), respectively, differences that were not significantly different. Similarly the mean consumption of maize-based foods was not significantly different between Chimaltenango and Escuintla or among males and females in both communities (Fig. 5B). The only statistically significant difference in urinary FB1 was that males in Escuintla had higher mean levels and a higher frequency of positive samples compared to females in either Chimaltenango or Escuintla or males in Chimaltenango (Fig. 5C). A total of 177 spot urine samples were analyzed from Chimaltenango and Escuintla and 107 were positive for FB1, but none contained detectable levels of FB2 or FB3 or hydrolyzed FB1 (Fig. 6 A–D).
Figure 5.
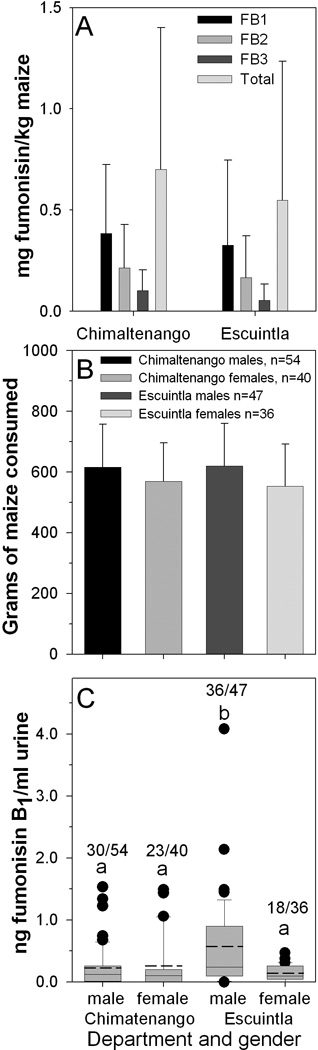
(A) The average (±SD) concentration (µg/g) of FB1, FB2, FB3 and total FB (FB1+FB2+FB3) in maize samples collected in Chimaltenango (n=5) and Escuintla (n=10) at the same time that urine samples were collected. (B) The average consumption (±SD) of maize-based foods by males and females. The estimated grams of dry maize equivalents are given in Supporting Information Table S7. (C) The box plot of the urinary FB1 concentration (ng/ml) in the spot urine samples from males and females in Chimaltenango and Escuintla. The numbers above each bar in (C) are the total FB1 positive urine samples over the total urine samples analyzed. The dashed line associated with each box is the mean value and the solid line is the median. The top of the error bars is the 95% confidence limit and the solid circles are outliers.
Figure 6.

Example of a urine sample from Guatemala showing clear evidence of FB1 but no evidence of FB2, FB3 or hydrolyzed FB1. (A) A composite chromatogram showing the separation and retention time of a 1 pg/µl standard of HFB1, U-[13C34] FB1 and FB1, FB2 and FB3. (B) U-[13C34] FB1 in a 9 ml urine sample spiked with 40 ng of U-[13C34] FB1 before extraction and clean-up on the C18-SPE cartridge. (C) The FB1 in the urine sample calculated to contain 2.4 ng FB1/ml based on recovery of U-[13C34] FB1. (D) TIC scanning at 406.4 m/z and 706.3 m/z showing there are no definitive peaks at the correct retention times (see (A)) or mass spectra (data not shown) for HFB1 (m/z 406.4) or FB2 or FB3 (m/z 706.3).
DISCUSSION
The kinetic studies in humans (Study 1, 2 and 3) showed that FB1 was rapidly absorbed following oral exposure. Once absorbed, FB1 levels remained elevated in the urine but the levels rapidly decreased after consumption of foods containing FB1 ceased. For example, after consuming FB contaminated diets for 3 or 6 days FB1 was not detectable in urine 5 days after intake ceased. Thus, urinary elimination is rapid with an apparent half-life (visually approximated) of < 48 h when consuming for 3 consecutive days and > 48 h but < 72 h when consuming for 6 consecutive days.
The total urinary excretion of FB1 was less than 1% of the cumulative dose ranging from 0.12% to 0.90% (n=10) which is greater than that reported in van der Westhuizen et al. [17] but at the lower range of values (0.25 to 2%) reported in animals (reviewed in [19, 20, 21]. FB1 was excreted in urine much more efficiently than FB2 or FB3. FB2 and FB3 were never convincingly detected in human urine samples. The LOD for FB2 in the urine was less than 0.04 ng/ml urine. In the maize and maize-based foods analyzed in this study the FB2 concentration was between 20% and 50% of the FB1 concentration (Figure 5A and Supporting Information Tables S4, S5 and S6). Thus, based on the LOD for FB1 and FB2, if FB1 and FB2 were absorbed and excreted with equal efficiency then FB2 should have been detected in urine samples that contained greater than 0.2 ng/ml of FB1. Assuming that fumonisins B1, B2 and B3 are absorbed equally well from the gastrointestinal tract, then FB1 would appear to be transferred to the urine much more efficiently than FB2 or FB3. Alternatively, relative to FB1, FB2 and FB3 may not be as well absorbed as suggested by the absence of toxicity or disruption of sphingolipid metabolism in liver of mice fed pure FB2 or FB3 [11]. Reduced absorption is also consistent with the observation that in rat serum, liver and kidney FB2 and FB3 were detected in lower levels than expected based on the levels of FB1, FB2 and FB3 in the diets [26]. Hydrolyzed FB1 was also never clearly detected in urine samples from Guatemala even when the urinary FB1 concentration was as high as 4 ng/ml (Fig. 5C). This result is also consistent with the reduced toxicity of hydrolyzed FB1 in animal models [11, 12] and may also reflect less efficient absorption from the gastrointestinal tract. This is of importance from the point of view of risk managers since the current established group PMTDI is for FB1, FB2 and FB3, alone or in combination [8].
The percentage of the predicted FB1 intake excreted in the urine of the Guatemalan consumers was similar to that seen in the controlled studies in the USA. Assuming that the mean levels of FB1 in the Guatemalan maize (Fig. 5A) are representative of the maize being used to produce nixtamalized maize-based foods and that traditional Mayan nixtamalization reduced the total fumonisins by 50% [10] and that FB1 constitutes 50% of the remaining fumonisins [10] then the calculated estimated mean FB1 intake for people in Chimaltenango and Escuintla would be 0.45 µg/kg b.w./day ((0.356 µg FB1/g maize × 337 g maize/day)/67 kg). The mean urinary FB1 for the Guatemalan consumers was 0.30 ng/ml. Assuming daily urine output of 1000 ml then on average the total amount of FB1 excreted in the urine was 0.3 µg/day. The calculated average FB1 intake/day/person is 30.0 µg (0.45 µg/kg b.w./day×67 kg) which is 1.0% of the calculated FB1 intake. For comparison the percentage the FB1 intake excreted in the urine by the volunteers in the controlled studies in the USA was 0.50%±0.24% (n=8).
The significantly higher level of urinary FB1 in males in Escuintla is difficult to explain since there was no difference between males and females in Chimaltenango and the amount of maize-based food consumed was the same. One possibility is that the source of the maize being consumed by males in Escuintla is not the same as for the females. One difference between Chimaltenango and Escuintla is that Escuintla is a much more rural population (Supporting Information Table S7) with many large sugar cane plantations. It is possible that some of the males recruited in Escuintla were migrant workers and were eating maize-based foods containing higher levels of FB1 the women living in Escuintla.
In conclusion, the levels of FB1 in the urine, even under controlled conditions, are highly variable suggesting that the processes regulating excretion of FB are complex. Nonetheless, the results provide support for the usefulness of urinary FB1 as a marker to assess ongoing exposure in population-based studies. In individuals consuming maize every day (as in Guatemala), the difference between AM and PM spot urine samples should be minimal if the level of exposure is relatively constant. However, relating the urinary FB1 concentration in spot urine samples to dietary intake of FB in individuals will be complicated due to inter-individual variability and the rapidity of clearance. Regardless, the monitoring of urinary FB1 levels in combination with more mechanism-based biomarkers has the potential for testing the likelihood of FB as a contributing factor in human diseases in areas of the world where maize is consumed in large amounts and FB exposure is likely.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Elizabeth Savage for technical assistance in preparing cooked materials and Adela Ruiz, Rosa Chovix and Waldemar González for the field work and sample collection in Guatemala, Marta María Méndez, Cecilia de Mayorga, Luis Rodríguez and Flor Días for the urine and maize extraction in Guatemala.
This work was supported by USDA-ARS NP108 in house project 6612-42000-012-00D and Award Number RC4HD067971-01 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health.
Abbreviations
- FB
fumonisins
- FB1
fumonisin B1
- FB2
fumonisin B2
- FB3
fumonisin B3
- HFB1
hydrolyzed fumonisin B1
- PMTDI
provisional maximum tolerable daily intake.
Footnotes
The authors have no conflict of interest to report. IRB-approved protocols were followed.
References
- 1.Wang E, Norred WP, Bacon CW, Riley RT, Merrill AH., Jr Inhibition of sphingolipid biosynthesis by fumonisins: Implications for diseases associated with Fusarium moniliforme. J. Biol. Chem. 1991;266:14486–14490. [PubMed] [Google Scholar]
- 2.Voss KA, Smith GW, Haschek WM. Fumonisins: toxicokinetics, mechanism of action and toxicity. Anim. Feed Sci. Technol. 2007;137:299–325. [Google Scholar]
- 3.International Agency for Research on Cancer (IARC) monographs on the Evaluation of Carcinogenic Risk to Humans: Fumonisin B1. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. 2002;82:275–366. [PMC free article] [PubMed] [Google Scholar]
- 4.Marasas WFO, Riley RT, Hendricks KA, Stevens VL, et al. Fumonisins disrupt sphingolipid metabolism, folate transport and development of neural crest cells in embryo culture and in vivo: A risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize? . J. Nutr. 2004;134:711–716. doi: 10.1093/jn/134.4.711. [DOI] [PubMed] [Google Scholar]
- 5.Kimanya ME, De Meulenaer B, Roberfroid D, Lachat C, Kolsteren P. Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Mol. Nutr. Food Res. 2010;54:1659–1667. doi: 10.1002/mnfr.200900483. [DOI] [PubMed] [Google Scholar]
- 6.Rheeder JP, Marasas WFO, Vismer HF. Production of fumonisin analogs by Fusarium species. Appl. Environ. Microbiol. 2002;68:2101–2105. doi: 10.1128/AEM.68.5.2101-2105.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartok T, Szecsi A, Szekeres A, Mesterhazy A, Bartok M. Detection of new fumonisin mycotoxins and fumonisin-like compounds by reversed-phase high-performance liquid chromatography/electrospray ionization ion trap mass spectrometry. Rapid Commun. Mass. Spectrom. 2006;20:2447–2462. doi: 10.1002/rcm.2607. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Evaluation of certain mycotoxins in food (Seventy fourth report of the Joint FAO/WHO Expert Committee on Food Additives). WHO Technical Report, 2011, Series No. 966. 2011:70–94.
- 9.Bressani R. Chemistry, technology, and nutritive value of maize tortillas. Food Rev. Int. 1990;6:225–264. [Google Scholar]
- 10.Palencia E, Torres O, Hagler W, Meredith FI, et al. Total fumonisins are reduced in tortillas using the traditional nixtamalization method of Mayan communities. J. Nutr. 2003;133:3200–3203. doi: 10.1093/jn/133.10.3200. [DOI] [PubMed] [Google Scholar]
- 11.Howard PC, Couch LH, Patton RE, Eppley RM, et al. Comparison of the toxicity of several fumonisin derivatives in a 28-day feeding study with female B6C3F1 mice. Toxicol. Appl. Pharmacol. 2002;185:153–165. doi: 10.1006/taap.2002.9529. [DOI] [PubMed] [Google Scholar]
- 12.Voss KA, Riley RT, Snook ME, Gelineau-van Waes J. Comparing the reproductive and sphingolipid metabolic effects of fumonisin B1 and its alkaline hydrolysis product in LM/Bc mice: hydrolyzed fumonisin B1 did not cause neural tube defects. Toxicol. Sci. 2009;112:459–467. doi: 10.1093/toxsci/kfp215. [DOI] [PubMed] [Google Scholar]
- 13.Torres O, Palencia E, Lopez de Pratdesaba L, Grajeda R, et al. Estimated fumonisin exposure in Guatemala is greatest in consumers of lowland maize. J. Nutr. 2007;137:2723–2729. doi: 10.1093/jn/137.12.2723. [DOI] [PubMed] [Google Scholar]
- 14.Chelule PK, Gqaleni N, Dutton MF, Chuturgoon AA. Exposure of rural and urban populations in KwaZulu Natal, South Africa, to fumonisin B1 in maize. Environ. Health Perspect. 2001;109:253–256. doi: 10.1289/ehp.01109253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gong YY, Torres-Sanchez L, Lopez-Carrillo L, Peng JH, et al. Association between tortilla consumption and human urinary fumonisin B1 levels in a Mexican population. Cancer Epidemiol. Biomarkers Prev. 2008;17:688–694. doi: 10.1158/1055-9965.EPI-07-2534. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Cai Q, Tang L, Wang S, et al. Evaluation of fumonisin biomarkers in a cross-sectional study with two high-risk populations in China. Food Addit. Contam. 2010;Part A 27:1161–1169. doi: 10.1080/19440049.2010.481638. [DOI] [PubMed] [Google Scholar]
- 17.Van der Westhuizen L, Shepard G, Burger HM, Rheeder JP, et al. Fumonisin B1 as a urinary biomarker of exposure in a maize intervention study among South African subsistence farmers. Cancer Epidemiol. Biomarkers Prev. 2011;20:483–489. doi: 10.1158/1055-9965.EPI-10-1002. [DOI] [PubMed] [Google Scholar]
- 18.Robinson AN, Johnson M, Strey A, Taylor JF, et al. Calcium montmorillonite clay reduces urinary biomarkers of fumonisin B1 exposure in rats and humans. Food Addit. Contam. 2012 doi: 10.1080/19440049.2011.651628. Part A DOI: 10.1080/19440049.2011.651628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolger M, Coker RD, Dinovi M, Gaylor D, et al. Fumonisins. In: Safety evaluation of certain mycotoxins in food. Food and Agriculture Organization of the United Nations, paper 74. World Health Organization Food Additives Series. 2001;47:103–279. [Google Scholar]
- 20.Bulder AS, Arcella D, Bolger M, Carrington C, et al. Fumonisins (addendum), In: Safety evaluation of certain food additives and contaminants. Geneva, World Health Organization. World Health Organization Food Additives Series. 2012;65:325–794. [Google Scholar]
- 21.Shephard GS, Van der Westhuizen L, Sewram V. Biomarkers of exposure to fumonisin mycotoxins: a review. Food Addit. Contam. Part A. 2007;24:1196–1201. doi: 10.1080/02652030701513818. [DOI] [PubMed] [Google Scholar]
- 22.Zitomer NC, Glenn AE, Bacon CW, Riley RT. A single extraction method for the analysis by liquid chromatography/tandem mass spectrometry of fumonisins and biomarkers of disrupted sphingolipid metabolism in tissues of maize seedlings. Anal. Bioanal. Chem. 2008;391:2257–2263. doi: 10.1007/s00216-008-2166-x. [DOI] [PubMed] [Google Scholar]
- 23.Zitomer NC, Riley RT. Extraction and analyses of fumonisins and compounds indicative of fumonisin exposure in plant and mammalian tissues and cultured cells. Methods Mol. Biol. 2011;739:171–185. doi: 10.1007/978-1-61779-102-4_15. [DOI] [PubMed] [Google Scholar]
- 24.Riley RT, Torres O, Palencia E. International shipping of fumonisins from maize extracts on C18 sorbent. Food Addit. Cont. 2006;23:826–832. doi: 10.1080/02652030600699650. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Regional Office for South-East Asia, Guidelines on SOP’s for Clinical Chemistry. 2006 Apr 27;
- 26.Riley RT, Voss KA. Differential sensitivity of rat kidney and liver to fumonisin toxicity: organ-specific differences in toxin accumulation and sphingoid base metabolism. Toxicol. Sci. 2006;92:335–345. doi: 10.1093/toxsci/kfj198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


