Abstract
All sexually reproducing animals exhibit gender differences in behavior. Such sexual dimorphisms in behavior are most obvious in stereotyped displays that enhance reproductive success such as mating, aggression, and parental care. Sexually dimorphic behaviors are a consequence of a sexually differentiated nervous system, and recent studies in fruit flies and mice reveal novel insights into the neural mechanisms that control these behaviors. In the main, these include a diverse array of novel sex differences in the nervous system, surprisingly modular control of various stereotyped dimorphic behavioral routines, and unanticipated sensory and central modulation of mating. We start with a brief overview to provide the appropriate conceptual framework so that the advances made by the newer studies discussed subsequently can be fully appreciated. We restrict our review to reporting progress in understanding the basis of mating and aggression in fruit flies and mice.
Introduction
Sexually dimorphic behaviors such as mating and aggression are instinctual in the sense that they can be displayed without prior training or social experience. Thus, the development and function of the underlying neural circuits is hard-wired into the genome. Such hard-wiring affords the use of molecular and genetic approaches to identify and experimentally manipulate the neuronal ensembles that influence sexually dimorphic social behaviors. The stereotyped nature of these mating and aggression routines also enables quantitative and, if desired, even automated analysis of these complex social behaviors. Such analyses can routinely detect even subtle changes in the behavior of experimentally manipulated animals.
Despite developmental programming of the underlying neural circuits, the display of mating and aggression is tightly regulated by sensory cues and internal physiological regulators. In both fruit flies (Drosophila melanogaster) and mice (Mus musculus), displays of mating and aggression are controlled by specific chemosensory cues emanating from conspecifics (Figure 1). These chemosensory cues, or pheromones, are species-specific odorants that signal social and reproductive status to conspecifics [1,2]. Recent studies have revealed the chemical nature of many of these cues [3–10,11•,12,13], the identities of the putative cognate receptors [5,11•,14••,15••,16•,17•,18••], and in some cases, the neural pathways that relay pheromone-evoked sensory neuron activation to the brain [19–22,23••,24].
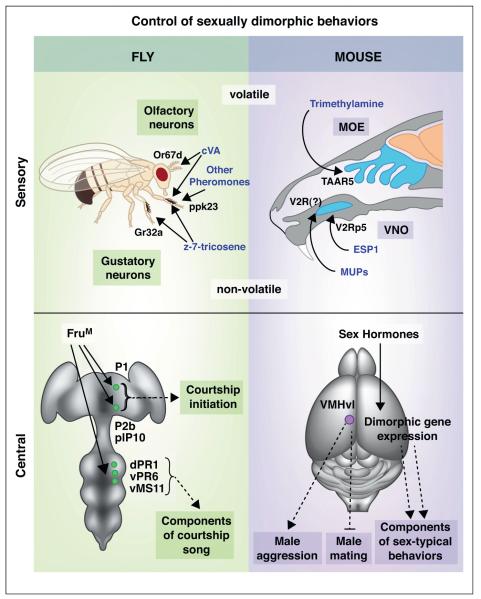
Figure 1.
Sensory and central control of sexually dimorphic behaviors. Both flies and mice respond to pheromones that trigger or inhibit particular behaviors. In fruit flies, pheromones are sensed by olfactory neurons as well as by gustatory neurons. In mice, volatile pheromones are sensed by the main olfactory epithelium (MOE) and non-volatile pheromones by the vomeronasal organ (VNO). In fruit flies, various components of male courtship are controlled by specific populations of FruM neurons. Activation of P1, P2b, or pIP10 neurons elicits the initiation of courtship behavior, while FruM ventral nerve cord neurons (dPR1, vPR6, vMS11) control specific elements of courtship song. In mice, gonadal sex hormones control the sexually dimorphic expression of many genes which regulate specific components of sex-typical behaviors. The VMHvl appears to contain neurons that inhibit male mating and activate male aggression. All neuronal clusters shown are bilateral but are depicted on one side for clarity.
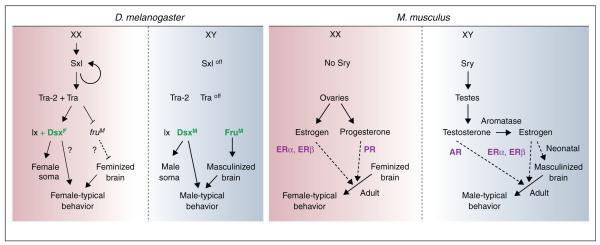
In contrast to the commonalities in the sensory control of sexually dimorphic displays between fruit flies and mice, there are clear differences between these animals in the internal physiological regulators that influence these behaviors (Figure 2) [25]. In fruit flies, the sex determination pathway ensures cell autonomous sexual differentiation of the nervous system via the expression of sex-specific splice-forms of two putative transcription factors, fruitless (fru) and doublesex (dsx). The expression of the sex-specific forms of Fru and Dsx marks the neuronal populations thought to underlie courtship and aggression [26–29]. The male-specific forms of Fru (FruM) are necessary and sufficient for male-pattern courtship behavior and aggression [30–32]. Strikingly, the activity of FruM neurons only appears to mediate male-typical behaviors because silencing synaptic transmission in these cells results in significant deficits in dimorphic behaviors but not in behaviors common to both sexes such as walking and flying. Fruit flies mutant for male-specific Dsx (DsxM) appear to have a subtle but reproducible deficit in courtship whereas the behavioral role of the female-specific Dsx (DsxF) is unclear at present [33].
Figure 2.
Sex determination and differentiation of the nervous system in fruit flies and mice. Sex determination in fruit flies is cell-autonomous, and sexually dimorphic behaviors are controlled by neurons expressing sex-specific splice forms of Dsx and Fru. In mice, the sex-determination pathway directs the differentiation of the bipotential gonad into ovaries or testes. Gonadal sex steroids and their cognate receptors control the sexual differentiation of nervous system and the expression of sex-typical behaviors in the adult.
The primary role of the sex determination pathway in mice is to drive sexual differentiation of the bipotential gonads at mid-gestation. Sex steroid hormones secreted by the testes and ovaries subsequently control sexual differentiation of the nervous system. These hormones bind to cognate nuclear hormone receptors that regulate gene expression, either by directly binding to DNA or as part of transcriptional complexes. Testosterone produced by the testes binds to the androgen receptor (AR), whereas the ovarian hormones estrogen and progesterone bind to estrogen receptors (ERα, ERβ) and progesterone receptor (PR), respectively [34]. These sex hormones and their receptors are essential for the sexually dimorphic patterns of mating and aggression. The role of other receptors of these sex hormones in these behaviors is unclear. In the male brain, testosterone also functions as a prohormone for estrogen, which is synthesized by discrete neuronal populations that express the converting enzyme aromatase [35–37]. Genetic studies from many groups show that the developmental or organizational effects of testosterone on masculinizing the brain are largely mediated by estrogen and the later, activational effects of testosterone on the expression of male behaviors require both testosterone and estrogen [37–40]. Females are protected from the early masculinizing effects of estrogen by multiple mechanisms, including the fact that the ovaries are quiescent perinatally, a critical window for masculinization in which a male-specific surge of circulating testosterone is converted into estrogen in the brain [41,42]. In contrast to the sex-specific functions of Fru and Dsx in controlling behavior exclusively in one or the other sex, estrogen and its nuclear hormone receptors control gender-typical behaviors in both sexes in mice. Moreover, sexual differentiation of the mouse brain is controlled non-autonomously by gonadal hormones, and sensory input as well as internal states such as stress can act on the brain to control secretion of these sex hormones. Common to both fruit flies and mice however is the notion that Dsx, FruM, AR, ERα, ERβ, and PR regulate the transcription of target genes to drive sexual differentiation of the brain and behavior. Although such transcriptional targets remain to be identified in the fly brain, recent studies in mice have identified many potential target genes that are also required for male or female-typical behaviors [43••].
Sensory control of sexually dimorphic behaviors
As mentioned earlier, sexually dimorphic behaviors such as mating and aggression are primarily triggered by pheromonal cues, and our discussion correspondingly focuses on advances in our understanding of the chemosensory control of these behaviors.
Insights from fruit flies
A great deal is already known about the chemosensory control of courtship and aggression in fruit flies. Previous work had identified specific olfactory or gustatory (contact-based) chemoreceptors that were required for observing the high, wild-type levels of male courtship toward females and female receptivity to male mating attempts [44–46]. One surprising finding from such studies was that some chemoreceptors function in assays of social behavior specifically to inhibit intermale courtship, suggesting the presence of evolutionary pressures to select against courtship of reproductively futile targets [47,48]. Such chemoreceptors are presumably essential in restricting courtship to female targets on food sources where flies of both sexes aggregate. These chemoreceptors also detect bitter chemorepellents such as quinine, indicating that aversive social cues may engage neural circuits generally used for avoidance. Recent studies provide evidence for a more complex role for individual pheromones or their cognate receptors in controlling courtship and aggression in flies.
The fly cuticle is coated with chemically diverse hydrocarbons that not only prevent dessication but also serve as contact-dependent pheromones [9]. These hydrocarbons and their cognate chemoreceptors regulate courtship and aggression in a complex manner. Several groups have identified chemosensory neurons expressing a Degenerin/Epithelial sodium channel class of ion channels, Pickpocket 23 (Ppk23), that is required to trigger male courtship of females and to inhibit intermale courtship [15••,16•,17•]. Strikingly, Ppk23 is required in non-overlapping male sensory neurons for their activation by specific female and male-enriched hydrocarbons and other pheromones [15••], suggesting that Ppk23 may transduce pheromone recognition by distinct chemoreceptors. It is also possible that Ppk23 functions as a co-receptor with other chemoreceptors to recognize these chemically diverse pheromones. Although there is a significant FruM-dependent sex difference in the central projections of ppk23 sensory neurons [15••,49], the role of these neurons in female flies is unclear.
The gustatory chemoreceptor Gr32a was previously shown to suppress intermale courtship, although the pheromone recognized by Gr32a was not identified [48]. A recent study showed that Gr32a was also essential for the normal, high levels of intermale aggression [11•]. In addition, this work identified a cuticular hydrocarbon, z-7-tricosene, that elicited Gr32a-dependent intermale aggression and suppressed intermale courtship. However, electrical activation of Gr32a sensory neurons only weakly elicits intermale aggression, indicating that additional chemosensory pathways that remain to be identified also promote this behavior.
The pheromone cis-vaccenyl acetate (cVA), recognized by the chemoreceptors Or67d and Or65a as well as ppk23-expressing neurons in the foreleg, is secreted into the male genital tract and serves to increase the receptivity of the female to male mating attempts and perhaps to decrease intermale courtship [13,15••,46,50]. During copulation, cVA is transferred to the female soma where it appears to inhibit subsequent male mating attempts. In addition, cVA also promotes intermale aggression acutely, albeit in a Gr32a-dependent manner, and it has been proposed that a high density of males increases aggressivity and may promote dispersal of males to other locations [10,11•,51]. These disparate and sexually dimorphic behavioral responses to cVA pose the question as to how neural pathways emanating from cVA-responsive sensory neurons transform cVA recognition into sexually dimorphic behavioral output. An elegant series of studies tracing the connectivity of Or67d sensory neurons reveals sex differences in at least the first three synaptic relays, starting with the synapse between Or67d neurons and their projection targets [23••,24]. Although it remains to be demonstrated, these anatomical sexual dimorphisms provide a potential substrate for the control of courtship in both males and females by cVA. In addition, these neurons, including Or67d sensory neurons, also express FruM, thereby suggesting a possible molecular basis for the sexual differentiation of this neural circuit.
Insights from mice
In contrast to the detailed insights offered by recent studies on the sensory control of mating and aggression in fruit flies, our understanding of how specific pheromones and their cognate receptors control these behaviors in mice is rather limited. However, work in mice has elucidated several general principles whereby pheromones control mating and aggression. Mice utilize two sensory epithelia in the nose, the main olfactory epithelium (MOE) and the vomeronasal organ (VNO), to detect volatile and contact-based pheromones, respectively [1,2]. These sensory epithelia express G-protein coupled chemosensory receptors encoded by large, distinct families of genes that bear little resemblance to the fruit fly chemoreceptors. Studies using mice with genetically disabled odor-evoked signaling by the MOE or VNO show that the normal display of aggression requires the coordinate function of both epithelia [52–54]. What has emerged from such studies however is the surprising finding that male sexual behavior requires a functional MOE [52,55]. Moreover, VNO neurons function to suppress atypical displays of male sexual behavior in both sexes. Wild-type male mice only rarely attempt to mate with males whereas the vast majority will mate with females. By contrast, wild-type female mice rarely, if ever, display male-typical sexual behavior toward males although a significant minority of females do show such male-pattern mating toward females. Strikingly, male or female mice null for Trpc2, a cation channel required for odor-evoked activity in the VNO, exhibit male-pattern sexual behaviors toward conspecifics of either sex with a high probability [53,54,56]. These studies with Trpc2 mutant females additionally show that the neural circuit for male mating is present in females, a conclusion supported by classical endocrinological studies that demonstrated activation of male sexual behavior at high frequency in adult females treated with testosterone [57]. It appears therefore that male sexual displays are non-redundantly inhibited in adult wild-type females by a functional VNO and by the absence of high, male-typical levels of testosterone.
Various small molecules and proteins found in mouse urine or other exocrine secretions have been assigned pheromonal activity [2], but the chemoreceptors that recognize these putative pheromones remain unknown for the most part. A recent study showed that trimethylamine is enriched in male urine and serves as a chemoattractant at physiological concentrations [5]. Trimethylamine is recognized by TAAR5, a member of the trace amine-associated receptor family that is expressed in a subset of MOE neurons, and mice null for TAAR5 are not attracted to trimethylamine in behavioral assays. The ethological significance of trimethylamine and TAAR5-mediated chemoattraction is unknown. It is possible that such volatile pheromones may bring conspecifics in proximity to each other, thereby facilitating social interactions regulated by other pheromones. Darcin is a major urinary protein (MUP) highly enriched in male urine that also mediates chemoattraction, albeit at shorter ranges [3,58]. The presence of darcin promotes association with volatile cues emanating from conspecifics and also elicits a long-lasting conditioned place preference in both sexes. Thus, a single pheromone can elicit an innate behavior acutely and influence behavior in the longer term by the formation of specific memories. Although the molecular identity of the chemoreceptors that recognize darcin is unknown, previous work shows that MUPs are recognized by VNO neurons expressing the V2R class of chemoreceptors [6]. A related set of MUPs, also enriched in male urine, is necessary and sufficient to elicit intermale aggression [6]. Whether female mice recognize and respond to these intermale aggression-eliciting MUPs is presently unclear. As is the case with darcin, the identity of the V2R receptors that recognize intermale aggression-eliciting MUPs is unknown.
In addition to urine, lacrimal secretions also provide pheromonal cues. In a series of spectacular studies [7,18••], Touhara’s group has identified a family of genes encoding small exocrine gland-secreted peptides (ESPs), some of which are found exclusively in male or female tears in a sex hormone-dependent manner. One such peptide, ESP1, is secreted by males and recognized by VNO neurons expressing the V2Rp5 chemoreceptor. ESP1 promotes female sexual receptivity, and females null for V2Rp5 show a significant reduction in sexual behavior with wild-type males. It is unclear whether male mice recognize and respond to ESP1 in a behaviorally meaningful manner.
Although these studies in mice provided insight into the regulation of social behaviors by pheromones, it was unclear why there are so many chemoreceptor-encoding genes. For example, there are ~250 distinct chemoreceptors expressed in sensory neurons in the VNO. A recent tour-de-force study by Dulac’s group addressed this issue by examining in an unbiased manner ethologically relevant sources of chemosensory cues that activate specific chemoreceptor-expressing VNO neurons [14••]. The authors used in situ hybridization to detect transcription of Egr1, an immediate early gene, in VNO neurons expressing one of 88 distinct VNO receptors of the V1R and V2R families. Remarkably, 17 of these receptors were activated solely by conspecific cues whereas 60 receptors were activated exclusively by heterospecific cues emanating from predator or non-predator species. In other words, a large majority of VNO neurons may be dedicated to recognizing individuals of other species. Of the receptors that recognized conspecific cues, the authors identified chemoreceptors that were activated exclusively by male or female-enriched pheromones as well as chemoreceptors that recognized sulfated steroids in urine that may signal endocrine state [4], but not membership of a species, of the animal. These studies open up fundamentally new avenues of investigating the role of specific chemoreceptors in the regulation of not only mating and aggression but also interactions with sympatric species, including predators. It will be interesting to determine whether a comparable fraction of fly chemoreceptors also functions to recognize heterospecific cues.
Central control of sexually dimorphic behaviors
Recent advances in fruit flies and mice provide complementary insights into the neural mechanisms that guide the display of mating and aggression. The facility of fruit fly molecular genetics has led to the identification of specific neuronal populations that control various elements of these behaviors. By contrast, reverse genetic approaches in mice have identified specific genes expressed in a sexually dimorphic manner that are essential for wild-type patterns of mating and aggression.
Insights from fruit flies
Functional studies in fruit flies have been aided by a pair of extremely informative publications that have mapped sexual dimorphisms in neurons marked by fruM [59,60]. These studies document virtually all sex differences in neuronal number and arborization for these neurons and provide a framework to study the neural circuits that control courtship and aggression. In addition, they reveal previously unappreciated, extensive sexual dimorphisms in the number and projections of fruM neurons. This map of sexual dimorphism in the fly brain has already proved invaluable in identifying the functional role of specific fruM neurons in male courtship. Several powerful studies from the groups of Baker, Dickson, and Yamamoto have focused on the role of a small, bilateral cluster of ~50 FruM-positive neurons, identified as P1 neurons, that are activated by female pheromonal cues and found exclusively in the male brain (Figure 1) [61•,62••,63•]. Remarkably, genetically targeted, electrical activation of P1 neurons elicits robust initiation of the male courtship ritual, including courtship song, even in the absence of a conspecific. The arbors of P1 neurons reside exclusively in the brain whereas the neurons that drive unilateral wing extension and vibration required for courtship song reside in the ventral nerve cord (analogous to the mouse spinal cord). Electrical activation of any one of two groups of neurons (P2b and pIP10) that reside in the brain but project to the ventral nerve cord also elicits courtship song in the absence of conspecific targets, suggesting a possible neural pathway that connects central P1 neurons with downstream neurons in the ventral nerve cord [61•,62••]. Strikingly, electrical activation of particular FruM-positive ventral nerve cord neurons (dPR1, vPR6, and vMS11) elicits specific elements of courtship song such as wing extension [62••]. Taken together, these studies nicely deconstruct the neuronal populations controlling courtship rituals and show that individual groups of FruM neurons may control specific components of a more complex courtship behavior such as singing. In future studies, it will be important to determine whether these neurons are synaptically linked or whether additional neurons that remain to be identified are interposed between these FruM neurons.
These studies in fruit flies have begun to elucidate the neural pathways that mediate mating and aggression. What is unclear is how FruM and Dsx in flies influence neurons to drive sexually dimorphic behaviors. Recent work has begun to reveal some of the transcriptional cofactors and regulatory proteins, including chromatin modifying enzymes, that coordinate sexual differentiation of the fruit fly brain in conjunction with FruM [64]. Nevertheless, the transcriptional targets of FruM (and Dsx) in the brain that regulate courtship or aggression remain unknown.
Insights from mice
The precision offered by fruit fly genetics in identifying specific FruM neuronal pools that regulate one or the other component of courtship or aggression has yet to be matched by studies in mice. Nevertheless recent work by Anderson and colleagues has identified a hypothalamic collection of neurons located within or in proximity to the ventrolateral compartment of the ventromedial nucleus (VMHvl) that is critical for the display of attack behavior in male mice (Figure 1) [65••]. In vivo recordings in freely moving male mice reveal a set of neurons whose activity increases during intermale aggression. Interspersed among these cells are neurons that are active during the early phases of mating with females as well as neurons whose activity is modulated during both mating and aggression. Optogenetic stimulation of these neurons elicits attacks toward conspecifics of either sex, and strikingly, even to an inflated glove. By contrast, pharmacogenetic silencing of these neurons inhibits intermale aggression but not sexual behaviors toward females. By rigorously localizing an aggression-eliciting center, these studies in mice very significantly extend the findings from experiments spanning almost a century that have ascribed a central role to the VMH or adjacent hypothalamic regions in modulating aggression in diverse species [66–69]. As is the case in the fruit fly, it will be interesting to delineate the connectivity of these neurons and to understand how their activity can override the onset of mating and trigger aggression. It will also be important to determine the molecular identity of these neurons, especially since the VMHvl also influences feeding and female sexual behavior [70,71]. These diverse behaviors may be regulated by molecularly separable VMHvl neurons or they may all be regulated by a single pool of neurons whose activity and function is context-dependent.
Studies in mice have revealed many sex differences in gene expression, including that of neuropeptides and biosynthetic enzymes for neurotransmitters [72], that are regulated by sex hormones. A recent study employed genome-wide expression profiling in conjunction with in situ hybridization and identified numerous, novel sex differences in gene expression patterns in the adult hypothalamus and amygdala (Figure 1) [43••]. These expression patterns highlight centers in the hypothalamus (such as the VMHvl) and amygdala previously implicated in sexually dimorphic behaviors [73]. Many individual genes are upregulated in different brain regions in the two sexes, indicating that the regulation of these dimorphic gene expression patterns is complex. Consistent with this notion, the authors showed that many, but not all, sex differences in gene expression were regulated by adult testosterone in males. By contrast, the dimorphic expression patterns in the female brain were, with rare exceptions, independent of adult ovarian hormones, suggesting developmental pre-patterning of a dimorphic transcriptional program in females. The authors explored the relevance of these genes in sexually dimorphic behaviors using mice bearing constitutively null alleles of individual genes. Strikingly, each of four mutant strains (Brs3, Cckar, Irs4, Sytl4) examined revealed specific deficits in one or more components of mating, aggression, or maternal care such that other dimorphic behaviors were unaffected. Thus, unlike castrates or animals mutant for sex hormone receptors that exhibit global deficits in dimorphic behaviors, mice mutant for genes downstream of sex hormone signaling exhibit restricted phenotypes in these behavioral displays. In other words, components or modules of various sexually dimorphic behaviors appear to be controlled by genetically separable pathways. In future studies, it will be important to determine whether these genes function in the adult brain to control these behaviors and to understand how the neuronal populations expressing these genes influence dimorphic behavioral displays. It is likely that more sensitive gene expression profiling approaches will reveal many additional sex differences in gene expression in the mammalian brain that control other components of sexually dimorphic behaviors.
Work over the past decade from many groups indicates that sex chromosomes may influence sexually dimorphic behaviors independent of sex hormones [74,75], but the underlying genetic loci on the sex chromosomes remain to be identified. Recent work shows that many genes are imprinted, including in a sexually dimorphic manner, in the mouse brain, although the extent of such imprinting is being actively pursued [76–78]. Given that some imprinted genes have previously been shown to regulate sexually dimorphic displays [79,80], this new set of imprinted genes could provide additional molecular control of these behaviors. Yet another axis of control of sexually dimorphic behaviors is the regulation of male mate choice by the neuromodulator serotonin. Male mice genetically engineered to lack serotonin in the brain attempt to mate with both males and females, with no discernible preference for females [81••]. This loss of preference does not reflect a developmental requirement for serotonin because it can be rescued by provision of serotonin precursors to adult mutant males. Whether serotonin signaling plays a similar role in females is an open question. How these sex chromosome, imprinting, and neuromodulation-based mechanisms intersect with sensory and hormonal signaling to regulate the genes and circuits underlying mating and aggression is an outstanding issue that is likely to be addressed in the near future.
Closing remarks
As the above discussion makes clear, these are exciting times that have yielded rapid advances in our understanding of how the brain encodes sexually dimorphic behaviors. We anticipate that studies in the near future will delineate synaptically connected neural pathways that mediate mating and aggressive behaviors. Although we have primarily discussed the sensory and central control of instinctual displays of mating and fighting, it is clear that the environmental and social context as well as past experience have a profound influence on these behaviors [82–88]. Thus we foresee that it will soon be feasible to address mechanistically at the level of specific neurons and genes how nature and nurture interact to influence genetically hard-wired behaviors.
Acknowledgements
We thank members of the Shah laboratory for comments on the manuscript and Liz Unger for artwork. This work was supported by the Ellison Medical Foundation (NMS), Brain and Behavior Research Foundation (DSM, NMS), NSF graduate fellowship (EJF), China Scholarship Council (PF), and the NIH (R01NS049488, DP1MH099900) (NMS).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- 2.Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nat Rev Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- 3.Roberts SA, Simpson DM, Armstrong SD, Davidson AJ, Robertson DH, McLean L, Beynon RJ, Hurst JL. Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 2010;8:75. doi: 10.1186/1741-7007-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nodari F, Hsu F-F, Fu X, Holekamp TF, Kao L-F, Turk J, Holy TE. Sulfated steroids as natural ligands of mouse pheromone-sensing neurons. J Neurosci. 2008;28:6407–6418. doi: 10.1523/JNEUROSCI.1425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q, Korzan WJ, Ferrero DM, Chang RB, Roy DS, Buchi M, Lemon JK, Kaur AW, Stowers L, Fendt M, et al. Synchronous evolution of an odor biosynthesis pathway and behavioral response. Curr Biol. 2012 doi: 10.1016/j.cub.2012.10.047. http://dx.doi.org/10.1016/j.cub.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, Cravatt BF, Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- 7.Kimoto H, Haga S, Sato K, Touhara K. Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature. 2005;437:898–901. doi: 10.1038/nature04033. [DOI] [PubMed] [Google Scholar]
- 8.Kimoto H, Sato K, Nodari F, Haga S, Holy TE, Touhara K. Sex- and strain-specific expression and vomeronasal activity of mouse ESP family peptides. Curr Biol. 2007;17:1879–1884. doi: 10.1016/j.cub.2007.09.042. [DOI] [PubMed] [Google Scholar]
- 9.Ferveur J-F. Cuticular hydrocarbons: their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005;35:279–295. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–231. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Wang L, Han X, Mehren J, Hiroi M, Billeter J-C, Miyamoto T, Amrein H, Levine JD, Anderson DJ. Hierarchical chemosensory regulation of male-male social interactions in Drosophila. Nat Neurosci. 2011;14:757–762. doi: 10.1038/nn.2800.. The authors find that z-7-tricosene is sufficient to elicit intermale aggression toward male flies otherwise depleted of cuticular hydrocarbons. The elicitation of aggression and suppression of intermale courtship by this pheromone requires Gr32a. Furthermore, they demonstrate that suppression of courtship by cVA requires Gr32a function, whereas the induction of courtship toward males in the absence of cuticular hydrocarbons requires the Or47b olfactory receptor.
- 12.Leinders-Zufall T, Brennan P, Widmayer PSPC, Maul-Pavicic A, Jäger M, Li X-H, Breer H, Zufall F, Boehm T. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 2004;306:1033–1037. doi: 10.1126/science.1102818. [DOI] [PubMed] [Google Scholar]
- 13.Van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Isogai Y, Si S, Pont-Lezica L, Tan T, Kapoor V, Murthy VN, Dulac C. Molecular organization of vomeronasal chemoreception. Nature. 2011;478:241–245. doi: 10.1038/nature10437. The authors use expression of Egr1 to identify VNO sensory neurons that respond to a wide array of ethologically relevant sources of chemosensory cues. They determine that distinct VR receptors are uniquely tuned to specific cues from male or female conspecifics and other species, including predators.
- 15••.Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male–male repulsion and male–female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. The authors identify the expression of Ppk23 in FruM gustatory neurons in the male foreleg. They demonstrate that Ppk23 is required in males for courtship of females and suppression of courtship towards conspecific males. Distinct classes of ppk23-expressing neurons in the foreleg respond to male and female-enriched cues respectively.
- 16•.Toda H, Zhao X, Dickson BJ. The Drosophila female aphrodisiac pheromone activates ppk23(+) sensory neurons to elicit male courtship behavior. Cell Rep. 2012;1:599–607. doi: 10.1016/j.celrep.2012.05.007. The authors demonstrate that ppk23-expressing neurons on the male foreleg respond to a female-enriched cuticular hydrocarbon. Electrical activation of ppk23-expressing neurons induces intermale courtship, demonstrating a central role of these gustatory neurons in courtship.
- 17•.Lu B, LaMora A, Sun Y, Welsh MJ, Ben-Shahar Y. ppk23-Dependent chemosensory functions contribute to courtship behavior in Drosophila melanogaster. PLoS Genet. 2012;8:e1002587. doi: 10.1371/journal.pgen.1002587. The authors show Ppk23 expression in specific FruM chemosensory neurons in the foreleg. Inhibiting electrical activity in ppk23 neurons or loss of Ppk23 function decreases male courtship of females.
- 18••.Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, Sakano H, Yoshihara Y, Kikusui T, Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466:118–122. doi: 10.1038/nature09142. The authors show that VNO neurons expressing the V2Rp5 chemoreceptor respond to the male-specific pheromone ESP1 and that female mice homozygous null for V2Rp5 show a diminution in sexual receptivity with males.
- 19.Ben-Shaul Y, Katz LC, Mooney R, Dulac C. In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proc Natl Acad Sci U S A. 2010;107:5172–5177. doi: 10.1073/pnas.0915147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meeks JP, Arnson HA, Holy TE. Representation and transformation of sensory information in the mouse accessory olfactory system. Nat Neurosci. 2010;13:723–730. doi: 10.1038/nn.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin DY, Zhang S-Z, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- 22.Luo M, Fee MS, Katz LC. Encoding pheromonal signals in the accessory olfactory bulb of behaving mice. Science. 2003;299:1196–1201. doi: 10.1126/science.1082133. [DOI] [PubMed] [Google Scholar]
- 23••.Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. These authors anatomically and physiologically map the neural circuitry downstream of Or67d sensory neurons into the fruit fly brain and ventral nerve cord. They identify significant sex differences at each of the first three synaptic relays.
- 24.Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- 25.Marín I, Baker BS. The evolutionary dynamics of sex determination. Science. 1998;281:1990–1994. doi: 10.1126/science.281.5385.1990. [DOI] [PubMed] [Google Scholar]
- 26.Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat Neurosci. 2010;13:458–466. doi: 10.1038/nn.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinett CC, Vaughan AG, Knapp J-M, Baker BS. Sex and the single cell. II. There is a time and place for sex. PLoS Biol. 2010;8:e1000365. doi: 10.1371/journal.pbio.1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- 29.Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, Yamamoto D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc Natl Acad Sci U S A. 1996;93:9687–9692. doi: 10.1073/pnas.93.18.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 31.Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121:785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Villella A, Hall JC. Courtship anomalies caused by doublesex mutations in Drosophila melanogaster. Genetics. 1996;143:331–344. doi: 10.1093/genetics/143.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ryan KJ, Naftolin F, Reddy V, Flores F, Petro Z. Estrogen formation in the brain. Am J Obstet Gynecol. 1972;114:454–460. doi: 10.1016/0002-9378(72)90204-9. [DOI] [PubMed] [Google Scholar]
- 36.Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- 37.Wu MV, Manoli DS, Fraser EJ, Coats JK, Tollkuhn J, Honda S-I, Harada N, Shah NM. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu MV, Shah NM. Control of masculinization of the brain and behavior. Curr Opin Neurobiol. 2011;21:116–123. doi: 10.1016/j.conb.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S, Honda S-I, Harada N, Shah NM. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66:260–272. doi: 10.1016/j.neuron.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raskin K, De Gendt K, Duittoz A, Liere P, Verhoeven G, Tronche F, Mhaouty-Kodja S. Conditional inactivation of androgen receptor gene in the nervous system: effects on male behavioral and neuroendocrine responses. J Neurosci. 2009;29:4461–4470. doi: 10.1523/JNEUROSCI.0296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 43••.Xu X, Coats JK, Yang CF, Wang A, Ahmed OM, Alvarado M, Izumi T, Shah NM. Modular genetic control of sexually dimorphic behaviors. Cell. 2012;148:596–607. doi: 10.1016/j.cell.2011.12.018. The authors identify many novel sex differences in gene expression patterns in the adult mouse hypothalamus and medial amygdala. These sexual dimorphisms are largely dependent on testosterone in males, whereas most such dimorphisms appear to be independent of adult ovarian hormones in females. Mice mutant for each of 4 of these genes exhibit specific behavioral deficits in mating (male or female), aggression (male or female), and maternal care of young. Together, these findings suggest that genes downstream of sex hormone signaling control sexually dimorphic behaviors in a modular manner.
- 44.Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe K, Toba G, Koganezawa M, Yamamoto D. Gr39a, a highly diversified gustatory receptor in Drosophila, has a role in sexual behavior. Behav Genet. 2011;41:746–753. doi: 10.1007/s10519-011-9461-6. [DOI] [PubMed] [Google Scholar]
- 46.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 47.Moon SJ, Lee Y, Jiao Y, Montell C. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship. Curr Biol. 2009;19:1623–1627. doi: 10.1016/j.cub.2009.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyamoto T, Amrein H. Suppression of male courtship by a Drosophila pheromone receptor. Nat Neurosci. 2008;11:874–876. doi: 10.1038/nn.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mellert DJ, Knapp J-M, Manoli DS, Meissner GW, Baker BS. Midline crossing by gustatory receptor neuron axons is regulated by fruitless, doublesex and the Roundabout receptors. Development. 2010;137:323–332. doi: 10.1242/dev.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ejima A, Smith BPC, Lucas C, Van der Goes van Naters W, Miller CJ, Carlson JR, Levine JD, Griffith LC. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu W, Liang X, Gong J, Yang Z, Zhang Y-H, Zhang J-X, Rao Y. Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in Drosophila. Nat Neurosci. 2011;14:896–902. doi: 10.1038/nn.2836. [DOI] [PubMed] [Google Scholar]
- 52.Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- 53.Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male–male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 55.Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 56.Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- 57.Edwards DA, Burge KG. Early androgen treatment and male and female sexual behavior in mice. Horm Behav. 1971;2:49–58. [Google Scholar]
- 58.Roberts SA, Davidson AJ, McLean L, Beynon RJ, Hurst JL. Pheromonal induction of spatial learning in mice. Science. 2012;338:1462–1465. doi: 10.1126/science.1225638. [DOI] [PubMed] [Google Scholar]
- 59.Cachero S, Ostrovsky AD, Yu JY, Dickson BJ. Jefferis GSXE: Sexual dimorphism in the fly brain. Curr Biol. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu JY, Kanai MI, Demir E, Jefferis GSXE, Dickson BJ. Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr Biol. 2010;20:1602–1614. doi: 10.1016/j.cub.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 61•.Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron. 2011;69:498–508. doi: 10.1016/j.neuron.2010.12.017. The authors use electrical activation of mosaic clones to show that activation of the P1 or P2b clusters of FruM neurons elicits initiation of male courtship in the absence of conspecifics. Using calcium imaging, they demonstrate that P1 neurons are activated by contact of the male foreleg with a female abdomen or cuticular compounds.
- 62••.Von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–522. doi: 10.1016/j.neuron.2011.01.011. The authors use electrical activation of subsets of FruM neurons to show that activation of P1 and pIP10 clusters triggers courtship song. Further studies demonstrate that the pIP10 cluster of neurons projects into the ventral cord, where they identify additional clusters of FruM neurons, dPR1, vPR6, and vMS11, that individually control specific components of courtship song.
- 63•.Pan Y, Meissner GW, Baker BS. Joint control of Drosophila male courtship behavior by motion cues and activation of male-specific P1 neurons. Proc Natl Acad Sci U S A. 2012;109:10065–10070. doi: 10.1073/pnas.1207107109. The authors show that DsxM is expressed in a subset of FruM P1 neurons. Activation of this subset of P1 neurons triggers male courtship of flies even in the absence of chemosensory cues as well as of moving, inanimate objects.
- 64.Ito H, Sato K, Koganezawa M, Ote M, Matsumoto K, Hama C, Yamamoto D. Fruitless recruits two antagonistic chromatin factors to establish single-neuron sexual dimorphism. Cell. 2012;149:1327–1338. doi: 10.1016/j.cell.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 65••.Lin D, Boyle MP, Dollar P, Lee H, Lein ES, Perona P, Anderson DJ. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. The authors identify neurons within or in proximity to the VMHvl whose activation is necessary and sufficient for aggression. Although wild-type male mice attack males but not females, activation of these neurons in males is sufficient to trigger attacks directed to males as well as females.
- 66.Hess WR, Akert K. Experimental data on role of hypothalamus in mechanism of emotional behavior. AMA Arch Neurol Psychiatry. 1955;73:127–129. doi: 10.1001/archneurpsyc.1955.02330080005003. [DOI] [PubMed] [Google Scholar]
- 67.Bard P. A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. Am J Physiol. 1928;84:490–515. [Google Scholar]
- 68.Kruk MR, Van der Poel AM, De Vos-Frerichs TP. The induction of aggressive behaviour by electrical stimulation in the hypothalamus of male rats. Behaviour. 1979;70:292–322. doi: 10.1163/156853979x00106. [DOI] [PubMed] [Google Scholar]
- 69.Olivier B, Wiepkema PR. Behaviour changes in mice following electrolytic lesions in the median hypothalamus. Brain Res. 1974;65:521–524. doi: 10.1016/0006-8993(74)90241-8. [DOI] [PubMed] [Google Scholar]
- 70.Musatov S, Chen W, Pfaff DW, Kaplitt MG, Ogawa S. RNAi-mediated silencing of estrogen receptor {alpha} in the ventromedial nucleus of hypothalamus abolishes female sexual behaviors. Proc Natl Acad Sci U S A. 2006;103:10456–10460. doi: 10.1073/pnas.0603045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Vries GJ. Sex differences in neurotransmitter systems. J Neuroendocrinol. 1990;2:1–13. doi: 10.1111/j.1365-2826.1990.tb00385.x. [DOI] [PubMed] [Google Scholar]
- 73.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 74.Arnold AP, Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Vries GJ, Rissman EF, Simerly RB, Yang L-Y, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gregg C, Zhang J, Butler JE, Haig D, Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010;329:682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gregg C, Zhang J, Weissbourd B, Luo S, Schroth GP, Haig D, Dulac C. High-resolution analysis of parent-of-origin allelic expression in the mouse brain. Science. 2010;329:643–648. doi: 10.1126/science.1190830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeVeale B, Van der Kooy D, Babak T. Critical evaluation of imprinted gene expression by RNA-Seq: a new perspective. PLoS Genet. 2012;8:e1002600. doi: 10.1371/journal.pgen.1002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lefebvre L, Viville S, Barton SC, Ishino F, Keverne EB, Surani MA. Abnormal maternal behaviour and growth retardation associated with loss of the imprinted gene Mest. Nat Genet. 1998;20:163–169. doi: 10.1038/2464. [DOI] [PubMed] [Google Scholar]
- 80.Garfield AS, Cowley M, Smith FM, Moorwood K, Stewart-Cox JE, Gilroy K, Baker S, Xia J, Dalley JW, Hurst LD, et al. Distinct physiological and behavioural functions for parental alleles of imprinted Grb10. Nature. 2011;469:534–538. doi: 10.1038/nature09651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81••.Liu Y, Jiang Y, Si Y, Kim J-Y, Chen Z-F, Rao Y. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature. 2011;472:95–99. doi: 10.1038/nature09822. The authors show that male mice lacking serotonin in the brain attempt to mate with males or females with no apparent sexual preference. Supplementation of such adult mutant males with a precursor to serotonin restores sexual preference for mating with females, thereby demonstrating that this neuromodulator controls sexual preference in adults.
- 82.Shohat-Ophir G, Kaun KR, Azanchi R, Mohammed H, Heberlein U. Sexual deprivation increases ethanol intake in Drosophila. Science. 2012;335:1351–1355. doi: 10.1126/science.1215932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim WJ, Jan LY, Jan YN. Contribution of visual and circadian neural circuits to memory for prolonged mating induced by rivals. Nat Neurosci. 2012;15:876–883. doi: 10.1038/nn.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grosjean Y, Rytz R, Farine J-P, Abuin L, Cortot J, Jefferis GSXE, Benton R. An olfactory receptor for food-derived odours promotes male courtship in Drosophila. Nature. 2011;478:236–240. doi: 10.1038/nature10428. [DOI] [PubMed] [Google Scholar]
- 85.Keleman K, Vrontou E, Krüttner S, Yu JY, Kurtovic-Kozaric A, Dickson BJ. Dopamine neurons modulate pheromone responses in Drosophila courtship learning. Nature. 2012;489:145–149. doi: 10.1038/nature11345. [DOI] [PubMed] [Google Scholar]
- 86.Wang L, Dankert H, Perona P, Anderson DJ. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc Natl Acad Sci U S A. 2008;105:5657–5663. doi: 10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Certel SJ, Leung A, Lin C-Y, Perez P, Chiang A-S, Kravitz EA. Octopamine neuromodulatory effects on a social behavior decision-making network in Drosophila males. PLoS ONE. 2010;5:e13248. doi: 10.1371/journal.pone.0013248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou C, Huang H, Kim SM, Lin H, Meng X, Han K-A, Chiang A-S, Wang JW, Jiao R, Rao Y. Molecular genetic analysis of sexual rejection: roles of octopamine and its receptor OAMB in Drosophila courtship conditioning. J Neurosci. 2012;32:14281–14287. doi: 10.1523/JNEUROSCI.0517-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]