Abstract
Romantic interest or rejection can be powerful incentives not merely for their emotional impact, but for their potential to transform, in a single interaction, what we think we know about another person—or ourselves. Little is known, though, about how the brain computes expectations for, and learns from, real-world romantic signals. In a novel “speed-dating” paradigm, we had participants meet potential romantic partners in a series of 5-min “dates,” and decide whether they would be interested in seeing each partner again. Afterward, participants were scanned with functional magnetic resonance imaging while they were told, for the first time, whether that partner was interested in them or rejected them. Expressions of interest and rejection activated regions previously associated with “mentalizing,” including the posterior superior temporal sulcus (pSTS) and rostromedial prefrontal cortex (RMPFC); while pSTS responded to differences from the participant's own decision, RMPFC responded to prediction errors from a reinforcement-learning model of personal desirability. Responses in affective regions were also highly sensitive to participants' expectations. Far from being inscrutable, then, responses to romantic expressions seem to involve a quantitative learning process, rooted in distinct sources of expectations, and encoded in neural networks that process both affective value and social beliefs.
Keywords: posterior superior temporal sulcus, rostromedial prefrontal cortex, social cognition, speed-dating, ventromedial prefrontal cortex
Introduction
Finding out whether another person likes or dislikes you can be one of the most powerful incentives that humans face, especially when that person is a potential romantic partner (Golightly and Byrne 1964; Turner et al. 1971; Buss 1983). Romantic interest and rejection do not merely elicit strong emotions, however; they can also transform how we view other people, befitting the impact that romantic relationships can have on our health and long-term happiness (Clark and Reis 1988; Myers and Diener 1995; Fisher 1998; Cohen 2004). Despite the personal and evolutionary significance of recognizing signals from potential romantic partners, however, we know very little about the neural systems that respond to expressions of romantic interest or rejection.
One challenge in understanding romantic expressions is that individuals vary enormously in their response to social incentives, in large part because of the important role of expectations (Chang and Sanfey 2013). For example, a simple theory might suggest that an expression of romantic interest from an unattached potential partner communicates an unambiguous message about that person's beliefs—they feel positively—and that this expression tells us something primarily about that partner, not ourselves. In the real world, however, what we learn about the person who expresses romantic interest or rejection—as well as what we learn about ourselves—varies enormously depending on what we believed about that partner and our goals with regard to that partner (Aron and Aron 1991).
One key question, then, is how romantic expressions—especially when unexpected—can lead us to update our beliefs about another person's thoughts and motivations. Regions including the rostromedial prefrontal cortex (RMPFC) and posterior superior temporal sulcus (pSTS) are thought to support incorporating new social information into beliefs about others (Behrens et al. 2008; Hampton et al. 2008; Young and Saxe 2008). Another related question is how basic neural systems for responding to rewards and punishments might be modulated by expectations and beliefs. Those modulations might affect responses even in primary networks for responding to social rewards (such as ventromedial prefrontal cortex [VMPFC] and ventral striatum; Aharon et al. 2001; Knutson and Cooper 2005; Montague et al. 2006; Somerville et al. 2006), or social punishments (such as anterior cingulate; Eisenberger et al. 2003; Fisher et al. 2010).
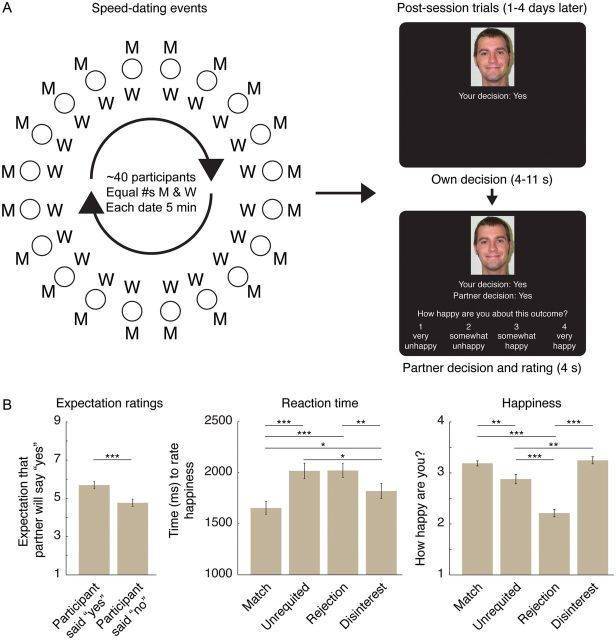
A second challenge for studying romantic expressions is how to fit genuine real-world feelings into the constraints of neuroimaging. Almost all neuroimaging studies of social incentives rely either on social stimuli without interaction (like photos of strangers), or on artificial, rule-based monetary transactions (like economic games). The current study thus used an entirely new paradigm that combined functional magnetic resonance imaging (fMRI) with face-to-face romantic interactions. Participants attended “speed-dating” events (Kurzban and Weeden 2005; Finkel and Eastwick 2008) where they met potential romantic partners and chose who they would be interested or not interested in seeing again (Fig. 1A). Following these events, participants were scanned while they found out, for the first time, each partner's decision about them.
Figure 1.
Experimental design and behavioral results. (A) Experimental design. Participants attended speed-dating events (∼20 of each gender at each) where participants met other participants for 5 min. conversations and made yes or no decisions about whether they wanted to see each partner again. Diagram shows event layout (each “date” at separate table, with 1 gender rotating after each date). Following the events (1–3 days), a subset of participants (N = 38) were scanned with fMRI while they learned about each partner's decision about them. On each trial, participants first saw a partner's face and their own decision about that partner as a reminder; following a randomized delay (4–11 s), participants were then shown the partner's decision, and rated how happy or unhappy they were about the decision (4 s). Following the postsessions, Match partners (mutual yes decisions) then received each other's contact information. Sample photo courtesy of Center for Vital Longevity Face Database. (B) Behavioral results. Left panel: Ratings during the speed-date events, following each date, of how likely that partner was to say yes to the participant (on 9-point Likert-type scale), split by whether the participant had said yes to that partner. Center panel: Reaction times to make a happiness rating during the postscan for each speed-date outcome (see Materials and Methods section for outcome details). Significant differences for reaction times are calculated on square-root-transformed reaction times to account for skew in distribution. Right panel: Happiness ratings during the postscan for each speed-date outcome. All error bars are standard error of the mean within-condition over scanned participants. ***P < 0.001; **P < 0.01; *P < 0.05.
We hypothesized that romantic interest and rejection would elicit responses in core networks for updating beliefs about others, such as the RMPFC and pSTS, and that these regions would especially reflect romantic expressions that violated participants' expectations. We also hypothesized that neural responses in basic affective systems, like the VMPFC and anterior cingulate cortex (ACC), would depend crucially on whether partners were desired or undesired.
Materials and Methods
Participants
One hundred fifty-one heterosexual student volunteers from Trinity College Dublin participated after providing informed consent for a study approved by the Research Ethics Committee of the Trinity College School of Psychology. Volunteers were ethnically representative of the student population (over 85% Irish).
Participants were assigned to separate scanning (N = 38; 18 women, 20 men; 19–31 years old, M = 21.47) or behavioral-only (N = 113; 53 men, 60 women; 18–32 years old, M = 20.45) pools at signup. Scanning participants were additionally screened for current psychiatric diagnoses, right-handedness, and MRI contraindications (e.g., claustrophobia). Scanning participants were paid €10 at signup, €20 for each speed-date event attended and €30 for the scanning session. Behavioral-only participants were paid €10 at signup, €20 for attending their speed-date event, and €5 for their post-task questionnaire.
Materials
Participants attended a signup session where they provided informed consent and had a digital photo taken in front of a neutral background. Participants were allowed to choose their expression and could repeat their photo until they approved it. Photos were cropped to a standard size (307 × 384 pixels) that showed only the face and hair.
Procedures
Speed-Date Events
We ran 6 speed-date events in total, each event including 31–40 participants (M = 36.83) with roughly equal numbers of men and women. Events took place mid-day in a large open classroom on campus.
Each participant was given a packet of blank date records and nametag (with a first name and unique identification number) on arrival. Date records included several Likert-type ratings of a partner's personality traits (not analyzed here), a 9-point rating of date success (“I was interested in getting to know this partner better,” anchored by “strongly disagree” and “strongly agree”), and a 9-point rating about expected partner decision (“This partner is likely to say ‘Yes’ to me,” anchored by strongly disagree and strongly agree). Each date record ended with a single “yes” or “no” question: “Would you be interested in seeing this partner again?”
During each date, participants had an unconstrained conversation with the partner across from them. Every 5 min, at an experimenter's signal, all of the men or all of the women (counterbalanced across events; Finkel and Eastwick 2009) would rotate one partner to their right; before beginning the new date, participants filled out a record for the date just completed, including their yes or no decision. To provide a minimum number of yes choices and matches, participants were asked to say yes to at least 50% of their partners at each event. (Every participant except one obeyed this instruction; that participant fell short in his responses to only 2 partners and so was left in the analyses). After all participants had met each opposite-sex partner, the event concluded; participants returned their date records to the experiment and were paid in cash.
To ensure that we had enough data for an event-related fMRI design, scanning participants each attended 3 speed-date events on 3 successive days (as a single event would allow for fewer than 20 trials and far fewer matches). Behavioral-only participants attended only a single event. All male scanning participants attended the first 3 events and met a new group of behavioral-only partners at each event, while all female scanning participants attended the second 3 events and met a new group of behavioral-only partners at each event (6 scanning participants (5 women, 1 men) missed 1 event and attended 2 events instead; results were qualitatively identical excluding these participants. One scanning participant, a woman, missed 2 events and is included in the behavioral-only results but not the scanning results.).
Because participants were asked to say yes to at least half of their partners, a rate above the average for students in similar published studies (∼40%; Finkel and Eastwick 2009), one potential concern is that some partners were chosen to pursue simply to follow instructions. To address this concern, we performed additional analyses using the 9-point rating of romantic desirability from the date, which was unconstrained by experimenter instruction, in place of the yes or no decision. These ratings were highly correlated with yes or no decisions; a hierarchical logistic regression using only this rating correctly classified 84.86% of decisions. Both the behavioral and neuroimaging results were qualitatively identical using the ratings instead of the decisions; thus, the relationship between brain activation and learning about romantic outcomes is unlikely to be significantly influenced by this experimental instruction.
Postsessions
Between 1 and 4 days following his or her final event (M = 2.50), each scanning participant attended a postsession in the laboratory, where they were scanned with event-related fMRI while they were shown the outcome of each date: each partner's yes or no decision about the participant. Stimuli were presented with Cogent 2000 (Wellcome Trust Centre for Neuroimaging; London).
Each trial had 2 phases (Fig. 1A). First, participants saw the face of a partner, with their own yes or no decision about that partner displayed below as a reminder. Faces remained onscreen for a short, jittered delay (4–11 s, randomly drawn from a truncated Poisson distribution, M = 6 s).
Next, the partner's yes or no decision about the participant was displayed below the face. At the same time, a 4-point rating scale was displayed with the question “How happy are you about this outcome?” (Scale points were “very unhappy,” “somewhat unhappy,” “somewhat happy,” and “very happy;” left and right sides were counterbalanced across participants.) Participants had 4 s to make their response with a button box. Trials were separated by an intertrial interval displaying a fixation cross (1–8 s, randomly drawn from a truncated Poisson distribution, M = 3 s ( for details on randomized intertrial intervals, see Henson 2007).
Experimental trials were randomly intermixed with control trials, which showed other faces from the study that the participant had not met. On these trials, both the participant's decision and the partner's were displayed as “Did Not Meet.” Control trials were otherwise identical, including the required happiness rating. Participants faced 33–56 experimental trials (the exact number varied depending on the number of partners at their events, and was lower for the small number of participants who missed 1 event; M = 51.42, SD = 7.15) and 25–51 control trials (the total was higher for participants who missed 1 event; M = 32.21, SD = 7.49), for a total of 63–88 trials (all but one >80; M = 83.63, SD = 3.86). One possible concern is that the slight variation in the number of trials per participant violates the assumption of identical distribution in the variance across participants required for the random effects analysis. To address this, we reanalyzed all imaging models using only 80 trials for each participant by randomly selecting 80 trials for each participant with >80 trials, and excluding the 1 participant with <80 trials. This version of the analysis yielded essentially identical results to the one where all trials were included; in particular, every cluster discussed in the results remains significant and of nearly identical size. For this reason, we report the analyses with all trials included in the Results section.
Participants were scanned with a Phillips 3-T MRI scanner using the standard head coil, padded to minimize head motion. Functional images covered the whole brain with 38 contiguous 3.2-mm-thick axial slices with gradient echo T2*-weighted echo-planar imaging (TR = 2 s, TE = 28 ms, 3 × 3-mm in-plane voxel size, 80 × 80 matrix). The acquisition plane was tilted about 30° to the anterior–posterior commissure plane to optimize sensitivity in the ventral prefrontal cortex (Deichmann et al. 2003). Each participant's scan consisted of a single functional run whose length varied depending on the number of trials (324–473 images, M = 443.5); the first 4 were discarded to account for magnetic equilibration. Almost all participants had a high-resolution structural image taken 1–2 weeks before the events as part of a separate study; for those that did not, a high-resolution structural image was acquired at this scan before the task (all structural scans: 3D acquisition; T1-weighted spoiled-gradient sequence; 0.9 × 0.9 × 0.9-mm voxel size; 256 × 256 × 180 matrix).
Following the scan, participants performed a separate self-paced multirating task outside the scanner at a computer. For each matched-partner only, participants rated their agreement with 2 statements: “I am very likely to initiate contact with this partner,” and “I hope that this partner initiates contact with me.” Ratings were made on 9-point Likert-type scales, anchored by strongly disagree, “neither,” and strongly agree.
Decision Emails
Immediately following the first set of postsessions (for male scanning participants, after the third speed-date event) and again following the second set (for female scanning participants, after the sixth event), both behavioral-only and scanning participants in that set of speed-date events were emailed a document containing photos of all of their partners, their first names and numbers from the events, and both the participant's and partner's decision for each date. For each match partner, an email address was also provided. To protect participants' privacy, every participant was assigned a unique email address for the study that forwarded to their personal address.
Statistical Analysis
Behavioral data were analyzed with MATLAB (The Mathworks, Inc.; Natick, MA, USA). Ratings and reaction times were analyzed with hierarchical models using nlmefit, nesting behavioral-only partners within scanning participants (Gelman and Hill 2006). Models included both fixed and random effects for each predictor, and were estimated with maximum likelihood and diagonal covariance for random effects. All predictors were centered on the group mean.
Imaging data were analyzed with SPM8 (Wellcome Department of Imaging Neuroscience; London). Functional images were preprocessed with standard parameters, including slice timing correction (to the center slice), realignment (to each participant's first image), co-registration of the high-resolution structural image, segmentation of the structural image into tissue types (using the “New Segment” routine with the default templates), spatial normalization of the functional images (into Montreal Neurological Institute [MNI] space, using parameters from segmentation and SPM8 defaults), and spatial smoothing (with a 4-mm full-width half-maximum Gaussian kernel).
A general linear model was created for each participant to estimate effects in response to partner decisions. This model included separate delta-function regressors (0 s duration) for the appearance of partners to whom the participant had said yes or “no,” as well as one for the appearance of control faces. It also included 4 delta-function regressors (0 s duration) for the appearance of partner decisions, one for each combination of partner and participant decision (yes from a yes partner, no from a no partner, etc.), as well as one for the decision phase of control trials (Reaction time was not included in this model.).
To investigate responses to learned expectations about partner decisions, a separate general linear model was estimated using parameters from a behavioral reinforcement learning (RL) model. This model assumed that participants learned during the scan how likely partners were to say yes to them, and included regressors to quantify how those expectations were violated (i.e., prediction errors). Learning was modeled with a simple Rescorla–Wagner rule (Rescorla and Wagner 1972): the estimate of a yes on each trial was updated according to the rule where PartnerDect is the current partner's actual decision (set to 1 for yes and 0 for no), α is a learning-rate parameter, and is the current estimate (from 0 to 1) of receiving a “yes.” ProbYes was initialized as 0.5 for all participants. To estimate α, we assumed that large absolute prediction errors (i.e., large violations in expectations, either positive or negative) would require greater cognitive processing and slow reaction times, and thus fit the absolute output of the model to participants' trial-to-trial reaction times (square-root-transformed to account for skew). The behavioral RL model was specified as:
PartnerDect and ParticipantDect were dummy variables set to 1 for yes and 0 for no for partner and participant decisions on that trial (grand-mean centered); Matcht was an interaction variable set to (grand-mean-centered versions of) PartnerDect ×ParticipantDect, while Trialt was set to the trial number (1, 2, etc.). δt represented the prediction error (and abs(δt) the absolute value of that error) on each trial (calculated by the Rescorla–Wagner update rule specified above. This model therefore estimated the learning constant α while controlling for the effects of participant and partner decisions, as well as expected speeding of reactions over time. All 6 β parameters and α were estimated in a mixed-effects (hierarchical) model by optimizing the likelihood function via a full parameter search (using MATLAB nlmefit, based on fminsearch), including both fixed and random effects in all parameters.
To estimate whether including this learning term improved the behavioral RL model, the full behavioral RL model above was compared with a reduced behavioral model that was identical except it did not include α, β6, or any prediction-error term (δt).
The imaging RL model included 4 delta-function regressors (0 s duration) for the appearance of partner faces, control faces, partner decision phases, and control decision phases. The partner decision regressor was modulated (in order) by contrast-coded regressors (1 for “yes,” −1 for no) for partner decision, participant decision, and their interaction. It was next modulated by the absolute value of the trial-by-trial-estimated prediction errors (abs(δt) above), then by the actual (i.e., signed) value of the prediction errors (δt above), and lastly by reaction time (SPM orthogonalizes each parameter against each parameter already entered in the model; for example, reaction time was orthogonalized against all other parameters, as it was the last entered.). Control decision regressors were modulated only by reaction time. Partner face regressors were modulated by the contrast-coded participant decision, the actual (i.e., signed) prediction, and the absolute value of the prediction (i.e., unsigned); control face regressors were not modulated.
Both models also included 6 regressors of no interest for estimated head motion and a constant term. Models were estimated using restricted maximum likelihood and an AR(1) model for temporal autocorrelation. A high-pass filter (cutoff 128 s) removed low-frequency noise. Beta-weight images for each regressor combined to form appropriate contrasts within participants, and contrast images were carried forward to group-level analyses. Significant effects were tested with 1-sample t-tests across the group. Activations were thresholded with a per-voxel significance of P < 0.001 and an extent threshold based on Gaussian random fields set to control the familywise error rate (FWE) at P < 0.05 (Worsley et al. 1996). Peaks are reported in ICBM/MNI coordinates.
For region-of-interest analysis (Fig. 3B), BOLD signal in an 8-mm-diameter sphere surrounding the peak of the VMPFC region for the “Match > Unrequited” contrast was extracted, averaged across the sphere, converted to percent signal change, high-pass filtered (cutoff 128 s), centered for the experiment mean, and window-averaged to create a measure of the average percent signal change between 4 and 6 s following the partner decision on each trial. Signal was then used as a fixed and random predictor in a hierarchical model for the desire for contact ratings for matched-partners only made after the scan. Importantly, because these desire for contact ratings were made only for matched partners (i.e., only within 1 cell of the contrast), this analysis was run only within the matched-partners trials; it was therefore orthogonal to the contrast used to select the functional region (which compared the average for all matched-partner trials to other trials), avoiding circularity in the model (Kriegeskorte et al. 2009).
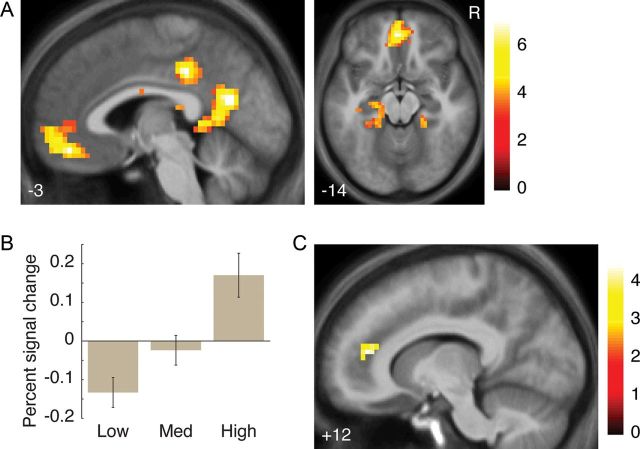
Figure 3.
Activations involved in matching and rejection. (A) Regions activated more for Match than for Unrequited outcomes. R indicates right. (B) Activation for Match outcomes in the VMPFC (average percent signal change 4–6 s following outcome appearance in an 8-mm-diameter sphere around the peak of the activation cluster for the Match > Unrequited contrast, x/y/z = 3/44/−15), split by ratings of desire for contact with each Match partner made after the postscan. Low = bottom third; Med = middle third; High = top third (within-participant). Error bars indicate standard error of the mean across participants; significant differences not shown. (C) Regions activated more for Rejection than for Disinterest outcomes. All images thresholded at P < 0.001 voxelwise with extent threshold set to control whole-brain FWE at P < 0.05 (25–30 voxels). Color bars indicate t-statistic. Coordinates in ICBM/MNI space.
Results
Note
Dates had 4 possible outcomes: each participant's yes or no decision crossed with each partner's yes or no decision. For clarity, we describe all outcomes below with labels from the participant's perspective. “Match” indicates dates where both participant and partner said “yes.” “Rejection” indicates dates where the participant said “yes,” but the partner said “no.” “Unrequited” indicates dates where the participant said “no,” but the partner said “yes.” “Disinterest” indicates dates where both participant and partner said “no.”
Behavioral
Yes Rates
Scanned participants said yes to 59.1% of their partners on average (SEM = 1.5%), with yes rates ranging from 47.2 to 96.2%. Yes rates for women and men did not differ significantly (Mann–Whitney U-test = 318, Z = 0.95, ns; women's M = 59.1%, SEM = 2.8%; men's M = 59.1%, SEM = 1.5%). Behavioral-only participants' yes rates (i.e., the yes rates of scanned participants' partners) did not significantly vary from scanned participants (behavioral-only M = 57.8%, SEM = 0.9%; Mann–Whitney U-test = 3097, Z = 0.89, ns).
Violations of Expectations and Learning
We first examined whether participants' expectations about partner decisions related to their own decisions about each partner, by examining the expectation rating made at the end of each date. Participants' expectations about their partners' decision were significantly correlated with their own decision (mean rating of expectation that the partner would say yes when the participant said yes = 5.69; mean rating when the participant said no = 4.76; paired SEM = 0.08, t(37) = 12.09; Fig. 1B), suggesting participants formed strong expectations that, in general, partners' decisions would match their own.
We also used reaction times for happiness ratings at the postsession as an index of how much cognitive processing was required to respond to each partner's decision. Reaction times (square-root transformed to account for skew) were modeled with a hierarchical model, including partner's decision, participant's own decision, their interaction, and the linear effect of time as predictors. Participants were significantly slower to respond both for receiving a no (Receiving no M = 1842.1 ms, SD = 678.5 ms; receiving yes M = 1723.1 ms, SD = 762.2 ms; model predictor t(1917) = 2.73, P = 0.006) and when they themselves had said no (Own no M = 1851.5 ms, SD = 765.5 ms; own yes M = 1721.4 ms, SD = 699.3 ms; model predictor t(1917) = 2.60, P = 0.009); this pattern matches the happiness ratings (Fig. 1B; see also Results section), suggesting that partners were slower to respond when they were less happy about the decision. Participants were also significantly slower when their decision did not match their partner's decision (mismatch trials M = 1928.4 ms, SD = 746.2; match trials M = 1633.1 ms, SD = 684.5 ms; model predictor t(1917) = 6.40, P < 0.001); these increased reaction times suggest mismatched decisions required additional cognitive processing, providing additional evidence that mismatched decisions violated participants' expectations.
Another potential source for participant expectations, however, was each participant learning, during the scan, how desirable he or she tended to be. Receiving a yes after the tenth no in a row, for example, might be more surprising than after the tenth yes in a row. To investigate how participants learned about their own desirability, we modeled reaction times with a simple RL algorithm that provided a trial-by-trial-predicted partner decision and prediction error (see Materials and Methods section). The absolute value of these prediction errors gives an estimate of the degree to which each partner's decision violated expectations based on the previous partners' decisions. When we added these unsigned prediction errors (i.e., the absolute value of the prediction error) to a reduced behavioral model of reaction time that did not include any learning over time, the prediction-error term significantly improved the model fit and correlated with significantly slower reaction time (likelihood-ratio compared with the reduced model: P = 0.002; predictor t(1913) = 5.93, P < 0.001; learning rate = 0.22). All other predictors remained significant. This model suggests that participants' expectations of partner decisions come both from their own decisions and from learning what partners tend to decide about them.
Emotional Response to Decisions
Scanning participants rated their happiness in the scanner with each partner's decision. We used a hierarchical linear model to predict happiness from whether the partner said “yes,” whether the participant said yes about that partner, and whether those decisions were the same as each other (Fig. 1B). Receiving a yes made participants significantly happier on average (t(1922) = 5.05, P < 0.001), while participants were less happy on average when they themselves said yes than when they said no (t(1922) = −3.88, P < 0.001), but these main effects were both driven by a significant interaction: participants were much happier when their decision was the same as their partner's than when the decisions were mismatched (t(1922) = 10.85, P < 0.001). More specifically, participants were significantly happier about Match outcomes (M = 3.19, SEM = 0.05) than Unrequited outcomes (M = 2.88, SEM = 0.09; t(36) = 2.88, P = 0.007). By contrast, participants were less happy about Rejection outcomes (M = 2.21, SEM = 0.07) than about Disinterest outcomes (M = 3.24, SEM = 0.07; t(37) = 14.06, P < 0.001). This interaction also highlighted the potential ambivalence of social incentives: Unrequited outcomes made participants significantly less happy than Disinterest outcomes (t(36) = 3.45, P = 0.001), even though Unrequited outcomes involved (theoretically) more positive social feedback.
Together, the results confirmed that participants' expectations were crucial drivers both of the cognitive processing involved in responding to partners' decisions and their emotional reactions to those decisions. Those expectations emerged both from participants' beliefs about that individual partner's likely choice, and from their expectations about their own desirability based on other partners' choices.
fMRI
Main Effect of Partner Decision
To investigate the neural response to potential romantic partner interest versus rejection, we examined the simple contrast of receiving a yes compared with a no (Match + Unrequited vs. Rejection + Disinterest outcomes). For receiving a “yes,” activation was greater across a large network of medial and lateral regions, prominently including VMPFC, medial parietal cortex, left parietal cortex and temporoparietal junction, nucleus accumbens, left dorsolateral prefrontal cortex and frontal pole, and left middle temporal gyrus (Table 1; Supplemental Fig. 1). By contrast, for receiving a “no,” no significant regions were more active.
Table 1.
Activations correlated with main effect of partner decision
| Region | Peak Z-score | X | Y | Z | Cluster size (vox) |
|---|---|---|---|---|---|
| Yes (Match + Unrequited) > No (Rejection + Disinterest) | |||||
| Dorsolateral PFC | 5.80 | −21 | 26 | 53 | 260 |
| Lateral parietal / temporoparietal cortex | 5.47 | −45 | −70 | 28 | 309 |
| Ventromedial PFC | 5.23 | −3 | 38 | 15 | 270 |
| Posterior middle temporal gyrus | 5.05 | −54 | −49 | −7 | 34 |
| Medial parietal cortex | 5.05 | −18 | −19 | 57 | 1021 |
| Rostral medial PFC | 4.75 | −15 | 65 | 17 | 67 |
| Ventral striatum | 4.54 | 9 | 14 | −4 | 68 |
| Lateral occipital cortex | 4.50 | 30 | −58 | −15 | 32 |
| Occipitotemporal junction | 4.35 | 42 | −61 | −4 | 63 |
| Lateral cerebellum | 4.30 | −42 | −64 | −25 | 114 |
| Rostral anterior cingulate | 4.09 | 6 | 29 | 14 | 36 |
| Medial cerebellum | 4.09 | 6 | −58 | −18 | 34 |
| Lateral cerebellum | 3.91 | 36 | −76 | −32 | 24 |
| No (Rejection + Disinterest) > Yes (Match + Unrequited) | |||||
| No regions active at this threshold | |||||
Note: Activations in table were thresholded voxelwise at P < 0.001 and with a cluster size set to control for multiple comparisons over whole brain at P < 0.05 (24 voxels). T-statistics were converted to Z-scores for reporting. Coordinates are reported in MNI space, as in SPM8. Voxel size was 3 × 3 × 3.2 mm.
PFC, prefrontal cortex.
Violations of Expectations
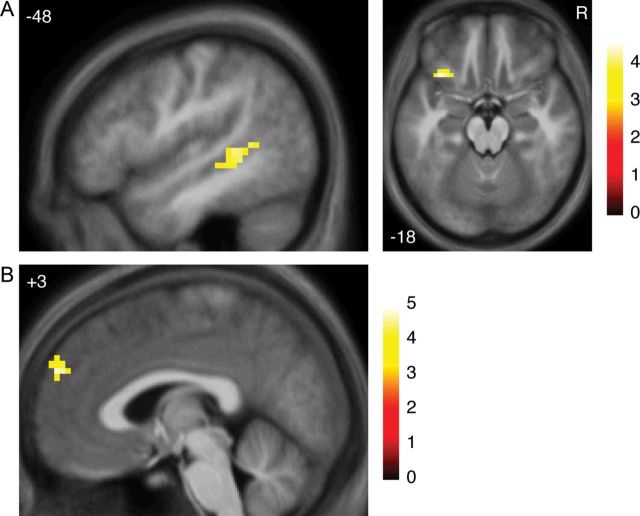
Participants expected that partners' decisions would tend to match their own, as indicated both by explicit expectations and implicit reaction times. Violations of expected partner decisions were also reflected in neural activation; mismatched decisions (Rejection + Unrequited outcomes), when compared with matched decisions (Match + Disinterest outcomes), elicited activation in significant clusters in left ventrolateral prefrontal cortex (VLPFC) and in left pSTS (Fig. 2A, Table 2).
Figure 2.
Activations involved in violations of expectations. (A) Regions activated more for receiving a mismatched decision (Rejection + Unrequited outcomes) compared with a matched decision (Match + Disinterest outcomes). R indicates right. (B) Region correlated with unsigned prediction errors about partner decisions, based on RL model of participant's own desirability. All images thresholded at P < 0.001 voxelwise with extent threshold set to control whole-brain FWE at P < 0.05 (27–33 voxels). Color bars indicate t-statistic. Coordinates in ICBM/MNI space.
Table 2.
Activations correlated with violations of expectations
| Region | Peak Z-score | X | Y | Z | Cluster size (vox) |
|---|---|---|---|---|---|
| Mismatched (Rejection + Unrequited) > matched (Match + Disinterest) | |||||
| Posterior superior temporal sulcus | 4.33 | −51 | −43 | 0 | 30 |
| Ventrolateral PFC | 4.15 | −36 | 20 | −18 | 27 |
| Unsigned prediction errors from RL model | |||||
| Rostromedial PFC | 4.60 | 9 | 53 | 25 | 33 |
Note: Activations in table were thresholded voxelwise at P < 0.001 and with a cluster size set to control for multiple comparisons over whole brain at P < 0.05 (27–33 voxels). T-statistics were converted to Z-scores for reporting. Coordinates are reported in MNI space, as in SPM8. Voxel size was 3 × 3 × 3.2 mm.
PFC, prefrontal cortex.
Next, to investigate violations of expectations based on participants' own learned desirability, we examined which regions were correlated with absolute prediction errors from an RL model that learned an expected partner decision based on observing other partners' decisions during the scan (see Materials and Methods section). Activation in response to a partner's decision was positively correlated with absolute prediction errors about that decision in a single cluster in the RMPFC (Fig. 2B, Table 2). No significant clusters were negatively correlated; as well, no significant clusters were correlated positively or negatively with the signed prediction error.
Matches and Reward Value
Both happiness and excitement ratings suggested that participants found Match outcomes to be more rewarding than Unrequited outcomes. To investigate regions that might be specifically involved in encoding social reward value, we examined the contrast of Match versus Unrequited outcomes (this contrast controlled for the partner's decision—yes in both cases). Activation was greater for Match outcomes in a network of brain regions largely overlapping those for the main effect, especially those linked to value like VMPFC and medial parietal cortex, as well as left posterior parietal cortex and medial temporal cortex (Fig. 3A; Table 3; also see Supplemental Fig. 1 for comparison to main effect.).
Table 3.
Activations correlated with matching and rejection
| Region | Peak Z-score | X | Y | Z | Cluster size (vox) |
|---|---|---|---|---|---|
| Match > Unrequited | |||||
| Posterior cingulate | 5.93 | −3 | −61 | 21 | 494 |
| Posterior cingulate | 5.60 | −3 | −34 | 39 | 96 |
| Ventromedial PFC | 5.56 | −3 | 44 | −15 | 242 |
| Medial temporal cortex | 4.93 | −33 | −49 | 0 | 125 |
| Medial temporal cortex | 4.52 | 33 | −34 | −4 | 25 |
| Dorsolateral PFC | 4.40 | −21 | 32 | 42 | 28 |
| Occipito-parietal junction | 4.36 | −33 | −70 | 35 | 60 |
| Dorsal caudate | 4.06 | 18 | −4 | 28 | 71 |
| Rejection > Disinterest | |||||
| Anterior cingulate | 4.25 | 12 | 41 | 14 | 31 |
Note: Activations in table were thresholded voxelwise at P < 0.001 and with a cluster size set to control for multiple comparisons over whole brain at P < 0.05 (25–31 voxels). T-statistics were converted to Z-scores for reporting. Coordinates are reported in MNI space, as in SPM8. Voxel size was 3 × 3 × 3.2 mm.
PFC, prefrontal cortex.
Even within Match partners, though, participants' preferences varied; some partners were highly desired while some were merely unobjectionable. A Match with a highly desired partner should have greater reward value than a Match with a less-desired partner, providing a finer-grained index of social reward value beyond decision category. Partner desirability was quantified with participants' self-reported desire for contact ratings, made after the scan about each Match partner (these 2 ratings were averaged together; a = 0.91). In the brain, we focused on the VMPFC as an a priori region of interest (ROI) due to its specific role in encoding reward value.
VMPFC activation for Match outcomes was significantly positively correlated with desire for partner contact in a hierarchical model (t(648) = 4.97, P < 0.001), suggesting that this region encoded not just the categorical partner decision, but a quantitative measure of that decision's reward value (Fig. 3B). An important question is whether VMPFC activation might encode reward value even accounting for the other self-report ratings. After including both happiness and excitement in the model, both were significant (happiness: t(644) = 5.87, P < 0.001; excitement: t(644) = 15.97, P < 0.001). VMPFC activation, however, remained significantly positively correlated even controlling for these ratings (t(644) = 2.12, P = 0.02), indicating that this region's response to a partner's decision captured variance in the desire for contact over and above what participants were able to provide in their other ratings.
Finally, if Match outcomes were rewarding, participants may have anticipated that reward even before knowing their partner's decision. To investigate signals for anticipated social reward, we examined the contrast of partners to whom the participant said yes versus no—at the appearance of their face, before their decision was revealed. Activation was greater for yes partners (i.e., potential Match or Rejection outcomes) in a network of regions linked to both anticipated value (like the ventral striatum and VMPFC) and anticipated risk (like the anterior insula), as well as regions linked to visual attention and motor preparation, like visual cortex and supplementary motor area (Table 4; Supplementary Fig. 2).
Table 4.
Activations correlated with anticipation of partner's decision
| Region | Peak Z-score | X | Y | Z | Cluster size (vox) |
|---|---|---|---|---|---|
| Partners who were given a yes > those given a no | |||||
| Lateral occipital cortex | 5.76 | −36 | −82 | 0 | 908 |
| Lateral occipital cortex | 5.65 | 39 | −79 | −14 | 999 |
| Anterior insula | 4.70 | 33 | 29 | 0 | 74 |
| Medial premotor cortex | 4.66 | 9 | 11 | 64 | 52 |
| Posterior parietal cortex | 4.58 | 24 | −46 | 46 | 32 |
| Ventral thalamus | 4.56 | −3 | −19 | 0 | 166 |
| Ventral striatum | 3.92 | −6 | 11 | −4 | * |
| Medial cerebellum | 4.37 | 6 | −43 | −43 | 30 |
| Medial cerebellum | 4.28 | −6 | −70 | −22 | 135 |
| Ventromedial PFC | 4.22 | −6 | 38 | −15 | 46 |
| Medial occipital cortex/posterior cingulate | 4.21 | −6 | −73 | 21 | 96 |
| Dorsolateral PFC | 4.18 | 48 | 8 | 18 | 30 |
| Dorsolateral PFC | 3.96 | 39 | −4 | 46 | 42 |
| Posterior cingulate | 3.83 | 9 | −34 | 42 | 37 |
| Partners who were given a no > those given a yes | |||||
| Right temporoparietal junction | 4.27 | 54 | −55 | 28 | 46 |
Note: Activations in table were thresholded voxelwise at P < 0.001 and with a cluster size set to control for multiple comparisons over whole brain at P < 0.05 (30–46 voxels). T-statistics were converted to Z-scores for reporting. Coordinates are reported in MNI space, as in SPM8. Voxel size was 3 × 3 × 3.2 mm.
PFC, prefrontal cortex.
*Subpeak in above cluster.
Rejections
Rejections (receiving a no from a partner to whom one had said yes) elicited the lowest happiness of all outcomes. To investigate regions that might be involved in encoding negative social feedback, we examined the contrast between Rejection outcomes versus Disinterest outcomes (this contrast controlled for the partner's decision—no in both cases). Activation was greater for Rejection outcomes in a single cluster in the rostral ACC (Fig. 3C, Table 3). This cluster was close to those activated in 2 previous studies on social exclusion in both romantic and nonromantic contexts (Eisenberger et al. 2003; Kross et al. 2011).
Discussion
An expression of romantic interest or rejection from a potential partner can be a powerful social incentive, but how we respond to that incentive depends importantly on what we believe and feel about that partner and ourselves. For the first time, we investigated the neural response to learning about romantic interest and rejection by utilizing a novel speed-dating design, in which participants met real-world potential romantic partners face-to-face and made real decisions about whether or not they were interested in seeing them again. After the speed-dating events, participants were scanned while they viewed photos of each partner and found out whether each partner expressed an interest in seeing them again (yes) or not (no).
The study implicated 2 distinct sets of brain areas. One set of areas was found to be involved in responding to unexpected partner decisions. These areas, including the RMPFC and pSTS, overlap substantively with a network of brain areas previously found to be involved in “mentalizing”: encoding and updating beliefs about the intentions and feelings of others (Frith 2007). A key feature of participants' expectations was that participants expected each partner, on average, to make the same decision that they made. In other words, participants tended to expect partners they liked to also like them (and partners they disliked to also dislike them), a strong and well-studied effect in interpersonal liking (Newcomb 1956; Backman and Secord 1959; Kenny 1994; Eastwick et al. 2007). This reciprocity in expectations was supported by the activation of reward systems like the VMPFC and ventral striatum for partners to whom the participant said yes, even before the partner's decision was shown; these systems are also activated for anticipated rewards (O'Doherty 2004; Knutson and Cooper 2005), and the behavioral data indicates participants were anticipating these desired partners to express reciprocal interest.
Receiving a mismatched decision from a partner, then, involved a significant violation of participants' expectations, and elicited activation in the left pSTS and VLPFC. While activation in the VLPFC might be involved in resolving ambivalence about the decision (or about which happiness rating to select; Aron 2008), activation in the left pSTS is more likely to relate to participants revising their beliefs about that partner thanks to their unexpected decision. This pSTS region has been associated in several recent studies with the process of updating representations of others' feelings and beliefs (Behrens et al. 2008; Hampton et al. 2008; Cooper et al. 2010), and may play a similar role in this paradigm. While these studies have focused primarily on changes to beliefs about partners in artificial economic game settings over multiple trials, the current study suggests that this region is engaged even in single-trial learning about real-world social information—at least if that information is salient enough. Participants' expectations about their partners' decisions are guided by what they thought those partners felt and believed, and discovering those expectations were wrong likely led to a substantial revision of how they perceived a partner.
Expectations about a partner's decision, however, did not come only from beliefs about that partner; they also were due to a participant's expectations about his or her own desirability in speed-dating. Activation in the RMPFC provides evidence that these expectations were shaped in part by on-line learning during the postsession. A simple RL model was fit for each participant that formed expectations based on the average partner decision—that is, whether most partners tended to say yes or no to that participant. The unsigned prediction error from the model—a measure of surprise about a partner's decision—correlated both with participant behavior (slowed reaction times in responding) and with increased activation in the anterior RMPFC. This region has been linked to the process of considering others' mental states during mentalizing (Amodio and Frith 2006), and specifically as a region that may encode social prediction errors about others' expected actions (Behrens et al. 2008; Hampton et al. 2008). The current data suggest that these prediction errors may be based not only on inferences about a single partner, but about expectations learned from other members of a social group (i.e., speed-dating partners). As well, this region has been particularly associated with considering and relating others' mental states to one's own self-image (Mitchell et al. 2006). In this case, the RMPFC might be specifically involved in encoding social prediction errors relevant to the personal self-image—each participant's expectation of how desirable others found him or her. Examining how this region's activation varies with individual differences in self-esteem could be a promising starting point for future research.
The second set of brain regions involved in responding to romantic interest and rejection were those areas involved in mediating affective responses to rewarding and punishing feedback. Importantly, activity in these brain areas was highly sensitive to participants' expectations and beliefs about their partners. Reward systems such as the VMPFC and ventral striatum were strongly activated by receiving a “yes,” consistent with the idea that expressions of romantic interest constitute a powerful social reward. These areas were significantly more activated, however, for Match outcomes (a yes from a desired partner) than for Unrequited outcomes (a yes from an undesired partner), consistent with increased self-reported happiness for Match outcomes. Moreover, activity in the VMPFC was even greater for Match partners whom participants expressed the greatest interest in contacting.
Together, the data suggest that the VMPFC in particular encodes a quantitative representation of how rewarding a particular expression of interest might be. This is consistent with its role in representing experienced utility in a variety of nonsocial and social domains (O'Doherty et al. 2003; McClure et al. 2004; O'Doherty 2004; Plassmann et al. 2007; Davey et al. 2009). The current findings argue that romantic rewards, instead of representing a qualitatively different kind of decision-making, are encoded, quantified, and compared in the same neural “common currency” that is thought to underlie other kinds of economic and social decision-making. An intriguing possibility for future study is how neural decision-making systems might directly trade off romantic rewards against other kinds of rewards, or particular costs in effort or time (cf. Deaner et al. 2005; Hayden et al. 2007).
Similarly, the anterior cingulate was activated by romantic rejection, consistent with its role in processing negative outcomes across many domains, and particularly consistent with earlier work suggesting it plays a specific role in responding to social rejection (Eisenberger et al. 2003; Eisenberger et al. 2007; Kross et al. 2011). Anterior cingulate activation was limited to Rejection outcomes (no from a desired partner), and was not involved in Disinterest outcomes (no from an undesired partner); self-reports indicated that Disinterest outcomes were considered relatively positive, perhaps due to avoidance of embarrassment or unreciprocated interest from another.
There are several caveats to our interpretation of the potential function of these networks of regions. We use slower reaction times as an index of greater cognitive processing in several cases; however, slower reaction times might also indicate avoidance (as opposed to approach) motivation. Our key behavioral and imaging models control for partner and own decision to help account for emotional differences between conditions—in other words, greater activations correspond with both positive and negative surprise—but more focused study will be needed to tease apart potential confounds between negative emotion and increased cognitive processing.
This study is the first to combine neuroimaging with real-world round-robin social interactions, and points toward a new path for further neuroimaging studies of more complex and unconstrained social decision-making. Social psychology has embraced round-robin designs for their utility in efficiently generating large numbers of interpersonal impressions that can be easily divided in smaller units of analysis (Kenny 1994; Kenny et al. 2006), which can help address the significant requirements for numbers of trials and participants imposed by neuroimaging technologies. Real-world social relationships can generate powerful and complex incentives that can broaden our understanding of the social brain beyond what we can learn from simpler games (Güroglu et al. 2008; Krienen et al. 2010; Redcay et al. 2010), and neuroimaging studies that utilize true face-to-face interactions as part of their design will play a major role in pushing our understanding forward.
Together, these findings reveal that responding to others' expressions of romantic interest involves a complex interplay of perceptions and expectations about the other person and oneself. A partner's romantic expression—especially when unexpected—resulted in activation consistent with updating representations of that partner's feelings and beliefs, but also involved significant computations involved in updating a participant's own beliefs about his or her own desirability. In addition, key systems involved in domain-general valuation-based decision-making, such as the VMPFC and ACC, were highly sensitive to the expectations that a participant had for a particular partner's decision, as well as a quantitative measure of how desirable the participant found that specific partner. Responding to real-world romantic expressions, then, elicits both learning and feeling; understanding this complex mix is crucial to future understanding of real-world social decisions in general.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by an Irish Research Council on Science, Engineering, and Technology Fellowship to J.C.C., a Wellcome Trust project grant (WT087388AIA), and a grant from the Gordon and Betty Moore Foundation to J.O.D.
Supplementary Material
Notes
The authors gratefully acknowledge technical assistance from Sojo Joseph and research assistance from Jamie Gallagher, Betsy Carroll, and the Science Gallery. Conflict of Interest: None declared.
References
- Aharon I, Etcoff NL, Ariely D, Chabris CF, O'Connor E, Breiter HC. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/S0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Aron A, Aron EN. Love and sexuality. In: McKinney K, Sprecher S, editors. Sexuality in close relationships. Hillsdale (NJ): Lawrence Erlbaum Associates; 1991. pp. 5–8. [Google Scholar]
- Aron AR. Progress in executive-function research: from tasks to functions to regions to networks. Curr Dir Psychol Sci. 2008:124–129. doi: 10.1111/j.1467-8721.2008.00561.x. [DOI] [Google Scholar]
- Backman CW, Secord PF. The effect of perceived liking on interpersonal-attraction. Hum Relations. 1959;12:379–384. doi: 10.1177/001872675901200407. [DOI] [Google Scholar]
- Behrens TEJ, Hunt LT, Woolrich MW, Rushworth MF. Associative learning of social value. Nature. 2008;456:245–249. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss AH. Social rewards and personality. J Pers Soc Psychol. 1983;44:553–563. doi: 10.1037/0022-3514.44.3.553. [DOI] [Google Scholar]
- Chang LJ, Sanfey AG. Great expectations: neural computations underlying the use of social norms in decision-making. Soc Cogn Affect Neurosci. 2013;8:277–284. doi: 10.1093/scan/nsr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Reis HT. Interpersonal processes in close relationships. Ann Rev Psychol. 1988;39:609–672. doi: 10.1146/annurev.ps.39.020188.003141. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. Am Psychol. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Kreps TA, Wiebe T, Pirkl T, Knutson B. When giving is good: ventromedial prefrontal cortex activation for others' intentions. Neuron. 2010;67:511–521. doi: 10.1016/j.neuron.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yucel M. Being liked activates primary reward and midline self-related brain regions. Hum Brain Mapp. 2010;31:660–668. doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage. 2003;19:430–441. doi: 10.1016/S1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Eastwick PW, Finkel EJ, Mochon D, Ariely D. Selective versus unselective romantic desire: not all reciprocity is created equal. Psychol Sci. 2007;18:317–319. doi: 10.1111/j.1467-9280.2007.01897.x. [DOI] [PubMed] [Google Scholar]
- Eisenberger N, Gable SL, Lieberman MD. Functional magnetic resonance imaging responses relate to differences in real-world social experience. Emotion. 2007;7:745–754. doi: 10.1037/1528-3542.7.4.745. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Finkel EJ, Eastwick PW. Arbitrary social norms influence sex differences in romantic selectivity. Psychol Sci. 2009;20:1290–1295. doi: 10.1111/j.1467-9280.2009.02439.x. [DOI] [PubMed] [Google Scholar]
- Finkel EJ, Eastwick PW. Speed-dating. Curr Dir Psychol Sci. 2008;17:193–197. doi: 10.1111/j.1467-8721.2008.00573.x. [DOI] [Google Scholar]
- Fisher HE. Lust, attraction, and attachment in mammalian reproduction. Hum Nat. 1998;9:23–52. doi: 10.1007/s12110-998-1010-5. [DOI] [PubMed] [Google Scholar]
- Fisher HE, Brown LL, Aron A, Strong G, Mashek D. Reward, addiction, and emotional regulation systems associated with rejection in love. J Neurophysiol. 2010;104:51–60. doi: 10.1152/jn.00784.2009. [DOI] [PubMed] [Google Scholar]
- Frith CD. The social brain? Philos Trans R Soc Lond. 2007;362:671–678. doi: 10.1098/rstb.2006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- Golightly C, Byrne D. Attitude statements as positive and negative reinforcements. Science. 1964;146:798–799. doi: 10.1126/science.146.3645.798. [DOI] [PubMed] [Google Scholar]
- Güroglu B, Haselager GJ, van Lieshout CF, Takashima A, Rijpkema M, Fernández G. Why are friends special? Implementing a social interaction simulation task to probe the neural correlates of friendship. Neuromage. 2008;39:903–910. doi: 10.1016/j.neuroimage.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Hampton AN, Bossaerts P, O'Doherty JP. Neural correlates of mentalizing-related computations during strategic interactions in humans. Proc Natl Acad Sci. 2008;105:6741–6746. doi: 10.1073/pnas.0711099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Parikh PC, Deaner RO, Platt ML. Economic principles motivating social attention in humans. Proc Biol Sci. 2007;274:1751–1756. doi: 10.1098/rspb.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN. Efficient experimental design for FMRI. In: Friston KJ, Ashburner JT, Kiebel S, Nichols T, Penny WD, editors. Statistical parametric mapping: the analysis of functional brain images. London: Elsevier; 2007. pp. 193–210. [Google Scholar]
- Kenny DA. Interpersonal perception: a social relations analysis. New York: Guilford Press; 1994. [PubMed] [Google Scholar]
- Kenny DA, West TV, Malloy TE, Albright L. Componential analysis of interpersonal perception data. Pers Soc Psychol Rev. 2006;10:282–294. doi: 10.1207/s15327957pspr1004_1. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, TU PC, Buckner RL. Clan mentality: evidence that the medial prefrontal cortex responds to close others. J Neurosci. 2010;30:13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci. 2011;108:6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzban R, Weeden J. HurryDate: mate preferences in action. Evol Hum Behav. 2005;26:227–244. doi: 10.1016/j.evolhumbehav.2004.08.012. [DOI] [Google Scholar]
- McClure SM, York MK, Montague PR. The neural substrates of reward processing in humans: the modern role of fMRI. Neuroscientist. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji M. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Montague PR, King-Casas B, Cohen JD. Imaging valuation models in human choice. Ann Rev Neurosci. 2006;29:417–448. doi: 10.1146/annurev.neuro.29.051605.112903. [DOI] [PubMed] [Google Scholar]
- Myers DG, Diener E. Who is happy? Psychol Sci. 1995;6:10–19. doi: 10.1111/j.1467-9280.1995.tb00298.x. [DOI] [Google Scholar]
- Newcomb TM. The prediction of interpersonal attraction. Am Psychol. 1956;11:575–586. doi: 10.1037/h0046141. [DOI] [Google Scholar]
- O'Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/S0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Plassmann H, O'Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci. 2007;27:9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Dodell-Feder D, Pearrow MJ, Mavros PL, Kleiner M, Gabrieli JDE, Saxe R. Live face-to-face interaction during fMRI: A new tool for social cognitive neuroscience. Neuroimage. 2010;50:1639–1647. doi: 10.1016/j.neuroimage.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: current research and theory. New York: Appleton Century Crofts; 1972. pp. 65–99. [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Turner J, Foa E, Foa U. Interpersonal reinforcers: classification, interrelationship, and some differential properties. J Pers Soc Psychol. 1971;19:168–180. doi: 10.1037/h0031278. [DOI] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant voxels in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Young L, Saxe R. The neural basis of belief encoding and integration in moral judgment. Neuroimage. 2008;40:1912–1920. doi: 10.1016/j.neuroimage.2008.01.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.