Abstract
Objective
We investigated temporal changes in overall survival among prostate cancer (PC) patients and the impact of comorbidity on all-cause mortality.
Methods
We conducted a population-based cohort study in the Central Denmark Region (1.2 million inhabitants). Using medical registries, we identified 7,654 PC patients with first-time PC diagnosis within the period 2000–2011 and their corresponding comorbidities within 10 years prior to the PC diagnosis. We estimated 1- and 5-year survival in four consecutive calendar periods using a hybrid analysis and plotted Kaplan–Meier survival curves. We used Cox proportional hazards regression to compute 1- and 5-year age-adjusted mortality rate ratios (MRRs) for different comorbidity levels. All estimates are reported with their corresponding 95% confidence intervals (CI).
Results
The annual number of PC cases doubled over the 12-year study period. Men aged <70 years accounted for the largest proportional increase (from 33% to 47%). The proportion of patients within each comorbidity category remained constant over time. One-year survival increased from 82% (CI: 80%–84%) in 2000–2002 to 92% (CI: 90%–93%) in 2009–2011, while 5-year survival increased from 43% (CI: 40%–46%) to 65% (CI: 62%–67%) during the same time intervals. Improvements in 5-year survival were most prominent among patients aged <80 years and among those with no comorbidity (from 51% to 73%) and medium comorbidity (from 32% to 54%). Improvements in survival were much smaller for those with high comorbidity (from 33% to 39%). The 1-year age-adjusted MRR for patients with high comorbidity (relative to patients with no comorbidity) increased over time from 1.84 (CI: 1.19–2.84) to 3.67 (CI: 2.49–5.41), while the 5-year age-adjusted MRR increased from 1.73 (CI: 1.34–2.23) to 2.38 (CI: 1.93–2.94).
Conclusion
Overall survival of PC improved substantially during 2000–2011, although primarily among men with low comorbidity. All-cause mortality was highest among PC patients with high comorbidity, and their relative 1- and 5-year mortality increased over time compared to those without comorbidity.
Keywords: prostate cancer, comorbidities, Charlson Index, survival
Introduction
Prostate cancer (PC) is a leading contributor to cancer prevalence and incidence among men worldwide. Consistent with global trends, the incidence of PC in Denmark continues to increase due to population aging and improvements in diagnostics.1,2 There has been a 53% increase in PC incidence during the period 2001–2010.2 In 2010, there were 4,060 new PC cases diagnosed in Denmark, which corresponds to an age-standardized incidence of 142 per 100,000 Danish men per year.2 Concurrent with the substantially increasing incidence, the 5-year survival of PC in Denmark has improved steadily over the past 2 decades from approximately 34% to 60%,1 and survival proportions for Denmark are now approaching those seen in other Nordic countries with similar demographics (eg, Norway, 78% and Sweden, 81%).3
The management of PC is often complicated by other age-related preexisting diseases or comorbidities such as cardiovascular disease, cerebrovascular disease, diabetes, and other primary cancers.4,5 It has therefore been projected that the majority of PC patients will die of other causes than the PC itself.6–12 Lund et al investigated comorbidity and survival among Danish PC patients from 1995–2006 and found that patients with a high comorbidity burden (corresponding to a Charlson Comorbidity Index [CCI] score ≥3) had more than a three-fold higher 1-year mortality than patients with no recorded comorbidity.13 Moreover, Lund et al showed that there were no temporal improvements in PC survival among those with high comorbidity, but reported improvements in survival among PC patients with low comorbidity.13
The aim of this study was to examine the impact of comorbidity on overall PC survival in the 12-year study period from 2000–2011. We investigated recent temporal changes in overall PC survival, divided into four calendar periods, and the impact of comorbidity on all-cause mortality within these periods.
Materials and methods
Study population
We conducted a population-based cohort study in the Central Denmark Region (1.2 million inhabitants). The Danish National Health Service provides universal, tax-financed health care with equal and free access to general practitioners and hospitals and partial reimbursement for prescribed medications. Accurate and unambiguous linkage of all registries at the individual level is possible in Denmark by means of the unique personal identification number assigned to each Danish resident upon birth or immigration.
Identification of prostate cancer patients
Using the Danish National Registry of Patients (DNRP), we identified all patients with a first-time diagnosis of prostate cancer (ie, registered with International Classification of Diseases 10th edition [ICD-10] code C61 “Malignant neoplasm of prostate”) within the period January 1, 2000 to December 31, 2011. The DNRP contains information on all hospital discharges in Denmark since 1977 and all emergency room and outpatient contacts since 1995.14,15 Each hospital discharge or outpatient contact is recorded in the DNRP with one primary diagnosis and one or more secondary diagnoses. Diagnoses have been classified according to the ICD-10 since 1994 (prior to 1994, the ICD-8 was used). The registration of incident PC in the DNRP has been shown to be valid with a registration completeness of 95% and a positive predictive value of 87%.16
Identification of comorbidities
For each patient included, we queried medical histories on comorbidity from the DNRP, and computed CCI scores17 based on all comorbidity diagnoses registered within 10 years preceding the date of first-time PC diagnosis (ie, index date). The CCI has been adapted and validated for use with DNRP hospital discharge data for the prediction of short- and long-term mortality18 and it has been shown to be highly applicable for outcome studies on prostate cancer.12,19,20 The following disease categories are included in the CCI: liver diseases; myocardial infarction; congestive heart failure; peripheral vascular disease; chronic pulmonary disease; cerebrovascular disease; hemiplegia; dementia; connective tissue disease; ulcer disease; type 1 and 2 diabetes; renal disease; hematological and solid tumor cancers; and HIV/AIDS. Any cancer registration occurring up to 60 days before the PC index date was excluded from the CCI scoring (in order to eliminate unspecific cancer registrations, which are likely to be related to the incident PC diagnosis). The diagnosis of PC itself was also excluded from the CCI; however, all other cancers diagnosed up to 10 years before the index date were included in the calculation of the CCI score. We categorized the comorbidity burden as none (CCI score = 0), medium (CCI score = 1–2), or high (CCI score ≥3).
Identification of vital status
We linked data to the Danish Civil Registration System (CRS)21 to obtain data on age and vital status. The CRS, which is electronically updated daily, has recorded vital status and migration status (amongst other variables) for the entire Danish population since 1968. Study subjects were followed until date of death, emigration, or end of follow-up (December 31, 2011), whichever occurred first.
Statistical analysis
We examined the distribution of comorbidity levels among PC patients during four consecutive calendar periods (2000–2002; 2003–2005; 2006–2008; 2009–2011). For each comorbidity level, we plotted Kaplan–Meier survival curves by calendar period. We used Cox proportional hazards regression to compute 1- and 5-year hazard ratios as a measure of relative mortality (ie, mortality rate ratio [MRR]) for each calendar period, adjusting for changes in age and comorbidity level. A similar Cox regression model was used to assess the association of comorbidity with all-cause mortality, using the CCI score of 0 as the reference category in each calendar period. In the latter periods, we predicted 1- and 5-year survival using a hybrid analysis in which survival was estimated using the survival experience of patients in the previous periods.22 This hybrid analysis, which combines traditional and period analyses, has the advantage of providing more up-to-date estimates of long-term survival.22 The proportional hazards assumption was assessed graphically and found to be appropriate. All estimates are reported with their corresponding 95% confidence intervals (CI). All analyses were performed using SAS statistical software (version 9.2; SAS Institute Inc, Cary, NC, USA).
Results
Descriptive data
We identified 7,654 men diagnosed with first-time PC between 2000 and 2011, and residing within the Central Denmark Region. The annual number of PC cases doubled over the 12-year study period from 1,219 new PC cases in 2000–2002 to 2,440 new cases in 2009–2011 (Table 1). This increase was most pronounced among patients <70 years old, constituting 33% (400/1,219) of new cases in 2000–2002 compared with 47% (1,155/2,440) of new cases in 2009–2011. Conversely, the annual number of first-time PC diagnoses among patients ≥80 years old remained unchanged over the 12-year study period. Consequently, the median age at time of PC diagnosis decreased from 74 years in 2000–2002 to 71 years in 2009–2011.
Table 1.
Distribution and median age of prostate cancer by calendar period of diagnosis, age group, and comorbidity level (CCI)
| Total period
|
Calendar period of prostate cancer diagnosis
|
||||
|---|---|---|---|---|---|
| 2000–2011 | 2000–2002 | 2003–2005 | 2006–2008 | 2009–2011 | |
| Total | 7,654 | 1,219 | 1,679 | 2,316 | 2,440 |
| Median age (years) | 72 | 74 | 73 | 71 | 71 |
| Age group | |||||
| 15–69 years | 3,254 (43) | 400 (33) | 644 (38) | 1,055 (46) | 1,155 (47) |
| 70–79 years | 2,993 (39) | 515 (42) | 709 (42) | 856 (37) | 913 (37) |
| ≥80 years | 1,407 (18) | 304 (25) | 326 (19) | 405 (18) | 372 (15) |
| Comorbidity level | |||||
| CCI = 0 (none) | 4,843 (63) | 726 (60) | 1,030 (61) | 1,505 (65) | 1,582 (65) |
| CCI = 1–2 (medium) | 2,194 (29) | 387 (32) | 504 (30) | 638 (28) | 665 (27) |
| CCI ≥ 3 (high) | 617 (8) | 106 (9) | 145 (9) | 173 (7) | 193 (8) |
| Median age (years) by CCI | |||||
| CCI = 0 (none) | 70 | 73 | 71 | 69 | 69 |
| CCI = 1–2 (medium) | 74 | 76 | 75 | 74 | 73 |
| CCI ≥ 3 (high) | 76 | 75 | 77 | 75 | 76 |
Note: Data are presented as n (%), except where indicated otherwise.
Abbreviation: CCI, Charlson Comorbidity Index.
Among all PC patients included in this study, 63% had no recorded comorbidity (ie, CCI score 0); 29% were categorized as comorbidity level “medium” (ie, CCI score 1–2); and 8% were categorized as comorbidity level “high” (ie, CCI ≥ 3). Despite an increasing proportion of younger men diagnosed with PC, the distribution of comorbidity level and individual comorbidities over the four calendar intervals remained relatively unchanged (Tables 1 and 2). The median age for CCI levels none, medium, and high was 70, 74, and 76 years old, respectively. Cerebrovascular disease (9.4%), chronic pulmonary disease (7.6%), previous cancer other than PC (6.2%), and prior myocardial infarction (6.2%) were the most prevalent comorbid diseases among all PC patients (Table 2).
Table 2.
Proportion of prostate cancer cases by individual Charlson comorbidities
| Charlson comorbidity diseases | Total period
|
Calendar period of registration of comorbidity diseases (%)
|
|||
|---|---|---|---|---|---|
| 2000–2011 | 2000–2002 | 2003–2005 | 2006–2008 | 2009–2011 | |
| Cerebrovascular disease | 717 (9.4) | 106 (8.7) | 165 (9.8) | 232 (10.0) | 214 (8.8) |
| Chronic pulmonary disease | 578 (7.6) | 126 (10.3) | 149 (8.9) | 151 (6.5) | 152 (6.2) |
| Any tumor | 472 (6.2) | 83 (6.8) | 114 (6.8) | 129 (5.6) | 146 (6.0) |
| Myocardial infarction | 472 (6.2) | 73 (6.0) | 108 (6.4) | 131 (5.7) | 160 (6.6) |
| Peripheral vascular disease | 406 (5.3) | 81 (6.6) | 84 (5.0) | 133 (5.7) | 108 (4.4) |
| Congestive heart failure | 378 (4.9) | 82 (6.7) | 95 (5.7) | 106 (4.6) | 95 (3.9) |
| Ulcer disease | 267 (3.5) | 52 (4.3) | 79 (4.7) | 68 (2.9) | 68 (2.8) |
| Diabetes (type I and II) | 251 (3.3) | 47 (3.9) | 55 (3.3) | 71 (3.1) | 78 (3.2) |
| Moderate-to-severe renal disease | 203 (2.7) | 32 (2.6) | 59 (3.5) | 41 (1.8) | 71 (2.9) |
| Diabetes with end organ disease | 179 (2.3) | 41 (3.4) | 31 (1.8) | 44 (1.9) | 63 (2.6) |
| Connective tissue disease | 182 (2.4) | 26 (2.1) | 38 (2.3) | 57 (2.5) | 61 (2.5) |
| Metastatic solid tumor | 51 (0.7) | 3 (0.2) | 9 (0.5) | 15 (0.6) | 24 (1.0) |
| Dementia | 46 (0.6) | 9 (0.7) | 9 (0.5) | 15 (0.6) | 13 (0.5) |
| Mild liver disease | 31 (0.4) | 5 (0.4) | 7 (0.4) | 16 (0.7) | 3 (0.1) |
| Lymphoma | 30 (0.4) | 6 (0.5) | 10 (0.6) | 7 (0.3) | 7 (0.3) |
| Leukemia | 11 (0.1) | 1 (0.1) | 5 (0.3) | 2 (0.1) | 3 (0.1) |
| Hemiplegia | 9 (0.1) | 0 | 1 (0.1) | 2 (0.1) | 6 (0.2) |
| Moderate-to-severe liver disease | 8 (0.1) | 1 (0.1) | 2 (0.1) | 3 (0.1) | 2 (0.1) |
| HIV/AIDS | 2 (0.0) | 0 | 0 | 0 | 2 (0.1) |
Note: Data are presented as n (%).
1-year and 5-year survival
Table 3 summarizes the 1-year and 5-year survival by calendar period of PC diagnosis, age group, and comorbidity level. The 1-year and 5-year survival of PC patients improved over time, with the most prominent advances occurring during the period 2000–2008. The 1-year survival increased from 82% (CI: 80%–84%) in 2000–2002 to 89% (CI: 87%–90%) in 2003–2005, and thereafter remained virtually unchanged at 92% (CI: 90%–93%) between 2006 and 2011. The 5-year survival improved from 43% (CI: 40%–46%) in 2000–2002 to 57% (CI: 55%–59%) in 2003–2005, then increased to a predicted 65% (CI: 62%–67%) and stayed at this level for the remainder of the study period (2006–2011). When stratified by age, the 5-year survival particularly improved for those aged <70 years, with a steady and large increase from 58% (CI: 53%–62%) in 2000–2002 to a predicted 80% (CI: 77%–82%) in 2009–2011. In contrast, those aged ≥80 years showed only a modest improvement in 5-year survival from 20% (CI: 16%–25%) to a predicted 28% (CI: 24%–32%) during the same calendar periods.
Table 3.
1-year and 5-year survival and MRRs, overall, by age group, comorbidity level (CCI), and calendar period of prostate cancer diagnosis
| Calendar period of prostate cancer diagnosis
|
||||
|---|---|---|---|---|
| 2000–2002 | 2003–2005 | 2006–2008 | 2009–2011 | |
| Overall estimates | ||||
| Overall 1-year estimates | ||||
| Survival % | 82 (80–84) | 89 (87–90) | 92 (91–93) | 92 (90–93) |
| Crude MRR | 1 (reference) | 0.61 (0.50–0.74) | 0.43 (0.35–0.52) | 0.44 (0.36–0.54) |
| Adjusted MRRa | 1 (reference) | 0.66 (0.54–0.80) | 0.50 (0.41–0.60) | 0.54 (0.44–0.65) |
| Overall 5-year estimates | ||||
| Survival % | 43 (40–46) | 57 (55–59) | 65 (62–66)* | 65 (63–67)* |
| Crude MRR | 1 (reference) | 0.67 (0.60–0.74) | 0.51 (0.46–0.57)* | 0.49 (0.45–0.55)* |
| Adjusted MRRa | 1 (reference) | 0.71 (0.64–0.79) | 0.57 (0.51–0.63)* | 0.56 (0.51–0.62)* |
| Age group | ||||
| 15–69 years | ||||
| 1-year survival | 90 (86–92) | 95 (93–96) | 97 (96–98) | 97 (96–98) |
| 5-year survival | 58 (53–62) | 75 (72–78) | 79 (76–81)* | 80 (77–82)* |
| 70–79 years | ||||
| 1-year survival | 84 (81–87) | 90 (88–92) | 91 (89–93) | 93 (91–94) |
| 5-year survival | 45 (41–50) | 55 (51–59) | 63 (60–67)* | 66 (62–69)* |
| ≥80 years | ||||
| 1-year survival | 67 (62–72) | 74 (69–78) | 80 (76–83) | 72 (67–77) |
| 5-year survival | 20 (16–25) | 26 (21–31) | 30 (26–35)* | 28 (24–32)* |
| Comorbidity level | ||||
| CCI = 0 (none) | ||||
| 1-year survival | 86 (84–89) | 93 (91–94) | 95 (93–96) | 95 (94–96) |
| 1-year crude MRR | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 1-year adjusted MRRb | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 5-year survival | 51 (47–54) | 66 (63–68) | 72 (69–74)* | 73 (71–76)* |
| 5-year crude MRR | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 5-year adjusted MRRb | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| CCI = 1–2 (medium) | ||||
| 1-year survival | 75 (71–79) | 85 (82–88) | 88 (85–91) | 88 (85–90) |
| 1-year crude MRR | 1.95 (1.47–2.58) | 2.23 (1.61–3.08) | 2.34 (1.71–3.21) | 2.65 (1.90–3.70) |
| 1-year adjusted MRRb | 1.77 (1.34–2.36) | 1.80 (1.29–2.51) | 1.76 (1.27–2.42) | 1.94 (1.38–2.73) |
| 5-year survival | 32 (27–36) | 47 (43–51) | 54 (49–57)* | 54 (50–58)* |
| 5-year crude MRR | 1.71 (1.46–2.00) | 1.81 (1.54–2.12) | 1.89 (1.62–2.20)* | 1.98 (1.71–2.30)* |
| 5-year adjusted MRRb | 1.61 (1.38–1.90) | 1.51 (1.29–1.78) | 1.50 (1.29–1.76)* | 1.56 (1.34–1.82)* |
| CCI ≥ 3 (high) | ||||
| 1-year survival | 75 (66–83) | 69 (61–76) | 81 (74–86) | 74 (67–80) |
| 1-year crude MRR | 1.95 (1.27–3.00) | 5.35 (3.68–7.78) | 3.94 (2.62–5.91) | 6.03 (4.13–8.80) |
| 1-year adjusted MRRb | 1.84 (1.19–2.84) | 3.68 (2.50–5.41) | 2.68 (1.77–4.06) | 3.67 (2.49–5.41) |
| 5-year survival | 33 (24–42) | 30 (23–38) | 42 (34–49)* | 39 (32–46)* |
| 5-year crude MRR | 1.80 (1.39–2.32) | 3.13 (2.51–3.91) | 2.72 (2.18–3.40)* | 3.34 (2.71–4.11)* |
| 5-year adjusted MRRb | 1.73 (1.34–2.23) | 2.25 (1.79–2.82) | 2.02 (1.61–2.53)* | 2.38 (1.93–2.94)* |
Notes: Data are presented as n (95% CI).
Age- and comorbidity-adjusted MRR;
age-adjusted MRR;
predicted values. The 1- and 5-year survival estimates were predicted using a hybrid analysis.25
Abbreviations: CCI, Charlson Comorbidity Index; CI, confidence interval; MRR, mortality rate ratio.
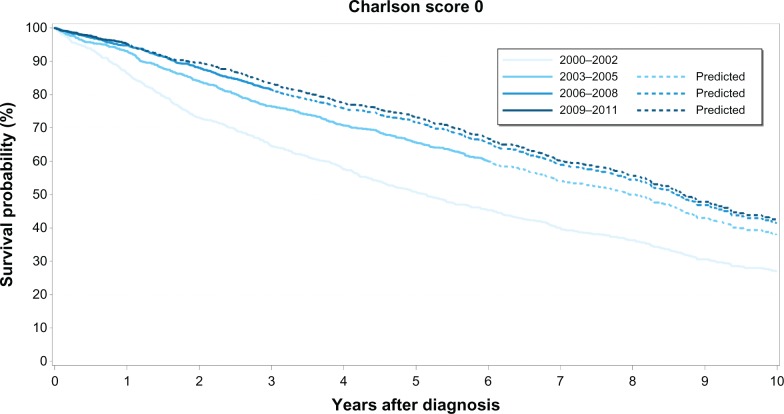
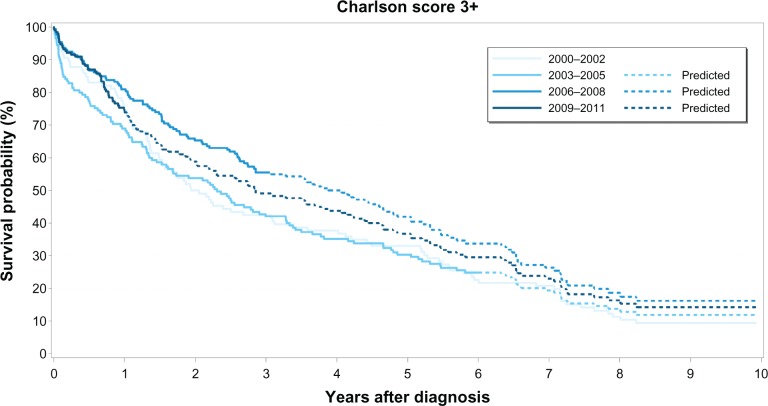
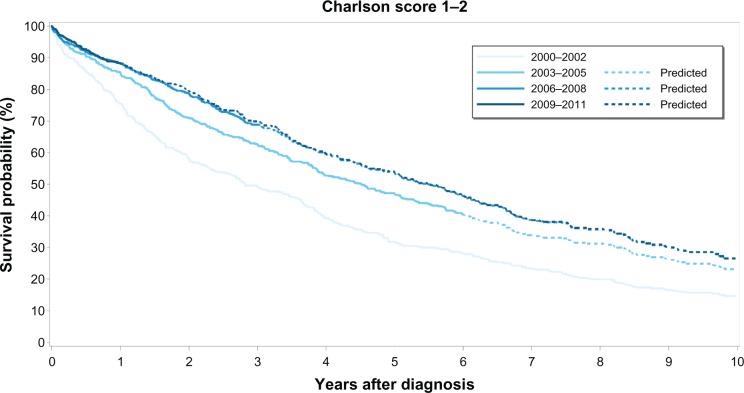
The overall median survival was 8 years for patients with no recorded comorbidity, 5 years for patients with medium comorbidity, and 3 years for patients with high comorbidity. Survival curves for each comorbidity level over time clearly show that PC survival improved in the latter half of our study period (ie, 2006–2011) compared to survival during the first 6 years (Figures 1–3). However, higher comorbidity level was consistently associated with relatively lower survival in all four calendar periods (Table 3). Among those with no comorbidity, 1-year and 5-year survival steadily increased over time from 86% to 95% and 51% to 73%, respectively. Likewise, for those with medium comorbidity, 1-year and 5-year survival improved substantially from 75% to 88%, and 32% to 54%, respectively. In contrast, for patients with high comorbidity, 1-year survival fluctuated between 69%–81%, whereas 5-year survival fluctuated between 30%–42% over the four calendar periods, with less improvement in survival over time compared with the other comorbidity groups.
Figure 1.
Kaplan–Meier survival curves for prostate cancer patients with no comorbidity (Charlson Comorbidity Index score 0) by calendar period of prostate cancer diagnosis.
Figure 3.
Kaplan–Meier survival curves for prostate cancer patients with high comorbidity (Charlson Comorbidity Index score ≥3) by calendar period of prostate cancer diagnosis.
The age-adjusted MRR increased with increasing comorbidity level (Table 3). Among PC patients with high comorbidity, the 1-year age-adjusted MRR increased from 1.84 (CI: 1.19–2.84) in 2000–2002 to 3.67 (CI: 2.49–5.41) in 2009–2011, relative to patients with no comorbidity. Likewise, the 5-year age-adjusted MRR increased from 1.73 (CI: 1.34–2.23) in the first period (2000–2002) to 2.38 (CI: 1.93–2.94) in the latest period (2009–2011) for PC patients with high comorbidity relative to those without comorbidity.
Discussion
Our population-based cohort study shows that survival among PC patients steadily improved over the 12-year study period, overall, and for all comorbidity levels. However, survival improvement was least prominent among those with high comorbidity. PC patients with high comorbidity continue to have a severe prognosis, and their risk of death (1-year and 5-year all-cause mortality) increased over time relative to those with no comorbidity.
There are three key findings in this study. First, although 5-year survival remains lowest among PC patients with high comorbidity, there was a modest but noteworthy improvement in their survival over time. Lund et al13 found that the 5-year survival in this patient group modestly decreased from 21% to 19% between 1995 and 2000, whereas the 5-year survival in our study period increased from approximately 30% in 2000–2005 to approximately 40% in 2006–2011. Second, we found a similar prevalence of comorbidity over the four calendar periods despite decreasing median age at PC diagnosis, which may, for example, suggest changes in the registration/reporting practices of comorbidities during the study period. Changes over time towards more complete comorbidity registration could have positively affected survival among those recorded with high comorbidity. Third, those <70 years old had the greatest improvement in 5-year survival during the 12-year study period, whereas the 5-year survival for those >80 years old increased only marginally in the same period. However, we observed a nearly three-fold increase in the annual number of men <70 years old who were diagnosed with PC, while the number of men >80 years old remained constant. These changes are likely explained by the increasingly widespread, albeit unsystematic, use of prostate-specific antigen (PSA) testing in Denmark among younger patients.23 It is therefore likely that our results are affected by lead- and length-time bias. If the increasing number of men diagnosed with PC under the age of 70 years old is predominantly due to higher detection of less aggressive tumors with better prognosis, then length-time bias would explain, at least in part, the improved survival seen within this patient group. It would have been ideal to include data on clinical cancer staging and grading to address this issue further. Also, during our study period, an increasing number of younger PC patients with no/low comorbidity were offered curative treatment in the Central Denmark Region (M Borre, personal communication, Aarhus University Hospital, January 3, 2013), which is consistent with Swedish practices described by Berglund et al.24 In this light, PC patients with moderate-to-high comorbidity may have, gradually over time, been treated more conservatively compared to patients with no comorbidity.
Our large-scale investigation extends the findings of previous smaller studies that the level of comorbidity burden is a negative prognostic factor among PC patients.7–13,24,25 Daskivich et al studied 1,482 nonmetastatic PC men using data from the Long Beach Veterans Affairs Medical Centers.25 They showed that for each CCI point increase there was a 2-fold increase in non-PC mortality.25 Tewari et al used PC diagnosis data from 1990–1997 from the Henry Ford Medical Group cancer registry (n = 1,611) and showed that a CCI score ≥2 was associated with increased risk of all-cause mortality (2.63 risk ratio), PC-specific mortality (1.43 risk ratio), and non-PC-specific mortality (3.03 risk ratio).10 Fowler et al showed similar findings for CCI ≥ 1 and all-cause mortality using data (n = 276) from a Veterans Affairs medical center in Mississippi, covering the period 1980–1991.26 Finally, Berglund et al used data from the National Prostate Cancer Register of Sweden and found that comorbidity was associated with all-cause, competing cause, and conditional PC-specific mortality.24
Several studies have proposed other influencing mechanisms on PC outcome.9,26–29 For example, PC and cardiovascular disease share common risk factors (eg, age, race, and family history), and biological interaction or shared etiologies may cause increased severity of both conditions.30 Also, comorbidity may be associated with delayed cancer diagnosis because comorbidities can mask early cancer symptoms. It has also been shown that older age and high comorbidity are associated with more conservative cancer treatments or even complete opting-out of treatments by both physicians and patients.9,26
The strengths of our study are its population-based approach, high-quality data from comprehensive medical registries, and the large sample size, which yields high precision in our estimates. Also, our registry-based approach to identifying PC patients minimized loss to follow-up. Unlike previous studies with limited and/or variable tracking periods,7,11 we were able to base our CCI scoring on medical histories up to 10 years prior to the incident PC diagnosis. However, as was mentioned earlier, our study is inherently limited by lead- and length-time bias (as a result of widespread PSA testing and the natural history of PC), and the magnitude of such bias on our observed improvements in survival is unknown. Other limitations include lack of data on clinical staging, Gleason scores, PSA values in accordance with the D’Amico risk stratification model for PC,31 and potential confounders such as smoking and obesity. Finally, we lacked data on cause of death. Since PC-specific mortality constitutes a relatively small proportion of the all-cause mortality,6,32 the changes in overall survival observed in this study most likely reflect changes in mortality from other, non-PC-specific causes of death. Unfortunately, since this study did not differentiate between non-PC mortality and PC-specific mortality, we could not determine which cause of mortality is driving these temporal changes. However, determining one sole underlying cause of death is complex in patients with several coexisting diseases, and the high prevalence of multimorbidity among prostate cancer patients creates major challenges in accurately identifying cause of death.
Conclusion
Overall survival has improved substantially over the past 12 years for Danish PC patients, including modest survival improvements among those with high comorbidity. Yet, despite these advances, the 1-year and 5-year risk of death remains 2- to 4-fold higher for PC patients with high comorbidity relative to those with no comorbidity.
Figure 2.
Kaplan–Meier survival curves for prostate cancer patients with medium comorbidity (Charlson Comorbidity Index score 1–2) by calendar period of prostate cancer diagnosis.
Acknowledgments
Cancer analyses in the Central Denmark Region were conducted as part of the Aarhus University Disease Epidemiology and Outcomes (AUDEO) Project at the Department of Clinical Epidemiology, Aarhus University Hospital.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Borre M, Erichsen R, Lund L, Larsen EH, Nørgaard M, Jacobsen JB. Survival of prostate cancer patients in central and northern Denmark, 1998–2009. Clin Epidemiol. 2011;3(Suppl 1):41–46. doi: 10.2147/CLEP.S20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancerregisteret 2010 [Cancer Registry 2010] [webpage on the Internet] Copenhagen: Sundhedsstyrelsen; 2011Available from: http://www.sst.dk/Udgivelser/2011/Cancerregisteret2010.aspxAccessed October 1, 2012Danish [Google Scholar]
- 3.Bray F, Klint A, Gislum M, et al. Trends in survival of patients diagnosed with male genital cancers in the Nordic countries 1964–2003 followed up until the end of 2006. Acta Oncol. 2010;49(5):644–654. doi: 10.3109/02841860903575315. [DOI] [PubMed] [Google Scholar]
- 4.Read WL, Tierney RM, Page NC, et al. Differential prognostic impact of comorbidity. J Clin Oncol. 2004;22(15):3099–3103. doi: 10.1200/JCO.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 5.Jespersen CG, Nørgaard M, Bjerklund Johansen TE, Søgaard M, Borre M. The influence of cardiovascular morbidity on the prognosis in prostate cancer. Experience from a 12-year nationwide Danish population-based cohort study. BMC Cancer. 2011;11:519. doi: 10.1186/1471-2407-11-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epstein MM, Edgren G, Rider JR, Mucci LA, Adami HO. Temporal trends in cause of death among Swedish and US men with prostate cancer. J Natl Cancer Inst. 2012;104(17):1335–1342. doi: 10.1093/jnci/djs299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29(10):1335–1341. doi: 10.1200/JCO.2010.31.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzpatrick JM. Management of localized prostate cancer in senior adults: the crucial role of comorbidity. BJU Int. 2008;101(Suppl 2):16–22. doi: 10.1111/j.1464-410X.2007.07487.x. [DOI] [PubMed] [Google Scholar]
- 9.Alibhai SM, Leach M, Tomlinson G, et al. 30-day mortality and major complications after radical prostatectomy: influence of age and comorbidity. J Natl Cancer Inst. 2005;97(20):1525–1532. doi: 10.1093/jnci/dji313. [DOI] [PubMed] [Google Scholar]
- 10.Tewari A, Johnson CC, Divine G, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol. 2004;171(4):1513–1519. doi: 10.1097/01.ju.0000117975.40782.95. [DOI] [PubMed] [Google Scholar]
- 11.Groome PA, Rohland SL, Siemens DR, Brundage MD, Heaton J, Mackillop WJ. Assessing the impact of comorbid illnesses on death within 10 years in prostate cancer treatment candidates. Cancer. 2011;117(17):3943–3952. doi: 10.1002/cncr.25984. [DOI] [PubMed] [Google Scholar]
- 12.Albertsen PC, Fryback DG, Storer BE, Kolon TF, Fine J. The impact of co-morbidity on life expectancy among men with localized prostate cancer. J Urol. 1996;156(1):127–132. [PubMed] [Google Scholar]
- 13.Lund L, Borre M, Jacobsen J, Sørensen HT, Nørgaard M. Impact of comorbidity on survival of Danish prostate cancer patients, 1995–2006: a population-based cohort study. Urology. 2008;72(6):1258–1262. doi: 10.1016/j.urology.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(Suppl 7):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 15.Sørensen HT, Christensen T, Kjeldahl Schlosser H, Pedersen L. Use of Medical Databases in Clinical Epidemiology. First Edition. Aarhus, Denmark: Aarhus University Press; 2009. p. 121. [Google Scholar]
- 16.Gammelager H, Christiansen CF, Johansen MB, Borre M, Schoonen M, Sørensen HT. Quality of urological cancer diagnoses in the Danish National Registry of Patients. Eur J Cancer Prev. 2012;21(6):545–551. doi: 10.1097/CEJ.0b013e328351c680. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall WH, Jani AB, Ryu JK, Narayan S, Vijayakumar S. The impact of age and comorbidity on survival outcomes and treatment patterns in prostate cancer. Prostate Cancer Prostatic Dis. 2005;8(1):22–30. doi: 10.1038/sj.pcan.4500772. [DOI] [PubMed] [Google Scholar]
- 20.Sarfati D. Review of methods used to measure comorbidity in cancer populations: no gold standard exists. J Clin Epidemiol. 2012;65(9):924–933. doi: 10.1016/j.jclinepi.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(Suppl 7):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 22.Brenner H, Rachet B. Hybrid analysis for up-to-date long-term survival rates in cancer registries with delayed recording of incident cases. Eur J Cancer. 2004;40(16):2494–2501. doi: 10.1016/j.ejca.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Dansk Urologisk Cancer Gruppe [webpage on the Internet] Available from: www.ducg.dkAccessed August 3, 2012
- 24.Berglund A, Garmo H, Tishelman C, Holmberg L, Stattin P, Lambe M. Comorbidity, treatment and mortality: a population based cohort study of prostate cancer in PCBaSe Sweden. J Urol. 2011;185(3):833–839. doi: 10.1016/j.juro.2010.10.061. [DOI] [PubMed] [Google Scholar]
- 25.Daskivich TJ, Chamie K, Kwan L, et al. Comorbidity and competing risks for mortality in men with prostate cancer. Cancer. 2011;117(20):4642–4650. doi: 10.1002/cncr.26104. [DOI] [PubMed] [Google Scholar]
- 26.Fowler JE, Jr, Terrell FL, Renfroe DL. Co-morbidities and survival of men with localized prostate cancer treated with surgery or radiation therapy. J Urol. 1996;156(5):1714–1718. [PubMed] [Google Scholar]
- 27.Ketchandji M, Kuo YF, Shahinian VB, Goodwin JS. Cause of death in older men after the diagnosis of prostate cancer. J Am Geriatr Soc. 2009;57(1):24–30. doi: 10.1111/j.1532-5415.2008.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riihimäki M, Thomsen H, Brandt A, Sundquist J, Hemminki K. What do prostate cancer patients die of? Oncologist. 2011;16(2):175–181. doi: 10.1634/theoncologist.2010-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newschaffer CJ, Otani K, McDonald MK, Penberthy LT. Causes of death in elderly prostate cancer patients and in a comparison nonprostate cancer cohort. J Natl Cancer Inst. 2000;92(8):613–621. doi: 10.1093/jnci/92.8.613. [DOI] [PubMed] [Google Scholar]
- 30.Thomas JA, 2nd, Gerber L, Bañez LL, et al. Prostate cancer risk in men with baseline history of coronary artery disease: results from the REDUCE Study. Cancer Epidemiol Biomarkers Prev. 2012;21(4):576–581. doi: 10.1158/1055-9965.EPI-11-1017. [DOI] [PubMed] [Google Scholar]
- 31.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 32.Daskivich TJ, Fan KH, Koyama T, et al. Effect of age, tumor risk, and comorbidity on competing risks for survival in a u.s. Population-based cohort of men with prostate cancer. Ann Intern Med. 2013;158(10):709–717. doi: 10.7326/0003-4819-158-10-201305210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]