Abstract
Despite the dramatic increase of global spending on drug discovery and development, the approval rate for new drugs is declining, due chiefly to toxicity and undesirable side effects. Simultaneously, the growth of available biomedical data in the post-genomic era has provided fresh insight into the nature of redundant and compensatory drug-target pathways. This stagnation in drug approval can be overcome by the novel concept of polypharmacology, which is built on the fundamental concept that drugs modulate multiple targets. Polypharmacology can be studied with molecular networks which integrate multidisciplinary concepts including cheminformatics, bioinformatics, and systems biology. In silico techniques such as structure and ligand-based approaches can be employed to study molecular networks and reduce costs by predicting adverse drug reactions and toxicity in the early stage of drug development. By amalgamating strides in this informatics-driven era, designing polypharmacological drugs with molecular network technology exemplifies the next generation of therapeutics with less off-target properties and toxicity. In this review, we will first describe the challenges in drug discovery, and showcase successes using multi-target drugs toward diseases such as cancer and mood disorders. We will then focus on recent development of in silico polypharmacology predictions. Finally, our technologies in molecular network analysis will be presented.
Keywords: Molecular networks, polypharmacology, drug discovery, in silico prediction
The first problem: lack of new drugs
Despite the remarkable increase in spending on drug discovery and development across the globe, the rate of new drug approval has been declining. From 1992 onward, the increase in industry R&D expense has become increasingly disproportional to approval rates.1 In the five years from 2002 to 2006, there were 43% fewer new chemical-based drugs than the years of 1995 to 1999, despite more than a doubling of R&D spending.2 The number of drug candidates that become effective therapies has encountered stagnation over this past decade, with 30% of drugs failures in phase II and phase III due to either lack of efficacy or off-target side effects.3 It was reported that lack of efficacy accounted for approximately 30% of all failures, and safety (clinical safety and toxicology) accounted for a further 30%.3
Studies published in 2003 reported an average pre-tax cost of approximately $800 million to bring a new drug (i.e. a drug with a New Chemical Entity) to market; current estimates place the value from $900 million to $1.7 billion (USD).4–6 Expiring patents on the current cycle of marketed drugs combined with the decreased productivity leads to a startling fact; pharmaceutical companies will face, for the first time in a four decade period, a projected fall in revenue this year.7 The average success rate for big pharma companies from first-in-man to registration has been reported from 1991–2000 at approximately 11%, with oncology and central nervous system (CNS) disorder treatments show ~5% and ~8% success, respectively.3 This rate increases in the later stages of development with the vast majority of attrition occurring in clinical development (Phases IIb and III), where the cumulative investment to that point is the highest. Even more worrisome is the fact that compounds with novel mechanisms of action experience higher attrition than those with previously acknowledged mechanisms5 (defined as hitting a known therapeutic target or showing proof of concept in late clinical trials).3, 8
The second problem: disease complexity
Stalling clinical approval rates are not the only problem plaguing the pharmaceutical industry; another problem is that the industry is attacking diseases of increasing complexity beyond a single target with a single drug for a single disease. Increasing evidence from large scale phenotypic deletion and functional genomics studies have shown in several model organisms that many biological systems are quite resilient to a single attacker.9, 10 Molecular network analysis of drug-target interactions has shown that over 40% of these disease targets are involved in more than one disease.11 In a number of model organisms, under ideal conditions, many single gene-knockout studies, when considered individually, have little or no effect on phenotype, with approximately 19% of genes proving essential (embryonic lethal).12, 13 Of the potential druggable gene targets in mice,14 deletion of each gene and profiling of each knockout under a variety of phenotypic assays has shown that the number of knockouts with corresponding phenotypes that could be valuable in target validation can be as little as 10%.9, 12, 15, 16
The staunch and robust nature of these phenotypic results can be understood when one considers the redundancy inherent in vital systems, such as alternative and complementary compensatory signaling in the genome. The scale-free nature of biological networks endows a high resilience to deletion of a random node, but high instability when the highly connected hubs are deleted.17, 18 It appears that organisms have developed compensatory signaling pathways to counteract mutation/deletion of a single node.19 The robustness inherent in signaling networks of complex diseases encourages a new thinking of drug discovery: to modify a phenotype, modulation of multiple targets is often required.17 The burgeoning field of network biology predicts that deletion of a single node will not affect a robust disease state; adjusting multiple proteins in the network is necessary to alter function and restore the normal phenotype.17, 20 Even if perfectly selective drugs could be developed towards a specific ligand, those targets are likely to be involved in multiple pathways, and thus target specificity does not guarantee a resulting “functional” specificity.
Motivation for a new approach
It can be argued that the bleak outlook for the pharmaceutical industry is founded in the social climate or the corporate structure of our time; regulatory authorities are more demanding of drug safety than ever before, and the standard of care is higher than ever. The true reason for this bleak outlook, however, could be more fundamental than the current climate; the basic conceptual manner in which drug discovery is executed might be flawed. It is difficult to ignore the increasing rate of drug failures over the last two decades correlated with adherence to the establishment of ‘one drug for one target for one disease’ model. This philosophy was harmonious with genetic reductionism and classification of ‘disease-causing’ genes.21 The goal of drug discovery at that time was to produce ‘clean’ drugs that minimized toxic side effects by making ligands as selective as possible for their respective targets. Polypharmacology, defined as “the specific binding of a compound to two or more molecular targets”,7 then was a property that was considered undesirable in the design of drugs. Now, however, it is considered to be helpful in the design of more effective and less toxic agents by targeting multiple protein families. It is worthy of note that polypharmacology design is still a very challenging task although some of the most successful drugs are actually hitting multiple protein families by chance (e.g, Gleevec).
Polypharmacology in cancer
In the area of cancer therapeutics, utilization of molecular network to study pharmacology/toxicology might prove pivotal in the next big breakthrough for treatment of this seemingly unstoppable disease. Cancer is poised to surpass heart disease to become the most common cause of death in the United States.22 It is no surprise that, when compared to other diseases afflicting the current population, cancer is best described as a disease “at best, minimally controlled by modern medicine”.23 While the mortality rates of cardiac, cerebrovascular, and infectious diseases have been brought down about two-thirds in the last 50 years, mortality rates from cancer have remained largely the same.22 The oncology market grew to $32 billion by 2005, aided by the fact that recent scientific discoveries in many diverse fields have yielded new targets for cancer drugs to attack.24 Also, the active cancer patient population demands the latest aggressive chemotherapy regiments that can easily command high prices due to unmet need. Patients whose survival is limited are desperate for treatments in order to extend their livelihood. These facts allow drug prices to reach record amounts, and demand for the next cancer breakthrough to reach unprecedented levels.
Multi-target kinase inhibitors are a specific subset of drugs that hold promise in overcoming the woes of the current attrition rate, and a rational approach to their design can bring the pipe dream of a cancer cure closer to reality. Kinases, or phosphotransferases, transfer a phosphate group from ATP to specific substrates, thereby altering their function. It is worth mentioning that while inhibition of kinases is a relatively easy endpoint to achieve, kinase inhibitors prove to be most clinically effective when specificity amongst kinases is shown. It should come as no surprise that because tyrosine kinases are involved in cell growth, survival, and proliferation, many model systems show that a perturbation of tyrosine kinase signaling results in malignant transformation and oncogenic mutations in tumors.25–27 Tyrosine kinase inhibitors with impressive clinical efficacy have been attributed to a selectivity profile that incorporates multiple targets.27, 28 Examples include: imatinib (Gleevec), used for chronic myelogenous leukemia (CML) and gastrointestinal stromal tumors (GIST), sunitinib (Sutent) for treatment of imatinib-resistant GIST and renal cell carcinoma (RCC), sorafenib (Nexavar) for RCC and hepatocellular carcinoma. Analysis of 974 anticancer agents from 1995 to 2007 in the area of oncology demonstrates an attrition rate from phase I to registration of 82% as a whole, but in the subset of multi-targeted kinase inhibitors, the observed attrition rate drops to only 53%.29
Along with tyrosine kinases, the phosphatidylinositol-3-OH kinase (PI3K) class of kinases are commonly mutated and activated in human cancers. While the tyrosine kinases and PI3Ks lack a primary sequence homology, they share a kinase domain structure, and one crucial function of tyrosine kinases is to activate the lipid kinases of the PI3K family.30 PI3Ks are also a good target of drug inhibition; the most frequently mutated kinase in human cancer, p110α, lies within the PI3K family.31, 32 As a result, kinase inhibitors have been the focus of intense efforts in recent years, with over 10,000 patents applications since 2001 in the US, primarily towards PI3K and tyrosine kinases.33 In the hopes of generating molecules that preferentially inhibit both PI3Ks and tyrosine kinases, Apsel et al.34 used kinome-level biochemical profiling of compounds sharing a pyrazolopyrimidine (PP) core combined with X-ray crystallography and iterative chemical synthesis to discover the small molecule PP121. PP121 (Figure 1) forms the same hydrogen bonds to kinases as the adenine of ATP, exploiting the conserved region in both tyrosine kinases and PI3Ks. The authors then showed that the selectivity of PP121 towards the PI3K/tyrosine kinases relative to serine/threonine kinases is due to a “gatekeeper residue” that is bulkier in serine/threonine. Given that PP121 can inhibit both tyrosine kinases and PI3Ks at nanomolar efficacy, they next examined its use in imatinib-resistant CML. Drug resistance in CML is due to a T315I mutation in Bcr-Abl, and is a major concern in clinical use of imatinib. PP121 was able to block proliferation by inducing G0/G1 cell cycle arrest, despite not inhibiting Bcr-AblT315I-mediated tyrosine phosphorylation. The authors attribute this effect to PI3K pathway inhibition. Their results show that consideration of potential inherent polypharmacological effects against two signaling pathways provides advantages over other treatments and promising new anti-cancer drugs.
Figure 1.

PP121, a dual-inhibitor of PI3Ks and tyrosine kinases
Successes of polypharmacology in mood disorder treatments
Another area that has seen the first hand benefits of polypharmacological drugs are the anti-psychotic and anti-dementia medications. Profitability and demand from this new market has exploded, with over 45 million annual prescriptions as of 2007, and worldwide sales are over $17 billion.35, 36 While the profitability is large, the success rate of CNS drugs, as stated above, is ~8% from first-in-man to registration. It has been well established that many mental illnesses are polygenic in origin and the embodiment of complexity, with substantial environmental and possibly even epigenetic components.37, 38 Although it has been well known that multiple brain diseases display dysregulation of multiple signaling pathways, drug mechanisms towards these diseases only focus on specific targets, such as dopamine transmission for psychosis, and acetylcholine and glutamate transmission for Alzheimer’s dementia.39
Atypical antipsychotics, also known as second generation antipsychotics, differentiate themselves from typical antipsychotics by their ability to bind to both serotonin and dopamine receptors and their decreased extrapyramidal side effects. The first atypical antipsychotic is Clozapine (Figure 2A), which remains the ‘gold-standard’ of atypical antipsychotic treatments. Interestingly, it was discovered through phenotypic screening/optimization and it was not originally designed to hit any one individual protein target. However, the pharmacological profile of Clozapine shows binding (with affinity values Ki < 100nM) to at least 26 receptors (Figure 2B).36 Even more interesting, almost all attempts to target a single ‘magic receptor’ thought to be involved in schizophrenia and related disorders have resulted in much lower efficacy than multi-target drugs like Clozapine.36 The pleiotypic nature of Clozapine is most likely accountable for its efficacy in treating schizophrenia and related disorders.40, 41 Examination of functional electrophysiology data demonstrated anti-psychotic and anti-dementia medications directly modulated a selection of ligand- and voltage- gated ion channels.39 A logical expansion of this fact is that enhanced understanding of the polypharmacological effects of these drugs can coincide with progress in disease sub-typing, leading to personalized drugs towards patients with unique polymorphisms of ion channels.
Figure 2.
A. Structure of Clozapine, an atypical antipsyphotic agent, B. Molecular network of Clozapine receptor targets for which the binding affinity (Ki) < 100nM. Octagon: serotonin receptors, Rhomb: muscarinic receptors, Circle: histamine receptors, Triangle: dopamine receptors, Trapezoid: adrenergic receptors.
Antidepressants are also trending towards polypharmacological effects. Genetic studies have shown that depression displays a complexity that spans many diverse genes42. Results show that ‘dual-action’ antidepressants (serotonin and noradrenaline reuptake inhibitors, SNRIs), which inhibit both 5-HT and dopamine and/or noradrenaline reuptake, are more effective than ‘single-action’ antidepressants (selective serotonin reuptake inhibitors, SSRIs).43, 44 Clearly, the most clinically effective treatments for depression, schizophrenia, and possibly other mood disorders display multiple mechanisms of action, working as non-specific ‘magic shotguns’ rather than ‘magic bullets’ on the CNS.36
Current development in molecular networks for drug discovery
Polypharmacology can be studied using molecular network analysis to investigate the complex ligand-target interactions. A cell’s response to treatments like the ones used in cancer and CNS disorders is the result of a complex cascade starting with receptors and signaling molecules, down to kinases, metabolites, and even transcriptional changes. The interplay between different chemicals and cellular entities can be characterized in the form of a network, for instance, drug-target network. RNAi-chemical sensitization screens are commonly used to deduce these networks; compound mixtures are treated with RNAi treated cell models in the hopes to show synthetically lethal results. The caveat of this technique, as with any model system, is that results obtained in this way do not necessarily translate into results that can be applied to the clinic.45 Even if this approach was clinically applicable, it does not provide useful clinical information on pharmacokinetics, dosing, or metabolism optimization. It becomes necessary then, to incorporate novel techniques to reduce the search space and expand the drug target network. In recent years, a number of studies have used both computational and experimental techniques to discover and map drug-target interactions on a genomic scale.34, 46–51 This target space can be considered to be comprised of three main components: protein-protein, metabolic and transcriptional interactions.5 Interestingly, the majority of clinical drugs belong to the protein kinase or receptor family, with enzymes and ion channels forming the second class.52
The vast amounts of biological data from mapping of these drug-target networks have been incorporated into publicly available databases. Chemical databases, such as PubChem,53 PDB (Protein Data Bank),54 MOAD (Mother Of All Databases),55 ChEMBL Database56, and ZINC (Zinc Is Not Commercial)57 are valuable resources in drug discovery. STITCH integrates information from metabolic pathways, crystal structures, binding experiments, and drug-target relationships.58 A database of 68,000 compounds and 2,200 drugs is available to users, along with similarity predictions based on text mining and chemical structure. A successor, STITCH 2, was recently announced that adds 6,000 more compounds and incorporates BindingDB, PharmGKB, and a Comparative Toxicogenomics Database.59
From this data, molecular interaction networks have been constructed by way of a systems biology approach.60–65 These databases have probed the relationships of their respective biological data in either a top-down or bottom-up approach. Another approach incorporating analytical and in silico techniques is to examine not only the biological targets themselves but also the ligands and their biology/chemistry.66 The underlying hypothesis is that molecules with similar structural features and descriptors will have similar properties, leading to binding of similar proteins.67 Occasionally, small modifications can result in a radical change in binding affinity, but these exceptions do not detract significantly from chemical similarity’s place as a driving principle in drug design.68
Hopkins and colleagues found that compound promiscuity and protein classification could be understood by connecting proteins in chemical space by polypharmacology interactions.46 This Hopkins group constructed linkage maps by comparing full chemical identity (chemical structure, protein sequence, and disease indication) among ligands that had two or more receptors in common and a protein interaction network was built based on polypharmacology. They showed that probabilistic models can make predictions of pharmacology that were derived from a large knowledge base. Vieth and colleagues were able to build a dendrogram of kinases based entirely on small molecule selectivity data.69 From this, they were able to organize the selectivity similarities among kinases, an important step towards the growing problem of achieving inhibitor selectivity for kinases that have high homology. Izrailev and Farnum used known binders towards an enzyme with unknown function to search a protein-ligand interaction database for classes of enzymes that are known to interact with a similar set of ligands.70 The enzyme’s function can be elucidated when the similarity between the sets of ligands (based upon similarity between individual compounds) is considered in conjunction with sequence and structural information.
Keiser and colleagues used a novel algorithm (which they called SEA: Similarity Ensemble Approach71) to assemble fingerprints (unique patterns) of chemical structures of the known ligands for each of the hundreds of drug targets considered.72 From these fingerprints, an accurate prediction can be made towards another structurally unrelated compound in regard to its chemical pattern.72, 73 Without using any biological data, they were able to quantitatively relate protein targets by their ligands, providing both sensible clusters and novel predictions that were later confirmed in vitro. The well-known antidepressants fluoxetine (Prozac) and paroxetine (Paxil) are selective serotonin reuptake inhibitors (SSRIs). The authors’ SEA predicted that they might also act as beta-blockers (binding to β-adrenergic receptors), which was validated experimentally. This fact does not come as a huge surprise because the biological similarities between the serotonin and β-adrenergic receptors and their ligands provided a logical conclusion. The authors also compared the difference between chemical similarity-based ligand networks and sequence-based bioinformatics networks, and concluded that ligand-based networks are more stable, organized and natural than their bioinformatics counterparts. This work demonstrated the importance of using small molecules to derive biological information for drug discovery and development. In particular, using cheminformatics approaches to relate ligands with their receptors is complementary to bioinformatics methods while it is also more intuitive.71
A tougher challenge is to predict polypharmacology for drug targets that have very little in common by way of sequence, structure, or ligands; Keiser and colleagues were able to beat this challenge as well, and confirm the predictions experimentally. Their machine-learning technique was able to predict the polypharmacological properties of delavirdine (Rescriptor, an HIV-1 reverse transcriptase inhibitor) in its binding affinity to histamine H4 receptors.73 These targets have virtually no commonalities in terms of structure, amino-acid residue sequence, or endogenous ligands. This result might explain why a common side effect of delavirdine is skin rashes. Finally, Bork and colleagues, building from the knowledge from Fliri et al. that drugs with similar in vitro protein binding profiles trend towards displaying similar side effects,74, 75 examined the side effects of 746 marketed drugs. 1018 drug-drug relations appeared, 261 of which exist from chemically dissimilar drugs in different therapeutic categories.76 Experimental testing validated 11 out of 13 of these implied relations, and these 11 relations had an inhibition constant equal or less than 10μM. Bork and colleagues showed the feasibility of inferring molecular interactions based on annotated, phenotypic information derived from the perturbations of a human system. Jenkins and colleagues were able to analyze compounds with similar toxic phenotypes and compare the pathways that they affect with the pathways modulated by nontoxic compounds. They were able to elucidate relevant pathways for rhabdomyolysis and hypotensive side effects for Cerivastatin, which was pulled from the market in 2001 for drug-induced rhabdomyolysis and its resultant renal failure. The advantage of their ‘systems chemical biology’ approach is that identical targets are not only considered byt rather that compounds may cause the same phenotype by affecting different targets in the same pathway.77 From these studies, it is plain to see that a careful and thorough examination of the chemistry of the ligands involved can yield fresh and/or complementary information that pertains to multiple endpoints.
Our new molecular network technology
For a given chemical molecule, molecular network analysis can help to identify all of its related molecules (interaction partners or functional/chemical similar agents). Therefore, this technology can be used to study the potential off-targets of new molecules for side effects/toxicities, find new indications for existing drugs, or guide the combined therapy for multiple drugs. During the past several years, our group has embarked the development of using molecular networks that allows users to visually and efficiently navigate publicly available information on drug properties (biological targets, pharmacology, etc) and can show relationships among drugs and their receptors based on their function annotation (biological targets, toxicity, side effects, metabolism, etc.) (Figures 3 and 4). or chemical similarity.78 Currently, this platform has been expanded to the analysis of more than 5,000 drugs and 10 million virtual library compounds, along with 56,000 biological macromolecules with available high resolution 3D structures. To fully explore the huge and complex molecular networks among ligand-ligand, ligand-receptor, and receptor-receptor molecules, integration of a diverse set of tools including: chemical similarity searches, text mining, cheminformatics mapping, and high-throughput docking approaches are being implemented for in silico polypharmacology prediction and design.79–83
Figure 3.
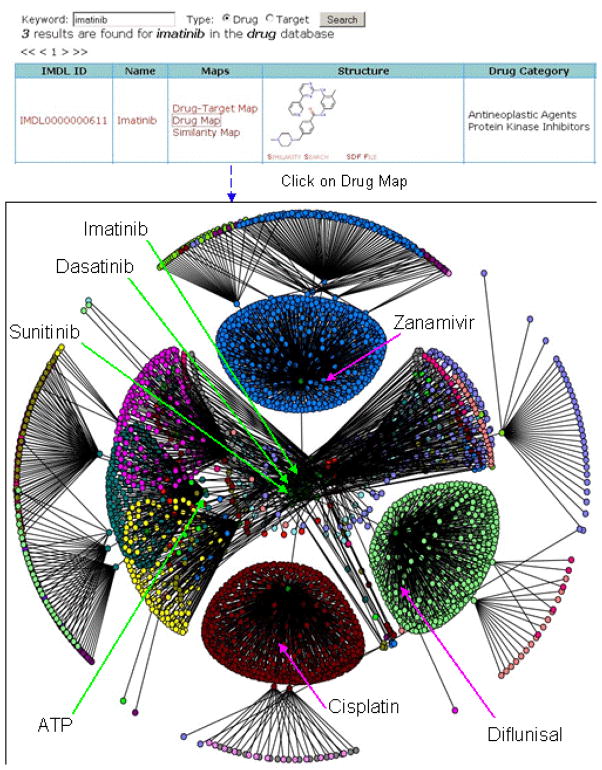
Features of molecular network analysis tool interface: Using keyword searches for drug or target (here with dasatinib as an example), users can perform a series of functions including: navigate through drug-target maps, visualize targets in Jmol, view structures and external resource pages, download SDF files for similar compounds, etc.
Figure 4.
The 3-level network of imatinib based on the target annotation of the drug. Using the same concept in Figure 3, we extended the networks of imatinib to multiple levels and it resulted in the network on the left. Those drugs at the same level (proximity to imatinib) are in the same colors. For example, the query compound in the center of the network in blue, and its inner most cluster (obscured) contains tyrosine kinase inhibitor such as dasatinib and sunitinib, whereas the blue, green, and red clusters contain the antiviral zanamivir, the anti-inflamitory difluisal, and the anti-cancer cisplatin, respectively.
In order to simplify the graph in Figure 3, we can hide the targets by looking at the networks of chemical molecules only. Figure 4 is such a network formed by all drugs as close neighbors of imatinib. Since such networks are usually very complex, we chose to display only three levels of the networks for visualization, which is still huge. With this network, it was found that multiple clusters of compounds have close relationships based on their shared targets. Some compounds are kinase inhibitors (e.g., dasatinib, sunitinib, etc.) but some of them are not (e.g., Zanamivir, cisplatin, etc.). As expected, ATP is among the molecules closely related to all of the kinase inhibitors and is also involved in a complex network.
Chemical similarity-based drug networks
Chemical similarity search can be performed to identify all similar compounds, and this has also been used to conduct the network analysis because it is known that structurally similar compounds tend to have similar properties and bind to the same group of proteins.67 The idea is that the chemically similar structures can be found for each drug and thus an extended network can be constructed for all of the drugs. In our implementation, chemcial similarity search is performed using ChemAxon built-in fingerprints and the Tanimoto coefficient for similarity measurement. For instance, based on chemical similarity search, we found that autoimmune drug prednisolone is involved in a network with many other steroid-like drugs as they have a similar chemical scaffold (e.g., four fused rings). This indicates that prednisolone, although a corticosteroid drug, maybe also interacts with targets of anabolic steroids including testosterone, and actually shares similar side effects with them such as causing hypertension and acne.
Summary of our molecular network technology
In summary, this tool will help significantly to identify molecular networks among ligands-ligands and ligands-receptors, and to derive the relationship of one drug with other drugs. Therefore, with this tool, it is possible to: predict polypharmacology, identify potential new indications of existing drugs, and guide combined therapies of multiple drugs. For instance, based on analysis of the network, dasatinib may interact with several proteins including Ephrin A2 (ephA2) receptor protein kinase (1MQB), SRC SH2 domain (1O4H), or even poly(A) polymerase (1Q78). On the other hand, quercetin based on chemical similarity with dasatinib, has the potential to be used as a tyrosine inhibitor or could be combined with dasatinib for cancer therapies. A recent study by Kuriyan et al. confirms this prediction, when comparing dasatinib to quercetin as a non-specific kinase inhibitor against quinone reductase 2 (NQO2) with an IC50 Of 42nM and >100nM for quercetin and dasatinib, respectively.84 These tools have been developed as web-based applications and will be freely available through our web sites to academia in the future.
Practical approaches to generate promiscuous drugs
While the development of a new promiscuous drug might be very costly, let alone development of a single-target drug, the molecular network technique of retrospectively analyzing older approved drugs in the hopes of discovering new therapeutic uses for these drugs can provide all the benefit of a new, multi-target entity without the risk and cost of an entire clinical trial. As described earlier, attrition rates for drugs are hitting worrisome levels, and companies that attempt to circumnavigate this problem by designing drugs with new mechanisms of action experience higher attrition than those with previously acknowledged mechanisms. This fact suggests that future focus shift toward existing, approved drugs and use them towards unique therapeutic endpoints.3 This concept has been coined drug “repurposing” or “repositioning” and has shown a high return for low cost/risk.85–87 One serendipitous discovery of new uses for old drugs was that of aspirin, which was originally designed for use in treatment of arthritis, but later was found to have antineoplastic, anti-platelet, anti-inflammatory, analgesic, antipyretic, to inhibit prothrombin, pro-apoptotic, and even to have preventative effects of cancer.88
The selective optimization of side activities (SOSA)89, 90 approach, incorporates the benefits of repurposing, and offers a validated alternative to high-throughput screening. This technique involves two basic steps. First, a database of structurally diverse drugs with bioavailability and toxicology studies is built (a smart-library). Since these compounds already have demonstrated efficacy and safety, all of the hits in this smart-library will be, by definition, drug-like. Next, these hits are optimized (by means of traditional, parallel, or combinatorial chemistry) to give increased affinity towards the new target, and decreased affinity to the original target. The result is that the initial ‘side activity’ becomes the ‘main activity’ and, conversely, the initial ‘main activity’ is strongly reduced or even abolished. An important advantage provided by molecules that undergo SOSA switches is that their inherent drug-like properties lend themselves to good absorption, distribution, metabolism, and excretion (ADME) profiles, as well as reduced toxicity.90
The most conventional clinical multi-target approach is to prescribe multiple individual medications, which is best demonstrated in the multidrug cocktails against HIV. This cocktail approach is especially useful in treatments where the target in question can generate mutant forms which might escape contact and become resistant. To this end, theoretical model formulations have been devised that optimize the design of drug cocktails for targeting molecular ensembles.91 Also, fixed-dose combination (FDC) drugs combine several biologically active compounds formulated in the same delivery device, for example, a single inhaler, or capsule.92 This concept is hardly unfamiliar, traditional Chinese medicine has used mixtures of herbs and their extracts in their therapy, and the activities of these therapies disappear when the components were fractionated into individual components.93 Well known successes of FDC drugs include the GlaxoSmithKline blockbuster Advair for asthma, which was found in at least one meta-analysis study to be synergistic in efficacy when compared with concomitant use of two separate inhalers.94 Another blockbuster success comes from the joint venture (Merck and Schering-Plough) against hyperlipidaemia called Vytorin (combining Zetia and Zocor), which works in two different mechanisms of action; Zetia blocks cholesterol absorption, and Zocor reduces cholesterol naturally produced in the body.
Challenges of molecular network studies in drug discovery
The path to rational design of multi-target drugs is not without caveats. One of the biggest hurdles comes from a lack of complete data on networks and their targets. A complete, systematic examination of the drug-target network, and its components: the target-target, drug-drug, and disease network space, will prove decisive in the use of multi-targeted drugs in the clinic.5 The differences in topology, stability and structure between the disease network and the normal network can be exploited to generate targets for multi-target drugs. If these differences are not fully understood, or the complete molecular network/polypharmacology profile of a particular drug is not understood, one cannot fully predict the efficacy of a treatment.
Molecular network approaches are best developed from integration or creation of databases with multiple annotations and descriptors towards the ligands and their receptors. In the ‘natural selection’ of increasing data collation and that exists among various sources, databases that combine more aspects of diverse information such as bioinformatics, cheminformatics, pharmacology, and pathway analysis will likely replace those with minimal annotations in the coming years. Also, incorporation of recent discoveries including the complete human interactome map into these databases will bolster existing data. Despite open sharing of medical advances in public organizations, there is a scarcity of public drug-screening data relative to proprietary data. Private drug companies should be made aware of the new market potential that sharing their data will provide, and that the incentives for contributing their information, far outweigh those for holding it close. Initiatives to private companies might aid in their willingness to share data, and the elucidation of new targets and drugs for these targets is to their long-term benefit.
Two of the key challenges in the progress of molecular network technology in drug development are identifying the central nodes of the network through which changes can result in the desired therapeutic effect, and the design of drugs with the right polypharmacology profile to affect said nodes. The means by which to address these challenges are based upon novel and unique knowledge of the targets. Mapping of ligand chemistry and 3D structures of the drug-receptor complexes is the foundation of understanding individual nodes. Mining of relevant text terms on a large scale from sources like PubMed can identify and rank implicit relationships and incorporate data from other fields of biology and chemistry to buttress existing network vigor and bridge centrality of the networks.95
Conclusion
After almost twenty years of high selectivity toward individual, central disease-causing genes, the number of druggable targets seem much less than we considered previously. Adherence to the ‘one-drug for one-disease, caused by one-gene’ model is a recipe for inevitable failure in modern pharmaceutical development, due to the interactions of the complex drug-target network environment. The discovery of redundant biological systems via large-scale gene knock-outs, along with detection of compensatory signaling pathways by way of systems biology begs for novel techniques in drug development. It is becoming increasingly clear, that for the next wave of pharmaceutical therapies to be successful against the multifaceted diseases that we face today, a more innovative approach is required. Effective drugs should be always considered for their potential off-target properties (side effects or toxicities) for “fail early, fail fast” through molecular network analysis, or even further they should not merely target a single but rather several nodes of a biological network, and comprehension of the robustness and redundancy present in the target network helps to achieve this goal.20, 28, 36, 92, 96
Confronting drug discovery as a probabilistic endeavor from a priori knowledge with consideration of the dynamic degrees of polypharmacological effects may prove significant in increasing productivity of pharmaceutical research. The novel concept of molecular network technology, which is built on the foundations of polypharmacology, can bridge the paradox of failing drugs yet increased biological data by utilizing the vast expanses of data that are available and using it to design drugs that bring decreased toxicity and higher efficacy. Molecular network technology is an approach that embraces diverse fields such as systems biology, network biology, chemoinformatics, and pharmacology for drug discovery and development. Numerous studies, only some of which have been presented here, show the power of molecular network analysis and can aid in curing complex biological diseases. As the drug design tools become more comprehensive in their breadth and their scope, more multi-target drugs enter into the market.
Reference list
- 1.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003 Mar;22(2):151–85. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 2.Martinez B, Goldstein J. Big Pharma Faces Grim Prognosis. Wall Street Journal. 2007 [Google Scholar]
- 3.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004 Aug;3(8):711–5. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 4.DiMasi JA. The value of improving the productivity of the drug development process: faster times and better decisions. Pharmacoeconomics. 2002;20( Suppl 3):1–10. doi: 10.2165/00019053-200220003-00001. [DOI] [PubMed] [Google Scholar]
- 5.Janga SC, Tzakos A. Structure and organization of drug-target networks: insights from genomic approaches for drug discovery. Mol Biosyst. 2009 Sep 4; doi: 10.1039/B908147j. [DOI] [PubMed] [Google Scholar]
- 6.Collier R. Drug development cost estimates hard to swallow. CMAJ. 2009 Feb 3;180(3):279–80. doi: 10.1503/cmaj.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008 Nov;4(11):682–90. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 8.Ma P, Zemmel R. Value of novelty? Nat Rev Drug Discov. 2002 Aug;1(8):571–2. doi: 10.1038/nrd884. [DOI] [PubMed] [Google Scholar]
- 9.Zambrowicz BP, Sands AT. Knockouts model the 100 best-selling drugs--will they model the next 100? Nat Rev Drug Discov. 2003 Jan;2(1):38–51. doi: 10.1038/nrd987. [DOI] [PubMed] [Google Scholar]
- 10.Wagner A. Robustness against mutations in genetic networks of yeast. Nat Genet. 2000 Apr;24(4):355–61. doi: 10.1038/74174. [DOI] [PubMed] [Google Scholar]
- 11.Yildirim MA, Goh KI, Cusick ME, Barabasi AL, Vidal M. Drug-target network. Nat Biotechnol. 2007 Oct;25(10):1119–26. doi: 10.1038/nbt1338. [DOI] [PubMed] [Google Scholar]
- 12.Zambrowicz BP. Modeling drug action in the mouse with knockouts and RNA interference. Drug Discov Today: Targets. 2004;3(5):198–207. [Google Scholar]
- 13.Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, et al. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999 Aug 6;285(5429):901–6. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002 Sep;1(9):727–30. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 15.Austin CP, Battey JF, Bradley A, Bucan M, Capecchi M, Collins FS, et al. The knockout mouse project. Nat Genet. 2004 Sep;36(9):921–4. doi: 10.1038/ng0904-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zambrowicz BP, Turner CA, Sands AT. Predicting drug efficacy: knockouts model pipeline drugs of the pharmaceutical industry. Curr Opin Pharmacol. 2003 Oct;3(5):563–70. doi: 10.1016/j.coph.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Barabasi AL, Oltvai ZN. Network biology: understanding the cell’s functional organization. Nat Rev Genet. 2004 Feb;5(2):101–13. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- 18.Kitano H. Towards a theory of biological robustness. Mol Syst Biol. 2007;3:137. doi: 10.1038/msb4100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartman JLt, Garvik B, Hartwell L. Principles for the buffering of genetic variation. Science. 2001 Feb 9;291(5506):1001–4. doi: 10.1126/science.291.5506.1001. [DOI] [PubMed] [Google Scholar]
- 20.Csermely P, Agoston V, Pongor S. The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol Sci. 2005 Apr;26(4):178–82. doi: 10.1016/j.tips.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Sams-Dodd F. Target-based drug discovery: is something wrong? Drug Discov Today. 2005 Jan 15;10(2):139–47. doi: 10.1016/S1359-6446(04)03316-1. [DOI] [PubMed] [Google Scholar]
- 22.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009 Apr 17;57(14):1–134. [PubMed] [Google Scholar]
- 23.Varmus H. The new era in cancer research. Science. 2006 May 26;312(5777):1162–5. doi: 10.1126/science.1126758. [DOI] [PubMed] [Google Scholar]
- 24.Booth B, Glassman R, Ma P. Oncology’s trials. Nat Rev Drug Discov. 2003 Aug;2(8):609–10. doi: 10.1038/nrd1158. [DOI] [PubMed] [Google Scholar]
- 25.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001 May 17;411(6835):355–65. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 26.Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005 Jul 14;353(2):172–87. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 27.Sebolt-Leopold JS, English JM. Mechanisms of drug inhibition of signalling molecules. Nature. 2006 May 25;441(7092):457–62. doi: 10.1038/nature04874. [DOI] [PubMed] [Google Scholar]
- 28.Petrelli A, Giordano S. From single- to multi-target drugs in cancer therapy: when aspecificity becomes an advantage. Curr Med Chem. 2008;15(5):422–32. doi: 10.2174/092986708783503212. [DOI] [PubMed] [Google Scholar]
- 29.Walker I, Newell H. Do molecularly targeted agents in oncology have reduced attrition rates? Nat Rev Drug Discov. 2009 Jan;8(1):15–6. doi: 10.1038/nrd2758. [DOI] [PubMed] [Google Scholar]
- 30.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006 May 25;441(7092):424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 31.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004 Apr 23;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 32.Samuels Y, Velculescu VE. Oncogenic mutations of PIK3CA in human cancers. Cell Cycle. 2004 Oct;3(10):1221–4. doi: 10.4161/cc.3.10.1164. [DOI] [PubMed] [Google Scholar]
- 33.Akritopoulou-Zanze I, Hajduk PJ. Kinase-targeted libraries: the design and synthesis of novel, potent, and selective kinase inhibitors. Drug Discov Today. 2009 Mar;14(5–6):291–7. doi: 10.1016/j.drudis.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Apsel B, Blair JA, Gonzalez B, Nazif TM, Feldman ME, Aizenstein B, et al. Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol. 2008 Nov;4(11):691–9. doi: 10.1038/nchembio.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snyder EM, Murphy MR. Schizophrenia therapy: beyond atypical antipsychotics. Nat Rev Drug Discov. 2008 Jun;7(6):471–2. doi: 10.1038/nrd2571. [DOI] [PubMed] [Google Scholar]
- 36.Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004 Apr;3(4):353–9. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 37.Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003 Jul;73(1):34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison PJ, Owen MJ. Genes for schizophrenia? Recent findings and their pathophysiological implications. Lancet. 2003 Feb 1;361(9355):417–9. doi: 10.1016/S0140-6736(03)12379-3. [DOI] [PubMed] [Google Scholar]
- 39.Bianchi MT. Promiscuous modulation of ion channels by anti-psychotic and anti-dementia medications. Med Hypotheses. 2009 Sep 26; doi: 10.1016/j.mehy.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988 Sep;45(9):789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 41.Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT) Arch Gen Psychiatry. 2003 Jan;60(1):82–91. doi: 10.1001/archpsyc.60.1.82. [DOI] [PubMed] [Google Scholar]
- 42.Zubenko GS, Maher B, Hughes HB, 3rd, Zubenko WN, Stiffler JS, Kaplan BB, et al. Genome-wide linkage survey for genetic loci that influence the development of depressive disorders in families with recurrent, early-onset, major depression. Am J Med Genet B Neuropsychiatr Genet. 2003 Nov 15;123B(1):1–18. doi: 10.1002/ajmg.b.20073. [DOI] [PubMed] [Google Scholar]
- 43.Anderson IM. Meta-analytical studies on new antidepressants. Br Med Bull. 2001;57:161–78. doi: 10.1093/bmb/57.1.161. [DOI] [PubMed] [Google Scholar]
- 44.Thase ME, Entsuah AR, Rudolph RL. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br J Psychiatry. 2001 Mar;178:234–41. doi: 10.1192/bjp.178.3.234. [DOI] [PubMed] [Google Scholar]
- 45.Dancey JE, Chen HX. Strategies for optimizing combinations of molecularly targeted anticancer agents. Nat Rev Drug Discov. 2006 Aug;5(8):649–59. doi: 10.1038/nrd2089. [DOI] [PubMed] [Google Scholar]
- 46.Paolini GV, Shapland RH, van Hoorn WP, Mason JS, Hopkins AL. Global mapping of pharmacological space. Nat Biotechnol. 2006 Jul;24(7):805–15. doi: 10.1038/nbt1228. [DOI] [PubMed] [Google Scholar]
- 47.Yamanishi Y, Araki M, Gutteridge A, Honda W, Kanehisa M. Prediction of drug-target interaction networks from the integration of chemical and genomic spaces. Bioinformatics. 2008 Jul 1;24(13):i232–40. doi: 10.1093/bioinformatics/btn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacob L, Vert JP. Protein-ligand interaction prediction: an improved chemogenomics approach. Bioinformatics. 2008 Oct 1;24(19):2149–56. doi: 10.1093/bioinformatics/btn409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brewerton SC. The use of protein-ligand interaction fingerprints in docking. Curr Opin Drug Discov Devel. 2008 May;11(3):356–64. [PubMed] [Google Scholar]
- 50.Hillenmeyer ME, Fung E, Wildenhain J, Pierce SE, Hoon S, Lee W, et al. The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science. 2008 Apr 18;320(5874):362–5. doi: 10.1126/science.1150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuhn M, Campillos M, Gonzalez P, Jensen LJ, Bork P. Large-scale prediction of drug-target relationships. FEBS Lett. 2008 Apr 9;582(8):1283–90. doi: 10.1016/j.febslet.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 52.Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008 Jan;36(Database issue):D901–6. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.http://pubchem.ncbi.nlm.nih.gov/
- 54.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The Protein Data Bank. Nucleic Acids Res. 2000 Jan 1;28(1):235–42. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu L, Benson ML, Smith RD, Lerner MG, Carlson HA. Binding MOAD (Mother Of All Databases) Proteins. 2005 Aug 15;60(3):333–40. doi: 10.1002/prot.20512. [DOI] [PubMed] [Google Scholar]
- 56.http://www.ebi.ac.uk/chembldb/
- 57.Irwin JJ, Shoichet BK. ZINC--a free database of commercially available compounds for virtual screening. J Chem Inf Model. 2005 Jan-Feb;45(1):177–82. doi: 10.1021/ci049714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuhn M, von Mering C, Campillos M, Jensen LJ, Bork P. STITCH: interaction networks of chemicals and proteins. Nucleic Acids Res. 2008 Jan;36(Database issue):D684–8. doi: 10.1093/nar/gkm795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuhn M, Szklarczyk D, Franceschini A, Campillos M, von Mering C, Jensen LJ, et al. STITCH 2: an interaction network database for small molecules and proteins. Nucleic Acids Res. 2010 Jan;38(Database issue):D552–6. doi: 10.1093/nar/gkp937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berg EL, Kunkel EJ, Hytopoulos E. Biological complexity and drug discovery: a practical systems biology approach. Syst Biol (Stevenage) 2005 Dec;152(4):201–6. doi: 10.1049/ip-syb:20050036. [DOI] [PubMed] [Google Scholar]
- 61.Boyle J, Cavnor C, Killcoyne S, Shmulevich I. Systems biology driven software design for the research enterprise. BMC Bioinformatics. 2008;9:295. doi: 10.1186/1471-2105-9-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mayburd AL, Martlinez A, Sackett D, Liu H, Shih J, Tauler J, et al. Ingenuity network-assisted transcription profiling: Identification of a new pharmacologic mechanism for MK886. Clin Cancer Res. 2006 Mar 15;12(6):1820–7. doi: 10.1158/1078-0432.CCR-05-2149. [DOI] [PubMed] [Google Scholar]
- 63.Splendiani A. RDFScape: Semantic Web meets systems biology. BMC Bioinformatics. 2008;9( Suppl 4):S6. doi: 10.1186/1471-2105-9-S4-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steinhauser D, Usadel B, Luedemann A, Thimm O, Kopka J. CSB.DB: a comprehensive systems-biology database. Bioinformatics. 2004 Dec 12;20(18):3647–51. doi: 10.1093/bioinformatics/bth398. [DOI] [PubMed] [Google Scholar]
- 65.Wierling C, Herwig R, Lehrach H. Resources, standards and tools for systems biology. Brief Funct Genomic Proteomic. 2007 Sep;6(3):240–51. doi: 10.1093/bfgp/elm027. [DOI] [PubMed] [Google Scholar]
- 66.Schreiber SL. Small molecules: the missing link in the central dogma. Nat Chem Biol. 2005 Jul;1(2):64–6. doi: 10.1038/nchembio0705-64. [DOI] [PubMed] [Google Scholar]
- 67.Johnson MA, Maggiora GM. Concepts and applications of molecular similarity. New York: John Wiley & Sons; 1990. [Google Scholar]
- 68.Whittle M, Gillet VJ, Willett P, Alex A, Loesel J. Enhancing the effectiveness of virtual screening by fusing nearest neighbor lists: a comparison of similarity coefficients. J Chem Inf Comput Sci. 2004 Sep-Oct;44(5):1840–8. doi: 10.1021/ci049867x. [DOI] [PubMed] [Google Scholar]
- 69.Vieth M, Higgs RE, Robertson DH, Shapiro M, Gragg EA, Hemmerle H. Kinomics-structural biology and chemogenomics of kinase inhibitors and targets. Biochim Biophys Acta. 2004 Mar 11;1697(1–2):243–57. doi: 10.1016/j.bbapap.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 70.Izrailev S, Farnum MA. Enzyme classification by ligand binding. Proteins. 2004 Dec 1;57(4):711–24. doi: 10.1002/prot.20277. [DOI] [PubMed] [Google Scholar]
- 71.Hert J, Keiser MJ, Irwin JJ, Oprea TI, Shoichet BK. Quantifying the relationships among drug classes. J Chem Inf Model. 2008 Apr;48(4):755–65. doi: 10.1021/ci8000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Keiser MJ, Roth BL, Armbruster BN, Ernsberger P, Irwin JJ, Shoichet BK. Relating protein pharmacology by ligand chemistry. Nat Biotechnol. 2007 Feb;25(2):197–206. doi: 10.1038/nbt1284. [DOI] [PubMed] [Google Scholar]
- 73.Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, et al. Predicting new molecular targets for known drugs. Nature. 2009 Nov 12;462(7270):175–81. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fliri AF, Loging WT, Thadeio PF, Volkmann RA. Analysis of drug-induced effect patterns to link structure and side effects of medicines. Nat Chem Biol. 2005 Dec;1(7):389–97. doi: 10.1038/nchembio747. [DOI] [PubMed] [Google Scholar]
- 75.Fliri AF, Loging WT, Volkmann RA. Analysis of system structure-function relationships. Chem Med Chem. 2007 Dec;2(12):1774–82. doi: 10.1002/cmdc.200700153. [DOI] [PubMed] [Google Scholar]
- 76.Campillos M, Kuhn M, Gavin AC, Jensen LJ, Bork P. Drug target identification using side-effect similarity. Science. 2008 Jul 11;321(5886):263–6. doi: 10.1126/science.1158140. [DOI] [PubMed] [Google Scholar]
- 77.Scheiber J, Chen B, Milik M, Sukuru SC, Bender A, Mikhailov D, et al. Gaining Insight into Off-Target Mediated Effects of Drug Candidates with a Comprehensive Systems Chemical Biology Analysis. J Chem Inf Model. 2009 Jan 13; doi: 10.1021/ci800344p. [DOI] [PubMed] [Google Scholar]
- 78.Tian L, Zhang S. Mapping drug-target interaction networks. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2336–9. doi: 10.1109/IEMBS.2009.5335053. [DOI] [PubMed] [Google Scholar]
- 79.Zhang S, Golbraikh A, Tropsha A. Development of quantitative structure-binding affinity relationship models based on novel geometrical chemical descriptors of the protein-ligand interfaces. J Med Chem. 2006 May 4;49(9):2713–24. doi: 10.1021/jm050260x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang S, Golbraikh A, Oloff S, Kohn H, Tropsha A. A novel automated lazy learning QSAR (ALL-QSAR) approach: method development, applications, and virtual screening of chemical databases using validated ALL-QSAR models. J Chem Inf Model. 2006 Sep-Oct;46(5):1984–95. doi: 10.1021/ci060132x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang S, Kumar K, Jiang X, Wallqvist A, Reifman J. DOVIS: an implementation for high-throughput virtual screening using AutoDock. BMC Bioinformatics. 2008;9:126. doi: 10.1186/1471-2105-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang S, Du-Cuny L. Development and evaluation of a new statistical model for structure-based high-throughput virtual screening. Int J Bioinform Res Appl. 2009;5(3):269–79. doi: 10.1504/IJBRA.2009.026419. [DOI] [PubMed] [Google Scholar]
- 83.Zhang S, Wei L, Bastow K, Zheng W, Brossi A, Lee KH, et al. Antitumor agents 252. Application of validated QSAR models to database mining: discovery of novel tylophorine derivatives as potential anticancer agents. J Comput Aided Mol Des. 2007 Jan-Mar;21(1–3):97–112. doi: 10.1007/s10822-007-9102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Winger JA, Hantschel O, Superti-Furga G, Kuriyan J. The structure of the leukemia drug imatinib bound to human quinone reductase 2 (NQO2) BMC Struct Biol. 2009;9:7. doi: 10.1186/1472-6807-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Connor KA, Roth BL. Finding new tricks for old drugs: an efficient route for public-sector drug discovery. Nat Rev Drug Discov. 2005 Dec;4(12):1005–14. doi: 10.1038/nrd1900. [DOI] [PubMed] [Google Scholar]
- 86.Uliana SR, Barcinski MA. Repurposing for neglected diseases. Science. 2009 Nov 13;326(5955):935. doi: 10.1126/science.326.5955.935-a. author reply. [DOI] [PubMed] [Google Scholar]
- 87.Boguski MS, Mandl KD, Sukhatme VP. Drug discovery. Repurposing with a difference. Science. 2009 Jun 12;324(5933):1394–5. doi: 10.1126/science.1169920. [DOI] [PubMed] [Google Scholar]
- 88.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009 Apr 11;373(9671):1301–9. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 89.Wermuth CG. Selective optimization of side activities: another way for drug discovery. J Med Chem. 2004 Mar 11;47(6):1303–14. doi: 10.1021/jm030480f. [DOI] [PubMed] [Google Scholar]
- 90.Wermuth CG. Selective optimization of side activities: the SOSA approach. Drug Discov Today. 2006 Feb;11(3–4):160–4. doi: 10.1016/S1359-6446(05)03686-X. [DOI] [PubMed] [Google Scholar]
- 91.Radhakrishnan ML, Tidor B. Optimal drug cocktail design: methods for targeting molecular ensembles and insights from theoretical model systems. J Chem Inf Model. 2008 May;48(5):1055–73. doi: 10.1021/ci700452r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Keith CT, Borisy AA, Stockwell BR. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov. 2005 Jan;4(1):71–8. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- 93.Foungbe S, Kouassi G, Kablan JB, Marcy R. Study of Costus lucanusianus: plant juice, fraction combinations and pharmacologic estimation of natural product total activity. J Ethnopharmacol. 1991 Jul;33(3):221–6. doi: 10.1016/0378-8741(91)90080-w. [DOI] [PubMed] [Google Scholar]
- 94.Nelson HS, Chapman KR, Pyke SD, Johnson M, Pritchard JN. Enhanced synergy between fluticasone propionate and salmeterol inhaled from a single inhaler versus separate inhalers. J Allergy Clin Immunol. 2003 Jul;112(1):29–36. doi: 10.1067/mai.2003.1558. [DOI] [PubMed] [Google Scholar]
- 95.Wren JD, Bekeredjian R, Stewart JA, Shohet RV, Garner HR. Knowledge discovery by automated identification and ranking of implicit relationships. Bioinformatics. 2004 Feb 12;20(3):389–98. doi: 10.1093/bioinformatics/btg421. [DOI] [PubMed] [Google Scholar]
- 96.Wermuth CG. Multitargeted drugs: the end of the “one-target-one-disease” philosophy? Drug Discov Today. 2004 Oct 1;9(19):826–7. doi: 10.1016/S1359-6446(04)03213-1. [DOI] [PubMed] [Google Scholar]