Abstract
Neuroinflammation through the cytokine, tumor necrosis factor-alpha (TNF-α) is thought to play an important role in the pathogenesis of amyotrophic lateral sclerosis (ALS). We conducted a preliminary phase II trial of thalidomide, which reduces levels of TNF-α pre-transcriptionally and post-transcriptionally in vivo and has been shown to prolong disease duration and extend the lifespan of transgenic animal models of ALS. Patients who met diagnostic criteria for ALS received thalidomide at escalating doses to a target dose of 400 mg/day. The primary endpoints in the trial were the ALS Functional Rating Scale (ALSFRS) and pulmonary function testing (PFT) curves after nine months of thalidomide treatment that were compared to historical controls. Secondary endpoints were: survival stratified for newly diagnosed and progressive disease, toxicity, quality of life, and serum cytokine measurements. Twenty-three patients were enrolled, but only 18 were evaluable for the primary outcome. There was no improvement in the ALSFRS or PFT compared to historical controls. Thalidomide had several side-effects in our ALS patients. There was no significant shift in cytokine profile after treatment compared to baseline. In conclusion, treatment of ALS with the TNF-α inhibitor, thalidomide, does not appear to effectively modulate disease progression and can cause adverse effects.
Keywords: Thalidomide, tumor necrosis factor-alpha, neuroinflammation, therapy, clinical trials, survival
Introduction
The pathogenesis of amyotrophic lateral sclerosis (ALS), a common fatal neurodegenerative disease, is unknown although it appears to be multifactorial, most likely reflecting a multifaceted interaction of genetic (1) and environmental (2) factors. Recently, inflammatory mechanisms have been implicated in the pathogenesis of ALS. Experimental animal models support a pathological process involving dysfunction not only of motor neurons (MN) but of surrounding cells in their micro-environment including: astrocytes, microglia, oligodendrocytes, and endothelial cells (3). Human post mortem studies show astrocytic and microglial activation (4-6), pro-inflammatory factors such as interleukin (IL)-6, IL-lβ, and elevated TNF-α in the cerebrospinal fluid, serum, and spinal cord tissue (7-9). In the mutant SOD1 transgenic model of ALS, IL-1β, TNF-α, and inducible nitric oxide synthase (iNOS) levels are increased (10-13).
TNF-α has been immunohistochemically localized to MN in an ALS mouse model (10). In the G93A-SOD1 mouse, the TNF-α receptor p55 is up-regulated between presymptomatic and symptomatic stages of disease suggesting that TNF-α and its receptors may connect inflammation to apoptosis in ALS (14). In the same mouse model, it has been shown that the messages and protein levels for TNF-α along with several other cytokines are simultaneously increased, leading to neuroinflammation principally driven by TNF-α, but potentiated by several costimulating cytokines and chemokines (13-15).
Kiaei et al. (16) more recently demonstrated that treatment of a transgenic mouse model of ALS with both thalidomide and its analog lenalidomide attenuated weight loss, enhanced motor performance, decreased motor neuron cell death, and significantly increased lifespan. The immunoreactivity of TNF-α and its superfamily member fibroblast-associated cell-surface ligand (FasL), markers of apoptosis, has been shown to be elevated early in disease in both ALS patients and in animal models (16). It is reduced in the MN of the lumbar spinal cord in mice treated with thalidomide and lenalidomide (16). Another interrelated study showed that G93A-SOD1 mutant mice with a homozygous FasL mutant showed a modest but statistically significant extension of survival, and reduced loss of motor neurons (17). A TNF-α knockout in a mutant SOD1 transgenic mouse demonstrated no effect on motor neuron degeneration or disease, calling into question TNF-α’s role alone in ALS pathogenesis (18).
Nevertheless, TNF-α also impairs axonal transport and causes cell death by affecting anterograde movement (19,20). We have found TNF-α to inhibit anterograde transport of mitochondria in vitro leading to clustering of the mitochondria in the cell body and axonal hillock areas (21), a histological pattern also seen in anterior horn cells in human ALS (22). Taken together, these findings may suggest an important mechanistic link between TNF-α signaling and MN degeneration. Such a finding provides a mechanistic link between cytokine activation and axonal transport in the pathogenesis of ALS.
In vitro and in vivo studies show that thalidomide selectively reduces levels of TNF-α by accelerating the degradation of TNF-α mRNA encoding protein and facilitating the increased turnover of TNF-α mRNA at the post-transcriptional level (23). We conducted a phase II study to evaluate the safety and efficacy of thalidomide in ALS patients. We hypothesized that thalidomide would be safe and would slow the rate of decline of ALS as measured by ALSFRS and forced vital capacity (FVC). We conducted a single arm open label phase II study to evaluate the safety and effect of thalidomide on ALS patients. We hypothesized that thalidomide would be safe and would slow the rate of decline of ALS as measured by ALSFRS and FVC.
Methods
Patient selection and study design
This was an open label single institution study of thalidomide for patients who had clinically proven disease according to the El Escorial criteria for ALS (24). Patients with significant sensory abnormalities, dementia, other serious neurological diseases, uncompensated medical illness, substance abuse and debilitating psychiatric illness were excluded. Patients with pre-existing pulmonary disorders not attributed to the ALS were excluded. FVC was greater than or equal to 65% of predicted value. Patients were not on concurrent investigational medication. ALS patients allowed to enroll were all between the ages of 18 and 80 years, had disease duration less than or equal to five years, and had stage I or II ALS as defined by Sinaki and Mulder (25).
An ALSFRS of ≥ 30 at the onset of the trial was required. Adequate bone marrow, hepatic and renal function documented within 14 days prior to study entry included: absolute neutrophil count (neutrophils and bands) ≥1.5 × 109/1, platelets ≥ 100 × 109/1, hepatic transaminases (AST or ALT) ≤ 5.0 times the upper limits of normal, total bilirubin ≤ 1.5 times the upper limits of normal, serum creatinine ≤1.5 times the upper limit of normal or estimated creatinine clearance ≥ 60 ml/min. Patients who used any medications for ALS were allowed to remain on them for the duration of the study.
The trial was approved by the Committee for the Protection of Human Subjects of Dartmouth College and reviewed quarterly by the data and safety monitoring committee of the Norris Cotton Cancer Center. Eligible patients who wished to participate in the study and signed informed consent had baseline evaluations including: 1) medical history and physical examination; 2) liver functions, complete blood count, thyroid stimulating hormone; 3) disease status by physical examination and electrodiagnostic studies as outlined in Table I.
Table I.
Timetable of baseline and subsequent evaluations in thalidomide trial.
| Observations | Initial visit | Month 1 | Month 2 | Month 3 | Month 4 | Month 5 | Month 6 | Month 7 | Month 8 | Month 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Interval history and PE | X | X | X | X | ||||||
| Weight, BP, Temp, Pulse rate |
X | X | X | X | X | X | X | X | X | X |
| ALSFRS | X | X | X | X | ||||||
| QoL-36 | X | X | X | X | ||||||
| Toxicity assessment (CTC) | X | X | X | X | ||||||
| PFTs (FVC) | X | X | X | X | ||||||
| Liver function tests | X | X | X | X | ||||||
| CBC, TSH | X | |||||||||
| Cytokine profile | X | X | X | X | ||||||
| Pregnancy testing1 | X | X | X | X | X | X | X | X | X | X |
| S.T.E.P.S. Program Survey (Birth Control Counseling) |
X | X | X | X | X | X | X | X | X | X |
women of childbearing potential.
Between February 2005 and November 2007 there were 21 patients with sporadic and two patients with familial ALS (FALS) included. Thalidomide was started at a dose of 100 mg/day, escalating every two weeks by 100 mg/day to a target dose of 400 mg/day as suggested in the literature for other diseases (26). All patients were required to enroll in the FDA-mandated System for Thalidomide Education and Prescription Safety (S.T.E.P.S.®) Program. When indicated, pregnancy testing was performed as outlined in S.T.E.P.S. Program (http://www.thaiomid.com/steps_program.aspx). A cycle was defined as 90 days of daily oral thalidomide. If a patient stopped thalidomide for 10 days or more during the first cycle they were considered unevaluable and were instructed to stop medication. If they finished one cycle they were considered to be evaluable. Patients were evaluated after each cycle and treated for three cycles at which time the primary outcome was evaluated (see Table I).
Patients with gastrointestinal abnormalities including inability to take oral medication, requirement for intravenous alimentation, prior surgical procedures affecting absorption, or active peptic ulcer disease, were excluded. Patients with dementia, significant cardiac disease and hypercoagulability were also excluded. Thalidomide has the tendency to cause constipation. Standard bowel regimen used in our clinic was instituted when there were any signs of constipation.
Concomitant medications
No medication other than that which the patients were taking at the beginning of the study or experimental anti-ALS medications for the primary disease were permitted while the subject was on study. Many patients were taking supplemental medications at the onset of the trial such as creatine, coenzyme Q, lipoic acid and N-acetyl-cysteine and were allowed to continue them during the trial. Several patients were on riluzole at the onset of the study and were allowed to continue the medication. Table I shows the schedule for evaluations.
Primary endpoints
The main endpoints were measured using functional testing ALSFRS and FVC every three months of receiving thalidomide for a total of nine months and compared to data on the natural history of ALSFRS and FVC in patients not receiving therapy, as has been reported in the literature (27). Datasets from these studies were kindly given to us by Merit Cudkowicz (Massachusetts General Hospital, Boston, MA).
Secondary endpoints
Safety was a secondary endpoint for this phase II clinical trial and any patient who received treatment on this protocol was evaluable for adverse events. Each patient was assessed for the development of any adverse events, and dose modifications were made based on these events. Toxicities were graded on a scale of 0 to 4, using the common toxicity criteria (CTC) of the National Cancer Institute. This study utilized the CTC scale version 3.0 for toxicity and adverse event reporting (http://ctep.info.nih.gov).
Survival and progression-free survival were also followed. Survival was assessed from the date of enrollment in this clinical trial to the date of patient death, due to any cause, or to the last date the patient was known to be alive. Progression-free survival was assessed for each patient as the time from enrollment in this clinical trial until the patient reached objective disease progression or began another chemotherapy regimen. All patients were followed during the study and then until death, although some patients who have gone through the trial have not yet died.
QoL-36
This simple questionnaire has been used effectively in previous studies as a means to monitor therapeutic response with ALS (28,29). This was a preliminary endpoint since there is no published adequate control group with which to compare our patients’ scores.
Cytokine analysis
Serum samples were obtained from patients at three-month intervais and stored at −140°C until the cytokine assay. Samples were centrifuged at 16,000 × g for 3 min at 4°C and the aqueous layer removed for the cytokine assay. Cytokines were analyzed by the Immune Monitoring Laboratory (IML) in the Norris Cotton Cancer Center (NCCC) at the Dartmouth-Hitchcock Medical Center using the Bio-Plex Cytokine Assay System (Bio-Rad Laboratories, Hercules, CA), a bead-based multiplex detection system for cytokine and other proteins. Cytokine concentrations were calculated by reference to a standard curve for each cytokine derived using various concentrations of the cytokine standards assayed in the same manner as the serum samples in triplicate. High and low spikes (supernatants from stimulated human lymphocytes and dendritic cells) were included to determine cytokine recovery. All assays were carried out directly in a 96-well filtration plate (Millipore, Billerica, MA) at room temperature and protected from light. The fluorescence intensity of the beads was measured using the Bio-Plex array reader. Bio-Plex Manager software with five-parametric-curve fittine (Bio-Rad technical note 2861 at www.bio-rad.com) was used for data analysis.
Statistical analysis
The continuons clinical endpoints in this study, ALSFRS and FVC, were measured longitudinally with changes from baseline tested for a mean of zero using a paired t-test. The changes over time (e.g. slope) were estimated using a mixed effects model. Comparisons were made between patients in this study and the controls in the topiramate trial for ALSFRS, using FVC at specific follow-up times using two-sample t-tests. Cytokines after baseline (i.e. 3, 6 and 9 months) were compared to baseline levels using paired t-tests.
Survival was estimated using a Kaplan-Meier method, and survival compared between patients in this study and the topiramate controls using a log-rank test. The response to treatment was evaluated at nine months based on the slope of the expected average decline compared to historical controls obtained from a previous study (27). A 95% CI for the response rate was constructed using exact methods. Adverse events were tabulated according to frequency and grade. For cytokine analysis, a sample of pooled human AB sera was evaluated to obtain reference of normal serum levels.
This open label phase II study with historical controls was designed to enroll 24 patients and perform follow-up every three months, for at least six months, in order to have sufficient power (i.e. 80%) to detect an improvement in the six-month change in the primary endpoint ALSFRS of 3 points compared to historical controls. This was based on a type 1 error rate of 5% and the finding in the historical controls of a six-month change in ALSFRS of mean −5.3 with a standard deviation of 4.1. The statistical software employed was R: A Language and Environment for Statistical Computing (http ://www.R-project.org) and GraphPad Prism 4.03 for Windows (www.graphpad.com).
Results
Clinical data
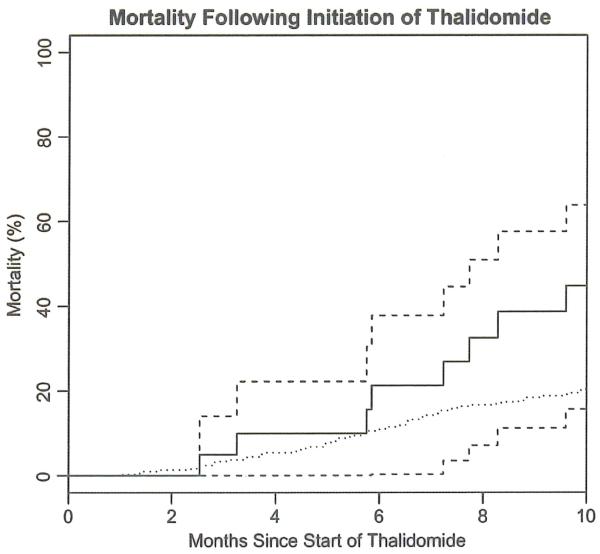
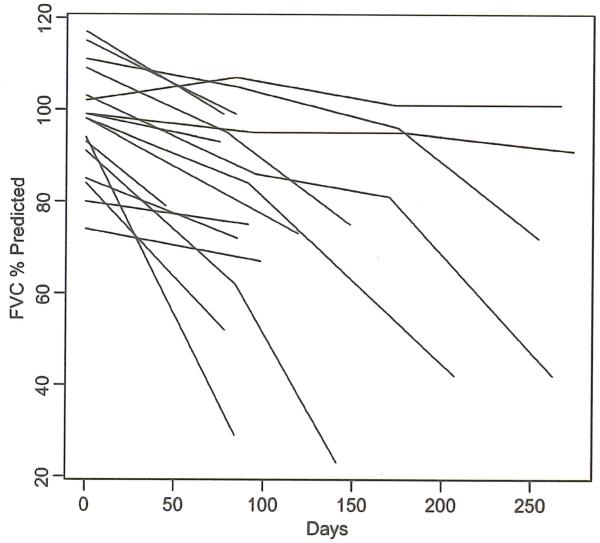
Twenty-three patients were enrolled (five of the 23 were not evaluable for any parameter). Age, gender, ALSFRS at onset of starting study, maximum doses of thalidomide achieved in each patient and adjuvant therapy (riluzole and NSAIDs) are all listed in Table II. The results of the ALSFRS, FVC, QoL-36 and survival outcomes are shown in Figures 1-4. Thalidomide did not provide any benefit for the ALS patients in this trial based on the chosen endpoints. Thalidomide subjects overall experienced a greater (but not statistically significant) decline in ALSFRS compared to control subjects from the topiramate trial (27). There was a significant decline in the SF-36 at three months after starting thalidomide (analyzed without a control group). Mortality was higher in the thalidomide group than in the control subjects (Figure 4) (27) (a significant difference in survival between the two groups (p =0.02)). Subjects in the thalidomide study had significant decline from baseline in FVC (Figure 2) compared to the control subjects (27). This decline was significantly greater at three months (p =0.007), calculated using means and standard errors from each trial and a t-distribution approximation using Satterthwaite’s method.
Table II.
Clinical summary of ALS patients.
| patient ID | Patient age (years) |
Patient gender |
Duration of symptoms before beginning thalidomide (months) |
ALSFRS score at onset |
Thalidomide (max. dose in mg/day)* |
Other therapy |
|
|---|---|---|---|---|---|---|---|
| Riluzole | NSAIDs | ||||||
| Patient 1 | 47 | M | 15 | 30 | 400 | No | No |
| Patient 2 | 63 | F | 36 | 35 | 400 | No | No |
| Patient 3 | 62 | M | 28 | 30 | 50 | No | No |
| Patient 4 | 38 | M | 4 | 37 | 400 | Yes | No |
| Patient 5 | 61 | F | 7 | 33 | 50 | No | Yes |
| Patient 6 | 68 | M | 36 | 36 | 200 | No | No |
| Patient 7 | 48 | F | 12 | 36 | 400 | Yes | No |
| Patient 8 | 46 | M | 7 | 30 | 300 | No | No |
| Patient 9 | 43 | F | 18 | 31 | 400 | Yes | No |
| Patient 10 | 74 | F | 36 | 30 | 50 | No | No |
| Patient 11 | 55 | M | 24 | 38 | 200 | No | No |
| Patient 12 | 74 | M | 11 | 32 | 50 | No | No |
| Patient 13 | 51 | F | 11 | 33 | 200 | No | No |
| Patient 14 | 41 | M | 9 | 39 | 400 | Yes | No |
| Patient 15 | 57 | M | 8 | 40 | 400 | Yes | No |
| Patient 16 | 58 | M | 9 | 34 | 400 | Yes | No |
| Patient 17 | 53 | M | 22 | 37 | 400 | Yes | No |
| Patient 18 | 61 | M | 12 | 36 | 300 | Yes | No |
| Patient 19 | 63 | F | 12 | 31 | 100 | No | No |
| Patient 20 | 49 | M | 21 | 34 | 100 | No | No |
| Patient 21 | 70 | M | 36 | 34 | 100 | No | No |
| Patient 22 | 62 | F | 34 | 34 | 100 | No | No |
| Patient 23 | 74 | M | 6 | 33 | 400 | No | No |
Mean age (years): 57.3+/−10.8
Mean ALSFRS-R: 34.0+/−3.0
Column reflects the highest dose taken by a patient for the longest period of time. The initial protocol stated that thalidomide would be administered as a daily dose in an escalating fashion starting at a dose of 100 mg/day. Doses would be escalated every two weeks by 100 mg/ day up to a target of 400 mg/day.
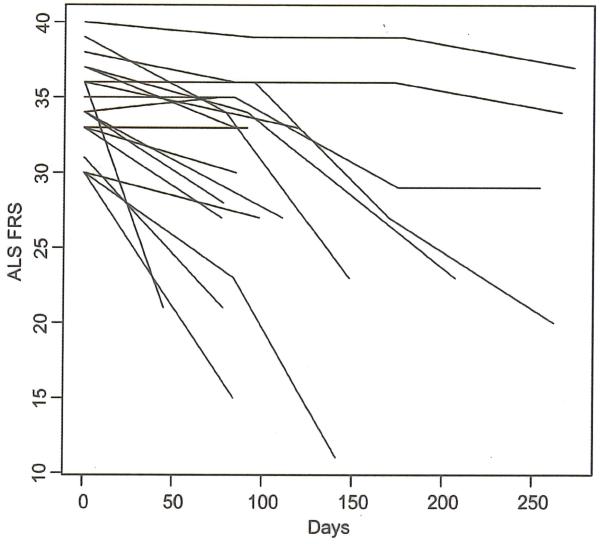
Figure 1.
ALSFRS over time for each of the 19 subjects in the study that had at least one post-baseline measurement for this parameter.
| Month | Mean changes from baseline |
P (t-test) |
p (Signed Wil- coxon) |
|---|---|---|---|
| 3 | −4.7 (95% CI: −6.9, −2.5) |
0.0003 | 0.0006 |
| 6 | −9.6 (95% CI: −16.0, −3.1) |
0.011 | 0.016 |
| 9 | −7.3 (95% CI: −16.7, +2.2) |
0.093 | 0.125 |
| In topiramate controls: Month Mean changes from baseline |
|||
| 3 | −2.2±0.2 | ||
| 6 | −5.0±0.5 | ||
| 9 | −6.5±0.5 | ||
Figure 4.
Mortality with treatment (dotted line is mortality for controls in the topiramate clinical trial (27)). There was a significant (p =0.02) difference in survival between the two groups. Dashed lines are confidence intervais. Bold line is Kaplan-Meier estimate.
Figure 2.
FVC at baseline: mean of 97, range 74–117. X axis is days. Y axis is percentage expected.
| Month | Mean changes from baseline |
P (t-test) |
p(Signed Wilcoxon) |
|---|---|---|---|
| 3 | −16.5 (95% CI: −24.5, −8.4) |
0.0005 | 0.0005 |
| 6 | −28.6 (95% CI: −52.3, −4.9) |
0.025 | 0.016 |
| 9 | −27.3 (95% CI: −71.7, 17.2) |
0.145 | 0.250 |
| In the MGH topiramate controls: Month Mean changes from baseline |
|||
| 3 | −6.0 ± 0.7 | ||
| 6 | −12.8 ± 1.0 | ||
| 9 | −17.2 ± 1.6 | ||
The characteristics of patients included in this trial and of the historical control can be observed in Table II. There are no significant differences in baseline clinical and PFT characteristics. In our trial, the average duration of illness before starting thalidomide was 17.8+/−11.5 months compared to 18.0 months +/−8.0 months for the topiramate control group (27). Other baseline characteristics from the thalidomide trial were a predicted FVC of 97+/−13.5% and ALSFRS of 34.0+/−3.0 compared to the topiramate control group where there was a predicted FVC of 86+/−24.5% and ALSFRS of 30.3+/−5.9. During the thalidomide trial 21.7% of patients began treatment with non-invasive positive pressure ventilation (NIPPV); 17.4%) underwent feeding tube placement and a single patient (4.4%) underwent a tracheostomy at the very end of his time in the trial. Similar values were seen in the topiramate placebo group where 20.6% began treatment with NIPPV; 20.6% underwent feeding tube placement and 3.1% underwent a tracheostomv.
Adverse events
A number of adverse events were documented during this trial (Table III). The more serious adverse events included bradycardia and possible thalidomide-related deep vein thrombosis (DVT), although this was not confirmed by autopsy. Some of the reported events may have had more to do with the disease process of ALS than the medication itself, such as a decline in FVC. The most common adverse events were rash, sedation and constipation. Sedation generally cleared as the patients adapted to the medication. Rash was temporary and, with a brief discontinuation of the thalidomide, the rash did not return with restarting the medication. Constipation was generally treatable with diet and medications, but one patient chose to discontinue the medication entirely because of this side-effect. Only 10 patients were able to tolerate a full dose of 400 mg/day and we attempted to maximize the dose tolerated in patients willing to continue thalidomide (Table II). Patients were followed for nine months or until death, whichever came first. Five patients took thalidomide for at least nine months. After the nine-month study, we did continue to follow patients and three patients stayed on thalidomide after the planned nine months.
Table III.
Adverse events.
| Adverse event | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
TOTAL |
|---|---|---|---|---|---|
| Ataxia | 4 | 4 | 0 | 0 | 8 |
| Constipation | 10 | 6 | 6 | 0 | 22 |
| Depression | 5 | 1 | 0 | 0 | 6 |
| DVT | 1 | 0 | 3 | 0 | 4 |
| Fatigue | 11 | 3 | 1 | 0 | 15 |
| Muscles weakness | 5 | 3 | 1 | 0 | 9 |
| Neuropathy | 9 | 1 | 0 | 0 | 10 |
| Pulmonary embolism |
0 | 0 | 0 | 1 | 1 |
| Rash | 6 | 4 | 1 | 0 | 11 |
| Sinus bradycardia | 9 | 0 | 0 | 0 | 9 |
| Sinus tachycardia | 2 | 0 | 0 | 0 | 2 |
| WBC (low) | 2 | 0 | 0 | 0 | 2 |
| Total adverse events |
64 | 22 | 12 | 1 | 99 |
Neuropathological examination
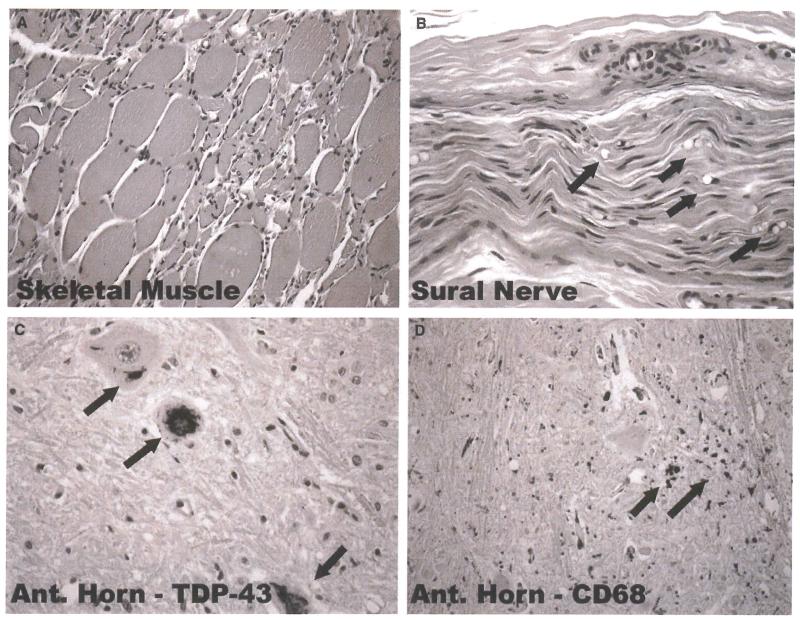
Similar pathological findings were observed for all three cases that underwent autopsy. Sural nerves were examined in two of the three cases, and spinal cords and brains were examined in three of the cases. Sural nerve histopathology demonstrated occasional fibers undergoing Wallerian degeneration (see Figure 5). This particular case was of a patient with FALS treated with thalidomide for 13 months, although it was not determined what genetic mutation this individual carried. Sections of motor and sensory cortex were examined in all three cases. Histopathological findings in the brain and spinal cord in these cases were similar to those seen in non-thalidomide-treated ALS patients at end-stage of disease (Figure 5).
Figure 5.
Representative histopathological and immunohistochemical changes observed at autopsy in ALS patient who had taken thalidomide for 13 months. Skeletal muscle displays typical grouped atrophy of myofibers (A). Sural nerve3 which usually shows no or minimal changes in most ALS patients, displays active Wallerian degeneration in patients who have received thalidomide (arrows point to digestion chambers of Cajal, B). Degenerating lower motor neurons within the spinal cord anterior horns display cytoplasmic skein-like aggregates (arrows) that immunostain for TDP-43 (C) and have surrounding microgliosis {CD-68 immunostaining} (D).
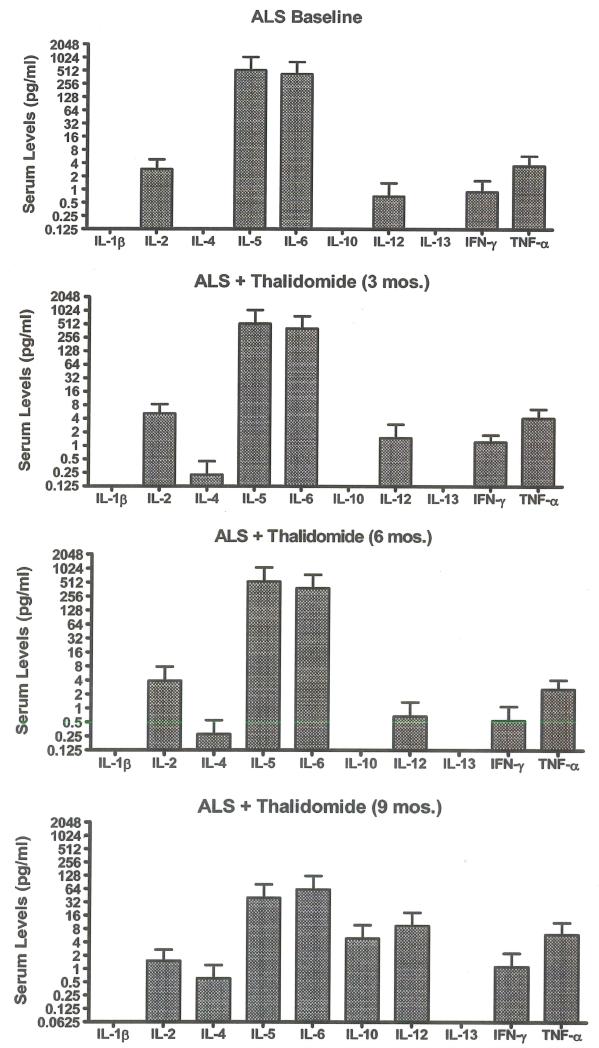
Serum cytokine profile
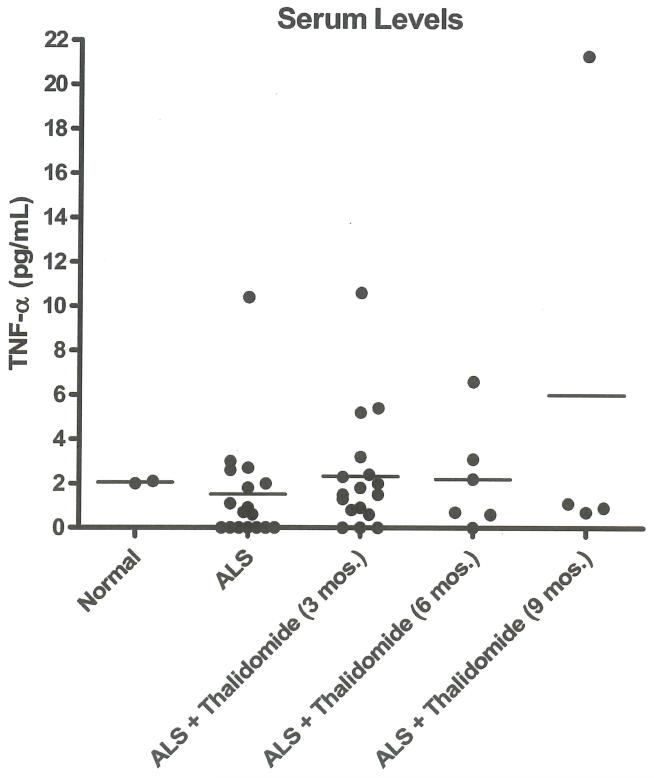
Four of 23 patients enrolled were able to provide serum samples at baseline and 3, 6, and 9 months after initiation of thalidomide treatment. No significant change in average cytokine concentration was determined among these patients (Figure 6). IL-lb values were all below the detection limit of analysis. Serum levels of TNF-α from all analyzed patients are represented in Figure 7, which shows the variability of serum TNF-α over nine months. There was a small increase in average TNF-α concentration from 17 patients analyzed at three months after initiation of thalidomide treatment, but this increase was not observed at six (six patients) or nine (four patients) months. The baseline level of regulated-upon-activation, normal T-cell expressed and secreted (RANTES) in serum from nine ALS patients (16754.0+/−1688.5 pg/ml) was 7.5-fold higher than in a pooled sample of human control serum (2240.5 +/− 21.5 pg/ml). No noticeable change in RANTES serum levels was observed in ALS patients with thalidomide treatment for three months (four patients) or 12 months (two patients).
Figure 6.
Average serum cytokine concentrations in four ALS patients who remained on thalidomide for nine months. Serum was collected prior to thalidomide treatment (baseline) and at 3, 6, and 9 months after thalidomide treatment. No cytokine had significantly higher or lower values compared to baseline values (paired t-test).
Figure 7.
TNF-α concentrations in pooled normal human sera and in sera from ALS patients taken prior to thalidomide treatment (baseline, n = 17), and at 3 (n = 17), 6 (n =6), and 9 (n =4) months after thalidomide treatment. There was only a small increase in TNF-α concentration at three months after thalidomide treatment compared to baseline (p =0.045, paired t-test).
Discussion
In this single-center phase II trial of thalidomide for ALS, we observed no significant effect in the course of the disease compared to historical controls. Furthermore, there appeared to be negative, albeit non consistently-significant, effects on survival, functional status, and pulmonary function compared to a historical control population. Although thalidomide was tolerated reasonably well, many patients were unable to reach the target dosage due to minor side-effects. Severe adverse events associated with thalidomide therapy included DVT and peripheral neuropathy, which are consistent with thalidomide’s known side-effect profile. Without the potential for a substantial positive impact in the course of the illness and the difficultes with compliance and side-effects observed in our study, any further clinical trials of thalidomide for ALS would seem unwarranted.
Thalidomide and its analog lenalidomide have been shown to have some benefit in the G93A-hmSODl ALS mouse model (16), but thalidomide did not show efficacy in our trial in human ALS patients. The lack of a beneficiai effect with thalidomide in human ALS patients could be related to a number of factors. One significant difference between animal studies and human studies is that animais are treated, relatively speaking, much sooner in their disease. Additionally, mouse and human ALS are likely quite different diseases. ALS animal models are of limited utility for extrapolating to human disease. Unfortunately, other than animal models, there are few alternatives for understanding human disease. The rodent model is a mutant SODl-related FALS (16) and while our cohort did include two familial cases, genetic testing was not positive for a SOD1 mutation by commercial testing. There are most certainly variations in the pathophysiology among sporadic ALS (SALS), non-mutant SODl-related FALS, and mutant SODl-related FALS diseases. A lack of response to therapeutic intervention for ALS with any treatment may also be related to the fact that the disease is very advanced by the time a diagnosis is made and the MN are markedly compromised, suggested both by previous histological (30-32) and electrodiagnostic (33-35) assessment.
The dosing of the thalidomide which was often at the low end of our target of 400 mg per day might be another reason for a lack of efficacy. The maximum dose in humans we used was 400 mg per day compared to that in mice (16) where thalidomide was used at a dose as high as 100 mg/kg per day, the positive efficacy of which on survival was shown to be in a dose-dependent manner (16). Thus, our patients, considering their average weight (60–70 kg) at the highest thalidomide dose were receiving at the very least 10 times less drug than the transgenic mice. It is clear that human subjects/patients would not tolerate a dose as high as 4000 mg per day. It is our experience with a previous clinical trial using thalidomide as a treatment for glioblastoma multiforme, that few patients were able to tolerate doses as high as 400 mg/day (36). It is possible that lenalidomide, which is more potent than thalidomide and has a different side-effect profile might be a more promising medication for ALS. Knowing the theoretical potential for such a medication in ALS suggests that a clinical trial using lenalidomide might be justified.
Very few of our patients were taking only thalidomide for ALS. Most were also taking creatine, coenzyme Q, lipoic acid, N-acetyl-cysteine and some were on riluzole prior to starting thalidomide and according to the protocol were allowed to continue it during the trial. Certainly in the realm of cancer therapy, a multiple drug approach has proven necessary for most successful therapeutic regimens. Some of the adjuvant medications taken by patients in our trial should have a neuroprotective effect while others might have anti-apoptotic effects and anti-inflammatory benefits. In any case, thalidomide, despite the fact that many of our patients were taking some of these or all of these medications, had no significant benefit, alone or in combination.
One could argue that using controls from a different trial makes this trial statistically weak. We recognize that this is a limitation of the trial and that double-blind placebo- controlled trials can more accurately assess therapeutic efficacy. Nevertheless, the clinical characteristics of our patients were very similar to the topiramate control patients. A prolonged duration of illness prior to starting a clinical trial might inadvertently select for patients with a milder form of ALS. Our patients had a similar duration of symptoms in relation to starting the thalidomide compared to the topiramate controls. Overall, there is no evidence to suggest that the thalidomide treated patients were treated in a more or less aggressive manner than the topiramate control patients.
Although many of the possible adverse events attributable to thalidomide may actually relate directly to the disease process in ALS, many adverse events did occur while our trial patients were on thalidomide (see Table III). Bradycardia in our trial patients was largely asymptomatic and hence we did not feel that the rhythm change was worrisome. A German thalidomide/ALS trial similar to our own trial was terminated because of the high incidence of bradycardia (37). Efficacy in this trial was not reported.
Peripheral neuropathy is a common side-effect of thalidomide therapy (38-40). Many patients developed some minor sensory changes while on thalidomide, and at least one autopsy case of a patient with FALS treated for 13 months demonstrated evidence of peripheral neuropathy on sural nerve biopsy (Figure 5). We did not follow these patients who developed sensory symptoms with electrodiagnostics studies. Over a period of 3–4 months this patient started to note painless, paresthesias in her distal lower extremities. Of interest, her spasticity improved. We noted painless, paresthesias isolated to the lower extremities in several patients in association with a reduction in lower extremity deep tendon reflexes and retention of positive Babinski responses when initially present. We attribute this observation to the development of peripheral neuropathy. We did not observe the peripheral neuropathy symptoms or findings to reverse during the time frame of the trial. The less severe side-effects of rash and sedation were generally reversible by lowering or stopping the thalidomide and then restarting at a lower dose.
There is an increased incidence of deep venous thrombosis (DVT) in patients with ALS (41,42), while thalidomide has also been associated with an increased risk. The incidence of DVT in our patients was 17%, which was significantly higher (p =0.003 by one-sample exact binomial test) than the rate of 2.7% in non-thalidomide-treated ALS patients reported by Qureshi et al. (42). The incidence of DVT and pulmonary embolism (PE) together in the control group of the topiramate trial was 1.0%, whereas it was 12% in those patients receiving topiramate, demonstrating that topiramate has the known potential to cause PE/DVT as well. The predisposition to DVT in ALS may be related to many factors: 1) muscular weakness and atrophy in the legs reducing venous return and hence increasing blood congestion; 2) stasis in the legs from lack of ambulation; 3) reduced fluid intake causing dehydration and hence facilitating thrombosis; and 4) hypoxemia leading to endothelial cell damage, increased free radicals, and venous wall relaxation, causing decreased flow leading to stasis (41,42).
The small sample size with which we had to work may have limited the power to detect significant changes in cytokine levels with respect to baseline. Already at extremely low serum levels, cytokines are difficult to measure given their variability. Additionally, we had neither adequate healthy control serum to serve as a standard nor a placebo arm in the study to monitor changes in cytokines from ALS patients not treated with thalidomide. Our pooled human serum served solely as a general reference point to where normal serum cytokine values would fall but precluded statistical comparisons with experimental samples. An increase in both serum and CSF levels of RANTES has been reported in ALS patients previously (43,44), and we also noted a higher level in our ALS population compared to controls. No noticeable change in RANTES serum levels was observed in our ALS patients treated with thalidomide.
Although our data do not have the power to assert statistical changes in TNF-α concentrations, TNF-α and its receptor have been shown to be elevated in the serum and plasma at later stages in ALS (9,45,18). One could reasonably conclude that, although thalidomide was not shown to significantly decrease serum TNF-α levels in our study, it may have prevented or delayed the usual increase in TNF-α levels over time in our ALS study patients. However, it cannot be confirmed that this is the case. Even if thalidomide does decrease serum levels of TNF-α, it is unclear what relevance this has, given the negative outcome of this trial. CSF levels of cytokines, while not monitored in this study, might be a more relevant source of cytokine biomarkers for future studies.
In conclusion, we found no beneficiai effect in treating ALS patients with thalidomide and a trend toward (not consistently significant for all parameters and time points measured) a negative effect with this agent. A similar lack of efficacy has been found in many other clinical ALS trials with other medications that initially showed promise in animal model studies. The lack of efficacy might be related to insufficient doses, a different pathologie mechanism of ALS in humans compared to animal models or a misguided target for ALS with thalidomide. It is likely that inflammation has variable roles at different stages of disease in ALS: 1) causing neurotoxicity, and 2) maintaining homeostasis as neurons die. A therapy more targeted at role 1 without compromising role 2 might be more effective, although further experimental evidence is crucial to understanding the pathophysiology of neuroinflammation and ALS.
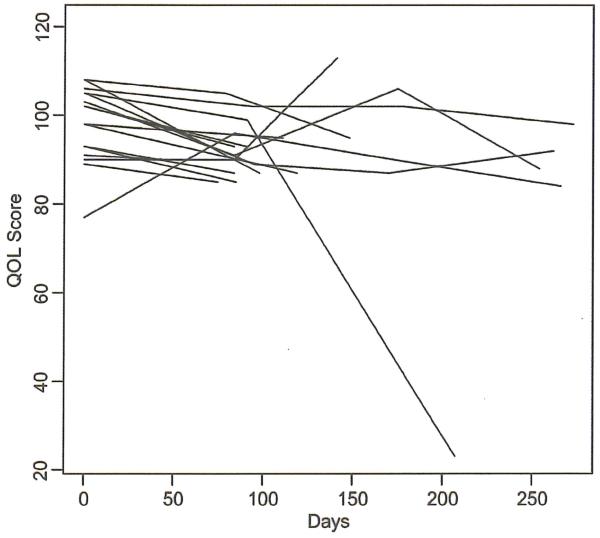
Figure 3.
SF 36 at baseline: mean of 98.0, range 77–108.
| Month | Mean changes from baseline |
P (t-test) |
p (Signed Wilcoxon) |
|---|---|---|---|
| 3 | −5.9 (95%CI: −10.5, −1.3) |
0.016 | 0.010 |
| 6 | −11.1 (95%CI: −42.9, 20.6) |
0.42 | 0.61 |
| 9 | −7.3 (95%CI: −23.1, 8.6) |
0.24 | 0.25 |
Acknowledgements
We are indebted to Celgene Corporation who supplied thalidomide and sponsored the trial. We are also indebted to Dr. Merit Cudkowicz (Massachusetts General Hospital, Boston, Massachusetts) for supplying us with the control data used in this trial. Cytokine assays were carried out by the Immune Monitoring Laboratory, which is supported in part by a Core Grant of the Norris Cotton Cancer Center and a COBRE award. We are also indebted to Patricia Alden for her administrative help. Finally we would like to thank all the ALS patients and their families who participated in this trial.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Brown RH., Jr. Amyotrophic lateral sclerosis: recent insights from genetics and transgenic mice. Cell. 1995;80:687–92. doi: 10.1016/0092-8674(95)90346-1. [DOI] [PubMed] [Google Scholar]
- 2.Cox PA, Sacks OW. Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology. 2002;58:956–9. doi: 10.1212/wnl.58.6.956. [DOI] [PubMed] [Google Scholar]
- 3.Boillee S5, van de Velde C, Cleveland DW. ALS: a disease of motor neurons and their non-neuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Hirano A. Neuropathology of ALS: an overview. Neurology. 1996 Oct;47(4 Suppl 2):S63–S66. doi: 10.1212/wnl.47.4_suppl_2.63s. [DOI] [PubMed] [Google Scholar]
- 5.Schiffer D, Cordera S, Cavalla P, Migheli A. Reactive astrogliosis of the spinal cord in amyotrophic lateral sclerosis. Journal of the Neurological Sciences. 1996;139:27–33. doi: 10.1016/0022-510x(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 6.Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249–56. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, et al. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model (see comment) Science. 2000;288:335–9. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- 8.Sekizawa T, Openshaw H, Ohbo K, Sugamura K, Itoyama Y, Niland JC. Cerebrospinal fluid interleukin 6 in amyotrophic lateral sclerosis: immunological parameter and comparison with inflammatory and non-inflammatory central nervous system diseases. Journal of the Neurological Sciences. 1998;154:194–9. doi: 10.1016/s0022-510x(97)00228-1. [DOI] [PubMed] [Google Scholar]
- 9.Poloni M, Facchetti D, Mai R, Micheli A, Agnoletti L, Francolini G, et al. Circulating levels of tumor necrosis factor-alpha and its soluble receptors are increased in the blood of patients with amyotrophic lateral sclerosis. Neuroscience Lett. 2000;287:214. doi: 10.1016/s0304-3940(00)01177-0. [DOI] [PubMed] [Google Scholar]
- 10.Ghezzi P, Bernardini R, Giuffrida R, Bellomo M, Manzoni C, Comoletti D, et al. Tumor necrosis factor is increased in the spinal cord of an animal model of motor neuron degeneration. European Cytokine Network. 1998;9:139–44. [PubMed] [Google Scholar]
- 11.Aimer G, Guegan C, Teismann P, Naini A, Rosoklija G, Hays AP, et al. Increased expression of the pro-inflammatory enzyme cyclooxygenase-2 in amyotrophic lateral sclerosis. Annals of Neurology. 2001;49:176–35. [PubMed] [Google Scholar]
- 12.Alaedini A, Latov N. Detection of anti-GMl ganglioside antibodies in patients with neuropathy by a novel latex agglutination assay. J Immunoassay. 2000;21:377–86. doi: 10.1080/01971520009349543. [DOI] [PubMed] [Google Scholar]
- 13.Elliott JL. Cytokine up-regulation in a murine model of familial amyotrophic lateral sclerosis. Brain Research, Molecular Brain Research. 2001;95:172–8. doi: 10.1016/s0169-328x(01)00242-x. [DOI] [PubMed] [Google Scholar]
- 14.Hensley K, Floyd RA, Gordon B, Mou S, Pye QN, Stewart C, et al. Temporal patterns of cytokine and apoptosis-related gene expression in spinal cords of the G93A-SOD1 mouse model of amyotrophic lateral sclerosis. Journal of Neurochemistry. 2002;82:365–74. doi: 10.1046/j.1471-4159.2002.00968.x. erratum appears in J Neurochem. 2002;82:1570. [DOI] [PubMed] [Google Scholar]
- 15.Yoshihara T, Ishigaki S, Yamamoto M, Liang Y, Niwa J, Takeuchi H, et al. Differential expression of inflammation-and apoptosis-related genes in spinal cords of a mutant SOD1 transgenic mouse model of familial amyotrophic lateral sclerosis. Journal of Neurochemistry. 2002;80:158–67. doi: 10.1046/j.0022-3042.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- 16.Kiaei M, Petri S, Kipiani K, Gardian G, Choi DK, Chen J, et al. Thalidomide and lenalidomide extend survival in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2006;26:2467–73. doi: 10.1523/JNEUROSCI.5253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petri S, Kiaei M, Wille E, Calingasan NY, Flint Beal M. Loss of Fas ligand-function improves survival in G93A-transgenic ALS mice. J Neurol Sci. 2006;251:44–9. doi: 10.1016/j.jns.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Gowing G, Dequen F, Soucy G, Julien JP. Absence of tumor necrosis factor-alpha does not affect motor neuron disease caused by superoxide dismutase 1 mutations. J Neurosci. 2006;26:11397–102. doi: 10.1523/JNEUROSCI.0602-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vos K, Severin F, van Herreweghe F, van Compernolle K, Goossens V, Hyman A, et al. Tumor necrosis factor induces hyperphosphorylation of kinesin light chain and inhibits kinesin-mediated transport of mitochondria. Journal of Cell Biology. 2000;149:1207–14. doi: 10.1083/jcb.149.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vos K, Goossens V, Boone E, Vercammen D, van Compernolle K, van Denabeele P, et al. The 55-kDa tumor necrosis factor receptor induces clustering of mitochondria through its membrane-proximal region. J Biol Chem. 1998;273:9673–80. doi: 10.1074/jbc.273.16.9673. [DOI] [PubMed] [Google Scholar]
- 21.Stommel EW, van Hoff RM, Graber DJ, Bercury KK, Langford GM, Harris BT. Tumor necrosis factor-alpha induces changes in mitochondrial cellular distribution in motor neurons. Neuroscience. 2007;146:1013–9. doi: 10.1016/j.neuroscience.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki S, Iwata M. Impairment of fast axonal transport in the proximal axons of anterior horn neurons in amyotrophic lateral sclerosis. Neurology. 1996;47:535–40. doi: 10.1212/wnl.47.2.535. [DOI] [PubMed] [Google Scholar]
- 23.Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993;177:1675–80. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial’Clinical limits of amyotrophic lateral sclerosis’ workshop contributors (comment) Journal of the Neurological Sciences. 1994;124(Suppl):96–107. doi: 10.1016/0022-510x(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 25.Sinaki M, Mulder DW. Rehabilitation techniques for patients with amyotrophic lateral sclerosis. Mayo Clin Proc. 1978;53:173–8. [PubMed] [Google Scholar]
- 26.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–39. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 27.Cudkowicz ME, Shefner JM, Schoenfeld DA, Brown RH, Jr, Johnson H, Qureshi M, et al. A randomized, placebo-controlled trial of topiramate in amyotrophic lateral sclerosis. Neurology. 2003;61:456–64. doi: 10.1212/wnl.61.4.456. [DOI] [PubMed] [Google Scholar]
- 28.Neudert C, Wasner M, Borasio GD. Individual quality of life is not correlated with health-related quality of life or physical function in patients with amyotrophic lateral sclerosis. J Palliat Med. 2004;7:551–7. doi: 10.1089/jpm.2004.7.551. [DOI] [PubMed] [Google Scholar]
- 29.Bongioanni P, Reali C, Sogos V. Ciliary neurotrophic factor (CNTF) for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD004302.pub2. CD004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsukagoshi H, Yanagisawa N, Oguchi K, Nagashima K, Murakami T. Morphometric quantification of the cervical limb motor cells in controls and in amyotrophic lateral sclerosis. J Neurol Sci. 1979;41:287–97. doi: 10.1016/0022-510x(79)90089-3. [DOI] [PubMed] [Google Scholar]
- 31.Atsumi T, Miyatake T. Morphometry of the degenerative process in the hypoglossal nerves in amyotrophic lateral sclerosis. Acta Neuropathol. 1987;73:25–31. doi: 10.1007/BF00695498. [DOI] [PubMed] [Google Scholar]
- 32.Sobue G, Hashizume Y, Sahashi K, Takahashi A, Mukai E, Matsuoka Y, et al. Amyotrophic lateral sclerosis: lack of central chromatolytic response of motor neurocytons corresponding to active axonal degeneration. Arch Neurol. 1983;40:306–9. doi: 10.1001/archneur.1983.04050050074011. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal A, Nicholson G. Detection of preclinical motor neuron loss in SOD1 mutation carriers using motor unit number estimation. J Neurol Neurosurg Psychiatry. 2002;73:199–201. doi: 10.1136/jnnp.73.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feeney SJ, McKelvie PA, Austin L, Jean-Francois MJ, Kapsa R, Tombs SM, et al. Presymptomatic motor neuron loss and reactive astrocytosis in the SOD1 mouse model of amyotrophic lateral sclerosis. Muscle Nerve. 2001;24:1510–9. doi: 10.1002/mus.1176. [DOI] [PubMed] [Google Scholar]
- 35.Shefner JM, Brown RH, Jr, Cole D, Chaturvedi P, Schoenfeld D, Pastuszak K, et al. Effect of neurophilin ligands on motor units in mice with SOD1 ALS mutations. Neurology. 2001;57:1857–61. doi: 10.1212/wnl.57.10.1857. [DOI] [PubMed] [Google Scholar]
- 36.Fadul CE, Kingman LS, Meyer LP, Cole BF, Eskey CJ, Rhodes CH, et al. A phase II study of thalidomide and irinotecan for treatment of glioblastoma multiforme. J Neurooncol. 2008;90:229–35. doi: 10.1007/s11060-008-9655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer T, Maier A, Borisow N, Dullinger JS, Splettstosser G, Ohlraun S, et al. Thalidomide causes sinus bradycardia in ALS. J Neurol. 2008;255:587–91. doi: 10.1007/s00415-008-0756-3. [DOI] [PubMed] [Google Scholar]
- 38.Cavaletti G, Beronio A, Reni L, Ghiglione E, Schenone A, Briani C, et al. Thalidomide sensory neurotoxicity: a clinical and neurophysiologic study. Neurology. 2004;62:2291–3. doi: 10.1212/wnl.62.12.2291. [DOI] [PubMed] [Google Scholar]
- 39.Plasmati R, Pastorelli F, Cavo M, Petracci E, Zamagni E, Tosi P, et al. Neuropathy in multiple myeloma treated with thalidomide: a prospective study. Neurology. 2007;69:573–81. doi: 10.1212/01.wnl.0000267271.18475.fe. [DOI] [PubMed] [Google Scholar]
- 40.Tosi P, Zamagni E, Cellini C, Plasmati R, Cangini D, Tacchetti P, et al. Neurological toxicity of longterm (> 1 year) thalidomide therapy in patients with multiple myeloma. Eur J Haematol. 2005;74:212–6. doi: 10.1111/j.1600-0609.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 41.Kimura F. Increased incidence of deep venous thrombosis in ALS. Neurology. 2007;68:2046–7. doi: 10.1212/01.wnl.0000268589.18238.01. author reply 2047. [DOI] [PubMed] [Google Scholar]
- 42.Qureshi MM, Cudkowicz ME, Zhang H, Raynor E. Increased incidence of deep venous thrombosis in ALS. Neurology. 2007;68:76–7. doi: 10.1212/01.wnl.0000250444.30622.ee. [DOI] [PubMed] [Google Scholar]
- 43.Hensley IC, Fedynyshyn J, Ferrell S, Floyd RA, Gordon B, Grammas P, et al. Message and protein-level elevation of tumor necrosis factor alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal cords of the G93A-SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Dis. 2003;14:74–80. doi: 10.1016/s0969-9961(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 44.Rentzos M, Nikolaou C, Rombos A, Boufidou F, Zoga M, Dimitrakopoulos A, et al. RANTES levels are elevated in serum and cerebrospinal fluid in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scier. 2007;8:283–7. doi: 10.1080/17482960701419232. [DOI] [PubMed] [Google Scholar]
- 45.Babu GN, Kumar A, Chandra R, Puri SK, Kalita J, Misra UK. Elevated inflammatory markers in a group of amyotrophic lateral sclerosis patients from northern India. Neurochem Res. 2008;33:1145–9. doi: 10.1007/s11064-007-9564-x. [DOI] [PubMed] [Google Scholar]
- 46.Moreau C, Devos D, Brunaud-Danel V, Defebvre L, Perez T, Destee A, et al. Elevated IL-6 and TNF-alpha levels in patients with ALS: inflammation or hypoxia? Neurology. 2005;65:1958–60. doi: 10.1212/01.wnl.0000188907.97339.76. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka M, Kikuchi H, Ishizu T, Minohara M, Osoegawa M, Motomura K, et al. Intrathecal up-regulation of granulocyte colony stimulating factor and its neuroprotective actions on motor neurons in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2006;65:816–25. doi: 10.1097/01.jnen.0000232025.84238.e1. [DOI] [PubMed] [Google Scholar]
- 48.Cereda C, Baiocchi C, Bongioanni P, Cova E, Guareschi S, Metelli MR, et al. TNF and sTNFRl/2 plasma levels in ALS patients. J Neuroimmunol. 2008;194:123–31. doi: 10.1016/j.jneuroim.2007.10.028. [DOI] [PubMed] [Google Scholar]