Abstract
Objectives:
Sensory over-responsivity (SOR), defined as a negative response to or avoidance of sensory stimuli, is both highly prevalent and extremely impairing in youth with autism spectrum disorders (ASD), yet little is known about the neurological bases of SOR. This study aimed to examine the functional neural correlates of SOR by comparing brain responses to sensory stimuli in youth with and without ASD.
Method:
Twenty-five high-functioning youth with ASD and 25 age- and IQ-equivalent typically developing (TD) youth were presented with mildly aversive auditory and visual stimuli during a functional magnetic resonance imaging (fMRI) scan. Parents provided ratings of children's SOR and anxiety symptom severity.
Results:
Compared to TD participants, ASD participants displayed greater activation in primary sensory cortical areas as well as amygdala, hippocampus, and orbital-frontal cortex. In both groups, the level of activity in these areas was positively correlated with level of SOR severity as rated by parents, over and above behavioral ratings of anxiety.
Conclusions:
This study demonstrates that youth with ASD show neural hyper-responsivity to sensory stimuli, and that behavioral symptoms of SOR may be related to both heightened responsivity in primary sensory regions as well as areas related to emotion processing, and regulation.
Keywords: amygdala, anxiety, autism spectrum disorders, functional magnetic resonance, imaging (fMRI), sensory over-responsivity
Children with autism spectrum disorders (ASD) often display impairments in responding to sensory stimuli, in addition to the core symptoms of ASD which include impairments in language and reciprocal social behavior. Sensory over-responsivity (SOR) is characterized by an extreme, negative response to, or avoidance of, sensory stimuli such as noisy or visually stimulating environments, sudden loud noises, seams in clothing, or being touched unexpectedly.1 About 56-70% of children with ASD meet criteria for SOR2,3 compared to 10–17% of typically developing (TD) children.3,4 SOR is associated with increased functional impairment in children with ASD, including lower levels of social and adaptive skills,1,5, negative emotionality,6 and anxiety.5,6
Despite the prevalence of and considerable impairment caused by SOR in children with ASD, there is a paucity of research on the neurobiological bases of SOR. Research in this area is critical to help explain heterogeneity within ASD, and can inform intervention targeted at specific subgroups of children with ASD. In one of the few functional MRI (fMRI) studies of response to nonsocial sensory stimuli in children with ASD, Gomot et al.7 found that early adolescents with ASD responded faster to novel sounds than TD controls did, and had higher activation in prefrontal and inferior parietal regions but no differences in activation of auditory cortex. The authors theorized that novel auditory stimuli are initially processed normally but receive differential attention from the novelty detection circuit. Similarly, Hadjikani et al.8 presented expanding circles of color to adults with and without ASD, and found no between-group differences in visual cortex retinotopic maps. However, some electroencephalography (EEG) studies have found group differences in event-related potentials (ERPs) in response to tones, which may suggest an atypical response to sound in the primary auditory cortex.9
The thalamus, which is considered the "gateway" that relays sensory information entering the brain to the cortex, could also be involved in SOR. For example, deficient thalamic gating could overload the sensory cortices; alternatively, thalamic dysfunction might result in a failure to integrate the sensory information appropriately. In support of this hypothesis, abnormally decreased metabolite (glutamate and glutamine) levels were found in the thalamus of individuals with ASD10 and these abnormalities related to sensory sensitivity. Although the thalamus has also been found to be smaller in high-functioning individuals with ASD compared to TD controls, 11functional connectivity between the thalamus and cortex has been shown to be greater in ASD.12 Mizuno et al. further suggest that thalamic hyperactivity during brain development may drive functional specialization in the cortex and could lead to cortical abnormalities such as reduced pruning and thalamo-cortical overconnectivity, which may ultimately put individuals at risk for SOR.
Other hypotheses on the neural basis of SOR posit heightened limbic responses to sensory stimuli, including the amygdala and hippocampus.3-15 A number of correlational studies have shown that children with ASD and SOR also have high rates of anxiety symptoms6,13,16. Because SOR co-occurs frequently with anxiety symptoms, theories related to abnormal amygdala and hippocampus functioning are particularly relevant given the role of these structures in anxiety. Functional MRI studies (fMRI) have consistently highlighted the amygdala's central role in detection and response to threat and fear conditioning.17-20 Similarly, the hippocampus is thought to be associated with anxiety through its role in context conditioning, memory of threat-related events, and orienting to situations that could be threatening.21,22 As discussed in a review of fMRI studies on the amygdala by Zald,19 the magnitude of amygdala activation in response to sensory input from the thalamus is found to correlate with the extent to which a stimulus is perceived as threatening or unpleasant. The amygdala can then trigger a response to these stimuli upon future exposure, including an enhanced sensory response that correlates with amygdala activation.
Limbic system abnormalities may increase the risk of SOR in children with ASD by decreasing ability to regulate in response to sensory input. There is evidence for functional amygdala abnormalities in ASD, though the evidence is mixed in terms of the direction of effect: early studies showed decreased amygdala activity in ASD23; however, Pierce et al.24 found no group differences in amygdala response to faces when stimuli were salient (e.g., family members). Furthermore, more recent studies have found that individuals with ASD show amygdala hyperactivity compared to TD controls during a face processing task25-27 and that the extent of activation was correlated with the amount of time ASD participants spent gazing at the eyes.25,26 Therefore, there is some evidence for abnormal amygdala function and possibly hyperactivity, but this has not been studied in the context of sensory sensitivity.
Few physiological or biological studies of sensory abnormalities in ASD have taken into account within-group heterogeneity in sensory symptoms, which may lead to null findings. For example, physiological studies examining a general hyperarousal in individuals with ASD have yielded few consistent findings,28 but the majority of these studies employed a small sample size and did not examine subgroups. Evidence from behavioral studies1,6 suggests the presence of SOR only in some children with ASD, whereas other children with ASD are actually under-responsive to sensory stimuli. Consistent with this, a recent study of electrodermal activity in children with ASD found 2 subgroups: one with high arousal and slow habituation and one with low arousal and fast habituation.29 Furthermore, higher baseline arousal in children with ASD is related to greater physiological response to sensory stimuli and higher anxiety levels.30 Similarly, the evidence for structural abnormalities in the amygdala and hippocampus in autism is mixed, with some studies finding smaller volumes31 and others finding larger volumes32,33 than in TD individuals. This inconsistency could again be due to the heterogeneity of the ASD phenotype, and indeed amygdala volume in children with ASD has been found to be positively correlated with anxiety.34 Therefore, it is important to account for within-group sensory characteristics when examining the neural bases of SOR, but as of yet there are no functional neuroimaging studies of response to sensory information in children who have both ASD and SOR.
It should be noted that, while physiological hyperarousal appears to be characteristic of both anxiety and SOR, these two conditions may be separate constructs. For example, in a large study of TD children, Carter et al.35 found that about 25% of the sample had elevated rates of SOR and 75% of this group exhibited SOR without any known co-occuring psychiatric diagnosis. However, because of the common overlap of anxiety and SOR, we took a conservative approach in this study and controlled for anxiety symptoms to examine the unique correlation between SOR symptom severity and brain function.
The goal of the current study was to use fMRI to a) examine differences in brain responses to mildly aversive sensory stimuli in youth with and without ASD, and b) identify the functional neural correlates of sensory over-responsivity in youth with and without ASD. Given the lack of research in this area, we took an exploratory, whole-brain approach, while also focusing on specific brain regions that have been implicated in anxiety and SOR. We hypothesized that, compared to TD controls, youth with ASD would display greater activation in areas related to sensory processing (thalamus and primary auditory and visual cortices) as well as areas related to anxiety (amygdala and hippocampus). Further, we predicted that amygdala and hippocampus activation would be correlated with severity of SOR symptoms within each group, given the role of these regions in processing threat-relevant stimuli.
Method
Participants
Participants were 25 youth with ASD and 25 TD matched controls recruited through flyers posted around the University of California Los Angeles (UCLA) campus as well as through referrals from the UCLA autism clinic. Participants ranged in age from 8–17 years (m=13.13; SD=2.29) and all had a full-scale IQ within the normal range based on an assessment with the Weschler Abbreviated Scales of Intelligence (WASI),36 or the Weschler Intelligence Scale for Chi|dren–4th Edition (WISC-IV).37 Original participants were 32 TD subjects and 35 ASD subjects, but 7 TD subjects and 10 ASD subjects were excluded due to maximum motion >2 mm. The final groups of 25 TD and 25 ASD did not differ significantly in age, full scale IQ (FSIQ), performance IQ, verbal IQ, and mean or maximum head motion during fMRI (see Table 1). All ASD participants had a prior diagnosis of an autism spectrum disorder (i.e., autistic disorder, pervasive developmental disorder not otherwise specified, or Asperger's disorder), which was confirmed using the Autism Diagnostic Interview–Revised (ADI-R)38 and the Autism Diagnostic Observation Schedule–Generic (ADOS-G).39 Two participants met criteria only on the ADI but met DSM-IV criteria based on clinical judgment. Two of the TD participants were taking psychoactive medications (psychostimulants), as were 7 of the ASD participants including atypical antipsychotics (n=2), selective serotonin reuptake inhibitors (n= 1), psychostimulants (n=2), and multiple medications (n=3). No participants reported loss of consciousness for longer than 5 minutes or any neurological (e.g., epilepsy), genetic (e.g., fragile X), or severe psychiatric disorder (e.g., schizophrenia) other than autism. T-tests were conducted comparing mean activation in children with and without medication in the a priori areas of interest (right and left hippocampus, amygdala, thalamus, and primary auditory [A1] and visual [V1] cortices). Out of 30 comparisons (the above 10 activations times 3 conditions), only 1 was significant (no more than would be expected by chance), indicating that medication status was unrelated to brain activation in response to the experimental task. T values ranged from –1.57 to 1.26; p= .07–.99, except for right thalamus in the auditory condition: T=–2.51; p=.016.
Table 1.
Descriptive Statistics
| ASD | TD | t or χ2 | |
|---|---|---|---|
| Age | 13.10 (2.47) | 13.15 (2.16) | 0.09 |
| Gender, male, n (%) | 21 (84) | 19 (76) | 0.50 |
| Handedness, right-handed, n (%) | 23 (92) | 24 (96) | 0.36 |
| FSIQ | 101.16 (15.95) | 106.20 (11.78) | 1.27 |
| VIQ | 102.00 (16.59) | 105.60 (11.74) | 0.89 |
| PIQ | 109.92 (15.27) | 107.32 (11.39) | −0.68 |
| Mean Absolute Motion | 0.23 (.16) | 0.22 (.18) | −0.12 |
| Max Absolute Motion | 0.58 (.40) | 0.63 (.51) | 0.40 |
| Mean Relative motion | 0.09 (.04) | 0.08 (.04) | −0.63 |
| Max Relative Motion | 0.54 (1.04) | 0.63 (.75) | −0.96 |
| SensOR visual count | 1.52 (1.83) | 0.36 (.81) | −2.90** |
| SensOR auditory count | 7.72 (6.67) | 1.60 (2.66) | −4.26*** |
| SSP auditory/visual | 18.09 (4.46) | 23.76 (1.74) | 5.60*** |
| SSP auditory filtering | 17.09 (5.08) | 26.12 (4.32) | 6.58*** |
| Auditory-Visual Composite | 3.23 (4.63) | −3.23 (1.75) | −6.52*** |
| CBCL Anxiety T-Score | 61.16 (9.67) | 51.56 (3.74) | −4.63*** |
Note: n=25 autism spectrum disorder (ASD), 25 typically developing (TD) except for Short Sensory Profile (SSP) analyses where n=22 ASD, 25 TD. CBCL = Child Behavior Checklist; FSIQ = full scale IQ; PIQ = performance IQ; SensOR = Sensory Over-Responsivity Inventory; VIQ = verbal IQ.
p<.01;
p<.001
fMRI Sensory Task Paradigm
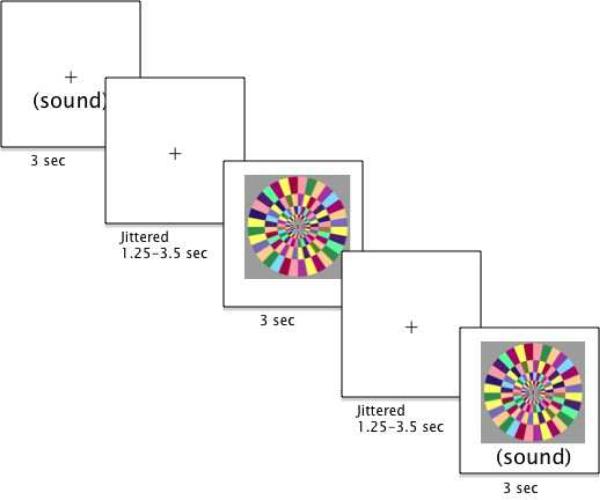
Participants were passively exposed to 3 mildly aversive stimulus conditions in an event-related paradigm (see Figure 1): an auditory stimulus, a visual stimulus, and the auditory and visual stimuli simultaneously (referred to as the "Joint" condition). The auditory stimulus was composed of white noise, which was set at the same volume for each participant. The volume increased linearly to the peak volume in the first .75 seconds of each 3-second presentation to minimize startle effects. The visual stimulus was a movie of a continually rotating color wheel (see Figure 1). Stimuli were chosen based on pilot testing with the Sensory Over-Responsivity Checklist indicating that these kinds of auditory and visual stimuli best differentiated the status groups. After completing the task, participants were asked to rate on a scale of 0–10 how "bad" each stimulus was. On average, both groups rated the auditory and joint conditions a 3 out of 10, and the visual condition a 2.2 out of 10. There were no significant group differences in aversiveness ratings. Each trial type was presented 12 times, in a randomized order, with each trial lasting 3 seconds. Inter-trial intervals were jittered between 1,250 and 3,500 ms. The total scan length was 3 minutes, 34 seconds including a 10-second final fixation.
Figure 1.
Experimental design.
MRI Data Acquisition
Scans were acquired on a Siemens Trio 3 Tesla magnetic resonance imaging scanner. A high-resolution structural T2-weighted echo-planar imaging volume (spin-echo, repetition time [TR]=5000 ms, time to echo [TE]=33 ms, 128×128 matrix, 20cm field of view [FOV], 36 slices, 1.56mm in-plane resolution, 3mm thick) was acquired coplanar to the functional scans in order to ensure identical distortion characteristics to the fMRI scan. Each functional run involved the acquisition of 107 EPI volumes (gradient-echo, TR=2000ms, TE=30ms, flip angle=90, 64×64 matrix, 20cm FOV, 33 slices, 3.125mm in-plane resolution, 3 mm thick). Visual and auditory stimuli were presented to the participant using 800×640 resolution magnet-compatible 3-D goggles and headphones under computer control (Resonance Technologies, Inc.). The stimuli were presented using E-Prime. Participants wore earplugs and headphones to reduce interference of the auditory stimuli from the scanner noise. Participants were instructed to focus on the center of the screen for the duration of the task.
Measures
The ADI-R, ADOS, WISC, and WASI were administered at a clinical assessment visit prior to the MRI scan. Parents completed the additional questionnaires and interviews listed below while the child was in the scanner.
Child Behavior Checklist for Ages 6–18 (CBCL).40
The CBCL is a parent-report measure of child problem behaviors. For the purposes of this study, the Anxiety Scale T-scores were used as a measure of severity of child anxiety symptoms.
Short Sensory Profile (SSP).41
The SSP is a widely used, 38-item parent report measure of youth sensory dysregulation across a number of sensory modalities. Parents rate the frequency with which their child responds in an atypical way to sensory stimuli on a 5-point Likert scale from "never" responds in this way to "always" responds in this way. This measure yields both a total score of sensory dysregulation as well as subscale scores for Tactile, Taste/Smell, Movement, and Auditory/Visual Sensitivity, Underresponsive/Seeks Sensation, Auditory Filtering, and Low Energy/Weak. For the purposes of this study, we used only the subscales relevant to the auditory and visual stimuli administered, namely the Auditory/Visual Sensitivity scores and the Auditory Filtering score. Higher scores on the SSP indicate lower impairment. On the Auditory/Visual Sensitivity subscale, a score of 19–25 is considered typical performance, a score of 16–18 is considered a "Probable Difference," and a score of 5–15 is considered a "Definite Difference." On the Auditory Filtering subscale, a score of 23–30 is considered typical performance, a score of 20–22 is considered a "Probable Difference," and a score of 6–19 is considered a "Definite Difference." This measure has strong reliability and validity (McIntosh et al., 1999a).
Sensory Over-Responsivity (SensOR) Inventory.42
The SensOR Inventory is a parent checklist of sensory sensations that bother their child. For the purposes of this study, only the visual, and auditory subcales were used. The number of items parents rate as bothering their child has been shown to discriminate between TD children and children with SOR (Schoen et al., 2008). The SensOR inventory has been found to best differentiate children with SOR from TD children when at least four tactile or auditory items are present (Schoen et al., 2008b).
fMRI Data Analysis
Analyses were performed using FSL Version 4.1.4 (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). Preprocessing included motion correction to the mean image, spatial smoothing (Gaussian Kernel FWHM = 5mm), and high-pass temporal filtering (t > 0.01 Hz). Functional data were linearly registered to a common stereotaxic space by first registering to the in-plane T2 image (6 degrees of freedom) then to the MNI152 T1 2mm brain (12 degrees of freedom).
FSL's fMRI Expert Analysis Tool (FEAT), Version 5.98 was used for statistical analyses. Fixed-effects models were run separately for each subject, then combined in a higher-level mixed effects model to investigate within and between-group differences. Each experimental condition (auditory, visual, or both together) was modeled with respect to the fixation condition (during ISIs and the final fixation). Higher-level group analyses were carried out using FSL's FLAME (FMRIB's Local Analysis of Mixed Effects State) stage 1 and stage 2.43-45 Within-group Z statistical images for each condition (vs. resting baseline) were thresholded at Z > 2.3 (p<.0l) to define contiguous voxel clusters. FSL's cluster correction for multiple comparisons (Gaussian-random field theory based) was set at p<.05, whole brain correction (http://www.fmrib.ox.ac.uk/fsl). Between-group comparisons were then performed and also thresholded at Z > 2.3 (p < .01). Given the exploratory nature of the study and the focus on a priori regions of interest, these comparisons were not corrected for multiple comparisons. To evaluate the correlation of SOR with blood-oxygen-ievel dependent contrast imaging (BOLD response, an SOR composite score was created by standardizing and averaging each relevant subscale of the SOR measures (SSP auditory/visual sensitivity, and auditory filtering scales and SensOR Inventory auditory and visual scores). To determine whether SOR predicted BOLD response over and above anxiety, regression analyses were performed with the de-meaned SOR composite as the independent variable and CBCL anxiety scores entered as covariates in the design matrix for the participants as a whole. These comparisons were also thresholded at Z>2.3, uncorrected. Parameter estimates for significant clusters in regions of interest (primary visual and auditory cortex, thalamus, amygdala, hippocampus, and orbitofrontal cortex), using functionally defined masks, were extracted from each participant and plotted in a graph to rule out the presence of outliers.
Results
Behavioral Results
Independent-sample t-tests were used to test for group differences in parent-reported SOR and anxiety data, including the SensOR Inventory visual and auditory scales, the Short Sensory Profile total and auditory/visual and auditory filtering subscales, as well as CBCL Anxiety T-scores. The ASD group was rated significantly higher on all of these measures (results are displayed in Table 2). The correlation between CBCL Anxiety T-scores and the SOR composite was significant in both groups (TD: r=.50, p=.011; ASD: r=.59, p=.002).
Table 2.
Montreal Neurological Institute (MNI) Coordinates for Auditory Condition as Compared to Baseline
| ASD | TD | ASD>TD | TD>ASD | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||
| MNI peak (mm) | Max | MNI peak (mm) | Max | MNI peak (mm) | Max | MNI peak (mm) | Max | |||||||||||||
| x | y | z | Z | vox | x | y | z | Z | vox | x | y | z | Z | x | y | z | Z | vox | ||
| Right Lateral Occipital Cortex inferior division | 38 | −74 | 6 | 2.92 | 50 | |||||||||||||||
| Left Supramarginal Gyrus | −60 | −40 | 20 | 3.68 | 726 | |||||||||||||||
| Left Angular Gyrus | −40 | −56 | 34 | 2.66 | 180 | |||||||||||||||
| Right Cingulate Gyrus, Posterior Division | 14 | −28 | 36 | 3.71 | 344 | |||||||||||||||
| Left Cingulate Gyrus, Posterior Division | −8 | −30 | 48 | 2.75 | 76 | |||||||||||||||
| Left Paracingulate Gyrus | −4 | 26 | 40 | 2.45 | 38 | |||||||||||||||
| Left Insular Cortex | −44 | 0 | 6 | 3.02 | 290 | |||||||||||||||
| Right Precentral Gyrus | 22 | −24 | 78 | 2.46 | 52 | 60 | −2 | 42 | 2.65 | 59 | ||||||||||
| Left Postcentral Gyrus | −36 | −34 | 58 | 3.39 | 683 | |||||||||||||||
| Left Amygdala | −24 | 0 | −18 | 2.54 | 34 | |||||||||||||||
| Right Amygdala | 20 | −4 | −22 | 3.14 | 2,834 | |||||||||||||||
| Right Supramarginal Gyrus | 62 | −34 | 36 | 3.35 | ||||||||||||||||
| Right Insular Cortex | 42 | −4 | −12 | 4.24 | ||||||||||||||||
| Right Inferior Temporal Gyrus | 44 | −54 | −6 | 3.64 | ||||||||||||||||
| Right Anterior Transverse Temporal Gyrus | 54 | 18 | −6 | 2.71 | ||||||||||||||||
| Right Superior Temporal Gyrus, | 54 | −32 | 12 | 5.85 | 60 | −40 | 10 | 5.31 | 2397 | |||||||||||
| Right Fusiform Gyrus | 38 | −54 | −14 | 3.81 | ||||||||||||||||
| Right Heschl’s Gyrus | 38 | −28 | 6 | 3.87 | 56 | −32 | 14 | 3.03 | 598 | |||||||||||
| Right Postcentral Gyrus | 48 | −20 | 38 | 2.99 | ||||||||||||||||
| Left Superior Temporal Gyrus | −64 | −30 | 22 | 3.78 | 1,864 | −66 | −16 | 2 | 3.16 | 95 | ||||||||||
| −4 | ||||||||||||||||||||
| Left Heschl’s Gyrus | −44 | −28 | 8 | 4.62 | 8 | −24 | 6 | 4.37 | 662 | |||||||||||
| −2 | ||||||||||||||||||||
| Left Thalamic Reticular Nucleus | 2 | −26 | 0 | 2.36 | ||||||||||||||||
| Right Middle Temporal Gyrus | 60 | −14 | −28 | 2.64 | 117 | 68 | −46 | 4 | 3.37 | 121 | ||||||||||
| Left Middle Temporal Gyrus | −60 | −48 | 4 | 2.57 | 109 | |||||||||||||||
| Left Inferior Temporal Gyrus | −48 | −66 | 0 | 2.70 | 46 | −54 | −44 | −20 | 2.44 | 47 | ||||||||||
| Left Temporal Pole | −40 | 4 | −34 | 3.12 | 155 | |||||||||||||||
| Left Superior Frontal Gyrus | −10 | 14 | 68 | 3.39 | 342 | |||||||||||||||
| Right Middle Frontal Gyrus | 44 | 18 | 50 | 2.68 | 53 | |||||||||||||||
| Left Middle Frontal Gyrus | −40 | 12 | 54 | 2.91 | 107 | |||||||||||||||
| Left Inferior Frontal Gyrus | −52 | 28 | 4 | 2.58 | 108 | |||||||||||||||
| Right Frontal Medial Cortex | 4 | 48 | −22 | 2.82 | 72 | |||||||||||||||
| Right Frontal Orbital Cortex | 20 | 26 | −22 | 2.71 | 58 | |||||||||||||||
| Left Frontal Orbital Cortex | −14 | 20 | 16 | 2.51 | 55 | |||||||||||||||
| Right Frontal Pole | 32 | 50 | 24 | 2.75 | 87 | |||||||||||||||
| Left Frontal Pole | −36 | 54 | −8 | 2.55 | 84 | |||||||||||||||
| Right Putamen | 36 | −12 | −2 | 2.62 | 46 | |||||||||||||||
| Right Caudate tail | 30 | −36 | 6 | 2.61 | 66 | |||||||||||||||
| Cerebellum | −8 | −62 | −18 | 3.09 | 80 | 34 | −4. | −32 | 2.75 | |||||||||||
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively. Z refers to the Z-score at those coordinates (local maxima or submaxima). k refers to cluster size in voxels; because FSL considers all contiguous voxels to be within the same cluster, some anatomical peaks fall within the same cluster size and are denoted with underlining the first peak listed in the cluster. Within-group analyses are cluster corrected for multiple comparisons, Z>2.3, p<.05; between-group analyses are thresholded at Z>2.3, uncorrected. A priori regions of interest are reported in bold font. ASD = autism spectrum disorder; TD = typically developing.
fMRI Results
Within-Group Results
We first examined activity within each group in each of the three conditions. Results are displayed in Tables 2-4 and Figure 2; while whole-brain results are reported in the tables, only a priori regions of interest are reported in the text that follows. In the Auditory condition, the TD group showed significant activation in primary auditory cortex; in the Visual condition, the TD group showed significant activation in primary visual cortex. In the Joint condition, the TD group showed significant activation in both visual and auditory cortices. The ASD group showed significant activation in amygdala and auditory cortex in the Auditory condition, amygdala, visual cortex, lateral geniculate nucleus (LGN), and orbital frontal cortex in the Visual condition, and amygdala, visual and auditory cortex, thalamus (pulvinar), and orbital frontal cortex in the Joint condition.
Table 4.
Montreal Neurological Institute (MNI) Coordinates for Joint Auditory+Visual Condition as Compared to Baseline
| ASD | TD | ASD>TD | TD>ASD | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||
| MNI peak (mm) | Max | MNI peak (mm) | Max | MNI peak (mm) | Max | MNI peak (mm) | Max | |||||||||||||
| x | y | z | Z | Vox | x | y | z | Z | Vox | x | y | z | Z | Vox | x | y | z | Z | Vox | |
| Right Occipital Pole | 32 | −90 | 14 | 6.58 | 18,101 | 10 | −96 | −4 | 9.13 | 16,254 | 8 | −88 | −4 | 2.53 | 61 | |||||
| Left Occipital Pole | −20 | −96 | 10 | 7.50 | −18 | −94 | −12 | 6.74 | ||||||||||||
| Left Lateral Occipital Cortex superior division | −28 | −72 | 26 | 3.42 | 0 | −88 | 44 | 2.62 | 68 | |||||||||||
| Left Lateral Occipital Cortex inferior division | −44 | −64 | −2 | 3.33 | −46 | −78 | −6 | 4.48 | ||||||||||||
| Right Lateral Occipital Cortex superior division | 26 | −58 | 32 | 2.96 | ||||||||||||||||
| Right Lateral Occipital Cortex inferior division | 48 | −72 | 2 | 5.60 | 36 | −72 | 12 | 2.87 | 82 | |||||||||||
| Right Fusiform Gyrus | 12 | −84 | −10 | 10.10 | 30 | −74 | −10 | 6.14 | ||||||||||||
| Left Fusiform Gyrus | −28 | −76 | −16 | 6.21 | −24 | −66 | −16 | 5.94 | −44 | −68 | −18 | 2.65 | 34 | |||||||
| Right Superior Temporal Gyrus, | 66 | −10 | 2 | 4.26 | 60 | −38 | 10 | 4.99 | ||||||||||||
| Right Heschl’s Gyrus | 42 | −30 | 12 | 4.70 | 36 | −26 | 6 | 3.31 | ||||||||||||
| Right Supramarginal Gyrus | 60 | −38 | 26 | 2.82 | ||||||||||||||||
| Right Frontal Orbital Cortex | 38 | 36 | −8 | 3.50 | 30 | 28 | −12 | 3.19 | 279 | |||||||||||
| Right Thalamus - pulvinar | 20 | −30 | −2 | 6.87 | ||||||||||||||||
| Right Amygdala | 28 | −2 | −14 | 4.16 | 18 | −2 | −18 | 2.56 | 117 | |||||||||||
| Cerebellum | −48 | −52 | −30 | 2.85 | 0 | −48 | −6 | 2.49 | 51 | |||||||||||
| Right Temporal Pole | 50 | 8 | −16 | 3.16 | ||||||||||||||||
| Left Temporal Pole | −40 | 4 | −34 | 3.16 | 131 | |||||||||||||||
| Right Insular Cortex | 40 | 2 | −16 | 3.30 | 46 | −4 | 4 | 2.87 | 59 | |||||||||||
| Right Middle Temporal Gyrus | 50 | −54 | −6 | 4.06 | 68 | −46 | 4 | 3.48 | 108 | |||||||||||
| Right Parahippocampal Gyrus | 24 | −34 | −16 | 3.51 | 24 | −28 | −20 | 2.71 | 47 | |||||||||||
| Precuneus | 18 | −54 | 12 | 3.03 | 38 | |||||||||||||||
| Left Heschl’s Gyrus | −42 | −20 | 8 | 4.65 | 1,101 | −42 | −30 | 8 | 4.48 | 508 | ||||||||||
| Left Supramarginal Gyrus | −66 | −42 | 22 | 3.29 | −60 | −40 | 20 | 3.33 | 533 | |||||||||||
| Right Cingulate Gyrus, Posterior Division | 4 | −40 | 8 | 2.46 | 95 | |||||||||||||||
| Left Insular Cortex | −32 | 20 | −4 | 2.76 | 138 | |||||||||||||||
| Left Postcentral Gyrus | −22 | −44 | 74 | 2.51 | 63 | |||||||||||||||
| Left Superior Temporal Gyrus | −64 | −14 | −4 | 3.09 | 257 | |||||||||||||||
| Right Superior Frontal Gyrus | 2 | 48 | −24 | 2.88 | 102 | |||||||||||||||
| Left Superior Frontal Gyrus | −22 | 62 | 18 | 2.56 | 190 | |||||||||||||||
| Right Middle Frontal Gyrus | 44 | 18 | 48 | 2.93 | 105 | |||||||||||||||
| Left Middle Frontal Gyrus | −40 | 12 | 56 | 2.79 | 97 | |||||||||||||||
| Right Frontal Pole | 28 | 56 | −6 | 2.55 | 195 | 16 | 72 | −2 | 2.55 | 33 | ||||||||||
| Left Frontal Pole | −20 | 68 | −4 | 3.12 | 32 | |||||||||||||||
| Left Putamen | −28 | −12 | 4 | 2.71 | 74 | |||||||||||||||
| Right Caudate | 16 | 18 | 12 | 2.99 | 102 | |||||||||||||||
| Right Caudate tail | 30 | −36 | 4 | 2.90 | 197 | |||||||||||||||
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively. Z refers to the Z-score at those coordinates (local maxima or submaxima). k refers to cluster size in voxels; because FSL considers all contiguous voxels to be within the same cluster, some anatomical peaks fall within the same cluster size and are denoted with underlining the first peak listed in the cluster. Within-group analyses are cluster corrected for multiple comparisons, Z>2.3, p<.05; between-group analyses are thresholded at Z>2.3, uncorrected. A priori regions of interest are reported in bold font. ASD = autism spectrum disorder; TD = typically developing.
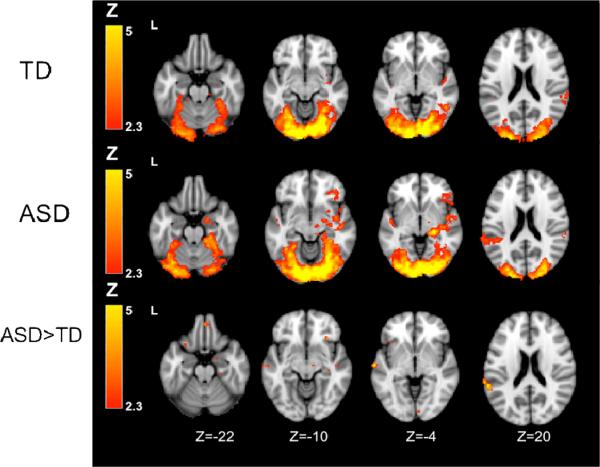
Figure 2.
Within- and between-group results: Joint auditory + visual condition. Note: Within-group constrats thresholded at Z>2.3, corrected (p<.05). Between-group contrasts thresholded at Z>2.3, uncorrected. ASD = autism spectrum disorder; TD = typically developing.
Between-Group Results
We then directly compared activation patterns between ASD and TD groups for each contrast (see Tables 2-4 and Figure 2). The between-group contrasts indicated that the ASD group showed greater activation in the amygdala in the Auditory and Joint conditions, and greater prefrontal cortex in all three conditions. The ASD group also had greater primary auditory activation in the Auditory and Joint conditions and greater primary visual activation in the Joint condition. No significant differences were observed for the opposite comparisons (TD > ASD) in any of the a priori regions of interest.
Correlation With Sensory Over-Responsivity Severity
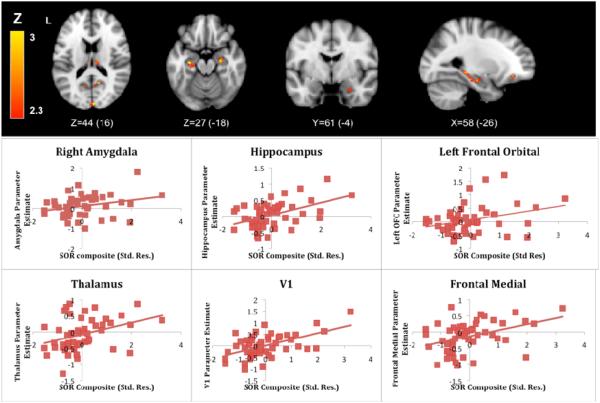
We examined SOR severity as a predictor of BOLD response above and beyond anxiety during the Joint condition by entering the SOR composite as a regressor of interest and CBCL anxiety T-scores as covariates. We examined significant correlations in our a priori areas of interest as well as in the frontal orbital and medial cortices given the significant group differences found in these regions. There were significant positive correlations between the SOR composite and signal increases during the Joint condition in the amygdala, hippocampus, left orbital frontal cortex, frontal medial cortex, thalamus, and primary visual cortex (Figure 3). While we present results for the full sample, these correlations held when examined in each group separately, though in the ASD group, the correlation with activity in the amygdala was only significantly correlated at a Z threshold of 1.7. These regression results indicate that the between-group differences are likely due to differences in SOR, and that anxiety alone did not account for these group differences in BOLD response to sensory stimuli. Significant areas along with graphs of the correlations are presented in Figure 3; the Montreal Neurological Institute (MNI) coordinates for all significant clusters are listed in Table 5.
Figure 3.
Sensory over-responsivity (SOR) severity as a predictor of blood-oxygen-level dependent (BOLD) response during the Joint condition. Note: The horizontal axis displays the standardized residual SOR composite score after regressing out Child Behavior Checklist (CBCL) anxiety T-scores. The vertical axis displays the parameter estimates extracted from areas where significant correlations between SOR severity and brain activity were observed. OFC = orbital frontal cortex.
Table 5.
Montreal Neurological Institute (MNI) Coordinates for Brain Areas Where Blood-Oxygen-Level Dependent (BOLD) Response Was Correlated With Sensory Over-Responsivity (SOR) Composite.
| MNI peak (mm) | Max | ||||
|---|---|---|---|---|---|
| x | y | z | Z | k | |
| Left Occipital Pole | −2 | −94 | 22 | 4.01 | 10,778 |
| Right Lateral Occipital Cortex superior division | 16 | −82 | 38 | 3.45 | |
| Left Lateral Occipital Cortex superior division | −46 | −62 | 24 | 3.79 | |
| Left Fusiform Gyrus | −32 | −42 | −24 | 2.32 | |
| Right Lingual Gyrus | 14 | −64 | −8 | 4.21 | |
| Left Lingual Gyrus | −22 | −56 | −4 | 2.60 | |
| Precuneus | −10 | −70 | 32 | 3.72 | |
| Right Cingulate Gyrus, Posterior Division | 10 | −36 | 38 | 3.17 | |
| Left Cingulate Gyrus, Posterior Division | −6 | −44 | 18 | 3.61 | |
| Left Middle Temporal Gyrus | −60 | 2 | −20 | 3.57 | |
| Left Inferior Temporal Gyrus | −52 | −20 | −22 | 3.33 | |
| Left Temporal Pole | −34 | 16 | −36 | 2.54 | |
| Left Hippocampus | −28 | −18 | −18 | 3.10 | |
| Left Parahippocampal Gyrus | −38 | −28 | −16 | 2.60 | |
| Left Lateral Occipital Cortex inferior division | −32 | −86 | −24 | 2.54 | 35 |
| Right Fusiform Gyrus | 42 58 | −48 | −24 | 2.89 3.11 | 80 |
| Right Angular Gyrus | −52 | 26 | 352 | ||
| Left Cingulate Gyrus, Anterior Division | 0 | 20 | 20 | 2.76 | 38 |
| Left Precentral Gyrus | −10 | −20 | 64 | 2.49 | 193 |
| Right Middle Temporal Gyrus | 48 | 4 | −30 | 3.02 | 198 |
| Right Superior Frontal Gyrus | 18 | 4 | 58 | 3.00 | 48 |
| Left Superior Frontal Gyrus | −16 | 22 | 54 | 2.98 | 455 |
| Left Inferior Frontal Gyrus | −36 | 4 | 20 | 3.66 | 104 |
| Right Inferior Frontal Gyrus, pars triangluaris | 50 | 26 | 0 | 2.94 | 65 |
| Left Inferior Frontal Gyrus, pars triiangularis | −50 | 22 | −4 | 2.95 | 163 |
| Right Frontal Medial Cortex | 4 | 26 | −28 | 3.02 | 96 |
| Left Frontal Medial Cortex | −8 | 36 | −24 | 2.83 | 34 |
| Left Frontal Orbital Cortex | −24 | 34 | −12 | 2.77 | 109 |
| Right Frontal Pole | 14 | 48 | 48 | 3.04 | 284 |
| Left Frontal Pole | −4 | 60 | −2 | 3.58 | 949 |
| Right Thalamus - Pulvinar | 8 | −22 | 16 | 2.63 | 102 |
| Left Thalamus - Pulvinar | −4 | −24 | 12 | 2.89 | 82 |
| Right Hippocampus | 26 | −14 | −18 | 3.14 | 913 |
| Right Parahippocampal Gyrus | 24 | −26 | −24 | 2.93 | |
| Right Amygdala | 26 | −2 | −24 | 2.91 | |
| Cerebellum | 10 | −46 | −30 | 3.47 | |
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively. Z refers to the Z-score at those coordinates (local maxima or submaxima). k refers to cluster size in voxels; because FSL considers all contiguous voxels to be within the same cluster, some anatomical peaks fall within the same cluster size and are denoted with indenting below the first peak listed in the cluster, which is underlined.. Analyses are thresholded at Z>2.3, uncorrected. A priori regions of interest are reported in bold font.
Discussion
The aim of this study was to examine the neural correlates of sensory over-responsivity in children with and without ASD, with a focus on brain areas related to primary sensory processing as well as those related to anxiety and emotion regulation, As predicted, we found evidence for increased neural responses to mildly aversive sensory stimuli in youth with ASD compared to TD youth. In particular, the ASD group displayed greater activation in primary sensory areas (auditory and visual cortices) as well as in emotion processing regions (amygdala, hippocampus, and prefrontal cortex).
In terms of the primary sensory processing areas, although both groups engaged the primary auditory and visual cortices, the ASD group displayed greater activity in both primary sensory cortices as well as the thalamus. For all participants, visual cortex and thalamic activity was significantly correlated with SOR severity over and above anxiety.
We hypothesized that the neural bases of SOR might be similar to those previously found to be related to anxiety (i.e., amygdala, hippocampus, and prefrontal cortex), due to the consistent finding that SOR frequently co-occurs with anxiety.6,13 Activity in these areas was also positively correlated with parent-rated SOR symptoms suggesting that group differences are related to greater SOR severity in the ASD group. Notably, SOR symptoms and brain activity were correlated over and above manifest anxiety symptoms, indicating that there may be a unique relationship between SOR and activity in these brain regions that is not fully mediated by anxiety level. This was a conservative approach, given the high co-occurrence of anxiety and SOR. This neural hyper-responsivity may reflect impairments in both bottom-up and top-down processing. The primary sensory cortices may be over-responsive to the stimuli and trigger an enhanced amygdala response, while simultaneously the amygdala may over-stimulate higher-level cortical regions. This is consistent with previous research showing that amygdala activation is correlated with level of behavioral response to sensory stimuli.19 The amygdala can then signal the hippocampus to retain memories of the stimuli, as well as the context in which the stimuli were presented, leading to context conditioning and generalization of the fear.46 Furthermore, Liss et ol.1 found that children with ASD and SOR had over-focused attention and "exceptional memory," which could also be related to a hyperactive hippocampus encoding threat-relevant events.
Contrary to the typical negative relationship seen between the amygdala and PFC,47 in the ASD group we found higher amygdala activity co-occurring with higher PFC activation, which may reflect an immature or dysfunctional regulatory system. It is possible that the PFC is inhibiting the amygdala, and the amygdala activation in the ASD group would be even stronger without modulation by the PFC. Alternatively, this finding could reflect a more immature connectivity pattern in the ASD group, as the negative connectivity between the amygdala and PFC develops with age.5 More research is needed on the development of the amygdala in ASD, especially given evidence that individuals with ASD have abnormally large amygdalae in childhood but not in adolescence, due to a lack of the typical amygdala volume increase normally seen in adolescence.33
To our knowledge, this is the first study to examine fMRI response to sensory stimuli in children with ASD while taking into account within-group heterogeneity in SOR severity and anxiety symptoms. Additionally, the stimuli presented in this study were rated by participants as being mildly aversive, as opposed to previous studies that failed to find group differences in response to more neutral stimuli, such as tones.28 Nevertheless, this study has a few limitations. The experimental paradigm included a limited number of trials per condition. For this reason, the power to find additional group differences may have been reduced. Despite this limitation, clear group differences were found in several a priori regions of interest; future studies should continue to examine how SOR severity relates to fMRI response in other brain areas. Another possible limitation is that participants who found the visual stimuli aversive could have shifted their gaze to avoid it, although we did find that all participants had significant increases in activation in visual cortex in the visual/both conditions compared to baseline. Future studies might combine the fMRI data with eye tracking to monitor participants' engagement with the stimuli. Additionally, it will be useful to examine brain response to tactile stimuli, which has been found to discriminate well between individuals with and without SOR.42
In addition, the findings of concurrent greater amygdala and PFC activity in the ASD group, which suggest a possible immature connectivity pattern in this group, need to be followed up on using functional connectivity analyses. Finally, future studies should examine the role that habituation in response to sensory stimuli may play in determining group differences. Evidence from the anxiety literature suggests that phobic subjects may have a more intense initial amygdala response to the feared stimulus and then look away, so their amygdala response quickly decreases, in comparison to control subjects who have a weaker but longer-lasting amygdala response.48 Additionally, Kleinhans et al.49 found reduced habituation in the amygdala in response to neutral faces. These findings highlight the importance of examining changes in the emotion regulation response across time, as averaging response over the entire task may mask important group differences in how the stimuli are processed.
In conclusion, we found that youth with ASD have a hyper-responsive BOLD response to mildly aversive sensory stimuli, particularly in areas related to sensory processing and emotion regulation. Activity in these regions was significantly related to parent-report symptoms of SOR in both groups even after controlling for anxiety, which indicates that group differences were not merely due to higher levels of anxiety in the ASD group. Overall, our findings suggest that SOR and anxiety may have a common neural basis in dysregulation of limbic system areas, particularly the amygdala and hippocampus. More research is needed to determine whether these neural abnormalities put youth with ASD at risk specifically for SOR and anxiety, or whether they simply contribute to overall emotional and behavioral dysregulation.
Table 3.
Montreal Neurological Institute (MNI) Coordinates for Visual Condition as Compared to Baseline.
| ASD | TD | ASD>TD | TD>ASD | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||
| MNI peak (mm) | Max | MNI peak (mm) | Max | MNI peak (mm) | Max | MNI peak (mm) | Max | |||||||||||||
| x | y | z | Z | Vox | x | y | z | Z | Vox | x | y | z | Z | Vox | x | y | z | Z | Vox | |
| Right Occipital Pole | 24 | −94 | −6 | 8.99 | 18,821 | 12 | −96 | −4 | 8.23 | 14,981 | ||||||||||
| Left Occipital Pole | −18 | −98 | 10 | 7.55 | −30 | −96 | 4 | 6.74 | ||||||||||||
| Right Lateral Occipital Cortex superior division |
38 | −86 | 12 | 6.69 | −28 | −70 | 24 | 3.58 | 32 | −76 | 26 | 2.79 | 96 | |||||||
| Right Lateral Occipital Cortex inferior division | 50 | −68 | −2 | 5.16 | 32 | −86 | 8 | 8.52 | 48 | −68 | 2 | 3.17 | 455 | |||||||
| Right Fusiform Gyrus | 30 | −48 | −16 | 7.72 | 30 | −70 | −10 | 6.14 | −20 | −46 | −16 | 2.95 | 91 | |||||||
| Left Fusiform Gyrus | −36 | −68 | −18 | 7.18 | −22 | −82 | −14 | 7.20 | ||||||||||||
| Left Parahippocampal Gyrus | −28 | −30 | −22 | 3.10 | ||||||||||||||||
| Left Lateral Occipital Cortex superior division | 30 | −78 | 40 | 3.09 | ||||||||||||||||
| Left Lateral Occipital Cortex inferior division | −42 | −64 | 8 | 3.24 | ||||||||||||||||
| Left Lingual Gyrus | 0 | −82 | −2 | 6.26 | ||||||||||||||||
| Right Insular Cortex | 34 | 14 | 0 | 2.84 | ||||||||||||||||
| Right Middle Temporal Gyrus | 48 | −16 | −14 | 3.84 | 68 | −46 | 4 | 3.06 | 126 | |||||||||||
| Right Thalamus - lateral geniculate nucleus | 22 | −28 | −2 | 6.60 | ||||||||||||||||
| Right Amygdala | 26 | −4 | −16 | 3.73 | ||||||||||||||||
| Right Frontal Orbital Cortex | 38 | 36 | −14 | 3.59 | 4 | 46 | −24 | 2.94 | 128 | |||||||||||
| Left Frontal Orbital Cortex | −38 | 32 | −12 | 3.04 | 90 | |||||||||||||||
| Right Lingual Gyrus | 2 | −70 | −4 | 2.82 | 137 | |||||||||||||||
| Right Precentral Gyrus | 52 | 2 | 44 | 2.70 | 87 | |||||||||||||||
| 3,33 | ||||||||||||||||||||
| Right Superior Temporal Gyrus, | 48 | −16 | −14 | 3.54 | 1 | |||||||||||||||
| Right Temporal Pole | 50 | 10 | −16 | 2.64 | ||||||||||||||||
| Precuneus | 12 | −50 | 18 | 2.72 | ||||||||||||||||
| Right Caudate tail | 30 | −36 | 6 | 3.14 | ||||||||||||||||
| Right Subthalamic Nucleus | 12 | −16 | −8 | 2.98 | ||||||||||||||||
| Cerebellum | 14 | −46 | −16 | 2.46 | 36 | −42 | −36 | 2.6 | 39 | |||||||||||
| Left Superior Temporal Gyrus | −66 | −14 | −4 | 2.61 | 56 | |||||||||||||||
| Right Inferior Temporal Gyrus | 48 | −44 | −24 | 2.60 | 61 | |||||||||||||||
| Left Temporal Pole | −40 | 4 | −34 | 2.49 | 95 | |||||||||||||||
| Left Temporal Pole | 1,80 | |||||||||||||||||||
| Right Superior Frontal Gyrus | 18 | 32 | 38 | 3.34 | 1 | |||||||||||||||
| Right Frontal Pole | 32 | 50 | 20 | 2.48 | " | |||||||||||||||
| Left Superior Frontal Gyrus | −10 | 14 | 70 | 2.90 | 138 | |||||||||||||||
| Left Middle Frontal Gyrus | 44 | 34 | 42 | 2.77 | 46 | |||||||||||||||
| Left Frontal Pole | −20 | 68 | −4 | 3.16 | 41 | |||||||||||||||
| Left Putamen | −28 | −10 | 6 | 2.83 | 87 | |||||||||||||||
Note: x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively. Z refers to the Z-score at those coordinates (local maxima or submaxima). k refers to cluster size in voxels; because FSL considers all contiguous voxels to be within the same cluster, some anatomical peaks fall within the same cluster size and are denoted with underlining the first peak listed in the cluster. Within-group analyses are cluster corrected for multiple comparisons, Z>2.3, p<.05; between-group analyses are thresholded at Z>2.3, uncorrected. A prioriregions of interest are reported in bold font. ASD = autism spectrum disorder; TD = typically developing.
Acknowledgments
This work was supported in part by grants from the National Institute of Child Health and Human Development (P50 HD055784) and the National Institute of Mental Health (1R01 HD065280-01) as well as a National Research Service Award predoctoral fellowship to S.G. (F31 MH093999-01A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Rudie, Wood, Shirinyan, Tottenham, Dapretto, and Bookheimer, Ms. Green, Ms. Colich, and Ms. Hernandez report no biomedical financial interests or potential conflicts of interest.
References
- 1.Liss M. Sensory and attention abnormalities in autistic spectrum disorders. Autism. 2006;10(2):155–172. doi: 10.1177/1362361306062021. [DOI] [PubMed] [Google Scholar]
- 2.Baranek GT, David FJ, Roe MD, Stone WL, Watson LR. Sensory experience questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry. 2006;(6):47, 591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Sasson A, Cermak SA, Orsmond GI, Carter AS, Kadlec MB, Dunn W. Extreme sensory modulation behaviors in toddlers with autism. American Journal of Occupational Therapy. 2007;61(5):584–592. doi: 10.5014/ajot.61.5.584. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Sasson A, Carter AS, Briggs-Gowan MJ. Sensory Over-Responsivity in Elementary School: Prevalence and Social-Emotional Correlates. Journal of Abnormal Child Psychology. 2009;37(5):705–716. doi: 10.1007/s10802-008-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeiffer B, Kinnealey M, Reed C, Herzberg G. Sensory modulation and affective disorders in children and adolescents with Asperger's disorder. Am J Occup Ther. 2005;(3):59, 335–345. doi: 10.5014/ajot.59.3.335. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Kadlec MB, Carter AS. Sensory clusters of toddlers with autism spectrum disorders: Differences in affective symptoms. Journal of Child Psychology and Psychiatry. 2008;49(8):817–825. doi: 10.1111/j.1469-7610.2008.01899.x. [DOI] [PubMed] [Google Scholar]
- 7.Gomot M, Belmonte MK, Bullmore ET, Bernard FA, Baron-Cohen S. Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain. 2008;131(9):2479–2488. doi: 10.1093/brain/awn172. [DOI] [PubMed] [Google Scholar]
- 8.Nouchine Hadjikhani CFC. Early visual cortex organization in autism: an fMRI study. Neuroreport. 2004;15(2):267–70. doi: 10.1097/00001756-200402090-00011. [DOI] [PubMed] [Google Scholar]
- 9.Marco EJ, Hinkley LBN, Hill SS, Nagarajan SS. Sensory Processing in Autism: A Review of Neurophysiologic Findings. Pediatric Research. 2011;69:48R–54R. doi: 10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardan AY, Minshew NJ, Melhem NM, et al. An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Research: Neuroimaging. 2008;163(2):97–105. doi: 10.1016/j.pscychresns.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsatsanis KD, Rourke BP, Klin A, Volkmar FR, Cicchetti D, Schultz RT. Reduced thalamic volume in high-functioning individuals with autism. Biological psychiatry. 2003;53(2):121–129. doi: 10.1016/s0006-3223(02)01530-5. [DOI] [PubMed] [Google Scholar]
- 12.Mizuno A, Villalobos ME, Davies MM, Dahl BC, Müller RA. Partially enhanced thalamocortical functional connectivity in autism. Brain research. 2006;1104(1):160–174. doi: 10.1016/j.brainres.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 13.Green SA, Ben-Sasson A. Anxiety Disorders and Sensory Over-Responsivity in Children with Autism Spectrum Disorders: Is There a Causal Relationship? Journal of Autism and Developmental Disorders. 2010;40(12):1495–1504. doi: 10.1007/s10803-010-1007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hitoglou M, Ververi A, Antoniadis A, Zafeiriou Dl. Childhood Autism and Auditory System Abnormalities. Pediatric Neurology. 2010;42(5):309–314. doi: 10.1016/j.pediatrneurol.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Waterhouse L, Fein D, Modahl C. Neurofunctional mechanisms in autism. Psychological Review. 1996;103(3):457–489. doi: 10.1037/0033-295x.103.3.457. [DOI] [PubMed] [Google Scholar]
- 16.Mazurek MO, Vasa RA, Kalb LG, et al. Anxiety, Sensory Over-Responsivity, and Gastrointestinal Problems in Children with Autism Spectrum Disorders. J Abnorm Child Psychol. 2013;41(1):165–176. doi: 10.1007/s10802-012-9668-x. [DOI] [PubMed] [Google Scholar]
- 17.Davis M. The Role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 18.Garakani A, Mathew SJ, Charney DS. Neurobiology of Anxiety Disorders and Implications for Treatment. Mount Sinai Journal of Medicine. 2006;73(7):941–949. [PubMed] [Google Scholar]
- 19.Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- 20.Rauch SL, Shin LM, Wright CI. Neuroimaging Studies of Amygdala Function in Anxiety Disorders. Annals of the New York Academy of Sciences. 2003;985(1):389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 21.Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in cognitive sciences. 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SCR. The amygdala theory of autism. Neuroscience & Biobehavioral Reviews. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- 24.Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127(12):2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- 25.Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tottenham N, Hertzig ME, Gillespie-Lynch K, Gilhooly T, Millner AJ, Casey BJ. Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc Cogn Affect Neurosci. 2013 doi: 10.1093/scan/nst050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng S- J, Carrasco M, Swartz JR, et al. Neural activation to emotional faces in adolescents with autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2011;52(3):296–305. doi: 10.1111/j.1469-7610.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers SJ, Ozonoff S. Annotation: What do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry. 2005;46(12):1255–1268. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- 29.Schoen SA, Miller U, Brett-Green B, Hepburn SL. Psychophysioiogy of children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2008;2(3):417–429. [Google Scholar]
- 30.Lane SJ, Reynolds S, Dumenci L. Sensory Overresponsivity and Anxiety in Typically Developing Children and Children With Autism and Attention Deficit Hyperactivity Disorder: Cause or Coexistence? Am J Occup Ther. 2012;66(5):595–603. doi: 10.5014/ajot.2012.004523. [DOI] [PubMed] [Google Scholar]
- 31.Aylward EH, Minshew NJ, Goldstein G, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53(9):2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- 32.Sparks BF, Friedman SD, Shaw DW, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59(2):184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 33.Schumann CM, Hamstra J, Goodlin-Jones BL, et al. The Amygdala Is Enlarged in Children But Not Adolescents with Autism; the Hippocampus Is Enlarged at All Ages. J. Neurosci. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juranek J, Filipek PA, Berenji GR, Modahl C, Osann K, Spence MA. Association Between Amygdala Volume and Anxiety Level: Magnetic Resonance Imaging (MRI) Study in Autistic Children. Journal of Child Neurology. 2006;21(12):1051–1058. doi: 10.1177/7010.2006.00237. [DOI] [PubMed] [Google Scholar]
- 35.Carter AS, Ben-Sasson A, Briggs-Gowan MJ. Sensory Over-Responsivity, Psychopathology, and Family Impairment in School-Aged Children. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(12):1210–1219. doi: 10.1016/j.jaac.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation: Harbourt Brace & Company; New York, NY: 1999. [Google Scholar]
- 37.Wechsler D. Wechsler Intelligence Scale for Children. 4th Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- 38.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 39.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 40.Achenbach TM, Rescoria LA. Manual for the ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. [Google Scholar]
- 41.Dunn W. The Sensory Profile: User's Manual. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 42.Schoen SA, Miller LI, Green KE. Pilot study of the sensory over-responsivity scales: Assessment and inventory. American Journal of Occupational Therapy. 2008;62:393–406. doi: 10.5014/ajot.62.4.393. [DOI] [PubMed] [Google Scholar]
- 43.Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neurolmage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- 44.Woolrich M. Robust group analysis using outlier inference. Neuroimage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- 45.Woolrich MW, Behrens TEJ, Beckmarin CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 46.Charney DS, Grillon C, Bremner JD. Review: The Neurobiological Basis of Anxiety and Fear: Circuits, Mechanisms, and Neurochemical Interactions (Part I. Neuroscientist. 1998;(1):4, 35–44. [Google Scholar]
- 47.Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. NeuroReport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- 48.Larson CL, Schaefer HS, Siegle GJ, Jackson CAB, Anderle MJ, Davidson RJ. Fear is fast in phobic individuals: amygdala activation in response to fear-relevant stimuli. Biological Psychiatry. 2006;60(4):410–417. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- 49.Kleinhans N, Johnson L, Richards T, et al. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. American Journal of Psychiatry. 2009;166(4):467–475. doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]