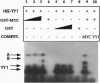
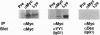Abstract
The c-Myc oncoprotein has previously been shown to associate with transcription regulator YY1 and to inhibit its activity. We show herein that endogenous c-Myc and YY1 associate in vivo and that changes in c-Myc levels, which accompany mitogenic stimulation or differentiation of cultured cells, affect the ratio of free to c-Myc-associated YY1. We have also investigated the mechanism by which association with c-Myc inhibits YY1's ability to regulate transcription. c-Myc does not block binding of YY1 to DNA. However, protein association studies suggest that c-Myc interferes with the ability of YY1 to contact basal transcription proteins TATA-binding protein and TFIIB.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atchison M. L., Meyuhas O., Perry R. P. Localization of transcriptional regulatory elements and nuclear factor binding sites in mouse ribosomal protein gene rpL32. Mol Cell Biol. 1989 May;9(5):2067–2074. doi: 10.1128/mcb.9.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin R. J., Biggin M. D. A domain of the even-skipped protein represses transcription by preventing TFIID binding to a promoter: repression by cooperative blocking. Mol Cell Biol. 1995 Sep;15(9):4683–4693. doi: 10.1128/mcb.15.9.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton C. H., Mann D. A., Walsh F. S. Characterization of the human N-CAM promoter. Biochem J. 1990 May 15;268(1):161–168. doi: 10.1042/bj2680161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauknecht T., Angel P., Royer H. D., zur Hausen H. Identification of a negative regulatory domain in the human papillomavirus type 18 promoter: interaction with the transcriptional repressor YY1. EMBO J. 1992 Dec;11(12):4607–4617. doi: 10.1002/j.1460-2075.1992.tb05563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Fernandez C., Packham G., Cleveland J. L. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7804–7808. doi: 10.1073/pnas.90.16.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenisty N., Leder A., Kuo A., Leder P. An embryonically expressed gene is a target for c-Myc regulation via the c-Myc-binding sequence. Genes Dev. 1992 Dec;6(12B):2513–2523. doi: 10.1101/gad.6.12b.2513. [DOI] [PubMed] [Google Scholar]
- Bird R. C., Kung T. Y., Wu G., Young-White R. R. Variations in c-fos mRNA expression during serum induction and the synchronous cell cycle. Biochem Cell Biol. 1990 May;68(5):858–862. doi: 10.1139/o90-127. [DOI] [PubMed] [Google Scholar]
- Chen S., Mills L., Perry P., Riddle S., Wobig R., Lown R., Millette R. L. Transactivation of the major capsid protein gene of herpes simplex virus type 1 requires a cellular transcription factor. J Virol. 1992 Jul;66(7):4304–4314. doi: 10.1128/jvi.66.7.4304-4314.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Kaestner K. H., Geiman D. E., Lane M. D. CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S., Perry R. P. The importance of downstream delta-factor binding elements for the activity of the rpL32 promoter. Nucleic Acids Res. 1993 Jul 11;21(14):3301–3308. doi: 10.1093/nar/21.14.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. D. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- Cory S. Activation of cellular oncogenes in hemopoietic cells by chromosome translocation. Adv Cancer Res. 1986;47:189–234. doi: 10.1016/s0065-230x(08)60200-6. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Littlewood T. D. The role of c-myc in cell growth. Curr Opin Genet Dev. 1993 Feb;3(1):44–49. doi: 10.1016/s0959-437x(05)80339-9. [DOI] [PubMed] [Google Scholar]
- Farnham P. J., Means A. L. Sequences downstream of the transcription initiation site modulate the activity of the murine dihydrofolate reductase promoter. Mol Cell Biol. 1990 Apr;10(4):1390–1398. doi: 10.1128/mcb.10.4.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. R., Becker K. G., Ennist D. L., Gleason S. L., Driggers P. H., Levi B. Z., Appella E., Ozato K. Cloning of a negative transcription factor that binds to the upstream conserved region of Moloney murine leukemia virus. Mol Cell Biol. 1992 Jan;12(1):38–44. doi: 10.1128/mcb.12.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaubatz S., Meichle A., Eilers M. An E-box element localized in the first intron mediates regulation of the prothymosin alpha gene by c-myc. Mol Cell Biol. 1994 Jun;14(6):3853–3862. doi: 10.1128/mcb.14.6.3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Grignani F., Lombardi L., Inghirami G., Sternas L., Cechova K., Dalla-Favera R. Negative autoregulation of c-myc gene expression is inactivated in transformed cells. EMBO J. 1990 Dec;9(12):3913–3922. doi: 10.1002/j.1460-2075.1990.tb07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W., Bhatia K., Magrath I. T., Dang C. V., Dalla-Favera R. Binding and suppression of the Myc transcriptional activation domain by p107. Science. 1994 Apr 8;264(5156):251–254. doi: 10.1126/science.8146655. [DOI] [PubMed] [Google Scholar]
- Guo B., Odgren P. R., van Wijnen A. J., Last T. J., Nickerson J., Penman S., Lian J. B., Stein J. L., Stein G. S. The nuclear matrix protein NMP-1 is the transcription factor YY1. Proc Natl Acad Sci U S A. 1995 Nov 7;92(23):10526–10530. doi: 10.1073/pnas.92.23.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan N., Kelley D. E., Perry R. P. Delta, a transcription factor that binds to downstream elements in several polymerase II promoters, is a functionally versatile zinc finger protein. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9799–9803. doi: 10.1073/pnas.88.21.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hateboer G., Timmers H. T., Rustgi A. K., Billaud M., van 't Veer L. J., Bernards R. TATA-binding protein and the retinoblastoma gene product bind to overlapping epitopes on c-Myc and adenovirus E1A protein. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8489–8493. doi: 10.1073/pnas.90.18.8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K. M., McMahon S. L., Farnham P. J. Multiple DNA elements are required for the growth regulation of the mouse E2F1 promoter. Genes Dev. 1994 Jul 1;8(13):1526–1537. doi: 10.1101/gad.8.13.1526. [DOI] [PubMed] [Google Scholar]
- Inghirami G., Grignani F., Sternas L., Lombardi L., Knowles D. M., Dalla-Favera R. Down-regulation of LFA-1 adhesion receptors by C-myc oncogene in human B lymphoblastoid cells. Science. 1990 Nov 2;250(4981):682–686. doi: 10.1126/science.2237417. [DOI] [PubMed] [Google Scholar]
- Inouye C. J., Seto E. Relief of YY1-induced transcriptional repression by protein-protein interaction with the nucleolar phosphoprotein B23. J Biol Chem. 1994 Mar 4;269(9):6506–6510. [PubMed] [Google Scholar]
- Kelly K., Siebenlist U. The regulation and expression of c-myc in normal and malignant cells. Annu Rev Immunol. 1986;4:317–338. doi: 10.1146/annurev.iy.04.040186.001533. [DOI] [PubMed] [Google Scholar]
- Koller B. H., Orr H. T. Cloning and complete sequence of an HLA-A2 gene: analysis of two HLA-A alleles at the nucleotide level. J Immunol. 1985 Apr;134(4):2727–2733. [PubMed] [Google Scholar]
- Lachman H. M., Skoultchi A. I. Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature. 1984 Aug 16;310(5978):592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Galvin K. M., See R. H., Eckner R., Livingston D., Moran E., Shi Y. Relief of YY1 transcriptional repression by adenovirus E1A is mediated by E1A-associated protein p300. Genes Dev. 1995 May 15;9(10):1188–1198. doi: 10.1101/gad.9.10.1188. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Galvin K. M., Shi Y. Evidence for physical interaction between the zinc-finger transcription factors YY1 and Sp1. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6145–6149. doi: 10.1073/pnas.90.13.6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. C., Shi Y., Schwartz R. J. Displacement of BrdUrd-induced YY1 by serum response factor activates skeletal alpha-actin transcription in embryonic myoblasts. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9814–9818. doi: 10.1073/pnas.89.20.9814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. H., Nerlov C., Prendergast G., MacGregor D., Ziff E. B. c-Myc represses transcription in vivo by a novel mechanism dependent on the initiator element and Myc box II. EMBO J. 1994 Sep 1;13(17):4070–4079. doi: 10.1002/j.1460-2075.1994.tb06724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Eisenman R. N. New light on Myc and Myb. Part I. Myc. Genes Dev. 1990 Dec;4(12A):2025–2035. doi: 10.1101/gad.4.12a.2025. [DOI] [PubMed] [Google Scholar]
- Maheswaran S., Lee H., Sonenshein G. E. Intracellular association of the protein product of the c-myc oncogene with the TATA-binding protein. Mol Cell Biol. 1994 Feb;14(2):1147–1152. doi: 10.1128/mcb.14.2.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai S., Jalava A. c-Myc binds to 5' flanking sequence motifs of the dihydrofolate reductase gene in cellular extracts: role in proliferation. Nucleic Acids Res. 1994 Jun 25;22(12):2264–2273. doi: 10.1093/nar/22.12.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B., Bossone S. A., Patel A. J. myc function and regulation. Annu Rev Biochem. 1992;61:809–860. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- Marcu K. B. Regulation of expression of the c-myc proto-oncogene. Bioessays. 1987 Jan;6(1):28–32. doi: 10.1002/bies.950060108. [DOI] [PubMed] [Google Scholar]
- Meier V. S., Groner B. The nuclear factor YY1 participates in repression of the beta-casein gene promoter in mammary epithelial cells and is counteracted by mammary gland factor during lactogenic hormone induction. Mol Cell Biol. 1994 Jan;14(1):128–137. doi: 10.1128/mcb.14.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyuhas O., Klein A. The mouse ribosomal protein L7 gene. Its primary structure and functional analysis of the promoter region. J Biol Chem. 1990 Jul 15;265(20):11465–11473. [PubMed] [Google Scholar]
- Natesan S., Gilman M. Z. DNA bending and orientation-dependent function of YY1 in the c-fos promoter. Genes Dev. 1993 Dec;7(12B):2497–2509. doi: 10.1101/gad.7.12b.2497. [DOI] [PubMed] [Google Scholar]
- Park K., Atchison M. L. Isolation of a candidate repressor/activator, NF-E1 (YY-1, delta), that binds to the immunoglobulin kappa 3' enhancer and the immunoglobulin heavy-chain mu E1 site. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9804–9808. doi: 10.1073/pnas.88.21.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear W. S., Nolan G. P., Scott M. L., Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B., Merezhinskaya N., Diffley J. F., Noguchi C. T. Protein-DNA interactions in the epsilon-globin gene silencer. J Biol Chem. 1993 Feb 15;268(5):3430–3437. [PubMed] [Google Scholar]
- Philipp A., Schneider A., Väsrik I., Finke K., Xiong Y., Beach D., Alitalo K., Eilers M. Repression of cyclin D1: a novel function of MYC. Mol Cell Biol. 1994 Jun;14(6):4032–4043. doi: 10.1128/mcb.14.6.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman D., Elkind N. B., Roy B., Beamon J., Rotter V. c-Myc trans-activates the p53 promoter through a required downstream CACGTG motif. Cell Growth Differ. 1993 Feb;4(2):57–65. [PubMed] [Google Scholar]
- Riggs K. J., Merrell K. T., Wilson G., Calame K. Common factor 1 is a transcriptional activator which binds in the c-myc promoter, the skeletal alpha-actin promoter, and the immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1991 Mar;11(3):1765–1769. doi: 10.1128/mcb.11.3.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs K. J., Saleque S., Wong K. K., Merrell K. T., Lee J. S., Shi Y., Calame K. Yin-yang 1 activates the c-myc promoter. Mol Cell Biol. 1993 Dec;13(12):7487–7495. doi: 10.1128/mcb.13.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. L., Carruthers C., Gutjahr T., Roeder R. G. Direct role for Myc in transcription initiation mediated by interactions with TFII-I. Nature. 1993 Sep 23;365(6444):359–361. doi: 10.1038/365359a0. [DOI] [PubMed] [Google Scholar]
- Rustgi A. K., Dyson N., Bernards R. Amino-terminal domains of c-myc and N-myc proteins mediate binding to the retinoblastoma gene product. Nature. 1991 Aug 8;352(6335):541–544. doi: 10.1038/352541a0. [DOI] [PubMed] [Google Scholar]
- Seto E., Lewis B., Shenk T. Interaction between transcription factors Sp1 and YY1. Nature. 1993 Sep 30;365(6445):462–464. doi: 10.1038/365462a0. [DOI] [PubMed] [Google Scholar]
- Shi Y., Seto E., Chang L. S., Shenk T. Transcriptional repression by YY1, a human GLI-Krüppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991 Oct 18;67(2):377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Shrivastava A., Calame K. An analysis of genes regulated by the multi-functional transcriptional regulator Yin Yang-1. Nucleic Acids Res. 1994 Dec 11;22(24):5151–5155. doi: 10.1093/nar/22.24.5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A., Saleque S., Kalpana G. V., Artandi S., Goff S. P., Calame K. Inhibition of transcriptional regulator Yin-Yang-1 by association with c-Myc. Science. 1993 Dec 17;262(5141):1889–1892. doi: 10.1126/science.8266081. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Tibensky D., Delovitch T. L. Promoter region of HLA-C genes: regulatory elements common to and different from those of HLA-A and HLA-B genes. Immunogenetics. 1990;32(3):210–213. doi: 10.1007/BF02114976. [DOI] [PubMed] [Google Scholar]
- Um M., Li C., Manley J. L. The transcriptional repressor even-skipped interacts directly with TATA-binding protein. Mol Cell Biol. 1995 Sep;15(9):5007–5016. doi: 10.1128/mcb.15.9.5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usheva A., Shenk T. TATA-binding protein-independent initiation: YY1, TFIIB, and RNA polymerase II direct basal transcription on supercoiled template DNA. Cell. 1994 Mar 25;76(6):1115–1121. doi: 10.1016/0092-8674(94)90387-5. [DOI] [PubMed] [Google Scholar]
- Vincent C. K., Gualberto A., Patel C. V., Walsh K. Different regulatory sequences control creatine kinase-M gene expression in directly injected skeletal and cardiac muscle. Mol Cell Biol. 1993 Feb;13(2):1264–1272. doi: 10.1128/mcb.13.2.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]