Abstract
Fibrolamellar carcinomas are a unique type of primary liver cancer. They occur most commonly in children and young adults. Their etiology remains a mystery, as they are not associated with chronic liver disease. Fibrolamellar carcinomas are not indolent tumors, but have an overall better prognosis than typical hepatocellular carcinomas, in part because of the younger age at presentation and the lack of cirrhosis. The most important prognostic feature is whether the tumor is resectable. Histologically, the tumor is made up of large cells that contain abundant mitochondria. The nuclei of the tumor cells have prominent nucleoli. The tumor cells induce the formation of extensive intratumoral fibrosis, which often grows in parallel, or lamellar bands. The tumor cells clearly show hepatocellular features but are also unique in showing both biliary and neuroendocrine differentiation. The uniqueness of fibrolamellar carcinoma extends to their molecular findings. While the genetic abnormalities that lead to fibrolamellar carcinomas are not yet known, studies have shown that they lack mutations in the genes most commonly mutated in typical hepatocellular carcinoma (TP53 and CTNNB1). In this paper, the clinical, pathological, and basic science literature on fibrolamellar carcinoma is comprehensively reviewed. Key areas of needed research are also discussed.
1. Introduction
Fibrolamellar carcinoma (FLC) was first described as a unique entity in 1956 by Edmondson [1], who described it as a unique form of primary hepatocellular carcinoma. Craig et al. further developed the striking clinical predilection for younger individuals and the characteristic histological features in 1980 [2]. This 1980 study was also the first to use the term “fibrolamellar carcinoma.” Subsequent case reports and cases series have confirmed and expanded the unique clinical and pathologic features of FLC. Major review articles on the clinical, pathological, and biological findings have been published in 2007 [3], 2009 [4], and 2011 [5].
While earlier editions of the series WHO Classification of Tumors recognized FLC as a unique histological pattern, it was not until the 2010 edition [6] that FLC was given its own WHO classification number. This was an important step because FLC is unique at the clinical, histological, and biological levels and they should be analyzed separately from typical hepatocellular carcinomas in all scientific studies.
2. Clinical Findings
2.1. Presentation
FLC generally present with vague, nonspecific clinical signs and symptoms, often which include elements of abdominal pain, weight loss, and malaise [2, 7–11]. Overall, the most common physical finding is an abdominal mass or hepatomegaly [9–11]. However, a wide variety of unusual presentations have also been described (Table 1). These include several reports of biliary obstruction secondary to direct tumor growth into the biliary tree or to compression by metastatic deposits in hilar lymph nodes. In fact, biliary obstruction is probably even more common than reported, as dilated intrahepatic bile ducts are found in 40% of cases by imaging studies [12]. Gynecomastia has also been reported by several authors [13–16].
Table 1.
Unusual presentations for fibrolamellar carcinoma.
| Category | Presentation |
|---|---|
| Vascular flow abnormalities | Budd-Chiari [17, 18] |
| Caval compression syndrome [19] | |
| Caval obstruction [20] | |
|
| |
| Systemic symptoms | Severe anemia hypoglycemia [21] |
| Increased beta hCG [22] | |
| Systemic AA amyloid deposition [23] | |
|
| |
| Biliary | Obstructive jaundice [24, 25] |
| Growth into bile duct [26] | |
| Background disease of ulcerative colitis and primary sclerosing cholangitis [27] | |
|
| |
| Miscellaneous | Nonbacterial thrombotic endocarditis [28] |
| Following autologous bone marrow transplant for lymphoma | |
| Extensive intraperitoneal metastases with ascites [29] | |
| Mimicking a liver abscess [30] | |
| Bone metastases [31] | |
| Gynecomastia [13–15] | |
| Pancreas tumor with liver metastasis [32] | |
| Cold agglutinin disease [33] | |
2.2. Serum Findings
Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) can be normal [11] or mildly elevated [2]. Alkaline phosphatase levels may be elevated [10, 32, 34], occasionally with levels greater than 1000 IU/mL [23], findings that most likely reflect growth into the biliary tree or obstruction of the biliary tree. Alpha-fetoprotein (AFP) levels are typically normal [35]. There is a subset of approximately 5–10% of reported cases in the literature that have serum AFP elevations in the 200 ng/mL or greater range. For example, in one of the larger series to date, 7% of the FLC had AFP levels greater than 200 ng/mL [36]. As a second example, 27% of cases in another study had elevated serum AFP levels [10]. However, overall it seems highly likely that a substantial proportion of the reported FLC cases with elevated serum AFP are misdiagnosed and should have been classified as typical hepatocellular carcinoma. While the histological features of FLC, as described in the Histology section, are quite distinctive, misclassification of typical hepatocellular carcinoma as FLC is a persistent and significant problem when evaluating the published literature.
Other serum findings in patients with FLC include elevated transcobalamin I levels [7, 37, 38], transcobalamin 2 [7], and vitamin B12 binding capacity [7, 19, 23, 37, 39, 40]. Transcobalamin I is also known as haptocorrin and is a glycoprotein that in normal physiology is made largely in the salivary glands, from where it is secreted into the digestive tract and protects vitamin B12 (also known as cobalamin) from degradation in the stomach. The reasons for the elevated B12 binding capacity and the elevated transcobalamin levels in FLC are not clear, but have been reported by multiple groups. Increased vitamin B12 binding capacity is more commonly seen in FLC than typical hepatocellular carcinoma [39] but is not exclusive to FLC. Serum transcobalamin I or haptocorrin levels can also be elevated in cases of typical hepatocellular carcinoma [41] and, in these cases, appear to be produced by tumor cells or have increased serum uptake by the tumor cells [42].
Other elevated serum proteins include fibrinogen [19]. Interestingly, fibrinogen can also be found within the “pale bodies” of FLC tumor cells (see below). Serum neurotensin levels can also be elevated in about 25% of FLC cases [43]. In one study on neurotensin, 20 patients with liver cancer were studied for serum neurotensin levels: five patients had elevated levels and four were diagnosed with FLC [44]. Finally, PIVKA-II (protein induced by vitamin K absence/antagonist-II), perhaps better known as Des-gamma carboxyprothrombin or DCP, was elevated in all cases of FLC in a USA-based study of 41 cases [45] and in approximately 70% of the cases in a report from Japan [19]. This protein is an abnormal form of the coagulation protein, prothrombin, and is also frequently elevated in typical HCC [46].
2.3. Demographics
One of the most distinctive and well-recognized features of FLC is its occurrence in younger individuals. To get a stronger sense of the age distribution in FLC, the age at diagnosis of FLC was extracted from the literature of 275 cases where individual ages were provided (data extracted from reports in the references list). It is recognized that a proportion of these cases were incorrectly diagnosed as FLC, and a few cases of obvious misdiagnosis were excluded. While this is a limitation, the data nonetheless provides the most comprehensive survey of age at diagnosis available for FLC. Based on these 275 cases, the age at presentation shows a unimodal distribution that rises sharply in the late teens and peaks in the early twenties, with an overall average age of 25.6 ± 12.6 years (mean ± standard deviation) and a median of 22 years and a mode of 21 years. In contrast, an average age of 39 ± 20 years was reported for 68 individuals from the Surveillance, Epidemiology, and End Results (SEER) database [47]. These latter results, while still younger than the average presenting age for typical hepatocellular carcinoma, are significantly older than the aggregate of cases reported in the literature and suggest that many of the cases in the SEER database are misclassified. Nonetheless, the strong pattern remains: FLC occurs at significantly younger ages than typical hepatocellular carcinoma, which has an average age at presentation of 65 years [47].
Based on extracted age information from 275 cases in the literature, FLC strongly clusters in the young with 50% of all cases occurring between the ages of 17 and 30 and 80% within the years 10 to 35. The youngest age reported in the literature is 7 months [48], but this case is such an exception that the diagnosis is strongly suspected. Likewise, the published images in another reported case of FLC in a 4-month-old infant do not strongly support the diagnosis of FLC [49]. However, FLC has been reported by several groups as early as 4 to 5 years of age [2, 50, 51]. Of note, FLC can occur in older individuals, including rare cases in those older than 60 years.
Overall, about 20% of typical hepatocellular carcinomas arise in noncirrhotic livers. Interestingly, these carcinomas share some broad overall similarities with FLC: they are often reported to present at a younger age [52], do not have as a strong of a skewed male : female ratio as hepatocellular carcinomas arising in cirrhotic livers [52], and frequently recur after surgery with curative intent [52].
2.4. Gender and Ethnicity
No strong gender predilection is apparent for FLC [8, 11, 45, 47]. Based on the SEER database, there appears to be a racial/ethnic predilection for Caucasians [47]. However, FLC has been reported from a wide variety of geographical locations including Mexico [53], Saudi Arabia [33], South Africa [54, 55], Japan [56], Taiwan [57, 58], Mainland China [59], Republic of Korea [60], Thailand [32], India [28, 61], and Europe [62], and the extent of the racial/ethnic bias remains poorly defined.
2.5. Frequency
Overall, FLC comprises approximately 5% of all hepatocellular carcinomas, but the exact proportion varies between less than 1% and 8% depending on study design and the patient population (Table 2).
Table 2.
Representative studies of the prevalence of FLC in various populations.
| Location | Prevalence | Comment |
|---|---|---|
| Ostra Sjukhuset, Sweden [63] | 2/532 (0.4%) | Based on cases from 1958 to 1979 |
| Songkhla, Thailand [64] | 1/180 (0.6%) | Southern Thailand, from 1991 to 1998 |
| USA [47] | 71/9,870 (0.7%) | From SEER database, based on cases from 1986 to 2000 |
| Columbus, OH, USA [65] | 3/58 (5%) | Ohio State, cases from 1966 to 1981 |
| Rochester, MN, USA [66] | 10/280 (4%) | Mayo clinic, cases from 1987 to 1993 |
| Pittsburgh, PA, USA [45] | 41/477 (8.9%) | Patients undergoing liver transplant for HCC between 1968 and 1995 |
| Rochester, MN, USA [66] | 10/280 (4%) | Mayo clinic, cases from 1987 to 1993 |
| Mexico City, Mexico [34] | 7/121 (5.8%) | Based on cases from 1987 to 2001 |
| Mexico City, Mexico [53] | 15/174 (8.6%) | Based on cases from 1990 to 2003 |
| Villejuif, France [52] | 5/68 (7.3%) | Frequency of HCCs in noncirrhotic livers |
| South Africa [54] | 9/274 (3%) | Data from pediatric cancer registry; includes all pediatric cancers and not just HCC |
| London, UK [39] | 8/107 (7%) | |
| Toronto, Canada [11] | 10/NS (9%) | Referrals to a tertiary care center for HCC with intent to treat from 1982 to 1995 |
Because of the well-known predilection of FLC for young individuals, there is a common misconception that FLC is the most frequent form of liver cancer in children and young adults. However, the most common form of liver cancer in children and young adults is in fact typical hepatocellular carcinoma, which still accounts for between 60% and 80% of liver cancers in this age group [48, 50, 67, 68].
2.6. Prognosis
The prognosis of FLC has been studied for many years. Earlier studies were often characterized by comparing FLC prognosis to that of typical hepatocellular carcinoma. Since most individuals with typical hepatocellular carcinoma are considerably older and have liver cirrhosis, as well as other comorbid conditions, patients with FLC appeared to do significantly better. However, in these cases, the better prognosis observed for FLC is in part due to the lack of cirrhosis and other comorbid conditions. Cirrhosis, for example, is a well-recognized independent risk factor for mortality. Thus, when FLC is compared only to those hepatocellular carcinomas that arise in the background of noncirrhotic livers, the survival advantage is less apparent, with no survival benefit seen in some case series [69], although a survival benefit was still evident in others [45, 67, 70].
2.7. Clinical and Pathology Prognostic Factors
Resectability is one of the most important prognostic features for FLC [36, 47, 48, 53]. For example, in one large study, the median 5-year survival for those who underwent resection was 76% with a median overall survival of 112 months [36]. In contrast, the median 5-year survival for those with unresectable disease was 0% with an overall median survival of 12 months [36]. Another study of FLC patients with unresectable liver disease due to regional node disease found a similar median survival of 14 months [71].
Other reported prognostic factors include gender [47], age at presentation [47, 53], lymph node [36, 45] or other metastatic disease [45] at the time of presentation, tumor stage [11, 45, 47], vascular invasion [45], absence of elevated liver enzymes [53], and absence of large vessel invasion or thrombosis [53]. However, most of these additional prognostic findings have not been well validated across multiple studies.
2.8. Treatment
As noted previously, surgical resection is a key first line treatment. However, even in those patients with resectable diseases, subsequent recurrent liver or metastatic tumor is seen in more than half of the cases, with a reported range from 36% to 100% [7, 36]. The median time to recurrence tends to be short, ranging from 10 to 33 months, though later recurrences have also been described. Aggressive surgical resection of metastatic disease, when possible, can be of benefit [7, 11].
In terms of systemic chemotherapy, data is limited but there have been reports of partial response to platinum-based therapy [7]. Overall, FLC does not respond better than typical HCC to cisplatin, vincristine, and 5-FU-based chemotherapy [48]. Others have found no clear benefit for chemotherapy in individuals as adjuvant therapy for FLC [7, 8, 45].
2.9. Section Summary and Key Remaining Questions
In sum, FLC occurs in younger individuals with no strong gender bias. FLC has been reported from many different ethnic backgrounds, but the overall current findings suggest a possible Caucasian predominance. FLC typically presents with advanced stage disease, is locally aggressive with a propensity to metastasize, and has a high relapse following resection. Cure rate is very low. Treatment remains focused on surgical resection with aggressive management of surgical disease, with either no or modest long-term benefit from systemic chemotherapy.
Key questions that remain to be answered or substantially clarified include the following: Question 1. One of the critical points to future progress is to ensure that reported cases of FLC actually meet the histological requirements for this diagnosis. It is a significant problem because studies that inadvertently include cases of typical hepatocellular carcinoma in studies of FLC confuse the literature and confound our ability to make future progress. As discussed below, there are now several histological immunostains that can be used to confirm the diagnosis of FLC and all authors should be strongly encouraged to use them. Question 2. Is there truly a Caucasian predominance? If so, can a more-specific-at-risk Caucasian population be identified? Question 3. Are there any regional risk factors for FLC that are not explained by ethnicity, such as urban versus rural, etc.? and as a related question, are there geographic or temporal clustering to cases of FLC? Question 4. What is the best currently available chemotherapeutic agent useful in patients with FLC?
3. Tumor Findings
3.1. Gross Findings
Grossly, FLCs are yellow to pale tan in color and their consistency can range from soft to firm and hard. A central scar is found in 60–70% of cases [10, 72]. The tumors tend to be more common in the left lobe of the liver [2, 12], but frequently involve both lobes [72]. At the time of resection, the average tumor diameter is large, ranging between 9 and 14 cm in greatest dimension [8, 10, 12, 36, 45]. In 80–90% of cases, a single large tumor is present [8, 12, 36, 45, 66]. Occasionally, the tumors contain small foci of necrosis as well as areas of hemorrhage [72]. Gross vascular invasion is seen in up to 25% of cases [45].
The background livers are noncirrhotic. While several cases of FLC in the setting of cirrhosis were included in the earliest descriptions of FLC [2] and have been occasionally reported subsequently [45], the diagnosis of FLC in the setting of cirrhosis should be carefully considered, as it seems likely that a large proportion of the FLCs reported in this context are misclassified.
3.2. Histology Findings
The tumor is made up of large polygonal cells with abundant eosinophilic cytoplasm, large vesiculated nuclei, and large nucleoli (Figures 1(a) and 1(b)). These three cytological findings in conjunction with the lamellar fibrosis Figure 1(b) are the defining features of FLC. FLC cells grown in culture retain their prominent nucleolus, though it may be found in several smaller structures that are likely distributed amongst different chromosomes (Figure 1(c)). The eosinophilic appearance of the tumor cells is a result of mitochondrial proliferation (see Ultrastructure section). In approximately 1/2 of cases, the tumor cells can have round amphophilic cytoplasmic inclusions, termed “pale bodies” [2] (Figure 1(d)). The exact composition of pale bodies is unclear, but they are immunoreactive for fibrinogen [10, 65, 73, 74], as well as other acute phase proteins [10] suggesting they contain a mixture of proteins that may reflect a fundamental defect in protein folding or secretion. Hyaline bodies (cytoplasmic inclusions that are eosinophilic and tend to be smaller than pale bodies) are also present in nearly half of FLC cases [2]. Neither pale bodies nor hyaline bodies are unique to FLC, and a diagnosis of FLC should not be made on the presence of these inclusions alone, as they can also be found in ordinary hepatocellular carcinoma. Mild macrovesicular steatosis can be seen in a minority of FLC cases [53].
Figure 1.
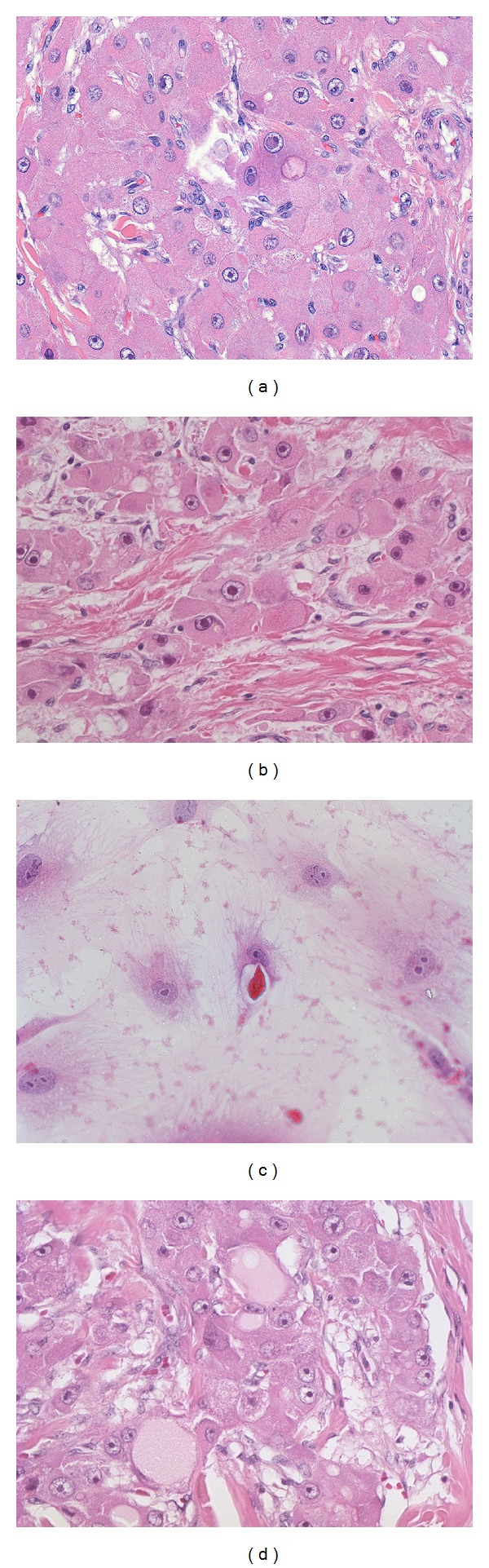
(a) Original magnification 160X. Fibrolamellar carcinomas are composed of large eosinophilic tumor cells with large vesiculated nuclei and large red nucleoli. (b) Original magnification 100X. Intratumoral fibrosis is a typical finding in fibrolamellar carcinoma. (c) Original magnification 160X. When FLC tumors grow in cell culture, they retain their large nucleoli and abundant cytoplasm. The nucleolus is often split into several larger subunits. (d) Original magnification 160X. Pale bodies are seen as large cytoplasmic inclusions with distinct borders and typically a “pale” grey color.
Mitotic figures are less common in FLC than in usual hepatocellular carcinoma [67]. Cholestasis is often present in FLC [10], with canalicular bile plugging the most common cholestatic pattern. Because of the frequent cholestasis, FLC commonly has copper deposition [2, 10, 69, 73, 75, 76]. Of note, copper accumulation is also common in ordinary HCC that is cholestatic and is not a defining feature of FLC [77]. Microscopic vascular invasion is present in 40–50% of FLC cases [36, 45, 67]. Given the very high rate of recurrence and distant metastasis after surgical resection, it appears very likely that angiolymphatic invasion is even more frequent than these numbers suggest. Occasionally, epithelioid granulomas can be found within the tumor [78] as well as the nonneoplastic liver tissue.
The tumor typically grows with broad pushing borders, but occasionally benign portal tracts can be found entrapped within the growing front of the tumor. One of the most characteristic low power features of the tumor is the presence of lamellar bands of fibrosis (Figure 2), which are present in essentially all primary tumors and can at times be seen in metastatic deposits. The bands of fibrosis are composed of type I, III, and V collagen and the collagen is produced by the stromal cells within the fibrous bands, although occasional tumor cells also produce collagen mRNA [79]. In about 60–70% of cases, the fibrosis will coalesce into larger central scars with radiating fibrous bands [78]. Calcifications are seen in 68% of FLC by CT studies [72] and small calcifications are not uncommon histologically, usually being seen in the central scar or fibrous bands.
Figure 2.
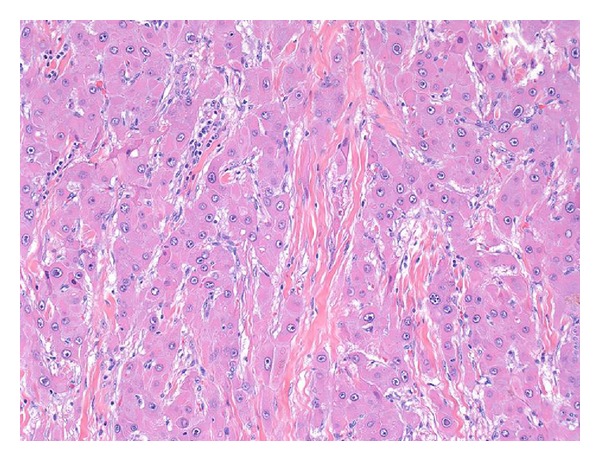
Original magnification 64X. A low power image demonstrates the characteristic lamellar fibrosis.
In terms of tumor grade, almost all FLCs are moderately differentiated. In fact, it is so unusual for FLC to be poorly differentiated that any poorly differentiated tumor should be carefully examined before making a diagnosis of FLC. Even in recurrent and metastatic FLC, the tumor grades are typically moderately differentiated.
Of note, some cases of FLC can demonstrate areas of gland-like (pseudoglands) formation with mucin production [3, 80–82]. The pseudoglands are circular to ovoid cystic structures lined by neoplastic cells. The lining cells may be somewhat smaller than the cells in the more typical areas of the tumor, but are otherwise morphologically similar (Figure 3). Mucin can be detected both within individual neoplastic cells as well as in the pseudoglands, and the mucin is positive for mucicarmine in just over half the cases and alcian blue in all cases [80, 82]. The cells lining the pseudoglands express biliary type cytokeratins and other proteins more typical of glandular differentiation. FLC with gland-like areas and mucin production has been called combined FLC and cholangiocarcinoma in the literature, but the best available evidence suggests that they are best considered as typical FLC. There is no strong evidence that they should be classified as true cholangiocarcinomas.
Figure 3.
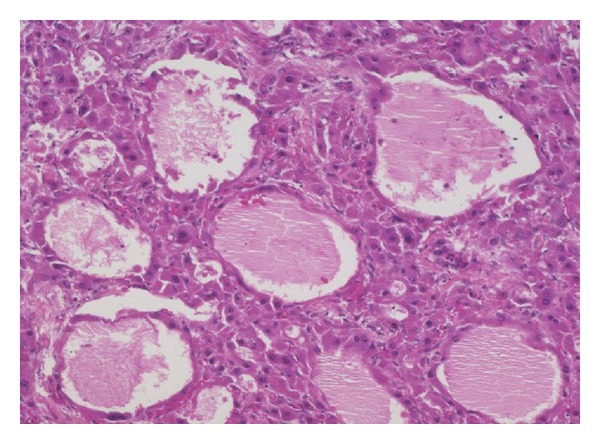
Original magnification 64X. An area of glandular-type differentiation with mucin production is shown.
3.3. Immunohistochemistry
Immunohistochemical studies of protein expression in FLC have shown the neoplastic cells are strongly HepPar positive [35, 68], even in areas with pseudoglandular differentiation and mucin production [80]. Glypican-3, however, is positive in only a subset of cases, ranging from 17% to 59% of cases [35, 83].
The neoplastic cells show expression of the expected hepatocellular cytokeratin 8 [84] and 18 [84], but also are strongly positive for cytokeratin 7 [35, 68, 83–86] and occasionally (between 5 and 25%) for cytokeratin 19 [35, 68, 83, 84]. These latter cytokeratins are normally expressed in biliary epithelium. Immunostains for AFP are negative [35, 60, 69]. The occasional reported cases with AFP positivity are likely misdiagnosed. It remains possible that rare FLC may express AFP, but there have been no convincing images published of AFP producing FLC. Ep-CAM is generally positive [35].
Neuroendocrine features in some FLCs have been noted by several authors [85, 87, 88]. Most FLCs are negative for chromogranin and synaptophysin by immunohistochemistry [35, 69], but occasional cases positive for chromogranin have been reported [23]. Of note, features of neuroendocrine differentiation are also seen in ordinary hepatocellular carcinoma, particularly in those that produce bile, and thus are not unique to FLC [89]. As noted previously, FLCs are also typically bile producing cancers and perhaps the bile production is a common denominator for neuroendocrine features.
3.4. Immunostains to Confirm the Diagnosis of FLC
Because some of the cases published in the literature do not appear to truly be FLC, it is clear that the H&E features alone are insufficient to make the diagnosis of FLC with sufficient specificity. The H&E findings appear to be very sensitive, but a significant proportion of typical HCC cases can have some areas that focally resemble FLC. This problem is underscored by reported cases of “mixed hepatocellular carcinoma and FLC” in the literature that are almost certainly not FLC. This problem with the specificity of the H&E findings was formally assessed in one study, which identified significant discrepancies in making the diagnosis of FLC amongst a group of experts in liver pathology [90]. These observations argue strongly for the use of additional tests to confirm the diagnosis of FLC. A recent study has found that FLCs uniformly are positive for CD68 [91]. When this observation is combined with the findings from many other studies that demonstrate that FLCs are strongly positive for CK7, a panel of markers becomes evident. All potential cases of FLC should be stained with CK7 and CD68 to confirm the H&E impression (Figures 4(a) and 4(b)). Those cases that are negative for CK7 and CD68 are most likely not FLC and consideration should be given to send them out for an expert opinion. CK7 and CD68 immunostains should be used together, as a small proportion of typical hepatocellular carcinomas can be CD68 positive [91], and a significant minority of typical hepatocellular carcinomas can also be CK7 positive, in particular hepatocellular carcinomas in children and young adults [68]. Thus, H&E findings should be used as a first screen for the diagnosis of FLC and all cases with H&E findings that suggest the diagnosis of FLC should then be confirmed by immunostaining [91]. At the diagnostic level, additional factors that strongly suggest a diagnosis of FLC may be incorrect include the following: the presence of significant fibrosis in the background liver; AFP elevations in the serum or AFP positivity in the tumor cells by immunostaining; and areas of tumor that lack the key histological features of FLC.
Figure 4.
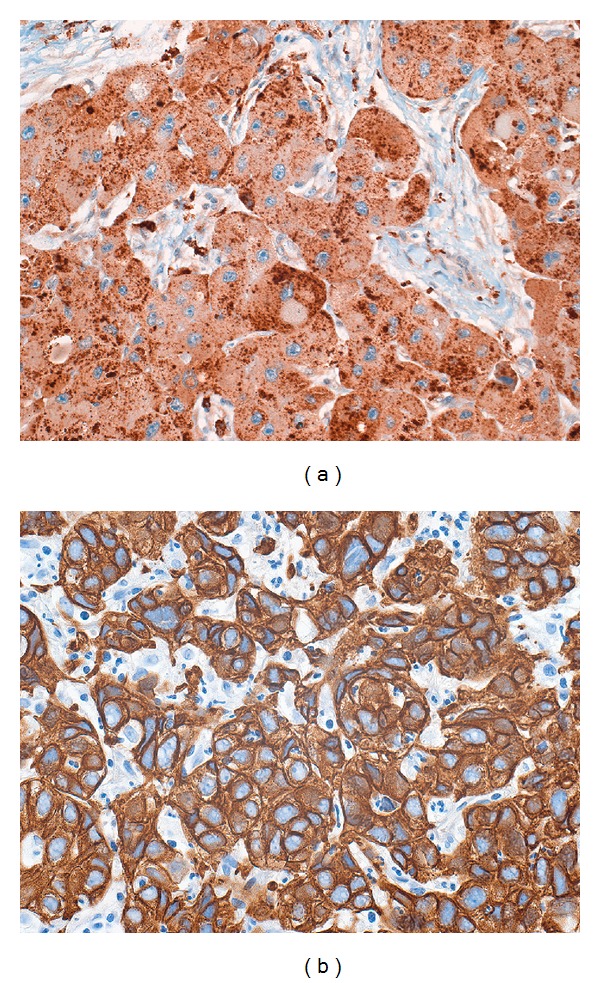
(a) Original magnification 100X. Fibrolamellar carcinomas express CD68 with a characteristic granular cytoplasmic staining pattern. CD68 stains lysosomes within the cytoplasm of the tumor cells. (b) Original magnification 160X. Fibrolamellar carcinomas strongly express cytokeratin 7.
3.5. Ultrastructural Findings
On ultrastructural examination, the neoplastic cells show abundant mitochondria [2, 19, 23, 74, 92]. Neurosecretory granules or other evidence of neuroendocrine differentiation can also be seen [23, 92]. Peroxisome-like bodies containing crystalloid material have been reported, along with filamentous material resembling Mallory hyaline [93]. Intracellular lumina, occasionally with bile, have been identified in some but not all cases [93–95]. Interestingly, FLC cells in cell culture may also have intracellular lumina (Figure 1(c)). By electron microscopy, some of the intercellular lumina contain microvilli [95, 96]. The pale bodies appear to correlate with larger endoplasmic vacuoles [74] or intracellular lumina containing numerous microvilli, with the largest ones developing a dense central hyaline core and eventually losing their microvilli [94].
3.6. Association with Other Liver Tumors
Rarely FLC and ordinary hepatocellular carcinoma or hepatic adenomas are found in the same liver. In such cases (Figure 5), the two components are typically adjacent but clearly separate [97, 98]. Others have reported FLC with a separate synchronous typical hepatocellular carcinoma [99] or cases in which the recurrence following resection for FLC was a typical hepatocellular carcinoma [100, 101]. In another case, a resected adenoma was followed five years later by an FLC [74]. Stipa et al. reported that 3/41 (7%) cases of FLC had areas resembling conventional hepatocellular carcinoma, but they are not illustrated and the significance and best histological diagnosis is not clear [36]. The intratumoral fibrosis in FLC can vary, and foci with less fibrosis in an otherwise typical FLC should not be misinterpreted as a mixed tumor.
Figure 5.
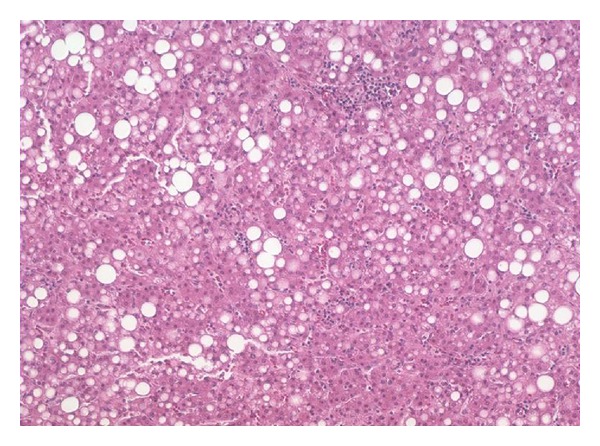
Original magnification 64X. This patient had a typical fibrolamellar carcinoma. Adjacent but clearly separate was a separate nodule of well-differentiated hepatocellular neoplasm with fatty change that lacked the features of fibrolamellar carcinoma.
FLCs have been also found in association with focal nodular hyperplasia [75, 102, 103]. Because of their shared central scar, copper accumulation, and other features, it was hypothesized that focal nodular hyperplasia may be a precursor lesion to FLC [75, 104]. However, at this time, there is no evidence to support an etiological association, and the nodular hyperplasia that surrounds a small subset of FLC is now recognized as a reaction to the tumor itself and not a precursor.
3.7. Tumor Spread
FLCs are locally aggressive and have a high frequency of distant metastases. Based on imaging findings, 42% of FLCs extend outside the liver into the adjacent fat planes [12]. Overall, lymph node and peritoneal metastases are more common in FLC than in typical hepatocellular carcinoma [2].
Approximately 50%–70% of individuals with FLC have positive lymph nodes at the time of presentation [7, 12, 36, 66]. FLCs commonly involve regional lymph nodes [23, 45, 92], including the celiac nodes [45, 92], gastric nodes [65], and para-aortic nodes [45, 65]. Direct extension into the stomach [65], diaphragm [12], and pancreas [12, 76] have all been reported. Metastatic disease to intra-abdominal organs, as well as peritoneal spread, is common [12, 19, 36, 45, 66, 71, 96]. Pulmonary metastases are frequently reported [11, 37, 45, 65, 71], and adrenal metastases are also common [12]. Rare metastatic sites include bone [11, 71] and ovary [105].
The distinctive tumor cytological findings (large pink cells with prominent nucleoli) are seen in most metastatic deposits. In addition, the distinctive lamellar fibrosis can also be seen in cases of metastatic disease [2, 105]. Because of these findings, most cases of FLC are readily diagnosed from metastatic sites. Rarely, tumor metastases can have a predominately glandular morphology (Figures 6(a)–6(d)), including mucin production, and be mistaken for a second primary. Immunostains can be very helpful in making the diagnosis of metastatic FLC (Figures 6(a)–6(d)).
Figure 6.
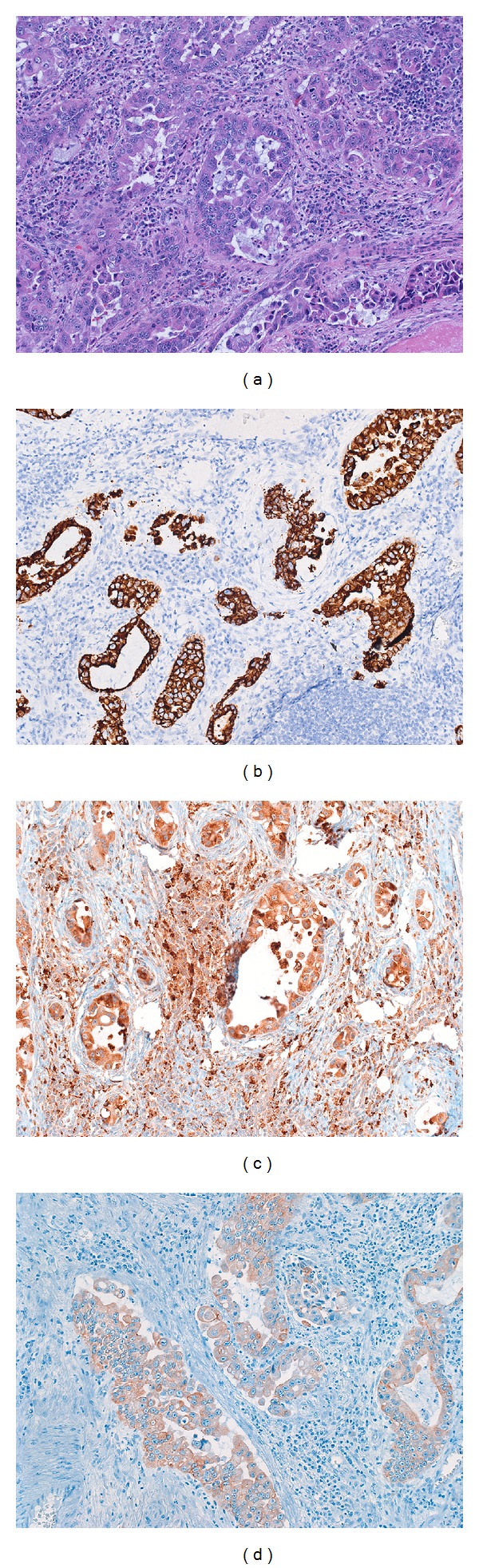
Original magnification 64X. A patient with a typical fibrolamellar carcinoma had a subsequent lymph node metastasis. The metastatic carcinoma had a distinctive glandular growth pattern that mimicked a cholangiocarcinoma. However, the metastatic tumor was still HepPar positive ((b), original magnification 64X), CD68 positive ((c), original magnification 64X). Cytokeratin 7 was also strongly positive (not shown), while cytokeratin AE1/3 was weakly positive ((d), original magnification 64X).
3.8. Section Summary and Key Questions
The gross and histological findings of FLC have been well studied, and the key findings are well established. These findings include tumor cells with abundant cytoplasm, large nucleoli, and extensive intratumoral fibrosis that can often coalesce into a central scar. While the H&E findings are distinctive, history has shown that they are not sufficiently specific across different study centers and immunohistochemical studies (CD68 and CK7) should be used to confirm the diagnosis. This is important to ensure that data results are accurate and can be compared across different study centers. Other immunohistochemical-based studies have been useful in highlighting the unique differentiation of FLC tumor cells—they clearly express features of hepatocytes, biliary cells, and neuroendocrine cells.
Key questions that remain to be answered or substantially clarified include the following: Key Question 1. What is the cell of origin of FLC? If a normal counterpart of FLC can be identified in the developing or mature liver, this may give important clues to etiology and to potential treatments. Key Question 2. While AFPs producing FLCs have been reported, it remains unclear if they are actually misclassified hepatocellular carcinoma. If there are truly AFP producing FLC, do they differ in clinical findings, histological findings, or prognosis? Key Question 3. In a similar fashion, the best classification of the reported cases of “mixed FLC and typical hepatocellular carcinoma” remains to be determined to clarify if this entity truly exists or if they are misclassified and are not actually FLCs. It seems clear from a few reported cases that FLC can very rarely coexist with a separate nodule of a typical hepatocellular neoplasm (see also Figure 5), but this phenomenon appears to be much more rare and quite different than the “mixed” tumors that have been reported by others.
4. Etiology
The etiology of FLC is unknown. Interestingly, FLCs are also reported to occur in other mammals and so are not unique to humans [106]. FLCs do not generally arise in the setting of any known chronic liver disease: the background livers typically lack significant inflammation or fibrosis [68]. While occasional reports have described the presence of hepatitis B viral proteins or DNA in FLC [2, 61, 107, 108], this appears to be by chance given the high worldwide prevalence of chronic hepatitis B infection and there is no data to suggest hepatitis B as an etiological agent. Likewise, FLCs have also developed in women using oral contraceptive pills [53, 109], but the association again appears to be by chance. No histological precursor lesion to FLC has been identified. In one study, occasional foci of altered hepatocytes were found in the background livers of resected FLC specimens [68], but the small number of cases and the fact that the background livers were not extensively sampled limits interpretation. Rare cases of FLC have been reported to arise in association with other malignancies or in the setting of well-defined inherited syndromes (Table 3). While FLCs are clearly not a major component of any of these inherited syndromes, their occurrence in these patients suggest the possibility of involvement by shared molecular pathways.
Table 3.
Reports of FLC in the setting of inherited syndromes or with other nonhepatic tumors.
5. Molecular Findings
The molecular basis for FLC remains largely unknown. Only a limited number of individual oncogenes and signaling pathways have been studied in FLC, but the results indicate that the uniqueness of FLC extends to the molecular level. As a caveat, a proportion of cases included in this literature may not be FLC, so the data should be interpreted with some caution.
5.1. Large Scale DNA Changes
DNA ploidy studies have shown that half of the FLC cases are aneuploid [74, 112] while the other half are tetraploid [112]. Despite these ploidy abnormalities, the tumor appears to be relatively chromosomally stable with few large chromosomal changes, compared to typical hepatocellular carcinoma [113], though recurrent FLC or metastases may show increased chromosomal abnormalities [114, 115]. Overall, the available data suggests the possibility of several recurrent cytogenetic abnormalities. For example, in one cytogenetic study of ten FLCs, three cases had no cytogenetic abnormalities, six cases had gains of chromosome 1q, and three cases had loss of chromosome 8p [113]. Others have also reported chromosome 1q abnormalities [15, 114]. The many cytogenetic studies on FLC are nicely summarized in the review article by Ward and Waxman [5]. The chromosomal stability of FLC is further supported by finding infrequent allelic loss using DNA probes [116]. Genomic homogeneity of FLC was also found by arbitrarily primed PCR [117].
5.2. Microsatellite Instability
Microsatellites are small segments of repetitive DNA that are present in normal genomic DNA. However, mutations in DNA repair genes can lead to widespread mutations, including changes in the length of the microsatellite DNA fragments. These mutations can lead to carcinoma. We studied three FLCs and found no microsatellite instability (unpublished observations).
5.3. Specific Mutations
5.3.1. TP53
While p53 protein is over-expressed in approximately 25% of typical HCC in the USA [118], it is only rarely over-expressed in FLC [119] and no TP53 mutations were found in FLC by denaturing gradient gel electrophoresis [120].
5.3.2. Beta Catenin
Mutations or overexpression of the beta catenin gene (CTNNB1), a key member of the Wnt signaling pathway, have been found in all other categories of hepatocellular neoplasms including hepatoblastomas, hepatic adenomas, and typical hepatocellular carcinoma [121]. While no mutations are found in FLC [122], and there is no nuclear accumulation of beta catenin by immunostain, there is some evidence that the Wnt signaling pathway may still be active in FLC [123].
5.4. Mitochondrial DNA Changes
As discussed previously, FLCs are characterized by increased mitochondria. However, careful analysis of the DNA content of the mitochondria found that the tumor cells are actually relatively depleted of mitochondrial DNA [124]. Complete sequencing of the mitochondrial DNA found no consistent mutation pattern to explain these changes in mitochondrial DNA [124].
5.5. Epigenetic Changes
Hepatocellular carcinomas frequently have hypermethylation of the promoters of tumor suppressor genes. FLCs also have abnormally methylated tumor suppressor gene promoters [125, 126], but the overall levels of hypermethylation are less than that of typical hepatocellular carcinomas arising in cirrhotic livers and more similar to those of hepatocellular carcinomas arising in noncirrhotic livers [125].
5.6. Specific Over-Expressed Pathways
5.6.1. Aromatase
The tumor cells of FLC overexpress aromatase [14, 15, 127] and individuals with FLC may present clinically with gynecomastia (Table 1). High serum estrone and estradiol levels are also found in these cases at presentation [14, 15]. Aromatase converts androgens to estrogens, more specifically androstenedione to estrone and testosterone to estradiol. Aromatase expression is under the control of several different tissue specific promoters. Aromatase levels are high in the developing liver but disappears in the postnatal liver. Interestingly, abnormal aromatase expression in FLC was not linked to abnormal activation of the fetal liver promoter, but instead to activation of promoters more commonly used in various endocrine cells [14].
5.6.2. Neurotensin
Other proteins that can also be expressed in FLC [128] but are normally restricted to the fetal liver include neurotensin [129], an endocrine agent.
5.6.3. mTOR
The mTOR pathway, important in energy metabolism and protein translation, is activated in 47% of FLCs, a finding similar to typical hepatocellular carcinoma [130].
5.6.4. EGFR
Epidermal growth factor receptor (EGFR) is over-expressed in the majority of FLCs by immunostaining [131, 132] as a result of polysomy of chromosome 7 [132].
5.6.5. AGR2
FLCs, but not typical hepatocellular carcinomas, also strongly overexpress anterior gradient 2 (Figure 7), [133]. Anterior gradient 2 is a protein normally expressed in the developing fetal gastrointestinal tract. In the fetal liver, anterior gradient 2 is expressed in the large size bile ducts and the gallbladder but not the smaller sized bile ducts in the periphery of the liver [134]. In the postnatal liver, anterior gradient 2 continues to be expressed in the gallbladder [134] and large intrahepatic bile ducts [133, 134]. Also of note, anterior gradient 2 is expressed in the postnatal gut, where it has an important role in regulating neuroendocrine cell specific lineage in the gastrointestinal tract and is found in all neuroendocrine cells that produce chromogranin as well as goblet cells [135].
Figure 7.
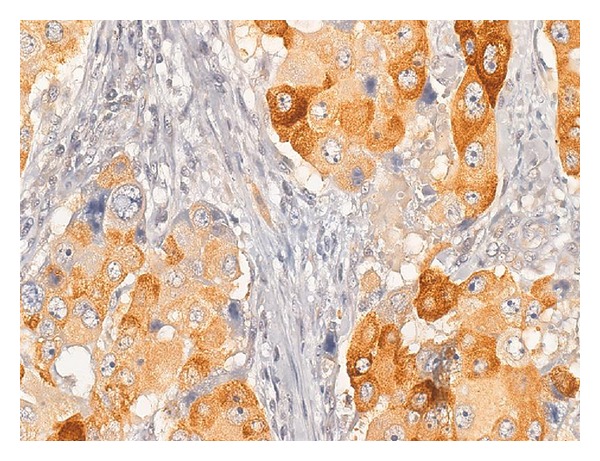
(Original magnification 100X). Fibrolamellar carcinomas are strongly AGR2 positive.
5.6.6. Miscellaneous
A wide variety of other proteins have been studied (Table 4). Despite the common overexpression of the antiapoptotic gene survivin in typical HCC, no [119] or limited [83] overexpression was found in FLC. Other studies have shown that the hepatocyte growth factor [136] and plasmin systems [136] as well as TFF-beta [137] are more frequently activated in HCC than in FLC.
Table 4.
Protein expression changes in FLC.
The tumors show overexpression of matrix metalloproteinase 2 [136] and the matrix itself shows increased expression of tenascin but limited expression of basement membrane components [142].
5.7. Section Summary and Key Questions
FLCs are relative chromosomal stable with frequent gains of chromosome 1q. They have epigenetic changes, but not as many as those found in typical hepatocellular carcinomas in cirrhotic livers. They lack mutations that are commonly found in typical hepatocellular carcinoma, such as CTNNB1 and TP53. FLCs do not have consistent or frequent mitochondrial mutations but do appear to have mitochondria biogenesis defects. Large numbers of lysosomes are found in their cytoplasm, suggesting a potential role for autophagy in tumorgenesis.
In keeping up with the histological findings discussed in the previous section, molecular studies confirm key aspects of neuroendocrine differentiation. FLCs frequently overexpress aromatase, perhaps through the use of a neuroendocrine specific promoter.
FLCs also show overexpression of the EGFR and the mTOR pathways, both pathways which are also commonly over-expressed in typically hepatocellular carcinomas and may have therapeutic possibilities.
Key questions that remain to be answered or substantially clarified include the following: Key Question 1. The most important question in this section is: what are the genetic changes that drive the formation of FLC? The typical homogenous appearance of FLC suggests that tumor formation may be largely driven by a relatively small number of key changes/mutations in a single pathway. Their unique age at presentation and the extensive histological data indicate that the tumors do not result from extended levels of low but persistent DNA damage due to chronic liver disease. They also do not have the classic pattern of neonatal tumor formation that can be seen in many cases of cancers resulting from inherited mutations or mutations acquired in utero (e.g., retinoblastoma and hepatoblastoma). There is no evidence of microsatellite instability, and they do not have unusually high levels of epigenetic instability. Nor do they manifest the “field effect” that can be seen in other forms of inherited cancer that can present in early adult years (e.g., colon cancer in the setting of familial adenomatosis polyposis). Thus, the genetic changes that lead to FLC appear to be relatively unique and their discovery may provide fundamental new insights into tumor genesis in general. Key Question 2. Since recurrent and metastatic disease is such a major problem after liver surgery, studies are needed that examine the genetic and epigenetic changes in primary versus metastatic disease. While a few studies have included metastatic tumors [124, 127], there is very little data on this topic. Yet, there could be important difference that would be relevant to chemotherapy treatments. Key Question 3. Critical basic science reagents are needed to advance the field, in particular robust cell lines and animal models.
6. Conclusions
FLCs are unique at the clinical, histological, and molecular levels. They are primary cancers of mixed differentiation (hepatocellular, biliary, and neuroendocrine) that occur in young individuals with no known liver disease and no precursor lesions. Their etiology is unknown and much of their molecular biology remains poorly described and awaits future investigation. FLCs are aggressive tumors with an overall low cure rate. Yet, there is hope that improvements in therapy will develop as advanced molecular biology tools are applied to the field and uncover the principle genetic lesions that drive tumor growth.
References
- 1.Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of the liver in infancy and childhood. A.M.A. Journal of Diseases of Children. 1956;91(2):168–186. doi: 10.1001/archpedi.1956.02060020170015. [DOI] [PubMed] [Google Scholar]
- 2.Craig JR, Peters RL, Edmondson HA, Omata M. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer. 1980;46(2):372–379. doi: 10.1002/1097-0142(19800715)46:2<372::aid-cncr2820460227>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Torbenson M. Review of the clinicopathologic features of fibrolamellar carcinoma. Advances in Anatomic Pathology. 2007;14(3):217–223. doi: 10.1097/PAP.0b013e3180504913. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Chan KW, Wang B, Qiao L. Fibrolamellar hepatocellular carcinoma. American Journal of Gastroenterology. 2009;104(10):2617–2625. doi: 10.1038/ajg.2009.440. [DOI] [PubMed] [Google Scholar]
- 5.Ward SC, Waxman S. Fibrolamellar carcinoma: a review with focus on genetics and comparison to other malignant primary liver tumors. Seminars in Liver Disease. 2011;31(1):61–70. doi: 10.1055/s-0031-1272835. [DOI] [PubMed] [Google Scholar]
- 6.Bosman FT, World Health Organization, International Agency for Research on Cancer . WHO Classification of Tumours of the Digestive System. 4th edition. Lyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 7.Maniaci V, Davidson BR, Rolles K, et al. Fibrolamellar hepatocellular carcinoma: prolonged survival with multimodality therapy. European Journal of Surgical Oncology. 2009;35(6):617–621. doi: 10.1016/j.ejso.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 8.El-Gazzaz G, Wong W, El-Hadary MK, et al. Outcome of liver resection and transplantation for fibrolamellar hepatocellular carcinoma. Transplant International. 2000;13(supplement 1):S406–S409. doi: 10.1007/s001470050372. [DOI] [PubMed] [Google Scholar]
- 9.Saab S, Yao F. Fibrolamellar hepatocellular carcinoma. case reports and a review of the literature. Digestive Diseases and Sciences. 1996;41(10):1981–1985. doi: 10.1007/BF02093600. [DOI] [PubMed] [Google Scholar]
- 10.Berman MA, Burnham JA, Sheahan DG. Fibrolamellar carcinoma of the liver: an immunohistochemical study of nineteen cases and a review of the literature. Human Pathology. 1988;19(7):784–794. doi: 10.1016/s0046-8177(88)80261-2. [DOI] [PubMed] [Google Scholar]
- 11.Hemming AW, Langer B, Sheiner P, Greig PD, Taylor BR. Aggressive surgical management of fibrolamellar hepatocellular carcinoma. Journal of Gastrointestinal Surgery. 1997;1(4):342–346. doi: 10.1016/s1091-255x(97)80055-8. [DOI] [PubMed] [Google Scholar]
- 12.Ichikawa T, Federle MP, Grazioli L, Marsh W. Fibrolamellar hepatocellular carcinoma: Pre- and posttherapy evaluation with CT and MR imaging. Radiology. 2000;217(1):145–151. doi: 10.1148/radiology.217.1.r00se46145. [DOI] [PubMed] [Google Scholar]
- 13.McCloskey JJ, Germain-Lee EL, Perman JA, Plotnick LP, Janoski AH. Gynecomastia as a presenting sign of fibrolamellar carcinoma of the liver. Pediatrics. 1988;82(3):379–382. [PubMed] [Google Scholar]
- 14.Agarwal VR, Takayama K, Van Wyk JJ, Sasano H, Simpson ER, Bulun SE. Molecular basis of severe gynecomastia associated with aromatase expression in a fibrolamellar hepatocellular carcinoma. Journal of Clinical Endocrinology and Metabolism. 1998;83(5):1797–1800. doi: 10.1210/jcem.83.5.4773. [DOI] [PubMed] [Google Scholar]
- 15.Hany MA, Betts DR, Schmugge M, et al. A childhood fibrolamellar hepatocellular carcinoma with increased aromatase activity and a near triploid karyotype. Medical and Pediatric Oncology. 1997;28(2):136–138. doi: 10.1002/(sici)1096-911x(199702)28:2<136::aid-mpo8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 16.Meriggi F, Forni E. Surgical therapy of hepatic fibrolamellar carcinoma. Annali Italiani di Chirurgia. 2007;78(1):53–58. [PubMed] [Google Scholar]
- 17.Asrani SK, LaRusso NF. Fibrolamellar hepatocellular carcinoma presenting with budd-chiari syndrome, right atrial thrombus, and pulmonary emboli. Hepatology. 2012;55(3):977–978. doi: 10.1002/hep.24811. [DOI] [PubMed] [Google Scholar]
- 18.Lamberts R, Nitsche R, de Vivie RE, et al. Budd-chiari syndrome as the primary manifestation of a fibrolamellar hepatocellular carcinoma. Digestion. 1992;53(3-4):200–209. doi: 10.1159/000200995. [DOI] [PubMed] [Google Scholar]
- 19.Kanai T, Takabayashi T, Kawano Y, Kuramochi S, Miyazawa N. A case of postoperative recurrence of fibrolamellar hepatocellular carcinoma with increased vitamin B12 binding capacity in a young Japanese female. Japanese Journal of Clinical Oncology. 2004;34(6):346–351. doi: 10.1093/jjco/hyh050. [DOI] [PubMed] [Google Scholar]
- 20.Knudson JD, Goldberg JF, Ayres NA. Fibrolamellar hepatocellular carcinoma with cardiac spread causing severe inferior vena cava obstruction in a 9-year-old child. Pediatric Cardiology. 2012;33(5):872–873. doi: 10.1007/s00246-012-0305-9. [DOI] [PubMed] [Google Scholar]
- 21.Tangkijvanich P, Thong-Ngam D, Kullavanijaya P, Suwangool P. Fibrolamellar hepatocellular carcinoma in a Thai man who presented with hypoglycemia: case report and review of literature. Journal of the Medical Association of Thailand. 2000;83(7):809–816. [PubMed] [Google Scholar]
- 22.Dahan MH, Kastell P. Fibrolamellar hepatic carcinoma with a presentation similar to that of septic pregnancy: a case report. Journal of Reproductive Medicine for the Obstetrician and Gynecologist. 2002;47(1):47–49. [PubMed] [Google Scholar]
- 23.Lloreta J, Vadell C, Fabregat X, Serrano S. Fibrolamellar hepatic tumor with neurosecretory features and systemic deposition of AA amyloid. Ultrastructural Pathology. 1994;18(1-2):287–292. doi: 10.3109/01913129409016302. [DOI] [PubMed] [Google Scholar]
- 24.Soyer P, Roche A, Levesque M. Fibrolamellar hepatocellular carcinoma presenting with obstructive jaundice. a report of two cases. European Journal of Radiology. 1991;13(3):196–198. doi: 10.1016/0720-048x(91)90028-t. [DOI] [PubMed] [Google Scholar]
- 25.Eckstein RP, Bambach CP, Stiel D, Roche J, Goodman BN. Fibrolamellar carcinoma as a cause of bile duct obstruction. Pathology. 1988;20(4):326–331. doi: 10.3109/00313028809085212. [DOI] [PubMed] [Google Scholar]
- 26.Kunz G, Jr., Chung J, Ali SZ. Hepatocellular carcinoma-fibrolamellar variant: cytopathology of an unusual case. Diagnostic Cytopathology. 2002;26(4):257–261. doi: 10.1002/dc.10088. [DOI] [PubMed] [Google Scholar]
- 27.Snook JA, Kelly P, Chapman RW, Jewell DP. Fibrolamellar hepatocellular carcinoma complicating ulcerative colitis with primary sclerosing cholangitis. Gut. 1989;30(2):243–245. doi: 10.1136/gut.30.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaideeswar P, Pandit MJ, Deshpande JR, Sivaraman A, Vora IM. Fibrolamellar carcinoma of the liver-an unusual presentation. Journal of Postgraduate Medicine. 1993;39(3):159–161. [PubMed] [Google Scholar]
- 29.Gupta P, Dhar S, Strickland NH. Fibrolamellar carcinoma: an unusual clinico-radiological presentation. European Journal of Radiology. 1999;32(2):119–123. doi: 10.1016/s0720-048x(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 30.Debray D, Pariente D, Fabre M, Foucaud P, Valayer J, Bernard O. Fibrolamellar hepatocellular carcinoma: report of a case mimicking a liver abscess. Journal of Pediatric Gastroenterology and Nutrition. 1994;19(4):468–472. [PubMed] [Google Scholar]
- 31.Tezer KM, Yalçín B, Büyükpamukçu N, Kale G, Büyükpamukçu M. Fibrolamellar hepatocellular carcinoma with skeletal metastases. Pediatric Hematology and Oncology. 2001;18(4):273–278. doi: 10.1080/088800101750238586. [DOI] [PubMed] [Google Scholar]
- 32.Thirabanjasak D, Sosothikul D, Mahayosnond A, Thorner PS. Fibrolamellar carcinoma presenting as a pancreatic mass: case report and review of the literature. Journal of Pediatric Hematology/Oncology. 2009;31(5):370–372. doi: 10.1097/MPH.0b013e3181984f7f. [DOI] [PubMed] [Google Scholar]
- 33.Al-Matham K, Alabed I, Zaidi SZA, Qushmaq KA. Cold agglutinin disease in fibrolamellar hepatocellular carcinoma: a rare association with a rare cancer variant. Annals of Saudi Medicine. 2011;31(2):197–200. doi: 10.4103/0256-4947.76409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arista-Nasr J, Gutierrez-Villalobos L, Nuncio J, Maldonaldo H, Bornstein-Quevedo L. Fibrolamellar hepatocellular carcinoma in Mexican patients. Pathology and Oncology Research. 2002;8(2):133–137. doi: 10.1007/BF03033723. [DOI] [PubMed] [Google Scholar]
- 35.Ward SC, Huang J, Tickoo SK, Thung SN, Ladanyi M, Klimstra DS. Fibrolamellar carcinoma of the liver exhibits immunohistochemical evidence of both hepatocyte and bile duct differentiation. Modern Pathology. 2010;23(9):1180–1190. doi: 10.1038/modpathol.2010.105. [DOI] [PubMed] [Google Scholar]
- 36.Stipa F, Yoon SS, Liau KH, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer. 2006;106(6):1331–1338. doi: 10.1002/cncr.21703. [DOI] [PubMed] [Google Scholar]
- 37.Wheeler K, Pritchard J, Luck W, Rossiter M. Transcobalamin I as a “marker” for fibrolamellar hepatoma. Medical and Pediatric Oncology. 1986;14(4):227–229. doi: 10.1002/mpo.2950140408. [DOI] [PubMed] [Google Scholar]
- 38.Lildballe DL, Nguyen KQT, Poulsen SS, Nielsen HO, Nexo E. Haptocorrin as marker of disease progression in fibrolamellar hepatocellular carcinoma. European Journal of Surgical Oncology. 2011;37(1):72–79. doi: 10.1016/j.ejso.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Paradinas FJ, Melia WM, Wilkinson ML, et al. High serum vitamin B12 binding capacity as a marker of the fibrolamellar variant of hepatocellular carcinoma. British Medical Journal. 1982;285(6345):840–842. doi: 10.1136/bmj.285.6345.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheppard KJ, Bradbury DA, Davies JM, Ryrie DR. High serum vitamin B12 binding capacity as a marker of the fibrolamellar variant of hepatocellular carcinoma. British Medical Journal. 1983;286(6358, article 57) doi: 10.1136/bmj.286.6358.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fremont S, Champigneulle B, Gerard P, et al. Blood transcobalamin levels in malignant hepatoma. Tumor Biology. 1991;12(6):353–359. doi: 10.1159/000217736. [DOI] [PubMed] [Google Scholar]
- 42.Boisson F, Fremont S, Migeon C, et al. Human haptocorrin in hepatocellular carcinoma. Cancer Detection and Prevention. 1999;23(2):89–96. doi: 10.1046/j.1525-1500.1999.09914.x. [DOI] [PubMed] [Google Scholar]
- 43.Soreide O, Czerniak A, Bradpiece H, Bloom S, Blumgart L. Characteristics of fibrolamellar hepatocellular carcinoma. a study of nine cases and a review of the literature. American Journal of Surgery. 1986;151(4):518–523. doi: 10.1016/0002-9610(86)90117-0. [DOI] [PubMed] [Google Scholar]
- 44.Collier NA, Weinbren K, Bloom SR, Lee YC, Hodgson HJ, Blumgart LH. Neurotensin secretion by fibrolamellar carcinoma of the liver. The Lancet. 1984;1(8376):538–540. doi: 10.1016/s0140-6736(84)90934-6. [DOI] [PubMed] [Google Scholar]
- 45.Pinna AD, Iwatsuki S, Lee RG, et al. Treatment of fibrolamellar hepatoma with subtotal hepatectomy or transplantation. Hepatology. 1997;26(4):877–883. doi: 10.1002/hep.510260412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bertino G, Ardiri AM, Calvagno GS, Bertino N, Boemi PM. Prognostic and diagnostic value of des-γ-carboxy prothrombin in liver cancer. Drug News and Perspectives. 2010;23(8):498–508. doi: 10.1358/dnp.2010.23.8.1444236. [DOI] [PubMed] [Google Scholar]
- 47.El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? a US population-based study. Hepatology. 2004;39(3):798–803. doi: 10.1002/hep.20096. [DOI] [PubMed] [Google Scholar]
- 48.Katzenstein HM, Krailo MD, Malogolowkin MH, et al. Fibrolamellar hepatocellular carcinoma in children and adolescents. Cancer. 2003;97(8):2006–2012. doi: 10.1002/cncr.11292. [DOI] [PubMed] [Google Scholar]
- 49.Cruz O, Laguna A, Vancells M, Krauel L, Medina M, Mora J. Fibrolamellar hepatocellular carcinoma in an infant and literature review. Journal of Pediatric Hematology/Oncology. 2008;30(12):968–971. doi: 10.1097/MPH.0b013e31818b0f82. [DOI] [PubMed] [Google Scholar]
- 50.Lack EE, Neave C, Vawter GF. Hepatocellular carcinoma. review of 32 cases in childhood and adolescence. Cancer. 1983;52(8):1510–1515. doi: 10.1002/1097-0142(19831015)52:8<1510::aid-cncr2820520830>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 51.Maitra A, Ramnani DM, Margraf LR, Gazdar AF. Synchronous Wilms tumor and fibrolamellar hepatocellular carcioma: report of a case. Pediatric and Developmental Pathology. 2000;3(5):492–496. doi: 10.1007/s100240010096. [DOI] [PubMed] [Google Scholar]
- 52.Bismuth H, Chiche L, Castaing D. Surgical treatment of hepatocellular carcinomas in noncirrhotic liver: experience with 68 liver resections. World Journal of Surgery. 1995;19(1):35–41. doi: 10.1007/BF00316977. [DOI] [PubMed] [Google Scholar]
- 53.Moreno-Luna LE, Arrieta O, Garcia-Leiva J, et al. Clinical and pathologic factors associated with survival in young adult patients with fibrolamellar hepatocarcinoma. BMC Cancer. 2005;5, article 142 doi: 10.1186/1471-2407-5-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore SW, Davidson A, Hadley GP, et al. Malignant liver tumors in South African children: a national audit. World Journal of Surgery. 2008;32(7):1389–1395. doi: 10.1007/s00268-008-9526-8. [DOI] [PubMed] [Google Scholar]
- 55.Bhaijee F, Locketz ML, Krige JEJ. Fibrolamellar hepatocellular carcinoma at a tertiary centre in South Africa: a case series. South African Journal of Surgery. 2009;47(4):108–111. [PubMed] [Google Scholar]
- 56.Hoshino H, Katada N, Nishimura D, et al. Case report: fibrolamellar hepatocellular carcinoma in a Japanese woman: a case report and review of Japanese cases. Journal of Gastroenterology and Hepatology. 1996;11(6):551–555. doi: 10.1111/j.1440-1746.1996.tb01701.x. [DOI] [PubMed] [Google Scholar]
- 57.Yuan CY, Yuan CC, Shiou GF, Tseng CH, Yau MT. Fibrolamellar variant of hepatocellular carcinoma report of a Chinese patient. Hepato-Gastroenterology. 1995;42(2):182–184. [PubMed] [Google Scholar]
- 58.Yen JB, Chang KW. Fibrolamellar hepatocellular carcinoma- report of a case. Chang Gung Medical Journal. 2009;32(3):336–339. [PubMed] [Google Scholar]
- 59.Liu S, Wah Chan K, Tong J, Wang Y, Wang B, Qiao L. PET-CT scan is a valuable modality in the diagnosis of fibrolamellar hepatocellular carcinoma: a case report and a summary of recent literature. QJM. 2011;104(6):477–483. doi: 10.1093/qjmed/hcr040. [DOI] [PubMed] [Google Scholar]
- 60.Haratake J, Horie A, Lee SD, Huh MH. Fibrolamellar carcinoma of the liver in a middle-aged Korean man. Journal of UOEH. 1990;12(3):349–354. doi: 10.7888/juoeh.12.349. [DOI] [PubMed] [Google Scholar]
- 61.Dadke D, Jaganath P, Krishnamurthy S, Chiplunkar S. The detection of HBV antigens and HBx-transcripts in an Indian fibrolamellar carcinoma patient: a case study. Liver. 2002;22(1):87–91. doi: 10.1046/j.0106-9543.2001.01602.x. [DOI] [PubMed] [Google Scholar]
- 62.Malouf GG, Brugieres L, Le Deley MC, et al. Pure and mixed fibrolamellar hepatocellular carcinomas differ in natural history and prognosis after complete surgical resection. doi: 10.1002/cncr.27520. Cancer. In press. [DOI] [PubMed] [Google Scholar]
- 63.Kaczynski J, Gustavsson B, Hansson G, Wallerstedt S. Fibrolamellar hepatic carcinoma in an area with a low incidence of primary liver cancer: a retrospective study. European Journal of Surgery. 1996;162(5):367–371. [PubMed] [Google Scholar]
- 64.Sooklim K, Sriplung H, Piratvisuth T. Histologic subtypes of hepatocellular carcinoma in the southern thai population. Asian Pacific Journal of Cancer Prevention. 2003;4(4):302–306. [PubMed] [Google Scholar]
- 65.Teitelbaum DH, Tuttle S, Carey LC, Clausen KP. Fibrolamellar carcinoma of the liver. review of three cases and the presentation of a characteristic set of tumor markers defining this tumor. Annals of Surgery. 1985;202(1):36–41. doi: 10.1097/00000658-198507000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stevens WR, Johnson CD, Stephens DH, Nagorney DM. Fibrolamellar hepatocellular carcinoma: stage at presentation and results of aggressive surgical management. American Journal of Roentgenology. 1995;164(5):1153–1158. doi: 10.2214/ajr.164.5.7717223. [DOI] [PubMed] [Google Scholar]
- 67.Farhi DC, Shikes RH, Murari PJ, Silverberg SG. Hepatocellular carcinoma in young people. Cancer. 1983;52(8):1516–1525. doi: 10.1002/1097-0142(19831015)52:8<1516::aid-cncr2820520831>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 68.Klein WM, Molmenti EP, Colombani PM, et al. Primary liver carcinoma arising in people younger than 30 years. American Journal of Clinical Pathology. 2005;124(4):512–518. doi: 10.1309/TT0R7KAL32228E99. [DOI] [PubMed] [Google Scholar]
- 69.Kakar S, Burgart LJ, Batts KP, Garcia J, Jain D, Ferrell LD. Clinicopathologic features and survival in fibrolamellar carcinoma: comparison with conventional hepatocellular carcinoma with and without cirrhosis. Modern Pathology. 2005;18(11):1417–1423. doi: 10.1038/modpathol.3800449. [DOI] [PubMed] [Google Scholar]
- 70.Houben KW, McCall JL. Liver transplantation for hepatocellular carcinoma in patients without underlying liver disease: a systematic review. Liver Transplantation and Surgery. 1999;5(2):91–95. doi: 10.1002/lt.500050201. [DOI] [PubMed] [Google Scholar]
- 71.Epstein BE, Pajak TF, Haulk TL, Herpst JM, Order SE, Abrams RA. Metastatic nonresectable fibrolamellar hepatoma: prognostic features and natural history. American Journal of Clinical Oncology. 1999;22(1):22–28. doi: 10.1097/00000421-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Ichikawa T, Federle MP, Grazioli L, Madariaga J, Nalesnik M, Marsh W. Fibrolamellar hepatocellular carcinoma: imaging and pathologic findings in 31 recent cases. Radiology. 1999;213(2):352–361. doi: 10.1148/radiology.213.2.r99nv31352. [DOI] [PubMed] [Google Scholar]
- 73.Stromeyer FW, Ishak KG, Berber MA, Mathew T. Ground-glass cells in hepatocellular carcinoma. American Journal of Clinical Pathology. 1980;74(3):254–258. doi: 10.1093/ajcp/74.3.254. [DOI] [PubMed] [Google Scholar]
- 74.Terracciano LM, Tornillo L, Avoledo P, Von Schweinitz D, Kühne T, Bruder E. Fibrolamellar hepatocellular carcinoma occurring 5 years after hepatocellular adenoma in a 14-year-old girl: a case report with comparative genomic hybridization analysis. Archives of Pathology and Laboratory Medicine. 2004;128(2):222–226. doi: 10.5858/2004-128-222-FHCOYA. [DOI] [PubMed] [Google Scholar]
- 75.Vecchio FM, Fabiano A, Ghirlanda G, Manna R, Massi G. Fibrolamellar carcinoma of the liver: the malignant counterpart of focal nodular hyperplasia with oncocytic change. American Journal of Clinical Pathology. 1984;81(4):521–526. doi: 10.1093/ajcp/81.4.521. [DOI] [PubMed] [Google Scholar]
- 76.Lefkowitch JH, Muschel R, Price JB, Marboe C, Braunhut S. Copper and copper-binding protein in fibrolamellar liver cell carcinoma. Cancer. 1983;51(1):97–100. doi: 10.1002/1097-0142(19830101)51:1<97::aid-cncr2820510120>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 77.Guigui B, Mavier P, Lescs MC, Pinaudeau Y, Dhumeaux D, Zafrani ES. Copper and copper-binding protein in liver tumors. Cancer. 1988;61(6):1155–1158. doi: 10.1002/1097-0142(19880315)61:6<1155::aid-cncr2820610616>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 78.Ishak KG, goodman ZD, Stocker JT. Armed Forces Institute of Pathology (U.S.). Tumors of the liver and intrahepatic bile ducts, Armed Forces Institute of Pathology : Supt. of Docs., U.S. G.P.O. For sale by the Armed Forces Institute of Pathology, Washington, DC, USA, 1999.
- 79.Nerlich AG, Majewski S, Hunzelmann N, et al. Excessive collagen formation in fibrolamellar carcinoma of the liver: a morphological and biochemical study. Modern Pathology. 1992;5(5):580–585. [PubMed] [Google Scholar]
- 80.Tanaka K, Honna T, Kitano Y, et al. Combined fibrolamellar carcinoma and cholangiocarcinoma exhibiting biphenotypic antigen expression: a case report. Journal of Clinical Pathology. 2005;58(8):884–887. doi: 10.1136/jcp.2004.021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ng IOL, Shek TWH, Nicholls J, Ma LT. Combined hepatocellular-cholangiocarcinoma: a clinicopathological study. Journal of Gastroenterology and Hepatology. 1998;13(1):34–40. doi: 10.1111/j.1440-1746.1998.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 82.Goodman ZD, Ishak KG, Langloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular cholangiocarcinoma. a histologic and immunohistochemical study. Cancer. 1985;55(1):124–135. doi: 10.1002/1097-0142(19850101)55:1<124::aid-cncr2820550120>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 83.Abdul-Al HM, Guanghua Wang, Makhlouf HR, Goodman ZD. Fibrolamellar hepatocellular carcinoma: an immunohistochemical comparison with conventional hepatocellular carcinoma. International Journal of Surgical Pathology. 2010;18(5):313–318. doi: 10.1177/1066896910364229. [DOI] [PubMed] [Google Scholar]
- 84.Van Eyken P, Sciot R, Brock P, Casteels-Van Daele M, Ramaekers FCS, Desmet VJ. Abundant expression of cytokeratin 7 in fibrolamellar carcinoma of the liver. Histopathology. 1990;17(2):101–107. doi: 10.1111/j.1365-2559.1990.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 85.Górnicka B, Ziarkiewicz-Wróblewska B, Wróblewski T, et al. Carcinoma, a fibrolamellar variant-immunohistochemical analysis of 4 cases. Hepato-Gastroenterology. 2005;52(62):519–523. [PubMed] [Google Scholar]
- 86.Kojima M, Kunimura T, Inagaki T, et al. A fibrolamellar carcinoma of the liver with a marked solid component. Journal of Gastroenterology. 2004;39(9):905–906. doi: 10.1007/s00535-003-1410-6. [DOI] [PubMed] [Google Scholar]
- 87.Garcia de Davila MT, Gonzalez-Crussi F, Mangkornkanok M. Fibrolamellar carcinoma of the liver in a child: ultrastructural and immunohistologic aspects. Pediatric Pathology. 1987;7(3):319–331. [PubMed] [Google Scholar]
- 88.Buscombe JR, Caplin ME, Hilson AJW. Long-term efficacy of high-activity 111in-pentetreotide therapy in patients with disseminated neuroendocrine tumors. Journal of Nuclear Medicine. 2003;44(1):1–6. [PubMed] [Google Scholar]
- 89.Zhao M, Laissue JA, Zimmermann A. ‘Neuroendocrine’ differentiation in hepatocellular carcinomas (HCCs): immunohistochemical reactivity is related to distinct tumor cell types, but not to tumor grade. Histology and Histopathology. 1993;8(4):617–626. [PubMed] [Google Scholar]
- 90.Malouf G, Falissard B, Azoulay D, et al. Is histological diagnosis of primary liver carcinomas with fibrous stroma reproducible among experts? Journal of Clinical Pathology. 2009;62(6):519–524. doi: 10.1136/jcp.2008.062620. [DOI] [PubMed] [Google Scholar]
- 91.Ross HM, Daniel HDJ, Vivekanandan P, et al. Fibrolamellar carcinomas are positive for CD68. Modern Pathology. 2011;24(3):390–395. doi: 10.1038/modpathol.2010.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Payne CM, Nagle RB, Paplanus SH, Graham AR. Fibrolamellar carcinoma of liver: a primary malignant oncocytic carcinoid? Ultrastructural Pathology. 1986;10(6):539–552. doi: 10.3109/01913128609007211. [DOI] [PubMed] [Google Scholar]
- 93.Caballero T, Aneiros J, Lopez-Caballero J, Gomez-Morales M, Nogales F. Fibrolamellar hepatocellular carcinoma. an immunohistochemical and ultrastructural study. Histopathology. 1985;9(4):445–456. doi: 10.1111/j.1365-2559.1985.tb02827.x. [DOI] [PubMed] [Google Scholar]
- 94.An T, Ghatak N, Kastner R, Kay S, Lee HM. Hyaline globules and intracellular lumina in a hepatocellular carcinoma. American Journal of Clinical Pathology. 1983;79(3):392–396. doi: 10.1093/ajcp/79.3.392. [DOI] [PubMed] [Google Scholar]
- 95.Sato SI, Masuda T, Oikawa H, et al. Bile canaliculi-like lumina in fibrolamellar carcinoma of the liver: a light- and electron-microscopic study and three-dimensional examination of serial sections. Pathology International. 1997;47(11):763–768. doi: 10.1111/j.1440-1827.1997.tb04454.x. [DOI] [PubMed] [Google Scholar]
- 96.Andreola S, Audisio RA, Lombardi L. A light microscopic and ultrastructural study of the two cases of fibrolamellar hepatocellular carcinoma. Tumori. 1986;72(6):609–616. doi: 10.1177/030089168607200612. [DOI] [PubMed] [Google Scholar]
- 97.Seitz G, Zimmermann A, Friess H, Bchler MW. Adult-type hepatocellular carcinoma in the center of a fibrolamellar hepatocellular carcinoma. Human Pathology. 2002;33(7):765–769. doi: 10.1053/hupa.2002.125380. [DOI] [PubMed] [Google Scholar]
- 98.Okano A, Hajiro K, Takakuwa H, Kobashi Y. Fibrolamellar carcinoma of the liver with a mixture of ordinary hepatocellular carcinoma: a case report. American Journal of Gastroenterology. 1998;93(7):1144–1145. doi: 10.1111/j.1572-0241.1998.00348.x. [DOI] [PubMed] [Google Scholar]
- 99.Okada K, Kim YI, Nakashima K, et al. Fibrolamellar hepatocellular carcinoma coexistent with a hepatocellular carcinoma of common type: report of a case. Surgery Today. 1993;23(7):626–631. doi: 10.1007/BF00311912. [DOI] [PubMed] [Google Scholar]
- 100.Yamamoto H, Watanabe K, Nagata M, et al. Transformation of fibrolamellar carcinoma to common hepatocellular carcinoma in the recurrent lesions of the rectum and the residual liver: a case report. Japanese Journal of Clinical Oncology. 1999;29(9):445–447. doi: 10.1093/jjco/29.9.445. [DOI] [PubMed] [Google Scholar]
- 101.Chang YC, Dai YC, Chow NH. Fibrolamellar hepatocellular carcinoma with a recurrence of classic hepatocellular carcinoma: a case report and review of oriental cases. Hepato-Gastroenterology. 2003;50(53):1637–1640. [PubMed] [Google Scholar]
- 102.Imkie M, Myers SA, Li Y, et al. Fibrolamellar hepatocellular carcinoma arising in a background of focal nodular hyperplasia: a report of 2 cases. Journal of Reproductive Medicine for the Obstetrician and Gynecologist. 2005;50(8):633–637. [PubMed] [Google Scholar]
- 103.Saxena R, Humphreys S, Williams R, Portmann B. Nodular hyperplasia surrounding fibrolamellar carcinoma: a zone of arterialized liver parenchyma. Histopathology. 1994;25(3):275–278. doi: 10.1111/j.1365-2559.1994.tb01328.x. [DOI] [PubMed] [Google Scholar]
- 104.Vecchio FM. Fibrolamellar carcinoma of the liver: a distinct entity within the hepatocellular tumors. a review. Applied Pathology. 1988;6(2):139–148. [PubMed] [Google Scholar]
- 105.Benito V, Segura J, Martinez MS, Arencibia O, Lubrano A. Fibrolamellar hepatocellular carcinoma metastatic to the ovary. Journal of Obstetrics and Gynaecology. 2012;32(2):200–202. doi: 10.3109/01443615.2011.621558. [DOI] [PubMed] [Google Scholar]
- 106.Bettini G, Marcato PS. Primary hepatic tumours in cattle. a classification of 66 cases. Journal of Comparative Pathology. 1992;107(1):19–34. doi: 10.1016/0021-9975(92)90092-9. [DOI] [PubMed] [Google Scholar]
- 107.Morize Z, Sugioka A, Mizoguchi Y, et al. Fibrolamellar carcinoma of the liver in a Japanese hepatitis B virus carrier. Journal of Gastroenterology and Hepatology. 2005;20(7):1136–1138. doi: 10.1111/j.1440-1746.2005.03835.x. [DOI] [PubMed] [Google Scholar]
- 108.Davison FD, Fagan EA, Portmann B, Williams R. HBV-DNA sequences in tumor and nontumor tissue in a patient with the fibrolamellar variant of hepatocellular carcinoma. Hepatology. 1990;12(4):676–679. doi: 10.1002/hep.1840120410. [DOI] [PubMed] [Google Scholar]
- 109.Malt RA, Galdabini JJ, Jeppsson BW. Abnormal sex-steroid milieu in young adults with hepatocellular carcinoma. World Journal of Surgery. 1983;7(2):247–252. doi: 10.1007/BF01656154. [DOI] [PubMed] [Google Scholar]
- 110.LeBrun DP, Silver MM, Freedman MH, Phillips MJ. Fibrolamellar carcinoma of the liver in a patient with Fanconi anemia. Human Pathology. 1991;22(4):396–398. doi: 10.1016/0046-8177(91)90088-7. [DOI] [PubMed] [Google Scholar]
- 111.Gruner BA, DeNapoli TS, Andrews W, Tomlinson G, Bowman L, Weitman SD. Hepatocellular carcinoma in children associated with gardner syndrome or familial adenomatous polyposis. Journal of Pediatric Hematology/Oncology. 1998;20(3):274–278. doi: 10.1097/00043426-199805000-00018. [DOI] [PubMed] [Google Scholar]
- 112.Orsatti G, Greenberg PD, Rolfes DB, Ishak KG, Paronetto F. DNA ploidy of fibrolamellar hepatocellular carcinoma by image analysis. Human Pathology. 1994;25(9):936–939. doi: 10.1016/0046-8177(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 113.Marchio A, Pineau P, Meddeb M, et al. Distinct chromosomal abnormality pattern in primary liver cancer of non-B, non-C patients. Oncogene. 2000;19(33):3733–3738. doi: 10.1038/sj.onc.1203713. [DOI] [PubMed] [Google Scholar]
- 114.Wilkens L, Bredt M, Flemming P, Kubicka S, Klempnauer J, Kreipe H. Cytogenetic aberrations in primary and recurrent fibrolamellar hepatocellular carcinoma detected by comparative genomic hybridization. American Journal of Clinical Pathology. 2000;114(6):867–874. doi: 10.1309/BMTT-JBPD-D13H-1UVD. [DOI] [PubMed] [Google Scholar]
- 115.Lowichik A, Schneider NR, Tonk’s V, Ansari MQ, Timmons CF. Report of a complex karyotype in recurrent metastatic fibrolamellar hepatocellular carcinoma and a review of hepatocellular carcinoma cytogenetics. Cancer Genetics and Cytogenetics. 1996;88(2):170–174. doi: 10.1016/0165-4608(95)00314-2. [DOI] [PubMed] [Google Scholar]
- 116.Ding SF, Delhanty JDA, Bowles L, Dooley JS, Wood CB, Habib NA. Infrequent chromosome allele loss in fibrolamellar carcinoma. British Journal of Cancer. 1993;67(2):244–246. doi: 10.1038/bjc.1993.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sirivatanauksorn Y, Sirivatanauksorn V, Lemoine NR, Williamson RCN, Davidson BR. Genomic homogeneity in fibrolamellar carcinomas. Gut. 2001;49(1):82–86. doi: 10.1136/gut.49.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Torbenson M, Kannangai R, Abraham S, Sahin F, Choti M, Wang J. Concurrent evaluation of p53, β-catenin, and α-fetoprotein expression in human hepatocellular carcinoma. American Journal of Clinical Pathology. 2004;122(3):377–382. doi: 10.1309/YH0H-3FKY-M4RM-U1JF. [DOI] [PubMed] [Google Scholar]
- 119.Kannangai R, Wang J, Liu QZ, Sahin F, Torbenson M. Survivin overexpression in hepatocellular carcinoma is associated with p53 dysregulation. International Journal of Gastrointestinal Cancer. 2005;35(1):53–60. doi: 10.1385/IJGC:35:1:053. [DOI] [PubMed] [Google Scholar]
- 120.Honda K, Sbisà E, Tullo A, et al. p53 mutation is a poor prognostic indicator for survival in patients with hepatocellular carcinoma undergoing surgical tumour ablation. British Journal of Cancer. 1998;77(5):776–782. doi: 10.1038/bjc.1998.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Torbenson M, Lee JH, Choti M, et al. Hepatic adenomas: analysis of sex steroid receptor status and the Wnt signaling pathway. Modern Pathology. 2002;15(3):189–196. doi: 10.1038/modpathol.3880514. [DOI] [PubMed] [Google Scholar]
- 122.Terris B, Pineau P, Bregeaud L, et al. Close correlation between β-catenin gene alterations and nuclear accumulation of the protein in human hepatocellular carcinomas. Oncogene. 1999;18(47):6583–6588. doi: 10.1038/sj.onc.1203051. [DOI] [PubMed] [Google Scholar]
- 123.Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SPS. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology. 2009;49(3):821–831. doi: 10.1002/hep.22695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vivekanandan P, Daniel H, Yeh MM, Torbenson M. Mitochondrial mutations in hepatocellular carcinomas and fibrolamellar carcinomas. Modern Pathology. 2010;23(6):790–798. doi: 10.1038/modpathol.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vivekanandan P, Torbenson M. Epigenetic instability is rare in fibrolamellar carcinomas but common in viral-associated hepatocellular carcinomas. Modern Pathology. 2008;21(6):670–675. doi: 10.1038/modpathol.2008.32. [DOI] [PubMed] [Google Scholar]
- 126.Tränkenschuh W, Puls F, Christgen M, et al. Frequent and distinct aberrations of DNA methylation patterns in fibrolamellar carcinoma of the liver. PLoS ONE. 2010;5(10) doi: 10.1371/journal.pone.0013688.e13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kannangai R, Vivekanandan P, Martinez-Murillo F, Choti M, Torbenson M. Fibrolamellar carcinomas show overexpression of genes in the RAS, MAPK, PIK3, and xenobiotic degradation pathways. Human Pathology. 2007;38(4):639–644. doi: 10.1016/j.humpath.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 128.Ehrenfried JA, Zhou Z, Thompson JC, Evers BM. Expression of the neurotensin gene in fetal human liver and fibrolamellar carcinoma. Annals of Surgery. 1994;220(4):484–491. doi: 10.1097/00000658-199410000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Evers BM, Rajaraman S, Chung DH, et al. Developmental expression of the neurotensin gene in the rat liver. Annals of Surgery. 1993;218(2):183–188. doi: 10.1097/00000658-199308000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sahin F, Kannangai R, Adegbola O, Wang J, Su G, Torbenson M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clinical Cancer Research. 2004;10(24):8421–8425. doi: 10.1158/1078-0432.CCR-04-0941. [DOI] [PubMed] [Google Scholar]
- 131.Kannangai R, Sahin F, Torbenson MS. EGFR is phosphorylated at Ty845 in hepatocellular carcinoma. Modern Pathology. 2006;19(11):1456–1461. doi: 10.1038/modpathol.3800665. [DOI] [PubMed] [Google Scholar]
- 132.Buckley AF, Burgart LJ, Kakar S. Epidermal growth factor receptor expression and gene copy number in fibrolamellar hepatocellular carcinoma. Human Pathology. 2006;37(4):410–414. doi: 10.1016/j.humpath.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 133.Vivekanandan P, Micchelli STL, Torbenson M. Anterior gradient-2 is overexpressed by fibrolamellar carcinomas. Human Pathology. 2009;40(3):293–299. doi: 10.1016/j.humpath.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lepreux S, Bioulac-Sage P, Chevet E. Differential expression of the anterior gradient protein-2 is a conserved feature during morphogenesis and carcinogenesis of the biliary tree. Liver International. 2011;31(3):322–328. doi: 10.1111/j.1478-3231.2010.02438.x. [DOI] [PubMed] [Google Scholar]
- 135.Wang Z, Hao Y, Lowe AW. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Research. 2008;68(2):492–497. doi: 10.1158/0008-5472.CAN-07-2930. [DOI] [PubMed] [Google Scholar]
- 136.Schoedel KE, Tyner VZ, Kim TH, Michalopoulos GK, Mars WM. HGF, MET, and matrix-related proteases in hepatocellular carcinoma, fibrolamellar variant, cirrhotic and normal liver. Modern Pathology. 2003;16(1):14–21. doi: 10.1097/01.MP.0000043521.96995.DB. [DOI] [PubMed] [Google Scholar]
- 137.Orsatti G, Hytiroglou P, Thung SN, Ishak KG, Paronetto F. Lamellar fibrosis in the fibrolamellar variant of hepatocellular carcinoma: a role for transforming growth factor beta. Liver. 1997;17(3):152–156. doi: 10.1111/j.1600-0676.1997.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 138.Cai L, Wang GJ, Mukherjee K, et al. Endothelins and their receptors in cirrhotic and neoplastic livers of Canadian and Chinese populations. Anticancer Research. 1999;19(3):2243–2247. [PubMed] [Google Scholar]
- 139.Li W, Tan D, Zenali MJ, Brown RE. Constitutive activation of nuclear factor-kappa B (NF-κB) signaling pathway in fibrolamellar hepatocellular carcinoma. International Journal of Clinical and Experimental Pathology. 2010;3(3):238–243. [PMC free article] [PubMed] [Google Scholar]
- 140.Dhingra S, Li W, Tan D, Zenali M, Zhang H, Brown RE. Cell cycle biology of fibrolamellar hepatocellular carcinoma. International Journal of Clinical and Experimental Pathology. 2010;3(8):792–797. [PMC free article] [PubMed] [Google Scholar]
- 141.Wilczek E, Szparecki G, Lukasik D, et al. Loss of the orphan nuclear receptor SHP is more pronounced in fibrolamellar carcinoma than in typical hepatocellular carcinoma. PLoS ONE. 2012;7(1) doi: 10.1371/journal.pone.0030944.e30944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Scoazec JY, Flejou JF, D’Errico A, et al. Fibrolamellar carcinoma of the liver: composition of the extracellular matrix and expression of cell-matrix and cell-cell adhesion molecules. Hepatology. 1996;24(5):1128–1136. doi: 10.1002/hep.510240525. [DOI] [PubMed] [Google Scholar]


