Abstract
The combination of paclitaxel and doxorubicin is among the most successful chemotherapy regimens in cancer treatment. CDK5RAP2, when mutated, causes primary microcephaly. We show here that inhibition of CDK5RAP2 expression causes chromosome mis-segregation, fails to maintain the spindle checkpoint, and is associated with reduced expression of the spindle checkpoint proteins BUBR1 and MAD2 and an increase in chromatin-associated CDC20. CDK5RAP2 resides on the BUBR1 and MAD2 promoters and regulates their transcription. Furthermore, CDK5RAP2-knockdown cells have increased resistance to paclitaxel and doxorubicin, and this resistance is partially rescued upon restoration of CDK5RAP2 expression. Cancer cells cultured in the presence of paclitaxel or doxorubicin exhibit dramatically decreased CDK5RAP2 levels. These results suggest that CDK5RAP2 is required for spindle checkpoint function and is a common target in paclitaxel and doxorubicin resistance.
Keywords: CDK5RAP2, primary microcephaly, spindle checkpoint, BUBR1, MAD2, drug resistance, paclitaxel, doxorubicin
Introduction
Abnormal chromosome content (cells that have a chromosome number other than 46), also known as aneuploidy, is the most common characteristic of human solid tumor, and can arise from errors during chromosome segregation. The mitotic spindle checkpoint prevents chromosome missegregation by delaying sister chromatid separation until all chromosomes have achieved bipolar attachment to spindle microtubules.1,2 The checkpoint is activated by kinetochores that lack bound microtubules or by lack of tension across attached sister kinetochores. Spindle checkpoint defects may result in chromosomal instability, aneuploidy and cancer predisposition. Though mutations in cancer critical genes (both oncogenes and tumor suppressors) are frequently detected in human tumor cells, mutations in mitotic checkpoint genes are not a common mechanism of checkpoint defects. Instead, checkpoint defects are often associated with changes in the mitotic checkpoint protein levels.3 In some tumor cells, these changes occur through abnormal transcriptional regulation by tumor suppressors or oncogene products.
Chromosome segregation is initiated by activation of the anaphase-promoting complex (APC).1,4 APC, the spindle checkpoint target, functions as an E3 ligase. When bound to its activator CDC20, APCCDC20 drives cells from metaphase into anaphase by inducing degradation of securin and mitotic cyclins. The spindle checkpoint ensures that the CDC20-dependent activation of APC does not occur until all chromosomes have achieved bipolar kinetochore-microtubule attachment. The spindle checkpoint proteins BUBR1 and MAD2 are part of an inhibitory complex for APC by sequestering CDC20 through directly binding and thus inhibiting APC activity.
Taxanes represent one of the most promising classes of anticancer agents.4 Paclitaxel (also known as taxol®) binds to β-tubulin in microtubules and stabilizes the polymer, thereby preventing chromosome segregation at mitosis through activation of the spindle checkpoint. While it is considered as one of the most effective anticancer drugs, acquired resistance often leads to treatment failure. Molecular mechanisms of taxane resistance have not been fully elucidated.
Anthracyclines rank among the most effective anticancer drugs ever developed.5 Doxorubicin was the first anthracycline isolated from the pigment-producing Streptomyces peucetius early in the 1960s. It is used extensively to treat a range of cancers, including leukemia, lymphomas, sarcomas and carcinomas.6 The cellular responses to doxorubicin are very complex. Its antitumor effect is mainly attributed to its DNA-binding and DNA-damaging capacities. Combination of paclitaxel and doxorubicin is among the very successful chemotherapy regimens used in treatment of early and advanced breast cancer.
CDK5RAP2 (cyclin dependent kinase 5 regulatory associated protein 2) was first identified as one of several binding proteins of the neuronal CDK5 activator (NCK5A).7,8 A homozygous T243A (S81X) nonsense mutation and an intronic mutation 15 bases upstream of the normal splice-acceptor site IVS126–15AG (E385fsX4) in the CDK5RAP2 gene were uncovered in primary microcephaly (MCPH, OMIM 251200) patients.9 MCPH is an autosomal recessive disorder characterized by reduced brain size without significant neurological deficits, other than variable degrees of mental retardation. It is a primary disorder of neurogenic mitosis.10
There are at least seven MCPH loci, four of which have been identified. These are MCPH1, encoding microcephalin (MCPH1); MCPH3, encoding CDK5RAP2; MCPH5, encoding ASPM; and MCPH6, encoding CENPJ. All of the four proteins localize to the centrosomes in interphase and to the spindle poles during mitosis. Like its Drosophila ortholog centrosomin (cnn), CDK5RAP2 is required for gamma-tubulin complex localization to the spindle pole body and formation of astral mitotic spindles.11 It was recently reported that MCPH1 was required for the spindle checkpoint function through negative regulation of Aurora A and PLK1.12
In this study, we report that CDK5RAP2 is required for spindle checkpoint function through positively regulating the BUBR1 and MAD2 promoters. Inhibition of CDK5RAP2 expression confers cancer cells resistance to paclitaxel and doxorubicin treatment. Cancer cells cultured in the presence of paclitaxel or doxorubicin decrease CDK5RAP2 protein levels. Thus, our data suggest that CDK5RAP2 is a common target of paclitaxel and doxorubicin and modulation of its expression may improve chemotherapy efficacy with paclitaxel and doxorubicin.
Results
Inhibition of CDK5RAP2 expression increases chromosome missegregation and leads to anastral mitotic spindles
All of the primary microcephaly genes identified so far encode proteins on spindle poles during mitosis.9,13,14 We sought to determine if CDK5RAP2 is involved in chromosome segregation during cell division. To this end, in collaboration with Bethyl Laboratories Inc., we produced five peptide antibodies against CDK5RAP2. We identified one antibody (BL2320) suitable for indirect immunofluorescence, immunoblotting and immunoprecipitation (data not shown). Consistent with earlier reports, indirect immunofluorescence staining in HeLa cells using this antibody demonstrated that CDK5RAP2 localized to the centrosomes in interphase and to the spindle poles during mitosis (Fig. 1).
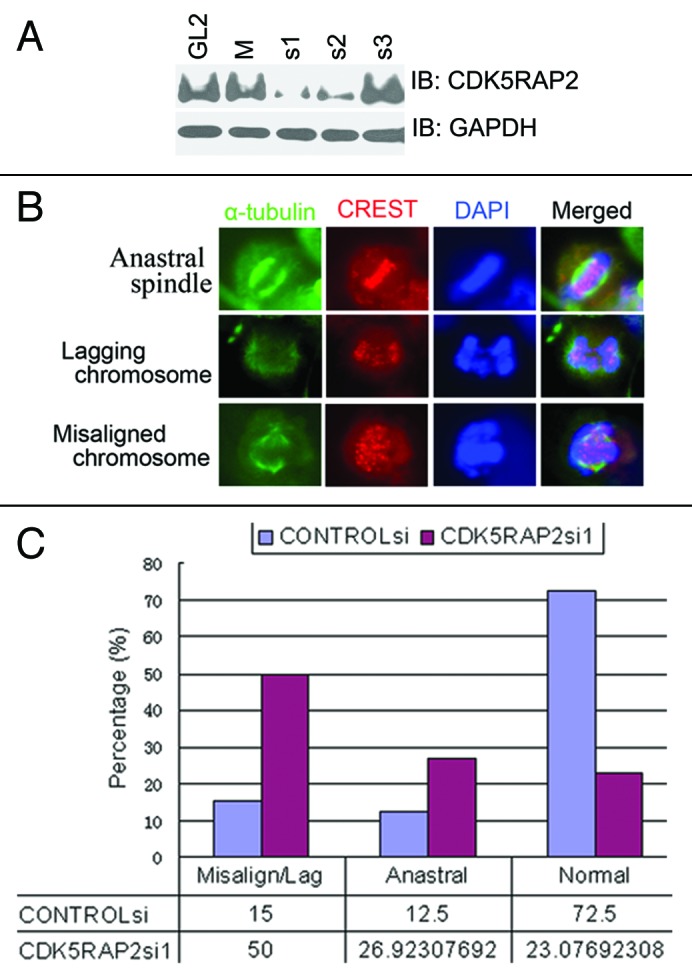
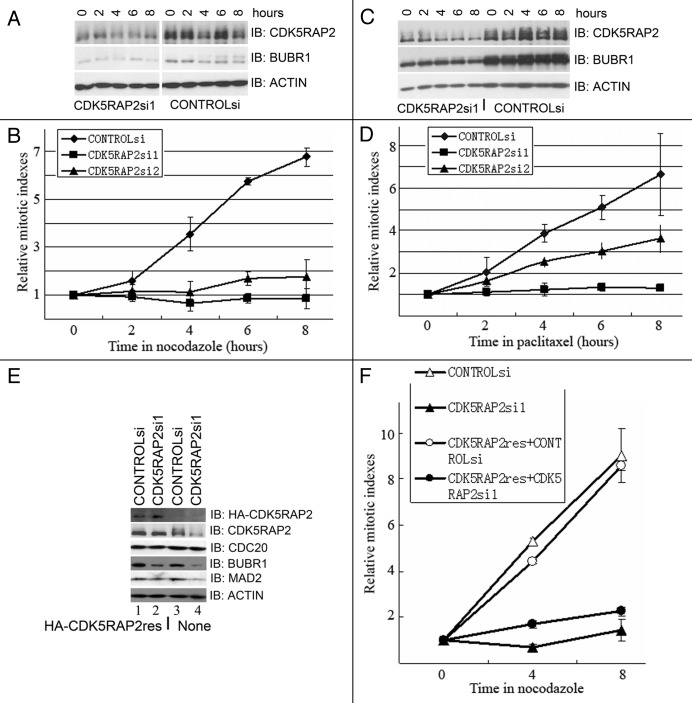
Figure 1. Inhibition of CDK5RAP2 expression leads to chromosome mis-segregation. (A) Inhibition of CDK5RAP2 expression by siRNA. HeLa cells were transfected with siRNA oligos as indicated twice (24 h apart) using Oligofectamine. Total lysates extracted 48 h after the second transfection were immunoblotted for CDK5RAP2 and GAPDH. (B and C) Increased chromosome mis-segregation in CDK5RAP2-inhibited cells. Mock- or CDK5RAP2-inhibited HeLa cells were immunostained with anti-α-tubulin (green) and the kinetochore marker CREST. Representative images of anastral spindle, lagging chromosome and misaligned chromosome in CDK5RAP2-inhibited cells are shown in (B).
To investigate functions of CDK5RAP2 by RNA interference, we tested three custom siRNA oligos (CDK5RAP2si1–3) and one predesigned siRNA oligo mixture (CDK5RAP2siM) (siGENOME ON-TARGETplus set of four duplexes, LQ-019154, Dharmacon) to knock down CDK5RAP2 expression in HeLa cells. We found that transfection with either CDK5RAP2si1 or CDK5RAP2si2 was able to inhibit expression of CDK5RAP2 to various degrees, while transfection with the CDK5RAP2si1 achieved better inhibition (Fig. 1A). Transfection with CDK5RAP2siM, CDK5RAP2si3, or a non-targeting control siRNA oligo CONTROLsi did not change CDK5RAP2 protein levels (Fig. 1A).
We then examined the role of CDK5RAP2 in the formation of mitotic spindles in HeLa cells. In two independent experiments, 30 fields (objective 60X) of images were randomly taken and all metaphase/anaphase cells were subject to further analysis. There were 120 metaphase/anaphase cells in CONTROLsi-transfected cells and 104 in CDK5RAP2si1-transfected cells. At metaphase/anaphase, cells transfected with CONTROLsi (87/120 or 72.5%) displayed a typical bipolar spindle structure with radial arrays of astral microtubules at the spindle poles, and only 15/120 or 12.5% cells exhibited anastral mitotic spindle poles. Meanwhile, inhibition of CDK5RAP2 expression exhibited anastral mitotic spindle poles in 28/104 or 26.9% cells (Fig. 1B and C and data not shown). This is consistent with the recent finding that CDK5RAP2 is a pericentriolar structural component that functions in gamma tubulin ring complex (TuRC) attachment and therefore in the microtubule organizing function of the centrosome.11,15
We further examined chromosome segregation during unperturbed mitosis in CDK5RAP2-depleted cells. Examination of CDK5RAP2-knockdown HeLa cells revealed that 52/104 or 50% of metaphase/anaphase cells showed misaligned or lagging chromosomes, compared with only 18/120 or 15% metaphase/anaphase cells transfected with CONTROLsi (Fig. 1B and C). This demonstrated that CDK5RAP2 is essential for proper chromosome segregation during mitosis.
Inhibition of CDK5RAP2 expression results in a defective spindle checkpoint
Chromosome mis-segregation in CDK5RAP2-inhibited cells promoted us to examine if CDK5RAP2 is required for the spindle checkpoint function. Paclitaxel or nocodazole was used to induce the spindle checkpoint in HeLa cells. Paclitaxel primarily interferes with tension at kinetochores through binding to β-tubulin in microtubules and stabilizing the polymer.4 Nocodazole, on the other hand, completely depolymerizes microtubules and abolishes both microtubule attachment and tension.4 Phosphorylation on serine 10 of histone H3 (pH3) is a distinctive marker for dividing cells since the metaphase chromosomes are heavily phosphorylated at this site, ultimately followed by a general decrease in the amount of phosphorylation during the progression through the cell cycle to anaphase and telophase.16 Mitotic index over time was determined by staining with an antibody specific for pH3 and propidium iodide (PI) for DNA contents. When HeLa cells were transfected with CONTROLsi, treatment of transfectants with either nocodazole or paclitaxel resulted in a significant increase of G2/M cells as well as mitotic indexes (pH3). When CDK5RAP2 expression was inhibited by transfection with CDK5RAP2si1 or CDK5RAP2si2 and transfectants were treated with nocodazole or paclitaxel, we found that, though a pronounced accumulation of G2/M cells was present, mitotic indexes over time in CDK5RAP2si2-transfected cells increased at a slower rate than those in CONTROLsi-transfected cells, and mitotic indexes overtime in CDK5RAP2si1-transfected cells did not have a significant increase (Fig. 2A–D and data not shown). These data demonstrate that CDK5RAP2 is required for the block of cell transition from metaphase to anaphase ensured by the spindle checkpoint activation induced in response to spindle poisons. A recent report indicated that CDK5RAP2 depletion did not lead to nocodazole-induced spindle checkpoint defect, but it led to centrosome and spindle defects in the osteosarcoma cell line U2OS.11 Spindle checkpoint assays were not described in detail and experimental data were not shown in that report.
Figure 2.CDK5RAP2 is required for spindle checkpoint activation induced by spindle poisons. (A and B) Inhibition of CDK5RAP2 expression confers a defect in nocodazole-induced spindle checkpoint function. Mock- or CDK5RAP2-inhibited HeLa cells were treated with nocodazole for different durations as indicated. One half of the cells were extracted for immunoblotting analysis in (A), the other half were harvested for analysis of mitotic indexes in (B). Three independent experiements were performed. (C and D) Inhibition of CDK5RAP2 expression confers a defect in paclitaxel-induced spindle checkpoint function. Cell treatment and assays were identical to those in (A and B) except that paclitaxel was used instead of nocodazole. Three independent experiments were performed. (E) Expression of CDK5RAP2si1-resistant HA-CDK5RAP2res. HeLa cells that stably expression of HA-CDK5RAP2res (revertant cells) and control cells were transfected with CONTROLsi or CDK5RAP2si1. Total cell lysates were extracted 48 h after transfection for immunoblotting analysis. (F) Expression of CDK5RAP2si1-resistant HA-CDK5RAP2res slightly rescues nocodazole-induced spindle checkpoint defect in CDK5RAP2si1-inhibited HeLa cells. Three independent experiments were performed.
To address the specificity of siRNA oligos against CDK5RAP2 and whether CDK5RAP2 is a core factor to induce the spindle checkpoint defect, we constructed a HA-tagged same-sense rescue mutant, HA-CDK5RAP2res, in which the recognition site by CDK5RAP2si1 was mutated. HeLa cells stably expressing HA-CDK5RAP2res (revertant cells) had low and detectable levels of HA-CDK5RAP2 immunoreactive to anti-HA antibody (Fig. 2E). When revertant cells were transfected with CDK5RAP2si1, HA-CDK5RAP2res expression was not inhibited in comparison to those transfected with control siRNA oligo (Fig. 2E). This demonstrated that HA-CDK5RAP2res was not targeted by CDK5RAP2si1. Expression of HA-CDK5RAP2res slightly rescued the spindle checkpoint defect in response to nocodazole treatment but not in response to paclitaxel treatment (Fig. 2F and data not shown). This weak or no rescue could be explained by low expression of HA-CDK5RAP2res. We failed to express either HA-CDK5RAP2 or HA-CDK5RAP2res to higher levels in a variety of cell lines including Ad293, HeLa and U2OS cells (data not shown). Our results here suggest that CDK5RAP2-dependent functions are required for the spindle checkpoint-signaling pathway.
CDC20 is enriched on chromatin upon spindle checkpoint activation in CDK5RAP2-depleted cells
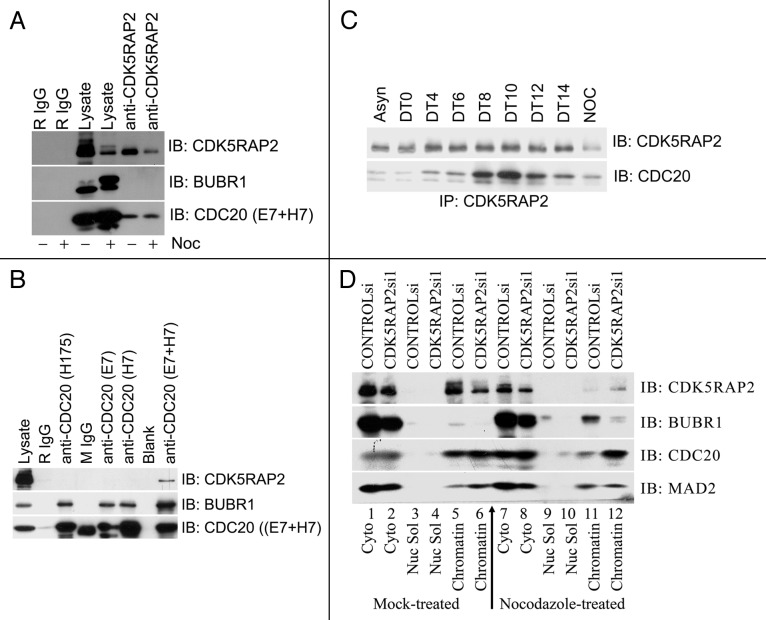
We then sought to determine the molecular mechanism how CDK5RAP2 is involved in the spindle checkpoint control. First, we screened for potential mislocalization of individual major players of the spindle checkpoint (MPS1, BUB1, BUBR1, MAD1, MAD2, CDC20, CDH1 and TPX2) in CDK5RPA2-inhibited HeLa cells by immunofluorescence staining, and we did not observe any obvious mislocalization of these proteins (data not shown). We then examined potential interactions between CDK5RAP2 and those spindle checkpoint proteins using co-immunoprecipitation assays. We found that the APC activator CDC20, but not others we examined, was present in the CDK5RAP2 immunocomplex in HeLa cell extracts (Fig. 3A). CDK5RAP2 was detected in the CDC20 immunocomplex as well (Fig. 3B). This interaction peaked during mitosis in synchronized HeLa cells (Fig. 3C).
Figure 3. CDK5RAP2 interacts with CDC20. (A) Endogenous CDC20 is present in the CDK5RAP2 immunoprecipitation complex. HeLa cells mock-treated or treated with nocodazole overnight were harvested for immunoprecipitation with anti-CDK5RAP2 or control rabbit IgG (R IgG). The CDK5RAP2 immunocomplex was blotted for CDK5RAP2, BUBR1 and CDC20 (mixture of two mouse monoclonal antibodies E7 and H7). (B) CDK5RAP2 is present in the CDC20 immunoprecipitation complex. Total HeLa cell lysates were precipitated with control IgG (mouse IgG or rabbit IgG) or CDC20 antibodies (rabbit polyclonal antibody H175 and mouse monoclonal antibodies E7 and H7). The immunocomplexes were blotted for different proteins as indicated. (C) Cell cycle dependent interaction between CDK5RAP2 and CDC20. HeLa cells were synchronized by double thymidine block and released for different durations (e.g., DT4 indicates 4 h after release). DT8 to DT12 contained the most mitotic cells. Asn, asnchronized; noc: single thymidine block followed by nocodazole treatment for 16 h. (D) CDC20 increased in the chromatin-enriched fraction after nocodazole treatment in CDK5RAP2-inhibited cells. Cyto, cytosolic fraction; Nuc Sol, nuclear soluble fraction, Chromatin, chromatin-enriched fraction.
Though we failed to collect any evidence that CDK5RAP2 enriched at kinetochores during the cell cycle, we did find that, similar to CDC20, CDK5RAP2 was mainly present in the cytosol, while a small fraction was present in the chromatin-enriched fraction (Fig. 3D). CDC20 has been reported to associate with centrosomes throughout the cell cycle and transiently bind kinetochores during prometaphase.17 Inhibition of CDC20 expression did not affect centrosome/spindle pole localization of CDK5RAP2, and inhibition of CDK5RAP2 expression did not change localization of CDC20 on centromeres during prometaphase (data not shown). Nevertheless, it was noted that inhibition of CDK5RAP2 expression led to an obvious increase of CDC20 in the chromatin-enriched fraction when treated with nocodazole for 16 h (Fig. 3D).
CDK5RAP2 is a transcriptional regulator of both BUBR1 and MAD2
We further examined protein levels and/or phosphorylation status of major players (MAD2, BUB1, BUBR1, MPS1, CDC20 and CDH1) of spindle checkpoint control in CDK5RAP2-inhibited cells. Inhibition of CDK5RAP2 expression did not change total CDC20 levels, and vice vs. (data not shown). We found that endogenous BUBR1 and MAD2 protein levels were reduced in HeLa cells transfected with CDK5RAP2si1 in comparison to those transfected with CONTROLsi (Fig. 2A, C and D), even in the absence of spindle poisons. This reduction was partially restored upon expression of CDK5RAP2si1-resistant HA-CDK5RAP2res in CDK5RAP2-inhibited cells (Fig. 2E, compare BUBR1, MAD2 and ACTIN levels between lanes 2 and 4).
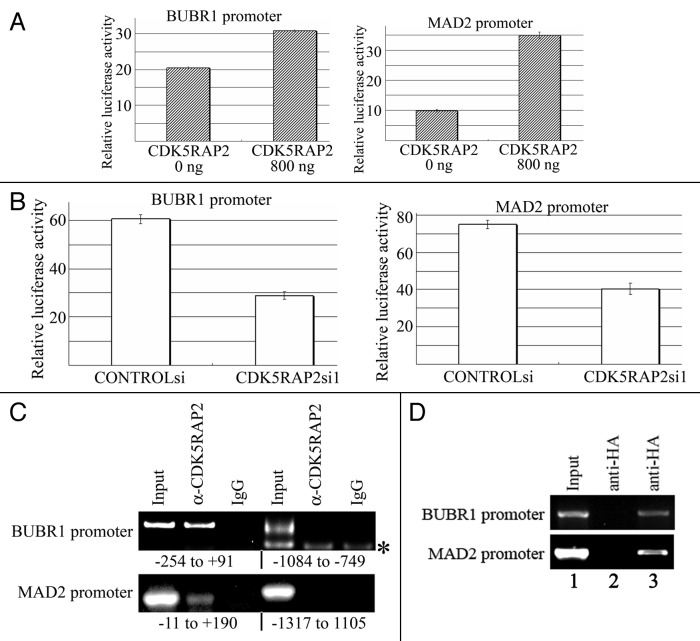
The common mechanisms of spindle checkpoint regulation include preoteasome-mediated protein degradation and transcriptional regulation. Treatment of CDK5RAP2-inhibited cells with the proteasome inhibitor MG-132 did not restore BUBR1 and MAD2 protein levels (data not shown). We then sought to determine if CDK5RAP2 is a regulator of the BUBR1 and MAD2 promoters. We cloned four overlapping putative BUBR1 promoter regions (relative to the putative transcription start site described in the Ensembl protein-coding gene ENSG00000156970, BUBR1–1k, from -998 to +91; BUBR1–2k, from -1987 to +91; BUBR1–3k, from -3045 to +91; BUBR1–4k, from -3954 to +91) and subcloned them individually into the pGL3 basic luciferase reporter vector. The pGL3-BUBR1–2k was found to have pronounced luciferase reporter activity in HeLa cells. Expression of exogenous HA-CDK5RAP2 in HeLa increased the BUBR1 promoter activity, while inhibition of CDK5RAP2 expression by RNA interference downregulated the BUBR1 promoter activity (Fig. 4A and B). We also cloned the putative MAD2 promoter region -1317 to +190 (relative to the putative transcription start site described in Jeong et al.18 sequence was retrieved from the Ensembl protein-coding gene ENSG00000164109) and subcloned it into the pGL3 basic luciferase reporter vector. The pGL3-MAD2 was found to have pronounced luciferase reporter activity in HeLa cells. We found that expression of exogenous HA-CDK5RAP2 in HeLa increased the MAD2 promoter activity, while inhibition of CDK5RAP2 expression by RNA interference downregulated the MAD2 promoter activity (Fig. 4A and B). These data indicate that CDK5RAP2 is a positive regulator of both the BUBR1 promoter and the MAD2 promoter.
Figure 4. CDK5RAP2 is a positive regulator of the BUBR1 and MAD2 promoters. (A) Overexpression of CDK5RAP2 in HeLa cells increases both the BUBR1 and MAD2 promoter activity. Quadruplicate samples were analyzed. (B) Inhibition of CDK5RAP2 expression by siRNA in HeLa cells decreases both the BUBR1 and MAD2 promoter activity. Quadruplicate samples were analyzed. (C) CDK5RAP2 binds to the BUBR1 promoter and the MAD2 promoter. ChIP assays were performed using IgG control or anti-CDK5RAP2 antibody. Numbers indicate positions of PCR fragments on the promoter relative to its transcriptional start site. (D) Exogenous CDK5RAP2 binds to the BUBR1 and MAD2 promoters. HeLa cells were co-transfected with HA-vector (lane 2) or HA-CDK5RAP2 (lane 3) along with the BUBR1 (or MAD2) promoter reporter construct. ChIP assays were performed using anti-HA antibody. The reverse primer for the ChIP PCR was specific to the backbone of the reporter vector.
We further examined if CDK5RAP2 resides on the BUBR1 and MAD2 promoters. The antibody against CDK5RAP2 was used for chromatin immunoprecipitation (ChIP) on cross-linked chromatin fragments prepared from HeLa cells. The ChIP-enriched DNA was subject to PCR analysis using 10 pairs of primers for amplification of 10 overlapping fragments within the BUBR1–2kb region or using 10 pairs of primers for amplification of 10 -overlapping fragments within the MAD2 promoter region. We found that the 10th fragment (from -254 to +91) of the BUBR1 promoter and the 10th fragment (from -11 to +190) of the MAD2 promoter were detected in the anti-CDK5RAP2 immunoprecipitates (Fig. 4C). To exclude the possibility of non-specific binding of the CDK5RAP2 antibody to DNA, we cotransfected HeLa cells with HA-vector or HA-CDK5RAP2 with the BUBR1 reporter or the MAD2 reporter respectively. The upstream primer for the ChIP PCR was specific to the 10th region of the BUBR1 or MAD2 promoter, and the downstream primer was specific to the reporter vector. Indeed, the anti-HA immunoprecipitate complex contained the CDK5RAP2 binding fragments identified in the BUBR1 and MAD2 promoters (Fig. 4D).
Inhibition of CDK5RAP2 expression renders cells resistant to both paclitaxel and doxorubicin treatment
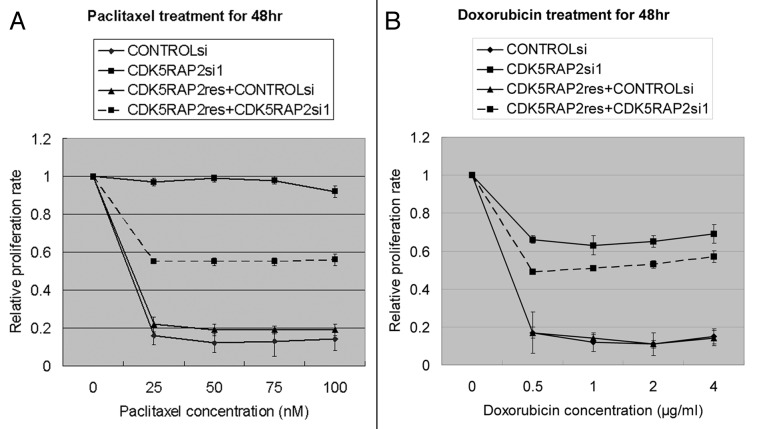
Combination of paclitaxel and doxorubicin is one of the most widely used chemotherapeutic drugs against many types of human cancer.19,20 To investigate if CDK5RAP2 inhibition affects killing of tumor cells by paclitaxel, HeLa cells transfected with CDK5RAP2si1 were treated with increasing concentrations of paclitaxel for 12 h and washed free of the drug. Strikingly, at all doses and time-points tested, CDK5RAP2-inhibited cells were more resistant to paclitaxel than HeLa cells transfected with CONTROLsi under these conditions, and expression of HA-CDK5RAP2res in CDK5RAP2-inhibited cells partially restored paclitaxel sensitivity (Fig. 5A). It was noted that CDK5RAP2 depletion reduced cell proliferation even in untreated cells (data not shown).
Figure 5. Inhibition of CDK5RAP2 expression confers cellular resistance to paclitaxel and doxorubicin treatment. HeLa cells or HeLa cells stably expressing HA-CDK5RAP2res were transfected with CONTROLsi or CDK5RAP2si1. Transfectants were treated with paclitaxel (A) or doxorubicin (B) at different concentrations for 48 h. Each concentration point was repeated at least 4 times. Cell proliferation assays were performed using the CellTiter 96® AQueous One Solution Cell Proliferation Assay system (Promega, Madison, WI) according the manufacturer’s instructions.
We then sought to determine if CDK5RAP2-inhibited HeLa cells are sensitive to doxorubicin treatment. It was unexpected that these cells were resistant to doxorubicin as well (Fig. 5B). This -resistance was also partially rescued by expression of siRNA-resistant CDK5RAP2res (Fig. 5B). These data demonstrate that CDK5RAP2-dependent functions are required for optimal tumor cell killing by both paclitaxel and doxorubicin.
CDK5RAP2 is a common target of paclitaxel and doxorubicin
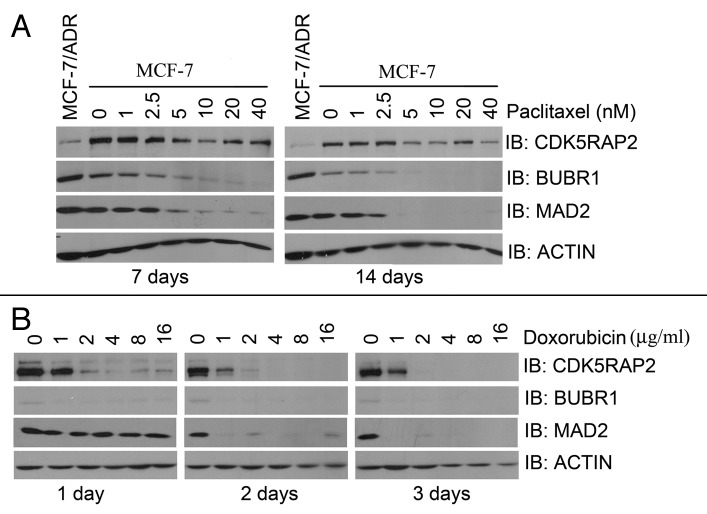
The fact that CDK5RAP2-inhibited HeLa cells were resistant to both paclitaxel and doxorubicin treatment prompted us to examine CDK5RAP2 expression status in MCF-7/ADR cells. MCF-7/ADR cells have been selected in the presence of stepwise increasing concentrations of doxorubicin over a period of more than one year and confer resistance to both doxorubicin and paclitaxel. Indeed, these cells had lower protein levels of CDK5RAP2 in comparison to its parental MCF-7 cells (Fig. 6A).
Figure 6. CDK5RAP2 is a common target of paclitaxel or doxorubicin. (A) MCF-7 or MCF-7/Adr cells were cultured in the presence of paclitaxel at different concentrations for 7 or 14 d. Total lysates were harvested and immunoblotted for proteins as indicated. (B) MCF-7 cells were cultured in the presence of doxorubicin at different concentrations for 1, 2 or 3 d. Total lysates were harvested and immunoblotted for proteins as indicated.
We reasoned that paclitaxel and/or doxorubicin treatment may modulate protein levels of CDK5RAP2. To this end, MCF-7 cells were cultured in the presence of paclitaxel for 1–2 weeks or doxorubicin for 1–3 d. We found that cultivation of MCF-7 in the presence of paclitaxel or doxorubicin resulted in dramatic reduction of protein levels of CDK5RAP2, BUBR1 and MAD2 (Fig. 6A and B). We noted that MCF-7/ADR cells had higher levels of BUBR1 and MAD2 than the parental MCF-7 cells (Fig. 6A). The simplest explanation is that these cells have disrupted the transcriptional regulation route of both BUBR1 and MAD2 by CDK5RAP2. Nevertheless, our results indicate that CDK5RAP2 is a common target of paclitaxel and doxorubicin.
Discussion
We demonstrate that CDK5RAP2 is essential for the spindle checkpoint function. This can be explained by the facts that (1) CDK5RAP2 is a positive transcriptional regulator of the essential inhibitor genes for the spindle checkpoint control BUBR1 and MAD2 (Figs. Two and 4); (2) Cytosolic CDK5RAP2 sequesters CDC20, the activator of the spindle checkpoint target, APC, from chromatin (Fig. 3D). Thus, we propose a model for CDK5RAP2-dependent function in spindle checkpoint control. Downregulation of CDK5RAP2 reduces its binding to CDC20, and inhibits transcription of BUBR1 and MAD2, consequently decreases BUBR1 and MAD2 protein level and releases BUBR1- and MAD2-sequestered CDC20. Collectively, more free CDC20 is available to bind to chromatin, activate the APC and thus allow transition from metaphase to anaphase in the presence of unattached kinetochores and/or kinetochores that lack tension in CDK5RAP2-inhibited cells.
We and other groups have demonstrated that all the identified primary microcephaly causative genes encode proteins localized on the centrosomes during interphase and the spindle poles during mitosis.9,13,14 In this report, we have revealed a novel function of CDK5RAP2 in the regulation of spindle checkpoint activation. We also found that MCPH1 and ASPM are required for spindle checkpoint function as well, though they may not use the same molecular mechanism (manuscript in preparation). A recent report also demonstrates that MCPH1 is required for spindle checkpoint activation.12 This indicates that a spindle checkpoint mechanism that controls the number of neurons generated by neural precursor cells directly contributes to microcephaly. We speculate that CENPJ may be required for spindle checkpoint function as well. It has been reported that depletion of CENPJ by RNA interference in HeLa cells disrupts centrosome integrity and induces mitotic arrest.21
During asymmetric cell division, spindle positioning is critical to ensure the unequal segregation of polarity factors and generate daughter cells with different sizes or fates.22 All four known MCPH genes are expressed in the neuroepithelium.9 The majority of neurons in the brain are thought to originate from neuronal progenitors that are the predominant cells in the neuroepithelium. Symmetric cell divisions in neural progenitor cells yield two neural progenitor cells, or asymmetric divisions give rise to one postmitotic neuron and one progenitor cell. Homozygous asp (its human ortholog is ASPM) mutations in Drosophila result in metaphase arrest during asymmetric cell division in larval brain23 and that cnn mutants result in deficient asymmetric cell division in the male germ line.24 It has been proposed that the function of MCPH genes during neurogenic mitosis involves the decision to switch from symmetric to asymmetric cell division.9 Spindle checkpoint function of the MCPH genes further strengthens this hypothesis.
It is not unexpected that CDK5RAP2 functions as a transcriptional regulator. CENPJ, encoded by one of the primary microcephaly causative genes, is a cofactor of STAT5- and NFkappaB-mediated transcription.25,26 A recent report showed that the mitotic BUBR1 gene transcription is activated through binding of the transcription factor hSTAF/ZNF143 to the repressor elements CDE and CHR.27 The CDK5RAP2 binding regions on both the BUBR1 and the MAD2 promoters contain the CDE/CHR elements, however, mutation of these elements did not affect CDK5RAP2 transcriptional activity (data not shown). To further understand CDK5RAP2-dependent spindle checkpoint functions, a genome-wide identification of genes targeted by CDK5RAP2 is warranted.
The commercially available taxanes (paclitaxel and docetaxel, also known as Taxotere) are widely recognized as extremely active chemotherapeutic agents in the treatment of a variety of cancers. Unfortunately, their clinical success has been limited by the insurgence of cellular resistance.20 This resistance is mainly mediated by the expression of the MDR (multidrug resistance) phenotype or by microtubule alterations. Microtubule alterations include altered expression or post-translational modifications of microtubule regulatory proteins. CDK5RAP2 is a microtubule regulatory protein because it functions in centrosomal attachment of the gamma-tubulin ring complex.11,15 We found in this study that CDK5RAP2 levels were downregulated upon paclitaxel treatment (Fig. 6A) and inhibition of CDK5RAP2 expression made cells resistant to paclitaxel treatment (Fig. 5A). This indicates that CDK5RAP2 is one of the targets of paclitaxel resistance.
Doxorubicin exhibits its antitumor effect mainly through its DNA binding capacity and induction of DNA damage and apoptosis. It was a surprise that cultivation of cancer cells in the presence of doxorubicin led to a decrease of CDK5RAP2 (Fig. 6B) and inhibition of CDK5RAP2 conferred resistance to doxorubicin treatment (Fig. 5B). Nevertheless, this is consistent with an earlier report that exposure of human hepatoma cell lines to doxorubicin led to downregualtion of multiple proteins with mitotic checkpoint function, including MAD2 and BUBR1.28 We have not found any evidence yet that CDK5RAP2 is directly involved in the cellular response to DNA damage (unpublished data). We speculate that CDK5RAP2 may have additional transcriptional targets that mediate cellular resistance to doxorubicin. Alternatively, the spindle checkpoint may be a bona fide target of doxorubicin.
Two evolutionarily conserved checkpoints, the DNA damage checkpoint and the spindle assembly checkpoint (SAC), had been thought to be independent from each other. There are indications, however, that they actually have overlapping functions. Microdissection laser beam-induced DNA damage during late prophase in some human cell lines delays migration into metaphase in a p53-independent manner and this delay is abrogated by inhibiting MAD2.29 The DNA damage checkpoint kinase CHK1 is required for activation and maintenance of paclitaxel-induced spindle checkpoint.30 In budding yeast, the DNA damaging agent methyl methane sulphonate (MMS) induced arrest prior to anaphase requires the SAC proteins Mad1, Mad2, Mad3, Bub1 and Bub3, and works through the APC/CDC20 in a Mec1/Tel1 (ATR/ATM)-dependent manner.31 Unlike the normal SAC, this arrest does not require a functional kinetochore.31
Combinations of paclitaxel and doxorubicin have been the preferred chemotherapy regimens for advanced breast cancer. However, recent clinical trials have shown that rapid development of drug resistance limits this treatment and this combination regimen, when compared with sequential single-agent regimens, which have a higher response rate, while overall survival is comparable.19,20 This is supported by the fact that both treatments lead to a decrease of CDK5RAP2 protein levels and this decrease may promote cancer cells resistant to both drugs. Additionally, high expression of CDK5RAP2 has been reported in breast cancer tissues.32 We therefore propose that monitoring expression levels of CDK5RAP2 before and during therapy receiving these two drugs could be an excellent marker for insurgence of drug resistance. Modulation of CDK5RAP2 protein levels could be a promising strategy to overcome resistance to both paclitaxel and doxorubicin.
Materials and Methods
For antibodies, cell lines and plasmids, see supplementary data.
Cell culture and treatments
Human HeLa cell line and breast cancer cell lines (MCF-7 and MCF-7/ADR) were grown in DMEM (Hyclone) containing 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C in a 5% CO2 incubator. Cells were treated with 300 nM nocodazole or 50 nM paclitaxel for various durations as appropriate.
Transfections and dual luciferase reporter assays
HeLa cells were transfected twice with a 24-h interval using oligofectamine (Invitrogen, Carlsbad, CA). Transfectants were used for further experiments 24 h after the second transfection. All siRNA oligo duplexes were purchased from Dharmacon (Lafayette, CO). Control siRNA oligonucleotide duplex was CONTROLsi: CGU ACG CGG AAU ACU UCG AdT dT. CDK5RAP2 siRNA oligonucleotide duplexes were CDK5RAP2si1, GGA CGU GUU GCU UCA GAA AdT dT; CDK5RAP2si2, GAG UCA GCC UUC UGC UAA AdT dT; CDK5RAP2si3, GAA AGA GCA GGA CAU CUA UdT dT; and CDK5RAP2siM (a mixture of 4 predesigned siRNA oligonucleotide duplexes, CAT# LQ-019154): GCA AAG AAG CUA CGA GAU Uuu, GCA CUG AAC AUA UCU ACA Auu, GCA AGG AUC UGA AUU UGU Uuu and CAG AUA CGA UUC AUU AGU Uuu.
On the day before transfection, 1.5 x 104 cells were seeded into each well of 24-well plates. A Firefly Luciferase reporter construct under the control of the BubR1 promoter (2 kb) (200 ng), an expression construct for HA-tagged CDK5RAP2 (200 ng, 400 ng or 800 ng), and a Renilla Luciferase reporter construct under the control of the TK promoter for normalization of transfection efficiency were cotransfected into cells in triplicate using FuGENE6 (Roche Molecular Biochemical) at a ratio of 1 μg of plasmid to 2.5 μl of FuGENE6. Luciferase activity was determined 48 h post-transfection with the dual luciferase assay system (Promega). Relative light units were determined using a luminometer (Microtiter Plate Luminometer). For siRNA reporter assays in Hela cells, siRNA oligonucleotide duplex at a final concentration of 40 μM was transfected using OligofectAMINE (Invitrogen) according to the manufacturer’s instructions. A Firefly luciferase reporter constructs under the control of the BubR1 promoter (2 kb) (1 μg) and a Renilla luciferase reporter construct under the control of the TK promoter (4 ng) were cotransfected into cells in triplicate using FuGENE6, 4 h after the secondary siRNA transfection. Luciferase activity was determined 48 h after the first siRNA transfection. Experiments were performed at least four times independently, and each combination was tested in triplicate or quadruplicate wells.
Indirect immunofluorescence microscopy
Cells were grown on coverslips in 6-well plates and fixed in ice-cold methanol at -20°C for 5 min. Fixed cells on a coverslip were incubated with blocking buffer (2% bovine serum albumin in PBST (PBS with 0.1% Tween 20)) for at least 30 min at room temperature, then with desired primary antibodies in blocking buffer for 1 h at room temperature, washed with PBST three times, and incubated with appropriate fluorescence-conjugated secondary antibodies (rhodamine-conjugated donkey anti-rabbit or mouse IgG (1:500) or fluorescein isothiocycanate-conjugated donkey anti-rabbit or mouse or human IgG (1:200)) for 1 h at room temperature. After washing, coverslips were incubated in 200 μl of DAPI (0.1 μg/ml) for 2 min and washed twice with PBS. Coverslips with cell-side down were mounted onto a glass slide with anti-fade mounting medium (Invitrogen) and sealed with nail polish. Dilutions of primary antibodies were: 1:1,000 for rabbit anti-CDK5RAP2 (Bethyl, BL2320), 1:1,000 for rabbit anti-BubR1 (Bethyl), 1:1,000 for rabbit anti-Aurora-A, 1:200 for mouse anti-CDC20, 1:1,000 for mouse anti-γ-tubulin (Sigma), 1:1,000 for human anti-centromere.
Immunoblotting and immunoprecipitation
Total cell lysate was extracted with NP-40 buffer (50 mM Tris-HCl (pH 7.5), 250 mM NaCl, 5 mM EDTA, 0.5% NP-40) and protease inhibitor mixture (Roche). Immunoprecipitation was performed using 2–4 μg of antibodies for 1 mg of total cell lysate and protein A sepharose (Amersham) to pull down immunocomplexes. Precipitates were washed with NP-40 buffer. Precipitates or total cell lysates were resolved in 5–15% gradient SDS-PAGE and transferred onto nitrocellulose membrane. Blots on nitrocellulose were blocked with 5% nonfat milk in PBST (PBS with 0.05% Tween 20) and sequentially incubated with primary antibodies and horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) in 5% nonfat milk in PBST. Blots were washed with PBST after each incubation for 1 h. The immunoreactive bands were visualized by Amersham Biosciences ECL reagents following the provided instructions.
Chromatin immunoprecipitation (ChIP)
ChIP assays were performed as described before.33 The ChIP-enriched DNA was amplified by PCR with primer pairs that are specific for the BubR1 or MAD2 promoter sequence. Primer sequences are described in the supplementary information.
Spindle checkpoint assays
HeLa cells with depletion of endogenous CDK5RAP2 by siRNA or mock treatment were treated with paclitaxel (50 nM) or nocodazole (300 nM) for different durations (0, 2, 4, 6 and 8 h). Cells at each time point were collected by trypsinization and washed once with PBS. Half of the cells were resolved in SDS sample buffer, the rest were fixed with cold 75% ethanol and stored at 4°C overnight. Fixed cells were blocked with 2% bovine serum albumin in PBST (PBS with 0.1% Tween 20) for 30 min at room temperature, incubated with anti-phosphorylated Histone 3 Serine 10 (1:10,000 in 2% bovine serum albumin in PBST) for 1 h at 37°C, washed with PBST, and incubated with FITC-conjugated donkey anti-rabbit IgG (1:150 in 2% bovine serum albumin in PBST) for 1 h at 37°C. After washing, samples were incubated with PBS containing PI (8 μg/ml) and RNaseA (1 μg/ml) at 37°C for 30 min, and then subjected to FACS analysis.
Cell proliferation assays. Cell proliferation assays were performed using CellTiter 96 AQueous one solution cell proliferation assay (MTS) system (Promega) essentially following the manufacturer’s instruction. Briefly, HeLa cells transfected with the CDK5RAP2si1 were treated with increasing concentrations of paclitaxel for 12 h, washed free of the drug, trypsinized, and seeded into a 96-well plate (5,000 cells in 100 μl per well) to continue cultivating at 37°C in a 5% CO2 incubator. After 24 h, 48 h, 72 h cultivation, adding substrate (CellTiter 96 AQueous) 20 μl per well, and back to the incubator for another 2 h, and then determined OD490 nm using a luminometer (Microtiter Plate Luminometer).
Acknowledgments
We thank Eric W. McIntush from the Bethyl Laboratories for CDK5RAP2 antibodies. We thank other members of the Xu’s laboratory for help. This work was supported by the startup fund from CNU, NSFC funds (30570371, 90608014 and 30711120570), the Program for New Century Excellent Talents in University (NCET-06–0187), Beijing Natural Science Foundation Program and Scientific Research Key Program of Beijing Municipal Commission of Education (KZ200810028014), and Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality [PHR(IHLB)] to X.X., and a National Institutes of Health grant (R01CA88873) to G.P.P. J.L is supported by NSFC fund 30700420 and Beijing Nova Program 2007B062.
Note
Recent work by Kumar et al. has identified a novel causative gene of primary microcephaly, namely STIL (SCL.TAL1 interrupting locus).34 This gene encodes a pericentriolar and centrosomal protein and is essential for taxol-induced block of mitotic entry.35
Glossary
Abbreviations:
- APC
anaphase-promoting complex
- CDK5RAP2
cyclin dependent kinase 5 regulatory associated protein 2
- ChIP
chromatin immunoprecipitation
- cnn
centrosomin
- MCPH1
primary microcephaly
- MDR
multidrug resistance
- MMS
methyl methane sulphonate
- pH3
phosphorylation on serine 10 of histone H3
- PI
propidium iodide
- SAC
spindle assembly checkpoint
- TuRC
tubulin ring complex
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/8205
References
- 1.Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–81. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 2.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–93. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 3.Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–85. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 4.McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta. 2008;1785:96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Weiss RB. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992;19:670–86. [PubMed] [Google Scholar]
- 6.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–41. doi: 10.1016/S0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Ching YP, Lam WH, Qi Z, Zhang M, Wang JH. Identification of a common protein association region in the neuronal Cdk5 activator. J Biol Chem. 2000;275:31763–9. doi: 10.1074/jbc.M004358200. [DOI] [PubMed] [Google Scholar]
- 8.Ching YP, Qi Z, Wang JH. Cloning of three novel neuronal Cdk5 activator binding proteins. Gene. 2000;242:285–94. doi: 10.1016/S0378-1119(99)00499-0. [DOI] [PubMed] [Google Scholar]
- 9.Bond J, Roberts E, Springell K, Lizarraga SB, Scott S, Higgins J, Hampshire DJ, Morrison EE, Leal GF, Silva EO, et al. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet. 2005;37:353–5. doi: 10.1038/ng1539. [DOI] [PubMed] [Google Scholar]
- 10.Woods CG, Bond J, Enard W. Autosomal recessive primary microcephaly (MCPH): a review of clinical, molecular, and evolutionary findings. Am J Hum Genet. 2005;76:717–28. doi: 10.1086/429930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fong KW, Choi YK, Rattner JB, Qi RZ. CDK5RAP2 is a pericentriolar protein that functions in centrosomal attachment of the gamma-tubulin ring complex. Mol Biol Cell. 2008;19:115–25. doi: 10.1091/mbc.E07-04-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rai R, Phadnis A, Haralkar S, Badwe RA, Dai H, Li K, Lin SY. Differential regulation of centrosome integrity by DNA damage response proteins. Cell Cycle. 2008;7:2225–33. doi: 10.4161/cc.7.14.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong X, Pfeifer GP, Xu X. Microcephalin encodes a centrosomal protein. Cell Cycle. 2006;5:457–8. doi: 10.4161/cc.5.4.2481. [DOI] [PubMed] [Google Scholar]
- 14.Zhong X, Liu L, Zhao A, Pfeifer GP, Xu X. The abnormal spindle-like, microcephaly-associated (ASPM) gene encodes a centrosomal protein. Cell Cycle. 2005;4:1227–9. doi: 10.4161/cc.4.9.2029. [DOI] [PubMed] [Google Scholar]
- 15.Graser S, Stierhof YD, Nigg EA. Cep68 and Cep215 (Cdk5rap2) are required for centrosome cohesion. J Cell Sci. 2007;120:4321–31. doi: 10.1242/jcs.020248. [DOI] [PubMed] [Google Scholar]
- 16.Prigent C, Dimitrov S. Phosphorylation of serine 10 in histone H3, what for? J Cell Sci. 2003;116:3677–85. doi: 10.1242/jcs.00735. [DOI] [PubMed] [Google Scholar]
- 17.Kallio MJ, Beardmore VA, Weinstein J, Gorbsky GJ. Rapid microtubule-independent dynamics of Cdc20 at kinetochores and centrosomes in mammalian cells. J Cell Biol. 2002;158:841–7. doi: 10.1083/jcb.200201135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeong SJ, Shin HJ, Kim SJ, Ha GH, Cho BI, Baek KH, Kim CM, Lee CW. Transcriptional abnormality of the hsMAD2 mitotic checkpoint gene is a potential link to hepatocellular carcinogenesis. Cancer Res. 2004;64:8666–73. doi: 10.1158/0008-5472.CAN-03-3455. [DOI] [PubMed] [Google Scholar]
- 19.Paridaens R, Biganzoli L, Bruning P, Klijn JG, Gamucci T, Houston S, Coleman R, Schachter J, Van Vreckem A, Sylvester R, et al. Paclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: a European Organization for Research and Treatment of Cancer Randomized Study with cross-over. J Clin Oncol. 2000;18:724–33. doi: 10.1200/JCO.2000.18.4.724. [DOI] [PubMed] [Google Scholar]
- 20.Piccart-Gebhart MJ, Burzykowski T, Buyse M, Sledge G, Carmichael J, Lück HJ, Mackey JR, Nabholtz JM, Paridaens R, Biganzoli L, et al. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol. 2008;26:1980–6. doi: 10.1200/JCO.2007.10.8399. [DOI] [PubMed] [Google Scholar]
- 21.Cho JH, Chang CJ, Chen CY, Tang TK. Depletion of CPAP by RNAi disrupts centrosome integrity and induces multipolar spindles. Biochem Biophys Res Commun. 2006;339:742–7. doi: 10.1016/j.bbrc.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy EK, Goldstein B. Asymmetric spindle positioning. Curr Opin Cell Biol. 2006;18:79–85. doi: 10.1016/j.ceb.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakefield JG, Bonaccorsi S, Gatti M. The drosophila protein asp is involved in microtubule organization during spindle formation and cytokinesis. J Cell Biol. 2001;153:637–48. doi: 10.1083/jcb.153.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–50. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 25.Koyanagi M, Hijikata M, Watashi K, Masui O, Shimotohno K. Centrosomal P4.1-associated protein is a new member of transcriptional coactivators for nuclear factor-kappaB. J Biol Chem. 2005;280:12430–7. doi: 10.1074/jbc.M410420200. [DOI] [PubMed] [Google Scholar]
- 26.Peng B, Sutherland KD, Sum EY, Olayioye M, Wittlin S, Tang TK, Lindeman GJ, Visvader JE. CPAP is a novel stat5-interacting cofactor that augments stat5-mediated transcriptional activity. Mol Endocrinol. 2002;16:2019–33. doi: 10.1210/me.2002-0108. [DOI] [PubMed] [Google Scholar]
- 27.Myslinski E, Gérard MA, Krol A, Carbon P. Transcription of the human cell cycle regulated BUB1B gene requires hStaf/ZNF143. Nucleic Acids Res. 2007;35:3453–64. doi: 10.1093/nar/gkm239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eom YW, Kim MA, Park SS, Goo MJ, Kwon HJ, Sohn S, Kim WH, Yoon G, Choi KS. Two distinct modes of cell death induced by doxorubicin: apoptosis and cell death through mitotic catastrophe accompanied by senescence-like phenotype. Oncogene. 2005;24:4765–77. doi: 10.1038/sj.onc.1208627. [DOI] [PubMed] [Google Scholar]
- 29.Mikhailov A, Cole RW, Rieder CL. DNA damage during mitosis in human cells delays the metaphase/anaphase transition via the spindle-assembly checkpoint. Curr Biol. 2002;12:1797–806. doi: 10.1016/S0960-9822(02)01226-5. [DOI] [PubMed] [Google Scholar]
- 30.Zachos G, Black EJ, Walker M, Scott MT, Vagnarelli P, Earnshaw WC, Gillespie DA. Chk1 is required for spindle checkpoint function. Dev Cell. 2007;12:247–60. doi: 10.1016/j.devcel.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim EM, Burke DJ. DNA damage activates the SAC in an ATM/ATR-dependent manner, independently of the kinetochore. PLoS Genet. 2008;4:e1000015. doi: 10.1371/journal.pgen.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Zhang TW, Yang Y, Li MH, Luo Y, Wei XY, et al. Evaluation of CDK5RAP2 expression in breast cancer by preparing its monoclonal antibody. Chin J Breast Dis. 2008;2:554–60. [Google Scholar]
- 33.Rauch T, Zhong X, Pfeifer GP, Xu X. 53BP1 is a positive regulator of the BRCA1 promoter. Cell Cycle. 2005;4:1078–83. doi: 10.4161/cc.4.8.1855. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Girimaji SC, Duvvari MR, Blanton SH. Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. Am J Hum Genet. 2009;84:286–90. doi: 10.1016/j.ajhg.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erez A, Castiel A, Trakhtenbrot L, Perelman M, Rosenthal E, Goldstein I, Stettner N, Harmelin A, Eldar-Finkelman H, Campaner S, et al. The SIL gene is essential for mitotic entry and survival of cancer cells. Cancer Res. 2007;67:4022–7. doi: 10.1158/0008-5472.CAN-07-0064. [DOI] [PubMed] [Google Scholar]