Background: The role of chaperones in extracellular space is important. Haptoglobin, an extracellular chaperone, is investigated in the context of β2-microglobulin amyloidosis.
Results: Haptoglobin interacts with prefibrillar species, facilitates intracellular degradation, and prevents formation of cytotoxic β2m fibrils. It exhibits pH-dependent chaperone activity.
Conclusions: Haptoglobin is an extracellular chaperone for β2-microglobulin under normal and inflammation-induced acidosis conditions.
Significance: Haptoglobin has promising therapeutic implications in extracellular protein deposition diseases.
Keywords: Acidosis, Inflammation, Lysosomes, Molecular Chaperone, Protein Degradation, Amyloidosis, β2-Microglobulin, Cytotoxicity, Haptoglobin
Abstract
Fibril formation of β2-microglobulin and associated inflammation occur in patients on long term dialysis. We show that the plasma protein haptoglobin prevents the fatty acid-promoted de novo fibril formation of β2-microglobulin even at substoichiometric concentration. The fibrils are cytotoxic, and haptoglobin abolishes the cytotoxicity by preventing fibril formation. Haptoglobin does not alleviate the cytotoxicity of preformed fibrils. Fibrillar β2-microglobulin is resistant to lysosomal degradation. However, the species of β2-microglobulin populated in the presence of haptoglobin is susceptible to degradation. We observed that haptoglobin interacts with oligomeric prefibrillar species of β2-microglobulin but not with monomeric or fibrillar β2-microglobulin that may underlie the molecular mechanism. 1,1′-Bis(4-anilino)naphthalene-5,5′-disulfonic acid cross-linking to haptoglobin significantly compromises its chaperone activity, suggesting the involvement of hydrophobic surfaces. Haptoglobin is an acute phase protein whose level increases severalfold during inflammation, where local acidosis can occur. Our data show that haptoglobin prevents fibril formation of β2-microglobulin under conditions of physiological acidosis (between pH 5.5 and 6.5) but with relatively decreased efficiency. However, compromise in its chaperone activity under these conditions is more than compensated by its increased level of expression under inflammation. Erythrolysis is known to release hemoglobin into the plasma. Haptoglobin forms a 1:1 (mol/mol) complex with hemoglobin. This complex, like haptoglobin, interacts with the prefibrillar species of β2-microglobulin, preventing its fibril formation and the associated cytotoxicity and resistance to intracellular degradation. Thus, our study demonstrates that haptoglobin is a potential extracellular chaperone for β2-microglobulin even in moderately acidic conditions relevant during inflammation, with promising therapeutic implications in β2-microglobulin amyloid-related diseases.
Introduction
Protein conformational diseases involve misfolding of proteins leading to either loss of crucial function of the protein or toxic gain of function of the misfolded species (1). Among such diseases, Alzheimer disease, prion disease, systemic amyloidosis, Finnish type amyloidosis, and dialysis-related amyloidosis involve extracellular amyloid deposition of the misfolded proteins/polypeptides that are characteristic of the disease (2, 3). Therefore, an understanding of factors in the extracellular environment that modulate amyloidogenicity of proteins is important and can be exploited for therapeutic development.
β2-Microglobulin (β2m),4 a component of the type I major histocompatibility complex, is present in low concentrations in circulating blood. Its turnover depends on the rate of its degradation in the kidney. However, defective homeostasis due to failure of kidney function and its inability to flow through a dialysis membrane leads to its accumulation in the blood. Eventually, fibrillar deposition of β2m occurs in the joints of patients undergoing dialysis for extended periods of time and leads to acute inflammation and tissue destruction by infiltrating macrophages, a pathological condition termed dialysis-related amyloidosis (4). Interestingly, amyloid fibril deposition did not occur in transgenic mice expressing elevated levels of human β2m either alone or when injected with the fibril seed (5). A critical balance of hydrophobic and hydrophilic interactions as modulated by environmental factors, for example anionic co-solutes, is important for the amyloid fibril formation of β2m (6). Therefore, understanding the factors (especially from plasma) that modulate the amyloid fibril formation of β2m is important in the context of β2m amyloidosis. Fatty acids, lysophospholipids (1, 7, 8), glycosaminoglycan, and proteoglycan are among the factors that are known to promote the fibril formation of β2m (9–11). The details of plasma factors that suppress or inhibit the fibril formation are not completely understood. However, a recent study by Ozawa et al. (12) has shown that α2-macroglobulin effectively suppresses the amyloid fibril formation of β2m. Because the quality control mechanism in the extracellular space is poorly understood, investigating factors that promote or inhibit the amyloid fibril formation of proteins is not only important in the context of fibril formation of β2m and dialysis-related amyloidosis but also in several amyloid-related diseases involving extracellular deposition of proteins/polypeptides.
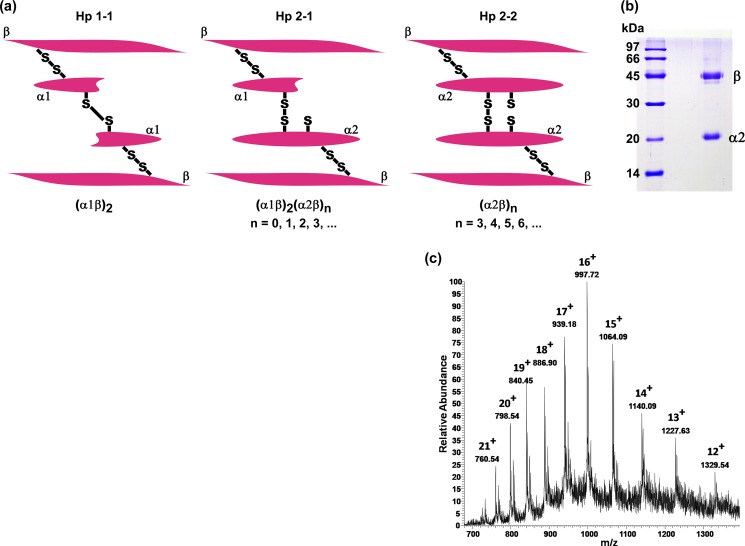
Haptoglobin, a glycosylated hemoglobin-binding protein, is abundantly present in the plasma (0.3–1.2 mg/ml; see Ref. 13). It is an acute phase protein, whose level is elevated in response to infection and a variety of injuries and inflammation (14) and is, thus, a useful marker for inflammation-related diseases (15–17). It possesses a tetrameric structure that is linked by disulfide bridges among two α chains and two β chains (18, 19). Based on the length of the α chain, three phenotypes of haptoglobin are known to occur in the human population: Hp1-1, Hp2-1, and Hp2-2. All of the phenotypes share the same 40-kDa β chain (243 amino acids), but there are two types of α chains: α1 (83 amino acids), and α2 (142 amino acids). The Hp1-1 phenotype has two β chains and two α1 chains; the Hp2-1 phenotype has two β chains, one α1 and one α2 chain, whereas the Hp2-2 phenotype has two β chains and two α2 chains (Fig. 1a). The three phenotypes of haptoglobin have been shown to exhibit chaperone activity in preventing the amorphous aggregation of target proteins (20). It has been proposed by Wilson's group that extracellular chaperones recognize exposed hydrophobic surfaces on non-native proteins in the extracellular space and mediate their clearance by targeting them for degradation in lysosomes via receptor-mediated endocytosis (21). Recent studies have shown that the Hp2-1 phenotype exhibits molecular chaperone activity in preventing the amyloid aggregation of some proteins (22).
FIGURE 1.
Phenotypes of human haptoglobin. a, schematic representation of the three phenotypes. b, SDS-PAGE of purified haptoglobin. Lane 1, molecular mass markers; lane 2, purified haptoglobin. The sample is homogeneous and exhibits bands corresponding to the β chain and α2 chain, indicating that it is Hp2-2. The α1 and α2 chains are distinguishable on SDS-PAGE because they exhibit apparent mobilities corresponding to ∼14 and ∼20 kDa, respectively (29). c, ESI mass spectrum of disulfide-reduced (by tris(2-carboxyethyl)phosphine hydrochloride) haptoglobin sample. The mass calculated from the m/z values of various charged species is around 15,945 Da, corresponding to the α2 chain.
Whether any of the isoforms of haptoglobin prevent the amyloid fibril formation of β2m, which is involved in dialysis-related amyloidosis and associated with inflammation, has not been adequately addressed. However, there are indications that haptoglobin (isoform not specified) can prevent the fibril formation of β2m (12). The Hp2-2 phenotype is found to be overrepresented in autoimmune and inflammatory diseases, and Hp2-2 subjects are characterized by higher immune reactivity (23). The Hp2-2 isoform has been shown to be more potent in macrophage activation than the Hp1-1 isoform (24). Considering the high levels of haptoglobin in plasma and its 3–8-fold increase under conditions of inflammation (14), it is important to study the role of haptoglobin in the amyloid fibril formation of β2m, which has relevance in β2m amyloid-related diseases. Because acidosis is associated with inflammation (25) and the acidic condition has a promoting effect on the amyloidosis of β2m (see Ref. 10; for a review, see Ref. 26), it is also important to investigate the effect of pH on the chaperone properties of haptoglobin with respect to β2m amyloidosis.
Fatty acids, such as palmitic acid, stearic acid, and oleic acid have been reported to be present in the serum in a molar ratio of 3:1:3 (2, 27, 28), and the mixture of these fatty acids is known to promote the amyloid fibril formation of β2m (7). In the present study, we have investigated the effect of the extracellular protein haptoglobin (Hp2-2 isoform) on the fatty acid-promoted de novo fibril formation of β2m. Our study indicates that extracellular factors (such as haptoglobin) have a potential role in the pathogenesis of dialysis-related amyloidosis. Our results show that haptoglobin prevents the amyloid fibril formation of β2m and the associated cytotoxicity. It plays an important role in the intracellular clearance of the β2m species. In addition, the results provide important insights into the molecular chaperone activity of haptoglobin itself, which is an emerging extracellular chaperone.
EXPERIMENTAL PROCEDURES
Materials
Thioflavin T (ThT), MES, hemoglobin, bovine serum albumin (BSA), N-acetyl-l-tryptophanamide, fluorescein isothiocyanate (FITC) isomer I, anti-hemoglobin antibody, and the sodium salts of the fatty acids palmitate, stearate, and oleate were purchased from Sigma-Aldrich. bis-ANS, LysoTracker Red, and Hoechst 33342 were purchased from Molecular Probes, Invitrogen. CNBr-activated Sepharose, Superdex G-75, and Superose 6 10/300 GL column were purchased from GE Healthcare. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Calbiochem. Ferritin was obtained from GE Healthcare. Human plasma was obtained from the Red Cross Society (Hyderabad, India) and stored frozen at −20 °C until use. All other chemicals used were of analytical grade.
Methods
Purification of Haptoglobin
Haptoglobin was purified by hemoglobin affinity chromatography essentially as described by Liau et al. (29). In order to remove traces of hemoglobin, we loaded haptoglobin purified by hemoglobin affinity chromatography onto an anti-hemoglobin antibody-Sepharose column to remove any haptoglobin-hemoglobin complex. The unbound flow-through, which contained haptoglobin, was found to be homogeneous by SDS-PAGE (Fig. 1b). The concentration of haptoglobin was determined using a molar extinction coefficient of 5.1 × 104 at 280 nm (20).
Preparation of α-Synuclein
α-Synuclein was expressed in Escherichia coli and purified as described previously by Ahmad et al. (30). Briefly, expression of α-synuclein in E. coli BL21 (DE3) cells transformed with the pRK172/α-synuclein plasmid was induced by 1 mm isopropyl 1-thio-β-d-galactopyranoside. Cells harvested 4 h after induction were suspended in 50 mm Tris-HCl buffer, pH 7.5, containing 1 mm EDTA, 100 mm NaCl, 0.1 mg/ml lysozyme, and 20 μm PMSF and lysed by sonication on ice. The sample was centrifuged at 5000 × g, and the supernatant containing α-synuclein was subjected to ammonium sulfate (15–45% saturation) fractionation. The precipitated protein was resolubilized in 50 mm Tris-HCl buffer, pH 7.5, containing 1 mm EDTA, 100 mm NaCl and subjected to gel filtration chromatography using a Sephadex G75 gel filtration column (150 cm × 1.8-cm diameter). Fractions containing α-synuclein were pooled, exchanged in 20 mm Hepes-NaOH buffer, pH 7.0, and concentrated by Amicon ultrafiltration. The protein preparation was found to be highly homogeneous as assessed by 15% SDS-PAGE. The concentration of α-synuclein was determined using an extinction coefficient of 0.354 at 280 nm for a 1 mg/ml solution.
Preparation of β2-Microglobulin
Human β2m was cloned by amplifying the β2m cDNA from total cDNA of HeLa cells using the primers TCT GGC CTG CAT ATG ATC CAG CGT and CCT CCA TGA AAG CTT TTA CAT GTC TGG and cloning the PCR product into the prokaryotic expression vector pET 21a using the NdeI and HindIII sites of the vector.
Expression of β2m in E. coli BL21 (DE3) cells transformed with the pET 21a-β2m plasmid was achieved by induction with 1 mm isopropyl 1-thio-β-d-galactopyranoside. The expressed β2m partitioned into inclusion bodies. Refolding and purification of β2m from the inclusion bodies was carried out by the method of Chiba et al. (31). Briefly, inclusion bodies were solubilized in 20 mm Tris-HCl (pH 8.0) containing 8 m urea and air-oxidized to form the intrachain disulfide bond. Disulfide bond formation was confirmed by reverse phase HPLC as described by Chiba et al. (31). β2m was refolded and loaded onto a DEAE-Sepharose column equilibrated with the same buffer. The protein was eluted by a linear concentration gradient of NaCl (0–200 mm). The fraction containing the major peak was concentrated and loaded onto a Sephadex G75 gel filtration chromatography column (150 cm × 1.8-cm diameter). The fractions corresponding to monomeric β2m were pooled and dialyzed against 10 mm sodium phosphate buffer, pH 7.5. Concentration of monomeric β2m was determined using an extinction coefficient at 280 nm of 1.69 for a 1 mg/ml solution. A micro-BCA protein assay kit from Pierce was used for determining the concentrations of fibrils and proteins.
De Novo Amyloid Fibril Formation of β2-Microglobulin
De novo β2m amyloid fibril formation was initiated essentially as described by Ozawa et al. (12). Briefly, monomeric human β2m (25 μm) was incubated at 37 °C with stirring at 500 rpm in phosphate-buffered saline (pH 7.5), containing a 250 μm concentration of the mixture of palmitate, stearate, and oleate in a 3:1:3 molar ratio (henceforth referred to as the fatty acid mixture). Similarly, we carried out fibril formation of β2m at different pH conditions (in 50 mm MES-Na2HPO4 buffers, pH 5.0–8.0, or in 50 mm sodium citrate buffers, pH 2.5–4.5) in the absence or in the presence of the indicated concentrations of haptoglobin or its complex with hemoglobin. Fibril formation was monitored by ThT fluorescence.
De Novo Amyloid Fibril Formation of α-Synuclein
De novo α-synuclein amyloid fibril formation was initiated by stirring monomeric human α-synuclein (20 μm) at 1000 rpm in PBS (pH 7.5), containing 250 μm fatty acid mixture at 37 °C, either in the absence or in the presence of the indicated concentrations of haptoglobin. Fibril formation was monitored by ThT fluorescence.
ThT Fluorescence
In order to measure fibril formation, an aliquot of 10 μl was taken from the sample and mixed with 1.0 ml of 10 μm ThT in 50 mm glycine-NaOH buffer, pH 8.5. ThT fluorescence intensity at 485 nm was measured upon excitation at 445 nm using a Hitachi F-4000 fluorescence spectrophotometer. The fluorescence intensity is proportional to the extent of fibril formation (32).
SDS-PAGE Analysis of β2m Species Formed in the Presence or Absence of Haptoglobin
To detect the nature of the species of β2m formed in the presence of haptoglobin, samples of β2m were agitated at 500 rpm and 37 °C in the presence or absence of haptoglobin, as described above. At the end point of the reaction, an aliquot of 10 μl of the sample was withdrawn from the 200-μl reaction mixture (total fraction). Thereafter the samples were centrifuged at 20,000 × g for 1 h to separate the supernatant and pellet fractions. The pellet was resuspended in 190 μl of PBS, pH 7.5. From the supernatant fraction and the PBS resuspended pellet fraction, an aliquot (10 μl) was withdrawn and dried in a ScanVac vacuum concentrator. The dried samples were boiled in SDS-PAGE sample loading buffer having 8 m urea and then subjected to 15% SDS-PAGE. The protein bands were visualized by Coomassie Brilliant Blue staining.
In another experiment, SDS-PAGE of the samples was performed as described above, and the protein bands from the SDS-polyacrylamide gel were transferred onto a nitrocellulose membrane using a semidry transfer apparatus (GE Healthcare). The nitrocellulose membrane was blocked with 5% BSA in PBS for 1 h. It was incubated for 2 h either with mouse monoclonal anti-β2m antibody (1:400) (sc-13565, Santa Cruz Biotechnology, Inc., Dallas, TX) or rabbit polyclonal anti-haptoglobin antibody (1:1000) (LS-B32, LifeSpan Biosciences, Seattle, WA). β2m and haptoglobin bands were detected with horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG (1:5000) (PerkinElmer Life Sciences), respectively.
Electron Microscopy
A portion of the sample of α-synuclein or β2m incubated alone or in the presence of haptoglobin was placed on Formvar/carbon-coated grids (300-mesh). Excess sample was removed by blotting with filter paper, and the grid was air-dried. The fibril-bearing grid was negatively stained with 2% (w/v) uranyl acetate solution for 1 min. Images from randomly selected areas were captured on a film at ×30,000–50,000 magnification.
Circular Dichroism Spectroscopy
Far-UV CD spectra of 0.2 mg/ml solutions of monomeric β2m and α-synuclein in PBS were recorded in a Jasco J-815 spectropolarimeter. Far-UV CD spectra of β2m (150 μg/ml) and α-synuclein (280 μg/ml) amyloid fibrils formed in the presence or absence of the indicated concentrations of haptoglobin in PBS were recorded using a 0.1-cm path length cell. All spectra shown are average of four scans taken at 37 °C. The signal contribution of haptoglobin was subtracted from samples of amyloid fibrils of β2m and α-synuclein formed in the presence of haptoglobin.
Preparation of the Haptoglobin-Hemoglobin Complex
Haptoglobin-hemoglobin complex was prepared by incubating haptoglobin (2 μm) with different molar ratios of hemoglobin in 50 mm MES-Na2HPO4 buffer, pH 7.5, containing 100 mm NaCl for 2 h at room temperature.
In order to study the stoichiometry of the complex, haptoglobin-hemoglobin complex formation was monitored by performing gel filtration chromatography on a Superose 12 10/300 GL column fitted to a Bio-Rad BioLogic DuoFlow chromatography system (Bio-Rad) previously equilibrated with 50 mm MES-Na2HPO4 buffer, pH 7.5, containing 100 mm NaCl. Protein were eluted at a flow rate of 0.5 ml/min. Molecular mass standards were used for calibration.
Chaperone Activity of the Haptoglobin-Hemoglobin Complex
To study the chaperone activity of the purified haptoglobin-hemoglobin complex, the complex was prepared by incubating haptoglobin (2 μm) with a 5-fold molar excess of hemoglobin under the conditions described above. The complex was purified using a Superose 12 gel filtration column.
To study pH-dependent changes in chaperone activity of the haptoglobin-hemoglobin complex, the complex was prepared by incubating haptoglobin (2 μm) with hemoglobin (2 μm) in 50 mm MES-Na2HPO4 buffers, pH 5.5–7.5, or in 50 mm sodium citrate buffers, pH 3.5–4.5, containing 100 mm NaCl for 2 h at room temperature.
Intrinsic Tryptophan Fluorescence
Intrinsic tryptophan fluorescence spectra of haptoglobin (2 μm), hemoglobin (2 μm) or haptoglobin-hemoglobin complex (each 2 μm) in 50 mm MES-Na2HPO4 buffer, pH 7.5, containing 100 mm NaCl were recorded in the corrected spectrum mode using a Hitachi F-4500 fluorescence spectrophotometer with the excitation wavelength set at 295 nm. The excitation and emission band passes were set at 5 nm.
Photochemical Cross-linking of Haptoglobin with Bis-ANS
Bis-ANS was photochemically cross-linked to haptoglobin essentially by the method of Poon et al. (33). Haptoglobin (40 μm) or haptoglobin-hemoglobin complex (each 40 μm) was incubated for 15 min with 800 μm bis-ANS. The samples were then dialyzed against PBS to remove unbound bis-ANS. Samples were then placed in a cuvette and illuminated with 254-nm light for 20 min in a F4500 fluorimeter under constant stirring. In controls, samples of haptoglobin or haptoglobin-hemoglobin complex were exposed to 254 nm light as described above in the absence of bis-ANS. Photochemical cross-linking of haptoglobin with bis-ANS was confirmed by the presence of a fluorescent band in SDS-PAGE. The chaperone activity of the cross-linked proteins against β2m amyloid fibril formation was assayed by monitoring ThT fluorescence as described above.
Electrospray ionization (ESI) Mass Spectrometry of Haptoglobin
Purified haptoglobin was reduced by 1 mm tris(2-carboxyethyl)phosphine hydrochloride. ESI mass spectra of reduced haptoglobin were recorded on LTQ-Orbitrap Velos (Thermo Fisher Scientific) equipped with a dual linear ion trap and Orbitrap mass analyzer. ESI mass spectra (direct injection) were recorded in positive ionization mode using acetonitrile/H2O/0.2% formic acid. The source was operated at 4.0 kV, with no sheath gas flow and with an ion transfer tube at 275 °C. The data were processed using Xcalibur (Thermo Fisher Scientific).
Immuno-dot-blot Assay
Aliquots of β2m (10 μl) withdrawn at the indicated time points were spotted on a nitrocellulose membrane (previously soaked in Tris/glycine transfer buffer containing 20% methanol) using a Millipore dot-blot apparatus. The nitrocellulose membrane was blocked with 5% BSA in PBS containing 250 μm fatty acid mixture. The nitrocellulose membrane was then incubated for 2 h with purified haptoglobin or haptoglobin-hemoglobin complex (7.5 μm each) in PBS containing 250 μm fatty acid mixture. The membrane was washed three times with PBS containing 250 μm fatty acid mixture. β2m-bound haptoglobin was detected using rabbit polyclonal anti-haptoglobin antibody (1:1000) (LS-B32, LifeSpan Biosciences) and horseradish peroxidase-conjugated anti rabbit IgG (1:5000) (PerkinElmer Life Sciences).
Effect of β2m Species on the Viability of Mouse Macrophage RAW 264.7 Cells
Cell viability was studied using the MTT cell viability assay. RAW 264.7 mouse macrophage cells were seeded in 96-well plates at a cell density of ∼8000 cells/well in serum-free AIM V medium (Invitrogen). After 18 h of incubation at 37 °C and in 5% CO2, PBS-dialyzed samples of β2m, formed in the absence or the presence of haptoglobin or haptoglobin-hemoglobin complex, were added to cells, and the cells were incubated in AIM V medium further for 24 h at 37 °C and in 5% CO2. The formazan crystals, formed after the incubation of cells with MTT (500 μg/ml in AIM V medium) for 2 h, were solubilized in DMSO, and absorbance was measured at 570 nm. In all of the samples, the final concentration of β2m corresponded to 18 μm monomeric β2m. The concentration of haptoglobin in samples of β2m incubated along with haptoglobin alone was 5.4 μm, and the concentrations of haptoglobin and hemoglobin in samples of β2m incubated with the haptoglobin-hemoglobin complex were 5.4 μm each. Final concentrations of haptoglobin or haptoglobin-hemoglobin complex were 5.4 μm in control samples that did not contain β2m.
FITC Labeling of β2m Samples
β2m samples formed in the presence or absence of haptoglobin were labeled with FITC according to the manufacturer's protocol. After labeling, the samples were dialyzed against PBS to remove excess unreacted FITC.
Live Cell Imaging of the Uptake and Localization of FITC-labeled β2m Species Formed in the Presence or Absence of Haptoglobin
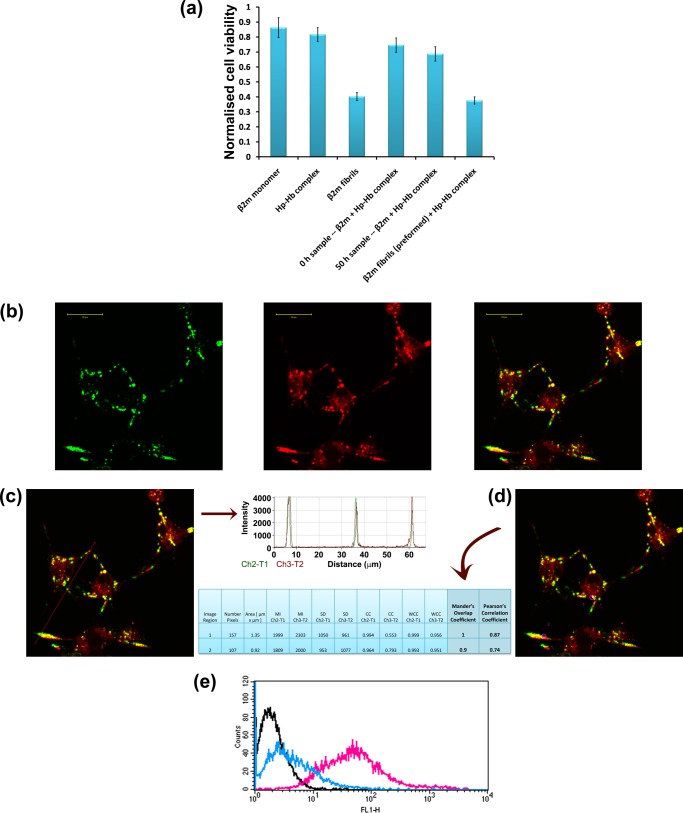
RAW 264.7 cells were seeded in Labtek 4-well glass chamber slides (Nunc, Thermo Fisher Scientific) in AIM V medium and incubated for 24 h at 37 °C and in 5% CO2. FITC-labeled β2m samples formed in the presence or absence of haptoglobin were added to the cells at a β2m monomer concentration of 30 μg/ml and incubated for 2.5 h at 37 °C and in 5% CO2. The cells were then washed twice with PBS. LysoTracker Red (25 nm) and Hoechst 33342 (2 μg/ml) were added to cells, and cell images were acquired using an LSM 510 Meta NLO confocal microscope (Carl Zeiss) with a ×63 oil immersion objective lens. Quantitative colocalization analysis was performed by calculating Pearson's correlation coefficient and Mander's overlap coefficient using the LSM 510 software.
Flow Cytometric Analysis of Cell-mediated Uptake and Degradation of β2m Species
Cell-mediated uptake and degradation of β2m species formed in the presence or absence of haptoglobin was studied by flow cytometry using the protocol essentially as described by Morten et al. (26). RAW 264.7 cells were seeded in 6-well plates in AIM V medium and incubated for 18 h at 37 °C and in 5% CO2. FITC-labeled β2m species formed in the absence or presence of either haptoglobin or haptoglobin-hemoglobin complex were added to the cells at a β2m concentration of 12.5 μm and incubated for 4 h at 37 °C and in 5% CO2. To monitor the cell-mediated degradation of β2m samples, the cells were washed twice with PBS and further incubated for 24 h at 37 °C in AIM V medium only (devoid of any FITC-labeled protein samples). Flow cytometric analyses were carried out after washing the cells three times with PBS. The intensity of cell-associated fluorescence was measured by BD FACSCalibur flow cytometer. Cells were gated on forward and side scatter to exclude cell debris, and 10,000 gated events were recorded. Data were analyzed using the Cell Quest software.
Analytical Ultracentrifugation
Sedimentation velocity measurements of β2m in the absence or presence of 250 μm fatty acid mixture were performed using an Optima XL-I analytical ultracentrifuge (Beckman Coulter, Fullerton, CA). The samples of monomeric β2m (300 μg/ml) in PBS (pH 7.5), β2m (300 μg/ml) in PBS (pH 7.5) containing 250 μm fatty acid mixture (taken immediately after adding the fatty acid mixture), and β2m (300 μg/ml) in PBS (pH 7.5) agitated in the presence of 250 μm fatty acid mixture for 10 h at 37 °C were centrifuged in an An-60 Ti rotor at 55,000 rpm, 37 °C. The molecular mass and the sedimentation coefficient s20,w was calculated using the software SEDFIT. We fitted the data with the continuous distribution c(S) Lamm equation model, which uses the weight-averaged frictional ratio. We also used the size and shape distribution model of the type continuous c(S, M), which allows the individual frictional ratios of the species to vary.
RESULTS
Effect of Haptoglobin on the Fibril Formation of β2m
We purified haptoglobin from human plasma by hemoglobin affinity chromatography and anti-hemoglobin antibody affinity chromatography as described under “Experimental Procedures.” Three phenotypes of haptoglobin are known to occur in the human population (schematically represented in Fig. 1a). The haptoglobin that we isolated from plasma was found to be Hp2-2, based on its mobility in SDS-PAGE (Fig. 1b). We further analyzed by mass spectrometry the haptoglobin preparation after reducing the disulfide bonds using 1 mm tris(2-carboxyethyl)phosphine hydrochloride. ESI mass spectrometry shows the presence of the α2 chain (charge states 12+ to 21+, yielding a mass of 15.94 kDa; Fig. 1c), whereas the m/z peak corresponding to the α1 chain could not be detected, confirming that the preparation was Hp2-2.
Whether the Hp2-2 isoform exhibits molecular chaperone activity against amyloid aggregation is not yet clear. We investigated the effect of Hp2-2 isoform (henceforth referred to as haptoglobin) on the fibril formation of human β2m promoted by physiologically relevant fatty acids. Fatty acids, such as palmitic acid, stearic acid, and oleic acid, have been reported to be present in the serum in a molar ratio of 3:1:3 (27, 28), and the mixture of these fatty acids is known to promote the fibril formation of β2m (7).
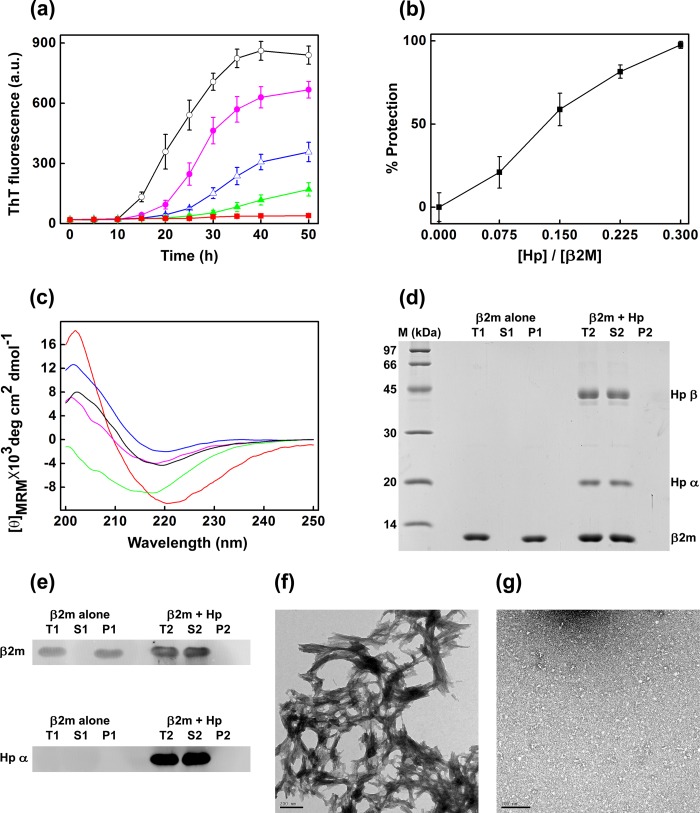
Fig. 2a shows the effect of haptoglobin on the de novo fibril formation of β2m promoted by 250 μm fatty acid mixture at pH 7.5, as monitored by ThT fluorescence. The fluorescence of ThT increases upon binding to amyloid fibrils (32). In the case of the β2m sample in the absence of haptoglobin, the ThT fluorescence increases with time and reaches a plateau at 40 h. Such increase in ThT fluorescence was progressively decreased in the presence of increasing concentrations of haptoglobin (Fig. 2a), indicating that haptoglobin prevents the fibril formation of β2m. At a low β2m/haptoglobin ratio (1:0.075, mol/mol), haptoglobin prevented the fibril formation to an extent of ∼21% (Fig. 2b). The percentage of prevention of fibril formation (% protection) increased with increasing concentrations of haptoglobin, and at a 1:0.3 (mol/mol) ratio of β2m/haptoglobin, the amyloid fibril formation of β2m was almost completely prevented (Fig. 2b).
FIGURE 2.
Haptoglobin prevents de novo amyloid fibril formation of β2m promoted by the fatty acid mixture known to be present in serum. a, de novo amyloid fibril formation of β2m in the presence of 250 μm serum fatty acid mixture (palmitate, stearate, and oleate in a 3:1:3 molar ratio) at pH 7.5, monitored by ThT fluorescence in the absence of haptoglobin, (○) and at β2m/haptoglobin molar ratios of 1:0.075 (●), 1:0.150 (▵), 1:0.225 (▴), and 1:0.30 (■). Data points represent a mean of ThT measurements from four samples. Error bars, S.E. b, percentage of prevention (% protection) of amyloid fibril formation of β2m in the presence of 250 μm fatty acid mixture (■) at different β2m/haptoglobin (Hp) molar ratios. The percentage of fibril formation was calculated by the formula, % protection = (1 − (FHp/F0)) × 100, where F0 and FHp are ThT fluorescence of the sample in the absence and in the presence of haptoglobin. c, far-UV CD spectra of native β2m (blue curve), β2m in the presence of the fatty acid mixture (black curve), amyloid fibrils of β2m (red curve), the sample of β2m incubated in the presence of haptoglobin at a molar ratio of 1:0.30 under the fatty acid mixture-induced fibril forming conditions (green curve), and the same sample after subtracting the signal contribution of haptoglobin (pink curve). MRM, mean residue mass ellipticity. The curves are representative of three independent experiments. d, SDS-PAGE analysis. Lane M, molecular mass markers; T1, S1, and P1, total sample, soluble fraction, and insoluble/pellet fraction of β2m species formed in the absence of haptoglobin; T2, S2, and P2, total sample, soluble fraction, and insoluble/pellet fraction of β2m species formed in the presence of haptoglobin. e, Western blot analysis of the β2m species formed in the presence or absence of haptoglobin. The top panel was probed for β2m, whereas the bottom panel was probed for haptoglobin. f, transmission electron microscope image of mature β2m amyloid fibrils formed in the presence of 250 μm fatty acid mixture at pH 7.5, in the absence of haptoglobin. g, transmission electron microscope image of the sample of β2m (plus 250 μm fatty acid mixture) incubated in the presence of haptoglobin at a molar ratio of 1:0.30. Scale bars in f and g, 200 nm. a.u., arbitrary units.
Amyloid fibril formation of proteins or peptides is accompanied by the generation of a well ordered cross-β-sheet structure (34). We investigated the effect of haptoglobin on the association-induced generation of the cross-β-sheet structure of β2m. The far-UV CD spectrum of native monomeric β2m exhibited a unique chiral structure (Fig. 2c, blue curve), which changed upon treatment with the fatty acid mixture (Fig. 2c, black curve), indicating a conformational change. The CD spectrum of β2m obtained in the presence of the fatty acid mixture is similar to the CD spectra of β2m reported earlier in the presence of the fatty acid, oleate (7), or in the presence of SDS (2, 35, 36). Under conditions that favor amyloid fibril formation (50-h incubation with stirring in the presence of the fatty acid mixture), β2m yielded a characteristic far-UV CD spectrum that showed the generation of cross-β-sheet structure (Fig. 2c, red curve). The far-UV CD spectrum (Fig. 2c, green curve) of the sample of β2m in the presence of haptoglobin showed much lower ellipticity in the region of 218–250 nm compared with that of the fibril alone. Subtracting the CD signal contribution of haptoglobin alone from that of the mixture (Fig. 2c, pink curve) did not show such a characteristic fibrillar β-sheet structure; rather, it resembled the CD spectrum of β2m in the presence of the fatty acid mixture (Fig. 2c, black curve). Thus, this result indicates that haptoglobin prevents the association-induced β-sheet generation of β2m.
The possibility of the loss of CD signal due to the formation of amorphous aggregates in the sample was analyzed by centrifuging the sample and subjecting the supernatant and the pellet to SDS-PAGE (Fig. 2d). Upon fibril formation (in the absence of haptoglobin), no detectable β2m was found in the supernatant (Fig. 2d, lane S1), and almost all β2m partitioned into the insoluble pellet fraction (Fig. 2d, lane P1). On the other hand, in the presence of haptoglobin, almost all of the β2m was recovered in the supernatant (Fig. 2d, lane S2), and no detectable β2m was found in the pellet fraction (Fig. 2d, lane P2). These results show that the loss of CD signal of β2m in the presence of haptoglobin is due to the formation of soluble β2m species and not due to the formation of amorphous aggregates. Analysis of the supernatant and pellet fractions on a Western blot, probed using antibodies to β2m and haptoglobin, showed the presence of β2m and haptoglobin in the supernatant, confirming the formation of soluble β2m species in the presence of haptoglobin (Fig. 2e).
The presence of matured fibrils could be seen in the electron micrograph of the β2m sample (Fig. 2f) but not in the presence of haptoglobin (Fig. 2g). Thus, all of these results show that haptoglobin not only prevents the amyloid fibril formation of β2m but also keeps it in a soluble state without forming detectable amorphous insoluble aggregates. It is also to be noted that haptoglobin, even at substoichiometric ratios, prevents the amyloid fibril formation of β2m at pH 7.5 almost completely.
We also tested the effect of the most abundant plasma protein, serum albumin, and the acute phase plasma protein, ferritin (whose molecular mass is comparable with that of haptoglobin), on the fibril formation of β2m. However, unlike haptoglobin, these proteins did not prevent the fatty acid mixture-induced fibril formation of β2m, although BSA delays the onset of fibril formation to some extent, as monitored by the ThT fluorescence (Fig. 3a). Transmission electron microscopy of the samples of β2m in the presence of BSA or ferritin revealed the presence of matured fibrils (Fig. 3, b and c). Interestingly, the ability of haptoglobin to prevent amyloid fibril formation also appears to be target protein-dependent because it did not prevent the fatty acid mixture-induced fibril formation of α-synuclein, as monitored by ThT fluorescence (Fig. 4a) and transmission electron microscopy (Fig. 4, b and c).
FIGURE 3.
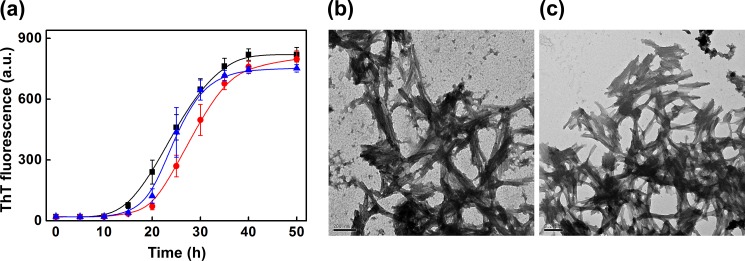
Effect of BSA and ferritin on the de novo fatty acid mixture promoted amyloid fibril formation of β2m. a, serum fatty acid mixture (250 μm) promoted de novo amyloid fibril formation of β2m alone at pH 7.5, monitored by ThT fluorescence, (■), in the presence of BSA (●), and in the presence of ferritin (▴). Molar ratios of β2m/BSA and β2m/ferritin were 1:0.30. Shown are transmission electron microscope images of the sample of β2m (plus 250 μm fatty acid mixture) incubated in the presence of BSA (b) and ferritin (c) at a molar ratio of 1:0.30. Scale bars in b and c, 200 nm. a.u., arbitrary units.
FIGURE 4.
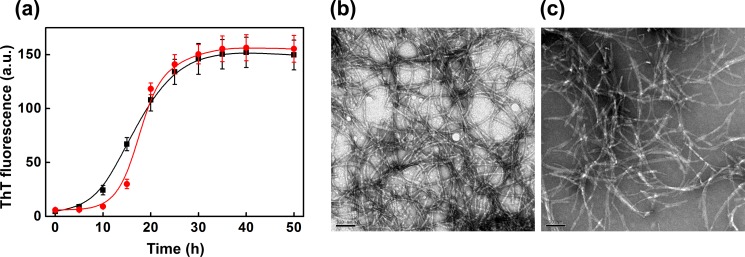
Effect of haptoglobin on the de novo fatty acid mixture promoted amyloid fibril formation of α-synuclein. a, serum fatty acid mixture (250 μm) promoted de novo amyloid fibril formation of α-synuclein alone at pH 7.5, monitored by ThT fluorescence (■) and in the presence of haptoglobin (●) at a molar ratio of 1:0.30. Shown are transmission electron microscope images of the sample of α-synuclein (plus 250 μm fatty acid mixture) incubated in the absence (b) and in the presence of haptoglobin (c) at a molar ratio of 1:0.30. Scale bars in b and c, 100 nm. a.u., arbitrary units.
Haptoglobin Prevents the Acquired Cytotoxicity of β2m upon Fibril Formation
It is generally believed that soluble oligomers are cytotoxic, whereas amyloid fibrils are less cytotoxic or relatively inert species (37). However, the fibrils of β2m have been reported to be cytotoxic (38). We investigated whether haptoglobin has any effect on such acquired cytotoxicity of β2m upon amyloid fibril formation. An MTT assay of cells after 24 h incubation with β2m fibrils or species formed in the presence of haptoglobin is shown in Fig. 5a. Incubation of mouse macrophage RAW 264.7 cells with monomeric β2m alone or haptoglobin alone did not affect the viability of the cells. In these cases, the cell viability was between 80 and 90%. However, when cells were incubated in the presence of β2m fibrils (corresponding to a monomeric β2m concentration of 18 μm), a drastic reduction in the viability of cells (∼40% cell viability; Fig. 5a) was observed. These results are in good agreement with the observation of Radford's group (38) that the β2m fibrils are highly cytotoxic.
FIGURE 5.
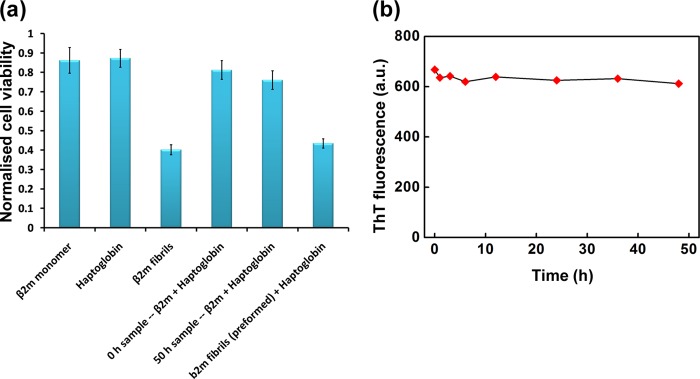
Cytotoxicity of β2m species formed in the presence and absence of haptoglobin in mouse macrophage RAW 264.7 cells. a, viability of RAW 264.7 cells upon treatment with the samples of β2m species formed in the presence or absence of haptoglobin as monitored by the MTT assay. Cell viability upon treating with preformed fibrils along with haptoglobin is also shown. Results are the average measurements of four independent samples in a given experiment; p < 0.001, one-way analysis of variance. Error bars, S.E. b, stability of the preformed mature β2m amyloid fibrils in the presence of haptoglobin. Haptoglobin was added to preformed mature amyloid fibrils at a β2m/haptoglobin ratio of 1:0.3 (mol/mol). Aliquots of the sample were withdrawn at the indicated time points, and ThT fluorescence was measured. a.u., arbitrary units.
When RAW 264.7 cells were treated with the sample of β2m incubated (for ∼50 h) in the presence of haptoglobin, there was only a marginal decrease in the cell viability to 76%, compared with 81% cell viability in control cells (Fig. 5a). Thus, haptoglobin could inhibit fibril formation and the acquired cytotoxicity. However, treating the preformed fibrils with haptoglobin neither led to dissociation of the fibrils (Fig. 5b) nor reduced their associated cytotoxicity (Fig. 5a).
β2m Fibrils Are Resistant to Lysosomal Degradation and Haptoglobin Populates β2m Species That Are Susceptible to Degradation
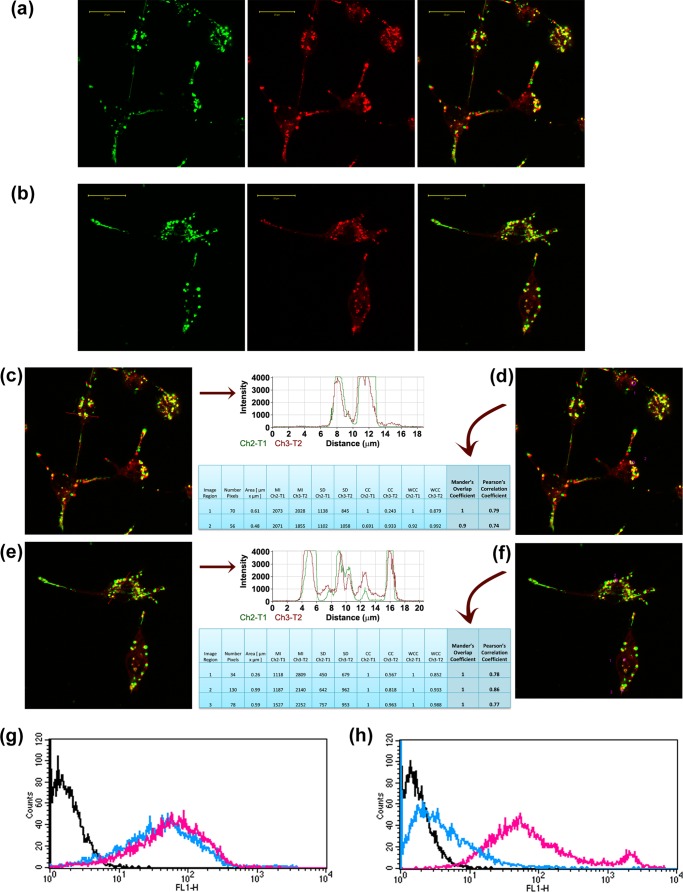
We next investigated whether the β2m species formed in the presence of haptoglobin can be internalized by RAW 264.7 cells and cleared from the extracellular space. We incubated RAW 264.7 cells with FITC-labeled samples of β2m fibrils or β2m species formed in the presence of haptoglobin. Confocal microscopy shows that FITC-labeled β2m fibrils colocalized with LysoTracker Red, showing that β2m fibrils were localized to the lysosomes of RAW 264.7 cells (Fig. 6a). The species of β2m formed in the presence of haptoglobin were also localized to the lysosomes (Fig. 6b). We performed quantitative estimation of colocalization of FITC-labeled β2m samples with LysoTracker Red by measuring Pearson's correlation coefficient and Mander's overlap coefficient. The fluorescence intensity profiles and colocalization results for β2m fibrils are shown in Fig. 6, c and d, and those for β2m species formed in the presence of haptoglobin are shown in Fig. 6, e and f. These results clearly demonstrate that the β2m fibrils as well as the β2m species formed in the presence of haptoglobin localize to the lysosomes.
FIGURE 6.
Uptake, intracellular localization, and degradation of β2m species formed in the presence and absence of haptoglobin in mouse macrophage RAW 264.7 cells. Uptake and intracellular localization of FITC-labeled β2m fibrils (a), and β2m species (b) formed in the presence of haptoglobin. The first panel in a and b shows the intracellular localization of FITC-labeled β2m samples (green), the second panel shows the LysoTracker Red staining, and the third panel shows the merged image (yellow), indicating the localization of FITC-labeled β2m samples to the lysosomes. Scale bars in a and b, 20 μm. c, colocalization profile of FITC-labeled β2m amyloid fibrils with LysoTracker Red dye in the pixels falling on the arrow shown in the figure. The fluorescence peaks of channel 2 (green) overlap with the fluorescence peaks of channel 3 (red), indicating colocalization. d, quantitative estimation of colocalization of β2m amyloid fibrils with LysoTracker Red was done by calculating Pearson's correlation coefficient and Mander's overlap coefficient. e, colocalization profile of FITC-labeled β2m species formed in the presence of haptoglobin with LysoTracker Red dye in the pixels falling on the arrow shown in the figure. The fluorescence peaks of channel 2 (green) overlap with the fluorescence peaks of channel 3 (red), indicating colocalization. f, quantitative estimation of colocalization of β2m species formed in the presence of haptoglobin with LysoTracker Red was done by calculating Pearson's correlation coefficient and Mander's overlap coefficient. In d and f, MI, mean intensity; SD, standard deviation; CC, colocalization coefficient; WCL, weighted colocalization coefficient. Shown is flow cytometry analysis of the FITC-labeled β2m samples formed in the absence of haptoglobin (g) and in the presence of haptoglobin (h). Black line, cell-associated fluorescence of control untreated cells; magenta line, cell-associated fluorescence after a 4-h incubation with FITC-labeled β2m samples. After a 4-h incubation with FITC-labeled β2m samples, the cells were washed with PBS and further incubated in AIM V medium for 24 h, and the cell-associated fluorescence was monitored as indicated by the blue line.
In order to address the susceptibility of the β2m species to lysosomal degradation, we monitored the cell-associated fluorescence using flow cytometry with time. Our study shows that the cell-associated fluorescence of β2m fibrils (FITC-labeled) persists in the RAW 264.7 cells over the chase period of 24 h, indicating that the fibrils are resistant to lysosomal degradation (Fig. 6g). This finding is consistent with the earlier observation of Morten et al. (26), that β2m fibrils are localized to the lysosomes and are resistant to lysosomal degradation (26). On the other hand, the cell-associated fluorescence of the species of β2m formed in the presence of haptoglobin decreased and came to the basal control level during the 24-h chase period (Fig. 6h). Our study shows for the first time that the species of β2m formed in the presence of haptoglobin are localized to the lysosomes and readily undergo lysosomal degradation during the chase period. Thus, haptoglobin has a potential role in β2m turnover by way of its ability to prevent the amyloid fibril formation of β2m and the consequently acquired cytotoxicity and resistance to intracellular degradation.
pH Dependence of the Chaperone Activity of Haptoglobin; Relevance to Inflammatory Conditions
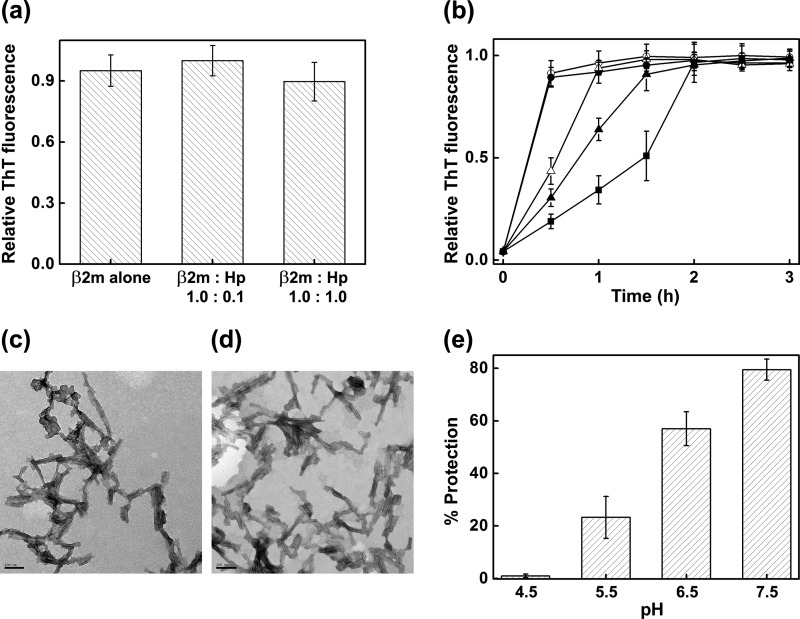
β2m amyloidosis leads to chronic inflammation (39, 40), which is known to be associated with a lowering of pH up to 5.5 (25). Morten et al. (26) have demonstrated that β2m undergoes fibril formation at lysosomal pH (pH 4.5) (41); it also undergoes fibril formation in much harsher acidic conditions (10, 42–44). We therefore studied the effect of haptoglobin on the fibril formation of β2m under different pH conditions. We found that in buffer alone, haptoglobin did not prevent the fibril formation significantly at either pH 4.5 (Fig. 7a) or at pH 2.5 (Fig. 7, b–d).
FIGURE 7.
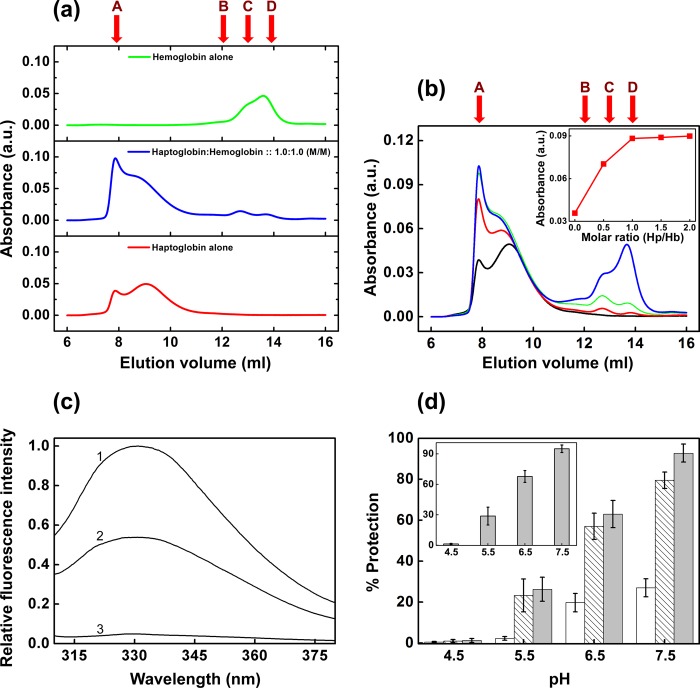
pH-dependent chaperone activity of haptoglobin toward the de novo amyloid fibril formation of β2m. a, amyloid fibril formation of β2m at pH 4.5 monitored by ThT fluorescence in the absence and in the presence of Hp. b, amyloid fibril formation of β2m at pH 2.5 monitored by ThT fluorescence in the absence of Hp (○) and at β2m/Hp molar ratios of 1:0.2 (●), 1:0.4 (▵), 1:1.0 (▴), and 1:2.0 (■). Data points represent a mean of ThT measurements from four samples. Error bars, S.E. c, transmission electron microscope image of mature β2m amyloid fibrils formed at pH 2.5, in the absence of Hp. d, transmission electron microscope image of the sample of β2m incubated at pH 2.5 in the presence of Hp at a molar ratio of 1:0.2. Scale bars in c and d, 100 nm. e, percentage of prevention of amyloid fibril formation of β2m (induced by the fatty acid mixture (250 μm)) by haptoglobin at a molar ratio of 1:0.225 at the indicated pH. The percentage of fibril formation was calculated by the formula, % protection = (1 − (FHp/F0)) × 100, where F0 and FHp are ThT fluorescence of the sample in the absence and in the presence of haptoglobin. Results are the average of ThT measurements of four independent samples in a given experiment; p < 0.0001, one-way analysis of variance. Error bars, S.E. The results shown are representative of three independent experiments.
At a β2m/haptoglobin ratio of 1:0.225 (mol/mol), haptoglobin did not prevent the fibril formation of β2m at pH 4.5 in the presence of the fatty acid mixture as well (Fig. 7e). However, it prevented the fibril formation significantly at moderately acidic conditions (pH 5.5 and 6.5) relevant to physiological acidosis (Fig. 7e). At pH 5.5, the prevention of amyloid fibril formation induced by the fatty acid mixture was 23%, whereas at pH 6.5, it was 57% (Fig. 7e). At pH 7.5, haptoglobin prevented the fibril formation of β2m very efficiently (Figs. 2 and 7e). Thus, the chaperone activity of haptoglobin is optimal at around neutral pH. It is important to note that under conditions of local acidosis that occur physiologically at sites of inflammation (between pH 5.5 and 6.5) (25), haptoglobin prevents the amyloid fibril formation of β2m significantly although relatively less than at normal physiological pH conditions.
Haptoglobin-Hemoglobin Complex Prevents β2m Fibril Formation and the Associated Cytotoxicity and Resistance to Lysosomal Degradation
Haptoglobin is known to form a complex with hemoglobin with high affinity (18), and this complex formation mediates the hemoglobin degradation (18, 45). We performed gel filtration chromatography on complexes of haptoglobin with hemoglobin mixed at various molar ratios. On a Superose 12 gel filtration column, haptoglobin and hemoglobin elute as distinct peaks. Thus, the complex formation of haptoglobin and hemoglobin could be readily monitored (Fig. 8a). We found that at a 1:1 mol/mol ratio of haptoglobin to hemoglobin, hemoglobin was completely complexed with haptoglobin (Fig. 8, b and inset), and any further increase in the concentration of hemoglobin led to saturation of the complex peak and increase in the free hemoglobin peak, indicating that the stoichiometry of the complex is 1:1 (Fig. 8, b and inset). Our preliminary study of isothermal titration calorimetry of the interaction of haptoglobin with hemoglobin showed a characteristic binding isotherm at pH 7.5 with a stoichiometry close to 1 (data not shown). Our results are in agreement with the stoichiometry found based on SPR results of haptoglobin-hemoglobin complex formation by Kristiansen et al. (18) and the crystal structure of the haptoglobin-hemoglobin complex by Andersen et al. (46), where the β chain of haptoglobin interacts with the αβ-dimer of hemoglobin (46).
FIGURE 8.
Complex formation of haptoglobin with hemoglobin and chaperone activity of the complex toward amyloid fibril formation of β2m. a, Superose 12 gel filtration chromatography elution profile of haptoglobin alone (2 μm), hemoglobin alone (2 μm) and haptoglobin-hemoglobin complex at 1:1 molar ratio (2 μm each). b, Superose 12 gel filtration chromatography elution profile of haptoglobin mixed with hemoglobin at various molar ratios. Shown are elution profiles of haptoglobin alone (black curve) and of haptoglobin mixed with hemoglobin at molar ratios of 1.0:0.5 (red curve), 1.0:1.0 (green curve), and 1.0:2.0 (blue curve). b (inset), plot of peak absorbance (at 8-ml elution volume) versus molar ratio of haptoglobin to hemoglobin complex. Molecular mass markers used for calibration are thyroglobulin (669 kDa) (A), conalbumin (75 kDa) (B), ovalbumin (43 kDa) (C), and carbonic anhydrase (29 kDa) (D). c, quenching of the fluorescence of haptoglobin upon complex formation with hemoglobin. Shown is intrinsic tryptophan fluorescence of 2 μm haptoglobin alone (curve 1) and in the presence of 2 μm hemoglobin (curve 2). Hemoglobin alone (2 μm) exhibits negligible fluorescence (curve 3). d, comparison of the pH-dependent variation of the chaperone activity of haptoglobin alone (hatched bars), hemoglobin alone (open bars) and the haptoglobin-hemoglobin complex (gray bars) toward the amyloid fibril formation of β2m. d (inset), chaperone activity of the purified haptoglobin-hemoglobin complex (using a Superose 12 gel filtration column as described under “Experimental Procedures”) toward amyloid fibril formation of β2m. Results are the average of ThT measurements of four independent samples in a given experiment; p < 0.0001, one-way analysis of variance. Error bars, S.E. The figures are representative of three independent experiments. a.u., arbitrary units.
Fluorescence of intrinsic or extrinsic probes of hemoglobin is known to be strongly quenched by resonance energy transfer to the heme (47). It is known that the intrinsic fluorescence of haptoglobin (Hp1-1 phenotype) is quenched upon complex formation with hemoglobin (48). We studied the quenching of intrinsic tryptophan fluorescence of haptoglobin upon adding hemoglobin at 1:1 (mol/mol) ratio. We found that the intrinsic fluorescence of haptoglobin was quenched to an extent of ∼50% in the presence of hemoglobin (Fig. 8c).
We mixed haptoglobin and hemoglobin at a 1:1 ratio by molarity and investigated the effect of the haptoglobin-hemoglobin complex on the amyloid fibril formation of β2m in the presence of the fatty acid mixture under different pH conditions. The percentage of prevention of fibril formation of β2m by the haptoglobin-hemoglobin complex was found to be ∼93%, 63, and 26% at pH 7.5, 6.5, and 5.5, respectively, which was higher than that found in the case of haptoglobin alone at the respective pH conditions (Fig. 8d). However, hemoglobin alone also offered some protection, although it was much lower than that offered by haptoglobin (Fig. 8d). At pH 4.5, neither haptoglobin, hemoglobin, nor their mixture prevented the fibril formation of β2m (Fig. 8d). Because free hemoglobin also exhibits some chaperone activity, we incubated haptoglobin with a 5-fold molar excess of hemoglobin and purified the complex using gel filtration chromatography. The results of the chaperone activity of the purified complex (Fig. 8d, inset) are also very similar to those shown in Fig. 8d.
As observed in the case of haptoglobin alone (Fig. 5a), the haptoglobin-hemoglobin complex could also prevent the acquired cytotoxicity (Fig. 9a). The β2m species formed in the presence of haptoglobin-hemoglobin complex localized to the lysosomes (Fig. 9, b–d) and underwent intracellular degradation (Fig. 9e).
FIGURE 9.
Cytotoxicity, uptake, intracellular localization, and fate of β2m species formed in the presence and absence of haptoglobin-hemoglobin complex in mouse macrophage RAW 264.7 cells. a, viability of RAW 264.7 cells upon treatment with the samples of β2m species formed in the presence or absence of haptoglobin-hemoglobin complex, as monitored by an MTT assay. Cell viability upon treatment with preformed fibrils along with haptoglobin-hemoglobin complex is also shown. Results are the average measurements of four independent samples in a given experiment; p < 0.001, one-way analysis of variance. Error bars, S.E. b, uptake and intracellular localization of FITC-labeled β2m species formed in the presence of haptoglobin-hemoglobin complex. The first panel shows the intracellular localization of FITC-labeled β2m samples (green); the second panel shows the LysoTracker Red staining; and the third panel shows the merged image (yellow), indicating the localization of the FITC-labeled β2m samples to the lysosomes. Scale bars in b, 20 μm. c, colocalization profile of FITC-labeled β2m species formed in the presence of haptoglobin-hemoglobin complex with LysoTracker Red dye in the pixels falling on the arrow shown in the figure. The fluorescence peaks of channel 2 (green) overlap with the fluorescence peaks of channel 3 (red), indicating colocalization. d, quantitative estimation of colocalization of β2m species formed in the presence of haptoglobin-hemoglobin complex with LysoTracker Red was done by calculating Pearson's correlation coefficient and Mander's overlap coefficient. In d, MI, mean intensity; SD, standard deviation; CC, colocalization coefficient; WCL, weighted colocalization coefficient. e, flow cytometry analysis of the FITC-labeled β2m samples formed in the presence of haptoglobin-hemoglobin complex. Black line, cell-associated fluorescence of control untreated cells; magenta line, cell-associated fluorescence after a 4-h incubation with FITC-labeled β2m samples. After a 4-h incubation with FITC-labeled β2m samples, the cells were washed with PBS and further incubated in AIM V medium for 24 h, and the cell-associated fluorescence was monitored as indicated by the blue line.
Involvement of Hydrophobic Surfaces in the Chaperone Activity of Haptoglobin or Haptoglobin-Hemoglobin Complex
To investigate the role of hydrophobic surfaces in the chaperone activity of haptoglobin, we used haptoglobin (or the haptoglobin-hemoglobin complex) photochemically cross-linked with bis-ANS. We studied the effect of cross-linked haptoglobin (or haptoglobin-hemoglobin complex) on the amyloid fibril formation of β2m at a β2m/haptoglobin ratio of 1:0.225 (mol/mol). Bis-ANS-cross-linked haptoglobin prevents β2m fibril formation to an extent of only ∼32% (Fig. 10a), a drastic decrease in chaperone activity when compared with that in the case of haptoglobin alone at this ratio (∼80%; Fig. 2a) or irradiated control (without bis-ANS) of haptoglobin (∼73%; Fig. 10a). A similar reduction in the chaperone activity was found for the haptoglobin-hemoglobin complex (from ∼83 to ∼51%; Fig. 10b) upon bis-ANS cross-linking. Thus, our results show that hydrophobic pockets (reported by the specific binding of bis-ANS) play an important role in the chaperone activity of haptoglobin.
FIGURE 10.
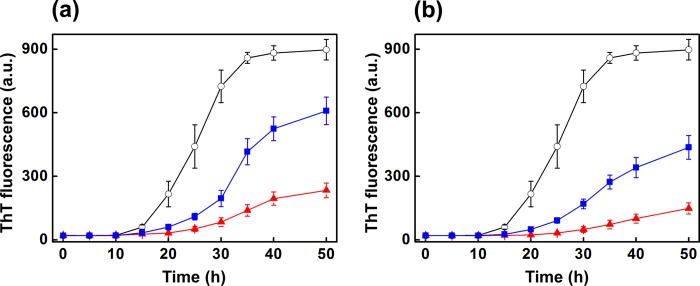
Role of hydrophobic surfaces in the chaperone activity of haptoglobin or haptoglobin-hemoglobin complex. Chaperone activity of bis-ANS-cross-linked haptoglobin and haptoglobin-hemoglobin complex toward de novo β2m amyloid fibril formation was monitored by ThT fluorescence. a, ThT fluorescence intensity of de novo β2m fibrils formed in the presence of fatty acid mixture (○), in the presence of bis-ANS cross-linked (by irradiation at 254 nm) to haptoglobin (■), and in the presence of irradiated (at 254 nm) haptoglobin control (▴). The molar ratio of β2m to haptoglobin was 1:0.225. b, ThT fluorescence intensity of de novo β2m fibrils formed in the presence of fatty acid mixture (○), in the presence of bis-ANS cross-linked (by irradiation at 254 nm) to haptoglobin-hemoglobin complex (■), and in the presence of irradiated (at 254 nm) haptoglobin-hemoglobin control (▴). The molar ratio of β2m to the haptoglobin-hemoglobin complex was 1:0.225. a.u., arbitrary units.
Prefibrillar Oligomeric Species of β2m Is Recognized by Haptoglobin and Haptoglobin-Hemoglobin Complex
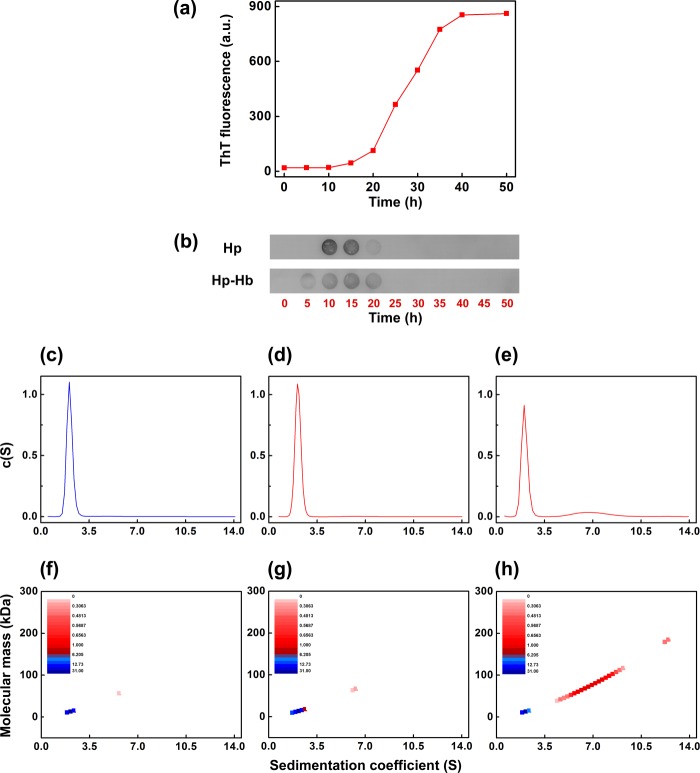
We performed dot-blot analysis to investigate the species of β2m on the pathway of its fibril formation recognized by haptoglobin. We initiated amyloid fibril formation of β2m in the presence of 250 μm fatty acid mixture, as mentioned under “Experimental Procedures.” There was no increase in the ThT fluorescence of the β2m sample until 10 h of incubation. The ThT fluorescence gradually increased beyond this time point and increased sharply after 20 h of incubation (Fig. 11a). During the course of β2m fibril formation, 10 μl of the β2m samples were withdrawn from the reaction mixture at the indicated time points. These samples were then spotted on the nitrocellulose membrane and incubated with either haptoglobin alone or with the haptoglobin-hemoglobin complex and subsequently washed with buffer. The blots were probed for haptoglobin using anti-haptoglobin antibodies. It was observed that haptoglobin interacted with the species formed at around 10–15 h during the course of the β2m fibril formation, where the ThT fluorescence is negligible (Fig. 11b). It neither recognized the β2m species at the zero time point nor recognized the fibrils that have high ThT fluorescence (beyond 25 h). A similar observation was made for the haptoglobin-hemoglobin complex as well (Fig. 11b).
FIGURE 11.
Haptoglobin or haptoglobin-hemoglobin complex interacts with the fatty acid mixture-induced prefibrillar oligomers of β2m. a, ThT fluorescence of fatty acid mixture-induced de novo β2m amyloid fibril formation; b, the corresponding dot-blot analysis of the species interacting with haptoglobin (top) and haptoglobin-hemoglobin complex (bottom). Shown is the distribution of sedimentation coefficients of monomeric β2m (c), β2m sample immediately after the addition of 250 μm fatty acid mixture (without incubation) (d), and fatty acid-induced species of β2m formed after 10-h agitation (e). The concentrations of the β2m samples are 300 μg/ml. A color temperature plot indicates the relative distribution of monomeric β2m (f), β2m sample immediately after the addition of fatty acid mixture (without incubation) (g), and fatty acid mixture-induced species of β2m formed after 10-h agitation (h). a.u., arbitrary units.
We also performed isothermal titration calorimetry of the interaction, if any, of haptoglobin with monomeric β2m or β2m fibrils and found that the results do not show a characteristic binding isotherm indicative of any stable interaction (data not shown). Thus, haptoglobin does not exhibit detectable interaction with monomeric β2m or β2m fibrils but interacts with certain prefibrillar species on the pathway of the fibril formation. Due to potential pH-dependent structural alteration in haptoglobin antibodies as well as conformational changes of haptoglobin potentially leading to differences in epitope exposures, we could not carry out dot-blot analysis at lower pH conditions.
In order to characterize the species that is recognized by haptoglobin, we performed sedimentation velocity measurements of the β2m samples in the absence and in the presence of the fatty acid mixture. We analyzed the sedimentation data using the program SEDFIT. We fitted the data with two different models: (i) continuous distribution c(S) Lamm equation model and (ii) the size and shape distribution model of the type continuous c(S, M). In both models of analysis, the sedimentation profile of native β2m gives an s20,w value of ∼2, corresponding to a molecular mass of 12,000 Da (Fig. 11, c and f), indicating that it is a monomer. The sedimentation velocity measurement of the β2m sample performed immediately after the addition of the fatty acid mixture also shows a sedimentation pattern similar to that of the native β2m, indicating a molecular mass corresponding to that of a monomer (Fig. 11, d and g).
Analysis of the sedimentation profile of the sample incubated with fatty acid mixture for 10 h (time point at which dot-blot indicates recognition of β2m species by haptoglobin) using the continuous distribution c(S) Lamm equation model yielded two peaks: one peak with a sedimentation coefficient value of ∼2 and a second relatively broad peak with a sedimentation coefficient value around 7 (Fig. 11e). The first peak yielded a molecular mass of ∼12 kDa, and the second peak gave an average molecular mass of 78 kDa, corresponding to hexamer (Fig. 11e). Similar results were obtained when the data were fitted with the size and shape distribution model of the type continuous c(S, M); in addition to a major monomeric β2m component, higher molecular mass species corresponding to oligomers (on average hexamers) were also revealed (Fig. 11h). Thus, our study for the first time shows that incubating β2m with the fatty acid mixture at 37 °C for 10 h results in a mixture of monomers and soluble higher oligomers on the pathway to β2m amyloid fibril formation. It is to be noted that both models used for analysis of the sedimentation data revealed the presence of hexameric species.
Because haptoglobin does not exhibit a detectable interaction with monomeric β2m, the results (Fig. 11) taken together show that haptoglobin or haptoglobin-hemoglobin complex interacts with the soluble oligomeric species formed on the pathway of the fatty acid-induced fibril formation of β2m.
The distribution plot (Fig. 11e) shows that a large proportion of β2m is monomeric (∼80%), whereas only about 20% exists as soluble oligomeric species. The fact that haptoglobin seems to bind to the oligomeric species may explain why a substoichiometric concentration of haptoglobin is sufficient for the complete prevention of β2m fibril formation.
DISCUSSION
Haptoglobin as a Potential Molecular Chaperone for β2m; Role in Preventing Amyloid Fibril Formation, Acquired Cytotoxicity, and Resistance to Intracellular Degradation
Amyloid deposits of β2m occur in pathological conditions, such as dialysis-related amyloidosis, primary localized cutaneous nodular amyloidosis, and plasma cell-associated systemic amyloidosis (49, 50). β2m is the major constituent protein of the amyloid fibrils in dialysis-related amyloidosis, whose major clinical manifestations are carpal tunnel syndrome and destructive arthropathy associated with cystic bone lesions (4).
Understanding factors that modulate the amyloid fibril formation of β2m is important to combat β2m-amyloid-related diseases. Interestingly, haptoglobin, an abundant plasma protein (0.3–1.2 mg/ml; see Ref. 13), is emerging as a molecular chaperone in the extracellular space (20, 21). However, the role of haptoglobin in β2m amyloidosis is not completely understood. A recent study by Ozawa et al. (12) provided indications that haptoglobin can prevent β2m fibril formation. Our study for the first time shows that substoichiometric concentrations of haptoglobin could prevent the fatty acid (relevant physiologically)-induced amyloid fibril formation, the consequent acquired cytotoxicity, and resistance to lysosomal degradation. Thus, haptoglobin plays a role in β2m turnover by preventing its amyloid fibril formation and populating the β2m species that are non-cytotoxic and readily undergo intracellular degradation. Haptoglobin is one of the few abundant proteins in the plasma. Its ability to prevent the amyloid fibril formation of β2m is important. Neither the most abundant plasma protein, BSA, nor the acute phase plasma protein, ferritin, offers any significant protection against the fibril formation of β2m.
As mentioned earlier, the level of haptoglobin increases severalfold during inflammation. Dialysis-related amyloidosis is also associated with chronic inflammation (39, 40). Local acidosis (pH as low as 5.5) is known to occur at the sites of inflammation (25). β2m amyloidosis is found to be promoted by acidic conditions (see Ref. 10; for review, see Ref. 26). Our study shows that, compared with its activity at neutral pH, haptoglobin prevents the fibril formation of β2m with decreased efficiency under moderately acidic conditions (pH 5.5–6.5). However, it is important to note that it can still significantly prevent amyloid fibril formation (at a level between 40 and 66% of that at neutral pH). This result suggests that in the cases of physiological acidosis, a characteristic feature of the inflammatory locus where the pH is known to drop to 5.5 (25), haptoglobin is also capable of chaperoning β2m, albeit with decreased efficiency. It appears that, although there is partial loss in the chaperone efficiency of haptoglobin under moderately acidic conditions, the available total capacity is much higher, due to as high as 8-fold increase in its level (14), under inflammatory conditions compared with normal conditions.
Haptoglobin-Hemoglobin Complex Exhibits Chaperone Activity toward Amyloid Fibril Formation of β2m
The formation of a high affinity complex of haptoglobin with hemoglobin (18) and subsequent targeting to lysosomal degradation is a well established mechanism of detoxification of circulating hemoglobin (18, 45). This complex is known to prevent amyloid fibril formation of Aβ peptide and ccβw (22). Our results demonstrate that the complex prevents the fibril formation of β2m, which is comparable with that of the haptoglobin alone. Therefore, it appears that hemoglobin complex formation with haptoglobin does not affect the chaperone activity of haptoglobin significantly either by masking of the chaperone sites or by an allosteric mechanism. Hemoglobin alone also offers some protection against fibril formation of β2m, which is much less compared with that offered by haptoglobin. However, it is important to note that the existence of free hemoglobin in circulation is less likely and negligible. Therefore, the chaperone activity of the complex, rather than that of free hemoglobin, is of physiological relevance.
Prefibrillar Oligomer of β2m Is Recognized by Haptoglobin and Haptoglobin-Hemoglobin Complex; Role of Native Hydrophobic Pockets
Our study, for the first time, shows that soluble oligomers (on average hexamers) are detectably populated on the pathway of fatty acid mixture-induced β2m amyloid fibril formation; haptoglobin interacts with these species, preventing amyloid fibril formation. Our study also shows that haptoglobin does not exhibit a stable and detectable interaction with either monomeric or fibrillar β2m. Because the oligomers that are recognized by haptoglobin constitute a relatively small population (∼20% under our experimental conditions), substoichiometric concentrations of haptoglobin are sufficient to prevent the fibril formation of β2m. Formation of soluble oligomers consisting of about five molecules (pentamers) has been reported on the SDS-induced amyloid fibril-forming pathway (12, 35, 36) of β2m, and formation of soluble oligomers of about four molecules (tetramers) has been reported on the acidic pH-induced amyloid fibril-forming pathway (51). Also, haptoglobin was earlier shown to recognize prefibrillar species of Aβ on the pathway of its amyloid fibril formation (22). Therefore, it appears that haptoglobin recognizes prefibrillar oligomers of some amyloidogenic proteins or polypeptides. Our study further shows that, whereas the β2m fibrils are cytotoxic (in agreement with the results of Xue et al. (38)), the soluble β2m species populated in the presence of haptoglobin are not cytotoxic and readily undergo lysosomal degradation.
Occupation of the native hydrophobic pocket(s) of haptoglobin by the hydrophobic dye, bis-ANS (cross-linked) leads to a drastic decrease in the protective ability toward the fibril formation of β2m. Thus, the hydrophobic pocket(s) on haptoglobin is important for its ability to prevent fibril formation and hence probably important for recognition of the prefibrillar oligomeric species.
CONCLUSIONS
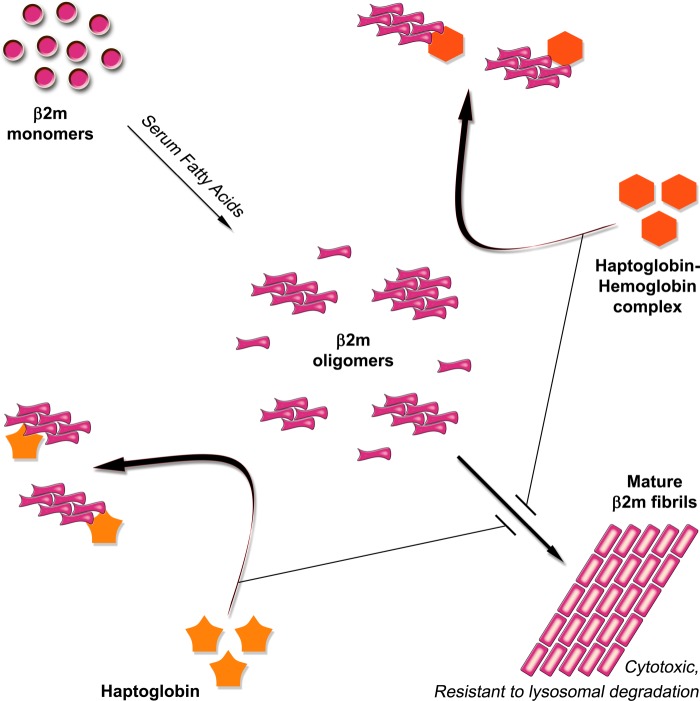
The role of haptoglobin as an extracellular molecular chaperone is emerging. Its role, particularly with respect to β2m amyloidosis, has not been given much attention previously. Our present study shows a potential chaperone role of haptoglobin for β2m, as depicted schematically in Fig. 12. It recognizes prefibrillar oligomeric species of β2m through its native hydrophobic pocket(s) and prevents the fibril formation and the cytotoxicity acquired upon fibril formation. Although the β2m fibrils are resistant to lysosomal degradation, the species of β2m populated in the presence of haptoglobin are soluble and susceptible to lysosomal degradation. Because some amount of haptoglobin could be complexed with hemoglobin under certain physiological conditions, our study also addressed the effect of this complex on the fibril formation of β2m. The complex, like haptoglobin, is capable of interacting with prefibrillar oligomeric species of β2m, preventing its fibril formation and the acquired cytotoxicity. Our study also shows that haptoglobin prevents the fibril formation of β2m significantly under conditions of physiological acidosis (pH 6.5–5.5) relevant to conditions of inflammation.
FIGURE 12.
Schematic representation of the chaperone activity of haptoglobin toward the formation of cytotoxic amyloid fibrils of β2m. The model shows that the serum fatty acid mixture (palmitate/stearate/oleate, 3:1:3 molar ratio) perturbs the β2m structure and promotes the formation of soluble prefibrillar oligomers (on average hexamers), which nucleates the formation of cytotoxic amyloid fibrils of β2m that are resistant to lysosomal degradation. Haptoglobin or the haptoglobin-hemoglobin complex interacts with the prefibrillar oligomers via hydrophobic interactions and prevents the fibril formation, thus populating β2m species in non-cytotoxic, lysosomal degradation-prone states.
All of these results indicate that haptoglobin is a potent molecular chaperone for β2m and exhibits a protective role in β2m amyloidosis. Exploring the levels of haptoglobin in the patients of β2m amyloid-related diseases and its age-related changes would be expected to provide valuable information in managing the pathology. Our present findings show the significance of haptoglobin as an extracellular molecular chaperone in the context of β2m amyloidosis and also contribute to a general understanding of the extracellular quality control system.
Acknowledgments
We thank Dr. Faiz Ahmad for the cloning of human β2m that was used in the study. We thank Dr. Suman Thakur for mass spectrometry of the haptoglobin preparation. We thank A. Harikrishna for technical help with the electron microscopy.
Footnotes
- β2m
- β2-microglobulin
- ThT
- thioflavin T
- bis-ANS
- 1,1′-bis(4-anilino)naphthalene-5,5′-disulfonic acid
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
REFERENCES
- 1. Naiki H., Nagai Y. (2009) Molecular pathogenesis of protein misfolding diseases. Pathological molecular environments versus quality control systems against misfolded proteins. J. Biochem. 146, 751–756 [DOI] [PubMed] [Google Scholar]
- 2. Aguzzi A., O'Connor T. (2010) Protein aggregation diseases. Pathogenicity and therapeutic perspectives. Nat. Rev. Drug Discov. 9, 237–248 [DOI] [PubMed] [Google Scholar]
- 3. Bellotti V., Chiti F. (2008) Amyloidogenesis in its biological environment. Challenging a fundamental issue in protein misfolding diseases. Curr. Opin. Struct. Biol. 18, 771–779 [DOI] [PubMed] [Google Scholar]
- 4. Gejyo F., Yamada T., Odani S., Nakagawa Y., Arakawa M., Kunitomo T., Kataoka H., Suzuki M., Hirasawa Y., Shirahama T. (1985) A new form of amyloid protein associated with chronic hemodialysis was identified as β2-microglobulin. Biochem. Biophys. Res. Commun. 129, 701–706 [DOI] [PubMed] [Google Scholar]
- 5. Zhang P., Fu X., Sawashita J., Yao J., Zhang B., Qian J., Tomozawa H., Mori M., Ando Y., Naiki H., Higuchi K. (2010) Mouse model to study human A beta2M amyloidosis. Generation of a transgenic mouse with excessive expression of human β2-microglobulin. Amyloid 17, 50–62 [DOI] [PubMed] [Google Scholar]
- 6. Raman B., Chatani E., Kihara M., Ban T., Sakai M., Hasegawa K., Naiki H., Rao Ch. M., Goto Y. (2005) Critical balance of electrostatic and hydrophobic interactions is required for beta 2-microglobulin amyloid fibril growth and stability. Biochemistry 44, 1288–1299 [DOI] [PubMed] [Google Scholar]
- 7. Hasegawa K., Tsutsumi-Yasuhara S., Ookoshi T., Ohhashi Y., Kimura H., Takahashi N., Yoshida H., Miyazaki R., Goto Y., Naiki H. (2008) Growth of β2-microglobulin-related amyloid fibrils by non-esterified fatty acids at a neutral pH. Biochem. J. 416, 307–315 [DOI] [PubMed] [Google Scholar]
- 8. Ookoshi T., Hasegawa K., Ohhashi Y., Kimura H., Takahashi N., Yoshida H., Miyazaki R., Goto Y., Naiki H. (2008) Lysophospholipids induce the nucleation and extension of β2-microglobulin-related amyloid fibrils at a neutral pH. Nephrol. Dial. Transplant. 23, 3247–3255 [DOI] [PubMed] [Google Scholar]
- 9. Borysik A. J., Morten I. J., Radford S. E., Hewitt E. W. (2007) Specific glycosaminoglycans promote unseeded amyloid formation from β2-microglobulin under physiological conditions. Kidney Int. 72, 174–181 [DOI] [PubMed] [Google Scholar]
- 10. Naiki H., Yamamoto S., Hasegawa K., Yamaguchi I., Goto Y., Gejyo F. (2005) Molecular interactions in the formation and deposition of β2-microglobulin-related amyloid fibrils. Amyloid 12, 15–25 [DOI] [PubMed] [Google Scholar]
- 11. Yamaguchi I., Suda H., Tsuzuike N., Seto K., Seki M., Yamaguchi Y., Hasegawa K., Takahashi N., Yamamoto S., Gejyo F., Naiki H. (2003) Glycosaminoglycan and proteoglycan inhibit the depolymerization of β2-microglobulin amyloid fibrils in vitro. Kidney Int. 64, 1080–1088 [DOI] [PubMed] [Google Scholar]
- 12. Ozawa D., Hasegawa K., Lee Y. H., Sakurai K., Yanagi K., Ookoshi T., Goto Y., Naiki H. (2011) Inhibition of β2-microglobulin amyloid fibril formation by α2-macroglobulin. J. Biol. Chem. 286, 9668–9676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bowman B. H., Kurosky A. (1982) Haptoglobin. The evolutionary product of duplication, unequal crossing over, and point mutation. Adv. Hum. Genet. 12, 189–261, 453–454 [DOI] [PubMed] [Google Scholar]
- 14. Dobryszycka W. (1997) Biological functions of haptoglobin. New pieces to an old puzzle. Eur. J. Clin. Chem. Clin. Biochem. 35, 647–654 [PubMed] [Google Scholar]
- 15. Arredouani M. S., Kasran A., Vanoirbeek J. A., Berger F. G., Baumann H., Ceuppens J. L. (2005) Haptoglobin dampens endotoxin-induced inflammatory effects both in vitro and in vivo. Immunology 114, 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Engström G., Stavenow L., Hedblad B., Lind P., Eriksson K. F., Janzon L., Lindgärde F. (2003) Inflammation-sensitive plasma proteins, diabetes, and mortality and incidence of myocardial infarction and stroke. A population-based study. Diabetes 52, 442–447 [DOI] [PubMed] [Google Scholar]
- 17. Sadrzadeh S. M., Bozorgmehr J. (2004) Haptoglobin phenotypes in health and disorders. Am. J. Clin. Pathol. 121, S97–S104 [DOI] [PubMed] [Google Scholar]
- 18. Kristiansen M., Graversen J. H., Jacobsen C., Sonne O., Hoffman H. J., Law S. K., Moestrup S. K. (2001) Identification of the haemoglobin scavenger receptor. Nature 409, 198–201 [DOI] [PubMed] [Google Scholar]
- 19. Wejman J. C., Hovsepian D., Wall J. S., Hainfeld J. F., Greer J. (1984) Structure and assembly of haptoglobin polymers by electron microscopy. J. Mol. Biol. 174, 343–368 [DOI] [PubMed] [Google Scholar]
- 20. Yerbury J. J., Rybchyn M. S., Easterbrook-Smith S. B., Henriques C., Wilson M. R. (2005) The acute phase protein haptoglobin is a mammalian extracellular chaperone with an action similar to clusterin. Biochemistry 44, 10914–10925 [DOI] [PubMed] [Google Scholar]
- 21. Yerbury J. J., Stewart E. M., Wyatt A. R., Wilson M. R. (2005) Quality control of protein folding in extracellular space. EMBO Rep. 6, 1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yerbury J. J., Kumita J. R., Meehan S., Dobson C. M., Wilson M. R. (2009) α2-Macroglobulin and haptoglobin suppress amyloid formation by interacting with prefibrillar protein species. J. Biol. Chem. 284, 4246–4254 [DOI] [PubMed] [Google Scholar]
- 23. Langlois M. R., Delanghe J. R. (1996) Biological and clinical significance of haptoglobin polymorphism in humans. Clin. Chem. 42, 1589–1600 [PubMed] [Google Scholar]
- 24. Asleh R., Marsh S., Shilkrut M., Binah O., Guetta J., Lejbkowicz F., Enav B., Shehadeh N., Kanter Y., Lache O., Cohen O., Levy N. S., Levy A. P. (2003) Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ. Res. 92, 1193–1200 [DOI] [PubMed] [Google Scholar]
- 25. Lardner A. (2001) The effects of extracellular pH on immune function. J. Leukoc. Biol. 69, 522–530 [PubMed] [Google Scholar]
- 26. Morten I. J., Gosal W. S., Radford S. E., Hewitt E. W. (2007) Investigation into the role of macrophages in the formation and degradation of β2-microglobulin amyloid fibrils. J. Biol. Chem. 282, 29691–29700 [DOI] [PubMed] [Google Scholar]
- 27. Püttmann M., Krug H., von Ochsenstein E., Kattermann R. (1993) Fast HPLC determination of serum free fatty acids in the picomole range. Clin. Chem. 39, 825–832 [PubMed] [Google Scholar]
- 28. Uji Y., Noma A., Shiraki M., Maeda M., Tsuji A., Okabe H. (1987) Separation and quantitation of plasma free fatty acids as phenacyl esters by HPLC. Biomed Chromatogr 2, 110–114 [DOI] [PubMed] [Google Scholar]
- 29. Liau C. Y., Chang T. M., Pan J. P., Chen W. L., Mao S. J. (2003) Purification of human plasma haptoglobin by hemoglobin-affinity column chromatography. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 790, 209–216 [DOI] [PubMed] [Google Scholar]
- 30. Ahmad M. F., Ramakrishna T., Raman B., Rao Ch. M. (2006) Fibrillogenic and non-fibrillogenic ensembles of SDS-bound human α-synuclein. J. Mol. Biol. 364, 1061–1072 [DOI] [PubMed] [Google Scholar]
- 31. Chiba T., Hagihara Y., Higurashi T., Hasegawa K., Naiki H., Goto Y. (2003) Amyloid fibril formation in the context of full-length protein. Effects of proline mutations on the amyloid fibril formation of β2-microglobulin. J. Biol. Chem. 278, 47016–47024 [DOI] [PubMed] [Google Scholar]
- 32. Naiki H., Higuchi K., Hosokawa M., Takeda T. (1989) Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal. Biochem. 177, 244–249 [DOI] [PubMed] [Google Scholar]
- 33. Poon S., Rybchyn M. S., Easterbrook-Smith S. B., Carver J. A., Pankhurst G. J., Wilson M. R. (2002) Mildly acidic pH activates the extracellular molecular chaperone clusterin. J. Biol. Chem. 277, 39532–39540 [DOI] [PubMed] [Google Scholar]
- 34. Dobson C. M. (2003) Protein folding and misfolding. Nature 426, 884–890 [DOI] [PubMed] [Google Scholar]
- 35. Kihara M., Chatani E., Sakai M., Hasegawa K., Naiki H., Goto Y. (2005) Seeding-dependent maturation of β2-microglobulin amyloid fibrils at neutral pH. J. Biol. Chem. 280, 12012–12018 [DOI] [PubMed] [Google Scholar]
- 36. Yamamoto S., Hasegawa K., Yamaguchi I., Tsutsumi S., Kardos J., Goto Y., Gejyo F., Naiki H. (2004) Low concentrations of sodium dodecyl sulfate induce the extension of beta 2-microglobulin-related amyloid fibrils at a neutral pH. Biochemistry 43, 11075–11082 [DOI] [PubMed] [Google Scholar]
- 37. Lee H. G., Casadesus G., Zhu X., Joseph J. A., Perry G., Smith M. A. (2004) Perspectives on the amyloid-β cascade hypothesis. J. Alzheimers Dis. 6, 137–145 [DOI] [PubMed] [Google Scholar]
- 38. Xue W. F., Hellewell A. L., Gosal W. S., Homans S. W., Hewitt E. W., Radford S. E. (2009) Fibril fragmentation enhances amyloid cytotoxicity. J. Biol. Chem. 284, 34272–34282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. García-García M., Argilés, Gouin-Charnet A., Durfort M., García-Valero J., Mourad G. (1999) Impaired lysosomal processing of β2-microglobulin by infiltrating macrophages in dialysis amyloidosis. Kidney Int. 55, 899–906 [DOI] [PubMed] [Google Scholar]
- 40. Hou F. F., Jiang J. P., Guo J. Q., Wang G. B., Zhang X., Stern D. M., Schmidt A. M., Owen W. F., Jr. (2002) Receptor for advanced glycation end products on human synovial fibroblasts. Role in the pathogenesis of dialysis-related amyloidosis. J. Am. Soc. Nephrol. 13, 1296–1306 [DOI] [PubMed] [Google Scholar]
- 41. Ohkuma S., Poole B. (1978) Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. U.S.A. 75, 3327–3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kad N. M., Thomson N. H., Smith D. P., Smith D. A., Radford S. E. (2001) β2-Microglobulin and its deamidated variant, N17D form amyloid fibrils with a range of morphologies in vitro. J. Mol. Biol. 313, 559–571 [DOI] [PubMed] [Google Scholar]
- 43. McParland V. J., Kad N. M., Kalverda A. P., Brown A., Kirwin-Jones P., Hunter M. G., Sunde M., Radford S. E. (2000) Partially unfolded states of β2-microglobulin and amyloid formation in vitro. Biochemistry 39, 8735–8746 [DOI] [PubMed] [Google Scholar]
- 44. Naiki H., Hashimoto N., Suzuki S., Kimura H., Nakakuki K., Gejyo F. (1997) Establishment of a kinetic model of dialysis-related amyloid fibril extension in vitro. Amyloid 4, 223–232 [Google Scholar]
- 45. Takami M. (1993) Catabolism of heme moiety of hemoglobin·haptoglobin in rat liver cells in vivo. J. Biol. Chem. 268, 20335–20342 [PubMed] [Google Scholar]
- 46. Andersen C. B., Torvund-Jensen M., Nielsen M. J., de Oliveira C. L., Hersleth H. P., Andersen N. H., Pedersen J. S., Andersen G. R., Moestrup S. K. (2012) Structure of the haptoglobin-haemoglobin complex. Nature 489, 456–459 [DOI] [PubMed] [Google Scholar]
- 47. Bucci E., Malak H., Fronticelli C., Gryczynski I., Lakowicz J. R. (1988) Resolution of the lifetimes and correlation times of the intrinsic tryptophan fluorescence of human hemoglobin solutions using 2-GHz frequency-domain fluorometry. J. Biol. Chem. 263, 6972–6977 [PubMed] [Google Scholar]
- 48. Benesch R. E., Ikeda S., Benesch R. (1976) Reaction of haptoglobin with hemoglobin covalently cross-linked between the αβ dimers. J. Biol. Chem. 251, 465–470 [PubMed] [Google Scholar]
- 49. Fujimoto N., Wada N., Akiyama M., Tajima S., Ishibashi A., Miyakawa S. (2002) Coexistence of β2 microglobulin and λ light chain in amyloid fibrils of dialysis-unrelated plasma cell dyscrasia-associated systemic amyloidosis. Br. J. Dermatol. 147, 549–553 [DOI] [PubMed] [Google Scholar]
- 50. Fujimoto N., Yajima M., Ohnishi Y., Tajima S., Ishibashi A., Hata Y., Enomoto U., Konohana I., Wachi H., Seyama Y. (2002) Advanced glycation end product-modified β2-microglobulin is a component of amyloid fibrils of primary localized cutaneous nodular amyloidosis. J Invest. Dermatol. 118, 479–484 [DOI] [PubMed] [Google Scholar]
- 51. Smith D. P., Radford S. E., Ashcroft A. E. (2010) Elongated oligomers in β2-microglobulin amyloid assembly revealed by ion mobility spectrometry-mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 107, 6794–6798 [DOI] [PMC free article] [PubMed] [Google Scholar]